Abstract
Background:
Guidelines on the management of aneurysmal subarachnoid hemorrhage (aSAH) recommend euvolemia, whereas hypervolemia may cause harm. We investigated whether high early fluid input is associated with delayed cerebral ischemia (DCI), and if fluid input can be safely decreased using transpulmonary thermodilution (TPT).
Methods:
We retrospectively included aSAH patients treated at an academic intensive care unit (2007-2011; cohort 1) or managed with TPT (2011-2013; cohort 2). Local guidelines recommended fluid input of 3 L daily. More fluids were administered when daily fluid balance fell below +500 mL. In cohort 2, fluid input in high-risk patients was guided by cardiac output measured by TPT per a strict protocol. Associations of fluid input and balance with DCI were analyzed with multivariable logistic regression (cohort 1), and changes in hemodynamic indices after institution of TPT assessed with linear mixed models (cohort 2).
Results:
Cumulative fluid input 0 to 72 hours after admission was associated with DCI in cohort 1 (n=223; odds ratio [OR] 1.19/L; 95% confidence interval 1.07-1.32), whereas cumulative fluid balance was not. In cohort 2 (23 patients), using TPT fluid input could be decreased from 6.0 ± 1.0 L before to 3.4 ± 0.3 L; P = .012), while preload parameters and consciousness remained stable.
Conclusion:
High early fluid input was associated with DCI. Invasive hemodynamic monitoring was feasible to reduce fluid input while maintaining preload. These results indicate that fluid loading beyond a normal preload occurs, may increase DCI risk, and can be minimized with TPT.
Keywords: aneurysmal subarachnoid hemorrhage, delayed cerebral ischemia, fluid management, transpulmonary thermodilution, hypervolemia
Introduction
Delayed cerebral ischemia (DCI) after aneurysmal subarachnoid hemorrhage (aSAH) affects approximately 30% of patients.1 DCI typically develops between days 4 and 14 after ictus2 and may progress to cerebral infarction which is associated with poor outcome.3,4 Since hypovolemia is associated with DCI, standard management includes maintenance of euvolemia.5,6 Nevertheless, ascertainment of euvolemia is problematic in clinical practice but highly relevant for several reasons. Guidelines recommend using meticulous fluid balance monitoring to guide fluid management. However, fluid balance has been shown to be poorly indicative of volume status.7 In addition, euvolemia as a fluid management goal is subject to interpretation, which is illustrated by highly variable maintenance fluid practices in aSAH across neurocritical care units.8–10 In clinical practice, many patients with aSAH receive excessive fluids, with the aim to maintain a positive fluid balance. However, excessive fluid administration may result in more systemic complications (eg, congestive heart failure and pulmonary deterioration).11–13 In addition, a recent overview of the current literature suggested that excessive fluids might also be detrimental to neurological outcomes.10 In contrast, hypervolemic therapy—as part of triple-H therapy—has long been regarded as beneficial rather than potentially harmful in aSAH.10 Hypervolemia, which may be defined as fluid input exceeding the amount necessary for adequate organ perfusion, may therefore be an ill-recognized cause of harm to the brain. Since fluid management in aSAH still relies importantly on fluid balance, it is clinically relevant to assess whether excessive fluids are beneficial or harmful with regard to neurological clinical course. When “hypervolemia” as defined above is a frequently occurring and undesirable consequence of aiming for positive fluid balances, one may hypothesize that hemodynamic monitoring may help restricting fluid input without compromising adequate cardiac preload and cerebral blood flow.
The main objective of this study was therefore to investigate whether high early fluid input and fluid balances within 72 hours after admission are associated with the occurrence of DCI in patients with aSAH. Our secondary objective was to report on the feasibility of decreasing fluid input guided by cardiac output monitoring with transpulmonary thermodilution (TPT).
Materials and Methods
Study Design and Population
In this report, we describe 2 thematically related but separate studies. A schematic representation of the design and aims of the separate cohorts is shown in Figure 1. The first study was a retrospective cohort study of consecutively admitted patients with aSAH (cohort 1) to the intensive care unit (ICU) of a university hospital (Erasmus MC, University Medical Center, Rotterdam, the Netherlands) between October 2007 and October 2011, aiming to investigate whether high early fluid input or positive fluid balances were associated with DCI. Because preliminary analyses of cohort 1 showed that high early fluid input was associated with DCI,14 we instituted a fluid management protocol using fluid responsiveness with TPT using the PiCCO device (Pulsion Medical Systems SE, Feldkirchen, Germany), assuming that a reduction in excessive fluid input while maintaining adequate cardiac preload might be possible. The second study (cohort 2) concerned the first series of patients with aSAH (April 2011-September 2013, admitted to the same unit) managed with this newly instituted TPT protocol (Supplemental File A) and was aimed at retrospectively assessing changes in fluid input and balances in the days before versus after TPT.
Figure 1.
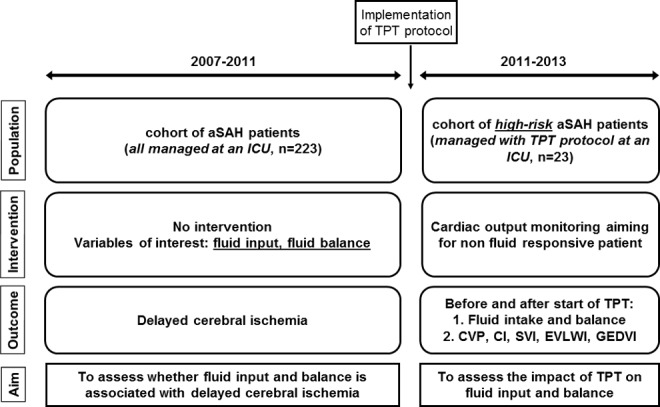
Schematic representation of the study design. TPT, transpulmonary thermodilution; aSAH, acute subarachnoid hemorrhage; ICU, intensive care unit; CVP, central venous pressure; CI, cardiac index; SVI, stroke volume index; EVLWI, extravascular lung water index; GEDVI, global end-diastolic volume index.
The inclusion criteria for cohort 1 were 18 years or older, aSAH, and admission to hospital ≤48 hours after ictus. The exclusion criteria were heart failure known from medical history, renal insufficiency (creatinine > 150 µmol/L), pregnancy, and death within 48 hours after admission. For cohort 2, the inclusion criteria were similar to the indications for TPT monitoring according to the fluid management protocol and concerned high-risk patients (detailed in Supplemental Files A and B). Briefly, these criteria concerned lower than expected blood pressure or highly negative fluid balance, signs of pulmonary or cardiac dysfunction, or progressive neurological deterioration due to DCI. Patients in both cohorts were identified through a hospital health service code indicating subarachnoid hemorrhage. Institutional medical ethics committee approval for both cohort studies was obtained and informed consent was not necessary, given the observational nature of the studies and anonymization of patient data in accordance with Dutch legislation. Due to the retrospective nature, we did not perform a power analysis and used a sample size of convenience.
Diagnosis and Patient Management
All patients were routinely managed at an ICU. In both cohorts, patients were evaluated with head computed tomography (CT) and CT angiography on admission. When no blood was seen on CT, a lumbar puncture was performed >12 hours after ictus for spectrophotometric analysis of cerebrospinal fluid (CSF). During the inclusion period for cohort 1, coiling procedures were performed by a regional team of interventional neuroradiologists. Stable patients were temporarily transferred to a different hospital for endovascular treatment when an interventional neuroradiologist was not available within 24 hours in the admitting hospital (Erasmus MC). A detailed description of patient management during the ICU admission in the 2 cohorts is given in Supplemental File B.
Data Collection and Outcomes
For both cohorts, data were collected from the ICU patient data management system and electronic patient records. Fluid input included all infusion fluids (including pharmaceuticals, blood products, and intraoperative fluids), tube feeding, and normal diet. Fluid losses included urine output, intraoperative blood loss, gastrointestinal losses, and CSF from intrathecal drains. Insensible loss was not accounted for in the analyses. Fluid balance was calculated by subtracting fluid loss from input.
In cohort 1, fluid input, loss, and balance were collected for the first 3 days after admission (day 1: 0-24 hours, day 2: 24-48 hours, and day 3: 48-72 hours). Admission CT scans were evaluated for Hijdra sum scores.15 The primary outcome was DCI defined by CT infarction, clinical deterioration, or both without other cause, according to recently proposed consensus criteria.1,16 Two authors (L.J.M.V. and M.v.d.J.) assessed the primary outcome. During outcome assessments, the authors were blinded to the daily fluid data. Consensus on the outcomes was obtained by discussion in case of initial disagreement. Glasgow outcome score (GOS) was assessed between 3 and 6 months after admission to the hospital as a secondary outcome. When GOS could not be retrieved from our electronic patient records, we sent a letter to the general practitioner to request the relevant information.
In cohort 2, fluid data and Glasgow coma score (GCS) were collected over a period from 3 days before until 3 days after TPT initiation. The TPT parameters—cardiac index (CI), stroke volume index (SVI), global end-diastolic volume index (GEDVI), and extravascular lung water index (EVLWI)—were collected during the study period and the daily average values recorded. Central venous pressure (CVP) measurements were collected at least from the day before TPT initiation until 3 days after. In this cohort, DCI was assessed as defined in cohort 1 by 1 author (M.E.) who was blinded for other clinical data. In contrast to cohort 1, the primary outcome was the difference in fluid parameters before versus after the start of TPT monitoring.
Statistical Analysis
Data were summarized as number with percentage (categorical), as median with interquartile range (ordinal), and as mean (standard error; continuous). Imputation of missing values in the fluid parameters and Hijdra sum scores in cohort 1 was performed with single imputation with regression based on relevant covariates and outcome (Supplemental File C). Patients with DCI and without DCI in cohort 1 were compared using the Student t test, Mann-Whitney U test, chi-square test, or Fisher exact test. In cohort 1, logistic regression models were created with cumulative fluid input or cumulative fluid balance during the first 24, 48, and 72 hours of ICU admission and previously identified independent predictors of DCI (age, gender, World Federation of Neurosurgical Societies [WFNS] grading score at admission, and Hijdra sum scores on initial CT scan)17,18 as covariables. Hijdra scores were dichotomized at their median, and WFNS was dichotomized into good (WFNS, 1-3) and poor (WFNS, 4-5) grades for the analyses. Sensitivity analyses with cerebral infarction on CT due to DCI with or without clinical signs and a secondary analysis with GOS as outcome were performed. Interaction between variables was assessed in each model. To assess whether the relation between fluid input and balance was nonlinear, that is, whether there was a specific cutoff in the effect of fluid on outcome, we did similar analyses with fluid input as covariables dichotomized on a cutoff of 3, 4, and 5 L daily. In cohort 2, fluid and hemodynamic parameters were compared before and after TPT using linear mixed models, with day 3 after initiation of TPT as the reference. The course of patients’ GCSs before and after TPT was assessed with Wilcoxon signed rank test. A 2-sided P value of<.05 was considered statistically significant. All statistical analyses were performed using SPSS 20.0.0 (IBM, Chicago, Illinois).
Results
Cohort 1
We included 223 consecutive patients with aSAH, of whom 91 (41%) developed DCI. General characteristics of patients with and without DCI are reported in Table 1. In total, 119 observations (18%) of fluid input data, 119 observations (18%) of fluid loss data, 8 (3.6%) Hijdra cistern sum scores, and 12 (5.4%) of Hijdra ventricle sum scores were missing and imputed. Mean hemoglobin (Hb) level at days 2 and 3 was lower, and blood pressure at day 1 was higher in patients who developed DCI versus those who did not.
Table 1.
General Characteristics of Cohort 1.a
| Variable | No DCI | DCI | P |
|---|---|---|---|
| N | 132 | 91 | |
| Female | 82 (62) | 61 (67) | .480 |
| Age (year) | 55 ± 1.1 | 57 ± 1.5 | .289 |
| Loss of consciousness at ictus | 53 (41) | 55 (61) | .003 |
| ICU admission within 24 hours | 125 (95) | 85 (94) | .761 |
| Admission GCS | 14 (13-15) | 13 (6-15) | .001 |
| Transferred for intervention within 72 hours | 59 (45) | 29 (32) | .054 |
| Aneurysm location | |||
| Anterior circulation | 101 (77) | 72 (79) | .744 |
| Posterior circulation | 23 (17) | 17 (19) | .860 |
| No aneurysm found | 8 (6) | 2 (2) | .206 |
| Hijdra cistern sum score | 16 (9-20) | 20 (14-23) | .001 |
| Hijdra ventricular sum score | 2 (0-4) | 3 (0-6) | .010 |
| Treatment day | 1.8 ± 0.2 | 2.0 ± 0.4 | .911 |
| Aneurysm treatment mode | |||
| Coiling | 85 (64) | 44 (48) | .019 |
| Clipping | 31 (24) | 25 (28) | .532 |
| No occlusion | 16 (12) | 22 (24) | .029 |
| Day of DCI diagnosis | 8 ± 0.5 | ||
| DCI diagnosis based on | |||
| CT only | 34 (37) | ||
| Clinical signs only | 31 (34) | ||
| Both CT and clinical signs | 26 (29) | ||
| Daily mean arterial pressure (mm Hg) | |||
| Day 1 | 93.7 ± 1.1 | 99.3 ± 1.4 | .002 |
| Day 2 | 97.2 ± 1.2 | 101.0 ± 1.8 | .072 |
| Day 3 | 101.0 ± 1.3 | 104.0 ± 1.7 | .170 |
| Mean hemoglobin (mmol/L) | |||
| Day 1 | 7.97 ± 0.08 | 7.82 ± 0.10 | .251 |
| Day 2 | 7.45 ± 0.08 | 7.15 ± 0.13 | .050 |
| Day 3 | 7.35 ± 0.09 | 6.95 ± 0.10 | .009 |
| Mean heart rate (beats per minute) | |||
| Day 1 | 71.8 ± 1.1 | 73.8 ± 1.5 | .303 |
| Day 2 | 69.7 ± 1.1 | 71.4 ± 1.7 | .389 |
| Day 3 | 71.5 ± 1.2 | 72.8 ± 1.7 | .535 |
| Lowest peripheral oxygen saturation (%) | |||
| Day 1 | 89.8 ± 0.95 | 89.6 ± 0.98 | .875 |
| Day 2 | 92.7 ± 0.52 | 91.7 ± 0.88 | .336 |
| Day 3 | 91.6 ± 0.58 | 91.6 ± 0.58 | .943 |
| GOS follow-up (months) | 3.5 ± 0.1 | 2.8 ± 0.2 | .008 |
| GOS | <.001 | ||
| Death | 11 (9) | 33 (39) | |
| Persistent vegetative state | 0 | 1 (1) | |
| Severe disability, dependent | 2 (2) | 16 (19) | |
| Moderate disability, independent | 21 (17) | 10 (12) | |
| Good recovery | 88 (72) | 25 (29) | |
| 6-month mortality | 11 (9) | 33 (38) | <.001 |
Abbreviations: DCI, delayed cerebral ischemia; ICU, intensive care unit; GCS, Glasgow coma score; CT, computed tomography; GOS, Glasgow outcome scale.
aData are reported as mean ± standard error, median (interquartile range), or number (percentage) where appropriate.
In patients who later developed DCI, fluid input was higher on day 1 (DCI: 4.9 ± 0.19 L; no DCI: 4.4 ± 0.13 L; P = .005) and day 2 after admission (DCI: 5.0 ± 0.21 L; no DCI: 4.2 ± 0.12 L; P = .004; Figure 2). Fluid balance did not differ between groups. In multivariable logistic regression models, cumulative fluid input was associated with an increased risk of DCI (0-24 hours: odds ratio [OR] 1.22/L, 95% confidence interval [CI] 1.01-1.46; 0-48 hours: OR 1.26/L, 95% CI 1.10-1.44; and 0-72 hours: OR 1.19/L, 95% CI 1.07-1.32; Table 2). There was no association between cumulative fluid balances and DCI (0-24 hours: OR 1.09/L, 95% CI 0.90-1.32; 0-48 hours: OR 1.07/L, 95% CI 0.95-1.21; and 0-72 hours: OR 1.06/L, 95% CI 0.97-1.17). The logistic regression models including fluid input dichotomized at 3, 4, or 5 L are reported in Supplemental File D. Higher fluid input was still associated with increased risk of DCI in these analyses, although specific cutoffs were not evident. The univariable analysis for the fluid variables is shown in Supplemental File E. Adding Hb level and blood pressure as covariables to the multivariable analyses did not change the associations between fluid intake and DCI (data not shown). No significant interactions were found between the independent variables.
Figure 2.
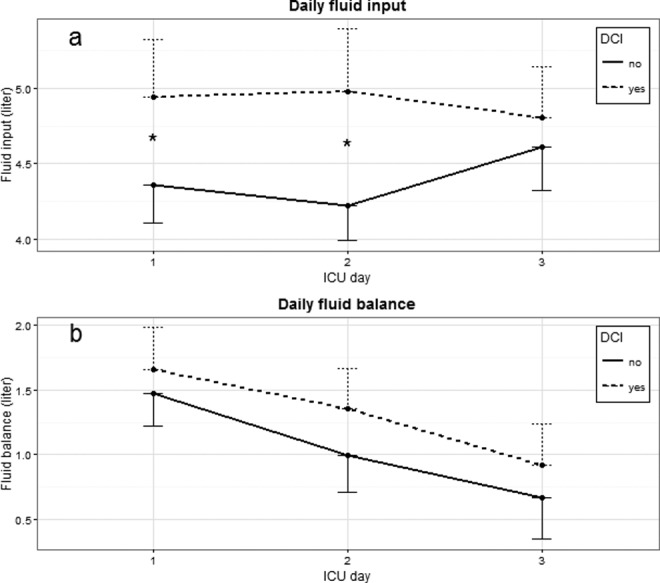
Daily fluid parameters in cohort 1. Data are represented as mean with 95% CI as 1-sided error bar. Differences between patients with and without DCI are indicated in figures: *P < .01. ICU, intensive care unit; DCI, delayed cerebral ischemia; CI, confidence interval.
Table 2.
Multivariable Logistic Regression Models for Cumulative Fluid Input and Balance Data From Cohort 1 with DCI as Outcome.
| Fluid Input | Fluid Balance | ||||
|---|---|---|---|---|---|
| Variable | OR | 95% CI | Variable | OR | 95% CI |
| Age (year) | 1.01 | 0.99-1.04 | Age (year) | 1.01 | 0.99-1.03 |
| Gender (female) | 1.33 | 0.73-2.43 | Gender (female) | 1.24 | 0.69-2.24 |
| Admission WFNS (>3) | 2.30 | 1.24-4.27 | Admission WFNS (>3) | 2.62 | 1.43-4.81 |
| Hijdra cistern score (≥17) | 2.23 | 1.25-3.98 | Hijdra cistern score (≥ 17) | 2.17 | 1.22-3.86 |
| Hijdra ventricular score (≥2) | 1.11 | 0.60-2.05 | Hijdra ventricular score (≥ 2) | 1.14 | 0.62-2.10 |
| Fluid input 0-24 hour L | 1.22 | 1.01-1.46 | Fluid balance 0-24 hour (L) | 1.09 | 0.90-1.32 |
| Age (year) | 1.01 | 0.99-1.04 | Age (year) | 1.01 | 0.99-1.03 |
| Gender (female) | 1.33 | 0.72-2.45 | Gender (female) | 1.24 | 0.68-2.23 |
| Admission WFNS (>3) | 2.25 | 1.21-4.20 | Admission WFNS (>3) | 2.54 | 1.39-4.66 |
| Hijdra cistern score (≥17) | 2.22 | 1.23-3.99 | Hijdra cistern score (≥17) | 2.15 | 1.21-3.81 |
| Hijdra ventricular score (≥2) | 1.14 | 0.61-2.12 | Hijdra ventricular score (≥2) | 1.15 | 0.63-2.10 |
| Fluid input 0-48 hour (L) | 1.26 | 1.10-1.44 | Fluid balance 0-48 hour (L) | 1.07 | 0.95-1.21 |
| Age (year) | 1.01 | 0.99-1.04 | Age (year) | 1.01 | 0.99-1.03 |
| Gender (female) | 1.39 | 0.75-2.58 | Gender (female) | 1.24 | 0.69-2.25 |
| Admission WFNS (>3) | 2.45 | 1.31-4.56 | Admission WFNS (>3) | 2.50 | 1.36-4.59 |
| Hijdra cistern score (≥17) | 2.27 | 1.26-4.08 | Hijdra cistern score (≥ 17) | 2.20 | 1.24-3.91 |
| Hijdra ventricular score (≥2) | 1.17 | 0.63-2.18 | Hijdra ventricular score (≥ 2) | 1.11 | 0.60-2.04 |
| Fluid input 0-72 hour (L) | 1.19 | 1.07-1.32 | Fluid balance 0-72 hour (L) | 1.06 | 0.97-1.17 |
Abbreviations: WFNS: World Federation of Neurosurgical Societies grading score; OR, odds ratio; CI, confidence interval.
The sensitivity analysis with DCI infarction as the outcome yielded similar results as those with DCI, but cumulative fluid balances at 0 to 48 and 0 to 72 hours after admission were associated with cerebral infarction due to DCI (0-48 hours: OR 1.15/L, 95% CI 1.00-1.33, and 0-72 hours: OR 1.12/L, 95% CI 1.01-1.25; Supplemental File F). Secondary outcome analysis for GOS showed results similar to the analysis for fluid input and DCI (Supplemental File G).
Cohort 2
We included the first 23 patients with aSAH who had an indication for TPT according to the protocol (Supplemental File A). Table 3 shows some pertinent characteristics on demographics, aneurysm management, and outcomes. Eleven patients (48%) died within 30 days after ICU admission, and this high mortality relates to the inclusion of high-risk patients for TPT, including those with recent signs of DCI. Fluid data from day −3 to day 3 relative to TPT initiation were available for 11, 10, 21, 23, 21, 20, and 20 patients, respectively (eg, only 11/23 patients had been admitted with available fluid data, 3 days before TPT initiation).
Table 3.
General Characteristics at Start of Invasive Hemodynamic Monitoring in Cohort 2.
| Variable | |
|---|---|
| N | 23 |
| Female | 19 (83) |
| Age (year) | 55 ± 3.4 |
| GCS | 8 (6-13) |
| DCI (infarction, clinical, and both) | 10 (44) |
| Hijdra cistern sum score | 14.6 ± 1.2 |
| Hijdra ventricular sum score | 4.4 ± 0.9 |
| Treatment mode | |
| Coiling | 9 (39) |
| Clipping | 11 (48) |
| No occlusion | 3 (13) |
| IHM initiated (days after admission) | 1.4 ± 0.4 |
| 30-day mortality | 11 (48) |
Abbreviations: DCI, delayed cerebral ischemia; GCS, Glasgow coma score; IHM, invasive hemodynamic monitoring by transpulmonary thermodilution.a Data are reported as mean ± standard error, median (interquartile range), or number (percentage) where appropriate.
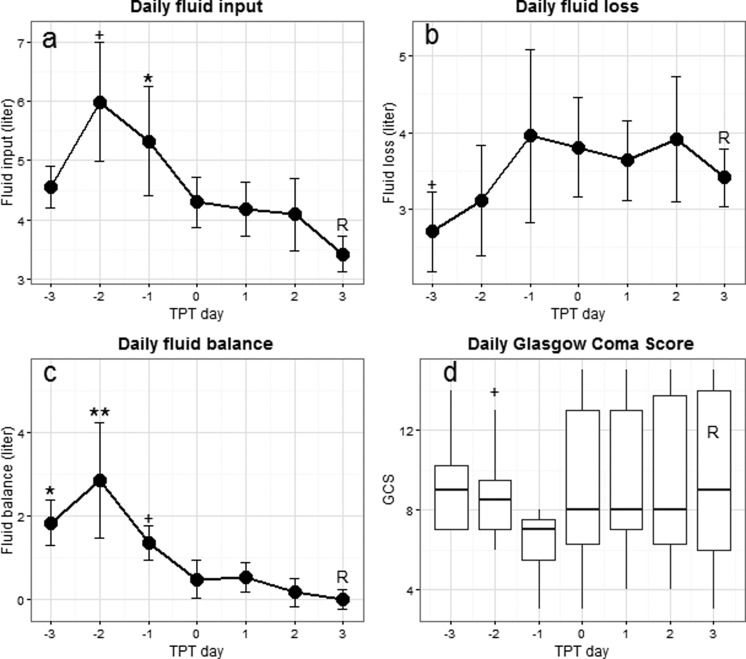
Daily fluid parameters and daily GCS are shown in Figure 3. Compared to day 3 (reference) after TPT initiation (fluid input: 3.4 ± 0.3 L), fluid input was higher on day −2 (6.0 ± 1.0 L; P = .012) and day −1 (5.3 ± 0.9 L; P = .008). Daily fluid loss was lower on day −3 when compared to day 3 (2.7 ± 0.5 L vs 3.4 ± 0.4 L; P = .049). As a result, daily fluid balance was higher on day −3 (1.8 ± 0.6 L; P = .003), day −2 (2.9 ± 1.4 L; P < .001), and day −1 (1.4 ± 0.4 L; P = .014) when compared to day 3 (0.0 ± 0.2 L). Median daily GCS on day −1 was not significantly lower than the median daily GCS on day 3 (GCS of 7 [5.5-7.5] vs 9 [6-14]; P = .18). Most hemodynamic parameters as measured during TPT (CVP, CI, and SVI) remained within the range of normal values in spite of significant reductions in fluid input and balances after start of TPT, except GEDVI and EVLWI which were higher than the normal ranges of 650 to 800 mL/m2 and 3.0 to 7.0 mL/kg, respectively (Supplemental File H).
Figure 3.
Daily fluid parameters and associated course of GCS in cohort 2. Data are represented as mean with standard error as 1-sided error bar and median with interquartile range for GCS. The TPT day 3 is used as the reference value (R) for the comparisons. + P < .05; *P < .01; **P < .001. TPT, transpulmonary thermodilution; GCS, Glasgow coma scale.
Discussion
The main finding of this study in patients with aSAH is that early high daily fluid input was independently associated with DCI and poor outcome. In addition, we showed that fluid loading beyond normal preload occurred in clinical practice and that it was feasible to significantly restrict fluid input while maintaining adequate preload with TPT in selected high-risk patients with aSAH. Taken together, these results corroborate the potential harm from fluid overload in patients with aSAH with regard to DCI and support further study on potential benefit of fluid restriction guided by hemodynamic monitoring.
We found that early high fluid input was associated with DCI. However, our cohort data, and specifically the analyses for fluid input of 3, 4, or 5 L daily, did not indicate a specific cutoff for fluid input beyond which DCI risk was increased. Therefore, firm conclusions about upper limits of fluid input for this cohort are not possible in spite of the robust finding that more fluids associate with DCI risk, indicating that fluid titration in aSAH should still be individualized. In a sensitivity analysis with cerebral infarction on CT as the outcome, positive net fluid balances also showed an association. Furthermore, the hemodynamic data in cohort 2 seem to indicate a state of hypervolemia, since GEDVI was higher than reference values. There are several possible explanations why high fluid input is associated with DCI.10 First, increased “fluid throughput” may cause fluids to accumulate in the interstitial space when the blood–brain barrier is damaged, which may impede local oxygen diffusion to neurons.19–21 Second, hemodilution as a consequence of fluid loading may decrease shear stress in the cerebral arteries, which may be detrimental to the integrity of the blood–brain barrier,22 and lower Hb levels may contribute to DCI due to decreased oxygen transport capacity of the blood, in line with our findings. Lastly, in cohort 1, as fluid input increased, fluid loss did not increase equally to match the higher fluid input (data not shown). Conversely, in cohort 2, when fluid input decreased, diuresis seemed to increase. Both observations may theoretically be explained by (renal) venous congestion (ie, high CVP).23 It has been postulated that venous congestion may also impede cerebral venous outflow and lead to intracranial pressure increase.24 Furthermore, several studies in brain injured critically ill patients have found CVP to be higher in patients with worse neurological outcomes.11,13,25 Similarly, a recent study found that higher CVP was associated with lower brain tissue oxygen saturation and worse outcome in postcardiac arrest patients.26 We acknowledge that these data do not provide proof and should be considered as hypothesis generating, with regard to the pathophysiologic role of venous congestion in DCI due to excessive fluids.
Our findings substantiate previous findings. Several investigators have described early mean daily fluid input varying from 3.3 to 6.6 L and daily fluid balances varying from −0.6 to +2.1 L.7,25,27–30 However, studies investigating the relation between fluid management and DCI are scarce.25,29,31,32 Two studies investigating the effect of early goal-directed hemodynamic management reported that mean daily fluid input was 2.7 L in the first 3 days with mean fluid balances between −0.5 and 0.5 L when using TPT.33,34 Using goal-directed hemodynamic management, less fluid was infused and fewer patients had DCI than in the conventional treatment group in line with our findings.34 The association of fluid input but not fluid balance with DCI is also in line with previous reports.28,33
Some limitations of our study should be considered. Due to the retrospective nature, potential unmeasured confounding factors and retrieval of relevant patient data are an inherent threat to the associations found in cohort 1. Consequently, causality between high fluid input and DCI cannot be proven with this retrospective study. Another limitation is the fact that we did not differentiate between or adjust for crystalloids versus synthetic colloids, since DCI has been associated with the use of synthetic colloids.29,35 However, these compounds were administered mainly in case of clinical deteriorations due to DCI as per our protocol at the time of the study period and not typically in the first 3 days when fluid data were collected for this study. Furthermore, we did not adjust fluid parameters for weight. In cohort 2, due to the variable start of the TPT protocol, in relation to clinical admission, missing/unavailable values were unavoidable (eg, when TPT was initiated soon after admission). Finally, our institutional fluid management protocol used in cohort 2 is based on our best clinical practice and the scarce literature available33,34 but has not been validated outside our ICU. The patients in cohort 2 are those more likely to develop DCI, so the generalizability of our protocol to low-risk patients with aSAH is uncertain. Because both cohorts are from a single center and due to the sample size used, the overall generalizability may be limited.
The strengths of our study include a consecutive series of patients managed at an ICU with detailed data on fluid management and prognostic factors as well as assessment of the primary outcome (DCI) according to recently proposed criteria.1,16 Second, we imputed missing data, with multiple imputations enhancing the statistical power. Finally, our data are in line with the recent notion that excessive fluid input is an established risk factor for adverse outcome in nonneurological critically ill patients36 and patients with aSAH in particular.11,29,33,34,37
A guideline endorsed by the American Heart Association advised to maintain euvolemia.5 However, establishing euvolemia is difficult when clear definitions are absent. This is reflected by the fact that mean daily fluid input and fluid balance in cohort 1 exceeded the predefined targets in our institutional protocol. Similarly, the hemodynamic data in cohort 2 suggested patients were hypervolemic when TPT was initiated. A multidisciplinary consensus statement recommended the use of hemodynamic monitoring devices in aSAH, only in hemodynamically unstable patients.38 A practical approach based on previous literature10,39 and our results may be to aim for euvolemia by giving 2.5 to 3.5 L/d in most patients with a fluid balance around 0. Invasive monitoring may then be considered in patients in whom deviations from euvolemia are suspected and considered highly detrimental, or in case of signs of stress cardiomyopathy, neurogenic pulmonary edema, excessive diuresis, or progressive DCI. Of note, with monitoring, the ambiguous term “euvolemia” might best be replaced by “adequate preload.”
Conclusion
High early daily fluid input is associated with DCI after aSAH. We showed the feasibility of a protocol using TPT to significantly reduce fluid input and balance without negatively impacting on preload parameters and GCS. Further study seems warranted to determine whether and when hemodynamic monitoring can help establishing both restricted fluid management and improved clinical outcomes.
Supplemental Material
Supplemental Material, 2017.08.01.JICM_Additional_files_B-H_(1) for High Early Fluid Input After Aneurysmal Subarachnoid Hemorrhage: Combined Report of Association With Delayed Cerebral Ischemia and Feasibility of Cardiac Output–Guided Fluid Restriction by Leonie J. M. Vergouw, Mohamud Egal, Bas Bergmans, Diederik W. J. Dippel, Hester F. Lingsma, Mervyn D. I. Vergouwen, Peter W. A. Willems, Annemarie W. Oldenbeuving, Jan Bakker and Mathieu van der Jagt in Journal of Intensive Care Medicine
Supplemental Material
Supplemental Material, Additional_file_A_(1) for High Early Fluid Input After Aneurysmal Subarachnoid Hemorrhage: Combined Report of Association With Delayed Cerebral Ischemia and Feasibility of Cardiac Output–Guided Fluid Restriction by Leonie J. M. Vergouw, Mohamud Egal, Bas Bergmans, Diederik W. J. Dippel, Hester F. Lingsma, Mervyn D. I. Vergouwen, Peter W. A. Willems, Annemarie W. Oldenbeuving, Jan Bakker and Mathieu van der Jagt in Journal of Intensive Care Medicine
Acknowledgments
The authors thank Dr J. Horn, Academic Medical Center, Amsterdam, and Dr P. J. W. Dennesen, Medisch Centrum Haaglanden, The Hague, the Netherlands, for their collection of data and contributions to an earlier version of the manuscript. They also thank Dr W. J. R. Rietdijk for his advice concerning the statistical analyses for cohort 2.
Footnotes
Authors’ Contribution: LJMV and ME collected, analyzed, and interpreted the data and wrote first drafts of the manuscript. BB collected the data for cohort 2 and interpreted the data. MDIV, PWAW, and AWO provided data and made substantial contributions to the manuscript. DWJD and HFL advised concerning the statistical analyses and HFL did the imputations. DWJD, HFL, and JB made substantial contributions to the intellectual content of the manuscript. MvdJ did the overall supervision of the study and contributed importantly to the design, analysis, interpretation, and writing up of the manuscript. All the authors read and approved the final manuscript. Leonie J. M. Vergouw and Mohamud Egal contributed equally to the manuscript. Research materials related to this paper may be available from the corresponding author.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. Vergouwen MD; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Vasospasm versus delayed cerebral ischemia as an outcome event in clinical trials and observational studies. Neurocrit Care. 2011;15(2):308–311. [DOI] [PubMed] [Google Scholar]
- 2. van Gijn J, Kerr RS, Rinkel GJ. Subarachnoid haemorrhage. Lancet. 2007;369(9558):306–318. [DOI] [PubMed] [Google Scholar]
- 3. Vergouwen MD, Etminan N, Ilodigwe D, Macdonald RL. Lower incidence of cerebral infarction correlates with improved functional outcome after aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2011;31(7):1545–1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vergouwen MD, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42(4):924–929. [DOI] [PubMed] [Google Scholar]
- 5. Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, et al. American Heart Association Stroke C, Council on Cardiovascular R, Intervention, Council on Cardiovascular N, Council on Cardiovascular S, Anesthesia, Council on Clinical C. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American heart association/American stroke association. Stroke. 2012;43(6):1711–1737. [DOI] [PubMed] [Google Scholar]
- 6. Hasan D, Vermeulen M, Wijdicks EF, Hijdra A, van Gijn J. Effect of fluid intake and antihypertensive treatment on cerebral ischemia after subarachnoid hemorrhage. Stroke. 1989;20(11):1511–1515. [DOI] [PubMed] [Google Scholar]
- 7. Hoff RG, van Dijk GW, Algra A, Kalkman CJ, Rinkel GJ. Fluid balance and blood volume measurement after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2008;8(3):391–397. [DOI] [PubMed] [Google Scholar]
- 8. Meyer R, Deem S, Yanez ND, Souter M, Lam A, Treggiari MM. Current practices of triple-H prophylaxis and therapy in patients with subarachnoid hemorrhage. Neurocrit Care. 2011;14(1):24–36. [DOI] [PubMed] [Google Scholar]
- 9. Velly LJ, Bilotta F, Fabregas N, Soehle M, Bruder NJ, Nathanson MH; European N, Critical Care Interest G. Anaesthetic and ICU management of aneurysmal subarachnoid haemorrhage: a survey of European practice. Eur J Anaesthesiol. 2015;32(3):168–176. [DOI] [PubMed] [Google Scholar]
- 10. van der Jagt M. Fluid management of the neurological patient: a concise review. Crit Care. 2016;20(1):126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Egge A, Waterloo K, Sjoholm H, Solberg T, Ingebrigtsen T, Romner B. Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery. 2001;49(3):593–605; discussion 605-596. [DOI] [PubMed] [Google Scholar]
- 12. Kim DH, Haney CL, Van Ginhoven G. Reduction of pulmonary edema after SAH with a pulmonary artery catheter-guided hemodynamic management protocol. Neurocrit Care. 2005;3(1):11–15. [DOI] [PubMed] [Google Scholar]
- 13. Lennihan L, Mayer SA, Fink ME, et al. Effect of hypervolemic therapy on cerebral blood flow after subarachnoid hemorrhage: a randomized controlled trial. Stroke. 2000;31(2):383–391. [DOI] [PubMed] [Google Scholar]
- 14. Vergouw L, Dippel DW, Bakker J, van der Jagt M. Association of fluid balance and delayed cerebral ischaemia after aneurysmal subarachnoid haemorrhage. Intensive Care Med. 2012;38(suppl 1): S111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hijdra A, Brouwers PJ, Vermeulen M, van Gijn J. Grading the amount of blood on computed tomograms after subarachnoid hemorrhage. Stroke. 1990;21(8):1156–1161. [DOI] [PubMed] [Google Scholar]
- 16. Vergouwen MD, Vermeulen M, van Gijn J, et al. Definition of delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage as an outcome event in clinical trials and observational studies: proposal of a multidisciplinary research group. Stroke. 2010;41(10):2391–2395. [DOI] [PubMed] [Google Scholar]
- 17. de Rooij NK, Greving JP, Rinkel GJ, Frijns CJ. Early prediction of delayed cerebral ischemia after subarachnoid hemorrhage: development and validation of a practical risk chart. Stroke. 2013;44(5):1288–1294. [DOI] [PubMed] [Google Scholar]
- 18. Dorhout Mees SM, Kerr RS, Rinkel GJ, Algra A, Molyneux AJ. Occurrence and impact of delayed cerebral ischemia after coiling and after clipping in the International Subarachnoid Aneurysm Trial (ISAT). J Neurol. 2012;259(4):679–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Altay O, Suzuki H, Hasegawa Y, et al. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke. 2012;43(9):2513–2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Orfanakis A, Brambrink AM. Long-term outcome call into question the benefit of positive fluid balance and colloid treatment after aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2013;19(2):137–139. [DOI] [PubMed] [Google Scholar]
- 21. Tranmer BI, Iacobacci RI, Kindt GW. Effects of crystalloid and colloid infusions on intracranial pressure and computerized electroencephalographic data in dogs with vasogenic brain edema. Neurosurgery. 1989;25(2):173–178; discussion 178-179. [DOI] [PubMed] [Google Scholar]
- 22. Cucullo L, Hossain M, Puvenna V, Marchi N, Janigro D. The role of shear stress in blood-brain barrier endothelial physiology. BMC Neurosci. 2011;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53(7):589–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Oddo M, Citerio G. ARDS in the brain-injured patient: what’s different? Intensive Care Med. 2016;42(5):790–793. [DOI] [PubMed] [Google Scholar]
- 25. Martini RP, Deem S, Brown M, et al. The association between fluid balance and outcomes after subarachnoid hemorrhage. Neurocrit Care. 2012;17(2):191–198. [DOI] [PubMed] [Google Scholar]
- 26. Ameloot K, Genbrugge C, Meex I, et al. Is venous congestion associated with reduced cerebral oxygenation and worse neurological outcome after cardiac arrest? Crit Care. 2016;20(1):146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Mori T, Katayama Y, Kawamata T, Hirayama T. Improved efficiency of hypervolemic therapy with inhibition of natriuresis by fludrocortisone in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 1999;91(6):947–952. [DOI] [PubMed] [Google Scholar]
- 28. Moro N, Katayama Y, Kojima J, Mori T, Kawamata T. Prophylactic management of excessive natriuresis with hydrocortisone for efficient hypervolemic therapy after subarachnoid hemorrhage. Stroke. 2003;34(12):2807–2811. [DOI] [PubMed] [Google Scholar]
- 29. Ibrahim GM, Macdonald RL. The effects of fluid balance and colloid administration on outcomes in patients with aneurysmal subarachnoid hemorrhage: a propensity score-matched analysis. Neurocrit Care. 2013;19(2):140–149. [DOI] [PubMed] [Google Scholar]
- 30. Lehmann L, Bendel S, Uehlinger DE, et al. Randomized, double-blind trial of the effect of fluid composition on electrolyte, acid-base, and fluid homeostasis in patients early after subarachnoid hemorrhage. Neurocrit Care. 2013;18(1):5–12. [DOI] [PubMed] [Google Scholar]
- 31. Hoff R, Rinkel G, Verweij B, Algra A, Kalkman C. Blood volume measurement to guide fluid therapy after aneurysmal subarachnoid hemorrhage: a prospective controlled study. Stroke. 2009;40(7):2575–2577. [DOI] [PubMed] [Google Scholar]
- 32. Katayama Y, Haraoka J, Hirabayashi H, et al. A randomized controlled trial of hydrocortisone against hyponatremia in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2007;38(8):2373–2375. [DOI] [PubMed] [Google Scholar]
- 33. Mutoh T, Kazumata K, Ajiki M, Ushikoshi S, Terasaka S. Goal-directed fluid management by bedside transpulmonary hemodynamic monitoring after subarachnoid hemorrhage. Stroke. 2007;38(12):3218–3224. [DOI] [PubMed] [Google Scholar]
- 34. Mutoh T, Kazumata K, Ishikawa T, Terasaka S. Performance of bedside transpulmonary thermodilution monitoring for goal-directed hemodynamic management after subarachnoid hemorrhage. Stroke. 2009;40(7):2368–2374. [DOI] [PubMed] [Google Scholar]
- 35. Tseng MY, Hutchinson PJ, Kirkpatrick PJ. Effects of fluid therapy following aneurysmal subarachnoid haemorrhage: a prospective clinical study. Br J Neurosurg. 2008;22(2):257–268. [DOI] [PubMed] [Google Scholar]
- 36. Genga K, Russell JA. Early liberal fluids for sepsis patients are harmful. Crit Care Med. 2016;44(12):2258–2262. [DOI] [PubMed] [Google Scholar]
- 37. Togashi K, Joffe AM, Sekhar L, et al. Randomized pilot trial of intensive management of blood pressure or volume expansion in subarachnoid hemorrhage (IMPROVES). Neurosurgery. 2015;76(2):125–134; discussion 134-125; quiz 135. [DOI] [PubMed] [Google Scholar]
- 38. Gress DR; Participants in the International Multi-Disciplinary Consensus Conference on the Critical Care Management of Subarachnoid Hemorrhage. Monitoring of volume status after subarachnoid hemorrhage. Neurocrit Care. 2011;15(2):270–274. [DOI] [PubMed] [Google Scholar]
- 39. Rinkel GJ. Medical management of patients with aneurysmal subarachnoid haemorrhage. Int J Stroke. 2008;3(3):193–204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, 2017.08.01.JICM_Additional_files_B-H_(1) for High Early Fluid Input After Aneurysmal Subarachnoid Hemorrhage: Combined Report of Association With Delayed Cerebral Ischemia and Feasibility of Cardiac Output–Guided Fluid Restriction by Leonie J. M. Vergouw, Mohamud Egal, Bas Bergmans, Diederik W. J. Dippel, Hester F. Lingsma, Mervyn D. I. Vergouwen, Peter W. A. Willems, Annemarie W. Oldenbeuving, Jan Bakker and Mathieu van der Jagt in Journal of Intensive Care Medicine
Supplemental Material, Additional_file_A_(1) for High Early Fluid Input After Aneurysmal Subarachnoid Hemorrhage: Combined Report of Association With Delayed Cerebral Ischemia and Feasibility of Cardiac Output–Guided Fluid Restriction by Leonie J. M. Vergouw, Mohamud Egal, Bas Bergmans, Diederik W. J. Dippel, Hester F. Lingsma, Mervyn D. I. Vergouwen, Peter W. A. Willems, Annemarie W. Oldenbeuving, Jan Bakker and Mathieu van der Jagt in Journal of Intensive Care Medicine