Abstract
Background and Objectives:
Currently, pancreatic cystic lesions (PCLs) are recognized with increasing frequency and have become a more common finding in clinical practice. EUS is challenging in the diagnosis of PCLs and evidence-based decisions are lacking in its application. This study aimed to develop strong recommendations for the use of EUS in the diagnosis of PCLs, based on the experience of experts in the field.
Methods:
A survey regarding the practice of EUS in the evaluation of PCLs was drafted by the committee member of the International Society of EUS Task Force (ISEUS-TF). It was disseminated to experts of EUS who were also members of the ISEUS-TF. In some cases, percentage agreement with some statements was calculated; in others, the options with the greatest numbers of responses were summarized.
Results:
Fifteen questions were extracted and disseminated among 60 experts for the survey. Fifty-three experts completed the survey within the specified time frame. The average volume of EUS cases at the experts' institutions is 988.5 cases per year.
Conclusion:
Despite the limitations of EUS alone in the morphologic diagnosis of PCLs, the results of the survey indicate that EUS-guided fine-needle aspiration is widely expected to become a more valuable method.
Keywords: EUS, pancreatic cystic lesion, survey
INTRODUCTION
Pancreatic cystic lesions (PCLs) are recognized with increasing frequency and have become a more common finding in clinical practice, because of the widespread use of advanced imaging modalities and the sharp drop in the mortality rate of pancreatic surgery.[1] The current consensus is that pancreatic cystic neoplasms (PCNs) constitute up to 60% of all PCLs, followed by injury-related and inflammation-related cysts (30%).[1] PCNs include intraductal papillary mucinous neoplasms (IPMNs), mucinous cystic neoplasms (MCNs), serous cystic neoplasms (SCNs), solid pseudopapillary neoplasms, cystic neuroendocrine neoplasms, ductal adenocarcinomas with cystic degeneration, and acinar cell cystic neoplasms.[2,3]
One study[4] found that 20% of resected PCNs are benign, even at a tertiary hospital, indicating the challenge involved in accurately diagnosing PCLs. Multiple modalities are widely used for detection and diagnosis, including computed tomography (CT), magnetic resonance imaging (MRI), endoscopic retrograde cholangiopancreatography, and EUS. EUS is currently accepted as the most valuable method in the detection and diagnosis of solid pancreatic lesions. However, for many aspects of the EUS management of cystic neoplasm of the pancreas, available data are currently insufficient to make evidenced-based decisions.[5] Here, we present the results of a survey of the use of EUS in the diagnosis of PCLs by a group of EUS experts, based on their own experience.
METHODS
A survey regarding the practice of EUS in the evaluation of PCLs was extracted from the published articles and drafted by the committee member of the International Society of EUS Task Force (ISEUS-TF).[6] We designed the survey to include the indications, complications, techniques, and yield of EUS-based diagnosis. The survey was disseminated to experts of EUS who are members of ISEUS-TF through the software SurveyMonkey (https://surveymonkey.com/). According to the guidance of the SurveyMonkey, we have limited the number of questions to 15 to ensure the response rates. Some questions were designed to assess the respondents' agreement with a statement, while the others were designed to assess the choices with the greatest numbers of responses. The responses were counted. The percentage agreements with statements were calculated in some cases; in others, the options with the greatest numbers of responses were summarized.
RESULTS
Fifteen survey questions were extracted and disseminated among 60 experts. Fifty-three experts completed the survey within the specified time frame. The geographical distribution of the experts is Asian group 58%, American group 19%, European group 11%, Oceania group 10%, and Africa group 2% [Figure 1]. The average volume of EUS cases at the experts' institutions is 988.5 cases per year. The results of the survey are listed in Figure 2.
Figure 1.
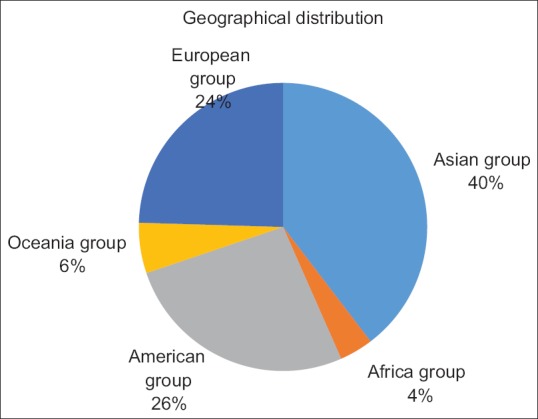
The reginal distribution of the responders
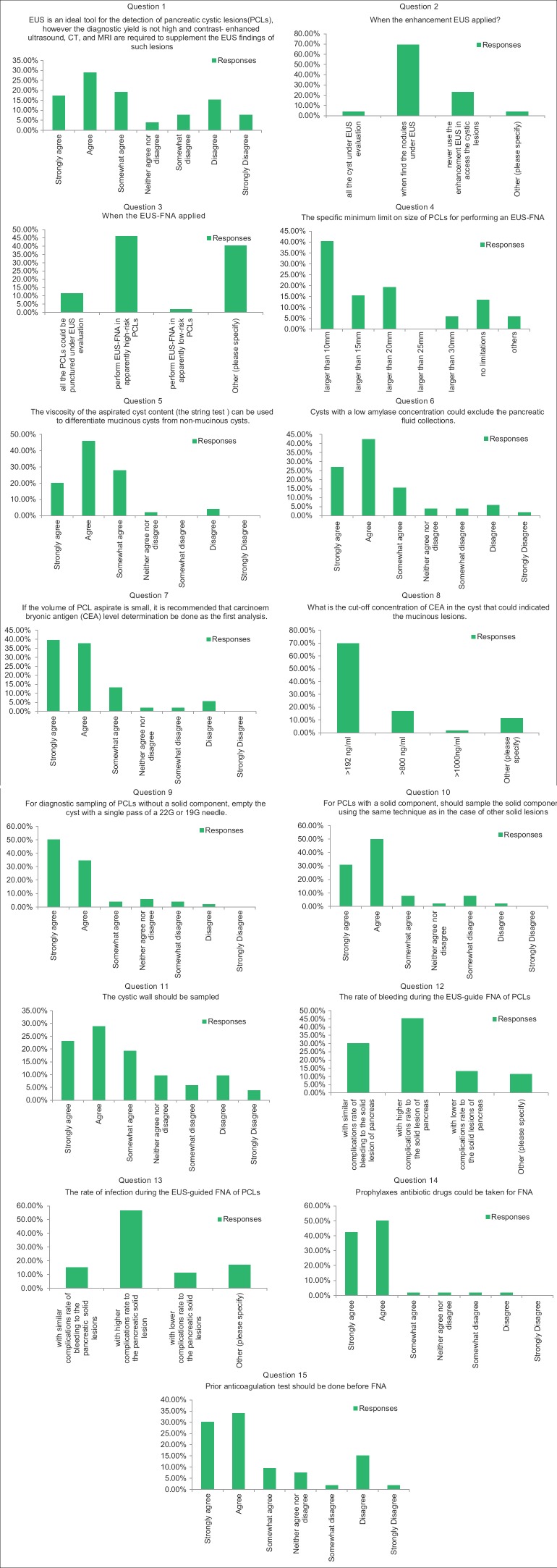
Figure 2.
The results of the survey
-
1.
EUS is an ideal tool for the detection of PCLs; however, the diagnostic yield is not high and contrast-enhanced ultrasound, CT, and MRI are required to supplement the EUS findings of such lesions.
Agreement: 65.39%; 34/52.
Supporting evidence and comments
In the consensus from European experts, it was stated that “CT and/or MRI imaging are indicated in all patients with cystic lesion of the pancreas for the differential diagnosis and for depicting signs suggestive of malignancy.”[7] Therefore, EUS is always performed as part of multi-modality diagnostic evaluation.
In 2004, Brugge et al. reported that the diagnostic yield of EUS imaging alone to differentiate mucinous versus nonmucinous cysts was not high; the sensitivity was 56% and specificity was 45%, resulting in an accuracy of 51%.[2] In 2015, the accuracies of EUS and cross-sectional imaging were compared for the preoperative diagnosis of PCLs.[8] The investigators concluded that MRI, when used as an additional diagnostic modality with multi-detector CT, increased the accuracy, sensitivity, and specificity, as well the ability to predict neoplastic cyst. In 2017, Zhang conducted a retrospective single-center study, using the new criteria to distinguish mucin and serum lesions; these new criteria demonstrated the accuracy of 82.93%.[9]
-
2.
When should EUS enhancement be applied?
Option with most responses: When nodules are found under EUS.
Agreement: 69.23%; 36/52.
Supporting evidence and comments
Some studies with limited numbers of patients have proven the usefulness of contrast enhanced (CE)-EUS in predicting the malignant potential of IPMN. According to Ohno et al., the mural nodules defined as blood flow-supplied protrusions were classified into four types; Type III and Type IV were highly associated with malignancy (88.9% and 91.7%, respectively). The diagnosis of malignancy in IPMNs with Types III or IV mural nodules showed a sensitivity of 60%, specificity of 92.9%, and accuracy of 75.9%.[10] Kurihara et al. found that the vessel shapes of the mural nodules depicted under CE-EUS were associated with size and pathological findings; these results suggested that CE-EUS with a contrast agent is a powerful modality with which to evaluate the malignancy potential of IPMN.[11] Harima et al. used CE-EUS to measure mural nodule height, which provided a highly accurate method for differentiating benign from malignant BD-IPMN (diagnostic accuracy increased to 98%).[12] In a prospective study, the diagnostic accuracy of CE-EUS in evaluating the mural nodes was as high as 94%, and the accuracy of severe atypical hyperplasia or invasive malignancy was 75%. Fujita et al. concluded that CE-EUS might be useful for avoiding overdiagnosis of BD-IPMN with mural nodule-like lesions. However, this method exhibited difficulty in distinguishing between clearly benign and clearly malignant lesions in BD-IPMN.[13] In addition, CH-EUS could help identify areas of malignant growth inside the cystic cavities, which could help in the targeting of EUS-guided fine-needle aspiration (FNA).[14]
-
3.
When should EUS-FNA be applied?
Option with most responses: EUS-FNA should be performed in apparently high-risk PCLs
Agreement: 46.15%; 24/52 (77.42%; 24/31).
Other responses
Furthermore, about 40.38% were responded with the choice of “Other (please specify).” According to the specify, 5 responses were mainly preferred to perform FNA in high-risk patients with the consideration of other conditions; 3 responses were never use the FNA in cystic lesions; 2 responses agreed to perform FNA in the solid part of the lesions; 2 responses agreed to perform FNA in all cysts and 5 responses considered to perform FNA depending on the clinical demanding.
Supporting evidence and comments
Japanese guidelines aim to directly subject high-risk lesions to surgery (to avoid neoplastic dissemination while performing EUS-FNA) and to perform EUS-FNA in apparently low-risk lesions. In contrast, American guidelines (both American Gastroenterological Association and American Society for Gastrointestinal Endoscopy) recommend EUS-FNA in apparently high-risk lesions, such that if they exhibit high-risk cytology, they can be subjected to surgery. Moreover, American guidelines permit lesions that appear to be low risk to simply be followed up (without performing EUS-FNA).
In 2015, a prospective study including 77 patients with PCLs found that the diagnostic accuracy of PCLs can be increased using FNA with analysis of cyst carcinoembryonic antigen (CEA) level, carbohydrate antigen 19-9 level, amylase level, mucin stain, and cytopathological examination.[15] In 2014, a prospective study that included 302 patients concluded that EUS-FNA impacts management in nearly 72% of IPCs, and has a large influence on the management strategy: either discharge rather than surgical resection, or surgery rather than the additional follow-up.[16] EUS-FNA increased diagnostic yield, compared with CT and MRI. The results of the EUS-guided sample could further alter the diagnosis and management of patients with PCLs. However, the specific role of EUS-FNA is unclear in some cases; some guidelines are critical of it, while others support it and enhance its use.
The American Gastroenterology Association suggests that pancreatic cysts with at least 2 high-risk features, such as size >3 cm, a dilated main pancreatic duct, or the presence of an associated solid component, should be examined with EUS-FNA (conditional recommendation, very low-quality evidence).[17]
-
4.
What is the specific minimum limit on the size of PCLs for performing EUS-FNA?
Option with most responses: Larger than 10 mm.
Agreement: 40.38%; 21/52.
Supporting evidence and comments
Aspirated samples from small cyst lesions were sufficiently limited that they were difficult to use for biochemical analyses, cytopathology examination, or other assays. From a 1-cm diameter cyst, a maximum of 0.5 ml sample could be aspirated. Thus far, no data are available regarding this issue. The option with the most responses is >10 mm in diameter. Therefore, patients with cyst lesions <10 mm in size may not benefit from FNA.
-
5.
The viscosity of the aspirated cyst content (the string test) can be used to differentiate mucinous cysts from nonmucinous cysts.
Agreement: 94%; 47/50.
Supporting evidence and comments
A simple approach to evaluate the viscosity of a sample is the “string sign,” which constitutes placement of a drop of aspirated fluid between two fingers, followed by spreading of those fingers. String sign positivity has been shown to be very specific for the diagnosis of mucinous lesions, with sensitivity and specificity values of 58% and 95%, respectively.[18]
-
6.
For cysts with a low amylase concentration, pancreatic fluid collection can be excluded.
Agreement: 84.62%; 44/52.
Supporting evidence and comments
A pooled review of 12 studies from 2005, all involving histologic diagnosis, found that cysts with amylase <250 U/L were serous cystadenoma, mucinous cystic adenoma (MCA), or mucinous cystadenocarcinoma (MCAC; sensitivity 44%, specificity 98%); this cutoff virtually excluded pseudocysts.[19] In one study from 2004, a cutoff value of >479 U/L for cyst amylase level showed a sensitivity of 73% and a specificity of 90% for distinguishing pseudocysts from cystic neoplasms.[20] In 2006, Linder et al. found that amylase levels were higher in pancreatic pseudocyst (7210 U/L), compared with cystic neoplasm (SCN, 679 U/L; MCN, 1605 U/L; MCAC, 569 U/L).[21] In a retrospective study published in 2015, Oppong et al. revealed that median amylase was significantly higher in benign cysts, compared with high-risk mucinous cysts (11429 IU/L vs. 113 IU/L; P <0.05).[22]
-
7.
If the volume of PCL aspirate is small, CEA level determination is recommended as an initial analysis.
Agreement: 90.57%; 48/53.
Supporting evidence and comments
Thus far, CEA is the most widely used and well-studied marker in pancreatic cyst fluid.[23] Approximately 0.2–1.0 mL of cyst fluid is required to assay for CEA. In 2004, a multi-center prospective study demonstrated that the accuracy of CEA (88 of 111, 79%) was significantly greater than the accuracy of EUS morphology (57 of 112, 51%) or cytology (64 of 109, 59%) (P < 0.05). There was no combination of tests (e.g., including CEA, CA 72-4, CA 125, CA 19-9, and CA 15-3) that provided greater accuracy than CEA alone (P < 0.0001). Of tested markers, cyst fluid CEA is the most accurate test available for the diagnosis of mucinous cystic lesions of the pancreas.[24]
-
8.
What is the cutoff concentration of CEA in a cyst that could indicate a mucinous lesion?
Option with most responses: CEA >192 ng/ml.
Agreement: 69.81%; 37/53.
Supporting evidence and comments
A multicenter prospective study by Brugge[19] found that CEA >192 ng/ml was diagnostic of a mucinous lesion. Alkaade, Chahla, and Levy[4] from the USA have extensively reviewed the accuracy, limitations, and issues with the use of cytology, viscosity, and CEA in the pancreatic cyst fluid. For CEA, pooled sensitivity was 63% (59%–67%) and specificity 88% (83%–91%).[25] Other investigations have described various cutoff values for CEA in the diagnosis of mucinous lesions. Van der Waaij et al. found that CEA >800 ng/ml is 79% accurate in the diagnosis of a mucinous lesion (benign and malignant). CEA >800 ng/mL strongly suggested MCA or MCAC (sensitivity 48%, specificity 98%). Some other values were mentioned by the experts, but these additional values have not been firmly established (e.g., CEA >1000 is highly indicative of a malignant cyst lesion; CEA 2-200 indicates inflammatory lesions such as pseudocysts).
-
9.
For diagnostic sampling of PCLs without a solid component, should the cyst be emptied with a single pass of a 22G or 19G needle?
Agreement: 88.46%; 46/52.
Supporting evidence and comments
Few studies have discussed whether the cyst should be emptied when performing diagnostic EUS-FNA of PCLs. The European Society of Gastrointestinal Endoscopy recommends that, for the diagnostic sampling of PCLs without a solid component, the cyst should be emptied with a single pass of a 22G or 19G needle (low-quality evidence, weak recommendation).[26]
-
10.
For PCLs with a solid component, should the solid component be sampled using the same technique as in other solid lesions?
Agreement: 88.46%; 46/52.
Supporting evidence and comments
In 2013, a multicenter Asian study revealed that the presence of solid cystic components and an increased number of needle passes during EUS-FNA were associated with a higher diagnostic yield of EUS-FNA. The investigators concluded that when a solid component was present in the cyst, use of more than one pass during EUS-FNA increased its diagnostic yield.[27] The statement was also weakly recommended by the European Society of Gastrointestinal Endoscopy, with low-quality evidence.[26]
-
11.
The cyst wall should be sampled.
Agreement: 71.15%; 37/52.
Supporting evidence and comments
From 2016 to 2018, three studies reported the value of sampling the cystic wall; all were retrospective studies. The success rate of the technique was 85.7%–100%; the clinical success rate was 71.4%. Notably, there were six cases of mild adverse events.[28,29,30]
-
12.
The rate of bleeding during the EUS-guide FNA of PCLs
Option with most responses: With a higher rate of bleeding complication than solid lesions of the pancreas.
Agreement: 45.28%; 24/53
-
13.
The rate of infection during the EUS-guided FNA of PCLs
Option with most responses: With a higher rate of infection complication than solid lesions of the pancreas.
Agreement: 56.60%; 30/53.
Supporting evidence and comments
The overall complication rate of EUS-FNA in prospective series ranges from 0% to 2.5%.[31] In 2005, Linda et al. performed a retrospective analysis of the complications.[32] A total of 603 patients with 651 pancreatic cysts were evaluated, complications were identified in 13 patients (2.2%, 13 of 603), with no patient requiring surgical management. Nearly half of the complications were pancreatitis; other rare complications included abdominal pain, infection, and retroperitoneal bleeding. EUS-guided pancreatic cyst aspiration exhibits a low complication rate, similar to that reported for solid pancreatic lesions.[32] No patient or cyst characteristics appear to be predictive of adverse events. In 2001, a total of 114 lesions were aspirated.[33] Complications were observed in 4 (1.2) patients with no severe or fatal incidents, acute pancreatitis in 3 patients, and pneumonia in 1 patient.
Yoon and Brugge[34] from the USA and Korea reviewed the safety of EUS-FNA of pancreatic cysts. The overall risk of complications was low, approximately 2%. They found that infectious complications can be mitigated by the use of prophylactic antibiotics.
-
14.
Prophylactic antibiotic drugs should be taken for FNA of PCLs
Agreement: 94.23%; 49/52.
Supporting evidence and comments
Both the American and European Societies of Gastrointestinal Endoscopy recommend antibiotic prophylaxis following EUS-FNA of cystic lesions to reduce the risk of infection.[4] In 2014, the UEG Journal published a cohort study, which concluded that single-dose piperacillin/tazobactam at the time of EUS-FNA of pancreatic cysts constitutes effective prophylaxis of septic cyst infection and can be conveniently provided as a single, periprocedural dose without further oral antibiotics.[35] However, routine use of prophylactic antibiotics for this indication is not free of adverse events and may not substantially reduce the risk of infection. Minimal data are available, including a lack of well-designed prospective trials, regarding the use of prophylactic antibiotic drug use in EUS-guided FNA of cystic lesions; moreover, the most efficacious route and regimen of administration are unknown. Thus, there remains controversy regarding antibiotic prophylaxis in EUS-FNA of PCLs.
-
15.
An anticoagulation test should be performed before FNA
Agreement: 73.58%; 39/53.
Supporting evidence and comments
Strong evidence is not yet available in support of coagulation tests before FNA. However, as recommended in all these guidelines, coagulation tests are recommended before EUS-FNA for patients with particular medical histories, such as a personal or family history suggestive of coagulation disorder, or those with a clear clinical indication. Furthermore, the incidence of severe bleeding in low-volume hospitals was five-fold higher than in medium-and high-volume hospitals (P = 0.045),[36] supporting the notion put forth previously that the frequencies of complications in such cases reflect a learning curve.[37] Thus, coagulation tests may be still needed before FNA in the low-volume hospitals. Although not evidence-based, platelet count and coagulation tests are performed before EUS-FNA in most centers, with platelet count <50,000/mm3 and international normalized ratio >1.5 considered contraindications for EUS-guided sampling.
DISCUSSION
EUS can scan proximally to the pancreas along the gastrointestinal tract, which enables high-resolution imaging of the pancreas. EUS provides an enhanced ability to study the cyst wall and the internal echo characteristics of a cystic pancreatic tumor. Septations, solid areas, mural nodules, and papillary projections, as well as connections to the main or side branches of the pancreatic duct, can be observed.[38,39] However, as noted in the survey, due to the overlapping morphological features among various cystic lesions, EUS morphological features did not appear sufficiently specific to differentiate between malignant and potentially malignant cysts. CT and MRI are required to supplement the EUS findings of such lesions.
EUS-FNA enables the use of aspirated samples for cytopathology examination and biochemical analyses, which provides an opportunity to further enhance diagnosis and medical decision-making.[40] When we survey about when to perform the FNA, we did not get the uniform responses. “To perform on the high-risk cyst” is the most voted one. Although it remains unsupported by the evidence, EUS-FNA has become the most highly anticipated technique in this field. Most exporters have agreed to sample the suspected cystic pancreatic neoplasm. Viscosity (string sign) and CEA levels in PCLs are the most accepted and widely used methods to identify mucinous lesions (with malignancy potential). The emerging application of cytology and various molecular markers, including KRAS and tumor suppressor gene mutations, GNAS oncogene, microRNAs, and various interleukins remain “hot” topics that require further proof. In addition to sampling cyst content, pathological study of the cystic wall provides further information for diagnosis. Most experts agreed with the use of cystic wall sampling to obtain pathological results. Traditional FNA sampling of the cystic wall is difficult with low technical and clinical success rates. Needle-based micro-forceps biopsy is a promising method, as is needle-based confocal laser endomicroscopy (CLE), which has enabled real-time imaging during EUS at the subcellular level, thus providing in vivo optical biopsy of the cystic wall. Needle-based cystoscopy (such as SpyGlass) could also be applied for cystic wall observation.[41] Although not mentioned in this survey, developments in this field are quite exciting and practical. Based on the FNA technique, minimally invasive nonsurgical methods may be applied for the treatment of PCLs.[42] The therapeutic aspects of EUS were not assessed in this study. The safety of EUS-FNA in PCLs has been reviewed. The overall risk of complications is low (approximately 2%), and is similar to that of solid lesions.
Pancreatitis is the main complication after aspiration; other complications include abdominal pain, infection, bleeding, and pneumonia. None of these complications are fatal or require surgical management. According to the guidelines, EUS-guided FNA of PCLs is considered a safe procedure, with a similar complication rate to that of solid lesions; the quality of this evidence is I (meta-analysis), and the recommendation is strong. In a systematic review by Zhendong, the overall morbidity as a result of adverse events of EUS-FNA was 2.66%. EUS-FNA is a safe procedure for the diagnosis of PCL and is associated with a relatively low incidence of adverse events. Most adverse events were mild, self-limiting, and did not require medical intervention.[43] However, in practice, as reported in the survey, most endoscopists puncture PCLs with caution. We selected bleeding and infection as representative complications in this survey. Prophylactic usage of antibiotic drugs is also accepted by most experts. Emptying of the cyst after puncture is another accepted way to avoid infection.
There are also some limitations in this study. The 15 questions did not cover all the issues referred to the EUS in the diagnosing of PCLs, and hence, another survey has been planned. The responses may vary between different regions of the experts, and this was not specifically analyzed in this study. Furthermore, surveys of what people think have their distinct limitations with regard to the actual practice.
CONCLUSION
According to the survey, it is widely accepted that despite the limitations of EUS alone for morphologic diagnosis of PCLs, EUS-FNA may become a more valuable method. The string sign and CEA level in cyst samples are used in the differential diagnosis of mucinous and nonmucinous lesions. Other EUS-FNA-based diagnosis methods, such as nCLE, cystoscopy, and micro-forceps, have shown great potential. Multicenter prospective studies with large samples are needed.
Financial support and sponsorship
This study was supported by the project of Technology engineering research center of Liaoning Province (Grant No. 2018225110); Natural Science Fund of Liaoning Province (Grant No. 20180530014).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Basturk O, Coban I, Adsay NV. Pancreatic cysts: Pathologic classification, differential diagnosis, and clinical implications. Arch Pathol Lab Med. 2009;133:423–38. doi: 10.5858/133.3.423. [DOI] [PubMed] [Google Scholar]
- 2.Brugge WR, Lauwers GY, Sahani D, et al. Cystic neoplasms of the pancreas. N Engl J Med. 2004;351:1218–26. doi: 10.1056/NEJMra031623. [DOI] [PubMed] [Google Scholar]
- 3.Sarno A, Tedesco G, De Robertis R, et al. Pancreatic cystic neoplasm diagnosis: Role of imaging. Endosc Ultrasound. 2018;7:297–300. doi: 10.4103/eus.eus_38_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adler DG, Jacobson BC, Davila RE, et al. ASGE guideline: Complications of EUS. Gastrointest Endosc. 2005;61:8–12. doi: 10.1016/s0016-5107(04)02393-4. [DOI] [PubMed] [Google Scholar]
- 5.Lennon AM, Ahuja N, Wolfgang CL. AGA guidelines for the management of pancreatic cysts. Gastroenterology. 2015;149:825. doi: 10.1053/j.gastro.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 6.Capurso G, Vanella G, Arcidiacono PG. Pancreatic cystic neoplasms in 2018: The final cut. Endosc Ultrasound. 2018;7:289–92. doi: 10.4103/eus.eus_48_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Del Chiaro M, Verbeke C, Salvia R, et al. European experts consensus statement on cystic tumours of the pancreas. Dig Liver Dis. 2013;45:703–11. doi: 10.1016/j.dld.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 8.Jang DK, Song BJ, Ryu JK, et al. Preoperative diagnosis of pancreatic cystic lesions: The accuracy of endoscopic ultrasound and cross-sectional imaging. Pancreas. 2015;44:1329–33. doi: 10.1097/MPA.0000000000000396. [DOI] [PubMed] [Google Scholar]
- 9.Zhang W, Linghu E, Chai N, et al. New criteria to differentiate between mucinous cystic neoplasm and serous cystic neoplasm in pancreas by endoscopic ultrasound: A preliminarily confirmed outcome of 41 patients. Endosc Ultrasound. 2017;6:116–22. doi: 10.4103/eus.eus_8_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ohno E, Hirooka Y, Itoh A, et al. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann Surg. 2009;249:628–34. doi: 10.1097/SLA.0b013e3181a189a8. [DOI] [PubMed] [Google Scholar]
- 11.Kurihara N, Kawamoto H, Kobayashi Y, et al. Vascular patterns in nodules of intraductal papillary mucinous neoplasms depicted under contrast-enhanced ultrasonography are helpful for evaluating malignant potential. Eur J Radiol. 2012;81:66–70. doi: 10.1016/j.ejrad.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 12.Harima H, Kaino S, Shinoda S, et al. Differential diagnosis of benign and malignant branch duct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J Gastroenterol. 2015;21:6252–60. doi: 10.3748/wjg.v21.i20.6252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fujita M, Itoi T, Ikeuchi N, et al. Effectiveness of contrast-enhanced endoscopic ultrasound for detecting mural nodules in intraductal papillary mucinous neoplasm of the pancreas and for making therapeutic decisions. Endosc Ultrasound. 2016;5:377–83. doi: 10.4103/2303-9027.190927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Serrani M, Lisotti A, Caletti G, et al. Role of contrast harmonic-endoscopic ultrasound in pancreatic cystic lesions. Endosc Ultrasound. 2017;6:25–30. doi: 10.4103/2303-9027.190931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Okasha HH, Ashry M, Imam HM, et al. Role of endoscopic ultrasound-guided fine needle aspiration and ultrasound-guided fine-needle aspiration in diagnosis of cystic pancreatic lesions. Endosc Ultrasound. 2015;4:132–6. doi: 10.4103/2303-9027.156742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ardengh JC, Lopes CV, de Lima-Filho ER, et al. Impact of endoscopic ultrasound-guided fine-needle aspiration on incidental pancreatic cysts. A prospective study. Scand J Gastroenterol. 2014;49:114–20. doi: 10.3109/00365521.2013.854830. [DOI] [PubMed] [Google Scholar]
- 17.Vege SS, Ziring B, Jain R, et al. American gastroenterological association institute guideline on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology. 2015;148:819–22. doi: 10.1053/j.gastro.2015.01.015. [DOI] [PubMed] [Google Scholar]
- 18.Bick BL, Enders FT, Levy MJ, et al. The string sign for diagnosis of mucinous pancreatic cysts. Endoscopy. 2015;47:626–31. doi: 10.1055/s-0034-1391484. [DOI] [PubMed] [Google Scholar]
- 19.van der Waaij LA, van Dullemen HM, Porte RJ. Cyst fluid analysis in the differential diagnosis of pancreatic cystic lesions: A pooled analysis. Gastrointest Endosc. 2005;62:383–9. doi: 10.1016/s0016-5107(05)01581-6. [DOI] [PubMed] [Google Scholar]
- 20.Ryu JK, Woo SM, Hwang JH, et al. Cyst fluid analysis for the differential diagnosis of pancreatic cysts. Diagn Cytopathol. 2004;31:100–5. doi: 10.1002/dc.20085. [DOI] [PubMed] [Google Scholar]
- 21.Linder JD, Geenen JE, Catalano MF. Cyst fluid analysis obtained by EUS-guided FNA in the evaluation of discrete cystic neoplasms of the pancreas: A prospective single-center experience. Gastrointest Endosc. 2006;64:697–702. doi: 10.1016/j.gie.2006.01.070. [DOI] [PubMed] [Google Scholar]
- 22.Oppong KW, Dawwas MF, Charnley RM, et al. EUS and EUS-FNA diagnosis of suspected pancreatic cystic neoplasms: Is the sum of the parts greater than the CEA? Pancreatology. 2015;15:531–7. doi: 10.1016/j.pan.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 23.Wang Y, Chai N, Feng J, et al. A prospective study of endoscopic ultrasonography features, cyst fluid carcinoembryonic antigen, and fluid cytology for the differentiation of small pancreatic cystic neoplasms. Endosc Ultrasound. 2018;7:335–42. doi: 10.4103/eus.eus_40_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brugge WR, Lewandrowski K, Lee-Lewandrowski E, et al. Diagnosis of pancreatic cystic neoplasms: A report of the cooperative pancreatic cyst study. Gastroenterology. 2004;126:1330–6. doi: 10.1053/j.gastro.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 25.Thornton GD, McPhail MJ, Nayagam S, et al. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology. 2013;13:48–57. doi: 10.1016/j.pan.2012.11.313. [DOI] [PubMed] [Google Scholar]
- 26.Polkowski M, Jenssen C, Kaye P, et al. Technical aspects of Endoscopic Ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline – March 2017. Endoscopy. 2017;49:989–1006. doi: 10.1055/s-0043-119219. [DOI] [PubMed] [Google Scholar]
- 27.Lim LG, Lakhtakia S, Ang TL, et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: A multicentre Asian study. Dig Dis Sci. 2013;58:1751–7. doi: 10.1007/s10620-012-2528-2. [DOI] [PubMed] [Google Scholar]
- 28.Nakai Y, Isayama H, Chang KJ, et al. A pilot study of EUS-guided through-the-needle forceps biopsy (with video) Gastrointest Endosc. 2016;84:158–62. doi: 10.1016/j.gie.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 29.Kovacevic B, Klausen P, Hasselby JP, et al. A novel endoscopic ultrasound-guided through-the-needle microbiopsy procedure improves diagnosis of pancreatic cystic lesions. Endoscopy. 2018;50:1105–11. doi: 10.1055/a-0625-6440. [DOI] [PubMed] [Google Scholar]
- 30.Kovacevic B, Karstensen JG, Havre RF, et al. Initial experience with EUS-guided microbiopsy forceps in diagnosing pancreatic cystic lesions: A multicenter feasibility study (with video) Endosc Ultrasound. 2018;7:383–8. doi: 10.4103/eus.eus_16_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Polkowski M, Larghi A, Weynand B, et al. Learning, techniques, and complications of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) technical guideline. Endoscopy. 2012;44:190–206. doi: 10.1055/s-0031-1291543. [DOI] [PubMed] [Google Scholar]
- 32.Lee LS, Saltzman JR, Bounds BC, et al. EUS-guided fine needle aspiration of pancreatic cysts: A retrospective analysis of complications and their predictors. Clin Gastroenterol Hepatol. 2005;3:231–6. doi: 10.1016/s1542-3565(04)00618-4. [DOI] [PubMed] [Google Scholar]
- 33.O'Toole D, Palazzo L, Arotçarena R, et al. Assessment of complications of EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:470–4. doi: 10.1067/mge.2001.112839. [DOI] [PubMed] [Google Scholar]
- 34.An W, Sun PB, Gao J, et al. Endoscopic submucosal dissection for gastric gastrointestinal stromal tumors: A retrospective cohort study. Surg Endosc. 2017;31:4522–31. doi: 10.1007/s00464-017-5511-3. [DOI] [PubMed] [Google Scholar]
- 35.Marinos E, Lee S, Jones B, et al. Outcomes of single-dose peri-procedural antibiotic prophylaxis for endoscopic ultrasound-guided fine-needle aspiration of pancreatic cystic lesions. United European Gastroenterol J. 2014;2:391–6. doi: 10.1177/2050640614544191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hamada T, Yasunaga H, Nakai Y, et al. Severe bleeding and perforation are rare complications of endoscopic ultrasound-guided fine needle aspiration for pancreatic masses: An analysis of 3,090 patients from 212 hospitals. Gut Liver. 2014;8:215–8. doi: 10.5009/gnl.2014.8.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eloubeidi MA, Tamhane A. EUS-guided FNA of solid pancreatic masses: A learning curve with 300 consecutive procedures. Gastrointest Endosc. 2005;61:700–8. doi: 10.1016/s0016-5107(05)00363-9. [DOI] [PubMed] [Google Scholar]
- 38.Lariño-Noia J, Iglesias-Garcia J, de la Iglesia-Garcia D, et al. EUS-FNA in cystic pancreatic lesions: Where are we now and where are we headed in the future? Endosc Ultrasound. 2018;7:102–9. doi: 10.4103/eus.eus_93_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Maggi G, Guarneri G, Gasparini G, et al. Pancreatic cystic neoplasms: What is the most cost-effective follow-up strategy? Endosc Ultrasound. 2018;7:319–22. doi: 10.4103/eus.eus_44_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ge N, Zhang S, Jin Z, et al. Clinical use of endoscopic ultrasound-guided fine-needle aspiration: Guidelines and recommendations from Chinese society of digestive endoscopy. Endosc Ultrasound. 2017;6:75–82. doi: 10.4103/eus.eus_20_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anand K, Kahaleh M, Tyberg A. Use of needle-based confocal laser endomicroscopy in the diagnosis and management of pancreatic cyst lesions. Endosc Ultrasound. 2018;7:306–9. doi: 10.4103/eus.eus_46_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moris M, Atar M, Kadayifci A, et al. Thermal ablation of pancreatic cyst with a prototype endoscopic ultrasound capable radiofrequency needle device: A pilot feasibility study. Endosc Ultrasound. 2017;6:123–30. doi: 10.4103/eus.eus_6_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhu H, Jiang F, Zhu J, et al. Assessment of morbidity and mortality associated with endoscopic ultrasound-guided fine-needle aspiration for pancreatic cystic lesions: A systematic review and meta-analysis. Dig Endosc. 2017;29:667–75. doi: 10.1111/den.12851. [DOI] [PubMed] [Google Scholar]