Abstract
Background and Objectives:
A needle with Franseen geometry for fine needle aspiration is now available. However, no reports have described prospective evaluations of the Franseen needle or comparisons with the standard needle. The aim of this comparative prospective study was to evaluate the histological diagnostic yield of the Franseen needle and the standard needle using tissue obtained by a single pass of each for the same lesion.
Patients and Methods:
In this study, only tissue obtained by the first pass using the Franseen needle was used. As a comparison group, only tissue obtained from the same lesion by a second pass using the standard needle was used. Evaluation of the histological diagnostic yield of the needles was based on tissue obtained by each single pass with no additional passes.
Results:
A total of 56 patients were prospectively enrolled. The rate of adequate tissue obtained was significantly higher for the Franseen needle than for the standard needle (89.4% vs. 62.5%, respectively; P < 0.05). The sensitivity and accuracy of the Franseen needle were 80.7%, and 84.6%, respectively, while those for the standard needle were 59.6% and 63.5%, respectively.
Conclusions:
The Franseen needle offers a better rate of obtaining adequate tissue and higher diagnostic accuracy than the standard needle.
Keywords: EUS, EUS-FNA, Franseen needle, pancreatic cancer
INTRODUCTION
Traditionally, histological diagnosis of pancreatic tumors has been based on sampling of pancreatic juice under ERCP guidance.[1,2,3] However, the diagnostic yield of this method is not very high. Moreover, acute pancreatitis, as one of the adverse events of ERCP, can be fatal. EUS-FNA has recently been developed as an alternative for the diagnosis of pancreatic tumors.[4] This method has various advantages, such as high diagnostic yield and a low rate of adverse events compared with ERCP. EUS-FNA is therefore considered the gold-standard method for obtaining histological evidence of pancreatic tumors.[5,6,7,8] The development of diagnostic imaging modalities, such as multidetector row computed tomography (CT), has facilitated the detection of more pancreatic lesions in the absence of any symptoms. To ensure diagnosis with EUS-FNA, various efforts have been reported such as on-site evaluation and technical methods including slow pull and wet suction techniques.[9,10,11,12] Obtaining a sufficient amount of tissue is essential for the assessment and subtyping of various neoplasms and for further immunohistochemical investigations of tumor type.[13,14] Moreover, an adequate amount of tissue will be needed to treat patients using an individualized approach, such as chemotherapy according to the tumor genotype.
An FNA needle with Franseen geometry (Acquire; Boston Scientific, Natick, MA, USA) is now available. According to a retrospective study, this novel FNA needle has the potential to obtain good core tissue.[15] However, no prospective evaluations of the Franseen needle or comparisons with the standard needle have been reported. The aim of this comparative prospective study was to evaluate the histological diagnostic yield of the Franseen needle and the standard needle using tissue obtained by a single pass of each.
PATIENTS AND METHODS
This prospective study was carried out at the Second Department of Internal Medicine of Osaka Medical College between January and May 2017. This study was approved by the Institutional Review Board of our hospital and registered with UMIN (registration number: UMIN000025708). Written, informed consent was obtained from all patients enrolled. All consecutive patients underwent noninvasive imaging, such as CT and EUS, and were diagnosed as having pancreatic tumors.
Technical tips for EUS-FNA
EUS-FNA was performed by two experienced endoscopists (T.O., D.M.) who were trained and experienced in diagnostic and therapeutic procedures under EUS guidance. EUS-FNA was performed at 7.5 MHz using a convex linear-array echoendoscope (UCT260; Olympus Optical, Tokyo, Japan) connected to an ultrasound device (SSD5500; Aloka, Tokyo, Japan). Patients received antibiotics before EUS-FNA, and EUS-FNA was then performed under midazolam sedation.
In this study, the first pass was performed using a 22G Franseen needle [Figure 1]. The second pass for the same lesion was performed using a 22G standard FNA needle (Expect, Boston Scientific) using the same technique as for the first puncture. The echoendoscope was advanced into the stomach or duodenum, and the pancreatic tumor was visualized. The tumor was then punctured while using color Doppler ultrasonography to avoid any intervening vessels. The stylet was then pulled out slowly without suction. During this procedure, approximately 20 strokes were performed within the tumor using a fanning technique. The material was then directly fixed in 10% formalin in a standard specimen bottle, centrifuged, and embedded in paraffin for histological analysis. Sections were visualized using hematoxylin and eosin staining, as well as immunohistochemical staining, if necessary [Figures 2 and 3]. Biopsy specimens were examined by an experienced pathologist (Y.K). The pathologist was blinded to which FNA needle had been used.
Figure 1.

The top of the Franseen needle has a crown shape with three symmetrical planes for tissue acquisition
Figure 2.
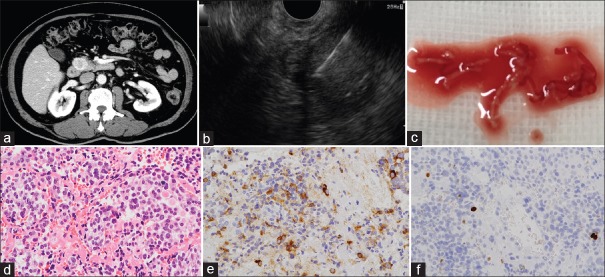
(a) Computed tomography shows a hypervascular tumor in the head of the pancreas. (b) EUS-FNA using the Franseen needle is performed for a tumor in the head of the pancreas. (c) Microscopic findings of the obtained tissue. (d) Tumor cells with oval nuclei and acidophilic granular cytoplasm show diffuse proliferation. (e) Tumor cells are chromogranin positive. (f) The Ki-67 index is around 4%, and the grade of neuroendocrine tumor is G2
Figure 3.
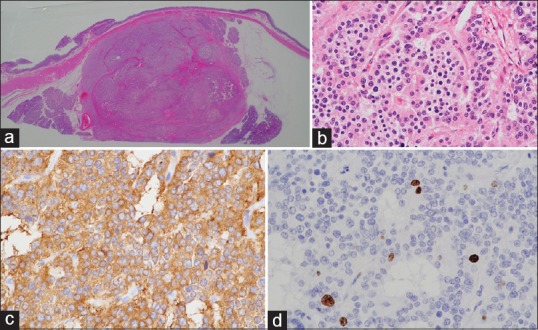
Resected specimen. This tumor is resected by pancreatoduodenectomy (a). This tumor is a neuroendocrine tumor (b) H and E staining; (c) chromogranin staining; (d) Ki-67 staining. The Ki-67 index is 4%, and the grade is therefore 2
Study endpoints and definitions
Sizes of all pancreatic tumors were measured using EUS, and the locations of lesions were defined by CT. Continuous variables are expressed as median values. All analyses were performed using SPSS version 13.0 statistical software (SPSS, Chicago, IL, USA). Adverse events were graded according to the American Society for Gastrointestinal Endoscopy lexicon's severity grading system.[16]
This prospective study was aimed primarily at investigating histological diagnostic yield, such as sensitivity, specificity, negative predictive value (NPV), positive predictive value (PPV), and accuracy compared with the final diagnosis from surgical or clinical follow-up. In this analysis, only tissue obtained by the first pass using the Franseen needle was used. For comparison, only tissue obtained by the second pass using the standard needle was used. Evaluation of the histological diagnostic yield of the needles was based on tissue obtained by each single pass with no additional passes.
Samples obtained by EUS-FNA were defined as adequate tissue if they could be histologically evaluated. Findings of suspected malignancy, serous cystic neoplasm, and neuroendocrine tumor (G1, G2) were considered benign, whereas pancreatic adenocarcinoma, neuroendocrine carcinoma, malignant lymphoma, and metastatic tumor were considered malignant. The final diagnosis was based on surgical pathology. However, if the patient did not undergo surgical resection, the final diagnosis was based on the presence or absence of new metastasis and/or vessel invasion during clinical follow-up.
Finally, a two-tailed sample size calculation was performed assuming a type I error rate (α) of 0.05 and power of 80% for detecting a difference in diagnostic accuracy between the Franseen and standard needle groups. The diagnostic accuracy of EUS-FNA was assumed to be 82%.[5] EUS-FNA using a Franseen needle was assumed to afford a diagnostic accuracy of 98% or higher. The calculation yielded a target sample size of 54 for each cohort. To avoid effects from differences in the patients'characteristics, EUS-FNA of the same lesions was performed using the two different needles.
RESULTS
A total of 56 patients (median age, 72.5 years; age range, 58–84 years; 30 men, 26 women) were prospectively enrolled. EUS-FNA was successfully performed using first the Franseen needle and then the standard needle (technical success rates, both 100%). The median duration of follow-up was 246 days (range, 166–255 days).
Table 1 shows the background characteristics of the patients. The median size of the target lesion was 20 mm (range, 8–55 mm). The location of the puncture site was the head (n = 27), body (n = 19), and tail (n = 10) of the pancreas. The final diagnosis was malignant disease, n = 52 (92.9%; including adenocarcinoma n = 47, metastatic tumor n = 2, neuroendocrine carcinoma n = 1, and malignant lymphoma n = 2) and benign disease, n = 4 (7.1%; including chronic pancreatitis n = 1, autoimmune pancreatitis n = 2, and neuroendocrine tumor n = 1). Among benign diseases, the final diagnosis was based on clinical follow-up without progressive disease and surgical resection (neuroendocrine tumor n = 1).
Table 1.
Patient’s characteristics
| Baseline Characteristics | |
|---|---|
| Total number of patients | 56 |
| Age (year), median (range) | 72.5 (58-84) |
| Gender, n (%) | |
| Male | 30 |
| Female | 26 |
| Size of tumor (mm), median (range) | 20 (8-55) |
| Location of tumor | |
| Head | 27 |
| Body | 19 |
| Tail | 10 |
| Rate of adequate tissue, n (%) | |
| Franseen needle | 50/56 (89.3) |
| Standard needle | 35/56 (62.5) |
| Final diagnosis | |
| Malignant disease, n (%) | 52 (92.9) |
| Adenocarcinoma | 47 |
| Metastatic tumor | 2 |
| Neuroendocrine carcinoma | 1 |
| Malignant lymphoma | 2 |
| Benign, n (%) | 4 (7.1) |
| Chronic pancreatitis | 1 |
| Autoimmune pancreatitis | 2 |
| Neuroendocrine tumor | 1 |
The rate of adequate tissue obtained by the Franseen needle was 89.3% (50/56). In contrast, the rate of adequate tissue obtained by the standard needle was significantly lower, at 62.5% (35/56; P < 0.05). Table 2 shows the diagnostic yield of the Franseen and standard needles. The sensitivity, specificity, PPV, NPV, and accuracy of the Franseen needle were 80.7%, 100%, 100%, 28.6%, and 84.6%, respectively. The sensitivity, specificity, PPV, NPV, and accuracy of with the standard needle were 59.6%, 100%, 100%, 16.0%, and 63.5%, respectively. Compared with the standard needle, the diagnostic yield and rate of adequate tissue obtained with the Franseen needle were good. Adverse events such as bleeding or pancreatitis were not seen in any patients.
Table 2.
Diagnostic yield among all tissue for histological diagnosis
| Franseen needle (n=56), n (%) | Standard needle (n=56), n (%) | P | |
|---|---|---|---|
| Sensitivity | 42/52 (80.7) | 31/52 (59.6) | 0.018 |
| Specificity | 4/4 (100) | 4/4 (100) | NS |
| PPV | 42/42 (100) | 31/31 (100) | NS |
| NPV | 4/14 (28.6) | 4/25 (16.0) | 0.351 |
| Accuracy | 44/52 (84.6) | 33/52 (63.5) | 0.014 |
PPV: Positive predictive value, NPV: Negative predictive value, NS: Not significant
DISCUSSION
Obtaining a sufficient amount of tissue is important not only for improving diagnostic yield but also for investigating tumor type immunohistochemically. One possible option to improve diagnostic yield is rapid on-site evaluation (ROSE). In a recent meta-analysis of 34 distinct studies covering 3644 patients,[17] meta-regression modeling showed that ROSE was a significant determinant of EUS-FNA accuracy after correcting for study population number and reference standard. Similarly, Matynia et al. reported that ROSE was associated with an improvement of up to 3.5% in adequacy rates for EUS-FNA of solid pancreatic lesions and affected the relationship between needle passes and per-case adequacy for EUS-FNA of solid pancreatic lesions.[18] However, because ROSE is not available in every institute, obtaining an adequate amount of tissue is still important for improving diagnostic yield.
Various types of EUS-FNA needles are currently available to obtain core tissue samples. For example, the ProCore fine needle biopsy (FNB) (ProCore; Cook Medical, Bloomington, IN, USA) needle with reverse-bevel technology has a clinical benefit for decreasing FNA passes, but a meta-analysis could not demonstrate any significant difference between the ProCore and standard needle in terms of sample adequacy, diagnostic accuracy, or acquisition of core tissue.[19] A novel fork-tip needle, the Shark Core (SC; Beacon Endoscopy/Medtronic, Boston, MA) is also available. Kandel et al. reported a retrospective case–control study of EUS-FNB-SC and EUS-FNA, with 39 patients in the EUS-FNB-SC group and 117 patients in the EUS-FNA group.[20] Histology cores were obtained from 95% of the EUS-FNB-SC group (35/37; 95% confidence interval [CI], 82%–99%) compared with 59% of the EUS-FNA group (67/114; 95% CI 49%–68%; P = 0.01). In addition, the EUS-FNB-SC group required significantly fewer passes (P = 0.01). More recently, Nayar et al. performed a comparison study of the ProCore needle and the SC needle.[21] In that study of 201 consecutive patients, 101 underwent EUS-FNA using an SC needle, and 100 underwent sampling using a ProCore needle. The SC needle had a significantly higher sensitivity (71.1% vs. 90.1%; P = 0.006) and overall accuracy (74% vs. 92%; P = 0.0006) compared with the ProCore needle. In addition, the proportion of samples classified as adequate for histologic analysis was 87% for the ProCore needle versus 99% for the SC needle (P = 0.002).
The Franseen needle was developed to obtain more core tissue. The top of the Franseen needle has a crown shape with three symmetrical planes for tissue acquisition. Bang et al. initially evaluated this needle in a retrospective study that included 30 patients with pancreatic cancer (n = 12), gastrointestinal stromal cell tumor (n = 5), or others.[15] Among them, the diagnostic accuracy of ROSE was 96.6%, and a histological diagnosis was established in 96.7%, although an adverse event (bleeding) was seen in one patient. Interestingly, median tissue area was 2.9 mm2 (interquartile range [IQR], 0.68–8.71 mm2), and the median tumor percentage in tissue was 73.9% (IQR, 44–97.6%). They therefore concluded that the Franseen needle yields sufficient diagnostic material for ROSE and histology in >95% of patients. Based on the results of that retrospective study, the Franseen needle may be clinically beneficial for obtaining a sufficient amount of tissue and improving diagnostic accuracy. However, no reports have described clinical studies of the Franseen needle.
In the present study, the diagnostic yield of the Franseen needle was evaluated. The rate of adequate tissue obtained with the standard needle was 62.5%. This rate is similar to that of a previous report, although EUS-FNA was performed with only one pass in the present study.[22] Therefore, the present results for EUS-FNA and diagnostic yield may be reliable. In contrast, the rate of adequate tissue obtained in the Franseen group from only one pass was relatively high, at 89.3%. This result was comparable to that for the ProCore needle. According to a meta-analysis of the ProCore needle,[19] the pooled rate of obtaining core tissue for pancreatic tumors was 79.2% (95% CI 69.6%–87.3%). Furthermore, the pooled rate for diagnostic accuracy for pancreatic tumors was 87.0% (95% CI 75.9%–95.1%). The present results thus suggest that the Franseen needle has the potential to obtain enough tissue to diagnose various pancreatic tumors. However, our study had several limitations. Our study used a nonrandomized design with a small sample size. Local bleeding that occurs in the lesion after puncturing it with the Franseen needle may have affected sampling in the second pass with the standard needle. Additional comparative studies should use a randomized design.
CONCLUSIONS
The present study may be the first prospective study to evaluate the Franseen needle. The Franseen needle has a better rate of obtaining adequate tissue and a higher diagnostic accuracy than the standard needle. A prospective randomized, controlled study with another FNA needle and a larger sample size is needed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Nakaizumi A, Tatsuta M, Uehara H, et al. Cytologic examination of pure pancreatic juice in the diagnosis of pancreatic carcinoma. The endoscopic retrograde intraductal catheter aspiration cytologic technique. Cancer. 1992;70:2610–4. doi: 10.1002/1097-0142(19921201)70:11<2610::aid-cncr2820701107>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 2.McGuire DE, Venu RP, Brown RD, et al. Brush cytology for pancreatic carcinoma: An analysis of factors influencing results. Gastrointest Endosc. 1996;44:300–4. doi: 10.1016/s0016-5107(96)70168-2. [DOI] [PubMed] [Google Scholar]
- 3.Talukdar R. Complications of ERCP. Best Pract Res Clin Gastroenterol. 2016;30:793–805. doi: 10.1016/j.bpg.2016.10.007. [DOI] [PubMed] [Google Scholar]
- 4.Turner BG, Cizginer S, Agarwal D, et al. Diagnosis of pancreatic neoplasia with EUS and FNA: A report of accuracy. Gastrointest Endosc. 2010;71:91–8. doi: 10.1016/j.gie.2009.06.017. [DOI] [PubMed] [Google Scholar]
- 5.Möller K, Papanikolaou IS, Toermer T, et al. EUS-guided FNA of solid pancreatic masses: High yield of 2 passes with combined histologic-cytologic analysis. Gastrointest Endosc. 2009;70:60–9. doi: 10.1016/j.gie.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 6.Itoi T, Sofuni A, Itokawa F, et al. Current status of diagnostic endoscopic ultrasonography in the evaluation of pancreatic mass lesions. Dig Endosc. 2011;23(Suppl 1):17–21. doi: 10.1111/j.1443-1661.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 7.Chen G, Liu S, Zhao Y, et al. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for pancreatic cancer: A meta-analysis. Pancreatology. 2013;13:298–304. doi: 10.1016/j.pan.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Hewitt MJ, McPhail MJ, Possamai L, et al. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: A meta-analysis. Gastrointest Endosc. 2012;75:319–31. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 9.Lin M, Hair CD, Green LK, et al. Endoscopic ultrasound-guided fine-needle aspiration with on-site cytopathology versus core biopsy: A comparison of both techniques performed at the same endoscopic session. Endosc Int Open. 2014;2:E220–3. doi: 10.1055/s-0034-1377611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakai Y, Isayama H, Chang KJ, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–85. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 11.Khan MA, Grimm IS, Ali B, et al. A meta-analysis of endoscopic ultrasound-fine-needle aspiration compared to endoscopic ultrasound-fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc Int Open. 2017;5:E363–75. doi: 10.1055/s-0043-101693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attam R, Arain MA, Bloechl SJ, et al. “Wet suction technique (WEST)”: A novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–7. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 13.Varadarajulu S, Fraig M, Schmulewitz N, et al. Comparison of EUS-guided 19-gauge trucut needle biopsy with EUS-guided fine-needle aspiration. Endoscopy. 2004;36:397–401. doi: 10.1055/s-2004-814316. [DOI] [PubMed] [Google Scholar]
- 14.Levy MJ, Jondal ML, Clain J, et al. Preliminary experience with an EUS-guided trucut biopsy needle compared with EUS-guided FNA. Gastrointest Endosc. 2003;57:101–6. doi: 10.1067/mge.2003.49. [DOI] [PubMed] [Google Scholar]
- 15.Bang JY, Hebert-Magee S, Hasan MK, et al. Endoscopic ultrasonography-guided biopsy using a Franseen needle design: Initial assessment. Dig Endosc. 2017;29:338–46. doi: 10.1111/den.12769. [DOI] [PubMed] [Google Scholar]
- 16.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 17.Hébert-Magee S, Bae S, Varadarajulu S, et al. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: A meta-analysis. Cytopathology. 2013;24:159–71. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matynia AP, Schmidt RL, Barraza G, et al. Impact of rapid on-site evaluation on the adequacy of endoscopic-ultrasound guided fine-needle aspiration of solid pancreatic lesions: A systematic review and meta-analysis. J Gastroenterol Hepatol. 2014;29:697–705. doi: 10.1111/jgh.12431. [DOI] [PubMed] [Google Scholar]
- 19.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–49. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 20.Kandel P, Tranesh G, Nassar A, et al. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: A case-control study. Gastrointest Endosc. 2016;84:1034–9. doi: 10.1016/j.gie.2016.03.1405. [DOI] [PubMed] [Google Scholar]
- 21.Nayar MK, Paranandi B, Dawwas MF, et al. Comparison of the diagnostic performance of 2 core biopsy needles for EUS-guided tissue acquisition from solid pancreatic lesions. Gastrointest Endosc. 2017;85:1017–24. doi: 10.1016/j.gie.2016.08.048. [DOI] [PubMed] [Google Scholar]
- 22.Bang JY, Hebert-Magee S, Trevino J, et al. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–7. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]