Abstract
Background and Objectives:
Biliary drainage (BD) under EUS guidance is usually indicated for malignant biliary obstruction. Recently, EUS-guided transluminal treatment has been applied to benign biliary disease (BBD). This multicenter retrospective study evaluated the clinical impact of EUS-guided transluminal stent deployment for BBD with long-term follow-up.
Patients and Methods:
This retrospective study investigated patients treated between September 2015 and October 2016 at participating hospitals in the therapeutic endoscopic group. The inclusion criteria comprised complications with BBD obstructive jaundice or cholangitis and failed endoscopic retrograde cholangiopancreatography or inaccessible ampulla of Vater.
Results:
Twenty-six patients underwent EUS-guided transluminal stent deployment. Indications for EUS-guided transluminal stent deployment comprised anastomotic biliary stricture (n = 17), bile duct stones (n = 5), inflammatory biliary stricture (n = 3), and acute pancreatitis prevention (n = 1). Thirteen of these 26 patients underwent scheduled reintervention, with technical success achieved in all 13 patients. None of the deployed stents became dysfunctional. Among the 13 patients who underwent reintervention on demand, stents had become dysfunctional in six patients (stent patency: 48, 90, 172, 288, 289, and 608 days). Reintervention was successfully performed in all patients. During follow-up (median, 749 days), severe adverse events were not seen in any patients.
Conclusion:
We concluded that EUS-guided transluminal stent deployment for BBD is feasible and safe. Because metal stent dysfunction was more frequent when deployed on demand, such stents should be exchanged for plastic stents in a scheduled manner if a metal stent is used.
Keywords: Benign disease, EUS, EUS biliary drainage, EUS-guided biliary drainage, EUS hepaticogastrostomy
INTRODUCTION
Endoscopic biliary drainage (BD) under ERCP guidance has been established for treating not only malignant biliary obstruction but also benign biliary stricture (BBS).[1,2,3,4,5] However, ERCP can be challenging when the biliary obstruction is complicated by duodenal obstruction or surgical anatomy. The alternative option of percutaneous transhepatic BD (PTBD) is associated with several complications such as cholangitis, bile leak, and pneumothorax. Moreover, the frequency of major complications such as prolonged hospital stay or permanent adverse sequelae is 4.6%–25% and that of procedure-related deaths is 0%–5.6%.[6,7,8] Cosmetic issues due to external drainage also impair quality of life. EUS-guided procedures have thus emerged as an alternative means of access to these biliary routes.[9,10,11,12,13] BD under EUS guidance is usually indicated for malignant biliary obstruction because a permanent fistula needs to be created. The EUS-guided rendezvous technique (RV) is preferred for BBS that is contraindicated or difficult to access by conventional means.[14,15,16,17] However, EUS-RV can be challenged by surgical anatomy preventing the endoscope reaching its target or difficulties with guidewire insertion into the intestine across the papilla. EUS-guided transluminal treatment has recently been applied to benign biliary diseases (BBDs) such as hepaticogastrostomy (HGS), hepaticojejunostomy (HJS), or choledochoduodenostomy (CDS).[18,19,20,21,22,23] However, those reports described small patient cohorts with a short follow-up period, especially after transluminal stent deployment. The present multicenter retrospective study evaluated long-term outcomes of EUS-guided transluminal stent deployment for BBD.
PATIENTS AND METHODS
This retrospective study investigated patients treated between September 2015 and October 2016 at participating hospitals in the therapeutic endoscopic group. In this study, patients in whom ERCP failed and who showed complicating BBD such as bile duct stones or BBS including anastomotic biliary stricture were consecutively enrolled. BBD was diagnosed from histological evidence or clinical follow-up for at least 1 year after enrollment in this study. The exclusion criteria comprised contraindications for endoscopic BD including ERCP and EUS guidance due to conditions such as massive ascites, or Eastern Cooperative Oncology Group performance status 3 or 4, other organ failure, prior antegrade stone removal without transluminal stenting, or lack of consent to participate. This retrospective study was approved by the institutional review boards of each participating hospital. Written, informed consent was obtained from all patients. The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the human research committees of each participating institution.
Technical tips for EUS-guided transluminal biliary drainage
All procedures were implemented by endoscopists who were trained and experienced in diagnostic and therapeutic procedures under ERCP guidance at the participating institutions. Patients received antibiotics before undergoing all procedures under sedation (Video 1).
A convex EUS probe (GF-UCT240 or 260; Olympus Optical, Tokyo, Japan) connected to an ultrasound device (SSD5500; Aloka, Tokyo, Japan) was initially advanced into the intestine to visualize the biliary tract, which was then punctured using fine needle aspiration with a Sono Tip Pro Control 19G needle (Medi-Globe GmbH; Medico's Hirata, Osaka, Japan) or an EchoTip Ultra (Cook Endoscopy, Bloomington, IN) via color Doppler ultrasonography to avoid intervening vessels. Bile juice was aspirated, contrast medium was injected [Figure 1a], then a 0.025-inch VisiGlide guidewire (Olympus Medical Systems, Tokyo, Japan) or RevoWave (PIOLAX Medical, Kanagawa, Japan) was inserted into the biliary tract. An ERCP catheter (MTW Endoskopie, Düsseldorf, Germany) was inserted into the biliary tract, and contrast medium was injected once again for cholangiography [Figure 1b]. The bile duct and intestinal wall were dilated using a 4-mm REN biliary dilation balloon catheter (KANEKA, Osaka, Japan), a 6- or 8-Fr Soehendra biliary dilation catheter (Cook Endoscopy), or a 6-Fr Cysto Gastro Set diathermic dilator (Endoflex, Voerde, Germany). Finally, a plastic FLEXMA biliary stent (Boston Scientific, Natick, MA), a Niti-S Biliary covered metal stent (TaeWoong Medical, Seoul, Korea), or a WallFlex stent (Boston Scientific) was deployed from the bile duct to the intestine [Figure 1c].
Figure 1.
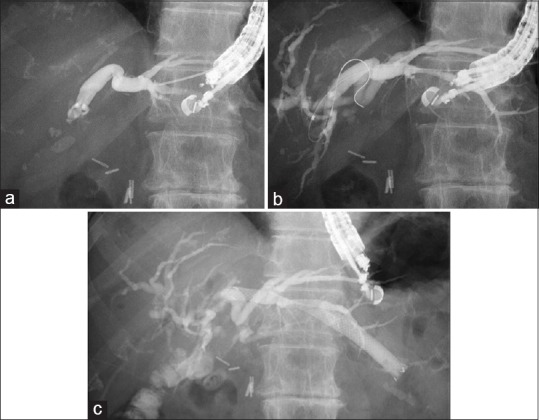
(a) The intrahepatic bile duct is punctured using a 19-G FNA needle, and the contrast medium is injected. (b) Hepaticojejunostomy stricture is seen. (c) A self-expandable metal stent is deployed from the intrahepatic bile duct to the stomach
Technical tips for reintervention
Reintervention for a dysfunctional stent was either scheduled or provided on demand. Scheduled transluminal stent exchange proceeded within 3–6 months. Figure 2 shows the reintervention for a HJS anastomotic biliary stricture. The covered metal stent was first removed under duodenoscopy (JF 260V; Olympus Medical Systems) as described [Figure 2a], and a guidewire was placed into the biliary tract [Figure 2b]. A Type IT plastic stent (Gadelius Medical, Tokyo, Japan) or a covered metal stent was then deployed from the intrahepatic bile duct to the stomach [Figure 2c]. Reintervention on demand involved balloon cleaning or deployment of an additional metal or plastic stent if the original metal stent could not be removed (Video 2).
Figure 2.
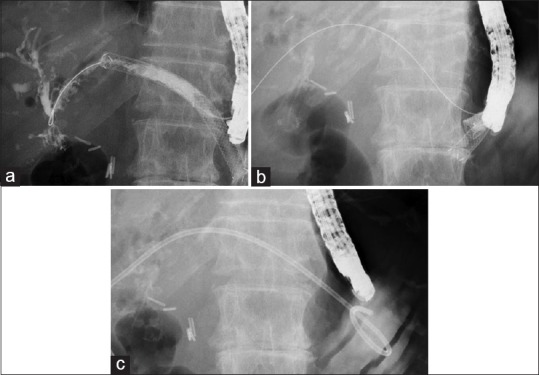
(a) The guidewire is placed in the biliary tract through a EUS-guided hepaticogastrostomy stent. (b) The covered metal stent is removed. (c) A plastic stent is deployed
Study endpoints and definitions
The primary endpoints of this study comprised early (≤14 days after initial deployment) and late (≥15 days after initial deployment) adverse events. Technical success was defined as stent deployment under EUS guidance. Clinical success was defined as a decrease in serum bilirubin concentration to <75% of preprocedural values within 30 days after stent deployment or resolved cholangitis. Adverse events were graded according to the severity grading system of the American Society for Gastrointestinal Endoscopy lexicon.[24]
Stent dysfunction was defined as cholangitis, stent migration, dislocation, or occlusion.
Clinical follow-up proceeded on an outpatient basis and comprised monitoring of clinical symptoms such as abdominal pain or fever and general blood tests including serum bilirubin concentration at least every 6 months. Follow-up was defined as the period from the day of EUS-guided transluminal stent deployment to the last follow-up or death of the patient.
Results are presented as median values and means. All data were statistically analyzed using SPSS version 13.0 software (SPSS, Chicago, IL, USA).
RESULTS
Table 1 shows the characteristics of the 26 patients (median age, 75 years; range, 48–88 years; 14 men, 12 women) who were categorized into on-demand (n = 13) and scheduled (n = 13) groups. Technical and clinical success was achieved in all patients. Four patients died during follow-up (median, 749 days; cause of death: lung cancer, n = 1; pneumonia, n = 1; heart failure, n = 2).
Table 1.
Patient’s characteristics
| Factors | Results |
|---|---|
| Total number of patients | 26 |
| Median age (range) | 75 (48-88) |
| Gender (male:female) | 14:12 |
| Reason for EUS-guided access | |
| Surgical anatomy | 21 |
| Roux-en Y | 6 |
| Pancreatojejunostomy | 14 |
| Billroth II | 1 |
| Failed ERCP | 4 |
| Other | 1 |
| Disease | |
| Benign biliary stricture | |
| Anastomotic | 17 |
| Other | 4 |
| Bile duct stones | 5 |
| Primary stent | |
| Plastic stent | 3 |
| Metallic stent | 23 |
| Early adverse events | 2 (abdominal pain) |
| Technical success | 100 (26/26) |
| Clinical success | 100 (26/26) |
| Median follow-up days (range) | 749 (400-1888) |
Outcomes of primary EUS-guided transluminal stent deployment
The 26 patients underwent EUS-guided transluminal stent deployment because of surgical anatomy (n = 21), difficult cannulation (n = 4), and due to complication with severe acute pancreatitis (n = 1). All cases of difficult cannulation involved complication with huge bile duct stones. Because huge bile duct stones were seen in the lower common bile duct, advancing the guidewire into the duodenum across the papilla may be challenging during EUS-guided RV procedures if the intrahepatic bile duct was not dilated, EUS-CDS was selected. Furthermore, the patient with severe acute pancreatitis was admitted with severe acute cholangitis due to a bile duct stone. If ERCP had been attempted, the acute pancreatitis may have been exacerbated. This patient therefore underwent EUS-CDS to avoid the risk of post-ERCP pancreatitis.
Underlying pathologies were anastomotic biliary stricture (n = 17), bile duct stones (n = 5), inflammatory biliary stricture complicated by chronic pancreatitis (n = 3), and acute pancreatitis prevention (n = 1). Adverse events associated with EUS-guided transluminal stent deployment developed in two patients and were treated conservatively in each case.
Outcomes of scheduled reintervention
Thirteen of the 26 patients underwent scheduled reintervention. All 13 patients underwent EUS-guided HGS (n = 8) or EUS-guided HJS (n = 5). Figure 3 shows a BBS caused by common bile duct stones with middle bile duct stricture in patient advanced lung cancer [Figure 3a]. He underwent EUS-HGS due to surgically altered anatomy with Roux-en-Y anastomosis [Figure 3b and c]. Three months later, the covered metal stent was replaced with a plastic stent [Figure 3d], which was subsequently replaced every 5 months. No adverse events were seen by the time he died of lung cancer after 711 days of follow-up. Technical success was achieved for all 13 patients who underwent scheduled reintervention, and none of the deployed stents became dysfunctional [Table 2].
Figure 3.
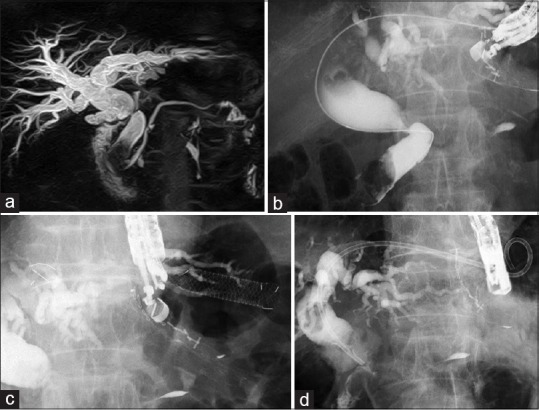
(a) Common bile duct stone and middle bile duct stricture are seen on magnetic resonance cholangiopancreatography. (b) Common bile duct stone and middle bile duct stricture are seen on cholangiography. (c) EUS-guided hepaticogastrostomy is performed. (d) After stent removal, plastic stent deployment is performed
Table 2.
Clinical outcome of patients in reintervention in the structured way group
| Factors | Results |
|---|---|
| Total number of patients | 13 |
| Access route | |
| Duodenal | 1 |
| Stomach | 8 |
| Jejunum | 5 |
| Technical success of reintervention | 100 (13/13) |
| Stent dysfunction (day) | None |
| Adverse events associated with reintervention | None |
Outcomes of reintervention on demand
Among the 13 patients who underwent reintervention on demand, EUS-CDS proceeded using plastic (n = 1) or covered metal (n = 2) stents, and 10 patients underwent EUS-HGS with a covered metal stent. Stents became dysfunctional in six patients (stent patency: 48, 90, 172, 288, 289, and 608, days) due to sludge (covered metal stents, n = 3), stent dislocation (plastic stent, n = 1; covered metal stent, n = 1), and obstruction (covered metal stent, n = 1). Reintervention for sludge consisted of endoscopic cleaning with a balloon catheter. Additional plastic stents were deployed to treat stent obstruction [Table 3].
Table 3.
Clinical outcome of patients in reintervention on demand group
| Factors | Results |
|---|---|
| Total number of patients | 13 |
| Access route | |
| Duodenal | 3 |
| Stomach | 10 |
| Jejunum | 0 |
| Technical success of reintervention | 100 (13/13) |
| Stent dysfunction (day) | Dislocation, n=2 (172, 608) Obstruction, n=1 (289) Sludge, n=3 (90, 48, 288) |
| Adverse events associated with reintervention | None |
DISCUSSION
Various situations can induce BBS, including chronic pancreatitis, cholelithiasis, and pancreatoduodenostomy.[25] Asano et al. evaluated the incidence of and risk factors for BBS after pancreaticoduodenectomy.[26] They found that 16 (8%) of 200 patients had BBS, and multivariate analysis revealed associations between BBS and both body mass index and preoperative biliary stenting. Reid-Lombardo et al. found that the 5- and 10-year cumulative probabilities of developing BBS after pancreaticoduodenectomy in 122 patients with benign disease were 8% (2%, 14%) and 13% (4%, 22%), respectively.[27] The incidence of postoperative BBS thus does not seem particularly high. However, thanks to improvements in diagnostic modalities such as EUS and surgical resection rates of pancreatic lesions such as intraductal mucinous neoplasm or pancreatic neuroendocrine tumors; BBS after procedures such as pancreatoduodenostomy might have increased.[28,29]
Stent deployment under ERCP guidance has been established for treating BBS.[2,5] Although this technique has clinical benefits, ERCP might be challenging when patients have a complicated surgical anatomy. One alternative is PTBD; but, this is associated with disadvantages such as hemorrhage, pneumothorax, cosmetic issues due to the external drainage, and risk of self-tube removal.[6,7,8] A recent development in BBS using ERCP is the use of various types of enteroscope, including a short double-balloon enteroscope (DBE). ERCP under DBE in expert hands[30] has shown success rates for reaching the blind end up to 99.1%, and the median time required was only 10 min, with a low adverse event rate. Mizukawa et al.[29] also achieved good technical and clinical success rates with this procedure in 46 patients without severe adverse events. However, the median time required to complete the procedure was 54 min (range, 37–82 min). ERCP under DBE might thus generally prove unnecessarily complex and laborious.
More recently, EUS-BD has emerged as an alternative approach, especially for treating malignant biliary obstruction. A systematic review and meta-analysis[8] found that technical success rates did not differ between EUS-BD and PTBD (odds ratio [OR], 1.78; 95% confidence interval [CI], 0.69–4.59; I2 = 22%), but EUS-BD was associated with better clinical success (OR, 0.23; 95% CI, 0.12–0.47; I2 = 57%) and lower rates of reintervention (OR, 0.13; 95% CI, 0.07–0.24; I2 = 0%). In addition, EUS-BD was more cost-effective, with a pooled standard mean difference of −0.63 (95% CI, −1.06 to −0.20). On the other hand, EUS-BD has been attributed in reports of benign disease, whereas EUS-RV and EUS-guided antegrade stone treatment have been relatively reported.[14,15,16,17] In contrast, very little has been reported regarding EUS-guided transluminal stent deployment for benign disease.[18] James et al. evaluated the clinical outcomes of EUS-guided transluminal stent deployment for BBD in 20 patients with surgical anatomy who underwent the procedure under transgastric (n = 15) or transjejunal (n = 5) approaches. All patients underwent EUS-guided transluminal stent deployment using covered metal stents, and 18 underwent antegrade, definitive endoscopic treatment. Metal stents were removed from 17 patients after a mean of 91 days without any adverse events. However, median follow-up was only 122 days. The present study followed up more patients for a median of 749 days and may represent the first evaluation of the clinical course of EUS-guided transluminal stent deployment for benign disease. Our findings indicated that metal stent dysfunction was more frequent when deployed on demand. We therefore recommend scheduled plastic stent placement within 6 months. However, because the present single-arm study was retrospective, our findings should be confirmed in a prospective, randomized controlled study.
CONCLUSION
EUS-guided transluminal stent deployment for BBD is feasible and safe. Because metal stent dysfunction was more frequent when deployed on demand, such stents should be exchanged for plastic stents in a scheduled manner.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Videos Available on: www.eusjournal.com
Acknowledgments
The present study was supported by grants from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Costamagna G, Pandolfi M, Mutignani M, et al. Long-term results of endoscopic management of postoperative bile duct strictures with increasing numbers of stents. Gastrointest Endosc. 2001;54:162–8. doi: 10.1067/mge.2001.116876. [DOI] [PubMed] [Google Scholar]
- 2.Draganov P, Hoffman B, Marsh W, et al. Long-term outcome in patients with benign biliary strictures treated endoscopically with multiple stents. Gastrointest Endosc. 2002;55:680–6. doi: 10.1067/mge.2002.122955. [DOI] [PubMed] [Google Scholar]
- 3.Pasha SF, Harrison ME, Das A, et al. Endoscopic treatment of anastomotic biliary strictures after deceased donor liver transplantation: Outcomes after maximal stent therapy. Gastrointest Endosc. 2007;66:44–51. doi: 10.1016/j.gie.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Poley JW, Cahen DL, Metselaar HJ, et al. A prospective group sequential study evaluating a new type of fully covered self-expandable metal stent for the treatment of benign biliary strictures (with video) Gastrointest Endosc. 2012;75:783–9. doi: 10.1016/j.gie.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Devière J, Nageshwar Reddy D, et al. Successful management of benign biliary strictures with fully covered self-expanding metal stents. Gastroenterology. 2014;147:385–95. doi: 10.1053/j.gastro.2014.04.043. [DOI] [PubMed] [Google Scholar]
- 6.Carrasco CH, Zornoza J, Bechtel WJ. Malignant biliary obstruction: Complications of percutaneous biliary drainage. Radiology. 1984;152:343–6. doi: 10.1148/radiology.152.2.6739796. [DOI] [PubMed] [Google Scholar]
- 7.Günther RW, Schild H, Thelen M. Percutaneous transhepatic biliary drainage: Experience with 311 procedures. Cardiovasc Intervent Radiol. 1988;11:65–71. doi: 10.1007/BF02577061. [DOI] [PubMed] [Google Scholar]
- 8.Winick AB, Waybill PN, Venbrux AC. Complications of percutaneous transhepatic biliary interventions. Tech Vasc Interv Radiol. 2001;4:200–6. doi: 10.1016/s1089-2516(01)90026-5. [DOI] [PubMed] [Google Scholar]
- 9.Teoh AY, Dhir V, Kida M, et al. Consensus guidelines on the optimal management in interventional EUS procedures: Results from the Asian EUS group RAND/UCLA expert panel. Gut. 2018;67:1209–28. doi: 10.1136/gutjnl-2017-314341. [DOI] [PubMed] [Google Scholar]
- 10.Boulay BR, Lo SK. Endoscopic ultrasound-guided biliary drainage. Gastrointest Endosc Clin N Am. 2018;28:171–85. doi: 10.1016/j.giec.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 12.Law R, Baron TH. Endoscopic ultrasound-guided biliary interventions: An update on recent developments. Curr Opin Gastroenterol. 2016;32:232–7. doi: 10.1097/MOG.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 13.Minaga K, Kitano M. Recent advances in endoscopic ultrasound-guided biliary drainage. Dig Endosc. 2018;30:38–47. doi: 10.1111/den.12910. [DOI] [PubMed] [Google Scholar]
- 14.Shiomi H, Yamao K, Hoki N, et al. Endoscopic ultrasound-guided rendezvous technique for failed biliary cannulation in benign and resectable malignant biliary disorders. Dig Dis Sci. 2018;63:787–96. doi: 10.1007/s10620-018-4908-8. [DOI] [PubMed] [Google Scholar]
- 15.Isayama H, Nakai Y, Kawakubo K, et al. The endoscopic ultrasonography-guided rendezvous technique for biliary cannulation: A technical review. J Hepatobiliary Pancreat Sci. 2013;20:413–20. doi: 10.1007/s00534-012-0577-8. [DOI] [PubMed] [Google Scholar]
- 16.Tsuchiya T, Itoi T, Sofuni A, et al. Endoscopic ultrasonography-guided rendezvous technique. Dig Endosc. 2016;28(Suppl 1):96–101. doi: 10.1111/den.12611. [DOI] [PubMed] [Google Scholar]
- 17.Iwashita T, Yasuda I, Mukai T, et al. EUS-guided rendezvous for difficult biliary cannulation using a standardized algorithm: A multicenter prospective pilot study (with videos) Gastrointest Endosc. 2016;83:394–400. doi: 10.1016/j.gie.2015.04.043. [DOI] [PubMed] [Google Scholar]
- 18.James TW, Fan YC, Baron TH. EUS-guided hepaticoenterostomy as a portal to allow definitive antegrade treatment of benign biliary diseases in patients with surgically altered anatomy. Gastrointest Endosc. 2018;88:547–54. doi: 10.1016/j.gie.2018.04.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iwashita T, Nakai Y, Hara K, et al. Endoscopic ultrasound-guided antegrade treatment of bile duct stone in patients with surgically altered anatomy: A multicenter retrospective cohort study. J Hepatobiliary Pancreat Sci. 2016;23:227–33. doi: 10.1002/jhbp.329. [DOI] [PubMed] [Google Scholar]
- 20.Nakai Y, Kogure H, Yamada A, et al. Endoscopic management of bile duct stones in patients with surgically altered anatomy. Dig Endosc. 2018;30(Suppl 1):67–74. doi: 10.1111/den.13022. [DOI] [PubMed] [Google Scholar]
- 21.Kawakami H, Kuwatani M, Kubota Y, et al. Endoscopic ultrasound-guided antegrade bile duct stone treatment followed by direct peroral transhepatic cholangioscopy in a patient with Roux-en-Y reconstruction. Endoscopy. 2015;47(Suppl 1):E340–1. doi: 10.1055/s-0034-1392507. [DOI] [PubMed] [Google Scholar]
- 22.Itoi T, Sofuni A, Tsuchiya T, et al. Endoscopic ultrasonography-guided transhepatic antegrade stone removal in patients with surgically altered anatomy: Case series and technical review (with videos) J Hepatobiliary Pancreat Sci. 2014;21:E86–93. doi: 10.1002/jhbp.165. [DOI] [PubMed] [Google Scholar]
- 23.Kamiyama R, Ogura T, Okuda A, et al. Electrohydraulic lithotripsy for difficult bile duct stones under endoscopic retrograde cholangiopancreatography and peroral transluminal cholangioscopy guidance. Gut Liver. 2018;12:457–62. doi: 10.5009/gnl17352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cotton PB, Eisen GM, Aabakken L, et al. A lexicon for endoscopic adverse events: Report of an ASGE workshop. Gastrointest Endosc. 2010;71:446–54. doi: 10.1016/j.gie.2009.10.027. [DOI] [PubMed] [Google Scholar]
- 25.Zheng X, Wu J, Sun B, et al. Clinical outcome of endoscopic covered metal stenting for resolution of benign biliary stricture: Systematic review and meta-analysis. Dig Endosc. 2017;29:198–210. doi: 10.1111/den.12742. [DOI] [PubMed] [Google Scholar]
- 26.Asano T, Natsume S, Senda Y, et al. Incidence and risk factors for anastomotic stenosis of continuous hepaticojejunostomy after pancreaticoduodenectomy. J Hepatobiliary Pancreat Sci. 2016;23:628–35. doi: 10.1002/jhbp.385. [DOI] [PubMed] [Google Scholar]
- 27.Reid-Lombardo KM, Ramos-De la Medina A, Thomsen K, et al. Long-term anastomotic complications after pancreaticoduodenectomy for benign diseases. J Gastrointest Surg. 2007;11:1704–11. doi: 10.1007/s11605-007-0369-7. [DOI] [PubMed] [Google Scholar]
- 28.Balcom JH, 4th, Rattner DW, Warshaw AL, et al. Ten-year experience with 733 pancreatic resections: Changing indications, older patients, and decreasing length of hospitalization. Arch Surg. 2001;136:391–8. doi: 10.1001/archsurg.136.4.391. [DOI] [PubMed] [Google Scholar]
- 29.Mizukawa S, Tsutsumi K, Kato H, et al. Endoscopic balloon dilatation for benign hepaticojejunostomy anastomotic stricture using short double-balloon enteroscopy in patients with a prior Whipple's procedure: A retrospective study. BMC Gastroenterol. 2018;18:14. doi: 10.1186/s12876-018-0742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shimatani M, Tokuhara M, Kato K, et al. Utility of newly developed short-type double-balloon endoscopy for endoscopic retrograde cholangiography in postoperative patients. J Gastroenterol Hepatol. 2017;32:1348–54. doi: 10.1111/jgh.13713. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


