Abstract
Background
Hepatocellular carcinoma (HCC) is a common malignancy, but the pathogenesis of HCC is unclear. TMUB1 has an inhibitory effect on normal hepatocytes, but its role in HCC has not been reported.
Material/Methods
We used immunohistochemistry to observe the expression of transmembrane and ubiquitin-like domain containing 1 protein (TMUB1) and signal transducer and activator of transcription 1 (STAT1) in 132 HCC tissue specimens. The expression of TMUB1, STAT1, and CCND1 in HCC cells were detected by quantitative polymerase chain reaction (qPCR) and western blotting. Cell Counting Kit-8 (CCK-8) and 5-ethynyl-2′-deoxyuridine (EdU) assays were used for detecting HCC cells proliferation, and Transwell assays were used for observing the invasion and migration of HCC cells.
Results
TMUB1 was negatively correlated with HCC pathological malignancy; low expression of TMUB1 indicated poor prognosis. TMUB1 inhibited proliferation but not metastasis in HCC cells. TMUB1 expression was positively correlated with STAT1 in 132 HCC tissues, TMUB1 promoted the expression of STAT1, and suppressed the expression of CCND1 in HCC cells.
Conclusions
TMUB1 negatively regulates hepatocellular carcinoma proliferation via regulating STAT1.
MeSH Keywords: Carcinoma, Hepatocellular; Cell Proliferation; STAT1 Transcription Factor
Background
Hepatocellular carcinoma (HCC) is a common malignant tumor whose global incidence rate ranks sixth among malignant tumors and whose mortality rate ranks second [1]. HCC is especially serious in People’s Republic of China, and the incidence and mortality has increased significantly in past decades [2,3]. HCC treatment includes mainly surgical resection, radiofrequency ablation, interventional therapy, and liver transplantation, of which surgical resection is the most commonly used [4,5]. However, because systemic chemotherapy drugs for HCC are ineffective, 70% of patients still have recurrence and metastasis 5 years after surgery [6,7]. Currently, the pathogenesis of HCC remains unclear, so finding a definitive and effective treatment is difficult [8]. Finding effective treatments through the in-depth study of the pathogenesis of HCC thus remains the focus of current HCC research.
TMUB1 (transmembrane and ubiquitin-like domain-containing protein 1) was first reported in 2005, and its expression in hepatocytes is significantly increased after hepatectomy [9]. The TMUB1 gene is located at the 4q11 position on chromosome 4 in rats (located on chromosome 7 in humans) and has a total length of 1381 bp. The TMUB1 protein contains 245 amino acids and includes a nuclear export signal (NES) at the amino terminus and a region containing a similar ubiquitin structure (UBL; 121–175 aa). TMUB1 is a transmembrane protein, acts as a ubiquitin-like protein participated in the ERAD (endoplasmic reticulum-associated protein degradation) pathway. As a nuclear-cytoplasmic shuttle protein, TMUB1 is released from the membrane and shuttles between the cytoplasm and the nucleus to exert its biological functions [10].
Early studies indicated that TMUB1 has an inhibitory effect on the proliferation of normal hepatocytes. Interleukin (IL)-6 and miR-27a/b can regulate the expression of TMUB1, as well as its post-transcriptional modification, thereby promoting its inhibition of hepatocyte proliferation [11–13]. TMUB1 regulates hepatocyte regeneration by inhibiting STAT3 phosphorylation and interfering with CAML binding to cyclophilin B [14,15]. TMUB1 also acts as an important factor in the p14arf-p53 pathway and participates in inhibiting tumorigenesis by repairing DNA [16]. However, whether TMUB1 plays a role in hepatocellular carcinoma is not clear.
STAT1 (signal transducer and activator of transcription 1) is a key member of the mammalian STAT family and transduces signals from the cytoplasm to the nucleus for regulating gene expression [17]. Current research indicates that STAT1 has inhibitory effects on a variety of tumors such as colon (rectal) cancer [18], HCC [19,20], soft tissue sarcoma, pancreatic cancer, esophageal cancer, and metastatic melanomas [21–24]. STAT1 not only inhibits tumorigenesis but also inhibits tumor cell proliferation, promotes apoptosis, inhibits angiogenesis, and regulates tumor immunity [24]. STAT1 exerts its function of inhibiting tumor progression by regulating cell cycle regulators (e.g., p21WAF1 and p27KIP1) [25,26], proapoptotic proteins (e.g., Bcl-2 family members) [27,28], death receptors (e.g., FAS) [29], and angiostatic chemokines (e.g., CXCL10 and CXCL11) [30,31]. Both STAT3 and STAT1 participate in the JAK/STAT signaling pathway and antagonize each other, and TMUB1 has an inhibitory effect on STAT3 [32,33]. Therefore, we speculate that TMUB1 may inhibit HCC by promoting STAT1.
In our research, we analyzed the correlation between TMUB1 expression in HCC tissues and the clinical pathological features and prognosis of HCC patients. We verified our results with cell experiments and conducted a preliminary molecular mechanism study.
Material and Methods
Medical record and follow-up
We gathered 132 paraffin specimens from HCC patients who have undergone partial hepatectomy at the Army Medical Center between January 2013 and December 2014. Clinical pathological information included gender, age, tumor size, tumor cells differentiation, TNM stage, extrahepatic metastasis, vascular invasion, lymph node metastasis, and other patient-related information. We followed the patients until January 1, 2019, and recorded relapse and deaths.
Immunohistochemical staining
We collected 132 paraffin-embedded HCC tissue specimens and 20 paraffin-embedded HCC paracancerous tissue specimens and used them to create 2 tissue microarrays. The tissue microarrays were deparaffinized, rehydrated in graded alcohols, placed in a sodium citrate solution and recovered antigen by microwave treatment for 10 minutes. After cooling naturally, the tissue microarrays were incubated in serum for 30 minutes, then incubation at 4°C with the primary antibody overnight. The results of immunohistochemical staining were scored of both the intensity of staining and the tumor cells positive rate. Each tissue microarray was scored by 2 pathologists independently who do not know the patient data in advance. For TMUB1 or STAT1, the final score (0~12) was determined by product of the score of intensity of intensity and the score of tumor cells positive rate. The intensity of staining defined as follow: 0 (negative), 1 (weak), 2 (moderate), and 3 (strong). The score of tumor cells positive rate was defined as follow: 0 (0%), 1 (1–24%), 2 (25–49%), 3 (50–74%), and 4 (75–100%). Total score=cell positive rate score×staining intensity score; and 0 point means negative, 1–4 points mean weakly positive (1+), 5–8 points mean moderately positive (2+), 9–12 points mean strong positive (3+). For statistical analysis, 0 and 1+ were defined as low expression, and 2+ and 3+ were defined as high expression.
Cell culture
MHCC97h and Huh7 cells were routinely cultured in Dulbecco’s Modified Eagle Medium (DMEM; Invitrogen) with 10% FBS (fetal bovine serum) and incubated at 5% CO2 and 37°C.
Plasmid extraction
The TMUB1 and STAT1 overexpression and shRNA vectors were purchased from Cyagen Biosciences. The TMUB1 shRNA sequence was 5′-GACACCATTGGCTCCTTGAAA-3′ and STAT1 shRNA sequence was 5′-CTGGAAGATTTACAAGATGAA-3′. Bacteria solution (100 μL), ampicillin sodium (100 μL) and LB medium (200 mL) were mixed in a conical flask, then shaken for 16 hours at 37°C. After the medium became turbid, the suspension was centrifuged, then collected the bacteria. The plasmid was extracted with an EndoFree Plasmid Maxi Kit (QIAGEN). The spectrophotometer (NV3000C) was used for detecting the plasmid concentration.
Cell transfection
MHCC97h or Huh7 cells were seeded onto 24-well culture plate. When the cells confluence reached 80%, they were transferred to new DMEM without penicillin-streptomycin. Plasmid DNA (4 μg) and Lipofectamine 3000 (5 μL) were diluted in serum-free medium for 20 minutes. The mixture solution was added to the cultured cells, and the plate was gently rotated for even distribution. The cells were further cultured for 6 hours, then the medium was changed with high-glucose DMEM with 10% FBS. After 36 hours, western blotting and qPCR were used for detecting the transfection efficiency.
Cell proliferation assay
When cells were successful transfected with plasmid, cells proliferation was detected by CCK-8 kit (Engreen) at 0, 24, 48, and 72 hours, and (EdU) DNA Proliferation In Vitro Detection kit (KeyGen Biotech) at 36 hours.
Migration and invasion assays
Basement Membrane Matrix (Corning Life Science) was spread on the bottom of the upper well of a Transwell chamber (Millipore) for the invasion assays. Cells were added to the upper wells, while DMEM with 10% FBS was added into the lower chamber. The cells in Transwell chambers were cultured at 37°C and 5% CO2. The cells had migrated or invaded into the lower chambers were fixed with paraformaldehyde, stained with crystal violet and counted.
Quantitative PCR and western blotting
TRIzol Reagent (TaKaRa) was used for extracting total RNA. The primers used to amplify TMUB1, STAT1, CCND1, and GAPDH (Table 1). The PrimeScript RT-polymerase (TaKaRa) was used for reverse transcription and amplification. The total protein was extracted using the RIPA lysate (TaKaRa,), the concentration was calculated by the BCA kit (CWBIO). Total proteins were separated by 10% SDS-PAGE, after that transferred to a NC membrane. The NC membrane was blocked by 5% skimmed milk, and incubated with anti-TMUB1, anti-STAT1, anti-CCND1 (Abcam) at 4°C overnight. Then, the membrane was incubated with secondary antibodies at room temperature for 2 hours.
Table 1.
Primers polymerase chain reaction amplification.
| Gene | Primers (5′-3′) |
|---|---|
| h-TMUB1-F | GCTACCGACAGTGAGAGG |
| h-TMUB1-R | ATTTCAGCCGTAGCACGAGG |
| h-STAT1-F | CGCTCTGCTGTCTCCGCTTCCACTCC |
| h-STAT1-R | AGCTGATCCAAGCAGCATTGG |
| h-Cyclin D1-F | CTGGCCATGAACTACCTGGA |
| h-Cyclin D1-R | GTCACATTGATCACTCTCC |
| h-GAPDH-F | AGGTGAAGGTCGGAGTCAAC |
| h-GAPDH-R | CGCTCCTGGAAGATGGTGAT |
Statistical analysis
Prism 6 (GraphPad) and SPSS 18.0 statistics software (SPSS Science) were used to perform the statistical analysis. All data were expressed 2-sided, unpaired Student’s t-test was used for statistical analysis between groups, and Chi-square test/Fisher exact test for processing count data. The P value <0.05 defined as statistical significance.
Results
Patient data statistics
The average age of patients was 51.2±11.0; 116 patients (87.9%) were males, and 16 patients (12.1%) were females. The average tumor size was 1.0–19.0 cm (5.8±2.5 cm). The patients were divided into 4 groups based on TNM staging: stage I (71.2%; n=94), stage II (17.4%; n=23), stage III (8.3%; n=11), and stage IV (3.1%; n=4). The proportion of multiple tumors was 23.5% (n=31). The patients were divided into 3 groups based on HCC cells differentiation: well differentiation (25.0%; n=33), moderate differentiation (50.0%; n=66), and poor differentiation (25.0%; n=33). In total, 2.3% (n=3) of patients suffering from extrahepatic metastasis, 3.0% (n=4) of patients suffering from lymph node metastasis, and 15.9% (n=21) of patients suffering from vascular invasion in our patient cohort. The recurrence rate was 66.7% (n=88), and the cancer-related mortality rate was 56.1% (n=74). (Table 2).
Table 2.
Clinical pathological and follow-up data from patients.
| Pathologic variables | No. of patients (%) |
|---|---|
| TNM stage | 132 |
| Stage I | 94 (71.2%) |
| Stage II | 23 (17.4%) |
| Stage III | 11 (8.3%) |
| Stage IV | 4 (3.1%) |
| HCC differentiation | 132 |
| WD | 33 (25.0%) |
| MD | 66 (50.0%) |
| PD | 33 (25.0%) |
| Number of tumors | 132 |
| Multiple tumors | 31 (23.5%) |
| Solitary tumor | 101 (76.5%) |
| Vascular invasion | 132 |
| Yes | 21 (15.9%) |
| No | 111 (84.1%) |
| Lymph node metastasis | 132 |
| Yes | 4 (3.0%) |
| No | 128 (97.0%) |
| Extrahepatic metastasis | 132 |
| Yes | 3 (2.3%) |
| No | 129 (97.7%) |
| Postoperative recurrence | 132 |
| Yes | 88 (66.7%) |
| No | 44 (33.3%) |
| Cancer related death | 74 (56.06%) |
| Survival | 53 (40.2%) |
| TMUB1 expression status | |
| 0 | 6 (4.5%) |
| 1+ | 47 (35.6%) |
| 2+ | 43 (32.6%) |
| 3+ | 36 (27.3%) |
HCC – hepatocellular carcinoma; WD – well differentiated; MD – moderately differentiated; PD – poorly differentiated; TMUB1 – transmembrane and ubiquitin-like domain-containing protein 1.
TMUB1 was negatively correlated with HCC pathological malignancy and low expression of TMUB1 indicated poor prognosis
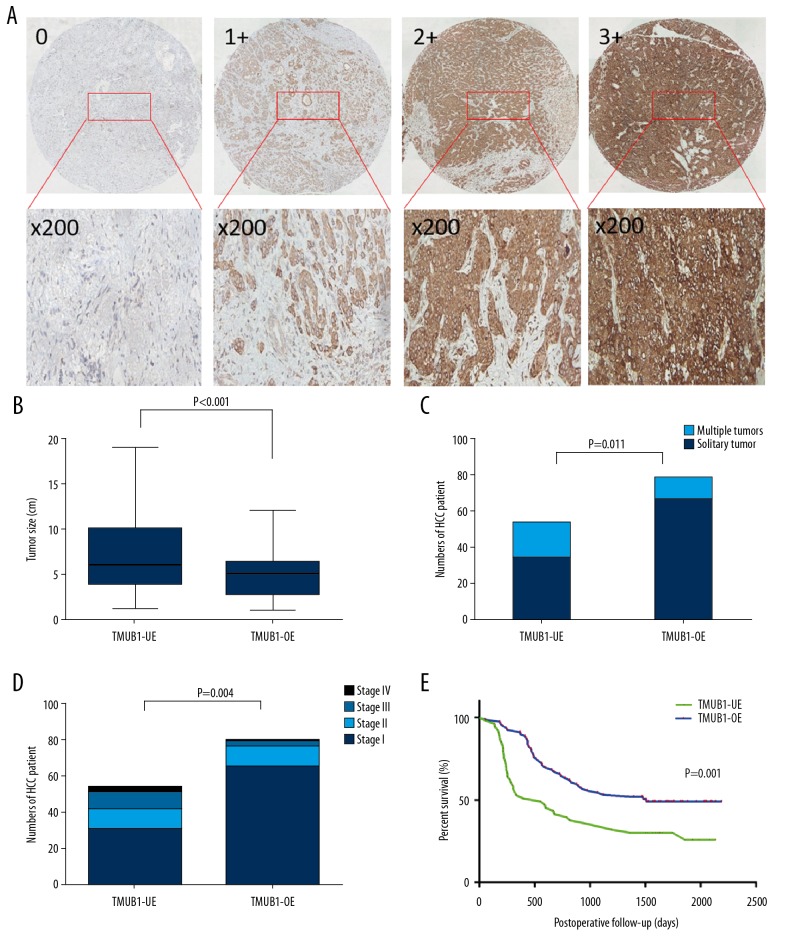
We analyzed differences in the tumor size, HCC differentiation, TNM stage, extrahepatic metastasis, lymph node metastasis, vascular invasion and prognosis of patients in TMUB1 high expression group and TMUB1 low expression group. The tumor sizes were significantly larger in TMUB1 low expression group (6.8±4.0 cm) than those in TMUB1 high expression group (5.2±2.5 cm) (P<0.001). The HCC differentiation in TMUB1 high expression group was better than those in TMUB1 low expression group (P=0.021). The proportion of multiple tumors in TMUB1 low expression group (14.4%, n=19) was significantly higher than that in TMUB1 high expression group (9.1%, n=12) (P=0.011). Regarding TNM stage, there were significantly more patients with stage III (6.8%) and stage IV (2.3%) in TMUB1 low expression group than patients with stage III (1.5%) and stage IV (0.8%) in TMUB1 high expression groups (P=0.004). But the vascular invasion or metastasis between the 2 groups had no statistically significant differences. In addition, the results of postoperative follow-up showed that the tumor-free survival time was obviously lower in TMUB1 low expression group than in TMUB1 high expression group (P=0.014), and the overall survival time was also obviously lower in TMUB1 low expression group than in TMUB1 high expression group (P=0.001). The mean postoperative survival time was lower in TMUB1 low expression group (828±102 days) than in TMUB1 high expression group (1180±150 days) (Table 3, Figure 1).
Table 3.
Comparison of the clinical pathological features and patient prognosis in the 2 groups.
| Biological characteristics | High TMUB1 expression (2+, 3+) | Low TMUB1 expression (0+, 1+) | P value |
|---|---|---|---|
| Mean tumor size (cm) | 5.2±2.5 | 6.8±4.0 | <0.001 |
| Numbers of tumors ≥2 | 12 (9.1%) | 19 (14.4%) | 0.011 |
| TNM stage | 0.004 | ||
| Stage I | 64 (48.5%) | 30 (22.7%) | |
| Stage II | 12 (9.1%) | 11 (8.3%) | |
| Stage III | 2 (1.5%) | 9 (6.8%) | |
| Stage IV | 1 (0.8%) | 3 (2.3%) | |
| HCC differentiation | 0.021 | ||
| WD | 25 (18.9%) | 8 (6.1%) | |
| MD | 42 (31.8%) | 24 (18.2%) | |
| PD | 12 (9.1%) | 21 (15.9%) | |
| Lymph node metastasis | 1 (0.8%) | 3 (2.3%) | >0.05 |
| Vascular invasion | 12 (9.1%) | 9 (6.8%) | >0.05 |
| Extrahepatic metastasis | 1 (0.8%) | 2 (1.5%) | >0.05 |
| Postoperative recurrence | 44 (33.3%) | 40 (30.3%) | 0.022 |
| Cancer related death | 38 (28.9%) | 36 (27.3%) | 0.031 |
Figure 1.
TMUB1 is negatively correlated with HCC pathological malignancy and positively correlated with patient prognosis. (A) The immunohistochemistry was used for detecting TMUB1 expression of tissue microarrays. TMUB1 expression was scored as described in the Methods. (B) The comparison of tumor size between the 2 groups. (C) The comparison of the proportion of multiple tumors between the 2 groups. (D) The comparison of TNM stage between the 2 groups. (E) The comparison of the mean postoperative survival time between the 2 groups. TMUB1 – transmembrane and ubiquitin-like domain-containing protein 1; HCC – hepatocellular carcinoma; TMUB1-OE – TMUB1 over-expression; TMUB1-UE – TMUB1 under-expression.
TMUB1 suppressed HCC proliferation but had no significant effect on HCC metastasis
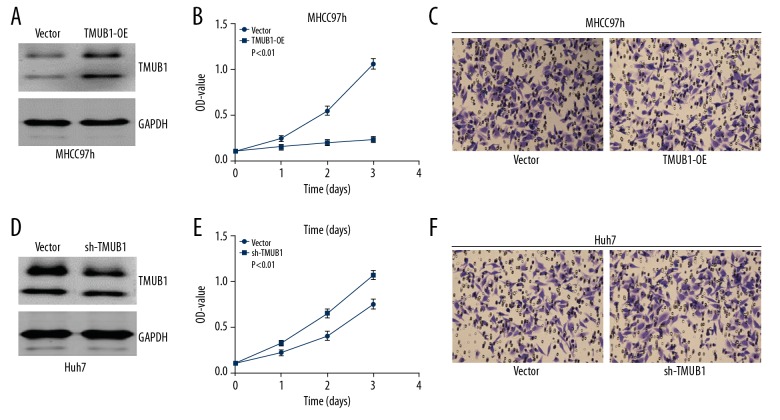
We transfected MHCC97h cells with a TMUB1 overexpression plasmid or control vector, and we transfected Huh7 cells with a TMUB1 interference plasmid or control vector. Then, we detected cells proliferation with CCK-8 kit, and invasion and migration with Transwell assays. We discovered that proliferation was suppressed in TMUB1-overexpressing MHCC97h cells compared with control MHCC97h cells, while proliferation was significantly enhanced in TMUB1-knockdown Huh7 cells compared with control Huh7 cells. There were no significant changes in migration and invasion in the 2 groups (Figure 2).
Figure 2.
TMUB1 expression controls HCC cell proliferation. (A, D) Western blotting detecting expression of TMUB1 in MHCC97h cells transfected by overexpression plasmid (A) and in Huh7 cells transfected by interference plasmid (D). (B, E) CCK-8 kit was used for detecting the proliferation of MHCC97h (B) and Huh7 cells (E). (C, F) Transwell assays were used for detecting the invasion and migration of MHCC97h cells (C) and Huh7 cells (F). The invasion and migration potential were not changed in neither MHCC97h cells treated by over-expression plasmid nor Huh7 cells treated by interference plasmid. TMUB1 – transmembrane and ubiquitin-like domain-containing protein 1; HCC – hepatocellular carcinoma; CCK-8 – Cell Counting Kit-8.
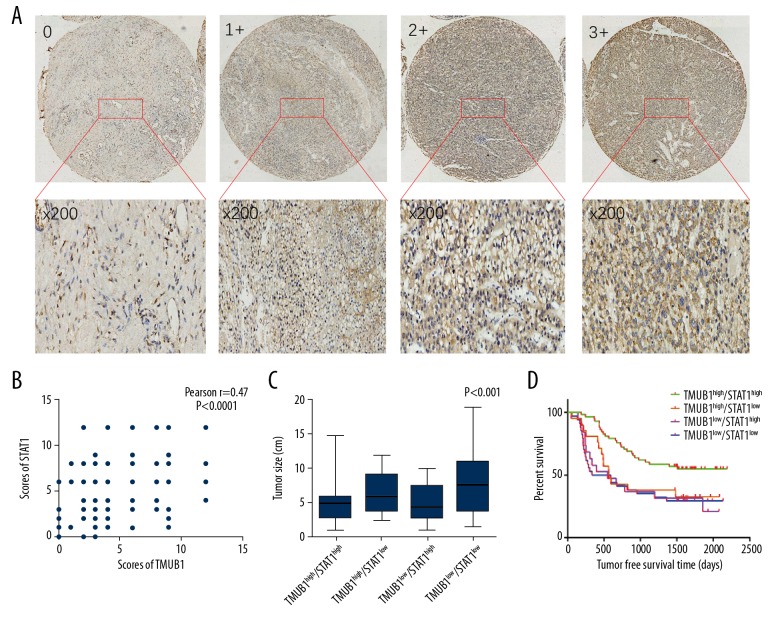
The expression of TMUB1 and STAT1 was positively correlated in surgically resected HCC specimens, TMUB1/STAT1 expression are negatively correlated with HCC pathological malignancy
TMUB1 can inhibit the proliferation of HCC cells, but its molecular mechanism is still unclear. Previous studies have shown that TMUB1 can inhibit STAT3 function by suppressing the phosphorylation of STAT3. STAT1 and STAT3 have antagonistic effects in promoting tumorigenesis and proliferation. High STAT1 expression indicates better prognosis of HCC patients. Therefore, we examined STAT1 expression in 132 HCC specimens and found that STAT1 expression and TMUB1 expression were positively correlated, P<0.0001, Pearson r=0.47. The tumor size of the TMUB1low/STAT1low group (7.8±4.4 cm) was significantly larger than that of the TMUB1high/STAT1low (6.7±3.0 cm), TMUB1low/STAT1high (5.3±2.7 cm), and TMUB1high/STAT1high (4.7±2.0 cm) groups (P<0.001). The proportion of multiple tumors in the TMUB1low/STAT1low group (8.3%, n=11) was significantly higher than that in the TMUB1high/STAT1high (5.3%, n=7), TMUB1high/STAT1low (3.0%, n=4) and TMUB1low/STAT1high groups (6.1%, n=8). Regarding TNM stage and HCC differentiation, the TMUB1high/STAT1high group was better than those of TMUB1high/STAT1low, TMUB1low/STAT1high, and TMUB1low/STAT1low groups (P=0.016). But there were no statistically significant differences in the 4 groups for vascular invasion or metastasis. The overall survival of the TMUB1high/STAT1high group were longer than those of TMUB1high/STAT1low, TMUB1low/STAT1high, and TMUB1low/STAT1low groups (P=0.004; Table 4, Figure 3).
Table 4.
Comparison of the clinical pathological features and patient prognosis in the TMUB1/STAT1 under-expression and over-expression.
| Biological characteristics | TMUB1high/STAT1hhigh | TMUB1high/STAT1low | TMUB1low/STAT1high | TMUB1low/STAT1low | P value |
|---|---|---|---|---|---|
| Numbers of patient | 58 | 21 | 19 | 34 | |
| Mean tumor size (cm) | 4.7±2.0 | 6.7±3.0 | 5.3±2.7 | 7.8±4.4 | <0.001 |
| Numbers of tumors ≥2 | 7 (5.3%) | 4 (3.0%) | 8 (6.1%) | 11 (8.3%) | 0.021 |
| TNM stage | 0.016 | ||||
| Stage I | 49 (37.1%) | 15 (11.3%) | 10 (7.6%) | 20 (15.2%) | |
| Stage II | 7 (5.3%) | 5 (3.8%) | 6 (4.5%) | 5 (3.8%) | |
| Stage III | 1 (0.8%) | 1 (0.8%) | 3 (2.3%) | 6 (4.5%) | |
| Stage IV | 1 (0.8%) | 1 (0.8%) | 0 (0.0%) | 3 (2.3%) | |
| HCC differentiation | 0.002 | ||||
| WD | 20 (15.2%) | 2 (1.5%) | 2 (1.5%) | 6 (4.5%) | |
| MD | 30 (22.7%) | 12 (9.1%) | 13 (9.8%) | 11 (8.3%) | |
| PD | 8 (6.1%) | 7 (5.3%) | 4 (3.0%) | 17 (12.9%) | |
| Lymph node metastasis | 1 (0.8%) | 0 (0.0%) | 0 (0.0%) | 1 (0.8%) | >0.05 |
| Vascular invasion | 6 (4.5%) | 6 (4.5%) | 2 (1.5%) | 6 (4.5%) | >0.05 |
| Extrahepatic metastasis | 1 (0.8%) | 0 (0.0%) | 1 (0.8%) | 2 (1.5%) | >0.05 |
| Postoperative recurrence | 35 (26.5%) | 6 (4.5%) | 14 (10.6%) | 26 (19.7%) | 0.003 |
| Cancer related death | 24 (18.2%) | 13 (9.8%) | 13 (9.8%) | 23 (17.4%) | 0.030 |
STAT1 – signal transducer and activator of transcription 1.
Figure 3.
Correlation of TMUB1 and STAT1 expression with clinical pathological features and patients’ survival. (A) The immunohistochemistry was used for detecting STAT1 expression of tissue microarrays. (B) STAT1 expression is positively correlated with TMUB1. (C) The comparison of tumor size between the 4 groups. (D) The comparison of the mean postoperative survival time between the 4 groups. TMUB1 – transmembrane and ubiquitin-like domain-containing protein 1; STAT1 – signal transducer and activator of transcription 1.
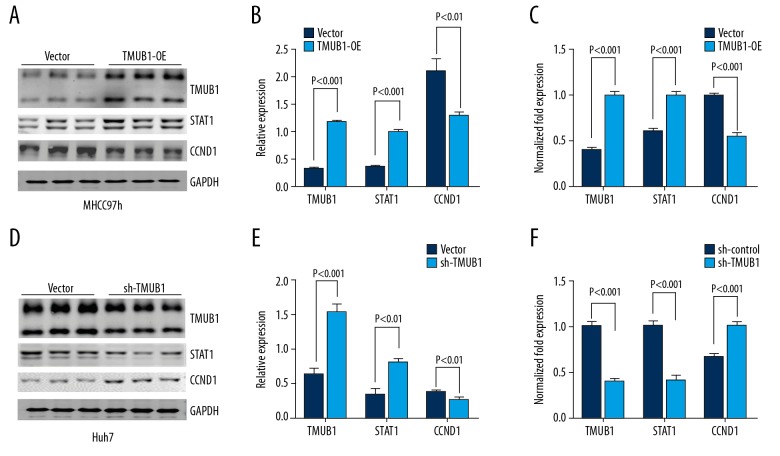
TMUB1 regulated the expression of STAT1
To further validate the regulation of TMUB1 on STAT1, we monitored the changes of STAT1 mRNA, and protein after interference and overexpression of TMUB1 expression, and we monitored the changes of cell cycle related protein CCND1. We transfected MHCC97h cells with the TMUB1 overexpression plasmid or control vector and then detected STAT1 and CCND1 mRNA changes with q-PCR and protein changes with western blot. When TMUB1 expression was upregulated, STAT1 mRNA and protein levels were both upregulated compared with control MHCC97h cells. CCND1 mRNA and protein levels were both downregulated compared with control MHCC97h cells. To further verify these effects, we transfected Huh7 cells with the TMUB1 interference plasmid or control vector and then detected changes in STAT1 and CCND1. We found that when TMUB1 expression was downregulated, STAT1 mRNA and protein expression levels were both downregulated compared with control Huh7 cells. CCND1 mRNA and protein levels were upregulated compared with control Huh7 cells (Figure 4).
Figure 4.
TMUB1 regulates STAT1 and CCND1 in HCC. (A, B) Following TMUB1 overexpression, the western blotting indicated the expression of STAT1 was upregulated following TMUB1 upregulation, and CCND1 was downregulated. (C) The q-PCR indicated the change of expression of STAT1 and CCND1 was consistent with the western blots. (D, E) Following TMUB1 downregulation, the western blotting indicated the expression of STAT1 was downregulated, and CCND1 was upregulated. (F) Following TMUB1 downregulation, the q-PCR indicated the change of expression of STAT1 and CCND1 was consistent with the western blots. TMUB1 – transmembrane and ubiquitin-like domain-containing protein 1; STAT1 – signal transducer and activator of transcription 1; HCC – hepatocellular carcinoma; q-PCR – quantitative polymerase chain reaction.
TMUB1 suppressed HCC proliferation via regulating STAT1 signaling
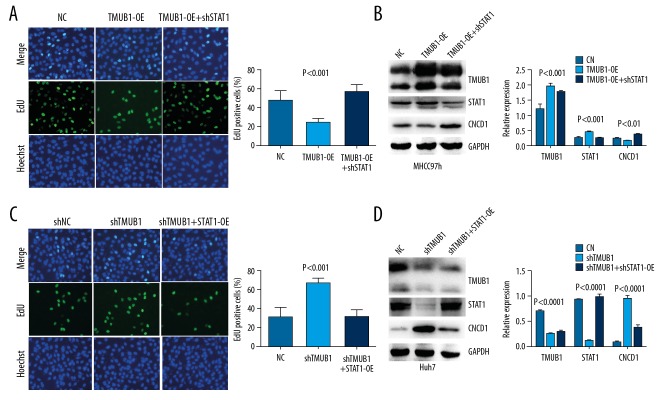
The expression of STAT1 were significantly reduced in MHCC97h cells by STAT1 interference plasmid. The STAT1 overexpression plasmid significantly increased STAT1 expression in Huh7 cells. An EdU assay indicated that MHCC97h cells proliferation were significantly suppressed by overexpression of TMUB1 and were rescued by STAT1 interference plasmid. In contrast, the promotive effect of TMUB1 knockdown on proliferation in Huh7 cells was abolished by STAT1 overexpression plasmid. These data demonstrate that TMUB1 suppresses HCC proliferation via regulating expression of STAT1 at least partially (Figure 5).
Figure 5.
TMUB1 suppresses HCC proliferation via regulating STAT1. (A) An EdU assay indicated that the STAT1 interference plasmid rescued the inhibition of TMUB1 on the proliferation of MHCC97h cells. (B) Western blotting detected TMUB1, STAT1 and CCND1 proteins in MHCC97h cells. (C) An EdU assay indicated that the STAT1 overexpression plasmid rescued the promotion of shTMUB1 on the proliferation of Huh7 cells. (D) Western blotting detected TMUB1, STAT1, and CCND1 proteins in Huh7 cells. TMUB1 – transmembrane and ubiquitin-like domain-containing protein 1; HCC – hepatocellular carcinoma; STAT1 – signal transducer and activator of transcription 1; EdU – 5-ethynyl-2′-deoxyuridine.
Discussion
The proliferation of normal cells is closely regulated by various mechanisms. When the mechanisms relating to the regulation of cell proliferation are disordered, abnormal proliferation may occur, which may cause tumorigenesis. Current studies indicate that cell proliferation is mainly regulated by the phosphatidylinositol [34], JAK-STAT [35,36], and PIK3-AKT-mTOR signaling pathways [37,38]. The signaling molecules inside and outside the cells initiate a proliferation-related signaling pathway and tightly regulate cell proliferation under the action of various positive and negative regulatory factors. When there exists an imbalance in this regulation, abnormal cell proliferation can manifest, which may cause tumorigenesis and development [39,40].
Hepatocytes have a strong ability to regenerate, and this ability is initiated when liver tissue is damaged. The regeneration process is closely regulated by various cytokines, growth factors and hormones. Our previous research had indicated that TMUB1 is a protein associated with cell cycle progressing during liver regeneration [41]. After 70% hepatectomy, TMUB1 expression was upregulated in regenerated liver tissue and suppressed hepatocyte proliferation. Recent studies have shown that TMUB1 overexpression suppresses hepatocyte proliferation by reducing the expression of cell cycle related genes. Conversely, interfering expression of TMUB1 obviously promoted proliferation of hepatocyte. However, whether the inhibitory effect of TMUB1 on hepatocytes is also present in HCC cells has not been reported.
We collected 132 cases of HCC and analyzed differences in the tumor size, HCC differentiation, TNM stage, extrahepatic metastasis, lymph node metastasis, vascular invasion, and prognosis of patients in the TMUB1 high expression group and the TMUB1 low expression group. We found that TMUB1 was negatively correlated with HCC malignancy, and low expression of TMUB1 indicated poor prognosis. Furthermore, cell experiments confirmed that TMUB1 expression suppressed the proliferation of HCC cells but not the metastasis of HCC cells. Recent research has indicated that TMUB1 can inhibit the biological activity of STAT3, and STAT3 and STAT1 have antagonistic effects. Therefore, we analyzed the correlation between the expression of TMUB1 and the expression of STAT1 in 132 cases of HCC. The expression of TMUB1 and STAT1 was positively correlated, and then confirmed by molecular experiments. TMUB1 promotes STAT1 expression while inhibiting the expression of the CCND1.
STAT1 is important part of JAK/STAT pathway and is a key molecule in various interferon-mediated biological processes. Current research has indicated that the expression of STAT1 is downregulated in a variety of tumor cells, and low STAT1 expression in tumor patients often indicates poor prognosis [42–44]. STAT3 and STAT1 have antagonistic effects, and the balance of STAT3 and STAT1 expression and activity is critical in maintaining the regulatory effect on downstream cytokines and growth factors [45,46]. STAT3 and STAT1 compete for binding the same receptor, target promoter and other cofactors. For example, competitive binding to IFN gamma receptor (IFNGR) contains an essential domain involved in JAK activation via tyrosine phosphorylation, and upregulation of STAT1 will affect STAT3 phosphorylation [47]. The DNA binding sequence of STAT1 and STAT3 is 72% identical. Upregulation of STAT1 expression will affect the binding of STAT3 to its downstream gene promoter and thus inhibit the expression of genes downstream of STAT3 [48,49]. Both STAT1 and STAT3 can be expressed in many isoforms, and these isoforms can affect the formation of STAT3 and antagonize STAT3 function. Previous studies have shown that TMUB1 can inhibit the phosphorylation but not the expression of STAT3 [50]. Our results reveal that TMUB1 affects STAT1 expression and can inhibit HCC cell proliferation by regulating 2 key molecules, STAT1 and STAT3, in JAK/STAT signaling pathways. However, the specific mechanism by which TMUB1 inhibits STAT3 phosphorylation and promotes STAT1 expression remains unclear and needs further study (Figure 6).
Figure 6.
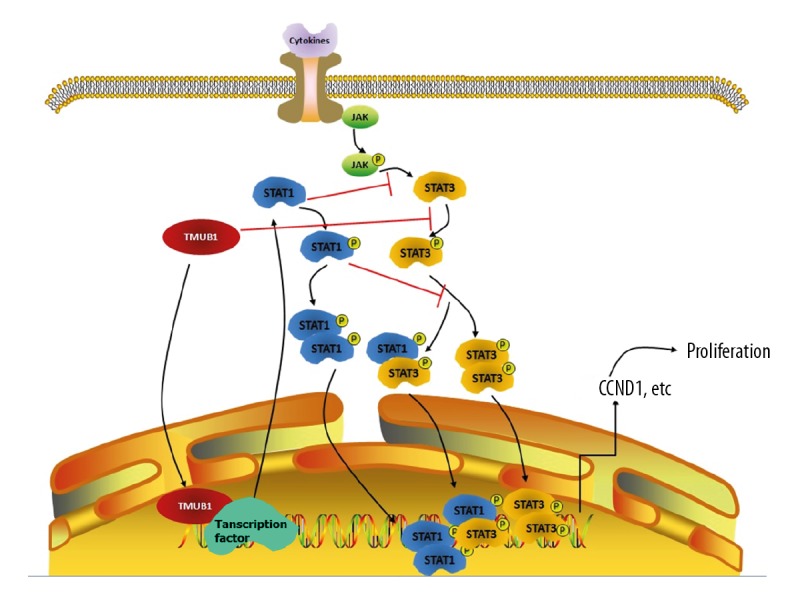
Mechanism diagram.
Conclusions
TMUB1 suppresses hepatocellular carcinoma proliferation via regulating STAT1.
Acknowledgments
We are particularly grateful to the Department of Hepatobiliary Surgery, Army Medical Center, for providing paraffin specimens of HCC and cell lines.
Footnotes
Source of support: Supported by the National Natural Science Foundation of China (grant number: 81270523)
References
- 1.Sun L-Y, Zhang H, Li ZL, et al. How to predict global trends in HCC mortality if neglecting more than half the world’s cases? J Hepatol. 2017;67(4):887–88. doi: 10.1016/j.jhep.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 2.Zhu RX, Seto WK, Lai CL, Yuen MF. Epidemiology of hepatocellular carcinoma in the Asia-Pacific region. Gut Liver. 2016;10(3):332–39. doi: 10.5009/gnl15257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen J, Wu G. Retrospective analysis of Chinese patients with hepatocellular carcinoma (HCC) undergoing transcatheter arterial chemoembolization (TACE) with or without prophylactic antibiotic therapy. J Coll Physicians Surg Pak. 2018;28(12):914–18. doi: 10.29271/jcpsp.2018.12.914. [DOI] [PubMed] [Google Scholar]
- 4.Kelley RK. Emerging role for systemic therapy in earlier stages of HCC. Int J Radiat Oncol Biol Phys. 2018;102(5):1407. doi: 10.1016/j.ijrobp.2018.08.062. [DOI] [PubMed] [Google Scholar]
- 5.Toyoda H, Kumada T, Tada T, et al. The impact of HCV eradication by direct-acting antivirals on the transition of precancerous hepatic nodules to HCC: A prospective observational study. Liver Int. 2019;39(3):448–54. doi: 10.1111/liv.13987. [DOI] [PubMed] [Google Scholar]
- 6.Kim DW, Talati C, Kim R. Hepatocellular carcinoma (HCC): Beyond sorafenib-chemotherapy. J Gastrointest Oncol. 2017;8(2):256–65. doi: 10.21037/jgo.2016.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perrella A, Izzi A, Punzi R, et al. Late HCC onset after direct antiviral agents therapy in patients with sustained virological response: Do we need to reconsider their efficacy according to long term follow-up? Scand J Gastroenterol. 2018;53(8):1025–26. doi: 10.1080/00365521.2018.1495260. [DOI] [PubMed] [Google Scholar]
- 8.Villani R, Vendemiale G, Serviddio G. Molecular mechanisms involved in HCC recurrence after direct-acting antiviral therapy. Int J Mol Sci. 2018;20(1) doi: 10.3390/ijms20010049. pii: E49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Della Fazia MA, Castelli M, Bartoli D, et al. HOPS: A novel cAMP-dependent shuttling protein involved in protein synthesis regulation. J Cell Sci. 118(Pt 14):3185–94. doi: 10.1242/jcs.02452. 200. [DOI] [PubMed] [Google Scholar]
- 10.Castelli M, Piobbico D, Bartoli D, et al. Different functions of HOPS isoforms in the cell: HOPS shuttling isoform is determined by RIP cleavage system. Cell Cycle. 2014;13(2):293–302. doi: 10.4161/cc.27054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu M, Liu H, Wang X, et al. IL-6 induction of hepatocyte proliferation through the Tmub1-regulated gene pathway. Int J Mol Med. 2012;29(6):1106–12. doi: 10.3892/ijmm.2012.939. [DOI] [PubMed] [Google Scholar]
- 12.Liu M, Yuan T, Liu H, Chen P. CCAAT/enhancer-binding protein beta regulates interleukin-6-induced transmembrane and ubiquitin-like domain containing 1 gene expression in hepatocytes. Mol Med Rep. 2014;10(4):2177–83. doi: 10.3892/mmr.2014.2457. [DOI] [PubMed] [Google Scholar]
- 13.Lan X, Li G, Liu H, et al. MiR-27a/b regulates liver regeneration by posttranscriptional modification of Tmub1. Dig Dis Sci. 2018;63(9):2362–72. doi: 10.1007/s10620-018-5113-5. [DOI] [PubMed] [Google Scholar]
- 14.Fu H, Dong R, Zhang Y, et al. Tmub1 negatively regulates liver regeneration via inhibiting STAT3 phosphorylation. Cell Signal. 2019;55:65–72. doi: 10.1016/j.cellsig.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Lan X, Fu H, Li G, et al. TMUB1 inhibits BRL-3A hepatocyte proliferation by interfering with the binding of CAML to cyclophilin B through its TM1 hydrophobic domain. Sci Rep. 2018;8(1):9917. doi: 10.1038/s41598-018-28339-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castelli M, Pieroni S, Brunacci C, et al. Hepatocyte odd protein shuttling (HOPS) is a bridging protein in the nucleophosmin-p19 Arf network. Oncogene. 2013;32(28):3350–58. doi: 10.1038/onc.2012.353. [DOI] [PubMed] [Google Scholar]
- 17.Meissl K, Macho-Maschler S, Müller M, Strobl B. The good and the bad faces of STAT1 in solid tumours. Cytokine. 2017;89:12–20. doi: 10.1016/j.cyto.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 18.Leon-Cabrera S, Vázquez-Sandoval A, Molina-Guzman E, et al. Deficiency in STAT1 signaling predisposes gut inflammation and prompts colorectal cancer development. Cancers (Basel) 2018;10(9) doi: 10.3390/cancers10090341. pii: E341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Xing L, Zhao N, et al. Furosine induced apoptosis by the regulation of STAT1/STAT2 and UBA7/UBE2L6 genes in HepG2 cells. Int J Mol Sci. 2018;19(6) doi: 10.3390/ijms19061629. pii: E1629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Wang H, Wang J, et al. STAT1 inhibits human hepatocellular carcinoma cell growth through induction of p53 and Fbxw7. Cancer Cell Int. 2015;15:111. doi: 10.1186/s12935-015-0253-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Y, Chen Y, Yun H, et al. STAT1beta enhances STAT1 function by protecting STAT1alpha from degradation in esophageal squamous cell carcinoma. Cell Death Dis. 2017;8(10):e3077. doi: 10.1038/cddis.2017.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sun Y, Yang S, Sun N, Chen J. Differential expression of STAT1 and p21 proteins predicts pancreatic cancer progression and prognosis. Pancreas. 2014;43(4):619–23. doi: 10.1097/MPA.0000000000000074. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi A, Nakayama R, Ishibashi N, et al. Analysis of gene expression profiles of soft tissue sarcoma using a combination of knowledge-based filtering with integration of multiple statistics. PLoS One. 2014;9(9):e106801. doi: 10.1371/journal.pone.0106801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Osborn JL, Greer SF. Metastatic melanoma cells evade immune detection by silencing STAT1. Int J Mol Sci. 2015;16(2):4343–61. doi: 10.3390/ijms16024343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimco G, Knight RA, Latchman DS, Stephanou A. STAT1 interacts directly with cyclin D1/Cdk4 and mediates cell cycle arrest. Cell Cycle. 2010;9(23):4638–49. doi: 10.4161/cc.9.23.13955. [DOI] [PubMed] [Google Scholar]
- 26.Wang S, Raven JF, Koromilas AE. STAT1 represses Skp2 gene transcription to promote p27Kip1 stabilization in Ras-transformed cells. Mol Cancer Res. 2010;8(5):798–805. doi: 10.1158/1541-7786.MCR-10-0027. [DOI] [PubMed] [Google Scholar]
- 27.Cao ZH, Zheng QY, Li GQ, et al. STAT1-mediated down-regulation of Bcl-2 expression is involved in IFN-gamma/TNF-alpha-induced apoptosis in NIT-1 cells. PLoS One. 2015;10(3):e0120921. doi: 10.1371/journal.pone.0120921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen Y, Fang L, Zhang J, et al. Blockage of glyoxalase I inhibits colorectal tumorigenesis and tumor growth via upregulation of STAT1, p53, and Bax and downregulation of c-Myc and Bcl-2. Int J Mol Sci. 2017;18(3) doi: 10.3390/ijms18030570. pii: E570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meyer Zu Horste G, Przybylski D, Schramm MA, et al. Fas promotes T helper 17 cell differentiation and inhibits T helper 1 cell development by binding and sequestering transcription factor STAT1. Immunity. 2018;48(3):556–69 e7. doi: 10.1016/j.immuni.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 30.Nam YR, Lee KJ, Lee H, Joo CH. CXCL10 production induced by high levels of IKKepsilon in nasal airway epithelial cells in the setting of chronic inflammation. Biochem Biophys Res Commun. 2019;514(3):607–12. doi: 10.1016/j.bbrc.2019.04.173. [DOI] [PubMed] [Google Scholar]
- 31.Arger NK, Ho M, Woodruff PG, Koth LL. Serum CXCL11 correlates with pulmonary outcomes and disease burden in sarcoidosis. Respir Med. 2019;152:89–96. doi: 10.1016/j.rmed.2019.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Z, Zhang Y, Chen Y, et al. STAT1 inhibits STAT3 activation in esophageal squamous cell carcinoma. Cancer Manag Res. 2018;10:6517–23. doi: 10.2147/CMAR.S182105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiong B, Qian H. Effects of Sijunzi decoction and Yupingfeng powder on expression of Janus kinase-signal transducer and activator of transcription signal pathway in the brain of spleen-deficiency model rats. J Tradit Chin Med. 2013;33(1):78–84. doi: 10.1016/s0254-6272(13)60105-3. [DOI] [PubMed] [Google Scholar]
- 34.Snoek GT. Phosphatidylinositol transfer proteins: Emerging roles in cell proliferation, cell death and survival. IUBMB Life. 2004;56(8):467–75. doi: 10.1080/15216540400012152. [DOI] [PubMed] [Google Scholar]
- 35.Bifulco M. Role of the isoprenoid pathway in Ras transforming activity, cytoskeleton organization, cell proliferation and apoptosis. Life Sci. 2005;77(14):1740–49. doi: 10.1016/j.lfs.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 36.Cook SJ, Lockyer PJ. Recent advances in Ca(2+)-dependent Ras regulation and cell proliferation. Cell Calcium. 2006;39(2):101–12. doi: 10.1016/j.ceca.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 37.Isenović ER, Kedees MH, Tepavcević S, et al. Role of PI3K/AKT, cPLA2 and ERK1/2 signaling pathways in insulin regulation of vascular smooth muscle cells proliferation. Cardiovasc Hematol Disord Drug Targets. 2009;9(3):172–80. doi: 10.2174/187152909789007034. [DOI] [PubMed] [Google Scholar]
- 38.De Santis MC, Sala V, Martini M, et al. PI3K signaling in tissue hyper-proliferation: from overgrowth syndromes to kidney cysts. Cancers (Basel) 2017;9(4) doi: 10.3390/cancers9040030. pii: E30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jarrett AM, Lima EABF, Hormuth DA, 2nd, et al. Mathematical models of tumor cell proliferation: A review of the literature. Expert Rev Anticancer Ther. 2018;18(12):1271–86. doi: 10.1080/14737140.2018.1527689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garay TM. [Oncogen dependent regulation of the migration and proliferation of human tumor cells]. Magy Onkol. 2016;60(4):339–42. [in Hungarian] [PubMed] [Google Scholar]
- 41.Fu H, Xu J, Chen J, et al. Microarray analysis reveals Tmub1 as a cell cycle-associated protein in rat hepatocytes. Mol Med Rep. 2018;17(3):4337–44. doi: 10.3892/mmr.2018.8451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Y, Liu Z. STAT1 in cancer: Friend or foe? Discov Med. 2017;24(130):19–29. [PubMed] [Google Scholar]
- 43.Gambin A, Charzyńska A, Ellert-Miklaszewska A, Rybiński M. Computational models of the JAK1/2-STAT1 signaling. JAKSTAT. 2013;2(3):e24672. doi: 10.4161/jkst.24672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shodeinde A, Ginjupalli K, Lewis HD, et al. STAT3 inhibition induces apoptosis in cancer cells independent of STAT1 or STAT2. J Mol Biochem. 2013;2(1):18–26. [PMC free article] [PubMed] [Google Scholar]
- 45.Avalle L, Pensa S, Regis G, et al. STAT1 and STAT3 in tumorigenesis: A matter of balance. JAKSTAT. 2012;1(2):65–72. doi: 10.4161/jkst.20045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wincewicz A, Sulkowska M, Rutkowski R, et al. STAT1 and STAT3 as intracellular regulators of vascular remodeling. Eur J Intern Med. 2007;18(4):267–71. doi: 10.1016/j.ejim.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 47.Concha-Benavente F, Srivastava RM, Ferrone S, Ferris RL. EGFR-mediated tumor immunoescape: the imbalance between phosphorylated STAT1 and phosphorylated STAT3. Oncoimmunology. 2013;2(12):e27215. doi: 10.4161/onci.27215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wan CK, Andraski AB, Spolski R, et al. Opposing roles of STAT1 and STAT3 in IL-21 function in CD4+ T cells. Proc Natl Acad Sci USA. 2015;112(30):9394–99. doi: 10.1073/pnas.1511711112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nivarthi H, Gordziel C, Themanns M, et al. Correction: the ratio of STAT1 to STAT3 expression is a determinant of colorectal cancer growth. Oncotarget. 2018;9(73):33865. doi: 10.18632/oncotarget.26156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Regis G, Pensa S, Boselli D, et al. Ups and downs: The STAT1: STAT3 seesaw of Interferon and gp130 receptor signalling. Semin Cell Dev Biol. 2008;19(4):351–59. doi: 10.1016/j.semcdb.2008.06.004. [DOI] [PubMed] [Google Scholar]