Abstract
Human trophoblast syncytialization is one of the most important yet least understood events during placental development. In this study, we found that detyrosinated α-tubulin (detyr-α-tub), which is negatively regulated by tubulin tyrosine ligase (TTL), was elevated during human placental cytotrophoblast fusion. Correspondingly, relatively high expression of TTL protein was observed in first-trimester human placental cytotrophoblast cells, but fusing trophoblast cells exhibited much lower levels of TTL. Notably, fusion of preeclamptic cytotrophoblast cells was compromised but could be partially rescued by knockdown of TTL levels. Mechanistically, chronic downregulation of TTL in trophoblast cells resulted in significantly elevated expression of detyr-α-tub. Restoration of detyr-α-tub thus contributed to the cell surface localization of the fusogenic protein Syncytin-2 and the gap junction protein Connexin 43 (Cx43), which in turn promoted successful fusion between trophoblast cells. Taken together, the results suggest that tubulin detyrosination plays an essential role in human trophoblast fusogenic protein aggregation and syncytialization. Insufficient tubulin detyrosination leads to defects in syncytialization and potentially to the onset of preeclampsia.
Keywords: TTL, detyrosination, syncytialization, preeclampsia, placenta, syncytin
Introduction
Cell–cell fusion processes are complex biological phenomena essential for placentation, fetal development, zygote formation (fertilization), skeletal muscle formation, and bone homeostasis (Aguilar et al., 2013). In the human placenta, trophoblast cell fusion results in the formation of the syncytiotrophoblast (STB), the site of many placental functions, such as exchange of nutrients and gases, elimination of wastes, and secretion of hormones required for pregnancy (Huppertz and Gauster, 2011). Trophoblast cell fusion is multifactorial and highly regulated. Various vital molecules, including membrane-localized proteins, have been described to be directly involved in this process. Syncytins, including Syncytin-1 and Syncytin-2 (Mi et al., 2000; Blaise et al., 2003), are bona fide glycoproteins encoded by the human endogenous retrovirus (HERV)-W and HERV-FRD envelope genes, respectively. Syncytin-2, which is mainly localized in fusing villous cytotrophoblast cells (CTBs), is thought to play a dominant role during syncytialization (Lu et al., 2017). Active caspase-8 , with a similar expression pattern as Syncytin-2, is essential for cytoskeletal reorganization during trophoblast syncytialization (Gauster et al., 2010). Connexin 43 (Cx43)/gap junction A1 (GJA1)-mediated gap junctional intercellular communication is also involved in controlling trophoblast cell fusion (Frendo et al., 2003; Dunk et al., 2012). Moreover, Cx43 could anchor the distal ends of microtubules to gap junctions through a tubulin-binding motif in its tail (Giepmans et al., 2001).
Microtubules, polymers of α-/β-tubulin heterodimers, interact with many microtubule-associated proteins to perform a variety of cellular functions, including intracellular transport and cell division. Posttranslational modifications on α-tubulin include acetylation, tyrosination, detyrosination, polyglutamylation, polyglycylation and phosphorylation, and these modifications are responsible for regulating various aspects of microtubule functions (Hammond et al., 2008). Tubulin tyrosine ligase (TTL) is the only known ligase to ligate a tyrosine to the C-terminal of α-tubulin and concurrently converts detyrosinated α-tubulin (detyr-α-tub) into tyrosinated α-tubulin (tyr-α-tub; Szyk et al., 2011). Detyrosination of α-tubulin may lead to stabilization of microtubules and inhibition of microtubule disassembly (Akhmanova and Steinmetz, 2008; Roll-Mecak and Vale, 2008; Sirajuddin et al., 2014). In our previous RNA-seq study, TTL was found to be significantly downregulated during the fusion of human trophoblast cells (Zheng et al., 2017). This observation raises an interesting question: whether and how the TTL-mediated tubulin tyrosination/detyrosination balance is involved in human trophoblast syncytialization.
Preeclampsia (PE) is a pregnancy-specific multisystem disorder characterized by gestational hypertension, proteinuria, and other systemic disturbances. PE affects up to 5%–8% of all pregnancies, and PE contributes substantially to maternal and perinatal morbidity and mortality worldwide (Ho et al., 2017). The pathophysiology of PE remains largely unknown. However, it is generally accepted that the placenta is the principal contributor to the pathogenesis of PE because the only curative treatment for PE is the delivery of the infant and placenta (Fisher, 2004). Mechanistically, shallow invasion of placental trophoblast cells and the release of excess quantities of antiangiogenic factors, such as soluble fms-like tyrosine kinase 1 and soluble endoglin, by the STB (Maynard et al., 2003; Venkatesha et al., 2006) may both contribute to the onset of this disease. Furthermore, Syncytin-1, Syncytin-2, and their transcription factor glial cells missing homolog 1 are decreased in preeclamptic placentae compared with normal placentae (Chen et al., 2004; Vargas et al., 2011; Zhuang et al., 2014). Similarly, electron microscopy technology has identified impairment of STBs in preeclamptic placentae; compared with those in normal placentae, the syncytia of preeclamptic placentae show discontinuation, have multiple vacuoles, and are thin (MacLennan et al., 1972). The above evidence implies that dysregulation of syncytialization may be associated with PE. However, the underlying mechanisms remain unclear.
In this study, we found that the detyrosination of microtubules was increased during human trophoblast syncytialization. Constant depletion of detyr-α-tub by overexpression of TTL resulted in compromised syncytialization, whereas enhancement of detyr-α-tub by knockdown of TTL led to increased syncytium formation. Additionally, mutations inactivating TTL attenuated its inhibitory effects on cell fusion. Perturbed detyrosination diminished the accumulation of cell surface membrane-localized Syncytin-2 and Cx43, while enhanced detyrosination promoted the enrichment of Syncytin-2 and Cx43 on the cell membrane. Furthermore, regulation of tubulin detyrosination was compromised in preeclamptic trophoblast syncytialization, and knockdown of TTL restored the fusion capacity of cytotrophoblast cells derived from preeclamptic placentae.
Results
Detyrosination of α-tubulin is markedly increased during spontaneous fusion of primary CTBs
To uncover unknown factors involved in the regulation of trophoblast cell–cell fusion, we performed RNA-seq analysis on human trophoblast cells (Zheng et al., 2017) that were induced to undergo syncytialization through treatment with forskolin (Figure 1A). Mononucleated BeWo cells (control, treated with DMSO) and fused multinucleated BeWo cells (treated with forskolin) were carefully collected and subjected to RNA-seq analysis. Among those genes whose mRNA levels showed significant expression changes during cell–cell fusion, TTL was dramatically decreased in fused cells compared to control cells.
Figure 1.
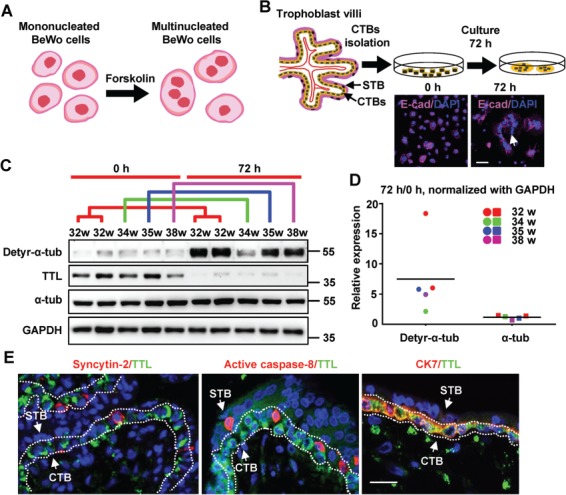
Augmented detyrosination of α-tubulin during spontaneous fusion of primary CTBs. (A) Illustrative model depicting BeWo cell fusion. (B) Upper panel: schematic illustration of spontaneous syncytialization of human primary CTBs after isolation. Lower panel: human trophoblasts stained at 0 and 72 h of culture for E-cadherin and nuclei. Scale bar, 50 μm. (C) Results of western blotting using the indicated antibodies on primary CTBs from the indicated gestational ages and their corresponding syncytia after 72 h of incubation. GAPDH was used as a loading control (here and hereafter). The colored lines represent groups of corresponding gestational ages. (D) Quantification of the western blot data in C. The band intensities detected with anti-α-tubulin and anti-detyr-α-tub antibodies were normalized to that of GAPDH as a control. The dots and squares representing individual samples from patients are colored according to the matching samples at different time points. The horizontal lines represent the means. (E) For confocal microscopy, Alexa Fluor 488 (green) was used to label TTL. Alexa Fluor 555 (red) was used to label Syncytin-2, active caspase-8, or CK7. Scale bar, 10 μm. The arrows indicate a layer of mononucleated CTBs and a layer of multinucleated STB.
To confirm the RNA-seq data, we next selectively isolated mononucleated CTBs from human term placental villi and carefully cultured these cells using standard complete culture medium in a 37°C incubator with CO2. As shown in Figure 1B, multinucleated syncytia began to form due to spontaneous cell–cell fusion. CTBs cultured in vitro for 0 or 72 h were harvested and subjected to western blotting assays using a monoclonal anti-TTL antibody to examine the protein expression levels of TTL during trophoblast cell fusion. Consistent with the results of the RNA-seq analysis in BeWo cells, while TTL protein was relatively highly abundant in control CTB lysate, it was hardly detected in fused CTB lysate (Figure 1C). TTL, a tyrosination ligase that mainly converts detyr-α-tub into tyr-α-tub, has been shown to regulate many cellular processes, such as cell cycle progression, protein translocation, and cell migration. To address whether the tyrosination and/or detyrosination levels of α-tubulin varied during the process of trophoblast syncytialization, we performed western blotting assays using a monoclonal anti-detyr-α-tub antibody. As shown in Figure 1D, the protein levels of detyr-α-tub were sharply increased (by 7- to 8-fold) in the syncytia compared to the initial CTBs. Correspondingly, no obvious changes in the expression of total α-tubulin were observed, suggesting that the upregulation of detyr-α-tub was due to a decrease in TTL protein and thus to prevention of tyrosination but not to de novo translation of tubulin protein during trophoblast cell–cell fusion. To further determine the exact expression pattern of TTL in fusion-competent CTBs ex vivo, we collected placental villi after 7 weeks of gestation for immunofluorescence microscopy. The CTBs were all positive for cytokeratin 7 (CK7) immunostaining (Figure 1E, right panel), and those exhibiting positive immunostaining for either Syncytin-2 or active caspase-8 were fusion-competent cells (Figure 1E, left and middle panels). Interestingly, we observed TTL immunostaining only in CTBs, not in fusion-competent cells or the STB (Figure 1E). These results indicate that during the formation of functional syncytia, the tyrosine ligase TTL is significantly downregulated, which in turn causes the apparent elevation of detyr-α-tub, implying a possible regulatory relationship among TTL, detyr-α-tub, and trophoblast cell fusion.
Decreased TTL expression and increased detyr-α-tub expression during BeWo cell fusion
Based on the dynamic changes in TTL and detyr-α-tub protein levels during trophoblast syncytialization, we next sought to determine whether precise regulation of the tyrosination modification pattern of α-tubulin by TTL is essential for trophoblast cell–cell fusion. To this end, we turned our attention to the in vitro BeWo cell system. First, we confirmed our previous conclusions that the levels of detyr-α-tub were increased during BeWo cell syncytialization and that the expression of TTL was significantly decreased in parallel with that of tyr-α-tub via western blotting (Figure 2A and B) and immunofluorescence analysis (Figure 2C). Moreover, as expected, the cellular localization of detyr-α-tub, to a certain extent, was complementary to that of TTL (Figure 2D) in BeWo cells.
Figure 2.
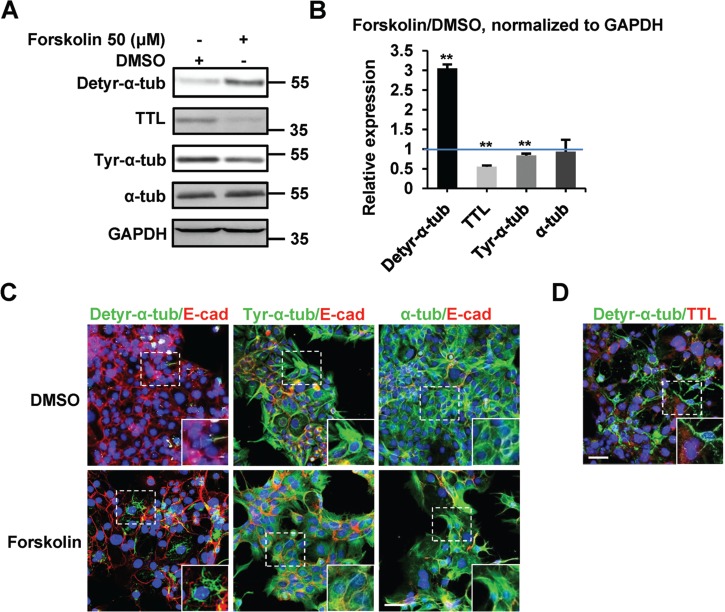
Levels of detyr-α-tub during BeWo syncytialization. (A) Western blotting of BeWo cells treated with DMSO (control) or forskolin for 48 h using the indicated antibodies. GAPDH was used as a loading control. (B) Quantification of the western blot data in A, shown as the relative expression levels of individual molecules normalized to the levels of GAPDH in DMSO-treated samples. The blue line indicates a relative expression level of 1. **P < 0.01, *P < 0.05, n = 3. (C and D) Confocal microscopy images of BeWo cells under the indicated treatments (48 h) using the indicated antibodies. E-cadherin (red) was used to label the boundaries of mononucleated or multinucleated cells. Nuclei were stained with DAPI (blue). All images are of the same magnification, as shown by the scale bar (20 μm).
TTL negatively regulates BeWo cell–cell fusion
Next, we aimed to enhance TTL expression in BeWo cell culture by establishing a stable cell line overexpressing TTL (BeWoTTL, with BeWoGFP serving as a control line) through lentivirus infection (Figure 3A). As shown in Figure 3C, upon forskolin treatment to induce cell fusion, we observed rapid formation of multinucleated syncytia in the control cells but not in the cells overexpressing TTL. As shown in Figure 3B, the total fusion index was significantly lower in forskolin-treated BeWoTTL cells than in treated control cells. Interestingly, compared with the controls, most of the fused BeWoTTL cells were restricted to 2N, suggesting that the elevation in TTL expression inhibited the process of BeWo cell fusion.
Figure 3.
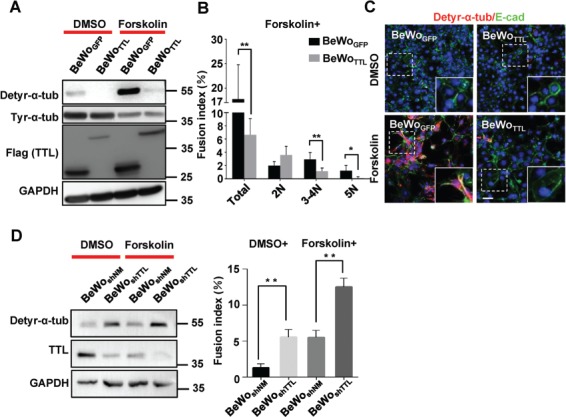
Fusion of human trophoblast BeWo cells is negatively regulated by TTL. (A) Results of western blotting for BeWoGFP and BeWoTTL cells treated with DMSO or forskolin for 48 h using anti-tyr-α-tub, anti-detyr-α-tub, anti-Flag, or anti-GAPDH antibodies. To detect each type of modified α-tubulin, the samples were blotted separately. (B) The fusion index was determined contemporaneously with the samples harvested for western blotting. The total and assorted fusion indices are shown in the bar graph. **P < 0.01, *P < 0.05, n = 3. (C) For confocal microscopy, Alexa Fluor 488 and Alexa Fluor 555 were used to label E-cadherin and detyr-α-tub, respectively, in BeWoGFP or BeWoTTL cells treated with DMSO or forskolin for 48 h. Nuclei were stained with DAPI (blue). Scale bar, 20 μm. (D) TTL was stably knocked down using lentiviral shRNA in cells without any treatment or with forskolin treatment for 48 h. The fusion index was determined contemporaneously with the samples harvested for western blotting. **P < 0.01, *P < 0.05, n = 3.
To further explore whether TTL negatively regulates forskolin-induced BeWo cell fusion, we transiently knocked down TTL expression via transfection of BeWo cells with siRNAs targeting TTL. As shown in Supplementary Figure S1A, a decrease in TTL led to a significant, but relatively moderate, increase in the fusion index of BeWo cells treated with forskolin compared to control cells. To abolish the endogenous cellular expression of TTL with higher efficiency, we established a stable TTL knockdown line of BeWo cells (BeWoshTTL, with BeWoshNM serving as a control line) and again cultured these cells with forskolin to induce syncytialization. As shown in Figure 3D, compared to the BeWoshNM control group, the BeWoshTTL group had a significantly higher total fusion index. Moreover, even without forskolin treatment, the spontaneous fusion of BeWo cells was increased in the BeWoshTTL group compared to the BeWoshNM group; indeed, it was hardly observed in the BeWoshNM group. We strongly suggest that the tyrosine ligase TTL plays a key role in negatively regulating the fusion process of BeWo cells.
The TTL-regulated tyrosination pattern of α-tubulin determines BeWo cell fusion fate
Given the findings that TTL controls the tyrosination of detyr-α-tub and the formation of multinucleated BeWo cells in the presence of forskolin, we hypothesized that inhibition of TTL would enhance BeWo cell fusion by promoting the expression of detyr-α-tub. While overexpression of TTL resulted in a substantial decrease in detyr-α-tub in both forskolin-treated and control (DMSO-treated) BeWo cells after 48 h and a significant inhibition of BeWo cell fusion (Figure 3A), depletion of TTL by siRNAs in BeWoTTL cells caused a significant elevation in detyr-α-tub and an increase in the fusion index (Figure 4A). To provide direct evidence that detyr-α-tub is indeed able to increase BeWo cell syncytialization, we overexpressed Vasohibin-2 (VASH2), a tubulin carboxypeptidase that cleaves the terminal tyrosine of α-tubulin, in BeWo cells to produce detyr-α-tub in vitro (Aillaud et al., 2017; Nieuwenhuis et al., 2017). As presented in Figure 4B, overexpression of VASH2 modestly but significantly increased the expression of detyr-α-tub and the fusion of BeWo cells. To verify the exact regulatory relationship between detyr-α-tub and BeWo cell syncytialization, we utilized a mutated TTL in which Arg202 of the tyrosination catalytic domain was substituted with alanine (TTL-R202A) (Supplementary Figure S1B; Szyk et al., 2011) and established a stable cell line, BeWoTTLmut, with the same procedure used to create the BeWoTTL cell line. Exogenous mutated TTL was expressed at levels similar to those of wild-type TTL, but the inhibition of detyr-α-tub and subsequent fusion ability were attenuated (Figure 4C) in the BeWoTTLmut group compared to the BeWoTTL group, confirming that catalytically deficient TTL had lost the ability to inhibit BeWo cell–cell fusion. Collectively, our findings support the idea that inhibition of TTL expression leads to high levels of detyr-α-tub, which in turn promotes trophoblast syncytialization.
Figure 4.
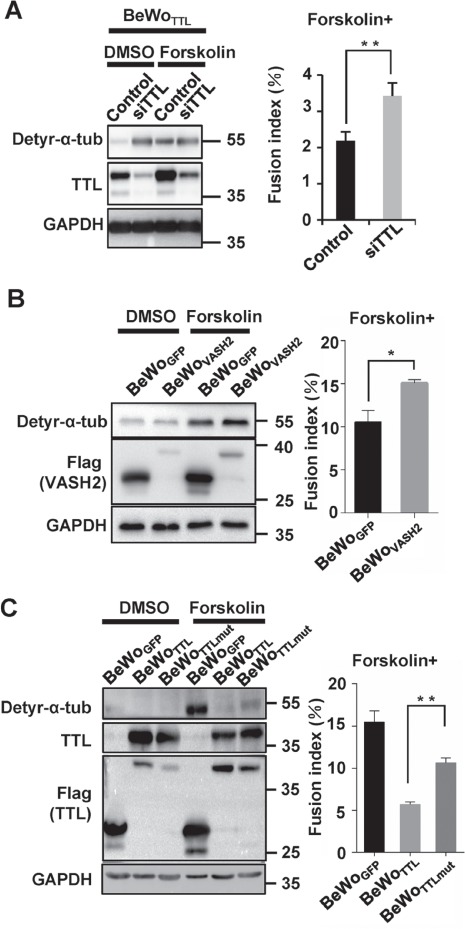
Reconstitution of the expression of detyr-α-tub attenuates the fusion deficiency caused by TTL overexpression. (A) TTL in BeWoTTL (TTL-overexpressing) cells was knocked down using siRNA. Western blotting was performed using anti-detyr-α-tub, anti-TTL, and anti-GAPDH antibodies. The fusion index was determined contemporaneously with the samples harvested for western blotting. **P < 0.01, *P < 0.05, n = 3. (B) Western blotting of BeWoVASH2 cells (with BeWoGFP serving as a control line) treated with DMSO (control) or forskolin for 48 h using anti-detyr-α-tub, anti-TTL, anti-Flag, or anti-GAPDH antibodies. The fusion index was determined contemporaneously with the samples harvested for western blotting. **P < 0.01, *P < 0.05, n = 3. (C) Western blotting of BeWoTTLmut cells (with BeWoGFP and BeWoTTL cells serving as controls) treated with DMSO or forskolin for 48 h. Western blotting was performed using anti-detyr-α-tub, anti-TTL, anti-Flag, or anti-GAPDH antibodies. The fusion index was determined contemporaneously with the samples harvested for western blotting. **P < 0.01, *P < 0.05, n = 3.
Altered expression of fusogenic proteins on the membranes of BeWo cells with various detyr-α-tub levels
To understand the molecular basis whereby the detyrosination of α-tubulin promotes trophoblast cell fusion, we traced the dynamics of α-tubulin during BeWo cell–cell fusion. Cells carrying EGFP-α-tubulin (displaying green fluorescence) or mCherry-α-tubulin (displaying red fluorescence) were mixed and subjected to forskolin treatment to trigger cell fusion. As shown in Figure 5A and Supplementary Movie S1, the labeled cells approached each other and subsequently formed some protrusions, as visualized by colored microtubules (the dotted line and the white arrows). The microtubules seemed to push the membrane forward to form these protrusions, similar to what has occasionally been found in TTL KO fibroblast cells (Peris et al., 2009). Finally, a syncytium formed, as indicated by the mixing of both red and green probes. Next, we performed superresolution structured illumination microscopy (SR-SIM) to better visualize the distribution patterns of detyr-α-tub and α-tubulin and to determine their correlation in BeWo cells. As depicted in Figure 5B, α-tubulin sections were disrupted by short segments of detyr-α-tub that were unevenly distributed along microtubules, with enrichment in certain regions. Considering that the protrusions were formed potentially by microtubules and more specifically by detyr-α-tub right before cell fusion, we hypothesized that detyr-α-tub is likely essential for the localization and aggregation of some membrane proteins that may be pivotal regulators in the process of cell–cell fusion. To detect the cell surface expression of membrane proteins such as Syncytin-2 and Cx43, we selectively harvested BeWoGFP, BeWoTTL, BeWoshNM, and BeWoshTTL cells for membrane protein purification assays. As shown in Figure 5C, the expression levels of Syncytin-2 and Cx43 in the cell membrane fraction were decreased when TTL was overexpressed (with detyr-α-tub reduced) and enriched when TTL was knocked down (with detyr-α-tub elevated). Notably, no significant changes in the expression of Syncytin-2 and Cx43 were observed in the total cell lysates, in terms of mRNA and protein levels (Figure 5C and Supplementary Figure S2A). We failed to observe any effects on the membrane delivery of several adherens junction proteins (E-cadherin, α-E-catenin, β-catenin, and γ-catenin) and most of the tight junction proteins (Occludin and Claudin-1) when TTL was either overexpressed or knocked down (Supplementary Figure S2B).
Figure 5.
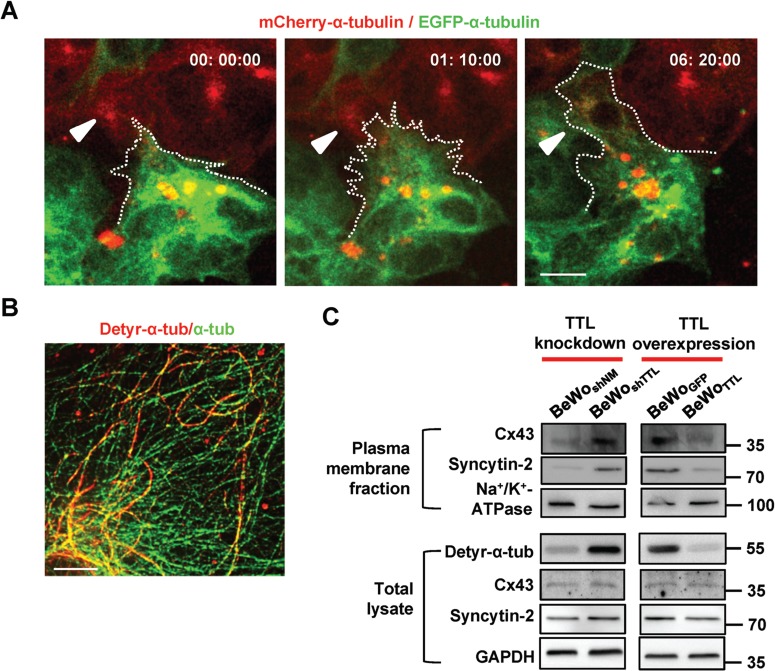
Membrane proteins required for fusion are enriched in detyr-α-tub-overexpressing cell membranes. (A) Time-lapse images of BeWo cells carrying EGFP-α-tubulin or mCherry-α-tubulin. The cells were treated with forskolin to induce fusion. The white arrowheads indicate where fusion occurred. Scale bar, 10 μm. (B) SR-SIM. Alexa Fluor 488 (green) and Alexa Fluor 555 (red) were used to label α-tubulin and detyr-α-tub, respectively, in cells after a 48-h forskolin treatment. Scale bar, 2.5 μm. (C) The influences of TTL overexpression or knockdown on the expression of cell surface proteins in different cell fractions of BeWo cells treated with forskolin. The cell surface proteins were isolated and examined with the indicated antibodies. Na+/K+-ATPase served as a plasma membrane loading control. Whole-cell lysates were harvested and examined with the indicated antibodies. GAPDH served as a loading control.
The levels of detyr-α-tub in CTBs affect syncytialization and pregnancy outcome
We further investigated whether detyrosination of α-tubulin is dysregulated under pathological conditions in pregnancy. To study whether CTBs from preeclamptic placentae have compromised syncytialization, CTBs from PE placentae and gestational age-matched control placentae were subjected to in vitro syncytialization. Western blotting (Figure 6A) and quantification (Figure 6B) revealed a decrease in TTL and an increase in detyr-α-tub expression during the spontaneous fusion of control CTBs, as expected, and the average ratio of detyr-α-tub enrichment in the PE group versus the control group was approximately 1:5. Moreover, the elevation of Syncytin-2 during the spontaneous fusion of CTBs was partially attenuated in the PE group (Figure 6A). These data suggest that trophoblast cell–cell fusion in preeclamptic placentae might be compromised due to abnormal regulation of detyrosination and aberrant expression of fusogenic proteins. We then determined the fusion ability of primary CTBs from PE patients by calculating the fusion index and found that the fusion capacities of CTBs from PE placentae were significantly lower than those of CTBs from age-matched control placentae (Figure 6C). Notably, after knockdown of TTL in CTBs from PE patients with siRNAs, detyr-α-tub was restored, and the defective fusion was significantly rescued (Figure 6D). Taken together, these data demonstrate that insufficient tubulin detyrosination due to high levels of TTL leads to defects in syncytialization and probably to the onset of PE.
Figure 6.
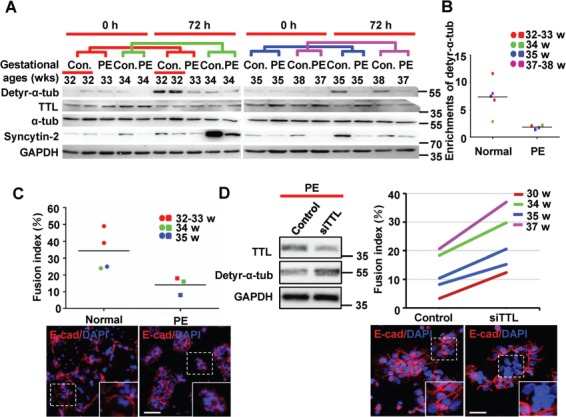
Detyrosination of α-tubulin varies during syncytialization of primary CTBs from normal and PE placentae. (A) Western blotting of primary CTBs isolated from both normal and PE placentae after spontaneous syncytialization for 72 h using anti-detyr-α-tub, anti-TTL, anti-α-tubulin, anti-Syncytin-2, and anti-GAPDH antibodies. The colored lines represent groups of corresponding gestational ages. (B) Quantification of detyr-α-tub from the western blotting data in A. The band intensities detected with anti-detyr-α-tub antibodies were normalized to the band intensity of GAPDH as the loading control. (C) The fusion index was determined contemporaneously with the samples harvested for western blotting in A. For confocal microscopy, Alexa Fluor 555 (red) was used to label E-cadherin to identify the cell boundaries. Nuclei were stained with DAPI (blue). The dots and squares representing individual samples from patients are colored according to the matched gestational ages. The horizontal lines represent the means. (D) Primary CTBs from preeclamptic placentae undergoing in vitro syncytialization were simultaneously treated with TTL siRNA and subjected to western blotting analysis using anti-detyr-α-tub, anti-TTL, and anti-GAPDH antibodies. The fusion indices were determined as in C. The lines in the enrichment and fusion index results represent groups of PE placentae and normal placentae of corresponding gestational ages. Scale bar, 20 μm.
Discussion
In the present study, we demonstrated that detyrosination of α-tubulin and relatively low levels of TTL are critical for human trophoblast syncytialization based on the following lines of evidence. First, fusion-competent cells exhibited lower levels of TTL than other cytotrophoblast cells. Second, detyrosination of α-tubulin was increased, and the levels of TTL were decreased during the fusion of primary CTBs or forskolin-induced BeWo cells. Third, enhancing the expression of TTL resulted in inhibition of α-tubulin detyrosination and cell–cell fusion. Fourth, inhibition of TTL function through multiple experimental approaches led to the promotion of α-tubulin detyrosination and trophoblast cell fusion.
Our work adds to the growing body of evidence that depletion of TTL and detyrosination of α-tubulin could contribute to cell differentiation. Previously, it has been demonstrated that TTL knockout in mice is lethal after birth due to neuronal disorganization and substantive accumulation of detyr-α-tub in the brain (Erck et al., 2005). In neuroblastoma cells, detyr-α-tub is restricted to the microtubules of elongated cell processes, while tyr-α-tub aggregates in the cell bodies (Wehland and Weber, 1987). In suspended mammary epithelial cells, detyr-α-tub accumulates in microtentacles, which facilitates the invasion of the epithelial cells (Mialhe et al., 2001). The invasion and metastasis of breast cancer cells are also related to unusual increases in detyr-α-tub resulting from the downregulated expression of TTL, and detyr-α-tub accumulation in microtentacles accelerates the reattachment of breast cancer cells (Whipple et al., 2013). Early studies on detyr-α-tub were mainly conducted via control of TTL. Recently, Aillaud et al. (2017) and Nieuwenhuis et al. (2017) reported that Vasohibins exhibit TCP functions and positively control the detyrosination of tubulin. In this study, we found that knockdown of TTL or overexpression of VASH2, either of which leads to elevation of detyr-α-tub, can promote cell–cell fusion. All these obsevations demonstrated the tight links between detyr-α-tub and trophoblast cell fusion.
PE is thought to be associated with shallow placental cytotrophoblast invasion and insufficient remodeling of the arterial side of the uterine vasculature (Zhou et al., 2013). Very few studies have focused on the syncytialization capacity of PE CTBs. In this study, we used age-comparable primary cultures of villous trophoblast cells isolated from control and PE placentae and confirmed that syncytialization was compromised in cells from PE patients and that detyrosination of α-tubulin was abnormal in PE placentae. Our results are consistent with previous observations from Langbein et al. (2008) and Li et al. (2003) showing that PE CTBs adhere and aggregate well in culture but fuse poorly. Moreover, we compared the variances in cell–cell fusion capacity between CTBs obtained from normal and PE pregnancies. Our results showing notably decreased expression of Syncytin-2 during spontaneous fusion of primary CTBs from PE patients are consistent with those of Lee et al. (2001) and Knerr et al. (2002). Furthermore, accumulation of detyr-α-tub due to TTL inhibition could partially restore the cell–cell fusion capacity of primary CTBs from PE patients. We have provided evidence that defective fusion and differentiation of PE trophoblasts might be able to be manipulated in vitro. Furthermore, we have elucidated the key roles of TTL and α-tubulin detyrosination in human trophoblast differentiation.
Microtubules are the basis for the intracellular transport system. Variations in the carboxy-terminal tail of tubulin result in unique interactions with microtubule-associated proteins, which regulate microtubule dynamics (Raybin and Flavin, 1975; Janke and Bulinski, 2011). For example, detyrosination of α-tubulin regulates the microtubule binding and motor activity of the ubiquitous kinesin1 and kinesin-2 proteins. Upon detyrosination, the increased affinity, velocity, and processivity of kinesins can greatly affect long-distance trafficking in the cell (Verhey and Hammond, 2009). In differentiated neurons, detyr-α-tub assists in the polarization of neurons by increasing specific motor binding to decorated microtubules and by guiding motor proteins to specific sites, probably for transport of particular cargoes (Janke and Bulinski, 2011). In HeLa and T51B rat liver epithelial cells, connexins are packaged in vesicular carriers traveling along microtubules from the Golgi to insertion sites all over the cell surface (Lauf et al., 2002). Interestingly, stable detyrosinated microtubules facilitate movement of recycling membrane from the cell center to the cell surface (Lin et al., 2002). TTL overexpression, which leads to downregulation of detyr-α-tub, may slow down membrane trafficking (Zink et al., 2012). In this study, Cx43 was shown to be enriched on the cell surface of fusion-competent trophoblast cells and was positively modulated by tubulin detyrosination. This result implies that in human trophoblast cells, Cx43 may be transported to the plasma membrane preferentially by detyr-α-tub, which subsequently contributes to gap junction communication and allows the exchange of second messengers to regulate STB-specific gene transcription and cell fusion. Syncytin-2 was also shown to accumulate on the membranes of detyr-α-tub-upregulated trophoblast cells. The membrane localization of Syncytin-2 could directly mediate fusion of the plasma membranes. Cx43 and Syncytin-2 may work together to guarantee the fusion of trophoblast cells; however, further studies are required to clarify the detailed mechanism.
In conclusion, we identified α-tubulin detyrosination as a key process essential for human trophoblast differentiation and revealed that unconventional detyrosination of α-tubulin is relevant to pregnancy disorders. We thus propose the following model: during normal placental development, the expression of TTL in fusion-competent cells is downregulated in cytotrophoblast cells, which elevates the levels of detyr-α-tub. The detyrosination of α-tubulin may promote the enrichment of Syncytin-2 and Cx43 on the cell membrane, which enhances the fusion of trophoblast cells. However, in PE placentae, cytotrophoblast cells exhibit relatively lower levels of detyr-α-tub, probably due to TTL upregulation, thereby causing trophoblast cell fusion deficiency (Figure 7).
Figure 7.
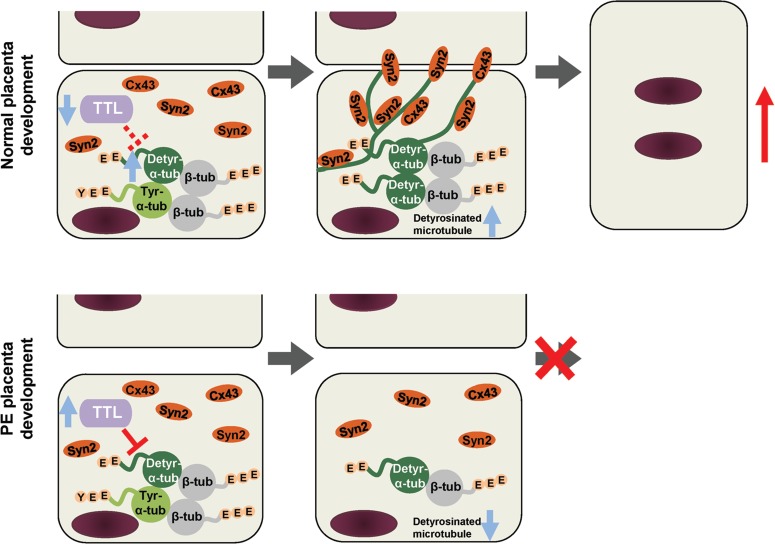
An illustrative model of the function of detyr-α-tub in trophoblast syncytialization during both normal and abnormal placentation. In normal placentae, when mononucleated cytotrophoblast cells become fusion-competent, the expression of TTL is decreased. The downregulation of TTL enhances the detyrosination of α-tubulin, which facilitates the aggregation of Syncytin-2 (Syn2) and Connexin 43 (Cx43) at the plasma membrane and thus initiates the fusion process to form the STB. Under pathological conditions such as PE, increased TTL downregulates the detyrosination of α-tubulin, which limits the aggregation of fusion-related proteins and leads to compromised syncytialization. Detyr-α-tub, detyrosinated α-tubulin; tyr-α-tub, tyrosinated α-tubulin; β-tub, β-tubulin.
Materials and methods
Tissue collection
Human placental tissues were collected in accordance with the policy of the Ethics Committee of the Beijing Obstetrics and Gynecology Hospital. Informed consent was obtained from all women who donated their placentae. Samples were used according to standard experimental protocols approved by the Ethics Committee of the Institute of Zoology, Chinese Academy of Sciences. For the spontaneous syncytialization study, placentae from pregnancies complicated by PE (n = 5) and placentae from gestation-matched controls (n = 5) and normal-term pregnant women were delivered by cesarean section and collected under sterile conditions. PE was characterized by increased blood pressure together with the emergence of proteinuria after 20 weeks of gestation. Hypertension was characterized as a systolic blood pressure >140 mm Hg and/or a diastolic blood pressure >90 mm Hg in a pregnant woman whose blood pressure was normal before 20 weeks of gestation. Proteinuria was characterized as the excretion of >0.3 g of protein in a 24-h urine specimen (Chen et al., 2004). For immunofluorescence staining, five normal human placentae at the gestational age of 6–8 weeks were collected from women undergoing legal abortion. After fixation with 4% paraformaldehyde, the placental tissues were subjected to paraffin embedding and cut into 5 μm-thick sections.
Constructs and lentivirus production
For overexpression experiments, cDNA encoding human TTL or VASH2 was obtained from BeWo cells and cloned into a CSII-CMV-MCS-IRES2-Venus lentiviral vector, a gift kindly provided by Dr Atsushi Miyawaki (RIKEN BSI, Japan) and Dr Hiroyuki Miyoshi (RIKEN Tsukuba Institute, Japan). A 3× Flag tag was added to the N-terminal of TTL or VASH2. For the control vector, EGFP was inserted instead of TTL or VASH2. For small hairpin RNA-mediated interference experiments, the shRNA sequences targeting TTL were 5′-CCGGGCTTTGAACATTACCCTAGAACTCGAGTTCTAGGGTAATGTTCAAAGCTTTTTG-3′ and 5′-AATTCAAAAAGCTTTGAACATTACCCTAGAACTCGAGTTCTAGGGTAATGTTCAAAGC-3′. As a control, an shRNA targeting no known mammalian genes (shNM) was used. Annealed shRNAs were cloned into a pLKO-RFP lentiviral vector derived from pLKO.1, in which the puromycin resistance gene was replaced with a red fluorescent protein, mRuby2, derived from pCAGGS-Raichu-RhoA-CR, a gift kindly provided by Dr Michael Lin (Stanford University, USA)(Lam et al., 2012).
Lentivirus production was performed as described previously (McMaster et al., 2004; Sakaue-Sawano et al., 2008). The lentiviral plasmid together with packaging plasmids (VSVG, pRSV-Rev, pMDL g/p RRE) was cotransfected into Lenti-X 293T cells (Clontech) using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The titer of the lentivirus was determined after transducing HT-1080 cells with different dilutions using flow cytometry, as described previously (Sastry et al., 2002).
Cell culture and establishment of the BeWoTTL, BeWoshTTL, and BeWoshVASH2 cell lines and their controls
The isolation and culture of CTBs from human term placentae were performed as previously described (Wang et al., 2014). More than 95% of the CTBs harvested stained positive for cytokeratin 7 (CK7) (ab9021, RRID: AB_306947, Abcam), a marker for CTBs. CTBs that spontaneously fused to form syncytia in vitro were identified by immunostaining for E-cadherin (sc-71008, RRID: AB_1121199, Santa Cruz Biotechnology, USA; 3195, RRID: AB_2291471, Cell Signaling Technology). The human choriocarcinoma cell line BeWo was obtained from the American Type Culture Collection. BeWo cells were cultured and treated with forskolin (F6886, Sigma-Aldrich) to induce cell–cell fusion as described previously (Wang et al., 2014). The resultant lentiviruses encoding EGFP (control), TTL, mutated TTL, VASH2, TTL shRNA, or shNM (control) were used to infect BeWo cells to establish stable cell lines (BeWoGFP, BeWoTTL, BeWoTTLmut, BeWoVASH2, BeWoshTTL, and BeWoshNM, respectively). The fluorescence-positive cells were sorted by fluorescence-activated cell sorting and cultured and maintained in Ham’s F-12K (Kaighn’s)/DMEM (1:1) containing 10% FBS, 100 U/ml penicillin, and 100 μg/ml streptomycin.
RNA interference
For the small RNA-mediated interference (RNAi) experiments, specific depletion of TTL was performed with the following duplex: 5′-CAGAGGCUUCAGAGCUUCUCGAUUU-3′/5′-AAAUCGAGAAGCUCUGAAGCCUCUG-3′. As a control, a nonspecific siRNA with medium GC content was used. BeWo cells were transfected with 100 nM siRNA with Lipofectamine 2000 according to the manufacturer’s instructions.
Immunofluorescence
Immunostaining of tissue slides was performed as previously described (Fu et al., 2010) using antibodies against the following proteins: TTL (66076-1-Ig, RRID: AB_11182924; 13618-1-AP, RRID: AB_2256858, Proteintech), Syncytin-2 (HPA011812, RRID: AB_1857702, Sigma-Aldrich), active caspase-8 (9496S, RRID: AB_561381, Cell Signaling Technology), and CK7. For α-tubulin (T9026, RRID: AB_477593, Sigma-Aldrich), detyr-α-tub (AB3201, RRID: AB_177350, Merck-Millipore), and E-cadherin staining, the cells were fixed with ice-cold methanol. After incubation at 4°C overnight with the primary antibodies, the cells were washed and incubated with the appropriate highly cross-adsorbed Alexa Fluor 488 goat anti-mouse IgG (A-11001, RRID: AB_2534069, Thermo Fisher Scientific), Alexa Fluor 555 goat anti-mouse IgG (A-21422, RRID: AB_2535844, Thermo Fisher Scientific), Alexa Fluor 488 goat anti-rabbit IgG (A-11034, RRID: AB_2576217, Thermo Fisher Scientific), or Alexa Fluor 555 goat anti-rabbit IgG (A-21428, RRID: AB_2535849, Thermo Fisher Scientific). Confocal images were acquired using a Carl Zeiss LSM 780 confocal laser-scanning microscope (Carl Zeiss MicroImaging GmbH). For SR-SIM, the cells were grown for 24 h after being seeded onto coverslips. The cells were then fixed with cold methanol at −20°C, incubated with anti-detyr-α-tub or anti-α-tubulin antibodies, and observed under an ELYRA superresolution microscope (Carl Zeiss MicroImaging GmbH).
Western blotting
Western blotting was performed as previously described (Wang et al., 2014). Briefly, the membranes were incubated with primary antibodies against TTL, detyr-α-tub, tyr-α-tub (T9028, RRID: AB_261811, Sigma-Aldrich), α-tubulin, Flag (F3165, RRID: AB_259529, Sigma-Aldrich), GAPDH (ab8245, RRID: AB_2107448, Abcam), E-cadherin, α-E-catenin (3236, RRID: AB_560918, Cell Signaling Technology), β-catenin (9562, RRID: AB_331149, Cell Signaling Technology), Claudin-1 (4933S, RRID: AB_823471, Cell Signaling Technology), Na+/K+-ATPase (3010, RRID: AB_2060983, Cell Signaling Technology), γ-catenin (610253, RRID: AB_397648, BD Biosciences), Connexin 43 (610061, RRID: AB_397473, BD Biosciences; 71-0700, RRID: AB_2533973, Thermo Fisher Scientific), Occludin (71-1500, RRID: AB_88065, Invitrogen), and Syncytin-2 (AP13018a, Abgent) and with horseradish peroxidase-conjugated secondary antibodies. The signals were developed using a GeneGnome Imaging System (Syngene Bio-imaging). The intensity of the detected bands was quantified with ImageJ (version 1.48) and is presented as the ratio of the relative optical density of the protein band to that of the GAPDH band.
Live-cell imaging
Live-cell imaging was performed as previously described (Wang et al., 2014). BeWo cells were plated onto 8-well chamber slides (Nunc; Thermo Fisher Scientific) in culture medium containing 10% FBS, 100 U/ml penicillin, and 100 mg/ml streptomycin and subjected to forskolin (50 μM) treatment after attachment. The cells were imaged using an Eclipse Ti model inverted microscope with a 20× objective lens (numerical aperture, 0.95; Nikon), an Orca ER model camera (Hamamatsu Photonics), and a Perfect Focus System. The microscope was surrounded by a custom-made enclosure to maintain a constant temperature (37°C) and atmosphere (5% CO2 and 95% air). Image acquisition was controlled by using Volocity software (PerkinElmer). Time-lapse images were recorded every 5 min for 48 h.
Isolation of cell surface proteins
Cell surface proteins were isolated in accordance with the instructions provided with the Qproteome Plasma Membrane Protein Kit (37601, QIAGEN Group). Briefly, BeWo cells treated with forskolin for 48 h were incubated with 250 μl of Lysis Buffer with protease inhibitors for 15 min on ice. Then, 2.5 μl of Lysis Solution PL was added, and the cells were incubated for another 5 min on ice. After disruption using a Dounce homogenizer, the lysates were incubated with 10 μl of reconstituted Binding Ligand PBL for 2 h at 4°C and with Strep-Tactin Magnetic Beads under gentle agitation on an end-over-end shaker for 4 h at 4°C. After incubation, 200 μl of sample buffer containing 50 mM DTT was added to elute the protein for 30 min at 70°C. The protein levels in the plasma membrane lysates were normalized to the quantitative levels of Na+/K+-ATPase.
Fusion index calculation
Fusion index calculation was performed as previously described (Lin et al., 2011; Wang et al., 2014). In brief, cell fusion was assessed by immunofluorescence using an anti-human E-cadherin antibody to show the cell membrane followed by staining of the nuclei with DAPI. The total fusion index was determined by (N-S)/T, where N is the number of nuclei in the syncytia, S is the number of syncytia, and T is the total number of nuclei. The grouped fusion indices (2-cell fusion, 3- to 4-cell fusion, and 5+-cell fusion) were calculated as the number of nuclei in multinucleated cells divided by the total number of nuclei.
Statistical analysis
Results were presented as means ± S.E.M. Statistical analysis was performed by paired-sample t-test, which was performed by using the Statistical Package for Social Science (SPSS for Windows package release 10.0; SPSS Inc.). Significance was accepted at P < 0.05.
Supplementary Material
Acknowledgements
We wish to acknowledge Dr Atsushi Miyawaki (RIKEN BSI, Japan) and Dr Hiroyuki Miyoshi (RIKEN Tsukuba Institute, Japan) for providing the CSII-CMV-MCS-IRES2-Venus plasmid. We also thank Dr Michael Lin (Stanford University, USA) for providing the pCAGGS-Raichu-RhoA-CR plasmid. We thank Dr Wenxiang Meng for analytic tools, Ms Shiwen Li for confocal microscopy, and Ms Hua Qin for flow cytometry experiments.
Funding
This work was supported by grants from the National Natural Science Foundation of China (81490740, 31501102 and 81671465) and the Ministry of Science and Technology of China (2016YFC1000208). X.L. was funded by the CPRIT Research Training Program (RP170067).
Conflict of interest: none declared.
References
- Aguilar P.S., Baylies M.K., Fleissner A., et al. (2013). Genetic basis of cell–cell fusion mechanisms. Trends Genet. 29, 427–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aillaud C., Bosc C., Peris L., et al. (2017). Vasohibins/SVBP are tubulin carboxypeptidases (TCPs) that regulate neuron differentiation. Science 358, 1448–1453. [DOI] [PubMed] [Google Scholar]
- Akhmanova A., and Steinmetz M.O. (2008). Tracking the ends: a dynamic protein network controls the fate of microtubule tips. Nat. Rev. Mol. Cell Biol. 9, 309–322. [DOI] [PubMed] [Google Scholar]
- Blaise S., de Parseval N., Benit L., et al. (2003). Genomewide screening for fusogenic human endogenous retrovirus envelopes identifies syncytin 2, a gene conserved on primate evolution. Proc. Natl Acad. Sci. USA 100, 13013–13018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.P., Chen C.Y., Yang Y.C., et al. (2004). Decreased placental GCM1 (glial cells missing) gene expression in pre-eclampsia. Placenta 25, 413–421. [DOI] [PubMed] [Google Scholar]
- Dunk C.E., Gellhaus A., Drewlo S., et al. (2012). The molecular role of connexin 43 in human trophoblast cell fusion. Biol. Reprod. 86, 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erck C., Peris L., Andrieux A., et al. (2005). A vital role of tubulin-tyrosine-ligase for neuronal organization. Proc. Natl Acad. Sci. USA 102, 7853–7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher S.J. (2004). The placental problem: linking abnormal cytotrophoblast differentiation to the maternal symptoms of preeclampsia. Reprod. Biol. Endocrinol. 2, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frendo J.L., Cronier L., Bertin G., et al. (2003). Involvement of connexin 43 in human trophoblast cell fusion and differentiation. J. Cell Sci. 116, 3413–3421. [DOI] [PubMed] [Google Scholar]
- Fu J., Lv X., Lin H., et al. (2010). Ubiquitin ligase cullin 7 induces epithelial-mesenchymal transition in human choriocarcinoma cells. J. Biol. Chem. 285, 10870–10879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauster M., Siwetz M., Orendi K., et al. (2010). Caspases rather than calpains mediate remodelling of the fodrin skeleton during human placental trophoblast fusion. Cell Death Differ. 17, 336–345. [DOI] [PubMed] [Google Scholar]
- Giepmans B.N., Verlaan I., Hengeveld T., et al. (2001). Gap junction protein connexin-43 interacts directly with microtubules. Curr. Biol. 11, 1364–1368. [DOI] [PubMed] [Google Scholar]
- Hammond J.W., Cai D., and Verhey K.J. (2008). Tubulin modifications and their cellular functions. Curr. Opin. Cell Biol. 20, 71–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho L., van Dijk M., Chye S.T.J., et al. (2017). ELABELA deficiency promotes preeclampsia and cardiovascular malformations in mice. Science 357, 707–713. [DOI] [PubMed] [Google Scholar]
- Huppertz B., and Gauster M. (2011). Trophoblast fusion. Adv. Exp. Med. Biol. 713, 81–95. [DOI] [PubMed] [Google Scholar]
- Janke C., and Bulinski J.C. (2011). Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell Biol. 12, 773–786. [DOI] [PubMed] [Google Scholar]
- Knerr I., Beinder E., and Rascher W. (2002). Syncytin, a novel human endogenous retroviral gene in human placenta: evidence for its dysregulation in preeclampsia and HELLP syndrome. Am. J. Obstet. Gynecol. 186, 210–213. [DOI] [PubMed] [Google Scholar]
- Lam A.J., St-Pierre F., Gong Y., et al. (2012). Improving FRET dynamic range with bright green and red fluorescent proteins. Nat. Methods 9, 1005–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langbein M., Strick R., Strissel P.L., et al. (2008). Impaired cytotrophoblast cell-cell fusion is associated with reduced Syncytin and increased apoptosis in patients with placental dysfunction. Mol. Reprod. Dev. 75, 175–183. [DOI] [PubMed] [Google Scholar]
- Lauf U., Giepmans B.N., Lopez P., et al. (2002). Dynamic trafficking and delivery of connexons to the plasma membrane and accretion to gap junctions in living cells. Proc. Natl Acad. Sci. USA 99, 10446–10451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee X., Keith J.C., Stumm N., et al. (2001). Downregulation of placental syncytin expression and abnormal protein localization in pre-eclampsia. Placenta 22, 808–812. [DOI] [PubMed] [Google Scholar]
- Li H.S., Dakour J., Kaufman S., et al. (2003). Adrenomedullin is decreased in preeclampsia because of failed response to epidermal growth factor and impaired syncytialization. Hypertension 42, 895–900. [DOI] [PubMed] [Google Scholar]
- Lin F.Y., Chang C.W., Cheong M.L., et al. (2011). Dual-specificity phosphatase 23 mediates GCM1 dephosphorylation and activation. Nucleic Acids Res. 39, 848–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.X., Gundersen G.G., and Maxfield F.R. (2002). Export from pericentriolar endocytic recycling compartment to cell surface depends on stable, detyrosinated (glu) microtubules and kinesin. Mol. Biol. Cell 13, 96–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X., Wang R., Zhu C., et al. (2017). Fine-tuned and cell-cycle-restricted expression of fusogenic protein syncytin-2 maintains functional placental syncytia. Cell Rep. 21, 1150–1159. [DOI] [PubMed] [Google Scholar]
- MacLennan A.H., Sharp F., and Shaw-Dunn J. (1972). The ultrastructure of human trophoblast in spontaneous and induced hypoxia using a system of organ culture. A comparison with uultrastructural changes in pre-eclampsia and placental insufficiency. J. Obstet. Gynaecol. Br. Commonw. 79, 113–121. [PubMed] [Google Scholar]
- Maynard S.E., Min J.Y., Merchan J., et al. (2003). Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J. Clin. Invest. 111, 649–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster M.T., Zhou Y., and Fisher S.J. (2004). Abnormal placentation and the syndrome of preeclampsia. Semin. Nephrol. 24, 540–547. [DOI] [PubMed] [Google Scholar]
- Mi S., Lee X., Li X., et al. (2000). Syncytin is a captive retroviral envelope protein involved in human placental morphogenesis. Nature 403, 785–789. [DOI] [PubMed] [Google Scholar]
- Mialhe A., Lafanechere L., Treilleux I., et al. (2001). Tubulin detyrosination is a frequent occurrence in breast cancers of poor prognosis. Cancer Res. 61, 5024–5027. [PubMed] [Google Scholar]
- Nieuwenhuis J., Adamopoulos A., Bleijerveld O.B., et al. (2017). Vasohibins encode tubulin detyrosinating activity. Science 358, 1453–1456. [DOI] [PubMed] [Google Scholar]
- Peris L., Wagenbach M., Lafanechere L., et al. (2009). Motor-dependent microtubule disassembly driven by tubulin tyrosination. J. Cell Biol. 185, 1159–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raybin D., and Flavin M. (1975). An enzyme tyrosylating α-tubulin and its role in microtubule assembly. Biochem. Biophys. Res. Commun. 65, 1088–1095. [DOI] [PubMed] [Google Scholar]
- Roll-Mecak A., and Vale R.D. (2008). Structural basis of microtubule severing by the hereditary spastic paraplegia protein spastin. Nature 451, 363–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaue-Sawano A., Kurokawa H., Morimura T., et al. (2008). Visualizing spatiotemporal dynamics of multicellular cell-cycle progression. Cell 132, 487–498. [DOI] [PubMed] [Google Scholar]
- Sastry L., Johnson T., Hobson M.J., et al. (2002). Titering lentiviral vectors: comparison of DNA, RNA and marker expression methods. Gene Ther. 9, 1155–1162. [DOI] [PubMed] [Google Scholar]
- Sirajuddin M., Rice L.M., and Vale R.D. (2014). Regulation of microtubule motors by tubulin isotypes and post-translational modifications. Nat. Cell Biol. 16, 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szyk A., Deaconescu A.M., Piszczek G., et al. (2011). Tubulin tyrosine ligase structure reveals adaptation of an ancient fold to bind and modify tubulin. Nat. Struct. Mol. Biol. 18, 1250–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas A., Toufaily C., LeBellego F., et al. (2011). Reduced expression of both syncytin 1 and syncytin 2 correlates with severity of preeclampsia. Reprod. Sci. 18, 1085–1091. [DOI] [PubMed] [Google Scholar]
- Venkatesha S., Toporsian M., Lam C., et al. (2006). Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat. Med. 12, 642–649. [DOI] [PubMed] [Google Scholar]
- Verhey K.J., and Hammond J.W. (2009). Traffic control: regulation of kinesin motors. Nat. Rev. Mol. Cell Biol. 10, 765–777. [DOI] [PubMed] [Google Scholar]
- Wang R., Dang Y.L., Zheng R., et al. (2014). Live cell imaging of in vitro human trophoblast syncytialization. Biol. Reprod. 90, 117. [DOI] [PubMed] [Google Scholar]
- Wehland J., and Weber K. (1987). Turnover of the carboxy-terminal tyrosine of α-tubulin and means of reaching elevated levels of detyrosination in living cells. J. Cell Sci. 88, 185–203. [DOI] [PubMed] [Google Scholar]
- Whipple R.A., Vitolo M.I., Boggs A.E., et al. (2013). Parthenolide and costunolide reduce microtentacles and tumor cell attachment by selectively targeting detyrosinated tubulin independent from NF-κB inhibition. Breast Cancer Res. 15, R83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng R., Li Y., Sun H., et al. (2017). Deep RNA sequencing analysis of syncytialization-related genes during BeWo cell fusion. Reproduction 153, 35–48. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Gormley M.J., Hunkapiller N.M., et al. (2013). Reversal of gene dysregulation in cultured cytotrophoblasts reveals possible causes of preeclampsia. J. Clin. Invest. 123, 2862–2872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X.W., Li J., Brost B.C., et al. (2014). Decreased expression and altered methylation of syncytin-1 gene in human placentas associated with preeclampsia. Curr. Pharm. Des. 20, 1796–1802. [DOI] [PubMed] [Google Scholar]
- Zink S., Grosse L., Freikamp A., et al. (2012). Tubulin detyrosination promotes monolayer formation and apical trafficking in epithelial cells. J. Cell Sci. 125, 5998–6008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


