Abstract
Background
It is common for people not to take antidepressant medication as prescribed, with around 50% of people likely to prematurely discontinue taking their medication after six months. Community pharmacists may be well placed to have a role in antidepressant management because of their unique pharmacotherapeutic knowledge and ease of access for people. Pharmacists are in an ideal position to offer proactive interventions to people with depression or depressive symptoms. However, the effectiveness and acceptability of existing pharmacist‐based interventions is not yet well understood. The degree to which a pharmacy‐based management approach might be beneficial, acceptable to people, and effective as part of the overall management for those with depression is, to date, unclear. A systematic review of randomised controlled trials (RCTs) will help answer these questions and add important knowledge to the currently sparse evidence base.
Objectives
To examine the effects of pharmacy‐based management interventions compared with active control (e.g. patient information materials or any other active intervention delivered by someone other than the pharmacist or the pharmacy team), waiting list, or treatment as usual (e.g. standard pharmacist advice or antidepressant education, signposting to support available in primary care services, brief medication counselling, and/or (self‐)monitoring of medication adherence offered by a healthcare professional outside the pharmacy team) at improving depression outcomes in adults.
Search methods
We searched the Cochrane Common Mental Disorders Controlled Trials Register (CCMD‐CTR) to June 2016; the Cochrane Library (Issue 11, 2018); and Ovid MEDLINE, Embase, and PsycINFO to December 2018. We searched theses and dissertation databases and international trial registers for unpublished/ongoing trials. We applied no restrictions on date, language, or publication status to the searches.
Selection criteria
We included all RCTs and cluster‐RCTs where a pharmacy‐based intervention was compared with treatment as usual, waiting list, or an alternative intervention in the management of depression in adults over 16 years of age. Eligible studies had to report at least one of the following outcomes at any time point: depression symptom change, acceptability of the intervention, diagnosis of depression, non‐adherence to medication, frequency of primary care appointments, quality of life, social functioning, or adverse events.
Data collection and analysis
Two authors independently, and in duplicate, conducted all stages of study selection, data extraction, and quality assessment (including GRADE). We discussed disagreements within the team until we reached consensus. Where data did not allow meta‐analyses, we synthesised results narratively.
Main results
Twelve studies (2215 participants) met the inclusion criteria and compared pharmacy‐based management with treatment as usual. Two studies (291 participants) also included an active control (both used patient information leaflets providing information about the prescribed antidepressant). Neither of these studies reported depression symptom change. A narrative synthesis of results on acceptability of the intervention was inconclusive, with one study reporting better acceptability of pharmacy‐based management and the other better acceptability of the active control. One study reported that participants in the pharmacy‐based management group had better medication adherence than the control participants. One study reported adverse events with no difference between groups. The studies reported no other outcomes.
Meta‐analyses comparing pharmacy‐based management with treatment as usual showed no evidence of a difference in the effect of the intervention on depression symptom change (dichotomous data; improvement in symptoms yes/no: risk ratio (RR), 0.95, 95% confidence interval (CI) 0.86 to 1.05; 4 RCTs, 475 participants; moderate‐quality evidence; continuous data: standard mean difference (SMD) ‐0.04, 95% CI ‐0.19 to 0.10; 5 RCTs, 718 participants; high‐certainty evidence), or acceptability of the intervention (RR 1.09, 95% CI 0.81 to 1.45; 12 RCTs, 2072 participants; moderate‐certainty evidence). The risk of non‐adherence was reduced in participants receiving pharmacy‐based management (RR 0.73, 95% CI 0.61 to 0.87; 6 RCTs, 911 participants; high‐certainty evidence). We were unable to meta‐analyse data on diagnosis of depression, frequency of primary care appointments, quality of life, or social functioning.
Authors' conclusions
We found no evidence of a difference between pharmacy‐based management for depression in adults compared with treatment as usual in facilitating depression symptom change. Based on numbers of participants leaving the trials early, there may be no difference in acceptability between pharmacy‐based management and controls. However, there was uncertainty due to the low‐certainty evidence.
Plain language summary
Pharmacies might be able to support people with their depression medicines
Background
Some people with depression find it difficult to take their depression medicines (often called 'antidepressants') as prescribed by their doctor. This can mean that the medicines do not work properly and people might not get better or might even get worse. It could be that pharmacists and their teams can help people with their depression treatment in ways that their family doctor (general practitioner (GP)) cannot. Pharmacies are based within the community, easier to get to, and people may feel more comfortable telling a pharmacist about their mood. However, there are not many studies to tell us if this works.
Study characteristics
We searched medical databases for well‐designed studies that compared a group of adults with depression who received additional help with their depression medicines from their pharmacy with a group of adults with depression who received their treatment as usual.
The evidence is current to 14 December 2018.
Key results and certainty of the evidence
We found 12 studies with over 2000 adults taking part. They compared pharmacy‐based support with treatment as usual, for example, basic information about their medicines or signposting to other services only. We found that additional support given by the pharmacist was no better at reducing people's depression than their treatment as usual. The studies also showed that people may have liked both approaches the same, although we are uncertain about the results as the evidence was of low certainty.
The studies did show that people who received support from their pharmacy were more likely to take their antidepressants as prescribed. We were not able to combine information from the included studies on other outcomes we were interested in (diagnosis of depression, frequency of healthcare appointments, quality of life, social functioning, or side effects).
We found no difference in effectiveness when people with depression received additional support from a pharmacist compared with treatment as usual.
Summary of findings
Summary of findings for the main comparison. Pharmacy‐based management (pharmacist with or without wider team) compared to treatment as usual for depression in adults.
| Pharmacy‐based management (pharmacist with or without wider team) compared to treatment as usual for depression in adults | |||||
| Patient or population: adults Setting: primary care practices, hospitals, and pharmacies Intervention: pharmacy‐based management (pharmacist with or without wider team) Comparison: treatment as usual | |||||
| Outcomes | № of participants (studies) | Certainty of the evidence (GRADE) | Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | |
| Risk with treatment as usual | Risk difference with pharmacy‐based management (pharmacist with or without wider team) | ||||
| Depression symptom level: improvement (intervention endpoint) | 475 (4 RCTs) | ⊕⊕⊕⊝ Moderatea | RR 0.95 (0.86 to 1.05) | Study population | |
| 345 per 1000 | 17 fewer per 1000 (48 fewer to 17 more) | ||||
| Depression symptom level: change from baseline mean depression score (intervention endpoint) | 718 (5 RCTs) | ⊕⊕⊕⊕ High | — | The mean change from baseline mean depression score (intervention endpoint) was 0 | SMD 0.04 lower (0.19 lower to 0.1 higher) |
| Acceptability of the intervention (as measured by participants not attending follow‐up) | 2072 (12 RCTs) | ⊕⊕⊝⊝ Lowb,c | RR 1.09 (0.81 to 1.45) | Study population | |
| 285 per 1000 | 26 more per 1000 (54 fewer to 128 more) | ||||
| Diagnosis of depression | 368 (3 RCTs) | — | — | We were unable to combine these studies in a meta‐analysis as they reported findings using different depression measures at different time points. The effectiveness of pharmacy‐based management on a diagnosis of depression (or depression remission) remains unclear. | |
| Non‐adherence to medication | 911 (6 RCTs) | ⊕⊕⊕⊕ High | RR 0.73 (0.61 to 0.87) | Study population | |
| 413 per 1000 | 112 fewer per 1000 (161 fewer to 54 fewer) | ||||
| Frequency of primary care appointments | 74 (1 RCT) | — | — | At 12 months, there were no significant differences between treatment groups in the number of visits to healthcare providers; this was evident in overall visits, and in subgroup analyses of specific healthcare providers, including those of primary care. | |
| Quality of life | 239 (1 RCT) | — | — | Overall, there was no significant differences in health‐related quality of life between the intervention group and the control group at 3 and 6 months. | |
| Social functioning | 125 (1 RCT) | — | — | At 6 months, there was no significant changes in WSDS scores between intervention and control groups. | |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio; SMD: standardised mean difference; WSDS: Work and Social Disability Scale. | |||||
| GRADE Working Group grades of evidence High certainty: we are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low certainty: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||||
aEffect estimates of individual studies on either side of the line of no effect. Pooled effect driven by the largest study (Kanwal 2018). bEffect estimates of individual studies on either side of the line of no effect, including several where the confidence interval did not cross the line of no effect in either direction. cSome concern around using number of participants not attending follow‐up as a measure of acceptability of the intervention.
Background
Some services provided by pharmacists may have positive effects on patient health, including improved management of blood pressure and physical function (de Barra 2018). In the UK, antidepressant management for depression is usually achieved through general practitioner (GP) contact and monitoring, which typically involves regular appointments (e.g. every two to four weeks within the first three months) to assess response and tolerance to treatment (NICE 2018). Community pharmacists may be well placed to have a role in antidepressant management because of their unique pharmacotherapeutic knowledge and ease of access for patients. In the UK, there have been efforts to raise public awareness about the role that pharmacists can play as part of multidisciplinary teams to better support people with managing their health conditions, including mental health problems (Royal Pharmaceutical Society 2018).
In England, an estimated 89.2% of the population have access to a community pharmacy within a 20‐minute walk; in the most deprived areas, this figure increases to 99.8% of the population (Todd 2014). Therefore, pharmacy teams (including pharmacists, pharmacy assistants, technicians, healthy living champions) working in community, general practice, or secondary care are ideally placed to offer proactive interventions to people with depression or depressive symptoms. A narrative evidence synthesis by Rubio‐Valera 2014 suggests that pharmacists, specifically, have skills in medication management, provision of drug information, supporting and advising patients about their medicines, and facilitating medication adherence strategies in mental health care. Although pharmacy‐based management interventions can vary in their components, there is scope for these approaches to be used in partnership with other healthcare professionals (e.g. as part of a collaborative care approach). Previous research has suggested that multiprofessional approaches (involving more than one type of health professional, using a structured management plan and scheduled follow‐ups, with enhanced interprofessional communication) have the potential to improve the management of depression in primary care settings (Archer 2012; Gilbody 2003; Gunn 2006). However, despite this potential, and the emerging evidence of the role of pharmacy‐based management interventions for depression, the effectiveness and acceptability of these interventions is not yet well understood.
Description of the condition
Depression can be characterised by low mood; markedly diminished interest or pleasure in activities; impaired cognitive function; fatigue; reduced or disturbed sleep; feelings of worthlessness; and significant decrease or increase in appetite (APA 2013; Otte 2016). The aetiology and maintenance of depression is complex and multifactorial, involving environmental and social factors, as well as genetic and biological factors affecting function changes and regions of the brain (Otte 2016). The condition can be recurrent or long‐term and chronic, and often results in debilitating burden that can interfere with family, home, social, and work responsibilities (Marquez 2016).
Depression is a common mental health problem, with more than 300 million people globally estimated to be living with the condition (WHO 2018). It is the leading cause of disability worldwide and a major contributor to the overall burden of disease (WHO 2018). In extensive global population research on Global Burden of Disease involving 188 countries between 1990 and 2013, depression was one of the top 10 causes of years lived with disability in every country studied, with higher rates of depression typically found in women (Vos 2015). The global point prevalence of depression is reported to be 3.2% for men and 5.5% for women (Ferrari 2013a; Ferrari 2013b; Whiteford 2013). It is estimated that annually 12 billion days of lost productivity (equivalent to 50 million years of work) are attributable to depressive and anxiety disorders (which are often comorbid with each other) combined, with an estimated cost of USD 925 billion, a cost that is anticipated to grow in coming years (Chisholm 2016).
Antidepressants have long been a mainstay of pharmacological treatment for depression (Taylor 2015), and have been reported to be more effective than placebo (Cipriani 2018). However, it is common for patients not to take antidepressant medication as prescribed, with around 50% of patients likely to prematurely discontinue taking their medication after six months. Premature discontinuation of antidepressant therapy has been linked to increased healthcare costs, poor treatment outcomes, and increased risk of relapse and recurrence (Chong 2011).
Description of the intervention
In the treatment as usual (TAU) offered to people with depression, the pharmacist generally has limited involvement with the individual beyond the dispensing of the prescribed antidepressant. Pharmacists or members of the wider pharmacy team (including pharmacy assistants, technicians, healthy living champions, and others who work within the community pharmacy setting) offer basic antidepressant information at the point of dispensing the medication, including information on dosage and important adverse events. They might also signpost the person (back) to primary care services that can provide additional support. Generally, in the UK, the majority of treatment for depression is delivered in primary or secondary care, rather than by the pharmacy team (NICE 2018). Even though there is variation between the standard care offered to people with depression (between, for example, different countries), a common characteristic is that the majority of the information is provided upon request by the person or contingent on the individual taking the initiative ('patient‐led') rather than being offered proactively by the pharmacist or a member of their team.
Pharmacy‐based management interventions can be delivered by a single pharmacist or the wider pharmacy team. In the context of depression, pharmacy‐based management interventions are often delivered in partnership with other healthcare professionals, usually as part of a collaborative care approach. This can be done in several ways: first, by providing patient support, counselling, and education; second, by monitoring or following up adverse effects of medications; and, third, under specific protocols, titrating doses of medications according to patient response (Brook 2005; Capoccia 2004a; Finley 2003a). These interventions can be provided face‐to‐face, using written support materials or visual information relating to medication, through telephone support, or via more formal 'counselling' strategies (Adler 2004; Al‐Saffar 2005), and they may happen alongside the involvement of care managers, mental health specialists, and primary care physicians (Aljumah 2015; Rickles 2005).
As the intervention can be delivered in multiple ways and, given the number of interacting components involved (including the number and difficulty of behaviours required by those delivering or receiving the intervention, the number and potential variability of outcomes, and the degree of flexibility or tailoring of the intervention permitted) it can be described as a complex intervention (Petticrew 2011).
How the intervention might work
A key aspect of effective collaborative care is 'case management' (Gilbody 2003), which has been described as a 'health worker taking responsibility for proactively following up patients, assessing patient adherence to psychological and pharmacological treatments, monitoring patient progress, taking action when treatment is unsuccessful, and delivering psychological support' (Von Korff 2001). Pharmacy‐based management interventions might also involve the delivery of direct psychological interventions to people with depression. An example of this is behavioural activation therapy, which uses principles of operant conditioning by encouraging people with depression to reconnect with environmental positive reinforcement (Ekers 2014a). Behavioural activation can be effective when delivered by paraprofessionals (Gilbody 2017), and current research is exploring if it can be delivered by community pharmacies to people with long‐term physical health problems and subthreshold depression (ISRCTN11290592).
Qualitative work has shown that people tend to form different relationships with a pharmacist compared with other healthcare professionals, such as primary care practitioners or GPs. When people consult with a pharmacist, they do not see themselves as 'patients' and, as such, are more likely to be open and honest in their discussions. In view of this, people might be more likely to discuss certain aspects of their health with a pharmacist compared with other healthcare professionals (Lindsey 2016). Previous research has demonstrated how pharmacist‐based management interventions, including providing patient support, counselling, or coaching patients about their medication and what to expect, can improve antidepressant adherence rates (Al‐Saffar 2005; Brook 2005). It is proposed that pharmacy‐based management interventions, such as engaging patients through face‐to‐face or remote counselling, education and advice (e.g. via teleconferencing, or 'take‐home' audio/visual materials) alongside prescribing and monitoring of antidepressant medication, can also improve depressive symptoms.
Why it is important to do this review
Pharmacists are now engaging with patients in different ways, and it is important to bring together the evidence from randomised controlled trials for pharmacy‐based approaches for depression to determine effectiveness on depressive symptoms and acceptability, as well as adherence levels, adverse effects of prescribed medication, quality of life, and levels of social functioning (Hanlon 2004; Holland 2008; Nkansah 2010; Readdean 2018; Royal 2006; Rubio‐Valera 2013; Yaghoubi 2017). Research involving community household surveys from 21 countries showed that only a minority of people received 'minimally adequate treatment' for depression. This finding equates to one in five people in high‐income countries, and one in 27 in low‐income or low‐ to middle‐income countries, highlighting the need to implement fundamental transformations involving community education and outreach, beyond that currently being offered in primary and secondary care services (Thornicroft 2017). Treatment and support for depression clearly extends beyond the pharmacological; however, due to inadequate resources, antidepressants are more often used than treatment alternatives, for example, psychological therapies (Cipriani 2018).
Against this backdrop, there are policy expectations for pharmacies to expand their professional responsibilities beyond retail and dispensing to encompass more patient‐centred services (Smith 2013), including counselling and support, education, monitoring adverse events, and advice relating to prescribed medication and medicines optimisation and titration, resulting in a trend for community pharmacy medicine management interventions being introduced globally (including in Australia, Canada, New Zealand, Switzerland, the US, and England; Latif 2018). Even with a stronger push for pharmacy‐based interventions, there remains ambivalence among pharmacists as to whether the public are willing to engage or would readily accept advice and support (Eades 2011; Rodgers 2016).
Pharmacy‐based management strategies have shown some promising effects in other areas of health care (de Barra 2018). The degree to which a pharmacy‐based management approach might be beneficial, acceptable to patients, effective, and cost‐effective as part of the overall management for those with depression is, to date, unclear. A systematic review of randomised controlled trials will help answer these questions and add important knowledge to the currently sparse evidence base. Our focus is on the impact on improvement in depression as well as acceptability of the intervention rather than medication adherence.
Objectives
To examine the effects of pharmacy‐based management interventions compared with active control, waiting list, or treatment as usual at improving depression outcomes in adults.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion all randomised controlled trials (RCTs) and cluster‐RCTs where a pharmacy‐based intervention was compared with treatment as usual (TAU), waiting list, or an alternative intervention ('active control', e.g. patient information materials or any other active intervention delivered by someone other than the pharmacist or the wider pharmacy team) in the management of depression. The intervention could have been delivered within the pharmacy or external to the pharmacy (e.g. in a hospital, clinic, online, etc.) or in the community, provided that a pharmacist/wider pharmacy team was involved, or both.
Types of participants
We included all trials of adults (defined as 16 years or over with no upper age limit), with a primary diagnosis of depression according to an international diagnostic classification, including for example, the Diagnostic and Statistical Manual (DSM) of Mental Disorders (APA 2013) or the International Classification of Diseases (ICD) (WHO 1992). Studies of participants with depressive symptoms diagnosed via self‐reported scales or questionnaires were also eligible. We included studies in which participants were prescribed an antidepressant (including but not limited to: selective serotonin reuptake inhibitors; serotonin noradrenaline (norepinephrine) reuptake inhibitors; tricyclic antidepressants; monoamine oxidase inhibitors; tetracyclic antidepressants; noradrenergic and specific serotonergic antidepressants) by their primary care physician for the treatment of their diagnosed depression or depressive symptoms.
We included adults as defined as 16 years and over. While we expected most studies to use a cut‐off point of 18 years and above, we note that people consent to take part in research from the age of 16 years. We judged those studies would be relevant to this review if identified and as such used the lower threshold. Furthermore, the age in which people transition from child services to adult services varies from 16 to 18 years across different countries.
Comorbidities
Studies including people with any type of physical (e.g. long‐term conditions, diabetes) or mental health comorbidity (e.g. anxiety) were also eligible, provided that the management of depression was the primary focus of the study.
Types of interventions
We included studies where the intervention was delivered by a pharmacist with or without the input of a (multidisciplinary) team. Studies were excluded if the involvement of the pharmacist was unclear or if they played a background role in the trial intervention, such as prescribing or advising, without coming in contact with the patient.
All comparator interventions were eligible. We grouped studies as follows according to their comparator.
Active control (e.g. other non‐pharmacy‐based management, or psychological intervention).
TAU (i.e. standard pharmacy interaction).
We did not identify any eligible trials that used a waiting list or no treatment as the comparator.
Accordingly, we assessed outcomes for two comparisons.
Pharmacy‐based management versus active control.
Pharmacy‐based management versus TAU.
We conducted subgroup analyses for the primary outcomes to investigate the impact of the involvement of a team (for example, pharmacy assistants, technicians, or healthy living champions; designated 'the wider pharmacy team' in this review) in the delivery of the intervention (see Subgroup analysis and investigation of heterogeneity). This was a pragmatic team decision based on the range of eligible studies identified. See Differences between protocol and review for a full explanation.
Types of outcome measures
All outcome measures were eligible as reported by study authors.
Primary outcomes
Depression symptom level: as measured using validated patient‐reported or clinician‐rated depression measures. Included studies used a range of outcome measures to describe symptom level including dichotomous outcomes (improvement in symptoms yes/no) and continuous outcomes (mean depression scores). We combined results from dichotomous outcome measures and results from continuous outcome measures in separate meta‐analyses (see Data synthesis).
Acceptability of the intervention: based on the number of people discontinuing the intervention by leaving the study early.
Secondary outcomes
Diagnosis of depression: as measured using validated clinician‐rated depression measures.
Non‐adherence to medication: the number of participants not taking antidepressant medication as prescribed, assessed via self‐report, through healthcare records, or clinician‐reported adherence scales.
Frequency of primary care appointments (e.g. GP): based on any data relating to primary care service use as reported in the trial.
Quality of life: as assessed using any validated quality of life measure.
Social functioning: as assessed using any validated social functioning measure.
Any adverse event: as reported in the trial.
Timing of outcome assessments
Where possible, we reported outcomes at the following prespecified time points:
intervention endpoint (regardless of length of intervention);
six to 12 months from intervention endpoint ('medium‐term'); and
12 months or more from intervention endpoint ('longer‐term').
We reported these results narratively when meta‐analysis was not possible.
Search methods for identification of studies
Cochrane Common Mental Disorders Controlled Trials Register (CCMD‐CTR)
The Cochrane Common Mental Disorders (CCMD) Group maintains an archived specialised register of RCTs, the CCMD‐CTR. This register contains over 40,000 reference records (reports of RCTs) for anxiety disorders, depression, bipolar disorder, eating disorders, self‐harm, and other mental disorders within the scope of this Group. The CCMD‐CTR is a partially studies‐based register with more than 50% of reference records tagged to about 12,500 individually PICO (Population, Intervention, Comparison, Outcome)‐coded study records. Reports of trials for inclusion in the register were collated from (weekly) generic searches of key bibliographic databases to June 2016, which included: Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE (1950 onwards), Embase (1974 onwards), and PsycINFO (1967 onwards), and review‐specific searches of additional databases. Reports of trials were also sourced from international trial registries; drug companies; the handsearching of key journals, conference proceedings and other (non‐Cochrane) systematic reviews; and meta‐analyses. Details of CCMD's core search strategies (used to identify RCTs) can be found on the Cochrane Common Mental Disorders website, with an example of the core MEDLINE search displayed in Appendix 1.
Electronic searches
Searches were developed and conducted in collaboration between the author (RS) and CCMD's information specialist, between the end of November and beginning of December 2018.
1. Cochrane Specialised Register (CCMD‐CTR)
CCMD's information specialist searched the Group's specialised register (CCMD‐CTR‐Studies and CCMD‐CTR‐References) (all years to June 2016), using the following terms (for setting/healthcare professional (only)):
pharmacy or pharmacies or pharmacist* [all fields].
2. Additional bibliographic database searches
We shared the searches of the following bibliographic databases, using relevant subject headings, keywords, and search syntax appropriate to each resource (Appendix 2):
CENTRAL (Issue 11 of 12, December 2018);
Ovid MEDLINE (1946 to 7 December 2019);
Ovid PsycINFO (1806 to December week 1 2018);
Ovid Embase (1974 to week 49 2018).
We applied no restrictions on date, language, or publication status to the searches.
3. International Trial Registries
We searched ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp/en) via CENTRAL on the Cochrane Registry of Studies (CRS) (27 August 2019) to identify ongoing or unpublished studies.
Two review authors (SJS, NW, AT, or JB) independently considered all abstracts retrieved from the search results for relevancy, and screened full‐text reports to identify studies for inclusion in the review using Covidence software (Covidence 2018). Any disagreements were managed through discussion and referred to a third review author (LW or DE).
Searching other resources
Grey literature
We searched the following databases to identify relevant theses (all available years to 27 August 2019):
DART‐Europe E‐theses Portal (www.dart‐europe.eu);
EThOS – the British Libraries e‐theses online service (ethos.bl.uk);
Open Access Theses and Dissertations (oatd.org);
ProQuest Dissertations and theses database (c/o dissexpress.umi.com).
Reference lists
We checked the reference list of all relevant reviews retrieved from this search together with reports of included studies to help identify additional research relevant to the review. All references identified as potentially relevant were discussed with a second author and deduplicated against records already retrieved through the electronic searches. We screened other systematic reviews and meta‐analyses identified from an earlier search of the Cochrane Library review databases (Issue 4, 2018) (Cochrane Database of Systematic Reviews (CDSR), Database of Reviews of Effects (DARE), Health Technology Assessments database (HTA)), and Ovid MEDLINE (from 1946), Embase (from 1974), and PsycINFO (from 1806) to 30 April 2018.
Personal communication
For studies where the published paper(s) did not contain sufficient detail to allow a definitive assessment of eligibility, we made reasonable efforts to obtain missing information from the study authors. Where necessary, we undertook several attempts to contact authors using a range of channels, including email and online research sharing platforms. If we had not received the requested information by the time of submission of the present review, we excluded the study due to 'insufficient data'.
Data collection and analysis
We used Covidence for managing citations, screening titles and abstracts, uploading, and screening full texts (Covidence 2018). We used Review Manager 5 (Review Manager 2014) and RevMan Web (RevMan Web 2019) alongside Covidence and Excel for data extraction and risk of bias assessments. We used GRADEpro to carry out GRADE assessments and to produce the 'Summary of findings' table (GRADEpro GDT). We conducted meta‐analyses in Review Manager 5 (Review Manager 2014).
Selection of studies
Two review authors independently screened titles and abstracts from the results of the literature searches, including trials potentially relevant to the review and excluding others based on the prespecified criteria. Disagreements were discussed with another review author. Two review authors independently assessed full texts: we obtained full publications of potentially relevant titles/abstracts and made the final eligibility decision. We recorded reasons for exclusion for all full texts that did not meet eligibility criteria. Any disagreements were resolved by discussion with another review author.
We documented the flow of studies through the review and presented a PRISMA flow chart in the Results of the search. We reported characteristics of included studies narratively and in tables to show that they met inclusion criteria and provided references to excluded studies alongside reasons for exclusion.
Data extraction and management
We extracted descriptive data pertaining to the methodological, participant, intervention, and outcome characteristics of included studies (including author details; country of study; study design; description of study setting; recruitment process; description of participants including any comorbidities; description of intervention and comparator; primary and secondary outcomes; outcomes reported but not included in this review; and funding and potential conflicts of interest of study authors).
We extracted quantitative data from each trial for the outcomes and time points prespecified in this review. One review author extracted data and another review author checked them, using Covidence (Covidence 2018) and Review Manager (Review Manager 2014; RevMan Web 2019). We resolved disagreements by discussion.
We assessed the usability of our data extraction form to ensure consistency in data extraction between reviewers.
Assessment of risk of bias in included studies
We assessed risk of bias in included studies using Cochrane's 'Risk of bias' tool (Higgins 2011a), which addresses the potential for bias in the following six domains:
random sequence generation;
allocation concealment;
blinding of outcome assessment;
blinding of participants and personnel;
incomplete outcome data;
selective reporting;
other sources of bias (e.g. funding, affiliations and declarations of interest of study authors).
One review author assessed risk of bias and another review author checked it. We resolved disagreements by discussion. Where detail reported in the study allowed, domains were rated as 'high' or 'low' risk of bias. The 'unclear' category was used when sufficient detail was not available in the study publication(s) or through contact with the authors.
As per the Cochrane Handbook for Systematic Reviews of Interventions, we used an amended risk of bias tool for any included cluster‐RCTs (Higgins 2019a).
Measures of treatment effect
We inputted all data into Review Manager 5 (Review Manager 2014) or RevMan Web (RevMan Web 2019). For dichotomous outcomes, we calculated the risk ratio (RR) with 95% confidence intervals (CI), a measure of the relative risk comparison between two groups. For continuous data, we used standardised mean differences (SMDs), where different standardised scales were used to measure the same outcome, and the mean difference (MD) when the same scale was used, with 95% CIs.
For depression symptom level, reporting and outcome measurement were inconsistent across studies (e.g. studies reported a combination of endpoint and change from baseline data). It was not possible to combine endpoint and change from baseline data in meta‐analyses of SMDs that synthesise data from a variety of depression scales. Therefore, where studies reported endpoint data we converted these to change scores (as most studies reported change from baseline).
Where mean change was not reported, we subtracted mean endpoint from mean baseline scores (negative scores indicated a reduction in depression). Similarly, if the standard deviation (SD) of mean change was not reported, we imputed it using the formula in Section 6.5.2.8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b). The formula requires entering a correlation coefficient. We calculated this correlation coefficient from data reported in Rubio‐Valera 2013 based on the appropriate formula also reported in Section 6.5.2.8 of the Cochrane Handbook for Systematic Reviews of Interventions. As Aljumah 2015 did not have the information required to calculate the relevant correlation coefficient, we applied the same correlation used in Rubio‐Valera 2013.
Unit of analysis issues
Cluster‐randomised trials
For any eligible cluster‐RCTs, we incorporated results into the review ensuring that data were analysed within the individual study with consideration of their clustering. As per the guidance of the Cochrane Handbook for Systematic Reviews of Interventions, we reported data where proper adjustment for the intracluster correlation coefficient (ICC) had been undertaken (Higgins 2011b).
Trials with multiple treatment arms
We included eligible trial with additional treatment arms. Guided by the effort to avoid double counting of participants, we treated these trials on a case‐by‐case basis when data extracting. Details are reported in Description of studies.
Dealing with missing data
When reported, we extracted data where appropriate imputation methods (e.g. multiple imputation, simple imputation methods with adjustment to the standard error (SE), etc.) or statistical models allowing for missing data have been used by the trialists. However, where such data were not available, we extracted observed case data. Where a combination of imputed and observed case data were available, we investigated the impact of excluding imputed data in a Sensitivity analysis.
Where data were missing, we contacted the trial authors to request further information and documented their responses. For dichotomous outcomes, we used intention‐to‐treat (ITT) analysis where this was reported. We recorded whether or not ITT analysis was done in the Characteristics of included studies table. We assumed that dropouts from treatment were treatment failures unless trialists expressly stated otherwise.
For continuous data, we contacted trial authors for any missing statistics or calculated them using available reported data. Rubio‐Valera 2013 only presented in 95% CIs, where there is a 95% likelihood that the effect size lies between the upper and lower interval, for depression symptoms at baseline and follow‐up, and these were converted to SD according to Section 6.5.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b). This was then converted to mean change and SD. Crockett 2006 did not present SD with mean change in K‐10 (the depression and anxiety checklist used by the study). The SE was calculated using the P value according to Section 6.5.2.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b), and the SD was calculated from the SE according to Section 6.5.2.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2019b).
Assessment of heterogeneity
We examined statistical heterogeneity using the I2 statistic for statistical variation across studies (Deeks 2011). The I2 statistic provides a measure of the proportion of dispersion of effects across studies that reflect real differences rather than random error. Benchmark values of 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% would represent considerable heterogeneity, and we reported 95% CIs. When we detected substantial levels of statistical heterogeneity (via visual inspection of graphs, and the presence of an I2 of 75% or greater, accompanied by a statistically significant Chi2), we closely inspected data to ensure they were inputted correctly. When making this inspection, to avoid imposing arbitrary thresholds, we took into account the magnitude and direction of the observed effect, and the strength of the evidence for heterogeneity (e.g. the P value from Chi2 test, or CI from the I2 test). We also judged clinical and methodological heterogeneity between included trials by inspecting for any outlying people, situations, or methods which we did not expect would arise. We documented and discussed these factors (see Subgroup analysis and investigation of heterogeneity and Sensitivity analysis).
Assessment of reporting biases
For all meta‐analyses including 10 or more trials, we used a funnel plot to help detect instances of reporting biases. We interpreted a symmetrical funnel plot as likely to indicate low publication bias while an asymmetric funnel plot may indicate likely publication bias in included trials (Sterne 2011).
Data synthesis
Previous systematic reviews have highlighted wide variation in pharmacist‐based interventions and reported outcomes, and have noted high levels of heterogeneity in their data (Bell 2005; Bower 2006; de Barra 2018). We have, therefore, used a random‐effects model for our analyses, taking account of both within‐ and between‐study variance and following the assumption that different studies are estimating different, yet related, intervention effects (Deeks 2011).
We tested heterogeneity using both the Chi2 test and the I2 statistic (as outlined above). If a meta‐analysis was not possible (e.g. due to insufficient data or high heterogeneity), we provided a narrative synthesis of the evidence (Noyes 2011).
Subgroup analysis and investigation of heterogeneity
Given the different models for delivering pharmaceutical care, we were interested to see if the method of delivery modified the magnitude of the intervention effect. In particular, we were interested to find out if the intervention had to be delivered face‐to‐face by the pharmacist, or if it could be delivered remotely (e.g. telephone). This will have potential implications for future service development. We were also interested if co‐morbidity or depression severity factors moderated the intervention effects. The rationale was that severe depression is often more challenging to treat from a pharmacological viewpoint, so we were interested to establish if this was the case for pharmacy‐based management interventions. Finally, often in a real word setting, people commonly have other comorbidities in addition to depression; in some cases, people have multimorbidity (two or more long‐term conditions); we were interested to establish how applicable our findings were to real world settings for people with other comorbidities.
We conducted the following prespecified subgroup analyses in order to reduce the likelihood of spurious findings on factors that may influence heterogeneity.
1. Participant characteristics
1.1 Participants with physical and mental health comorbidities
Subgrouping trials according to whether or not they provided data for participants either with no comorbidities, or with physical or mental (or both) health comorbidities.
1.2 Baseline severity of depression
As defined as mild, moderate, or severe in individual studies, or using cut‐points for validated rating scales, such as the Patient Health Questionnaire 9 (PHQ‐9; Kroenke 2001), or the Beck Depression Inventory, second edition (BDI‐II; Beck 1996).
2. Intervention characteristics: delivery method
We conducted a subgroup analysis grouping trials based on the delivery method of the intervention: remote (telephone) or in person. Data were not available for more detailed subgroup analyses.
In addition, we conducted an ad hoc subgroup analysis grouping those trials where the pharmacist was working alone and those where they were working with a team. This analysis was included in the review as part of the decision to analyse studies that included a team together with those that did not, rather than having separate comparisons for each type of study (see Differences between protocol and review).
Subgroup analyses were conducted for primary outcomes only with the exception of the subgroup analysis for presence or absence of a wider pharmacy team, which was also carried out for adherence.
Sensitivity analysis
We conducted the following prespecified sensitivity analyses.
1. Risk of bias
If an included trial was rated as a high risk of bias on two or more of the risk of bias domains, we removed this trial to see whether removal would make a substantive difference to the results.
2. Assumptions for missing data
Where meta‐analyses included data from a combination of imputed and completer data, we conducted sensitivity analyses excluding imputed data. We compared these estimates with the main analyses to assess any important differences.
3. Classification of depression
Where a trial did not report how depression is classified, we removed this trial to see whether removal would make a substantive difference to the results.
Summary of findings and assessment of the certainty of the evidence
To assess the certainty of the evidence available for each outcome across studies (rather than within individual studies), we produced a 'Summary of findings' table using GRADEpro GDT (GRADEpro GDT). The GRADE framework addresses the following five domains:
risk of bias (across studies, for each outcome);
imprecision (between the effect estimates of the studies reporting each outcome);
inconsistency (in the effect estimates of the studies reporting each outcome);
indirectness (of measurement in the studies reporting each outcome);
other (including publication bias).
We chose the following outcomes at intervention endpoint as the most important.
-
Depression symptom level (patient‐reported):
dichotomous outcome: improvement in symptoms yes/no;
continuous outcome: change from baseline in mean depression score.
Acceptability of intervention.
Diagnosis of depression (clinician‐rated).
Non‐adherence to medication.
Frequency of primary care appointments (e.g. GP).
Quality of life.
Social functioning.
One review author performed the GRADE assessment, which was checked by another review author. We resolved disagreements by discussion.
Results
Description of studies
See below for a description of the studies found in our literature searches. Details are reported in the Characteristics of included studies; Characteristics of excluded studies; and Characteristics of ongoing studies tables.
Results of the search
The main database searches identified 1594 records with a further 298 records identified from other sources. After removing duplicates we screened 1435 records for eligibility. We excluded 1362 records after screening titles and abstracts as they did not meet the inclusion criteria. We assessed 73 records at full‐text stage and at this point we combined multiple reports of the same study and began working at study rather than record level. We formally excluded 14 studies (reported in 34 papers) at the full‐text stage. The findings of this review are based on 12 studies (reported in 31 papers). The flow of studies through the review process is illustrated in Figure 1.
1.
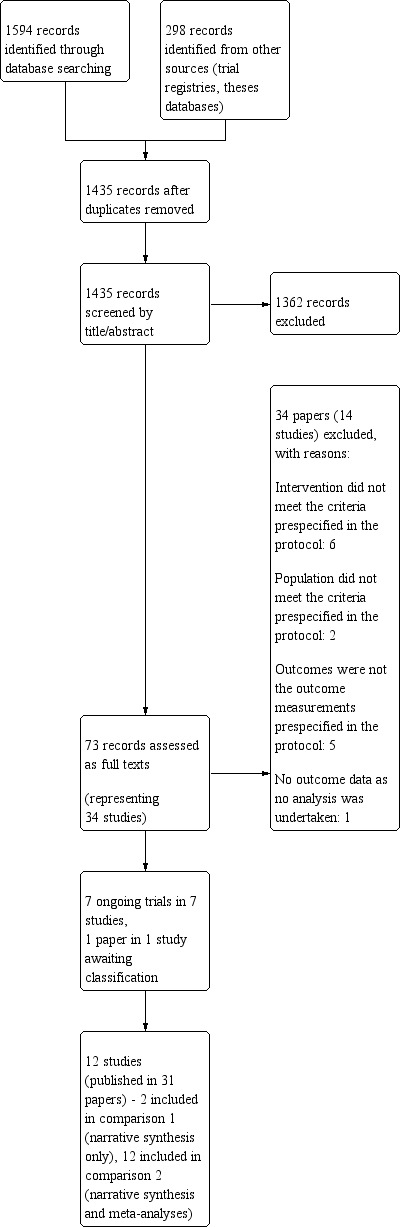
Study flow diagram.
We found one potentially relevant unpublished PhD thesis during reference checking of relevant systematic reviews (Harris 2005). Unfortunately, we were unable to obtain this work and consequently any results have not been included in this review. Details of this study can be found in the Characteristics of studies awaiting classification table.
We identified a conference abstract describing an eligible ongoing study (Phung 2013). Our searches of the trial registers identified six further potentially relevant, ongoing trials (ACTRN12618001105235; ChiCTR‐TRC‐08000726; ISRCTN11290592; NCT01188135; NCT02027259; NCT03591224; see Characteristics of ongoing studies table). Results of these will be included as appropriate when this review is updated.
We contacted the authors of several studies with requests for clarification of their methods or results, or both. This was done either to determine eligibility of a study or to obtain missing data or clarification on a study that was already deemed eligible for inclusion.
Included studies
Twelve studies met the inclusion criteria, 11 of these were individually randomised RCTs. Crockett 2006 was the only cluster‐RCT and the study authors appeared to have adjusted their analyses appropriately to account for clustering. The included studies were published between 2002 and 2016. The majority were carried out in high‐income countries (five in the USA, two in Kuwait, one in Australia, one in the Netherlands, one in Saudi Arabia, and one in Spain) with only one study carried out in an upper‐ to middle‐income country (Brazil). Settings included: primary care (Adler 2004; Capoccia 2004b; Rubio‐Valera 2013); outpatients clinics (Al‐Saffar 2005; Al‐Saffar 2008; Marques 2013); hospital (Aljumah 2015); community pharmacies (Brook 2005; Crockett 2006; Rickles 2005); medical centres (Finley 2003b); and Veteran Affairs clinics (Kanwal 2018). Crockett 2006 was the only study that conducted an intervention in a rural setting.
The 12 studies included 2215 participants. Study samples varied from 63 to 533 participants, with most studies randomising between 100 and 300 participants. One study specified only women (Marques 2013), all other studies included both men and women. Four studies measured comorbidities: Capoccia 2004b; Finley 2003b; and Rubio‐Valera 2013 measured the comorbidities associated with the participants while the intervention presented in Kanwal 2018 was aimed at participants who also had hepatitis C.
Nine studies recruited participants aged 18 years and over (Adler 2004; Aljumah 2015; Al‐Saffar 2008; Brook 2005; Capoccia 2004b; Crockett 2006; Marques 2013; Rickles 2005; Rubio‐Valera 2013). Several studies specified age ranges with both a lower and upper age limits of between 18 and 60 years (Aljumah 2015); 18 and 65 years (Marques 2013); and 18 and 75 years (Rubio‐Valera 2013). One study recruited participants with an age range of 17 to 70 years (Al‐Saffar 2005), and two studies did not report an age range in their inclusion criteria but the mean ages were about 54 to 59 years (Finley 2003b; Kanwal 2018).
In addition to pharmacists, several studies included primary care providers and psychiatrists (Capoccia 2004b), care managers and psychiatrists (Finley 2003b), and nurse depression care manager and psychiatrist (Kanwal 2018). The other nine studies were based on an intervention delivered by a pharmacist only. Capoccia 2004b; Finley 2003b; Kanwal 2018; and Rickles 2005 presented remotely delivered interventions (i.e. contact via telephone); the interventions in the other studies were conducted face‐to‐face.
All interventions were centred around patient education of the condition and the medication that participants were taking: the nature of the depression; possible adverse events; benefits of treatment; and remaining adherent to treatment. Crockett 2006 achieved this by providing participants with an educational video. Management of adverse effects was also an aim of all the interventions; pharmacists were able to alter dosages/titrate based on the information that they were given by participants. Marques 2013 based their intervention on the Dáder Method, a standardised systematic approach aimed at drawing out information relating to patients' health problems and pharmacotherapy.
Self‐management was a key focus of the pharmacy‐based management. Al‐Saffar 2005 and Al‐Saffar 2008 included counselling where patients could talk freely about their concerns. Crockett 2006 emphasised assessing how participants "were going". Rubio‐Valera 2013 additionally encouraged participants to follow GP advice and Adler 2004 aimed to facilitate communication between participants and PCPs. Kanwal 2018 sought to refer participants to speciality health services when necessary. The intervention described in Finley 2003b specifically encouraged participants to continue undertaking activities that they enjoyed. Aljumah 2015 was based on shared decision making designed to assess participant beliefs and their knowledge about medication.
All 12 studies had a TAU arm that was used as a control group, which involved receiving standard communication when collecting their medication, this usually reinforced labelling instructions on the medication, as well as answering any question participants may have had. TAU was viewed as being consistent with the patient education routinely delivered when prescriptions were collected by outpatients. Additionally, Adler 2004 provided participants with the results of the depressive screener that indicated their diagnosis; Kanwal 2018 included referral to speciality mental health clinics; and Rickles 2005 included education and patient monitoring. Al‐Saffar 2005 and Al‐Saffar 2008 had an active control as a third arm, both were based on participant information leaflets (PILs).
All studies used a validated measure of depression to assess participant eligibility for inclusion in the trial. Four studies based diagnosis on the DSM‐IV (APA 2000) (Adler 2004; Aljumah 2015; Capoccia 2004b; Rubio‐Valera 2013); and one study used the ICD‐10 (International Classification of Diseases 10th edition) (Marques 2013). Two studies used the HAMD‐17 (Hamilton Depression Rating) scale (Al‐Saffar 2005; Al‐Saffar 2008). Other measures included: BDI‐II (Rickles 2005); BIDS (Brief Index for Depression Symptoms) (Finley 2003b); PHQ‐9 (Kanwal 2018); SCL‐13 (Symptom Checklist‐13) (Brook 2005); and K‐10 (anxiety and depression checklist) (Crockett 2006).
The primary outcomes of this study were change in depressive symptoms and acceptability of the intervention. The majority of studies measured depression outcomes, either as a symptom change using a change in mean depression score or by means of cut‐off scores on depression measures presented as dichotomous measures. Eleven studies did not present change in depressive symptoms (Adler 2004; Aljumah 2015; Al‐Saffar 2005; Al‐Saffar 2008; Brook 2005; Capoccia 2004b; Finley 2003b; Kanwal 2018; Marques 2013; Rickles 2005; Rubio‐Valera 2013). Adler 2004 did not provide usable depression symptom change data and correspondence with the authors did not provide the necessary information. For all studies, we assessed the acceptability of the intervention based on the number of participants discontinuing the intervention by leaving the study early as specified in our protocol.
Secondary outcome measures included: diagnosis of depression (presented as remission of depression); non‐adherence to medication; frequency of primary care appointments; quality of life; social functioning; and any adverse event. Three studies measured depression remission (Finley 2003b; Kanwal 2018; Marques 2013). Eight studies included a measure of medication adherence (Adler 2004; Al‐Saffar 2005; Aljumah 2015; Capoccia 2004b; Crockett 2006; Finley 2003b; Kanwal 2018; Rubio‐Valera 2013). Two studies measured use of primary care providers and resource utilisation (Capoccia 2004b; Finley 2003b). Five studies presented quality of life (Adler 2004; Aljumah 2015; Brook 2005; Capoccia 2004b; Rubio‐Valera 2013). One study measured social functioning (Finley 2003b). Four studies measured adverse events, including adverse effects and death (Adler 2004; Al‐Saffar 2005; Kanwal 2018; Rubio‐Valera 2013).
Beyond this, most studies reported outcomes that were not prespecified in our protocol. See the Characteristics of included studies table for further details.
Excluded studies
We formally excluded 14 studies. We excluded six studies as the intervention did not meet the criteria prespecified in our protocol (Sampson 2019), often this was due to the pharmacist's involvement being unclear or less than our protocol specification. Two studies had an illegible population. Five studies did not measure our outcomes. One study had only three participants who finished phase one of the intervention and the outcome data were not analysed. Each excluded study and the reason for its exclusion are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
As per the methods prespecified in our protocol (Sampson 2019), we investigated the impact of risk of bias in included studies in sensitivity analyses (see Sensitivity analysis). We excluded any studies that were at high risk of bias in two or more domains from the analyses (Adler 2004; Finley 2003b; Marques 2013). Results of the sensitivity analyses are reported in the Effects of interventions section.
A visual summary of the risk of bias in the included studies is presented in Figure 2 and Figure 3.
2.
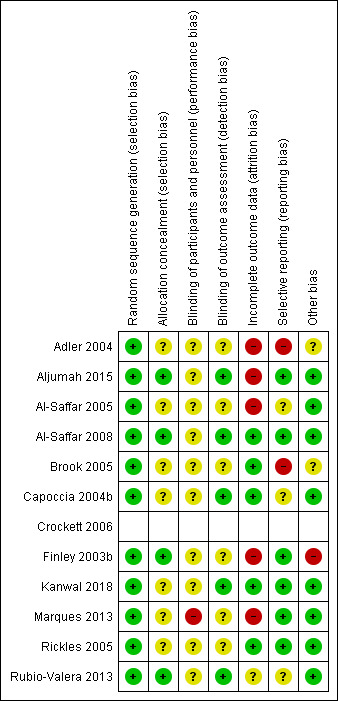
Risk of bias summary.*
*Risk of bias assessment for Crockett 2006 is missing from this graph as the risk of bias assessment for this cluster‐RCT was conducted separately, see Additional tables.
3.
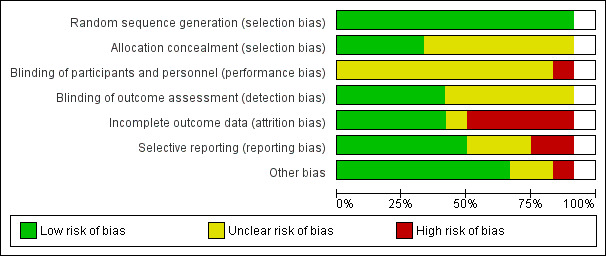
Risk of bias graph*
*Risk of bias assessment for Crockett 2006 is missing from this graph as the risk of bias assessment for this cluster‐RCT was conducted separately, see Additional tables.
Crockett 2006 was the only cluster RCT in the included studies. It was not possible to analyse the risk of bias of a cluster RCT in the same table as the other 11 studies; Table 2 presents the risk of bias for Crockett 2006. The study was at low risk for bias arising from the randomisation process. However, there were some concerns of bias arising from the timing of identification and recruitment of participants in relation to timing of randomisation as there was no mention of blinding in either papers. Bias due to deviations from intended interventions was high as the authors stated that "four of the control pharmacists were identified (after randomisation) as delivering a service to their patients, which paralleled that being provided by the intervention pharmacists. This had the potential to confound the results" (p. 267). Bias due to missing outcome data was at low risk. There was a low risk of bias for measurement of the outcome. Finally, there was no evidence of selective reporting so the risk of reporting bias was low.
1. Crockett 2006 risk of bias tablea.
| Bias domain | Judgement | Support for judgement |
| 1. Bias arising from the randomisation process | Low | Quote: "recruited pharmacies were grouped into clusters by geographical area; the clusters were randomly allocated to one of two groups: control and intervention" (p. 264). |
| 2. Bias arising from the timing of identification and recruitment of individual participants in relation to timing of randomisation | Some concerns | No blinding mentioned. |
| 3. Bias due to deviations from intended interventions | High | Quote: "four of the control pharmacists were identified (after randomisation) as delivering a service to their patients, which paralleled that being provided by the intervention pharmacists. This had the potential to confound the results" (p. 267). |
| 4. Bias due to missing outcome data | Low | Complete data were obtained on 106 participants, 60 control and 46 intervention. |
| 5. Bias in measurement of the outcome | Low | 1 measure, set measures; no evidence of bias |
| 6. Bias in selection of reporting | Low | No evidence of selective reporting |
aAs Crockett 2006 was a cluster RCT a custom 'Risk of bias' table was required.
Allocation
Overall, all studies were at low risk of selection bias (random sequence generation). Allocation concealment was unclear for most studies (Adler 2004; Al‐Saffar 2005; Brook 2005; Capoccia 2004b; Crockett 2006; Kanwal 2018; Marques 2013; Rickles 2005), with four having a low risk of bias (Aljumah 2015; Al‐Saffar 2008; Finley 2003b; Rubio‐Valera 2013).
Blinding
Regarding blinding of participants and personnel, 11 studies were at unclear risk (Adler 2004; Aljumah 2015; Al‐Saffar 2005; Al‐Saffar 2008; Brook 2005; Capoccia 2004b; Crockett 2006; Finley 2003b; Kanwal 2018; Rickles 2005; Rubio‐Valera 2013). Due to the nature of the studies, it was difficult to blind either those participating or delivering the interventions. One study was at high risk as the authors reported five participants in the control group received pharmaceutical guidance that was not characterised as the intervention (Marques 2013).
The risk of detection bias (blinding of outcome and assessment) was low for five studies (Al‐Saffar 2008; Aljumah 2015; Capoccia 2004b; Kanwal 2018; Rubio‐Valera 2013), and unclear for seven studies (Adler 2004; Al‐Saffar 2005; Brook 2005; Crockett 2006; Finley 2003b; Marques 2013; Rickles 2005).
Incomplete outcome data
The risk of attrition bias (incomplete outcome data) was low for six studies (Al‐Saffar 2008; Brook 2005; Capoccia 2004b; Crockett 2006; Kanwal 2018; Rickles 2005), unclear for one study (Rubio‐Valera 2013), and high risk for five studies (Adler 2004: high proportion of missing data, participant numbers at enrolment/follow‐up unclear/inconsistent; Al‐Saffar 2005: high rate of unexplained withdrawals; Aljumah 2015: reported completer data only; Finley 2003b: follow‐up surveys returned primarily by participants who were doing well; Marques 2013: high rate of unexplained withdrawals/dropouts).
Selective reporting
The risk of reporting bias was low for seven studies (Al‐Saffar 2008; Aljumah 2015; Crockett 2006; Finley 2003b; Kanwal 2018; Marques 2013; Rickles 2005), unclear for three studies (Al‐Saffar 2005; Capoccia 2004b; Rubio‐Valera 2013), and high for two studies (Adler 2004; Brook 2005: several prespecified outcomes not reported).
Other potential sources of bias
In nine studies, we did not find any other potential sources of bias (Aljumah 2015; Al‐Saffar 2005; Al‐Saffar 2008; Capoccia 2004b; Crockett 2006; Kanwal 2018; Marques 2013; Rickles 2005; Rubio‐Valera 2013). Two studies were at an unclear risk of bias from other sources (Adler 2004: unexplained differences in the effectiveness of the intervention dependent on timing of enrolment; Brook 2005: funded by a pharmaceutical company). Finley 2003b was at high risk of bias; the corresponding author confirmed that there was a high risk of selection bias in the return of the survey questionnaires at follow‐up.
Effects of interventions
See: Table 1
Comparison 1: pharmacy‐based management (pharmacist with or without wider team) versus active control
Two studies (291 participants) compared pharmacy‐based management with an active control as part of three‐armed trials (Al‐Saffar 2005; Al‐Saffar 2008). In both trials, the active control treatment involved participants receiving a PIL. It was not possible to combine any of the reported outcome data in meta‐analyses.
Primary outcomes
1. Depression symptom level
Neither study reported depression symptom level.
2. Acceptability of the intervention
Al‐Saffar 2005 reported that 44/98 (44.9%) participants assigned to the pharmacy‐based management arm did not attend the five‐month follow‐up. Of the 93 participants assigned to the active control arm, 51 (54.8%) did not attend follow‐up. Al‐Saffar 2008 reported that 33/50 (66.0%) participants assigned to the pharmacy‐based management arm did not attend the six‐week follow‐up. For the active control arm, 21/50 (42.0%) participants did not attend six‐week follow‐up.
This shows that for Al‐Saffar 2005 the pharmacy‐based management arm was more acceptable to participants, but for Al‐Saffar 2008, the active control arm was more acceptable. Therefore, it is unclear whether active controls or pharmacy‐based management are more acceptable for participants.
Secondary outcomes
1. Diagnosis of depression
Neither study reported diagnosis of depression.
2. Non‐adherence to medication
Al‐Saffar 2005 measured adherence by self‐report and tablet counting as the number of participants who reported that they were taking their tablets exactly as prescribed and whose 'tablet‐count ratio' was 80–100% inclusive. At two months, people who received the educational intervention had higher odds of being 'adherent' (for counselling and leaflet: OR 5.09, 95% CI 2.35 to 11.03; for leaflet: OR 3.15, 95% CI 1.42 to 6.99). At five months, people who received the educational intervention had higher odds of being 'adherent' (for counselling and leaflet: OR 6.02, 95% CI 2.79 to 13.00; for leaflet: OR 2.83, 95% CI 1.26 to 6.32). Overall, people who received the pharmacist intervention were more likely to be adherent to their antidepressant medication.
3. Frequency of primary care appointments (e.g. GP)
Neither study reported frequency of primary care appointments.
4. Quality of life
Neither study reported quality of life.
5. Social functioning
Neither study reported social functioning.
6. Any adverse event
In Al‐Saffar 2005, the majority of people in the study reported an adverse effect at two months, which appeared to be related to the pharmacology of the antidepressant medication. The number of reported adverse effects appeared to be similar across all groups. The group receiving the leaflet and pharmacist counselling reported fewer adverse effects. However, there were insufficient data reported to judge whether this difference was clinically meaningful.
Comparison 2: pharmacy‐based management (pharmacist with or without wider team) versus treatment as usual
Twelve studies (2072 participants) compared pharmacy‐based management with TAU (Adler 2004; Aljumah 2015; Al‐Saffar 2005; Al‐Saffar 2008; Brook 2005; Capoccia 2004b; Crockett 2006; Finley 2003b; Kanwal 2018; Marques 2013; Rickles 2005; Rubio‐Valera 2013). This section includes the TAU arms of Al‐Saffar 2005 and Al‐Saffar 2008. TAU included advice from the pharmacist reiterating the instructions in the PIL of the antidepressant; standard communication; signposting; routine patient education; and standard patient monitoring. Wherever possible, we combined outcome data in meta‐analyses.
Primary outcomes
1. Depression symptom level
Due to the wide range of measures used in the primary studies to assess depression, we had to make pragmatic decisions weighing up the statistical possibilities of combining continuous and dichotomous data with the desire to produce clinically meaningful results. We outline the methods used to combine studies for this outcome in Data synthesis. In short, we decided to analyse separately improvement in depression (dichotomous data, Analysis 1.1) and change from baseline mean depression score (continuous data, Analysis 1.2).
1.1. Analysis.
Comparison 1 Pharmacy‐based management (pharmacist with or without wider team) versus treatment as usual, Outcome 1 Depression symptom level: improvement (intervention endpoint).
1.2. Analysis.
Comparison 1 Pharmacy‐based management (pharmacist with or without wider team) versus treatment as usual, Outcome 2 Depression symptom level: change from baseline mean depression score (intervention endpoint).
At intervention endpoint, four studies (475 participants) contributed to Analysis 1.1 (Capoccia 2004b; Finley 2003b; Kanwal 2018; Rickles 2005). There was probably no difference in effectiveness between the pharmacy‐based management intervention group and the TAU controls (RR 0.95, 95% CI 0.86 to 1.05; I2 = 0%, moderate‐certainty evidence; Figure 4). GRADE assessment of the certainty of the evidence included in this analysis revealed concerns about imprecision in the analyses (the CI was not sufficiently narrow to rule out a difference between interventions and the summary estimate was driven by the largest study, Kanwal 2018).
4.
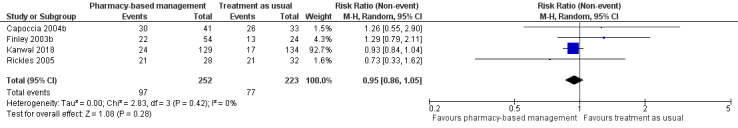
Primary outcome 1: Improvement in depression (intervention endpoint).
Five studies (718 participants) contributed to Analysis 1.2 (Aljumah 2015; Brook 2005; Crockett 2006; Finley 2003b; Rubio‐Valera 2013). This meta‐analysis found no evidence of a difference in effectiveness between the two groups in change in mean depression score from baseline (SMD ‐0.04, 95% CI ‐0.19 to 0.10; I2 = 0%, high‐certainty evidence; Figure 5).
5.
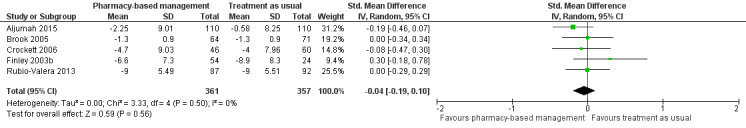
Primary outcome 1: Change from baseline mean depression score (endpoint).
There was no statistical heterogeneity in these analyses although studies used interventions of different duration.
None of the included studies reported depression outcomes at follow‐up.
2. Acceptability of the intervention
Data for this outcome were extracted from trial publications based on the number of people discontinuing the intervention by leaving the study early using participant numbers at intervention endpoint as defined by the authors. Meta‐analysis of 12 studies (2072 participants) showed there may have been no effect for pharmacy‐based management compared with TAU on the acceptability of the intervention (RR 1.09, 95% CI 0.81 to 1.45; I2 = 79%; low‐certainty evidence; Analysis 1.3). The considerable heterogeneity observed in this analysis might be explained by the difference in treatment duration (six weeks to 12 months). However, regardless of treatment duration, most studies reported equivocal results, with wide CIs often spanning the line of no effect (see Figure 6). We comment on the issues around using number of participants leaving the trial as a measure of acceptability in the Discussion. Given the concerns about imprecision and indirectness in this analysis, we cannot be certain whether or not there is a difference in effectiveness between pharmacy‐based management and TAU.
1.3. Analysis.
Comparison 1 Pharmacy‐based management (pharmacist with or without wider team) versus treatment as usual, Outcome 3 Acceptability of the intervention (as measured by participants not attending follow‐up).
6.
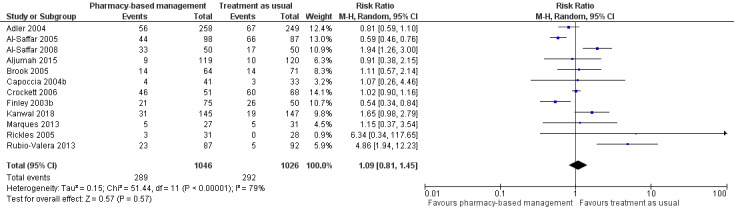
Primary outcome 2: Acceptability of the intervention.
As specified in Assessment of reporting biases, we created and scrutinised a funnel plot for this analysis (Figure 7). Visual inspection of this plot suggested that bigger studies with non‐significant results are relatively well balanced. However, as is often the case, smaller studies with non‐significant findings may be missing. Overall, we do not feel that the shape of the funnel plot gives reason to assume a high risk of reporting bias within the included studies.
7.
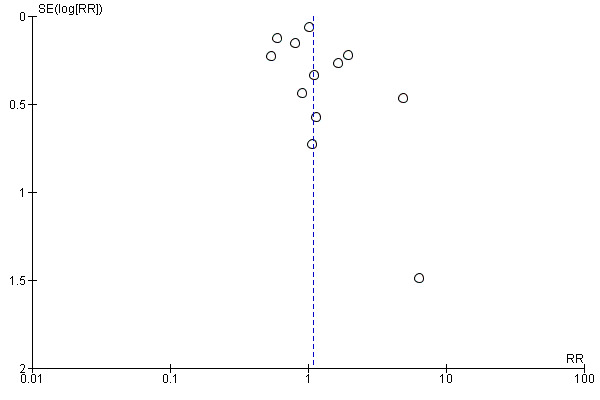
Funnel plot for Primary outcome 2: Acceptability of the intervention (Analysis 2.3).
Secondary outcomes
1. Diagnosis of depression
Three studies (368 participants) reported diagnosis of depression or remission (Finley 2003b; Kanwal 2018; Marques 2013). This was defined as a participant scoring below a certain cut‐off point on a depression scale. We were unable to combine these studies in a meta‐analysis as they reported findings using different depression measures at different time points. The effectiveness of pharmacy‐based management on a diagnosis of depression (or depression remission) remains unclear.
At intervention endpoint, Finley 2003b reported that 30/54 (55.6%) participants in the pharmacy‐based management arm had a score of less than 9 on the BIDS depression scale which indicated remission. For the TAU arm, this proportion was 14/24 (58.3%). This suggests a slightly higher proportion of participants in the TAU achieved remission by the end of the intervention than the pharmacy‐based management arm. However, this difference might not be clinically significant as there was a high risk of attrition bias in this trial. The paper did not conduct a statistical test to compare groups for this outcome.
Kanwal 2018 reported at intervention endpoint that 22/114 (19.3%) participants in the pharmacy‐based management arm had a score of less than 9 on the BIDS depression scale. For the TAU arm this proportion was 9/128 (7.0%). A Chi2 test showed that intervention participants were more likely to experience remission at 12 months (P = 0.004).
Marques 2013 reported that 7/22 (31.8%) participants in the pharmacy‐based management arm achieved remission, defined by the study authors as a score of less than 11 on the BDI. For the TAU arm, this proportion was 4/26 (15.4%) participants indicating that a higher proportion of those in the pharmacy‐based management arm experienced remission at intervention endpoint. However, the authors did not perform a statistical test for differences between groups.
2. Non‐adherence to medication
Most of the included studies reported adherence rather than non‐adherence so we extracted these data as reported in the papers. We used the function in Review Manager to swap events and non‐events so that the RR reflected a reduction in non‐adherence (Review Manager 2014). This has the advantage of presenting the forest plots consistently across outcomes so that a pooled effect to the left of the line of no effect indicated benefit for the intervention group.
Al‐Saffar 2005 measured medication adherence by self‐report and tablet counting, presented as a dichotomous variable. Crockett 2006 measured adherence based on a self‐reported dichotomous variable of whether participants were still taking the medication or not. Rubio‐Valera 2013 used computerised pharmacy records to measure non‐adherence defined as refilling less than 80% of the prescribed dosages and thus presented non‐adherence as a dichotomous measure. Capoccia 2004b measured adherence through a self‐reported telephone interview based on the number of days participants said they took the antidepressant medication in the past month. Finley 2003b expressed adherence both as a continuous and dichotomous measure using medication possession ratio (MPR), participants were defined as adherent if they had an MPR of 0.83 and above, as there was no SD provided in the results we opted to use the dichotomous measure. Kanwal 2018 also used MPR to measure adherence but classified a participant as adherent if they had an MPR greater than 90% and presented the data as a dichotomous measure. It was deemed suitable to perform a meta‐analysis.
Aljumah 2015 measured adherence using the Morisky Medication Adherence Scale (MMAS), a continuous scale with scores ranging from 0 to 8, when higher scores represented better adherence; a score of less than 6 indicated poor adherence. Brook 2005 measured adherence using an electronic tablet dispenser called an eDEM that recorded each opening of the box by the day, hour, and minute, this was combined with pharmacy record data and presented as a continuous measure. Rickles 2005 measured medication non‐adherence using pharmacy refill records over a six‐month period (i.e. measured up to three months after the intervention concluded); it was presented continuously as percentage of dosages missed per participant.
At intervention endpoint, the six studies (911 participants) with dichotomous measures contributed to the analysis (Al‐Saffar 2005; Capoccia 2004b; Crockett 2006; Finley 2003b; Kanwal 2018; Rubio‐Valera 2013). The meta‐analysis suggests a 27% reduced risk of non‐adherence in the pharmacy‐based management group compared with TAU (RR 0.73, 95% CI 0.61 to 0.87; I2 = 45%; high‐certainty evidence; Analysis 1.4; Figure 8). The heterogeneity in this analysis might have been related to the difference in adherence measures used or some uncertainty (due to unclear reporting) about when exactly adherence was measured, or both.
1.4. Analysis.
Comparison 1 Pharmacy‐based management (pharmacist with or without wider team) versus treatment as usual, Outcome 4 Non‐adherence to medication.
8.
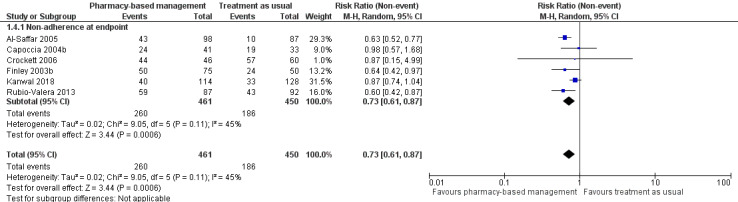
Secondary outcome 2: Non‐adherence.
3. Frequency of primary care appointments
Capoccia 2004b followed the number of clinic visits accessed by study participants over 12 months. Clinic visits included primary care providers, psychiatrists, emergency department, counsellors, and alternative medicine providers. At 12 months, there were no significant differences between treatment groups in the number of visits to healthcare providers; this was evident in overall visits, and in subgroup analyses of specific healthcare providers, including those of primary care.
4. Quality of life
Aljumah 2015 assessed health‐related quality of life at baseline, three months, and six months using the Arabic version of the EQ‐5D. Overall, there was no significant differences in health‐related quality of life between groups at three months (P = 0.971), and six months (P = 0.722).
5. Social functioning
Finley 2003b assessed social functioning using the Work and Social Disability Scale (WSDS), a one‐item, 5‐point scale used to assess the degree of disability ranging from absent to severe. At six months, there was no significant changes in WSDS scores between intervention and control groups (P = 0.357).
6. Any adverse event
None of the studies reported adverse events.
Subgroup analyses
1. Participant characteristics
1.1 Participants with physical and mental health comorbidities
We investigated the impact of comorbidities on the effectiveness of the intervention by means of prespecified subgroup analyses. The two studies comparing pharmacy‐based management with an active control did not report any comorbidities (comparison 1; Al‐Saffar 2005; Al‐Saffar 2008). Therefore, we were unable to conduct subgroup analyses for this comparison.
In comparison 2 (pharmacy‐based management compared with TAU), four studies reported comorbidities (Capoccia 2004b; Finley 2003b; Kanwal 2018; Rubio‐Valera 2013). For improvement in depression (dichotomous measure of depression symptom change) three studies (415 participants) reported and measured comorbidities (Capoccia 2004b; Finley 2003b; Kanwal 2018). Subgroup analysis did not reveal a difference in effectiveness of the intervention dependent on the presence or absence of comorbidities. There was no statistically significant difference between the subgroups (test for subgroup differences: P = 0.83; see Figure 9). The same pattern was true for change from baseline mean depression score (continuous measure of depression symptom change) where two studies (257 participants) reported comorbidities (test for subgroup differences: Chi2 = 1.43, degrees of freedom (df) = 1, P = 0.23; see Figure 10) (Finley 2003b; Rubio‐Valera 2013).
9.
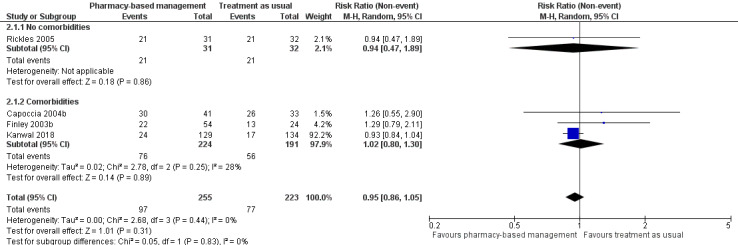
Subgroup analysis 1.1: Improvement in depression (endpoint).
10.
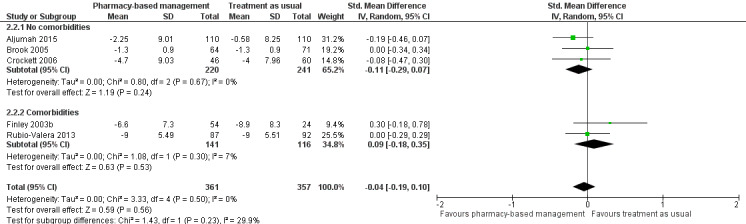
Subgroup analysis 1.1: Change from baseline mean depression score (endpoint).
For acceptability of the intervention, four studies (670 participants) reported comorbidities (Capoccia 2004b; Finley 2003b; Kanwal 2018; Rubio‐Valera 2013). There was no evidence of difference in the effectiveness of the intervention associated with the presence or absence of comorbidities and there was no statistically significant difference in acceptability between the subgroups (test for subgroup differences: Chi2 = 0.48, df = 1, P = 0.49; see Figure 11).
11.
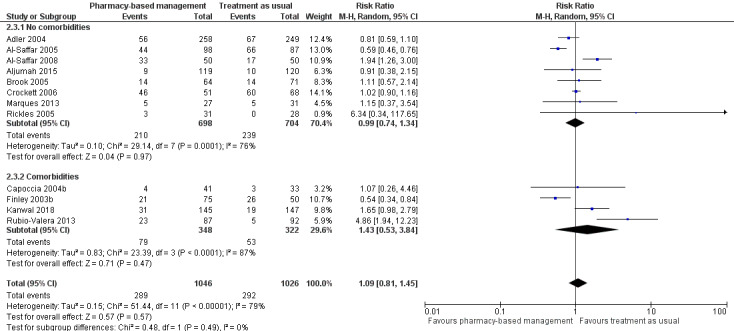
Subgroup analysis 1.1: Acceptability of intervention (as measured by participants not attending follow‐up).
1.2 Baseline severity of depression
We had planned to investigate the impact of the severity of depression at baseline on the effectiveness on the intervention. Not all studies reported baseline severity; however, all studies recruited participants with all levels of depression (mild to severe) and did not report outcome data separately for groups of participants based on baseline depression severity. We were unable to conduct these subgroup analyses.
2. Intervention characteristics: delivery method
We had specified in the protocol that we would conduct subgroup analyses to investigate the impact of different types of "in‐person" and "remote" delivery. Our suggested approach was to compare, for example, one‐to‐one appointments with group sessions or telephone‐based support with a digital technology supported programme ("app"). However, the studies using an "in‐person" approach did not report sufficient details to allow these subgroup analyses. Of the studies using a remotely delivered intervention, all used telephone calls. We conducted a subgroup analysis to investigate the impact of the delivery method ("in‐person" or "remote") on the effectiveness of the intervention on the primary outcomes.
The two studies comparing pharmacy‐based management with an active control (comparison 1) were both delivered in person (Al‐Saffar 2005; Al‐Saffar 2008). Therefore, it was not possible to conduct subgroup analyses.
In comparison 2 (pharmacy‐based management compared with TAU), eight studies were delivered in person (Adler 2004; Aljumah 2015; Al‐Saffar 2005; Al‐Saffar 2008; Brook 2005; Crockett 2006; Marques 2013; Rubio‐Valera 2013); the remaining four used a telephone‐delivered intervention (Capoccia 2004b; Finley 2003b; Kanwal 2018; Rickles 2005). For improvement in depression (dichotomous measure of depression symptom change), all four studies used a remotely delivered intervention (Capoccia 2004b; Finley 2003b; Kanwal 2018; Rickles 2005). A subgroup analysis was not possible. For change from baseline mean depression score (continuous measure of depression symptom change), all studies were delivered in person apart from apart from Finley 2003b, which used a telephone‐based intervention (78 participants). Subgroup analysis did not reveal a difference in effectiveness of the intervention dependent on the delivery method of the intervention. There was no statistically significant difference between the subgroups (test for subgroup differences: P = 0.14; see Figure 12).
12.
Subgroup analysis 2: Change from baseline mean depression score (endpoint).
For acceptability of the intervention, four studies (550 participants) delivered the intervention remotely (Capoccia 2004b; Finley 2003b; Kanwal 2018; Rickles 2005). There was no difference in the effectiveness of the intervention associated with the way the intervention was delivered and there was no difference in effectiveness between the subgroups (test for subgroup differences: P = 0.99; see Figure 13).
13.
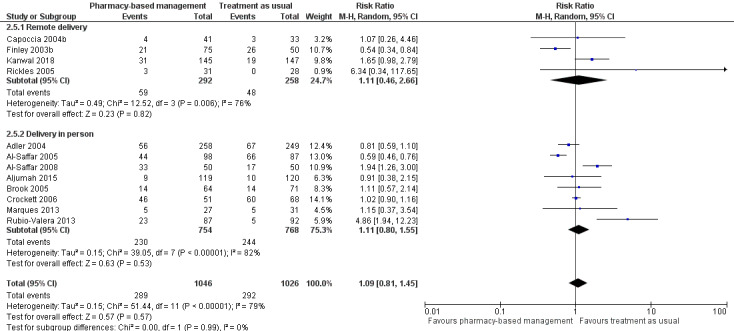
Subgroup analysis 2: Acceptability of the intervention
3. Ad‐hoc analysis: involvement of wider pharmacy team
Due to a scarcity of data in the included studies, we deviated from our initial plan to analyse studies where the wider pharmacy team was involved with the delivery of the intervention separately from those where one pharmacist delivered the intervention on their own. In the interest of producing the most usable and informative systematic review and meta‐analysis, we made a pragmatic team decision to combine those two groups of studies and investigate the impact of the involvement of a team in subgroup analyses (see Differences between protocol and review). None of the included studies involved a collaborative care team outside of the pharmacy in the intervention. The presence or absence of a "wider team" refers exclusively to the pharmacy team, for example the involvement of pharmacy assistants, technicians, healthy living champions or other roles based within community pharmacies.
In the two studies comparing pharmacy‐based management with an active control (comparison 1; Al‐Saffar 2005; Al‐Saffar 2008), the intervention was delivered by a pharmacist alone.
In comparison 2 (pharmacy‐based management compared with TAU), three studies used an intervention that was delivered by a pharmacist plus the wider pharmacy team (Capoccia 2004b; Finley 2003b; Kanwal 2018). For improvement in depression (dichotomous measure of depression symptom change), three studies (415 participants) used an intervention that was delivered by a pharmacist plus the wider pharmacy team (Capoccia 2004b; Finley 2003b; Kanwal 2018). Subgroup analysis did not reveal a difference in the effectiveness of the intervention dependent on the involvement of the wider pharmacy team. There was no statistically significant difference between the subgroups (test for subgroup differences: Chi2 = 0.05, df = 1, P = 0.83; see Figure 14). The same pattern was true for change from baseline mean depression score (continuous measure of depression symptom change), where Finley 2003b (78 participants) used an intervention that was delivered by a pharmacist plus the wider pharmacy team (test for subgroup differences: Chi2 = 2.14, df = 1, P = 0.14; see Figure 15).
14.
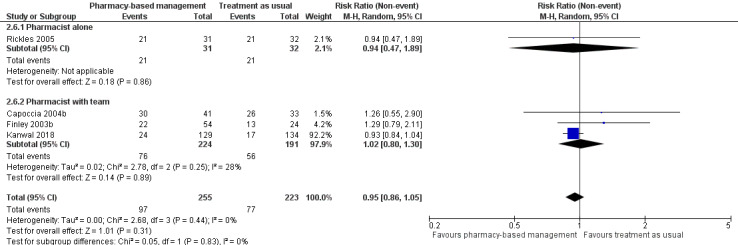
Subgroup analysis 3: Improvement in depression (endpoint).
15.
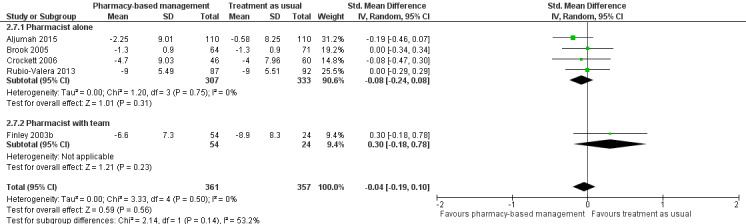
Sensitivity analysis 3: Change from baseline mean depression score (endpoint).
For acceptability of the intervention, three studies (419 participants) included the wider pharmacy team (Capoccia 2004b; Finley 2003b; Kanwal 2018). There was no difference in the effectiveness of the intervention associated with the way the intervention was delivered and there was no statistically significant difference in effectiveness between the subgroups (test for subgroup differences: Chi2 = 0.12, df = 1, P = 0.73; see Figure 16).
16.
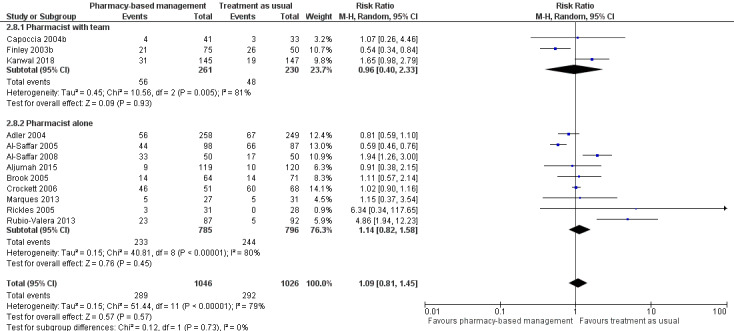
Subgroup analysis 3: Acceptability of the intervention.
We decided to investigate the impact of the presence or absence of the wider pharmacy team in the delivery of the intervention on the effectiveness of pharmacy‐based management in improving medication adherence. This was a pragmatic ad hoc team decision that acknowledges the potential importance of this question to practitioners and policy makers. We also judged it important to have a more thorough assessment as we had originally proposed to analyse pharmacist alone and wider pharmacy team interventions separately.
For non‐adherence, three studies (419 participants) included the wider pharmacy team (Capoccia 2004b; Finley 2003b; Kanwal 2018). While the direction of effect was the same for both subgroups, in that it favoured the intervention group, the test for subgroup differences suggested that there was a statistically significant difference between interventions delivered by a pharmacist alone and those delivered with involvement by the wider pharmacy team (test for subgroup analyses: Chi2 = 5.58, df = 1, P = 0.02). The effect in the 'pharmacist only' studies was larger (RR 0.63, 95% CI 0.53 to 0.74; I2 = 0%) than in the 'pharmacist plus wider team' studies (RR 0.84, 95% CI 0.71 to 1.00; I2 = 8%) and also larger than the overall effect estimate (RR 0.73, 95% CI 0.63 to 0.87; I2 = 45%; Analysis 2.9; Figure 17).
2.9. Analysis.
Comparison 2 Subgroup analyses, Outcome 9 Non‐adherence to medication.
17.
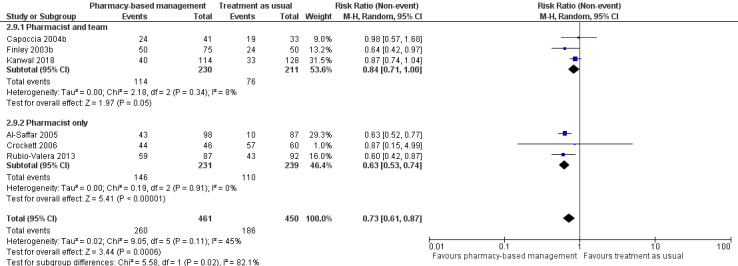
Subgroup analysis 3: Non‐adherence.
Sensitivity analyses
As per our protocol, we conducted the following sensitivity analyses for the primary outcomes.
1. Risk of bias
We removed all trials with a high risk of bias in two or more domains from each analysis.
Improvement in depression (dichotomous measure of depression symptom change): removing Finley 2003b (78 participants; high risk of attrition and selection bias) from this analysis did not make a substantive change to the results (RR 0.94, 95% CI 0.85 to 1.04; I2 = 0%; 3 studies, 400 participants; Analysis 3.1).
3.1. Analysis.
Comparison 3 Sensitivity analyses, Outcome 1 Sensitivity analysis 1: improvement in depression (endpoint).
Change from baseline mean depression score (continuous measure of depression symptom change): removing Finley 2003b (78 participants; high risk of attrition and selection bias) from this analysis did not make a substantive change to the results (SMD ‐0.08, 95% CI ‐0.24 to 0.08, I2 = 0%; 4 studies, 640 participants; Analysis 3.2).
3.2. Analysis.
Comparison 3 Sensitivity analyses, Outcome 2 Sensitivity analysis 1: change from baseline mean depression score (endpoint).
Acceptability of the intervention: removing Adler 2004 (high risk of attrition and reporting bias), Finley 2003b (high risk of attrition and selection bias), and Marques 2013 (high risk of performance and attrition bias; 690 participants in total) from this analysis did not make a substantive difference to the results (RR 1.29, 95% CI 0.88 to 1.90, I2 = 83%; 9 studies, 1382 participants; Analysis 3.3).
3.3. Analysis.
Comparison 3 Sensitivity analyses, Outcome 3 Sensitivity analysis 1: acceptability of the intervention (as measured by participants not attending follow‐up).
2. Assumptions for missing data
We removed trials that used methods of imputation to account for missing data.
Improvement in depression (dichotomous measure of depression symptom change: removing Capoccia 2004b and Kanwal 2018 (337 participants in total) from this analysis did not make a substantive change to the results (RR 1.16, 95% CI 0.78 to 1.74; I2 = 0%; 2 studies, 141 participants; Analysis 3.4).
3.4. Analysis.
Comparison 3 Sensitivity analyses, Outcome 4 Sensitivity analysis 2: improvement in depression (intervention endpoint).
Change from baseline mean depression score (continuous measure of depression symptom change): removing Brook 2005 and Rubio‐Valera 2013 (314 participants in total) from this analysis did not make a substantive change to the results (SMD ‐0.05, 95% CI ‐0.31 to 0.21; I2 = 35%; 3 studies, 404 participants; Analysis 3.5).
3.5. Analysis.
Comparison 3 Sensitivity analyses, Outcome 5 Sensitivity analysis 2: change from baseline mean depression score (intervention endpoint).
Acceptability of the intervention: removing Brook 2005; Capoccia 2004b; Kanwal 2018; and Rubio‐Valera 2013 (680 participants in total) from this analysis did not make a substantive change to the results (RR 0.91, 95% CI 0.66 to 1.24, I2 = 81%; 8 studies, 1392 participants; Analysis 3.6).
3.6. Analysis.
Comparison 3 Sensitivity analyses, Outcome 6 Sensitivity analysis 2: acceptability of intervention (as measured by participants not attending follow‐up).
3. Classification of depression
All included trials reported how depression was assessed, see Characteristics of included studies table. We did not conduct a sensitivity analysis based on the classification of depression.
Discussion
Summary of main results
Our ability to analyse results from studies comparing pharmacy‐based management with active control was limited due to a scarcity of available evidence. Narrative synthesis of results from two studies on the acceptability of the intervention proved inconclusive.
We found no evidence of a difference in depression symptom change between pharmacy‐based management and TAU based on moderate certainty evidence of improvement in depression symptoms and high certainty evidence on mean change in depression score. Similarly, we found there was probably no difference in acceptability of the intervention between the two groups. This was based on low certainty evidence.
The risk of non‐adherence to medication was lower (i.e. adherence was better in participants who had received pharmacy‐based care than in those who received TAU). This was based on high certainty evidence. We were unable to synthesise data on the other outcomes we had prespecified, including adverse events.
These findings were based on evidence from 12 trials (just over 2215 participants), some of which were methodologically flawed. Overall, the certainty of the evidence was variable. While our review included all available evidence found by a comprehensive search, we do believe that the conclusions of this review might change in future as new evidence emerges. In addition, given the heterogeneity for both interventions and controls across trials, further work is needed in this area to explore what are the active ingredients of pharmacy‐based management.
We used prespecified subgroup analyses to investigate the potential impact of salient characteristics of the patient population and the intervention and its delivery. In addition, we also conducted an ad‐hoc subgroup analysis to evaluate the impact of the presence or absence of the wider pharmacy team (e.g. pharmacy assistants, technicians, healthy living champions) in the delivery of the intervention. None of these analyses revealed substantive differences between subgroups. However, missing details from study publications limited the depth and breadth of our analyses to some extent.
We scrutinised the robustness of our results by means of several prespecified sensitivity analyses, excluding studies at high risk of bias in two or more domains as well as those that had relied on imputation of missing data (using statistical methods to estimate outcomes for people who did not provide data to the study) from the meta‐analyses for the primary outcomes. None of the sensitivity analyses revealed a substantive change to the results when those studies were removed. However, we cannot rule out the impact of these factors on our data as most analyses included a small number of studies across subgroup and sensitivity analyses which limited our ability to detect any differences.
Overall completeness and applicability of evidence
Strengths
We are confident that this review includes all available evidence in the area of pharmacy‐based management for depression in adults, spanning a range of interventions that are likely to be accessible to people in practice. By including intervention programmes irrespective of their duration or delivery method, this review presents a high‐quality and up‐to‐date summary and evaluation of the evidence. However, including a wide range of eligible interventions introduces heterogeneity to the analyses. We have taken every precaution and have paid due diligence in our decision making whether or not to combine studies statistically. We believe the decision to conduct meta‐analyses is appropriate and justifiable, but we are aware that there might be fundamental differences between studies that cannot be explained by chance.
Limitations
We acknowledge that defining acceptability of the intervention as the number of people discontinuing the treatment by leaving the study early has important methodological and conceptual limitations. Leaving the study early does not necessarily mean that these participants also discontinued the treatment. However, this was a pragmatic decision based on the information available in the trial publications. All included trials reported dropout rates, none specified if there were any participants who left the trial but continued with the intervention. This information might be difficult to obtain as follow‐up of participants who have left the trial might be unethical.
Overall, the evidence base is complex and often poorly reported, making it challenging and time‐consuming not only to identify eligible studies but also to determine details of the intervention, for example the extent of the pharmacist's involvement. We acknowledge that there is some considerable variation in the comparator treatments we have combined in our meta‐analysis of pharmacy‐based management compared with TAU. However, while the comparator interventions vary in terms of their intensity and content, they share a number of common features: 1. they were delivered by a healthcare professional outside the pharmacy team (GPs, nurses, mental health practitioners) and 2. any contact with the pharmacist (beyond dispensing) was minimal and initiated by the patient. Some studies, for example, reported that pharmacists would answer factual questions about the prescribed antidepressant (TAU) but would not offer advice or counselling (pharmacy‐based management).
Similarly, due to the wide range of depression and adherence measures used in research and clinical practice, it can be difficult to synthesise evidence in a clinically meaningful way that will help practitioners and policy makers in their decision making. Searching the entirety of the medical literature using a very comprehensive and multilevel search strategy, we found 12 trials, some of them very small, reporting data on 2215 participants. Many of the trials were at unclear risk of bias, often due to incomplete reporting of methods, and some of the key outcomes we had specified as most relevant and important, including adverse events, were reported very sparsely.
Outcome data beyond the endpoint of the intervention was very scarcely reported, which has left us unable to conduct meta‐analyses of the effectiveness of pharmacy‐based management on long‐term depression symptom change, depression diagnosis, quality of life/well‐being, health resource use, or adverse events. This is a pattern that is frequently observed, not only in research published in this area but also in clinical guidelines (NICE 2018). However, as depression is known to be a relapsing condition, it is crucially important that these gaps in the literature are highlighted and communicated in an effort to facilitate high‐quality new research that can address them.
We appreciate that the potential implications of the findings of this review might vary between countries, regions, and healthcare systems. Interestingly, we did not identify any trials from the UK that were eligible for inclusion in this review. Consequently, the comparator interventions used by the trials we did include do not align with the UK guideline recommendation of regular check‐up appointments with the person's GP (NICE 2018). Therefore, our findings are likely to be more applicable to health systems where TAU is less intensive than that recommended in the UK. It is less likely that pharmacy‐based management is more effective than TAU in the UK. However, there is greater potential for pharmacy‐based management to be cost‐effective if found to be as clinically effective as TAU.
In addition, there is evidence that pharmacies are very accessible in England (Todd 2014), particularly in deprived areas. However, this might not be the case in other countries where higher percentages of the population live in more rural/remote areas. A related consideration is that the embedding of pharmacists within general practices might not be feasible in all healthcare/health insurance systems. As such, care will need to be taken when applying the findings of this review to contexts outside England/the UK.
Quality of the evidence
Incomplete reporting of trial methods and intervention components made study selection and analyses challenging; however, this may partly reflect the complexity of interventions.
GRADE assessment revealed concerns about imprecision in the evidence base for improvement in depression (Analysis 1.1) where point estimates of the individual studies varied widely and the summary estimate of effect was heavily influenced by one study. The certainty of the evidence for this outcome was judged to be moderate. Similarly, the analysis for acceptability of the intervention (Analysis 1.4) was affected by imprecision (wide CIs). Indirectness was also a concern here given the difficulties to appropriately define acceptability (see above). The evidence for this outcome was considered to be of low certainty.
Change from baseline depression data (Analysis 1.2) and non‐adherence (Analysis 1.3) were based on high‐certainty evidence.
Potential biases in the review process
While we are confident in the quality of our search and review methods, a small risk of bias being introduced during the review process remains. Studies might have been missed by the search (we identified one potentially eligible unpublished thesis we were unable to access) and we had to exclude potentially eligible trials due to insufficient data when we were unable to reach the authors. In line with best practice, we took steps to reduce review author bias and error, especially at the critical stages of study selection, data extraction, and risk of bias assessment. We made some pragmatic team decisions that changed the analysis methods from what we had initially specified in the protocol (Sampson 2019). These decisions were based on the data we had extracted from the included studies but they were taken in the interest of presenting our evidence synthesis in the most accessible and meaningful way.
Agreements and disagreements with other studies or reviews
The present review goes some way to filling the gap that has been identified in the literature of pharmacy‐based interventions, especially when applied in the context of mental, rather than physical, health problems (Thomson 2019). To our knowledge, it is the first comprehensive systematic review to address the role community pharmacists, with or without their teams, can play in the provision of safe and acceptable depression care. However, the findings of the present review are far from conclusive, and high‐quality primary research, such as planned in the current ISRCTN11290592 study, is needed urgently to gain a better understanding of this promising area.
Authors' conclusions
Implications for practice.
Currently, there is no evidence of a difference in acceptability or improvement in depression for pharmacy‐based management compared with treatment as usual (TAU). However, this does not necessarily rule out a more active role for pharmacists in the treatment of depression.
There was considerable variability between studies in both interventions and TAU included in trials. Therefore, there is need for further work to explore active ingredients of pharmacy‐based management to inform what a reliable and effective intervention might be.
In addition, if further support from pharmacists can reduce general practitioner time needed to manage depression care, without a detrimental effect on outcomes, this has potentially important implications for cost‐effectiveness. Although it should be emphasised we did not assess the impact of pharmacy‐based management on general practitioner time but future work should investigate this further.
Furthermore, improvements in non‐adherence observed for pharmacy‐based management has the potential to lead to improvements in depression long term. However, this cannot yet be established based on the current data in our review.
Regardless of the effectiveness and acceptability of pharmacy‐based management of depression, the cost and resource implications of providing training and supervision to pharmacists and their team (which may include pharmacy assistants, technicians, healthy living champions and others working within the community pharmacy setting) to offer this care will need to be evaluated and taken into consideration when making decisions about the feasibility of rolling this care model out more widely.
Implications for research.
The present systematic review was limited by incomplete or unclear (or both) reporting of included studies. This affected our assessment of trial methodology and conduct but also our understanding of the components of the interventions used in the individual trials. Future research should be rigorously designed and conducted. It is encouraging that there are trials ongoing in this area that will add substance to the evidence base. Researchers might like to focus on the mechanism underlying the pharmacy‐based depression management or explore its effectiveness in other populations (e.g. children/adolescents or people affected by comorbidities) or other mental health conditions (e.g. anxiety).
Our findings suggest that there is no difference in effectiveness between pharmacy‐based management and TAU. In the context of the current policy landscape in the UK and the drive towards increased embedding of pharmacists within primary care, for example, this finding raises important questions around the cost effectiveness of pharmacy‐based management compared with TAU. A robust, well‐designed review of cost effectiveness is needed to address this gap in the evidence and provide further details on the potentially important implications of our review findings.
Sufficiently powered randomised controlled trials with follow‐up beyond the intervention endpoint are also needed to establish whether the improvement in medication adherence translates into greater reduction of depressive symptoms in people receiving pharmacy‐based care. Future trials should take greater care to report components of both the treatment and the control interventions in sufficient detail to enable readers, and potential future systematic reviewers, to make informed decisions about the applicability of the trial to their given context. The evidence base would also benefit from future trials reporting depression outcomes using validated scales commonly in use in clinical practice.
In the UK, increased involvement of pharmacists in patient care is attractive due to the better accessibility of pharmacies for the majority of people (Todd 2014). It would be informative if studies would evaluate if pharmacy‐based interventions for depression and other (mental health) conditions reach the people who need them most as a function of this better accessibility.
What's new
| Date | Event | Description |
|---|---|---|
| 6 February 2020 | Amended | A typographical error has been corrected in the abstract. |
History
Protocol first published: Issue 4, 2019 Review first published: Issue 12, 2019
| Date | Event | Description |
|---|---|---|
| 23 December 2019 | Amended | New review. Searches were conducted between the end of November and beginning of December 2018. |
Acknowledgements
We thank the editorial team of the Cochrane Common Mental Disorders (CCMD) Group including Sarah Dawson (Information Specialist) for her support with the electronic literature searches and study selection.
We thank all authors of primary studies who replied to our requests for additional information or provided unpublished data, or both.
The review authors and the CCMD Editorial Team are grateful to the following peer reviewers for their time and comments: Cathal Cagogan, Philip Kerrigan, Virginia Minogue, and Gill Worthy. They would also like to thank Copy Editor, Anne Lawson, and Cochrane Copy Edit Support for the team's help.
CRG funding acknowledgement: the National Institute for Health Research (NIHR) is the largest single funder of the CCMD Group.
Disclaimer: the views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the NIHR, the National Health Service or the Department of Health and Social Care.
Appendices
Appendix 1. Specialised Register: CCMD's core MEDLINE search strategy
The search strategy listed below is the weekly Ovid MEDLINE search which was used to inform Cochrane Common Mental Disorders specialised register. It was based on a list of terms for all conditions within the scope of Cochrane Common Mental Disorders plus a sensitive RCT filter
1. [MeSH Headings]: eating disorders/ or anorexia nervosa/ or binge‐eating disorder/ or bulimia nervosa/ or female athlete triad syndrome/ or pica/ or hyperphagia/ or bulimia/ or self‐injurious behavior/ or self mutilation/ or suicide/ or suicidal ideation/ or suicide, attempted/ or mood disorders/ or affective disorders, psychotic/ or bipolar disorder/ or cyclothymic disorder/ or depressive disorder/ or depression, postpartum/ or depressive disorder, major/ or depressive disorder, treatment‐resistant/ or dysthymic disorder/ or seasonal affective disorder/ or neurotic disorders/ or depression/ or adjustment disorders/ or exp antidepressive agents/ or anxiety disorders/ or agoraphobia/ or neurocirculatory asthenia/ or obsessive‐compulsive disorder/ or obsessive hoarding/ or panic disorder/ or phobic disorders/ or stress disorders, traumatic/ or combat disorders/ or stress disorders, post‐traumatic/ or stress disorders, traumatic, acute/ or anxiety/ or anxiety, castration/ or koro/ or anxiety, separation/ or panic/ or exp anti‐anxiety agents/ or somatoform disorders/ or body dysmorphic disorders/ or conversion disorder/ or hypochondriasis/ or neurasthenia/ or hysteria/ or munchausen syndrome by proxy/ or munchausen syndrome/ or fatigue syndrome, chronic/ or obsessive behavior/ or compulsive behavior/ or behavior, addictive/ or impulse control disorders/ or firesetting behavior/ or gambling/ or trichotillomania/ or stress, psychological/ or burnout, professional/ or sexual dysfunctions, psychological/ or vaginismus/ or Anhedonia/ or Affective Symptoms/ or *Mental Disorders/
2. [Title/ Author Keywords]: (eating disorder* or anorexia nervosa or bulimi* or binge eat* or (self adj (injur* or mutilat*)) or suicide* or suicidal or parasuicid* or mood disorder* or affective disorder* or bipolar i or bipolar ii or (bipolar and (affective or disorder*)) or mania or manic or cyclothymic* or depression or depressive or dysthymi* or neurotic or neurosis or adjustment disorder* or antidepress* or anxiety disorder* or agoraphobia or obsess* or compulsi* or panic or phobi* or ptsd or posttrauma* or post trauma* or combat or somatoform or somati#ation or medical* unexplained or body dysmorphi* or conversion disorder or hypochondria* or neurastheni* or hysteria or munchausen or chronic fatigue* or gambling or trichotillomania or vaginismus or anhedoni* or affective symptoms or mental disorder* or mental health).ti,kf.
3. [RCT filter]: (controlled clinical trial.pt. or randomised controlled trial.pt. or (randomi#ed or randomi#ation).ab,ti. or randomly.ab. or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or distribut* or expose* or fashion or number* or place* or recruit* or subsitut* or treat*)).ab. or placebo*.ab,ti. or drug therapy.fs. or trial.ab,ti. or groups.ab. or (control* adj3 (trial* or study or studies)).ab,ti. or ((singl* or doubl* or tripl* or trebl*) adj3 (blind* or mask* or dummy*)).mp. or clinical trial, phase ii/ or clinical trial, phase iii/ or clinical trial, phase iv/ or randomised controlled trial/ or pragmatic clinical trial/ or (quasi adj (experimental or random*)).ti,ab. or ((waitlist* or wait* list* or treatment as usual or TAU) adj3 (control or group)).ab.)
4. (1 and 2 and 3)
Records were screened for reports of RCTs within the scope of the Cochrane Common Mental Disorders Group. Secondary reports of RCTs are tagged to the appropriate study record.
Appendix 2. Review searches
Summary of search results
Ovid MEDLINE (1946 to 21 November 2018): 176 records
The Cochrane Library (Issue 11 of 12, November 2018): 4
CENTRAL: 312
CCMDCTR (all years to June 2016): 215
Ovid PsycINFO (all years to December week 1 2018): 198
Ovid Embase (1974 to 2018 week 49): 689
Total: 1594 Duplicates removed: 457 Total screened: 1137
Search strategies
Ovid MEDLINE(R) and Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations and Daily <1946 to 21 November 2018> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ [Intervention/Setting] 1 COMMUNITY PHARMACY SERVICES/ 2 PHARMACISTS/ 3 PHARMACISTS' AIDES/ 4 (pharmacy or pharmacies or pharmacist*).ti,kf,hw. 5 ((pharmacy or pharmacies or pharmacist*) adj3 (advis* or advice or care or healthcare or coach* or commun* or counsel* or intervention* or model* or program* or service* or support* or treat* or therap* or psychotherap* or prevent*)).ab. 6 (pharmacy or pharmacies or pharmacist*).af. 7 ((collaborat* or coordinat* or co‐ordinat* or shared or integrat* or interdisciplinary or inter‐disciplinary or stepped) adj3 (care or healthcare or coach* or counsel* or working or service or model or effort* or intervention* or manage* or program* or treat* or therap* or psychotherap* or prevent*)).ti,ab,kf. 8 ((treatment or drug* or medicat* or pharmacotherap* or pharmaceutical* or therap* or psychotherap*) adj2 (adher* or comply or complian* or nonadher* or noncomplian* or collabor$ or commun* or educat* or advis* or advice or direct* or coach* or counsel* or support* or proactive* or pro‐active* or follow‐up or collaborat* or coordinat* or co‐ordinat* or shared or integrat* or stepped)).ti,ab,kf. 9 (6 and (7 or 8)) 10 or/1‐5,9 [Population‐1] 11 (depress$ or dysthymi$ or mood or affective disorder* or affective symptom*).ti,ab,kf. 12 ((mental* or psychiatr*) adj1 (ill* or disorder* or health or disease or diagnos* or condition)).ti,kf,hw. 13 exp DEPRESSIVE DISORDER/ 14 DEPRESSION/ 15 or/11‐14 [Population‐2] 16 exp ANTIDEPRESSIVE AGENTS/ 17 exp NEUROTRANSMITTER UPTAKE INHIBITORS/ 18 exp MONOAMINE OXIDASE INHIBITORS/ 19 PATIENT COMPLIANCE/ or MEDICATION ADHERENCE/ 20 (or/16‐18) and 19 21 ((adheren* or compliance or nonadheren* or noncompliance or collabor$ or communic* or educat* or advis* or advice or direct* or counsel* or support* or proactive* or pro‐active* or follow‐up) adj3 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) adj3 (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or psychotropic* or psychoactive*)).ti,ab,kf. 22 (20 or 21) [RCT Filter] 23 controlled clinical trial.pt. 24 randomized controlled trial.pt. 25 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kf. 26 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or cluster or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or pragmatic or quasi or recruit* or split or subsitut* or treat*))).ti,ab,kf. 27 placebo*.ab,ti,kf. 28 trial.ab,ti,kf. 29 (control* and (trial or study or group*) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kf,hw. 30 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,kf. 31 double‐blind method/ or random allocation/ or single‐blind method/ 32 exp animals/ not humans.sh. 33 (or/23‐31) not 32 34 (10 and 15 and 33) 35 (10 and 22 and 33) 36 (34 or 35) ***************************
The Cochrane Library (Issue 11 of 12, November 2018) #1MeSH descriptor: [Community Pharmacy Services] this term only #2MeSH descriptor: [Pharmacists] this term only #3MeSH descriptor: [Pharmacy Technicians] this term only #4(pharmacy or pharmacies or pharmacist*):ti,kw #5((pharmacy or pharmacies or pharmacist*) near (advis* or advice or care or healthcare or coach* or commun* or counsel* or intervention* or model* or program* or service* or support* or treat* or therap* or psychotherap* or prevent*)):ab #6(pharmacy or pharmacies or pharmacist*):ti,ab,kw #7((collaborat* or coordinat* or co‐ordinat* or shared or integrat* or interdisciplinary or inter‐disciplinary or stepped) near (care or healthcare or coach* or counsel* or working or service or model or effort* or intervention* or manage* or program* or treat* or therap* or psychotherap* or prevent*)):ab #8((treatment or drug* or medicat* or pharmacotherap* or pharmaceutical* or therap* or psychotherap*) near/2 (adher* or comply or complian* or nonadher* or noncomplian* or collabor* or commun* or educat* or advis* or advice or direct* or coach* or counsel* or support* or proactive* or pro‐active* or follow‐up or coordinat* or co‐ordinat* or shared or integrat* or stepped)):ti,ab,kw #9#6 AND (#7 OR #8) #10#1 OR #2 OR #3 OR #4 OR #5 OR #9 #11(depress* or dysthymi* or mood or moods or (affective next disorder*) or (affective next symptom*)):ti,ab,kw #12((mental* or psychiatr*) NEAR/2 (ill* or disorder* or health or disease or diagnos*)):ti,ab,kw #13MeSH descriptor: [Depression] this term only #14MeSH descriptor: [Depressive Disorder] explode all trees #15#11 OR #12 OR #13 OR #14 #16#10 AND #15 #17MeSH descriptor: [Antidepressive Agents] explode all trees #18MeSH descriptor: [Neurotransmitter Uptake Inhibitors] explode all trees #19MeSH descriptor: [Monoamine Oxidase Inhibitors] explode all trees #20MeSH descriptor: [Patient Compliance] this term only #21MeSH descriptor: [Medication Adherence] this term only #22(#17 OR #18 OR #19) AND (#20 OR #21) #23((adheren* or compliance or nonadheren* or noncompliance or collabor* or communic* or educat* or advis* or advice or direct* or counsel* or support* or proactive* or pro‐active* or follow‐up) NEAR (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) NEAR (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or psychotropic*)):ti,ab,kw #24#22 OR #23 #25#10 AND #24 #26#16 OR #25
*************************** Ovid PsycINFO <1806 to December week 1 2018> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 pharmacy/ or pharmacists/ 2 (pharmacy or pharmacies or pharmacist*).ti,id. 3 ((pharmacy or pharmacies or pharmacist*) adj3 (advis* or advice or care or healthcare or coach* or commun* or counsel* or intervention* or model* or program* or service* or support* or treat* or therap* or psychotherap* or prevent*)).ab. 4 (pharmacy or pharmacies or pharmacist*).af. 5 ((collaborat* or coordinat* or co‐ordinat* or shared or integrat* or interdisciplinary or inter‐disciplinary or stepped) adj3 (care or healthcare or coach* or counsel* or working or service or model or effort* or intervention* or manage* or program* or treat* or therap* or psychotherap* or prevent*)).ti,ab,id. 6 ((treatment or drug* or medicat* or pharmacotherap* or pharmaceutical* or therap* or psychotherap*) adj2 (adher* or comply or complian* or nonadher* or noncomplian* or collabor* or commun* or educat* or advis* or advice or direct* or coach* or counsel* or support* or proactive* or pro‐active* or follow‐up or coordinat* or co‐ordinat* or shared or integrat* or stepped)).ti,ab,id. 7 (4 and (5 or 6)) 8 or/1‐3,7 9 (depress$ or dysthymi$ or mood or affective disorder* or affective symptom*).ti,ab,id. 10 ((mental* or psychiatr*) adj1 (ill* or disorder* or health or disease or diagnos*)).ti,id,hw. 11 depression.hw. 12 or/9‐11 13 Psychopharmacology/ or Neuropsychopharmacology/ 14 "3340".cc. 15 exp Antidepressant Drugs/ 16 Neurotransmitter Uptake Inhibitors/ or exp Serotonin Norepinepherine Reuptake Inhibitors/ or exp Serotonin Reuptake Inhibitors/ 17 exp Monoamine Oxidase Inhibitors/ 18 exp Tricyclic Antidepressant Drugs/ 19 treatment compliance/ or client education/ or client participation/ or disease management/ or coaching/ or counseling/ 20 (or/13‐18) and 19 21 ((adheren* or compliance or nonadheren* or noncompliance or collabor* or communic* or educat* or advis* or advice or direct* or counsel* or support* or proactive* or pro‐active* or follow‐up) adj3 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit* or ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) adj3 (uptake or reuptake or re‐uptake)) or noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or psychotropic*)).ti,ab,id. 22 20 or 21 23 clinical trials.sh. 24 (randomi#ed or randomi#ation or randomi#ing).ti,ab,id. 25 (RCT or at random or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or split or subsitut* or treat*))).ti,ab,id. 26 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,id,hw. 27 ((single or double or triple or treble) adj2 (blind* or mask* or dummy)).ti,ab,id. 28 trial.ti. 29 placebo.ti,ab,id,hw. 30 treatment outcome.md. 31 treatment effectiveness evaluation.sh. 32 mental health program evaluation.sh. 33 or/23‐32 34 (8 and 12 and 33) 35 (8 and 22 and 33) 36 (34 or 35) *************************** Ovid Embase <1974 to 2018 week 49> Search strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ Intervention/Setting [Pharmacist/pharmacy services‐1] 1 PHARMACY/ or PHARMACY TECHNICIAN/ 2 PHARMACIST/ 3 PHARMACIST ATTITUDE/ 4 (pharmacy or pharmacies or pharmacist*).ti,kw,hw. 5 ((pharmacy or pharmacies or pharmacist*) adj3 (advis* or advice or care or healthcare or coach* or commun* or counsel* or intervention* or model* or program* or service* or support* or treat* or therap* or psychotherap* or prevent*)).ab. [Pharmacist/pharmacy services‐2] 6 (pharmacy or pharmacies or pharmacist*).af. 7 ((collaborat* or coordinat* or co‐ordinat* or shared or integrat* or interdisciplinary or inter‐disciplinary or stepped) adj3 (care or healthcare or coach* or counsel* or working or service or model or effort* or intervention* or manage* or program* or treat* or therap* or psychotherap* or prevent*)).ti,ab,kw. 8 ((treatment or drug* or medicat* or pharmacotherap* or pharmaceutical* or therap* or psychotherap*) adj2 (adher* or comply or complian* or nonadher* or noncomplian* or collabor$ or commun* or educat* or advis* or advice or direct* or coach* or counsel* or support* or proactive* or pro‐active* or follow‐up or collaborat* or coordinat* or co‐ordinat* or shared or integrat* or stepped)).ti,ab,kw. 9 (6 and (7 or 8)) 10 or/1‐5,9 Population‐1 11 (depress* or dysthymi* or mood or affective disorder* or affective symptom*).ti,ab,kw. 12 ((mental* or psychiatr*) adj1 (ill* or disorder* or health or disease or diagnos* or condition*)).ti,kw,hw. 13 exp DEPRESSION/ 14 or/11‐13 Population‐2 15 exp ANTIDEPRESSIVE AGENT/ 16 exp NEUROTRANSMITTER UPTAKE INHIBITORS/ 17 exp MONOAMINE OXIDASE INHIBITORS/ 18 exp TETRACYCLIC ANTIDEPRESSANT AGENT/ or exp TRICYCLIC ANTIDEPRESSANT AGENT/ or exp SEROTONIN UPTAKE INHIBITOR/ 19 PATIENT COMPLIANCE/ or MEDICATION COMPLIANCE/ or MEDICATION THERAPY MANAGEMENT/ or PATIENT EDUCATION/ or COUNSELING/ or *PHARMACEUTICAL CARE/ 20 (or/15‐18) and 19 21 ((adheren* or compliance or nonadheren* or noncompliance or collabor$ or communic* or educat* or advis* or advice or direct* or counsel* or support* or proactive* or pro‐active* or follow‐up) adj3 (antidepress* or anti depress* or MAOI* or monoamine oxidase inhibit*)).ti,ab. 22 ((adheren* or compliance or nonadheren* or noncompliance or collabor$ or communic* or educat* or advis* or advice or direct* or counsel* or support* or proactive* or pro‐active* or follow‐up) adj3 ((serotonin or norepinephrine or noradrenaline or nor epinephrine or nor adrenaline or neurotransmitt* or dopamine*) adj3 (uptake or reuptake or re‐uptake))).ti,ab. 23 ((adheren* or compliance or nonadheren* or noncompliance or collabor$ or communic* or educat* or advis* or advice or direct* or counsel* or support* or proactive* or pro‐active* or follow‐up) adj3 (noradrenerg* or antiadrenergic or anti adrenergic or SSRI* or SNRI* or TCA* or psychotropic* or psychoactive*)).ti,ab. 24 (20 or 21 or 22 or 23) [RCT Filter] 25 randomized controlled trial/ 26 randomization.de. 27 controlled clinical trial/ and (Disease Management or Drug Therapy or Prevention or Rehabilitation or Therapy).fs. 28 *clinical trial/ 29 placebo.de. 30 placebo.ti,ab. 31 trial.ti. 32 (randomi#ed or randomi#ation or randomi#ing).ti,ab,kw. 33 (RCT or "at random" or (random* adj3 (administ* or allocat* or assign* or class* or control* or determine* or divide* or division or distribut* or expose* or fashion or number* or place* or recruit* or split or subsitut* or treat*))).ti,ab,kw. 34 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$ or dummy)).mp. 35 (control* and (trial or study or group) and (placebo or waitlist* or wait* list* or ((treatment or care) adj2 usual))).ti,ab,kw,hw. 36 or/25‐35 37 ((animal or nonhuman) not (human and (animal or nonhuman))).de. 38 (36 not 37) 39 (10 and 14 and 38) or (10 and 24 and 38) ***************************
Data and analyses
Comparison 1. Pharmacy‐based management (pharmacist with or without wider team) versus treatment as usual.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression symptom level: improvement (intervention endpoint) | 4 | 475 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 2 Depression symptom level: change from baseline mean depression score (intervention endpoint) | 5 | 718 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.10] |
| 3 Acceptability of the intervention (as measured by participants not attending follow‐up) | 12 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.45] |
| 4 Non‐adherence to medication | 6 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.87] |
| 4.1 Non‐adherence at endpoint | 6 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.87] |
Comparison 2. Subgroup analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Depression symptom level: improvement (endpoint) | 4 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 1.1 No comorbidities | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.47, 1.89] |
| 1.2 Comorbidities | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.80, 1.30] |
| 2 Depression symptom level: change from baseline mean depression score (endpoint) | 5 | 718 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.10] |
| 2.1 No comorbidities | 3 | 461 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.29, 0.07] |
| 2.2 Comorbidities | 2 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.18, 0.35] |
| 3 Acceptability of the intervention (as measured by participants not attending follow‐up) | 12 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.45] |
| 3.1 No comorbidities | 8 | 1402 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.74, 1.34] |
| 3.2 Comorbidities | 4 | 670 | Risk Ratio (M‐H, Random, 95% CI) | 1.43 [0.53, 3.84] |
| 4 Depression symptom level: change from baseline mean depression score (endpoint) | 5 | 718 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.10] |
| 4.1 In person | 4 | 640 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.24, 0.08] |
| 4.2 Remotely | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.18, 0.78] |
| 5 Acceptability of the intervention (as measured by participants not attending follow‐up) | 12 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.45] |
| 5.1 Remote delivery | 4 | 550 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.46, 2.66] |
| 5.2 Delivery in person | 8 | 1522 | Risk Ratio (M‐H, Random, 95% CI) | 1.11 [0.80, 1.55] |
| 6 Improvement in depression (endpoint) | 4 | 478 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.05] |
| 6.1 Pharmacist alone | 1 | 63 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.47, 1.89] |
| 6.2 Pharmacist with team | 3 | 415 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.80, 1.30] |
| 7 Change from baseline mean depression score (endpoint) | 5 | 718 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.19, 0.10] |
| 7.1 Pharmacist alone | 4 | 640 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.24, 0.08] |
| 7.2 Pharmacist with team | 1 | 78 | Std. Mean Difference (IV, Random, 95% CI) | 0.30 [‐0.18, 0.78] |
| 8 Acceptability of the intervention (as measured by participants not attending follow‐up) | 12 | 2072 | Risk Ratio (M‐H, Random, 95% CI) | 1.09 [0.81, 1.45] |
| 8.1 Pharmacist with team | 3 | 491 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.40, 2.33] |
| 8.2 Pharmacist alone | 9 | 1581 | Risk Ratio (M‐H, Random, 95% CI) | 1.14 [0.82, 1.58] |
| 9 Non‐adherence to medication | 6 | 911 | Risk Ratio (M‐H, Random, 95% CI) | 0.73 [0.61, 0.87] |
| 9.1 Pharmacist and team | 3 | 441 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.71, 1.00] |
| 9.2 Pharmacist only | 3 | 470 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.53, 0.74] |
2.1. Analysis.
Comparison 2 Subgroup analyses, Outcome 1 Depression symptom level: improvement (endpoint).
2.2. Analysis.
Comparison 2 Subgroup analyses, Outcome 2 Depression symptom level: change from baseline mean depression score (endpoint).
2.3. Analysis.
Comparison 2 Subgroup analyses, Outcome 3 Acceptability of the intervention (as measured by participants not attending follow‐up).
2.4. Analysis.
Comparison 2 Subgroup analyses, Outcome 4 Depression symptom level: change from baseline mean depression score (endpoint).
2.5. Analysis.
Comparison 2 Subgroup analyses, Outcome 5 Acceptability of the intervention (as measured by participants not attending follow‐up).
2.6. Analysis.
Comparison 2 Subgroup analyses, Outcome 6 Improvement in depression (endpoint).
2.7. Analysis.
Comparison 2 Subgroup analyses, Outcome 7 Change from baseline mean depression score (endpoint).
2.8. Analysis.
Comparison 2 Subgroup analyses, Outcome 8 Acceptability of the intervention (as measured by participants not attending follow‐up).
Comparison 3. Sensitivity analyses.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Sensitivity analysis 1: improvement in depression (endpoint) | 3 | 400 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.85, 1.04] |
| 2 Sensitivity analysis 1: change from baseline mean depression score (endpoint) | 4 | 640 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.24, 0.08] |
| 3 Sensitivity analysis 1: acceptability of the intervention (as measured by participants not attending follow‐up) | 9 | 1382 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.88, 1.90] |
| 4 Sensitivity analysis 2: improvement in depression (intervention endpoint) | 2 | 141 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.78, 1.74] |
| 5 Sensitivity analysis 2: change from baseline mean depression score (intervention endpoint) | 3 | 404 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.31, 0.21] |
| 6 Sensitivity analysis 2: acceptability of intervention (as measured by participants not attending follow‐up) | 8 | 1392 | Risk Ratio (M‐H, Random, 95% CI) | 0.91 [0.66, 1.24] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adler 2004.
| Methods | Design: RCT, parallel group Country: USA Setting: 9 Eastern Massachusetts primary care practices |
|
| Participants | Inclusion criteria: diagnosis for MDD or dysthymia (or both) according to DSM‐IV criteria, received care from a PCP in any site, aged ≥ 18 years, could read and understand English. Lifetime alcoholism, long‐term/chronic depression (people with ≥ 4 MDD episodes in their lifetime plus their first diagnosis > 10 years ago), anxiety disorders, likely personality disorders (as indicated by NEO scores ≥ 17) or comorbid medical conditions were not excluded. Exclusion criteria: aged < 18 years, acute or life‐threatening condition with terminal prognosis, pregnant or had given birth in the past 6 months, current alcoholism (defined as > 1 positive response on the CAGE, plus 1 item assessing current usage), diagnosed with bipolar or psychotic disorders (or both). Mean age (years): overall: 42.3 (SD 13.9); intervention group: 42.9 (SD 13.8); control group: 41.7 (SD 14.0) Sex: 364 women; 143 men Severity of depression (mBDI): intervention group (n = 258): 23.2; control group (n = 249): 23.2 AD use status: current AD use at initial time point: overall: 49.5%; intervention group: 50.6%; control group: 48.3% Comorbidities: mentioned but not detailed |
|
| Interventions | Sample size (total randomised): 533 Length of trial: 6 months Intervention: in‐person intervention guided by a protocol based on clinical pharmacy principles and AHCPR guidelines. Protocol emphasised: 1. obtaining a thorough medication history; 2. assessing patient medication for drug‐related problems (such as adverse effects or interactions with other drugs); 3. monitoring efficacy and toxicity, especially for the symptoms of depression; 4. patient education for depression and ADs; 5. encouraging participants to start and maintain AD therapy; and 6. facilitating communication with patient's PCP. This was done over a minimum of 9 interactions over 18 months, each interaction averaged 15 minutes per participant over 6 months with each participant having a mean of 4 contacts. Pharmacists contacted the participants initially by telephone to set up an appointment, subsequent physician contacts with pharmacists were based on individual preferences and information was reported via a template to PCPs by the pharmacists. Additionally, pharmacists fulfilled some basic patient needs, such as general social support and overcoming system inadequacies as well as encouraging and facilitating referrals to the mental health speciality sector. Enrolled: n = 268; randomised: n = 258 Control: PCPs who saw the participants received the results of the depressive screener indicating the diagnosis; other than this the participants received TAU. Enrolled: n = 265; randomised: n = 249 |
|
| Outcomes | Adherence (measured by AD use at 3 and 6 months); severity in depression (mBDI at 3 and 6 months); health status (MHI = 5 from the SF‐36 generic health instrument at 3 and 6 months); changes in Physical and Mental Component Summaries from SF‐12; adverse events (death); acceptability of intervention (measured by those leaving the study). | |
| Notes | Sponsorship source: supported by the National Institute of Mental Health under grant RO1 MH56214. Trial registration identifier: not stated Date of recruitment: not stated Declarations of interest from primary researchers: not stated Correspondence: we thank Kathy Bungay and David Adler for agreeing to provide additional information. We will extract data on AD adherence, depression severity (mBDI), MHI, MCS, and PCS when this information becomes available. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised to control or intervention arm regardless of whether taking ADs or not, participants were randomised using a 'computerised coin flip' built into the screener. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data and where results were presented, it was unclear how many participants were in each treatment group (e.g. Table 2 and Table 3). We contacted the authors to request clarification. |
| Selective reporting (reporting bias) | High risk | Did not report MHI, MCS, or PCS at 3 months. Presented limited data for MHI, PCS, and MCS at 6 months. We contacted the authors to request clarification. |
| Other bias | Unclear risk | Quote: "After controlling for site, patients who started the study later (after the median entry time) had a higher chance of being on medications at both the time of the initial screening period (P .08) and after 6 months (P .05). The impact of the experiment was larger (and in fact, was significant earlier in the study) for both AD use and mBDI outcomes." |
Al‐Saffar 2005.
| Methods | Design: RCT, parallel group Country: Kuwait Setting: hospital outpatient clinic |
|
| Participants | Inclusion criteria: people with a diagnosis of depressive or persistent mood affective disorder, aged ≥ 17 years; in receipt of a prescription for a single AD; fluent in Arabic; and willing to commit their time so that follow‐up questionnaires on treatment could be completed. Exclusion criteria: people with suicidal tendencies and those with a limited mental capacity, psychotic disorders, deafness, addiction, or organic brain disease. Age range (years): 17–70 Sex: 92 women; 186 men Severity of depression: as measured by HAMD‐17 subclinical: n = 22 (score 10–17); mild: n = 152 (score 18–25); moderate: n = 88 (score 26–38). AD use status: n = 207 'newly treated' participants (i.e. receiving a new prescription for AD medication, not taken within 1 month before entering the study); n = 71 receiving 'ongoing' treatment (i.e. had been taking the AD continuously for ≥ 1 month before entering the study). n = 163 prescribed TCAs for the trial; n = 115 prescribed SSRIs for the trial. Comorbidities: not detailed |
|
| Interventions | Sample size: n = 300 (total originally randomised); n = 22 withdrew after randomisation but before treatment began. Length of trial: 18 months Intervention: in‐person intervention (pharmacist counselling + participant information leaflet (PIL)). Counselling protocol: it was emphasised that the counselling was not intended to replace, but rather to strengthen, the advice previously given by the doctor. Quote: "The pharmacist wished: to make sure the patient knew why their medication had been prescribed; to make sure the patient understood how they were meant to take their medication; to give the patient an opportunity to talk freely about any concerns they might have about their illness or their medication." "The pharmacist then made the following observations: the depression was neither the patient's fault, nor was it a weakness of character but it would not go away of it [its] own accord; the patient needed to take the medication because they were suffering from an illness; the medication they had been prescribed would help their problem if they took it as directed and for a sufficient period of time." PIL sheet: using non‐medical terms the leaflet detailed: "why the medicine has been prescribed; how an antidepressant works; the time required for symptoms to improve; how long treatment should be continued to prevent relapse; why patients should not vary the dose of their own accord; what to do if a dose is missed; common side‐effects that might occur and how they should be managed; how medicine should be stored; when patients should inform their doctor of concurrent disease or medication. The content of the leaflets went through a quality assessment process to gauge suitability by 20 patients with participants and 20 health volunteers. The pharmacist would hold discussions with the participant specifically regarding: dosing regimen; individual patient concerns (e.g. their thoughts about taking the medication, concerns of adverse effects); storage of medication; when to report allergies and side effects to a doctor. For each patient, the mean length of the first counselling session was 17 (SD 3) minutes. No session lasted longer than 23 minute," n = 98. Active control (PIL only): participants received the same PIL as the intervention group, n = 93. Control: TAU. Members of the control group received advice from the dispensing pharmacist that merely reinforced the labelling instructions on the medication, n = 87. |
|
| Outcomes | Acceptability of intervention (defined as participants attending follow‐up clinics); non‐adherence to medication (defined as participants who took their tablets exactly as prescribed and whose 'tablet‐count ratio' was 80–100% inclusive on an ITT basis); any adverse event (as listed) at 2 and 5 months. Outcomes not used: participant knowledge of therapy (not an outcome defined in our protocol). |
|
| Notes | Sponsorship source: the study was funded by the Kuwait Foundation for the Advancement of Sciences (KFAS) and a postgraduate scholarship from the Government of the State of Kuwait. Trial registration identifier: not detailed; "protocol written in Arabic" (p. 124). Date of recruitment: not stated Declarations of interest from primary researchers: not stated Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "recruited patients were randomised sequentially by day of recruitment into a control and two treatment groups" (p. 125). |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: after randomisation, "100 patients were allocated to the control group and to each of the intervention groups. Because of the time necessary to complete the attitudinal survey, 22 patients withdrew after this stage had been completed. There remained 87 patients in the control group, 93 in the 'leaflet‐only' group and 98 in the counselling plus patient information leaflet group" (p. 126). Comment: 22 who withdrew did not receive any intervention/control. Authors stated that not all participants provided data on outcome of adherence (p. 128, Table 3). Only 152 participants attending follow‐up provided adverse event data. |
| Selective reporting (reporting bias) | Unclear risk | Study reported outcomes at 2 and 5 months. Trial ran for 18 months. Unclear why longer‐term outcomes were not reported. |
| Other bias | Low risk | None detected. |
Al‐Saffar 2008.
| Methods | Design: RCT, parallel group Country: Kuwait Setting: outpatient department of psychiatric hospital |
|
| Participants | Inclusion criteria: aged ≥ 18 years who had been diagnosed as having unipolar depression in accordance with the ICD‐10 mood disorders criteria and who had been prescribed any 1 of the TCA or SSRI ADs. Exclusion criteria: people with psychotic disorders, mental retardation, deafness, addiction, or organic brain diseases; prescribed psychiatric medications other than TCAs and SSRIs; receiving a combination of TCAs and SSRIs ADs; and considered at a significant risk of suicide. Mean age (years): 34.5 (SD 9.5) Sex: 45 women; 105 men Severity of depression: measured by HAMD‐17: subclinical: n = 6; mild: n = 38; moderate: n = 34 (only numbers for those attending follow‐up clinics reported). AD use status: participants were prescribed either an SSRI or TCA AD; not stated whether participants received any form of AD medication before the trial. Comorbidities: not detailed |
|
| Interventions | Sample size (total): 150 Length of trial: 6–8 weeks Intervention: in‐person intervention (pharmacist counselling + participant information leaflet (PIL)): intensive drug‐related counselling sessions were delivered by a professionally trained senior pharmacist. Counselling sessions, which were carried out in a private counselling room, were 10–15 minutes in length. Participants were encouraged to contact the counselling pharmacist if any clarification about their medication was required. A standardised counselling format was planned. The counselling sessions were intended to help participants understand the nature of their depressive illness and to reinforce that taking medications in the way they were prescribed would be of benefit to them. Advice was also provided on adverse effects and their management. PIL: PILs were designed to contain information about the drug, its therapeutic group, and its mechanism of action. The importance of treatment continuation was stressed with emphasis on the drugs' non‐addictive properties, precautions, and adverse effects. Advice on drug storage was given based on knowledge of local climatic conditions in Kuwait, n = 50. Active control (PIL only): participants received the same PIL as used in the intervention group, n = 50. Control (TAU): received advice from the dispensing pharmacist that merely reinforced the labelling instructions on the medication, n = 50. |
|
| Outcomes | Acceptability of intervention (defined as participants attending follow‐up clinics). Outcomes not used: patient preferences on how to receive information relating to medication (not an outcome defined in our protocol). |
|
| Notes | Sponsorship source: unclear ‐ project "facilitated" by Director of Pharmaceutical Services, Ministry of Health, Kuwait. No further detail reported. Trial registration identifier: not detailed Date of recruitment: not stated Declarations of interest from primary researchers: not detailed Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "a contracted medical secretary for the research project in the PMH [Public Ministry of Health] obtained patients informed consent to participate in the study and then randomised the patients sequentially by day of recruitment into three study groups" (p. 96). |
| Allocation concealment (selection bias) | Low risk | Quote: "to maintain blinding, the randomisation key was concealed from the interviewer. A formal test of blindness was not conducted." (p. 96). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details given. Due to the nature of the intervention, it would be difficult to establish and maintain blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "To maintain blinding, the randomisation key was concealed from the interviewer. A formal test of blindness was not conducted. A contracted interviewer with a pharmacy background administered a validated questionnaire …" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None detected. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | Quote: "The researchers recognized that the survey instrument has not been previously validated for Kuwaiti patients. Those [sic] may be potential for a cultural bias in answers to some questions. However, our main comparison was between the intervention groups, and any potential bias would be non‐differential in nature, and would therefore have little or no effect on the intervention effects." |
Aljumah 2015.
| Methods | Design: RCT, parallel group Country: Saudi Arabia Setting: Riyadh, Al‐Amal Hospital |
|
| Participants | Inclusion criteria: aged 18–60 years; newly diagnosed with an MDD, according to DSM‐IV; 1994); no history of psychosis or bipolar disorders; no drug or dependency history; no cognitive impairment that may hinder the assessment. Exclusion criteria: no response at any level to the AD within 8 weeks of recruitment. Age range (years): 18–60 Sex: 120 women; 100 men Severity of depression: MADRS score at baseline, mean: intervention group: 22.90 (SD 13.27); control 21.89 (SD 12.89) Moderate‐to‐severe depression AD use status: number of ADs prescribed: intervention group: 1 AD: 86 (78.2%); 2 ADs: 24 (21.8); control group: 1 AD: 95 (86.4%); 2 ADs: 15 (13.6%) Comorbidities: not detailed. |
|
| Interventions | Sample size (total): 239 randomised Length of trial: 6 months (follow‐up) Intervention: usual pharmacy services plus pharmacist intervention based on SDM. This in‐person intervention involved using an SDM decision aid designed for Arabic‐speaking participants. The intervention focused on enhancing patients' involvement in decision making by assessing their beliefs and knowledge about ADs. The mean duration of the first SDM session (baseline) was 15 minutes, and the second session (final session) was 10 minutes (at 3‐month follow‐up), n = 119. Control: usual pharmacy services without SDM‐based pharmacist intervention, i.e. standard communication regarding AD medication, n = 120. |
|
| Outcomes | Medication adherence (Morisky Medication Adherence Scale, higher scores = better adherence on a per‐protocol basis)); acceptability of intervention (as measured by participants not attending follow‐up visits at 3 and 6 months); severity of depression – clinician rated (MADRS, lower scores = lower symptom severity on a per‐protocol basis); health‐related quality of life (EQ‐5D, Arabic version, self‐reported, higher score = better). Outcomes not used: patients beliefs about medication; patient involvement in decisions; satisfaction of treatment (not stated in our protocol). |
|
| Notes | Sponsorship source: no details Trial registration identifier: ISRCTN34879893 Date assigned: 30 December 2014 Date of recruitment: February to July 2014 Declarations of interest from primary researchers: authors declare no competing interests. Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study participants were individually randomised to one of two parallel groups with an allocation ratio of 1:1 using a computer‐generated list. The computer‐generated allocation was done by a research assistant with no clinical involvement in the trial" (p. 3). |
| Allocation concealment (selection bias) | Low risk | Quote: "Pharmacists and psychiatrists were not blinded to the patients' group allocation but the research assistant who collected all data was blinded to group allocation" (p. 3). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Authors stated that pharmacists and psychiatrists were not blinded to group allocation; the researcher who collected all data was blind to group allocation (see p. 3). However, authors later stated (p. 7) that "researchers who were not blind to intervention group may have biased the results found." They claimed that any impact of this would have been minimal on their results. Due to the nature of the intervention, it would be difficult to maintain blinding; however, it is unclear what impact on the results the unblinded researchers had. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "assessments were conducted by independent raters (two trained nurses) who were blinded to patients' group allocation" (p. 3). |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Nineteen patients dropped out of the study during the follow‐up phase and were not included in the final analysis" (p. 4). Comment: completer‐only data reported |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
Brook 2005.
| Methods | Design: RCT, parallel group Country: Netherlands Setting: 19 community pharmacies |
|
| Participants | Inclusion criteria: aged ≥ 18 years; attending the pharmacy with a 'new episode' prescription for a non‐tricyclic AD medication; from their GP, i.e. not having used an AD in a 6‐month period before the inclusion; ability to understand Dutch; taking ADs due to depressive complaints. ADs included SSRIs, RIMAs, NaSSAs, and SNRIs. Exclusion criteria: not stated Mean and median age (years): 42 Sex: 96 women; 39 men Severity of depression: mild: n = 25; moderate: n = 87; severe: n = 23 AD use status: participants were required to have not used an AD in the 6‐month period before study inclusion. Comorbidities: not detailed |
|
| Interventions | Sample size (total): 151 (authors only presented data analysed for 135). Length of trial: 6 months Intervention: pharmaceutical care; in‐person intervention that involved 3 coaching sessions during the study. In first contact, participants were informed about appropriate use, benefits, and adverse effects of their medication; participants were encouraged to take their medication daily, continue taking their medication even if feeling better, to not stop taking medication without checking with their PCP, and to not hesitate to ask the pharmacist/PCP about ADs. Participants also received a take home educational video of 25 minutes, reflecting previous points. Second contact took place within 2 weeks before prescription ended and third contact 3 months from baseline. The second visit involved discussion concerns relating to adverse effects. The third visit involved evaluation of the pharmacist intervention, n = 61. Control: usual oral information provided by pharmacists when collecting their prescriptions at the pharmacy, n = 74. |
|
| Outcomes | Primary outcomes: medication adherence (intake frequency and discontinuation rates measured using electronic drug container monitors). Secondary outcomes: self‐rated mental health (Hopkins Symptom Checklist SCL‐90, lower = better on an ITT basis); self‐rate quality of life; drug attitude; attrition (acceptability) (as defined as participants not returning their questionnaires at follow‐up); cost‐effectiveness. Outcomes not used: drug attitude; cost‐effectiveness (not specified in our protocol); quality of life (no data reported). |
|
| Notes | Sponsorship source: Organon (sponsor of the International Health Foundation); and 'unconditional funding' was received from GlaxoSmithKline. Participants received Euro 10 for completing questionnaires; pharmacists received Euro 30 for each participant they enrolled onto the study. Trial registration identifier: not provided Date of recruitment: April 2000 to April 2001 Declarations of interest from primary researchers: none stated Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation occurred at patient level and with a one to one ratio, using block randomisation to ensure equal numbers of intervention and control patients per pharmacy. In order to avoid crossover contamination of usual care with elements of the intervention during randomisation, all pharmacists attended a pre‐trial meeting at which they were instructed how to approach eligible patients, how to randomise them and how to use different protocols for control and intervention patients. The data administration forms of the whole sample were randomised before delivery to the pharmacies. These forms were pre‐coded and delivered in sealed envelopes. After receiving written informed consent of the patient, the pharmacist learned the group assignment by opening the envelope" (pp. 349–350). |
| Allocation concealment (selection bias) | Unclear risk | Quote: "neither patients nor pharmacists were blinded for group assignment" (p. 348). See above. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See above. This could have contaminated the usual care of participants in the control group. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors performed ITT analysis with LOCF and GMI. Authors stated that different conclusions were reached with each imputation method. Quote: "The LOCF method revealed that at the 6‐month follow‐up, the intervention patients were less depressed and less anxious than the controls. The intervention was particularly effective in patients with lower levels of education who received pharmacist's coaching. However, ITT with the GMI method showed no differences in psychological symptoms. Differences between LOCF and GMI were explained by the selective attrition in the intervention arm (attrition intervention patients had lower initial SCL‐item scores on depression and anxiety than the completers) and by the higher attrition rate in controls" (p. 347). Data from 135 participants presented (originally 151 were randomised, 4 left immediately after randomisation. From the remaining 147, 12 did not return their questionnaires, resulting in a baseline data set of 135). The analyses were performed both for participants who completed the study and for the whole group (by using imputed data). In the case of participants with missing data at 3‐month follow‐up (n = 29) and at 6‐month follow‐up (n = 38), we used GMI within the intervention group and the control group, separately. |
| Selective reporting (reporting bias) | High risk | Reported outcomes seemed consistent across publications and abstracts, with the exception of the outcome of drug attitude, which is not listed as an outcome on a couple of the supplement abstract publications but presented in separate papers. Quality of life outcomes not reported in any identified publications. Authors stated that the Hopkins SCL‐90 self‐reported depressive symptom measure was used, but conflictingly reported the SCL‐13 was used in different publications relating to the same trial. |
| Other bias | Unclear risk | Unconditional funding was received from GlaxoSmithKline; authors stated that the study was carried out without 'any interference' from the funding source. |
Capoccia 2004b.
| Methods | Design: RCT, parallel group Country: USA Setting: UWFMC, primary care clinic |
|
| Participants | Inclusion criteria: people diagnosed with a new episode of depression (DSM‐IV) and started on an AD medication. Exclusion criteria: aged < 18 years; terminal illness; psychosis; recent (within the past 3 months) alcohol (AUDIT score > 8) or substance abuse; > 2 suicide attempts; pregnancy or nursing; limited command of the English language; unwillingness to use UWFMC as a source of care for the next 12 months. Mean age (years): 39 (SD 13.5) Sex: 57 women; 17 men Severity of depression: not detailed AD use status: 32 participants had received prior AD medication for depression. Comorbidities: panic disorder: enhanced care group: 9 (22%); usual care group: 5 (15%); dysthymic disorder: enhanced care: 23 (56%); usual care: 16 (48%) |
|
| Interventions | Sample size (total): 74 Length of trial: 1 year Intervention: 'enhanced care' – a remotely delivered intervention consisting of a pharmacist collaborating with PCP and psychiatrist to facilitate patient education, initiation and adjustment of AD dosages, monitoring of patient adherence to the regimen, management of adverse reactions, and prevention of relapse. Follow‐up included weekly telephone calls for the first 4 weeks, followed by telephone contact every 2 weeks until week 12, after which point participants received a telephone call every other month. Support included addressing depression symptom and medication‐related concerns; support and education; medication dosage adjustment and management of adverse effects; change or discontinuation of ADs; additional pharmacotherapy for insomnia and sexual dysfunction; appointments with mental health providers were facilitated; included usual care, n = 41. Control: usual care (encouraged to use available resources such as PCPs, pharmacists, nurses, and mental health providers), n = 33. |
|
| Outcomes | Depression outcomes (20‐item Hopkins Symptom Checklist, SCL‐20 on an ITT basis); health‐related quality of life; medication adherence (measured by asking participants the number of days they took the AD medication in the past month on an ITT basis); patient satisfaction; use of depression‐related healthcare services (reported as median and range); cost‐effectiveness. Outcomes not used: patient satisfaction; cost‐effectiveness (not specified in our protocol); quality of life (not reported). |
|
| Notes | Sponsorship source: supported by Aetna Quality of Care Foundation Trial registration identifier: not detailed Date of recruitment: November 1999 to March 2001 Declarations of interest from primary researchers: none stated Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Study subjects were randomly assigned to either enhanced care (EC) or usual care (UC) using simple computer randomization" (p. 586). Comment: no further details. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Due to the nature of the intervention, the clinical pharmacist, PharmD resident, and patients became aware of the randomization status once the EC or UC began" (p. 586). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No details provided. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "A research assistant who is blinded to the patients' randomization status conducts all baseline and follow‐up interviews over the telephone" (p. 587). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "all enhanced care patients were included in the analysis, regardless of whether they completed the interventions" (p. 367). Comment: ITT used. |
| Selective reporting (reporting bias) | Unclear risk | Quality of life outcome data not reported. |
| Other bias | Low risk | None detected. |
Crockett 2006.
| Methods | Design: cluster RCT, parallel groups Country: Australia Setting: 32 community pharmacies in rural and remote New South Wales Australia |
|
| Participants | 160 participants Inclusion criteria: presented a prescription for ADs at a participating community pharmacy and replied using the word 'depression' when asked; aged ≥ 18 years; unaware of history of psychosis and a resident in that area for the following 3 months. Exclusion criteria: aged ≤ 17 years, not going to be a resident of the area for the following 3 months, aware of a history of psychosis. Mean age (years): intervention group: 46 (SD 12); control group: 46 (SD 15) Sex: intervention group: 35 women and 11 men; control group: 49 women and 11 men Severity of depression: not reported; previous episode of depression (years): intervention group: 27; control group: 37 AD use status: part of inclusion so assumed to be 100% Comorbidities: not reported |
|
| Interventions | Sample size (total randomised): 119 Length of trial: 2 months Intervention: intervention pharmacists were given video‐conference training on the nature and management of depression by a psychiatrist, psychologist and GP and were then asked to dispense medication with extra advice and support. This in person intervention involved giving participants information at recruitment (including brochures on depression provided to them by SANE Australia, a national charity helping people affected by mental illness, and the offer of a video on depression (on loan), also provided by SANE Australia) and checking "how they were going" at subsequent visits to the pharmacy, n = 46 analysed. Control: control pharmacists were asked to provide usual care, n = 60 analysed. |
|
| Outcomes | Adherence (measured by self‐report on an ITT basis); well‐being (measured using K10 on an ITT basis); acceptability of intervention (as measured by participants not attending follow‐up) Outcomes not used: Drug Attitude Index |
|
| Notes | Sponsorship source: quote: "This research was funded by a grant from the Rural and Remote Pharmacy Infrastructure Grants Scheme." Trial registration identifier: none mentioned Date of recruitment: not mentioned Declarations of interest from primary researchers: none mentioned Correspondence: none |
|
Finley 2003b.
| Methods | Design: RCT, parallel design Country: USA Setting: Kaiser Permanente Medical Center, San Rafael, CA, US |
|
| Participants | Inclusion criteria: people referred by their PCP immediately after starting AD therapy for the expressed purpose of treating depressive symptoms. Exclusion criteria: evidence that participants had received an AD during the preceding 6 months; concurrent psychiatric or psychological treatment; current symptoms of mania or bipolar disorder; psychotic symptoms; eminent suicidality; and active substance abuse or dependence. Mean age (years): intervention group: 54.4 (SD 14.1); control group: 54.1 (SD 17.3) Sex: 106 women; 19 men Severity of depression: mean score using BIDS: intervention group: 18.7 (SD 5.8); control group: 18.3 (SD 5.8) AD use status: participants were required to not have received an AD during the preceding 6 months. Comorbidities: chronic disease score, mean: intervention group: 606.5 (SD 363.8); control group: 664.3 (SD 428.4) |
|
| Interventions | Sample size (total): 125 Length of trial: 6 months Intervention: collaborative care treatment – remotely delivered intervention that involved care managers, clinical pharmacists, and psychiatrists. Medical, psychiatric, and drug therapy histories were taken. Patient education was delivered involving discussion on symptoms, aetiology, prognosis of depression, and detailed description of ADs' therapeutic and adverse effects provided. Clinical pharmacists were permitted to titrate doses in line with practice and prescribing guidelines; pharmacists could prescribe ancillary drugs (e.g. for insomnia), but PCP approval was needed to change AD medication. Pharmacists made telephone calls to participants on weeks 1, 2, 4, 10, and 16 to assess drug adherence, therapeutic effects, adverse effects, and other social/medical factors. Pharmacists were trained to detect activities that participants neglected during their illness and provided encouragement to resume these activities. Pharmacists also provided brief clinic visits on weeks 6 and 24 to assess clinical progress face‐to‐face. A psychiatrist provided supervision and mentoring to the pharmacists approximately 1 hour per week, and was available for consultation with the pharmacists during clinic hours, n = 75. Control: usual care – involved brief counselling on the prescribed drug, therapeutic endpoints, and adverse effects in a manner consistent with patient education routinely delivered to members receiving prescriptions from the outpatient pharmacy, n = 50. |
|
| Outcomes | Adherence (measured by computerised prescription refill records on an ITT basis); clinical and functional severity (using BIDS, patient‐rated survey ranking symptoms on a per‐protocol basis); resource utilisation (measured by change in all clinic visits between the 12 months preceding randomisation and the 12 months immediately after; work and social functioning. Acceptability (as measured by number of participants failing to return surveys at follow‐up at 6 months). Depression remission (score < 9 using BIDS) on a per‐protocol basis. Outcomes not used: patient satisfaction (not prespecified in our protocol); service utilisation (only reported by percentage increase, not usable data). |
|
| Notes | Sponsorship source: funded in part by a grant from the Sidney Garfield Memorial Fund (as part of the Interregional Depression Initiative) and by an unrestricted educational grant from Pfizer Inc., New York, NY. Trial registration identifier: not specified Date of recruitment: not specified Declarations of interest from primary researchers: none stated Correspondence: contacted authors to clarify their methods, who provided further details as to data collection and potential sources of bias (this has been included in the risk of bias assessment). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomly assigned to the collaborative care model or back to usual care in a 3:2 ratio" (p. 1177). |
| Allocation concealment (selection bias) | Low risk | Quote: "After the patients completed a brief survey to assess baseline depression severity (Brief Inventory for Depressive Symptoms [BIDS]) and functional impairment (Work and Social Disability Scale [WSDS]), the investigators opened a sealed envelope that determined study group assignment (intervention vs usual care)" (p. 1177). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "the study was not double‐blinded (i.e., subjects and providers were presumably aware of study group assignments after randomization)" (p. 1183). Comment: due to the nature of the intervention, it was unclear how this may have effected any results. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | See above. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Many surveys on outcomes not returned, with more than half not accounted for in the control group on depression symptoms using BIDS and the Work and Social Disability Scale. ITT used for the outcome of adherence only. See author correspondence below on missing data. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | High risk | Quote: "Our primary outcome was Medication Adherence. While we did plan on looking at clinical outcomes... there were comparatively few surveys returned by the control group and the subjects who did return their survey from this cohort appeared to be those who were doing well. In other words, there was a substantial selection bias at work here, rendering any comparison of these clinical outcomes meaningless." – from P. Finley, email communication, 5 March 2019. |
Kanwal 2018.
| Methods | Design: RCT, parallel group Country: USA Setting: 4 Veteran Affairs clinics |
|
| Participants | Inclusion criteria: people with HCV infection (positive HCV ribonucleic acid test) who were seen in HCV clinics and who screened positive for major depression on the PHQ‐9 depression screening instrument (score > 10). Exclusion criteria: participants already receiving HCV treatment, no access to telephone, PHQ‐9 score < 10, currently in bereavement, current suicidal ideation, significant cognitive impairment (score > 10 on the Blessed Orientation Memory and Concentration Test), in care of court‐appointed guardian, diagnosis of schizophrenia, and bipolar disorder with admission for psychiatric diagnosis in the previous 12 months. Mean age (years): intervention group: 59 (SD 6); control group: 59 (SD 5) years) at 6 months; intervention group: 59 (SD 5.6); control group: 59.4 (SD 4.9) years at 12 months. Sex: 11 women; 252 men at 6 months; 9 women; 233 men at 12 months. Severity of depression: participants had a mean score 2 (SD 1) on the Hopkins Symptom Checklist at baseline. AD use status: 137 participants had received 'any depression treatment' in the past 6 months before study commencement; 133 were on a current AD prescription at the start of the trial. Comorbidities: hepatitis C |
|
| Interventions | Sample size (total): 309 enrolled; 292 completed baseline assessment interviews; only data for 263 presented by study authors. Length of trial: 12 months (6 and 12 months' follow‐up data presented). Intervention: collaborative care – remotely delivered intervention. Depression care team consisted of a nurse depression care manager, pharmacist, and psychiatrist – located off site and convened once a week and as needed by telephone or in person. Model adopted a 5‐step care model for depression focusing with the following components: self‐management education, depression care team treatment suggestions (counselling or pharmacotherapy, with consideration of participant preference), pharmacotherapy suggestions after review of depression treatment history by the clinical pharmacist, combination pharmacotherapy and speciality mental health counselling, and referral to speciality mental health. Depression care manager conducted telephone‐based monitoring every 2 weeks during acute treatment and every 4 weeks during continuation treatment, n = 156 (n = 129 reported). Control: usual care – participants received "standard of care" depression treatment, which typically included referral to speciality mental health clinics or depression treatment at integrated primary care – mental health clinics where patients had access to evidence‐based psychotherapy and pharmacotherapy available at each site. Usual care participants did not have interactions with the depression care manager or depression care team, n = 153 (n = 134 reported). |
|
| Outcomes | Depression symptom severity (Hopkins Symptom Checklist, SCL‐20, higher score = worse symptoms on a per‐protocol basis); depression response (defined as ≥ 50% decrease in the mean SCL‐20 score compared with baseline); depression remission (defined as a mean SCL‐20 score < 5 on a per‐protocol basis); depression‐free days; acceptability (defined as participants not attending follow‐up); death. Outcomes not used: depression‐free days; hepatitis C outcomes (not included in our protocol). |
|
| Notes | Sponsorship source: supported by grant SDP 10‐044 from the Veterans Health Administration, Health Services Research and Development Service. Trial registration identifier: NCT01143896 Date of recruitment: 2012–2014 Declarations of interest from primary researchers: the authors reported no financial relationships with commercial interests. Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "participants were randomly assigned to the intervention or to usual care in a 1:1 ratio according to a computer generated random assignment sequence stratified by clinic and generated in advance" (p. 1077). |
| Allocation concealment (selection bias) | Unclear risk | No details. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "Clinicians were not blinded to the depression care received by the patient" (p. 2550). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Baseline and six‐month data were collected by a telephone interviewer who was blinded to treatment assignment" (p. 1078). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors used ITT analysis on participants. Quote: "A total of 309 eligible patients were enrolled in the study. Of these, 292 patients completed baseline assessment interviews. Follow‐up data‐collection interviews were completed for 263 participants (90%) at six months" (p. 1076). Comment: data for 263 participants presented by study authors at 6 months in 1 published report, with data for 242 participants presented by study authors at 12 months in another published report. ClinicalTrials.gov (NCT01143896) details numbers lost to follow‐up and reasons. |
| Selective reporting (reporting bias) | Low risk | Outcomes seem to have reported consistently between NCT‐provided data and published articles. |
| Other bias | Low risk | None detected. |
Marques 2013.
| Methods | Design: RCT, parallel design Country: Brazil Setting: outpatient clinic of Alzira Velano Hospital, University of Alfenas |
|
| Participants | Inclusion criteria: women aged 18–65 years, a diagnosis of depression at the initial stage of treatment (first treatment, no previous AD), or who were prescribed a new AD. Exclusion criteria: insurmountable difficulties in scheduling visits (lack of a telephone line or living in rural area or in a neighbourhood that was difficult to access), BDI < 11 points, dependence on illicit drugs, schizophrenia diagnosis, and presence of patient cognitive impairment that might affect the patient's ability to complete the research instruments. Mean age (years): intervention group: 40.8 (SD 12.2); control group: 44.2 (SD 13.9) Sex: 48 women Severity of depression: mild: n = 12; moderate: n = 26; severe: n = 10 AD use status: most participants in the control (76.9%) and intervention (68.2%) groups had started AD treatment within the last 60 days. Comorbidities: none detailed |
|
| Interventions | Sample size (total): 68 randomised; data for 48 reported by study authors. Length of trial: 3‐month follow‐up Intervention: in‐person pharmacotherapy follow‐up intervention (PF) using the Dáder Method (a standardised, documented and systematic PF approach based on information about health problems and the patient's pharmacotherapy) – participants received PF visits approximately every 30 days from the clinical pharmacist; the intervals between visits could be shorter according to the patient's needs. Quote: "These patients were given oral and written information about the treatment and educational lectures about disease and treatment; interventions with the psychiatrist were performed as needed. The aim of the Dáder Method was to identify drug‐related problems (DRPs) and drug‐related negative clinical outcomes (DNOs). The DRPs are ''those situations where the use of the drug causes or may cause a negative clinical outcomes associated with drug." The DNOs are situations associated with the use or misuse of drugs. These are classified into 6 categories according to the III Consensus of Granada (2007): (a) untreated health problems, (b) effect of unnecessary medications, (c) qualitative ineffectiveness of drugs, (d) quantitative ineffectiveness of drugs, (e) qualitative unsafety of drugs, (f) quantitative unsafety of the drug. After the problems related to pharmacotherapy were identified, the patients were duly informed about the problems, and pharmaceutical intervention was performed via oral and/or written communication between the pharmacist and patient or between the pharmacist, patient, and doctor. All interventions were recorded on the intervention form. Interventions included strategies to improve compliance with treatment, orientation regarding the disease and the patient's medications, dose adjustment, substitutions of ADs, and the addition of medications, among other features. A portion of the interventions included the prescribing physician's participation. The aim of the third visit was to assess the results of the intervention or multiple interventions. Pharmaceutical interventions included both actions designed to solve problems and health education actions—advice on hygienic and dietary habits—using oral and/or written communication as tools. The assessment of the results of the pharmaceutical interventions took place after a period that was predefined with each subject to establish whether her problems had been solved." n = 22. Control: TAU (did not receive any orientation or intervention that might have altered their response to treatment), n = 26. |
|
| Outcomes | Depression symptoms (BDI self‐report); remission (BDI scores < 11 on a per‐protocol basis); anxiety symptoms (Beck BDA); patient satisfaction with pharmaceutical assistance and care; acceptability of intervention (measured as participants not returning for follow‐up). Outcomes not used: Beck Anxiety inventory; patient satisfaction with pharmaceutical assistance and care (not specified in our protocol). |
|
| Notes | Sponsorship source: none stated Trial registration identifier: none stated Date of recruitment: 2010–2012 Declarations of interest from primary researchers: authors reported no conflicts of interest. Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The allocation of individuals to the control (CG) and intervention (IG) groups was performed via random sampling stratified according to age, medication type, severity of depression, and the presence or absence of recurrence according to Bernoulli's method" (p. 219). Allocation was made "according to stratification by age (18 to 65 years); severity (mild/moderate/severe), based on the BDI; medication type (tricyclic antidepressants [TCAs], selective serotonin reuptake inhibitors [SSRIs], other); and whether they were new cases (first treatment) or recurrences" (p. 220). |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Five patients in the control group, for ethical reasons, received some timely pharmaceutical guidance" (p. 220). Comment: however, the authors emphasise that this approach was not characterised as the intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No details provided. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "After the first visit, 4 patients were excluded because they did not score higher than 11 on the BDI, and 6 others were excluded because of insurmountable difficulties in scheduling visits (lack of a telephone line or living in rural area or in a neighborhood that was difficult to access). The 58 remaining participants were allocated to the control and intervention groups" (p. 220). Comment: in the analysis, authors presented data from 48 participants, detailing that 10 were either withdrawn or excluded. Authors did not state how they dealt with missing data. |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
Rickles 2005.
| Methods | Design: RCT, parallel group Country: USA Setting: 14 pharmacists; 8 Wisconsin community pharmacies within a large managed care organisation. |
|
| Participants | Inclusion criteria: no AD use in the past 4 months, aged ≥ 18 years, were willing to pick up their AD from a study pharmacy during the next 4 months, were not receiving medications for a psychotic or bipolar disorder, had a BDI‐II score ≥ 16, had no hearing impairment, planned to be in the local area during the next 4 months, and consented to participation. Exclusion criteria: required a translator, known or suspected physical condition that requires additional caution with the prescribed AD; currently pregnant; breastfeeding. Mean age (years): intervention group: 37.8 (SD 10.7); control group: 37.5 (SD 13.4) Sex: 53 women; 10 men Severity of depression: BDI‐II baseline mean score: intervention group: 28.9 (SD 8.15); control group: 27.0 (SD 8.40) AD use status: 45 participants had no history of psychiatric medication use; 18 recorded past use of psychiatric medication use. Comorbidities: none stated |
|
| Interventions | Sample size (total): 63 randomised; authors presented results for 60 (completer only) Length of trial: 6 months (intervention lasted for 3 months) Intervention group: remotely delivered pharmacist‐guided education and monitoring – involved training pharmacists for 90 minutes, then monthly telephone calls from pharmacists to participants providing structured education and monitoring. The first telephone call generally took place within 2–3 weeks of when the patient initially presented the new AD prescription. Using a monitoring tool the pharmacist was guided to assess the patient's AD knowledge and beliefs, adverse effects, and other concerns, treatment goals or areas in which they hoped the medication would help, and how the medication was being used during the week before the telephone call. The monitoring tool directed the pharmacists to clarify, probe, or explain issues that were not understood by patients. The tool guided the pharmacists to ask patients about their concerns, make recommendations to manage adverse reactions and other concerns, and follow‐up on any indication of medication non‐adherence. Pharmacist recommendations on the management of adverse reactions might include changing the timing of a dose to reduce insomnia or fatigue, use of food to reduce stomach upset or sugarless candy for dry mouth, and referral to prescriber for severe or bothersome reactions. Pharmacists could also recommend discussions with a prescriber to select lower cost alternatives, medications with better adverse effect profiles (e.g. less weight gain, headaches, sexual dysfunction, or easier to swallow). Follow‐up calls reviewed current adherence, determined whether any new adverse effects and concerns had developed, and asked whether participants had perceived any progress in their medication goals. The pharmacist made new recommendations to participants as needed, n = 28. Control group: usual care involved education and patient monitoring (no telephone follow‐up or special monitoring), n = 32. |
|
| Outcomes | Frequency of patient feedback to pharmacists; AD knowledge, beliefs; percentage of missed doses; depression symptom scores (BDI‐II self‐report on a per protocol basis), and perceptions of progress; adherence to medication (calculated by multiplying the number of prescribed doses per day by the number of days late between refills for the period and dividing by the number of days in the period on a per‐protocol basis); acceptability of treatment (number of participants not returning for follow‐up). Outcomes not used: frequency of patient feedback to pharmacists; AD knowledge, beliefs, and perceptions of progress. |
|
| Notes | Sponsorship source: sponsored by a dissertation grant award from the Sonderegger Research Center (Madison, WI) and a predoctoral National Research Service Award (1 F31 MH65833‐01) through the National Institute of Mental Health. Trial registration identifier: none stated. Date of recruitment: October 1, 2001 to September 30, 2002 Declarations of interest from primary researchers: authors declared no conflicts of interest. Correspondence: none. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization involved the researcher preparing 10 pieces of paper with sequential numbers for each participating pharmacist at the site (i.e., sites with two pharmacists would have 20 potential numbers, three pharmacists at a site would have 30 numbers). Each of the eight pharmacies had a different cluster of numbers; the first site had numbers beginning with 100, the second site had numbers with 200, and so forth. Since the first site had three participating study pharmacists, 30 slips of paper numbered from 100 to 130 were prepared and placed into an envelope. When a patient was enrolled from that site, the researcher would randomly select a number out of the envelope. Selection of an odd or even number meant the patient was assigned to the usual care or PGEM [pharmacist‐guided education and monitoring] group, respectively" (p. 346). Also, "despite randomization, [intervention] patients were more likely than usual care patients to have a history of psychotropic medication use (P ≤ .05)" (p. 349). |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were unblinded to their group assignment since they could figure out that if they did not receive telephone calls, they were likely assigned to the usual care group. No "dummy" telephone calls (calls that did not involve the content of the intervention) were made to the usual care group to simulate the intervention" (p. 346). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | See above. Due to the nature of the intervention is was not possible to use double blinding. It is not clear whether this affected any outcomes. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Both study groups may have reported greater clinical improvement since they wanted to meet the expectations of interested pharmacists (especially since patients were unblinded to group assignment). This phenomenon may explain why study groups had greater antidepressant adherence than has been reported in the literature" (p. 352). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 63 participants originally randomised, 60 completed the study; 3 participants lost to follow‐up, all from the intervention group. Authors stated in Table 2 (p. 351) "data shown are based on a per‐protocol analyses and excludes 3 dropouts. An intention‐to‐treat analysis indicates no significant differences between study groups on adherence at 3 and 6 months. Number of cases varies due to missing data." |
| Selective reporting (reporting bias) | Low risk | None detected. |
| Other bias | Low risk | None detected. |
Rubio‐Valera 2013.
| Methods | Design: RCT, parallel group Country: Spain Setting: 4 primary healthcare centres, Barcelona, Spain |
|
| Participants | Inclusion criteria: aged 18–75 years; clinical diagnosis of depression from a GP; beginning a pharmacological AD treatment; attending 1 of the participant community pharmacies. Exclusion criteria: used AD medication in the past 2 months; had an appointment with an specialist in mental disorders in the past 2 months; history of psychotic or bipolar episodes; history of drug abuse or dependency; cognitive impairment that did not allow assessment. Mean age (years): intervention group: 46.9 (95% CI 44.0 to 48.6); control group: 46.3 (95% CI 43.3 to 49.2) Sex: 135 women; 44 men Severity of depression: mean baseline severity of 15.9 (PHQ‐9) – moderately severe symptoms. AD use status: not stated. Comorbidities: 69 had over the median of 3 comorbidities (not specified). |
|
| Interventions | Sample size (total): 179 Length of trial: 3 months (6 months' follow‐up). Intervention: medical and pharmaceutical care plus a pharmaceutical support programme – pharmacists participating in the study received 8 hours of training about PRODEFAR prior to the study. The training followed a manual created for the study and was accredited by the Catalonian council of continuous pharmaceutical training (Consell Català de la formació farmacèutica contínua) (Reference Number: 09F00676). Quote: "Participants received the intervention on visiting the pharmacy where they received their first prescription of the 6‐months antidepressant course. The intervention consisted of an educational in person delivered intervention centred on improving patients' knowledge of antidepressants and awareness of the importance of adherence. In patients with a sceptical attitude towards the medication, the intervention aimed to reduce stigma, reassure the patient about possible side‐effects, and stress the importance of following GPs' advice. As patients were beginning treatment with antidepressants, the first contact was considered crucial. During the first visit, the pharmacist provided the patient with information about the medicine and briefly discussed various aspects of the illness to improve understanding of the treatment, eliminate erroneous preconceptions and reinforce the concept of illness to the patient. In subsequent visits, the pharmacist conducted a short review of some points covered in the first visit and checked patient progress (improvement, appearance of side‐effects, or queries). First and subsequent contacts took a mean of 14.4 and 7.7 min, respectively" n = 87. Control: usual care – usual pharmaceutical care and treatment considered most appropriate by their primary care physician, n = 92. "Usual care varied between pharmacies but mainly consisted of dispensing the medication; answering patients' questions, and giving some basic advice about how to take the medication" (p. 1059). |
|
| Outcomes | Adherence to AD medication (measured by pharmacy records defined as refilling < 80% of the prescribed doses on an ITT basis); clinical severity of depression (PHQ‐9 on an ITT basis), anxiety (STAI‐S), health‐related quality of life (EQ‐5D), satisfaction with the treatment received, adverse effects; cost‐effectiveness; acceptability of intervention (as measured by participants not returning for follow‐up). Outcomes not used: satisfaction; anxiety; cost‐effectiveness (not specified in our protocol); quality of life (data not usable). |
|
| Notes | Sponsorship source: funded by Carlos III Health Institute Grant (Spanish Ministry of Health and Consumer Affairs) (FISPI070546); the Carlos III Health Institute had no further role in study design. Trial registration identifier: ClinicalTrials.gov Identifier: NCT00794196 Date of recruitment: October 2008 to May 2011 Declarations of interest from primary researchers: all authors declared that they had no conflicts of interest. Correspondence: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomisation was generated at the patient level by a computerised random‐number generator following a permuted block design (1:1). As patients were enrolled, the GP sequentially stapled one of the envelopes to the prescription" (p. 1058). |
| Allocation concealment (selection bias) | Low risk | Quote: "to ensure allocation concealment, every GP received a set of 10 sequentially numbered, opaque, sealed envelopes generated by an external investigator (MRV) containing patient assignment" (p. 1058). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote in full publication: "Blinding of participants and pharmacists was not possible but outcome assessors were blind to the allocation. Patients were asked to avoid discussing the study among them" (p. 1058). |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote in protocol: "double blinding of investigator and outcomes assessor." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Sample sizes differ between protocol (n = 194) and final published report (n = 179). Quote: "for the main analyses, all participants were included as randomised regardless of whether they received the intervention or had incomplete follow‐up data" (p. 1059). Authors did not impute missing data, but use multi‐level analysis. Quote: "179 patients were randomised to UC [usual care] (92) and CPI [medical and pharmaceutical care] (87), were evaluated at baseline and included in the main analysis. Only 87 (95%) and 64 (74%) in the control and intervention group, respectively, received the intervention as allocated and were included in the PP [per protocol] analysis" (p. 1059). We used data from the authors main (multilevel analysis) for this review. |
| Selective reporting (reporting bias) | Unclear risk | Adverse effects data not reported by rate (a general list without data was provided by study authors). |
| Other bias | Low risk | None detected. |
AD: antidepressant; AHCPR: Agency for Health Care Policy and Research; AUDIT: Alcohol Use Disorders Identification Test; BDI: Beck Depression Inventory; BIDS: Brief Inventory for Depressive Symptoms; CAGE: Cutting down, Annoyance by criticism, Guilty feeling, and Eye‐openers; CI: confidence interval; DSM‐IV: Diagnostic and Statistical Manual of Mental Disorders 5th edition; EQ‐5D: EuroQol‐5D; GMI: group mean imputation; GP: general practitioner; HAMD‐17: 17‐item Hamilton Depression Rating Scale; HCV: hepatitis C virus; ICD‐10: International Classification of Diseases 10th edition; ITT: intention‐to‐treat; LOCF: last observation carried forward; MADRS: Montgomery–Åsberg Depression Rating Scale; mBDI: modified Beck Depression Inventory; MCS: Mental Composite Score; MDD: major depressive disorder; MHI: Mental Health Inventory; n: number of participants; NaSSA: noradrenergic and specific serotonergic antidepressant; NEO: Neuroticism‐Extraversion‐Openness; PCP: primary care provider; PCS: Physical Composite Score; PHQ‐9: 9‐item Patient Health Questionnaire; RCT: randomised controlled trial; RIMA: reversible inhibitors of monoamine oxidase A; SCL‐90: Symptom Checklist‐90; SD: standard deviation; SDM: shared decision‐making; SF‐12: 12‐item Short Form; SF‐36: 36‐item Short Form; SNRI: serotonin‐noradrenaline reuptake inhibitor; SSRI: selective serotonin reuptake inhibitor; STAI‐S: State‐Trait Anxiety Inventory; TCA: tricyclic antidepressant; TAU: treatment as usual; UWFMC: University of Washington Family Medical Center.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Akinbosoye 2016 | Intervention outside the scope of this review as the pharmacist only made contact with the participants once after they started taking new medication (within 3 days), this intervention did not constitute depression management. Although the accompanying abstract stated the study was a prospective randomised trial, the primary reference stated it was a retrospective cohort analysis, both analyses were based on the same dataset. |
| Bertsche 2015 | Abstract did not contain enough detail to clearly establish the involvement of the pharmacist in the intervention. Assessment of the full text revealed that the pharmacist (or a pharmacy team) did not play a leading role in the delivery of the intervention. |
| Fortney 2007 | Abstract did not specify the role that pharmacists/pharmacy teams played in the delivery of the collaborative care model. Outcomes and population seemed highly relevant. Assessment of the full text revealed that pharmacists/pharmacy teams were not primarily involved with the delivery of the intervention. |
| Fortney 2013 | Abstract did not specify the role that pharmacists/pharmacy teams played in the delivery of the collaborative care model. Outcomes and population seemed highly relevant. Assessment of the full text revealed that pharmacists/pharmacy teams were not primarily involved with the delivery of the intervention. |
| Kawaguchi 2018 | Japanese study with English Abstract. The abstract looked highly relevant. However, translation of the full text revealed that participants did not receive antidepressants. |
| Kooij 2016 | Assessment of the full text revealed that this study did not measure any of the outcome measures relevant to this review. |
| Liekens 2014 | Assessment of the full text revealed that this study presented outcomes of the study pharmacists rather than the study participants. |
| Lin 2018 | Study appeared relevant from the abstract. The full text revealed that people with 'psychological problems', including depression, were not eligible to participate in the trial. |
| Matzke 2018 | Assessment of the full text revealed that this study did not measure or report any relevant outcomes. |
| Moride 2013 | We received an unpublished report (which is not available in the public domain) from the study investigators via email. Assessment of this unpublished full text revealed that the intervention did not meet our inclusion criteria. |
| NCT00356655 | Study protocol appeared highly relevant. We requested further information. The investigators responded as follows: quote: "data collection ended in 2008 and there were only 3 patients. No analysis were even conducted. 3 patients completed Phase 1 of the study. Only 1 of those patients completed Phase 2 (randomized portion of the study)." Excluded due to absence of outcomes data. |
| Pyne 2011 | Although there was a pharmacist as part of the wider depression management team the pharmacist was located off‐site and was used primarily for information on drug interactions and relaying pharmacotherapy information to the care team. The pharmacist was not involved directly with participants' depression management. |
| Shaw 2000 | Study did not record outcome measures relevant to this review: it only measured median knowledge scores and readmission to hospital of postdischarge patients. |
| Zermansky 2001 | We contacted authors to verify eligibility for inclusion. The authors replied as follows: "I don't think our paper would be suitable for you to include in your systematic review. We did a medication review of all of the patients medicines but did not collect any separate data on antidepressants. Our outcome measures were not patient orientated i.e. we looked at number of medicine changes of any type." |
Characteristics of studies awaiting assessment [ordered by study ID]
Harris 2005.
| Methods | Design: RCT Country: UK |
| Participants | Older people with mental health problems |
| Interventions | Pharmaceutical care and adherence support by community pharmacists for older people with mental health problems in the community. Domiciliary visiting service by train community pharmacist and key worker, adherence judged by key worker. |
| Outcomes | Adherence and medicine storage |
| Notes | Unpublished thesis, we contacted the University of Nottingham repository but they could offer no details for Harris, and could not send the thesis without their permission. Hattingh was contacted, as the study was identified through a systematic review published by them, there was no response. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12618001105235.
| Trial name or title | Enhanced intervention to improve medication adherence in a community pharmacy setting |
| Methods | Randomised controlled trial, parallel |
| Participants | Inclusion criteria: aged > 18 years Signed consent form Signed data linkage consent form Able to complete questionnaires Prescribed medication for hypertension, cholesterol, depression, anxiety, COPD, or a combination of these Eligibility identified via GuildCare software Medical possession ratio < 70% Exclusion criteria: collecting someone else's medication Communication limitations or any other impairment the recruiting pharmacist considers could preclude them from participating Gender: men and women |
| Interventions | 3‐arm comparison of an enhanced tailored adherence intervention to an educational adherence programme and usual care in a community pharmacy setting: a cluster randomised controlled trial Arm 1: patient‐tailored brief complex interventions with monthly follow‐up over 12 months to improve, reinforce, and maintain adherence behaviour and their impact in clinical, economical, and humanistic outcomes. Arm 2: GuildLink software educational intervention as a guided counselling session to improve adherence behaviour and their impact in clinical, economical, and humanistic outcomes. Arm 3: The enhanced intervention is a brief complex intervention delivered once to eligible patients as identified through the GuildCare software which aims to optimise medicines use on a systematic and regular basis. Community pharmacists will be trained in 2 × 4‐hour sessions on the study process, data collection, and behavioural change theoretical framework by study investigators trained in practice change facilitation. The training will be delivered via power point with printed materials and motivational interviewing practice sessions. The face‐to‐face pharmacist‐delivered adherence service (evidenced‐based intervention adapted from interventions for enhancing medication adherence a systematic review by Cochrane) has several interacting components based on a sequence of principles that imply over an estimated 10‐minute session:
Pharmacists will determine barriers based on asking the patient questions based on the Moresby 4‐item questionnaire. These questions address patients' beliefs about their medication, knowledge of the medication, and if they stop or forget taking their medication ever. Additional questions based on pharmacist discretion (i.e. how often do you forget to take your medication?) will be asked to further evaluate reasons for non‐adherence. Pharmacist‐delivered interventions will include ≥ 1 of the following based on what barriers were discovered for non‐adherence:
Readiness to change will be based on the pharmacist's discretion, which we will address in the training sessions using the Transtheoretical Model. The participant will only be involved in short, 10‐minute monthly sessions when at the pharmacy to pick up their medications. If they so choose, they can opt into pharmacy repeat fill reminders to send an SMS or phone call monthly when their prescription is ready. Arm 2: 2 software programs: NTT and MedScreen Compliance guide an estimated 1 × 10‐minute face‐to‐face pharmacist–patient interactions to enhance adherence. The NTT program is a pharmacist intervention provided to patients who are initiating on a medication for the first time. It provides information on how to take/use the medication to achieve ultimate health outcomes as well as medication adherence guidance with an optional monthly follow‐up reminder to refill the medication. A software program guides a pharmacist and allows documentation of a patient's response and understanding, made from readily available resources already used in pharmacies subscribed to the GuildCare software. In addition, a pharmacist can document a clinical intervention, including compliance or education issues, and recommendation made. Under the Community Pharmacy Agreement Pharmacy Practice Incentive Programme, pharmacies may be eligible to receive an incentive payment for conducting clinical interventions. This is already included in standard dispensing for pharmacies prescribed to the GuildCare software. See clinical interventions listed on the Pharmaceutical Society of Australia website (www.psa.org.au/practice‐standards/pharmacists‐performing‐clinical‐interventions). The MedScreen Compliance and Compliance program creates a guided face‐to‐face pharmacist and patient interaction to target non‐adherent patients and increase medication adherence. The software calculates the patient's MedsIndex score, an already readily available resource from the dispensing data collected in the GuildCare software, which represents how much medicine the doctor intended the patient to take. |
| Outcomes | Primary outcomes: calculation of percentage of proportion of days covered (PDC) in all arms. These data will be collected from the GuildCare software dispensing records (prescription dispense dates). 'Days covered' are determined by the formula in PDC, adding total days dispensed over a period of time. This accounts for gaps in the dispense records if patients pick up their dispensed medication early; medication adherence: medication possession ratio (MPR) in all arms. These data will be collected from the GuildCare software dispensing records (prescription dispense dates); self‐report of adherence by Morisky 4‐item questionnaire. Secondary outcomes: number of hospital admissions will be measure with Australian Medicare data to determine the intervention effects on cost to the healthcare system; for COPD, a Clinical COPD Questionnaire (CCQ) will be asked to patients; for depression: Psychological Health Questionnaire (PHQ‐9) categorises severity; for humanistic outcomes: quality of life will be measured by the EuroQol‐5D‐5L; for hyperlipidaemia, total cholesterol will be measured using blood test with a cholesterol meter (total cholesterol levels); for hypertension, control of the disease will be done by the measurement of blood pressure levels using a digital blood pressure monitor; number of medications and their cost based on Australian Medicare data will be used to assess costs associated with non‐adherence and the intervention effects; time of training and mean time spent for the pharmacist on the interview and mean time PCF supporting interventional pharmacists will be measured by pharmacy and researcher records to measure the cost associated with the intervention. |
| Starting date | Not yet recruiting |
| Contact information | Dr Elyssa Wiecek, University of Technology Sydney Graduate School of Health, NSW, Australia Tel: +61 (02) 9514 9223 E‐mail: elyssa.wiecek@uts.edu.au |
| Notes |
ChiCTR‐TRC‐08000726.
| Trial name or title | Impact of an interdisciplinary approach on symptom management of newly diagnosed depressive patients |
| Methods | Randomised parallel controlled trial |
| Participants | Inclusion criteria: adults, who are newly diagnosed with depressive disorders, able to communicate in Cantonese or English, have no previous exposure (within the past 6 months) to any antidepressants, and will start a treatment with an antidepressant, will be included. Exclusion criteria: dementia or any type of significant cognitive impairment, hearing impairment, terminal illnesses, psychosis, bipolar disorder, history of alcohol or any type of substance abuse; high suicidal risks, and pregnant or nursing, illiterate, taking St John's Wort or any other traditional Chinese medicines for depression Age minimum: 18 years Age maximum: 80 years Gender: male |
| Interventions | Pharmacist education vs usual care |
| Outcomes | Primary outcomes: proportion of participants with ≥ 50% improvement in the Chinese version of 13‐item BDI score; % participants with HAMD ≤ 7 Secondary outcomes: mean change in BDI score at 6 months compared to baseline; improvement in health‐related quality of life assessed by The Chinese (Hong Kong) SF‐36 at baseline and 6‐month; baseline and 6‐month CGI‐S and CGI‐I scales; % participants compliant with antidepressant treatment at 3 and 6 months; participants who consumed > 80% of tablets dispensed during the prescribed period will be categorised as compliant with antidepressant treatment; cost‐utility analyses to estimate the ratio between the cost of intervention and the potential benefit in symptom improvement observed. |
| Starting date | 2008 |
| Contact information | Lee Chui Ping Room 622B, 6/F, Li Choh Ming BMSB, CUHK Tel: +852 26096819 E‐mail: cplee@cuhk.edu.hk |
| Notes |
ISRCTN11290592.
| Trial name or title | Community pharmacies mood intervention study (CHEMIST) |
| Methods | Randomised controlled trial Country: UK |
| Participants | Inclusion criteria: adults (male or female, aged 18 years and over; ≥ 1 long‐term conditions (arthritis, cancer, cardiovascular conditions, diabetes, respiratory conditions, stroke); subthreshold depression (screen positive with 2–4 symptoms confirmed by diagnostic assessment tool) |
| Interventions | Pilot RCT Participants randomised in a 1:1 ratio using the independent online randomisation service provided by the York Trials Unit to 1 of 2 group. Intervention group: ESI over 4–6 sessions in a 4‐month period either by telephone or face‐to‐face in the privacy of pharmacy consulting rooms, as in the feasibility study. Control group: usual primary care management of subthreshold depression offered by the GP or other local community provision. Participants in both groups followed up at 4 months' after randomisation. |
| Outcomes | Primary outcomes Feasibility study: recruitment and attrition rates; quality of data collection at baseline and 4 months; ESI adherence; process evaluation undertaken through qualitative interviews with participants, ESI facilitators, and pharmacy staff. Pilot RCT: self‐reported depression severity measured by the PHQ‐9 at baseline and 4 months. Secondary outcomes Pilot RCT: prevention of depression measured by binary depression scores on the PHQ9 at baseline and 4 months; anxiety is measured using the GAD‐7 at baseline and 4 months; health‐related quality of life measured by the SF‐12v2 at baseline and 4 months; health state utility measured by the EQ‐5D at baseline and 4 months; health service use, collected by a bespoke questionnaire (adapted Adult Service Use Schedule) at baseline and 4 months; participant's use of ESI, collected from intervention facilitator records at 4 months; process evaluation is undertaken using qualitative interviews with participants, pharmacy staff, and GPs at 4 months. |
| Starting date | 1 January 2017 |
| Contact information | Dr Liz Littlewood (public) E‐mail: liz.littlewood@york.ac.uk Mental Health and Addiction Research Group, Department of Health Sciences, University of York, York, UK Tel: +44 1904 321828 Dr David Ekers (scientific) E‐mail: david.ekers@york.ac.uk Mental Health and Addiction Research Group, Department of Health Sciences, University of York, York, UK Tel: +44 1904 321638 |
| Notes |
NCT01188135.
| Trial name or title | Antidepressant adherence via AD_IVR (AD_IVR) |
| Methods | Randomised controlled trial Country: US Setting: Kaiser Permanente Center for Health Research Portland, Oregon, PA, US |
| Participants | Inclusion criteria: Kaiser Permanente NW Region health plan members aged 21–75 years and members for ≥ 6 months prior to the initial antidepressive medications dispense; must have an EMR chart diagnosis or presenting complaint of a unipolar mood diagnosis, anxiety disorder, or any subclinical or "not otherwise categorized" variant of these; participants' providers must give permission to study staff for their patients' enrolment in the study. There must be no indication of pending HMO disenrollment in the membership data; must have an initial dispense of an antidepressant medication, with no dispense of any of these agents in the prior 6 months. Exclusion criteria: participants must have no EMR chart diagnosis that is likely to impair participant ability to complete evaluations or take part in the intervention. These include psychiatric diagnoses such as bipolar disorder I (BP II is acceptable), schizophrenia, schizo‐affective disorder, or similar diagnoses indicating psychosis; investigators will also exclude people with any chart diagnosis indicating significant intellectual impairment, such as any dementia disorder, mental retardation, or profound developmental disorder such as autism. |
| Interventions | Intervention 1: interactive voice messaging (3 types of interventions calls from the IVR telephone system). 1. The first call is to orient the participant to the IVR system, ask permission to leave detailed messages in the future, and to encourage adherence in this initial period of great risk for premature discontinuation. 2. The Refill Reminder Call is to remind patients that a refill their antidepressant medications is due and occurs approximately 6 days before the prior dispense of medication is due to run out. 3. The "Tardy" Refill Call is made to participants for whom EMR records indicate that a scheduled refill was missed. Control: usual care arm with no interactive telephone reminder calls Intervention 2: IVR messaging plus psycho‐educational materials IVR messaging as per Intervention 1 plus educational material about antidepressant medication. |
| Outcomes | Primary outcome: antidepressant persistence, measured as estimated level of persistence with therapy. Secondary outcomes: patient and provider satisfaction; healthcare utilisation; patient self‐reported depression and anxiety at end of week 40; cost‐effectiveness analyses. |
| Starting date | 2011 |
| Contact information | Greg N Clarke, PhD Kaiser Permanente Center for Health Research, Portland, Oregon, PA, US |
| Notes |
NCT02027259.
| Trial name or title | NCT02027259 |
| Methods | Design: RCT (pilot), parallel group Country: US Setting: Providence VA Medical Center |
| Participants | Inclusion criteria: aged ≥ 18 years, clinical diagnosis of type 2 diabetes; clinical diagnosis of depression; PHQ‐9 score indicating depressive symptoms; most recent haemoglobin A1c ≥ 8.0% within the previous 12 months in the chart and currently have ≥ 1 of the following modifiable CVD risk factors not at target goals: current smoker (any cigarette smoking < 30 days), blood pressure > 130/80 mmHg, document at least twice in the last 6 months or LDL cholesterol > 100 mg/dL within the last 12 months. Exclusion criteria: aged ≤ 17 years; inability to attend group sessions active psychosis of any type or organic brain injury that precludes diabetic management self‐care; type 1 diabetes as documented in the medical chart; pregnant; actively suicidal; end‐stage medical illness or people currently enrolled in diabetic management group programmes that include medication titration within the group setting would not be eligible due to cointervention. |
| Interventions | Sample size (total randomised): 50 Length of trial: 6 months Intervention: group visits with behavioural activation, consisting of 4 weekly group visits of a 2‐hour duration followed by monthly booster group visits for 6 months to prevent relapse. Enrolled: 25 Control: no intervention, standard group visits consisting of 4 weekly group visits of 2‐hour duration followed by monthly booster group visits for 6 months to prevent relapse Enrolled: 25 |
| Outcomes | Risk of future coronary events (change in United Kingdom Prospective Diabetes Study risk engine); depression symptoms (PHQ‐9) |
| Starting date | |
| Contact information | |
| Notes | Sponsorship source: Providence VA Medical Center Trial registration identifier: NCT02027259 (ClinicalTrials.gov Identifier) and 12CRP9840018 (other study ID Numbers) Correspondence: corresponded with the PI in July 2019 and were informed that "the manuscript is still under revision." We were unable to obtain unpublished results. |
NCT03591224.
| Trial name or title | Pharmacogenomic testing to optimize antidepressant drug therapy |
| Methods | Design: RCT Country: Canada |
| Participants | |
| Interventions | Intervention: pharmacist optimising antidepressant therapy using the patient's personalised pharmacogenomic report to make recommendations. Comparator: pharmacist optimising antidepressant therapy based on standard of care |
| Outcomes | Primary outcomes: patient satisfaction using a brief, self‐administered multidimensional generic questionnaire comprising 17 items; severity if depression using PHQ‐9; generalised anxiety using GAD‐7; functional impairment in work/school, social life, and family life using Sheehan Disability Scale from v=baseline to 6 months. Secondary outcomes: number of pharmacist identified drug therapy problems; prescriber acceptance rate of pharmacist's recommendations at completion of study (about 1 year) |
| Starting date | 2018 |
| Contact information | John Papastergiou Pharmacy Ltd, Ontario, Canada Tel: 416‐461‐2453 ext 33 E‐mail: asdm500@shoppersdrugmart.ca Wilson Li Tel: 416‐461‐7533 ext 33 Email: psdm994@shoppersdrugmart.ca |
| Notes |
Phung 2013.
| Trial name or title | Phung 2013 (pilot) |
| Methods | Design: randomised controlled trial, pilot Setting: Tulalip Health Clinic Country: US |
| Participants | Inclusion criteria: Native American, fluent in English, > 4 diagnosed chronic conditions, aged ≥ 18 years, and hospital length of stay > 3 days, with admission through the emergency department or directly by an outpatient provider. |
| Interventions | Control group: standard care Intervention 1: weekly home visits for 1 month Intervention 2: home visits twice a month for 3 months following hospital discharge. Each home visit will include an in‐home assessment including medication optimisation and assessment of readmission risk factors. A care plan will be developed, which will have participants follow‐up with their primary care provider if appropriate or continue with pharmacist monitoring, with duration of visits determined by assigned treatment group. |
| Outcomes | Participants' understanding about their medications and other health‐related concerns; participants' satisfaction with home visits; hospital readmissions; duration of hospital stay; clinical indication upon admission; participant satisfaction and education upon completion of study. |
| Starting date | |
| Contact information | |
| Notes |
AD‐SUS: Adult Service Use Schedule ; BDI: Beck Depression Inventory; CGI‐I: Clinical Global Impression – Improvement; CGI‐S: Clinical Global Impression – Severity of Illness; COPD: chronic obstructive pulmonary disease; CVD: cardiovascular disease; EMR: electronic medical record; EQ‐5D: EuroQol‐5D; ESI: Enhanced Support Intervention; GAD‐7: Generalized Anxiety Disorder‐7; GP: general practitioner; HAMD: Hamilton Depression Rating Scale; IVR: Interactive voice response; LDL: low‐density lipoprotein; PHQ‐9: 9‐item Patient Health Questionnaire; SF‐12v2; 12‐item Short Form version 2; SF‐36: 36‐item Short Form.
Differences between protocol and review
Objectives
We stated in the protocol that we would examine the effects of pharmacy‐based management interventions compared to an active control, waiting list/no treatment, or treatment as usual, at improving depression outcomes in adults (Sampson 2019). However, as no eligible studies compared pharmacy‐based management with waiting list or no treatment, we were unable to include this comparison in our review.
Methods – selection criteria – eligible interventions
In the protocol, we specified that we would group studies based on the person(s) delivering the team (Sampson 2019):
Delivery of the pharmacy‐based management intervention.
Pharmacist only.
Pharmacist plus the wider pharmacist team.
Multidisciplinary care group (involving the pharmacist with or without the wider care team, and other members of the healthcare team).
Comparator interventions were divided into studies using an active control and those usingtreatment as usual which would have led to four distinct comparisons.
Pharmacy‐based management (pharmacist only) versus active control.
Pharmacy‐based management (pharmacist only) versus treatment as usual.
Pharmacy‐based management (pharmacist plus wider care team) versus active control.
Pharmacy‐based management (pharmacist plus wider care team) versus treatment as usual.
Following data extraction, it became apparent that numbers of studies for each of these comparisons were small and that such a fine‐grained approach was not feasible. We came to a pragmatic consensus decision to reduce the number of comparisons to two based on active control or treatment as usual and decided to investigate the impact of the involvement of a team in the delivery of the intervention in subgroup analyses. See Types of interventions.
Contributions of authors
RS developed and conducted the electronic literature searches in collaboration with Sarah Dawson (Cochrane Common Mental Disorders Group Information Specialist).
SJS, NW, LW, AT, DE, RS, and JB selected studies for inclusion or extracted data, or both.
RS managed the references and contributed to the PRISMA flow diagram.
NW, JB, and NM conducted the statistical analyses.
NW, LW, AT, DE, JB, and NM made substantial contributions to the final review.
DM, SG, and RC contributed to the conceptualisation and design of the review.
All authors had an opportunity to review the final draft prior to publication.
Sources of support
Internal sources
-
Tees, Esk and Wear Valleys NHS Foundation Trust (TEWV), UK.
RS and RC time on this project is funded by TEWV as a matched contribution to a University of York ESRC Impact Acceleration Account award.
University of York, UK.
External sources
-
Economic and Social Research Council (ESRC), UK.
NW is funded by a PhD Studentship from the ESRC Northern Ireland and North East Doctoral Training Partnership ("NINE DTP").
SJS time on this project was part funded by the University of York ESRC Impact Acceleration Account (ES/M500574/1).
-
Northumberland, Tyne and Wear NHS Foundation Trust (NTW), UK.
SJS time on this project was part funded by NTW as a matched contribution to a University of York ESRC Impact Acceleration Account award.
-
National Institute for Health Research (NIHR), UK.
JB & NM time on this project was supported by Cochrane Infrastructure funding to the Common Mental Disorders Cochrane Review Group.
Declarations of interest
JB: none.
NW: none.
NM: none.
AT: none.
LW: none.
RS: none.
SJS: none.
RC: leads and has responsibility for Cochrane Common Mental Disorders, which has supported parts of the review process and is largely funded by a grant from the National Institute of Health and Research (NIHR) in the UK.
DM: none.
SG: none.
DE: Chief Investigator of the Community Pharmacy Mood Intervention Feasibility and Pilot Study funded by the National Institute for Health Research. This study is relevant to the subject matter of the review; however, the review author has no associated financial or commercial conflicts of interest.
These authors contributed equally to this work
These authors contributed equally to this work
Edited (no change to conclusions)
References
References to studies included in this review
Adler 2004 {published data only (unpublished sought but not used)}
- Adler DA, Bungay KM, Wilson IB, Pei Y, Supran S, Peckham E, et al. The impact of a pharmacist intervention on 6‐month outcomes in depressed primary care patients. General Hospital Psychiatry 2004;26(3):199‐209. [DOI] [PubMed] [Google Scholar]
- Bungay KM, Adler DA, Rogers WH, McCoy C, Kaszuba M, Supran S, et al. Description of a clinical pharmacist intervention administered to primary care patients with depression. General Hospital Psychiatry 2004;26(3):210‐8. [DOI] [PubMed] [Google Scholar]
Aljumah 2015 {published data only}
- Aljumah K, Hassali M. CP‐015 Impact of pharmacist intervention using shared decision making on adherence and measurable depressed patient outcomes. European Journal of Hospital Pharmacy 2015; Vol. 22:A6‐7.
- Aljumah K, Hassali MA. Impact of pharmacist intervention on adherence and measurable patient outcomes among depressed patients: a randomised controlled study. BMC Psychiatry 2015;15(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aljumah K, Nwaf A, Amed A, Alhoutan A, Aldhiab M. CP‐021 the impact of a decision aid on depressed patient's involvement in shared decision making: a pilot randomised controlled double blind study. European Journal of Hospital Pharmacy 2016; Vol. 23:A9‐10.
Al‐Saffar 2005 {published data only}
- Al‐Saffar N, Deshmukh AA, Carter P, Adib SM. Effect of information leaflets and counselling on antidepressant adherence: open randomised controlled trial in a psychiatric hospital in Kuwait. International Journal of Pharmacy Practice 2005;13(2):123‐31. [Google Scholar]
Al‐Saffar 2008 {published data only}
- Al‐Saffar N, Abdulkareem A, Abdulhakeem A, Salah AQ, Heba M. Depressed patients' preferences for education about medications by pharmacists in Kuwait. Patient Education and Counseling 2008;72(1):94‐101. [DOI: 10.1016/j.pec.2008.01.027] [DOI] [PubMed] [Google Scholar]
Brook 2005 {published data only}
- Bosmans JE, Brook OH, Hout HP, Bruijne MC, Nieuwenhuyse H, Bouter LM, et al. Cost effectiveness of a pharmacy‐based coaching programme to improve adherence to antidepressants. PharmacoEconomics 2007;25(1):25‐37. [DOI: 10.2165/00019053-200725010-00004] [DOI] [PubMed] [Google Scholar]
- Brook O, Hout H, Haan M, Nieuwenhuyse H. Pharmacist coaching of antidepressant users, effects on adherence, depressive symptoms and drug attitude; a randomized controlled trial. European Neuropsychopharmacology 2002;12(Suppl 3):S201. [Google Scholar]
- Brook O, Hout H, Haan M, Nieuwenhuyse H, Goodwin G. Medication coaching by community pharmacists of antidepressant users: a randomized controlled trial. XII World Congress of Psychiatry; 2002 Aug 24‐29; Yokohama (Japan). 2002.
- Brook O, Hout H, Nieuwenhuyse H, Heerdink E. Impact of coaching by community pharmacists on drug attitude of depressive primary care patients and acceptability to patients; a randomized controlled trial. European Neuropsychopharmacology 2003;13(1):1‐9. [DOI] [PubMed] [Google Scholar]
- Brook OH, Hout H, Stalman W, Nieuwenhuyse H, Bakker B, Heerdink E, et al. A pharmacy‐based coaching program to improve adherence to antidepressant treatment among primary care patients. Psychiatric Services 2005;56(4):487‐9. [DOI: 10.1176/appi.ps.56.4.487] [DOI] [PubMed] [Google Scholar]
- Brook OH, Hout HP, Nieuwenhuysea H, Haan M. Effects of coaching by community pharmacists on psychological symptoms of antidepressant users; a randomised controlled trial. European Neuropsychopharmacology 2003;13(5):347‐54. [DOI] [PubMed] [Google Scholar]
- Hout HP, Heerdink ER, Goodwin GM, Bakker BB, Nieuwenhuyse H. Improving adherence of antidepressants by pharmacies. 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans (LA). 2001.
- Hout HP, Heerdink ER, Goodwin GM, Nieuwenhuyse H, et al. Improving adherence to antidepressants by video and pharmacist coaching: study design. 154th Annual Meeting of the American Psychiatric Association; 2001 May 5‐10; New Orleans (LA). 2001.
Capoccia 2004b {published data only}
- Boudreau DM, Capoccia KL, Sullivan SD, Blough DK, Ellsworth AJ, Clark DL, et al. Collaborative care model to improve outcomes in major depression. Annals of Pharmacotherapy 2002;36(4):585‐91. [DOI: 10.1345/aph.1A259] [DOI] [PubMed] [Google Scholar]
- Capoccia KL, Boudreau DM, Blough DK, Ellsworth AJ, Clark DR, Stevens NG, et al. Randomized trial of pharmacist interventions to improve depression care and outcomes in primary care. American Journal of Health‐system Pharmacy 2004;61(4):364‐72. [DOI] [PubMed] [Google Scholar]
Crockett 2006 {published data only}
- Crockett J, Taylor S. Rural pharmacist perceptions of a project assessing their role in the management of depression. Australian Journal of Rural Health 2009;17(5):236‐43. [DOI: 10.1111/j.1440-1584.2009.01084.x] [DOI] [PubMed] [Google Scholar]
- Crockett J, Taylor S, Grabham A, Stanford P. Patient outcomes following an intervention involving community pharmacists in the management of depression. Australian Journal of Rural Health 2006;14(6):263‐9. [DOI: 10.1111/j.1440-1584.2006.00827.x] [DOI] [PubMed] [Google Scholar]
Finley 2003b {published data only}
- Finley PR, Rens HR, Gess S, Louie, C. Case management of depression by clinical pharmacists in a primary care setting. Formulary 1999; Vol. 34, issue 10:864‐70.
- Finley PR, Rens HR, Pont JT, Gess SL, Louie C, Bull SA, et al. Impact of a collaborative care model on depression in a primary care setting: a randomized controlled trial. Pharmacotherapy 2003;23(9):1175‐85. [DOI: 10.1592/phco.23.10.1175.32760] [DOI] [PubMed] [Google Scholar]
Kanwal 2018 {published data only}
- Kanwal F, Pyne JM, Tavakoli‐Tabasi S, Nicholson S, Dieckgraefe B, Storay E, et al. A randomized trial of off‐site collaborative care for depression in chronic hepatitis C virus. Health Services Research 2018;53(4):2547‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanwal F, Pyne JM, Tavakoli‐Tabasi S, Nicholson S, Dieckgraefe B, Storay E, et al. Collaborative care for depression in chronic hepatitis C clinics. Psychiatric Services 2016;67(10):1076‐82. [DOI: 10.1176/appi.ps.201400474] [DOI] [PubMed] [Google Scholar]
Marques 2013 {published data only}
- Marques LA, Galduroz JC, Fernandes MR, Oliveira CC, Beijo LA, Noto AR. Assessment of the effectiveness of pharmacotherapy follow‐up in patients treated for depression. Journal of Managed Care Pharmacy 2013;19(3):218‐27. [DOI: 10.18553/jmcp.2013.19.3.218] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rickles 2005 {published data only}
- Rickles NM. A randomized, controlled study evaluating the impact of an antidepressant monitoring program on consumer outcomes. Dissertation Abstracts International: Section B: the Sciences and Engineering 2003; Vol. 64, issue 5‐B:2145.
- Rickles NM, Svarstad BL, Statz‐Paynter JL, Taylor LV, Kobak KA. Improving patient feedback about and outcomes with antidepressant treatment: a study in eight community pharmacies. Journal of the American Pharmacists Association 2006;46(1):25‐32. [DOI] [PubMed] [Google Scholar]
- Rickles NM, Svarstad BL, Statz‐Paynter JL, Taylor LV, Kobak KA. Pharmacist telemonitoring of antidepressant use: effects on pharmacist–patient collaboration. Journal of the American Pharmacists Association 2005;45(3):344‐53. [DOI] [PubMed] [Google Scholar]
Rubio‐Valera 2013 {published data only}
- Rubio‐Valera M, Bosmans J, Fernández A, Peñarrubia‐María M, March M, Travé P, et al. Cost‐effectiveness of a community pharmacist intervention in patients with depression: a randomized controlled trial (PRODEFAR Study). PLoS One 2013;8(8):e70588. Erratum in: PLoS One 2016;11(1):e0147459. [DOI: 10.1371/journal.pone.0070588] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio‐Valera M, March Pujol M, Fernández A, Peñarrubia‐María MT, Travé P, López Del Hoyo Y, et al. Evaluation of a pharmacist intervention on patients initiating pharmacological treatment for depression: a randomized controlled superiority trial. European Neuropsychopharmacology 2013;23(9):1057‐66. Erratum in: European Neuropsychopharmacology 2016;26(6):1085. [DOI] [PubMed] [Google Scholar]
- Rubio‐Valera M, Peñarrubia‐María MT, Fernández‐Vergel R, Carvajal Tejadillo AC, Fernández Sánchez A, Aznar‐Lou I, et al. Impact of pharmaceutical intervention in preventing relapses in depression in primary care. Atencion Primaria 2016;48(5):308‐15. [DOI: 10.1016/j.aprim.2015.05.009] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio‐Valera M, Serrano‐Blanco A, Travé P, Peñarrubia‐María MT, Ruiz M, Pujol MM. Community pharmacist intervention in depressed primary care patients (PRODEFAR study): randomized controlled trial protocol. BMC Public Health 2009;9:284. [DOI: 10.1186/1471-2458-9-284] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Akinbosoye 2016 {published data only}
- Akinbosoye OE, Taitel MS, Grana J, Hill J, Wade RL. Improving medication adherence and health care outcomes in a commercial population through a community pharmacy. Population Health Management 2016;19(6):454‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taitel M, Jiang JZ, Rudkin K. Impact of pharmacist‐call intervention program on new‐to‐therapy patients' medication adherence. Value in health 2014;17(3):A138‐9. [Google Scholar]
Bertsche 2015 {published data only}
- Bertsche T, Lindner S, Damm M, Frontini R, Exner C, Himmerich H. An interdisciplinary concept to optimize patient safety – a pilot study [Ein interdisziplinäres Konzept zur Optimierung der Patientensicherheit – Eine Pilotstudie]. Psychiatrische Praxis 2015;42(4):216‐20. [DOI] [PubMed] [Google Scholar]
Fortney 2007 {published data only}
- Fortney JC, Pyne JM, Edlund MJ, Robinson DE, Mittal D, Henderson KL. Design and implementation of the Telemedicine‐Enhanced Antidepressant Management Study. General Hospital Psychiatry 2006;28:18‐26. [DOI] [PubMed] [Google Scholar]
- Fortney JC, Pyne JM, Edlund MJ, Stecker T, Mittal D, Robinson DE, et al. Reasons for antidepressant nonadherence among veterans treated in primary care clinics. Journal of Clinical Psychiatry 2011;72(6):827‐34. [DOI] [PubMed] [Google Scholar]
- Fortney JC, Pyne JM, Edlund MJ, Williams DK, Robinson DE, Mittal D, et al. A randomized trial of telemedicine‐based collaborative care for depression. Journal of General Internal Medicine 2007;22(8):1086‐93. [DOI: 10.1007/s11606-007-0201-9] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fortney 2013 {published data only}
- Fortney JC, Pyne JM, Mouden SB, Mittal D, Hudson TJ, Schroeder GW, et al. Practice‐based versus telemedicine‐based collaborative care for depression in rural federally qualified health centers: a pragmatic randomized comparative effectiveness trial. American Journal of Psychiatry 2013;170(4):414‐25. [DOI: 10.1176/appi.ajp.2012.12050696] [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00439452. Telemedicine‐based collaborative care to reduce rural disparities. clinicaltrials.gov/show/nct00439452 (first received 23 February 2007).
- Pyne JM, Fortney JC, Tripathi SP, Maciejewski ML, Edlund MJ, Williams DK. Cost‐effectiveness analysis of a rural telemedicine collaborative care intervention for depression. Archives of General Psychiatry 2010;67(8):812‐21. [DOI] [PubMed] [Google Scholar]
Kawaguchi 2018 {published data only}
- Kawaguchi F, Watanabe J, Tsuneki T, Kikkawa A. The significance of pharmacist intervention focused on improvement of adverse events and cancer pain. Gan to Kagaku Ryoho. Cancer & Chemotherapy 2018;45(3):443‐7. [PubMed] [Google Scholar]
Kooij 2016 {published data only}
- Kooij MJ, Heerdink ER, Dijk L, van, Geffen EC, van, Belitser SV, Bouvy ML. Effects of telephone counselling intervention by pharmacists (TelCIPI on medication adherence; results of a cluster randomized trial [Telefonische startbegeleiding door apothekers verbetert de therapietrouw; een cluster‐gerandomiseerd onderzoek]. Pharmaceutisch Weekblad 2017;152(37):19‐26. [Google Scholar]
- Kooij MJ, Heerdink ER, Dijk L, Geffen EC, Belitser SV, Bouvy ML. Effects of telephone counseling intervention by pharmacists (TelCIP) on medication adherence; results of a cluster randomized trial. Frontiers in Pharmacology 2016;7:269. [DOI: 10.3389/fphar.2016.00269] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy MJ, Heerdink ER, Geffen EC, Geers HC, Bouvy ML. Effects telephone counselling intervention by pharmacist on beliefs about medicines for patients starting treatment: Results of an interim analysis of a cluster randomized controlled trial. International Journal of Clinical Pharmacy 2013;35(5 Suppl 2):896‐7. [Google Scholar]
- Kooy MJ, Geffen EC, Heerdink ER, Dijk L, Bouvy ML. Effects of a TELephone Counselling Intervention by Pharmacist (TelCIP) on medication adherence, patient beliefs and satisfaction with information for patients starting treatment: study protocol for a cluster randomized controlled trial. BMC Health Services Research 2014;14:219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooy MJ, Geffen EC, Heerdink ER, Dijk L, Bouvy ML. Patients' general satisfaction with telephone counseling by pharmacists and effects on satisfaction with information and beliefs about medicines: results from a cluster randomized trial. Patient Education and Counseling 2015;98:797‐804. [DOI] [PubMed] [Google Scholar]
Liekens 2014 {published data only}
- Liekens S, Boedry O, Hendrickx E, Vandael E, Broecke I, Roter D, et al. Analysis of interactions between pharmacists and patients starting antidepressant therapy. International Journal of Clinical Pharmacy 2013;35(5 Suppl 2):879. [Google Scholar]
- Liekens S, Smits T, Laekeman G, Foulon V. A depression training session with consumer educators to reduce stigmatizing views and improve pharmacists' depression care attitudes and practices. American Journal of Pharmaceutical Education 2013;77(6):Article 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liekens S, Smits T, Laekeman G, Foulon V. Depression training for pharmacists significantly improves patients' satisfaction, concerns and feelings about side effects regarding antidepressant therapy. International Journal of Clinical Pharmacy 2015;37(2):424. [Google Scholar]
- Liekens S, Vandael E, Roter D, Larson S, Smits T, Laekeman G. Impact of training on pharmacists' counseling of patients starting antidepressant therapy. Patient Education and Counseling 2014;94(1):110‐5. [DOI] [PubMed] [Google Scholar]
- NCT02187380. Studying the impact of medication counselling by community pharmacists in patients starting a treatment with antidepressants. clinicaltrials.gov/show/nct02187380 (first received 11 July 2014).
- Wouters M, Foulon V. Studying the impact of medication counselling for patients starting a treatment with antidepressant drugs. Pharmacy World and Science 2009;31(4):506‐7. [Google Scholar]
Lin 2018 {published data only}
- Lin HW, Lin CH, Chang CK, Chou CY, Yu IW, Lin CC, et al. Economic outcomes of pharmacist‐physician medication therapy management for polypharmacy elderly: a prospective, randomized, controlled trial. Journal of the Formosan Medical Association 2018;117:235‐43. [DOI] [PubMed] [Google Scholar]
- Lin HW, Lin CH, Chang CK, Yu W, Lin CC, Li TC, et al. Economic, clinical and humanistic outcomes of a collaborative pharmacist‐physician medication therapy management service for polypharmacy elderly. Value in Health 2012;15(4):A24. [Google Scholar]
Matzke 2018 {published data only}
- Matzke GR, Moczygemba LR, Williams KJ, Czar MJ, Lee WT. Impact of a pharmacist‐physician collaborative care model on patient outcomes and health services utilization. American Journal of Health‐system Pharmacy 2018;75(14):1039‐47. [DOI] [PubMed] [Google Scholar]
Moride 2013 {published and unpublished data}
- Moride Y, Du Fort GG, Rossignol M, Collet JP, Lelorier J, Lachaine J, et al. A patient‐centered follow‐up program for improving persistence to antidepressant treatment and impact on clinical and patient reported outcomes. Final Report version 1.2 2013.
- Moride Y, Du Fort GG, Tournier M, Alary ME, Rossignol M, Ducruet T, et al. Effectiveness of the persistence telephone assessment and communication program for patients with treated depression: pragmatic randomized controlled trial. Pharmacoepidemiology and Drug Safety 2013;22:113‐4. [DOI: 10.1002/pds.3512] [DOI] [Google Scholar]
NCT00356655 {published data only}
- NCT00356655. The ACHIEVA study of enhanced pharmacist care on antidepressant use and response. clinicaltrials.gov/show/nct00356655 (first received 26 July 2006).
Pyne 2011 {published data only}
- Curran GM, Pyne J, Fortney JC, Gifford A, Asch SM, Rimland D, et al. Development and implementation of collaborative care for depression in HIV clinics. AIDS Care 2011;23(12):1626‐36. [DOI] [PubMed] [Google Scholar]
- NCT00304915. HIV Translating Initiatives for Depression Into Effective Solutions (HI‐TIDES). clinicaltrials.gov/ct2/show/NCT00304915 (first received 17 September 2014).
- Painter JT, Fortney JC, Gifford AL, Rimland D, Monson T, Rodriguez‐Barradas MC, et al. Cost‐effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Journal of Acquired Immune Deficiency Syndromes 2015;70(4):377‐85. [DOI: 10.1097/QAI.0000000000000732] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyne JM, Fortney JC, Curran GM, Tripathi S, Atkinson JH, Kilbourne AM, et al. Effectiveness of collaborative care for depression in human immunodeficiency virus clinics. Archives of Internal Medicine 2011;171(1):23‐31. [DOI: 10.1001/archinternmed.2010.395] [DOI] [PubMed] [Google Scholar]
Shaw 2000 {published data only}
- Shaw H, Mackie CA, Sharkie I. Evaluation of effect of pharmacy discharge planning on medication problems experienced by discharged acute admission mental health patients. International Journal of Pharmacy Practice 2000;8(2):144‐53. [Google Scholar]
Zermansky 2001 {published data only}
- Zermansky AG, Petty DR, Raynor DK, Lowe CJ, Freemantle N, Vail A. Clinical medication review by a pharmacist of patients on repeat prescriptions in general practice: a randomised controlled trial. Health Technology Assessment 2002;6:20. [DOI] [PubMed] [Google Scholar]
- Zermansky AG, Petty DR, Raynor DK, Freemantle N, Vail A, Lowe CJ. Randomised controlled trial of clinical medication review by a pharmacist of elderly patients receiving repeat prescriptions in general practice. BMJ 2001;323:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies awaiting assessment
Harris 2005 {unpublished data only}
- Harris DJ. Pharmaceutical Care and Adherence Support by Community Pharmacists for Older People with Mental Health Problems in the Community: a Randomised Controlled Trial [unpublished PhD]. Nottingham (UK): The University of Nottingham, 2005. [Google Scholar]
References to ongoing studies
ACTRN12618001105235 {unpublished data only}
- ACTRN12618001105235. Enhanced intervention to improve medication adherence in a community pharmacy setting. www.anzctr.org.au/ACTRN12618001105235.aspx (first received 3 July 2018).
ChiCTR‐TRC‐08000726 {published data only}
- ChiCTR‐TRC‐08000726. Impact of an interdisciplinary approach on symptom management of newly diagnosed depressive patients. www.chictr.org.cn/showproj.aspx?proj=8809 (first received 14 October 2008).
ISRCTN11290592 {published data only}
- ISRCTN11290592. Community pharmacies mood intervention study (CHEMIST). www.isrctn.com/ISRCTN11290592 (first received 8 March 2017).
NCT01188135 {published data only}
- NCT01188135. Antidepressant adherence via AD_IVR (AD_IVR). clinicaltrials.gov/ct2/show/NCT01188135 (first received 23 August 2010).
NCT02027259 {published data only}
- NCT02027259. Behavioral activation therapy for both depression and diabetes vs. diabetes alone delivered via group visits. clinicaltrials.gov/show/nct02027259 (first received 12 November 2013).
NCT03591224 {published data only}
- NCT03591224. Pharmacogenomic testing to optimize antidepressant drug therapy. clinicaltrials.gov/ct2/show/NCT03591224 (first received 25 June 2018).
Phung 2013 {published data only}
- Phung J. Reducing hospital readmissions: a pilot study of pharmacists' role on an American Indian reservation. Journal of the American Pharmacists Association 2013;53(2):e41. [Google Scholar]
Additional references
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). 4th Edition. Washington (DC): American Psychiatric Association, 2000. [Google Scholar]
APA 2013
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐V). 5th Edition. Washington (DC): American Psychiatric Association, 2013. [Google Scholar]
Archer 2012
- Archer J, Bower P, Gilbody S, Lovell K, Richards D, Gask L, et al. Collaborative care for depression and anxiety problems. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD006525.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beck 1996
- Beck AT, Steer RA, Ball R, Ranieri WF. Comparison of Beck Depression Inventories‐IA and ‐II in psychiatric outpatients. Journal of Personality Assessment 1996;67(3):588‐97. [DOI] [PubMed] [Google Scholar]
Bell 2005
- Bell S, McLachlan AJ, Aslani P, Whitehead P, Chen TF. Community pharmacy services to optimise the use of medications for mental illness: a systematic review. Australia and New Zealand Health Policy 2005;2(1):29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bower 2006
- Bower P, Gilbody S, Richards D, Fletcher J, Sutton A. Collaborative care for depression in primary care: making sense of a complex intervention: systematic review and meta‐regression. British Journal of Psychiatry 2006;189(6):484‐93. [DOI] [PubMed] [Google Scholar]
Capoccia 2004a
- Capoccia KL, Boudreau DM, Blough DK, Ellsworth AJ, Clark DR, Stevens NG, et al. Randomized trial of pharmacist interventions to improve depression care and outcomes in primary care. American Journal of Health‐System Pharmacy 2004;61(4):364‐72. [DOI] [PubMed] [Google Scholar]
Chisholm 2016
- Chisholm D, Sweeny K, Sheehan P, Rasmussen B, Smit F, Cuijpers P, et al. Scaling‐up treatment of depression and anxiety: a global return on investment analysis. Lancet 2016;3(5):415‐24. [DOI] [PubMed] [Google Scholar]
Chong 2011
- Chong WW, Aslani P, Chen TF. Effectiveness of interventions to improve antidepressant medication adherence: a systematic review. International Journal of Clinical Practice 2011;65(9):954‐75. [DOI] [PubMed] [Google Scholar]
Cipriani 2018
- Cipriani A, Furukawa TA, Salanti G, Chaimani A, Atkinson LZ, Ogawa Y, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta‐analysis. Lancet 2018;391(10128):1357‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Covidence 2018 [Computer program]
- Veritas Health Innovation. Covidence. Version accessed 1 October 2018. Melbourne, Australia: Veritas Health Innovation.
de Barra 2018
- Barra M, Scott CL, Scott NW, Johnston M, Bruin M, Nkansah N, et al. Pharmacist services for non‐hospitalised patients. Cochrane Database of Systematic Reviews 2018, Issue 9. [DOI: 10.1002/14651858.CD013102] [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Eades 2011
- Eades CE, Ferguson JS, O'Carroll RE. Public health in community pharmacy: a systematic review of pharmacist and consumer views. BMC Public Health 2011;11(1):582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ekers 2014a
- Ekers D, Webster L, Straten A, Cuijpers P, Richards D, Gilbody S. Behavioural activation for depression; an update of meta‐analysis of effectiveness and sub group analysis. PloS One 2014;9(6):e100100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrari 2013a
- Ferrari A, Charlson F, Norman R, Patten S, Freedman G, Murray CL, et al. Burden of depressive disorders by country, sex, age, and year: findings from the Global Burden of Disease Study 2010. PLoS Medicine 2013;10.11(e1001547):1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferrari 2013b
- Ferrari AJ, Somerville AJ, Baxter AJ, Norman R, Patten SB, Vos T, et al. Global variation in the prevalence and incidence of major depressive disorder: a systematic review of the epidemiological literature. Psychological Medicine 2013;43(3):471‐81. [DOI] [PubMed] [Google Scholar]
Finley 2003a
- Finley PR, Crismon ML, Rush AJ. Evaluating the impact of pharmacists in mental health: a systematic review. Pharmacotherapy 2003;23(12):1634‐44. [DOI] [PubMed] [Google Scholar]
Gilbody 2003
- Gilbody S, Whitty P, Grimshaw J, Thomas R. Educational and organizational interventions to improve the management of depression in primary care: a systematic review. JAMA 2003;289(23):3145‐51. [DOI] [PubMed] [Google Scholar]
Gilbody 2017
- Gilbody S, Lewis H, Adamson J, Atherton K, Bailey D, Birstwistle J, et al. Effect of collaborative care vs. usual care on depressive symptoms in older adults with subthreshold depression: the CASPER randomised controlled trial. JAMA 2017;317:728‐37. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- McMaster University (developed by Evidence Prime). GRADEpro GDT. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015.
Gunn 2006
- Gunn J, Diggens J, Hegarty K, Blashki G. A systematic review of complex system interventions designed to increase recovery from depression in primary care. BMC Health Services Research 2006;6(1):88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hanlon 2004
- Hanlon JT, Lindblad CI, Gray SL. Can clinical pharmacy services have a positive impact on drug‐related problems and health outcomes in community‐based older adults?. American Journal of Geriatric Pharmacotherapy 2004;2(1):3‐13. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JP, Altman DG, Sterne JC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JP, Deeks JJ, Altman DG. Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2019a
- Higgins JP, Eldridge S, Li T. Chapter 23: Including variants on randomized trials. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Higgins 2019b
- Higgins JP, Li T, Deeks JJ. Chapter 6: Choosing effect measures and computing estimates of effect. In: Higgins JP, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al, editor(s). Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). The Cochrane Collaboration, 2019. Available from www.training.cochrane.org/handbook.
Holland 2008
- Holland R, Desborough J, Goodyer L, Hall S, Wright D, Loke YK. Does pharmacist‐led medication review help to reduce hospital admissions and deaths in older people? A systematic review and meta‐analysis. British Journal of Clinical Pharmacology 2008;65(3):303‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kroenke 2001
- Kroenke K, Spitzer RL, Williams JB. The PHQ‐9: validity of a brief depression severity measure. Journal of General Internal Medicine 2001;16(9):606‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Latif 2018
- Latif A, Waring J, Watmough D, Boyd MJ, Elliott RA. 'I expected just to walk in, get my tablets and then walk out': on framing new community pharmacy services in the English healthcare system. Sociology of Health & Illness 2018;40(6):1019‐36. [DOI] [PubMed] [Google Scholar]
Lindsey 2016
- Lindsey L, Husband A, Steed L, Walton R, Todd A. Helpful advice and hidden expertise: pharmacy users' experiences of community pharmacy accessibility. Journal of Public Health 2016;39(3):609‐15. [DOI] [PubMed] [Google Scholar]
Marquez 2016
- Marquez PV, Saxena S. Making mental health a global priority. Cerebrum 2016;Jul‐Aug:10‐6. [PMC free article] [PubMed] [Google Scholar]
NICE 2018
- National Institute for Health and Care Excellence. Antidepressant treatment in adults. pathways.nice.org.uk/pathways/depression (accessed prior to 8 March 2019).
Nkansah 2010
- Nkansah N, Mostovetsky O, Yu C, Chheng T, Beney J, Bond CM, et al. Effect of outpatient pharmacists' non‐dispensing roles on patient outcomes and prescribing patterns. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD000336.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Noyes 2011
- Noyes J, Popay J, Pearson A, Hannes K, Booth A. Chapter 20: Qualitative research and Cochrane Reviews. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Otte 2016
- Otte C, Gold SM, Penninx BW, Pariante CM, Etkin A, Fava M, et al. Major depressive disorder. Nature Reviews Disease Primers 2016;2:16065. [DOI] [PubMed] [Google Scholar]
Petticrew 2011
- Petticrew M. When are complex interventions 'complex'? When are simple interventions 'simple'?. European Journal of Public Health 2011;21(4):397‐9. [DOI] [PubMed] [Google Scholar]
Readdean 2018
- Readdean KC, Heuer AJ, Parrott JS. Effect of pharmacist intervention on improving antidepressant medication adherence and depression symptomology: a systematic review and meta‐analysis. Research in Social and Administrative Pharmacy 2018;14:321‐31. [DOI] [PubMed] [Google Scholar]
Review Manager 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
RevMan Web 2019 [Computer program]
- The Cochrane Collaboration. Review Manager Web (RevMan Web). The Cochrane Collaboration, 2019.
Rodgers 2016
- Rodgers RM, Gammie SM, Loo RL, Corlett SA, Krska J. Comparison of pharmacist and public views and experiences of community pharmacy medicines‐related services in England. Patient Preference and Adherence 2016;10:1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
Royal 2006
- Royal S, Smeaton L, Avery AJ, Hurwitz B, Sheikh A. Interventions in primary care to reduce medication related adverse events and hospital admissions: systematic review and meta‐analysis. BMJ Quality & Safety 2006;1(15):23‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Royal Pharmaceutical Society 2018
- Royal Pharmaceutical Society. No health without mental health: how can pharmacy support people with mental health problems?. www.rpharms.com/Portals/0/Documents/RPS%20mental%20health%20roundtable%20report%20June%202018_FINAL.pdf?ver=2018‐06‐04‐100634‐577 (accessed prior to 8 March 2019).
Rubio‐Valera 2014
- Rubio‐Valera M, Chen T, O'Reilly C. New roles for pharmacists in community mental health care: a narrative review. International Journal of Environmental Research and Public Health 2014;11(10):10967‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 2013
- Smith J, Picton C, Dayan M. Now or never: shaping pharmacy for the future. The Report of the Commission on future models of care delivered through pharmacy November 2013. www.rpharms.com/Portals/0/RPS%20document%20library/Open%20access/Publications/Now%20or%20Never%20‐%20Report.pdf (accessed prior to 17 December 2019).
Sterne 2011
- Sterne JA, Egger M, Moher D. Chapter 10: Addressing reporting biases. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Taylor 2015
- Taylor D, Paton C, Kapur S. The Maudsley Prescribing Guidelines in Psychiatry. 12th Edition. Chichester (UK): John Wiley & Sons, Inc, 2015. [Google Scholar]
Thomson 2019
- Thomson K, Hillier‐Brown F, Walton N, Bilaje M, Bambra C, Todd A. The effects of community pharmacy‐delivered public health interventions on population health and health inequalities: a review of reviews. Preventive Medicine 2019;124:98‐109. [DOI] [PubMed] [Google Scholar]
Thornicroft 2017
- Thornicroft G, Chatterji S, Evans‐Lacko S, Gruber M, Sampson N, Aguilar‐Gaxiola S, et al. Undertreatment of people with major depressive disorder in 21 countries. British Journal of Psychiatry 2017;210(2):119‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Todd 2014
- Todd A, Copeland A, Husband A, Kasim A, Bambra C. The positive pharmacy care law: an area‐level analysis of the relationship between community pharmacy distribution, urbanity and social deprivation in England. BMJ Open 2014;1;4(8):e005764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Von Korff 2001
- Korff M, Goldberg D. Improving outcomes in depression: the whole process of care needs to be enhanced. BMJ 2001;323(7913):948‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vos 2015
- Vos T, Barber RM, Bell B, Bertozzi‐Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990‐2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386(9995):743‐800. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whiteford 2013
- Whiteford HA, Degenhardt L, Rehm J, Baxter AJ, Ferrari AJ, Erskine HE, et al. Global burden of disease attributable to mental and substance use disorders: findings from the Global Burden of Disease Study 2010. Lancet 2013;382(9904):1575‐86. [DOI] [PubMed] [Google Scholar]
WHO 1992
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Clinical Descriptions and Diagnostic Guidelines. Geneva: World Health Organization, 1992. [Google Scholar]
WHO 2018
- World Health Organization. Depression. www.who.int/news‐room/fact‐sheets/detail/depression (accessed prior to 8 March 2019).
Yaghoubi 2017
- Yaghoubi M, Mansell K, Vatanparastc H, Steeves M, Zeng W, Farag M. Effects of pharmacy‐based interventions on the control and management of diabetes in adults: a systematic review and meta‐analysis. Canadian Journal of Diabetes 2017;41(6):628‐41. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ekers 2014b
- Ekers D, Todd A, McMillan D, Webster L, Steele R, Husband A, et al. Clinical effectiveness of pharmacist management of depression in adults. PROSPERO 2014; Vol. CRD42014013517, issue www.crd.york.ac.uk/PROSPERO/display_record.php?ID=CRD42014013517.
Sampson 2019
- Sampson SJ, Todd A, Walton N, Steele R, Webster L, Churchill R, et al. Pharmacy‐based management for depression in adults. Cochrane Database of Systematic Reviews 2019, Issue 4. [DOI: 10.1002/14651858.CD013299] [DOI] [PMC free article] [PubMed] [Google Scholar]


