Abstract
BACKGROUND
Advanced age, frailty, low education level, and impaired cognition are generally reported to be associated with postoperative cognitive complications. To translate research findings into hospital-wide preoperative assessment clinical practice, we examined the feasibility of implementing a preoperative frailty and cognitive assessment for all older adults electing surgical procedures in a tertiary medical center. We examined associations among age, education, frailty, and comorbidity with the clock and 3-word memory scores, estimated the prevalence of mild to major cognitive impairment in the presurgical sample, and examined factors related to hospital length of stay.
METHODS
Medical staff screened adults ≥65 years of age for frailty, general cognition (via the clock-drawing test command and copy, 3-word memory test), and obtained years of education. Feasibility was studied in 2 phases: (1) a pilot phase involving 4 advanced nurse practitioners and (2) a 2-month implementation phase involving all preoperative staff. We tracked sources of missing data, investigated associations of study variables with measures of cognition, and used 2 approaches to estimate the likelihood of dementia in our sample (ie, using extant data and logistic regression modeling and using Mini-Cog cut scores). We explored which protocol variables related to hospital length of stay.
RESULTS
The final implementation phase sample included 678 patients. Clock and 3-word memory scores were significantly associated with age, frailty, and education. Education, clock scores, and 3-word scores were not significantly different by surgery type. Likelihood of preoperative cognitive impairment was approximately 20%, with no difference by surgery type. Length of stay was significantly associated with preoperative comorbidity and performance on the clock copy condition.
CONCLUSIONS
Frailty and cognitive screening protocols are feasible and provide information for perioperative care planning. Challenges to clinical adaptation include staff training, missing data, and additional administration time. These challenges appear minimal relative to the benefits of identifying frailty and cognitive impairment in a group at risk for negative postoperative cognitive outcome.
Research repeatedly shows that predictors of delirium, cognitive decline, and mortality after surgical procedures with anesthesia include (1) frailty,1 (2) fewer years of formal education,2,3 and (3) reduced preoperative cognitive abilities,1 particularly in the domains of memory and executive function.1,4,5 These findings are concerning due to the rate of frailty and cognitive impairment in our communities and preoperative anesthesia settings.6 Frailty syndrome, as defined as an increased vulnerability or an age-related decline in physiological reserve across multiple physiological systems, has been reported to be present in 9.2% of adults ≥60 years of age.7 Cognitive impairment is also present in ≥18% of community-dwelling older adults,8 and the rate of impairment can be higher in preoperative settings.9 These rates are concerning given that low- and high-risk surgical procedures are performed annually on more than half a million patients ≥65 years of age.10
For these reasons, researchers encourage frailty and cognitive screening measures in preoperative anesthesia or primary care settings.11 While frailty assessment has become more commonplace within presurgical settings,12 cognitive screening or even routine recording of years of education rarely occurs. Researchers are addressing the viability of cognitive screening in preoperative settings; however, research by Culley et al9 helped this initiative by showing that screening tools can be implemented in a preoperative anesthesia setting and identified impairment in 23% of people enrolled.9 They further demonstrated that screening could predict adverse events after orthopedic surgery.13 Feasibility for hospital-wide presurgical center implementation has yet to be shown. It is also unknown how different surgical patient groups (eg, orthopedic, cardiac, and abdominal) differ on the frailty–cognitive metrics and frequency of dementia.
Toward the overall goal of bridging research to practice, the current investigation examined the feasibility of implementing a combined frailty and cognitive screening protocol to all adults ≥65 years of age entering a tertiary preoperative anesthesia clinic. We had 5 aims: (1) to examine the frequency of missing data from the protocol; (2) to examine associations among age, education, frailty, and comorbidity with the clock and 3-word memory scores; (3) to provide summary statistics of demographic and protocol scores for the entire surgery sample and by surgery type (abdominal, orthopedic, gastrointestinal, neurosurgery, cardiothoracic, and other); (4) to assess likelihood of mild to major cognitive impairment within the preoperative sample, overall, and by surgery type; and (5) to explore the association of the preoperative cognitive screening measures with length of stay.
METHODS
Design and Setting
The University of Florida Institutional Review Board-01 approved this investigation, and the requirement for a written informed consent was waived by the Institutional Review Board. Electronic health records data were acquired through an institutional review board–approved process involving deidentified data provided via an honest broker. This report is based on our implementation of a Frailty–Cognitive Screener for patients ≥65 years of age in 2 phases: (1) a pilot phase involving 4 of 10 nurse practitioners working in the preoperative clinic and (2) a final implementation phase involving all nurse practitioners, nurses, and rotating residents and physicians within the University of Florida Preoperative Anesthesia Clinic. We conducted the pilot phase (August 24 to December 3, 2015) to identify pitfalls that would hinder a more comprehensive implementation phase with all preoperative anesthesia medical staff members. The implementation phase occurred between May 2 and July 1, 2016. Data were prospectively collected during both the pilot and implementation phases of the study. Patients are triaged for an in-person visit within the University of Florida Preoperative Anesthesia Clinic using the Patient-Centered Anesthesia Triage System criteria as defined by Enneking et al.14 Surgical specialties frequently using the Preoperative Anesthesia Clinic include abdominal surgery (included urology, pancreas and biliary surgery, colorectal surgery, and transplant surgeries), cardiothoracic surgeries (vascular and thoracic/cardiovascular surgeries), orthopedic surgeries (elective joint surgeries), gastroenterology (colonoscopies and sigmoidoscopies), neurological (laminectomies, craniectomies, deep brain stimulation, etc), and other (cataract removal, breast surgery, otolaryngology, and plastic reconstructive surgeries). Medical staff members did not complete the cognitive screening protocol with patients who were non-English speaking unless a certified interpreter was available during the testing or if they had marked visual or auditory deficits precluding testing.
Training and Monitoring
Licensed neuropsychologists and doctoral-level neuropsychology students trained preoperative anesthesia medical staff, including resident and attending physicians, advanced registered nurse practitioners, and registered nurses on administration of the Preoperative Frailty–Cognitive Protocol, with particular attention paid to reliable clock drawing, 3-word memory administration, and years of education. Training occurred in 2 segments. Group-based education involved separate team meetings to explain the necessity for reliability and accuracy. Individual trainings involved having a trained instructor administering the protocol to each staff member and then requiring the staff members to administer the protocol to another team member with the instructor present. Each day, a neuropsychology instructor visited each team member to assess protocols for administration problems, train new staff members (rotating residents), and troubleshoot questions.
Preoperative Cognitive Screener Measures
See Supplemental Digital Content 1, Document 1, http://links.lww.com/AA/C797, and Supplemental Digital Content 2, Document 2, http://links.lww.com/AA/C798, for protocol instructions. In addition to these measures, we acquired information on demographics (age, sex, and race) and comorbidity via the Charlson comorbidity index.15
Each preoperative testing room contained a binder with a packet of materials for reference and administration, as well as a hand dynamometer for frailty assessment. Packets included instructions for frailty assessment, how to record education, clock-drawing test administration, and 3-word recall. The clock drawing to command was administered followed by the copy test condition. Each staff member placed a patient identifying sticker on the paper containing patient name, medical record number, date of birth, age, sex, appointment encounter number, and admission date. Completed protocols, including the digital clock-drawing data, were transferred into the patient’s electronic health record, with electronic flags communicated ahead of time to the institutional review board honest data broker to enable record retrievals according to institutional review board approval.
Frailty Index
Frailty is a clinical syndrome, which is an increased vulnerability or age-related decline in physiological reserve across different systems. Frailty includes weakness, low energy, slowed walking speed, decreased physical activity, and weight loss. Higher frailty is associated with delirium and increased mortality rates.16 In this study, frailty was defined using the criteria from Fried et al.17 A patient qualified as frail if he/she exhibited/reported ≥3 of the following: (1) unintended weight loss of ≥10 pounds within the last 6 months; (2) subjective exhaustion, defined as endorsing moderate feelings that everything he/she did was an effort over the last week or moderate feelings that he/she could not “get going” in the last week; (3) slow walking speed (15 feet in ≥7 seconds for men below 68 inches and women below 63 inches; 15 feet in >6 seconds in men above 68 inches and women above 63 inches); (4) grip strength below a normative cutoff defined by the Geriatrics Evaluation and Management Tools18; and (5) low physical activity as defined by the Dukes Activity Status Index.19 The final outcome variable was scored of 0–5, where 0–1 = no frailty, 2–3 = prefrailty, and 4–5 = frail.
Education
Years of formal education is a predictor of postoperative cognitive outcome2,3 and is easily acquired via questionnaire. It is an adequate measure of premorbid intellect and cognitive reserve, which proposes that higher lifetime cognitive stimulation is protective against neurological insult.20,21 Administrators recorded education as the number of years of formal education completed. Skipped years counted toward the total number of years. Repeated years did not add additional years. If the patient dropped out of high school, staff recorded how many full years of school the patient completed. For example, a high school graduate counted as 12 years; a bachelor’s degree counted as 16 years, and so forth.
General Cognition
In contrast to education, general cognition represents “current” cognitive function. Here, we assessed general cognition with the clock-drawing test using procedures established and researched extensively by Kaplan22 and others.23,24 For the command condition, patients are told to “draw the face of a clock, put in all of the numbers, and set the hands for 10 after 11.” This is immediately followed by the copy condition in which patients were instructed to copy a model of a clock provided on a sheet of paper. The command condition requires language, memory, visuospatial, inhibition, and planning and associates highly with other general cognitive measures, such as the Mini-Mental State Examination.25 The copy condition, by contrast, provides information on frontal function and visual planning abilities; patients with copy errors show compromised attention and disinhibition on neuropsychological assessments and large-scale cortical–subcortical neural network disruption.23,24,26 Comparisons between command and copy test conditions provide clinical information about cognitive strengths and weaknesses and can assist with diagnostics.22–24,26
For the current report, we report on 2 separate clock-drawing score procedures, so that data are applicable to teams using either scoring scheme. First, we scored clocks with criteria suggested by the Montreal Cognitive Assessment,27 where scores range from 0 to 3 (3 = best) with 1 point each assigned for: (1) “contour”: the clock face is complete with only minor distortions (eg, the circle is only slightly elongated or there is a small imperfection on closing the circle); (2) “numbers”: all numbers are present in the correct clockwise sequence, located in their correct quadrant, and not repeated; and (3) “hands”: both hands are set to the correct time, the hour hand is distinctively smaller than the minute hand, and both hands join together near the center of the clock face. Second, we scored each clock using Mini-Cog criteria,28 with the goal of comparing our rate of impairment to other published reports of preoperative impairment.9 With the Mini-Cog, clock drawing is scored as normal (2 points) or abnormal (0 points) based on hand placement and accurate placement of numbers within 4 quadrants. The clock face was not included in our scoring because Mini-Cog test administration provides the patient with a clock face template. All clocks were scored by the same rater with excellent intrarater reliability and checked using an electronic scoring algorithm.29
Memory
Memory is one of the key cognitive domains to change postoperatively,30 and even a 3-word memory assessment is associated with delirium.5 We assessed recall for new information with 3-word recall from the Mini-Mental State Examination.25 Before administration of the clock-drawing test, patients were instructed to repeat 3 words (“apple, table, penny”). After completing clock drawing (approximately a 2- to 3-minute delay), the patient was instructed to recall the 3 words. The outcome variable was the total number of words recalled.
Statistical Analysis
Data were included in the final analyses if the administrator had appropriately labeled the data collection form and had correctly administered the clock-drawing test. Patients in the feasibility and pilot phases were analyzed separately. The Spearman rank correlation coefficient was calculated to examine relationships among numerical and ordinal variables. Surgical subtypes were compared using Kruskal–Wallis tests for numerical or ordinal data and χ2 tests for categorical data.
Likelihood of Cognitive Impairment
We estimated the incidence of cognitive impairment in the current preoperative sample using 2 different approaches. In the first approach, we combined 2 existing data sets to form a convenience sample with data from 402 individuals (210 individuals with documented mild or major neurocognitive disorder [dementia] through a memory disorder center that included a neuropsychologist, geriatrician, and neurologist, and 192 nondemented older adults without indicators of mild cognitive impairment or dementia based on an interview and a comprehensive neuropsychological protocol). Using the convenience sample data, we performed a multiple logistic regression where the outcome variable of impairment (yes or no) was modeled by the explanatory variables of age, sex, years of education, and clock-drawing Montreal Cognitive Assessment command score. Odds ratios and corresponding 95% CIs were computed for each variable in the logistic regression model. The area under the curve for the logistic regression model was 0.94. From the logistic regression model, regression coefficients for impairment likelihood were estimated. We then estimated the probability of impairment in the preoperative sample by applying the logistic regression coefficients to the patients with complete age, sex, years of education, and clock-drawing Montreal Cognitive Assessment command score data. In the second approach, we identified the likelihood of cognitive impairment in the preoperative sample via a frequency count of patient clocks with a Mini-Cog score of ≤2.28
The distribution of the length of hospital stay (in hours) variable was highly skewed. This variable was log-transformed and used as the outcome variable in a multivariable linear regression model to explore the predictive value of the preoperative cognitive screening measures on length of stay. The 2 variables, which were found to be significantly correlated with length of stay, comorbidity, and the clock-drawing Montreal Cognitive Assessment copy score, were included in the regression analysis as predictors. All data were analyzed using SAS 9.4 (SAS Institute, Cary, NC). Missing data were handled using listwise deletion. The level of significance was set at .05; all testing was 2-sided.
RESULTS
Aim 1: Missing Data
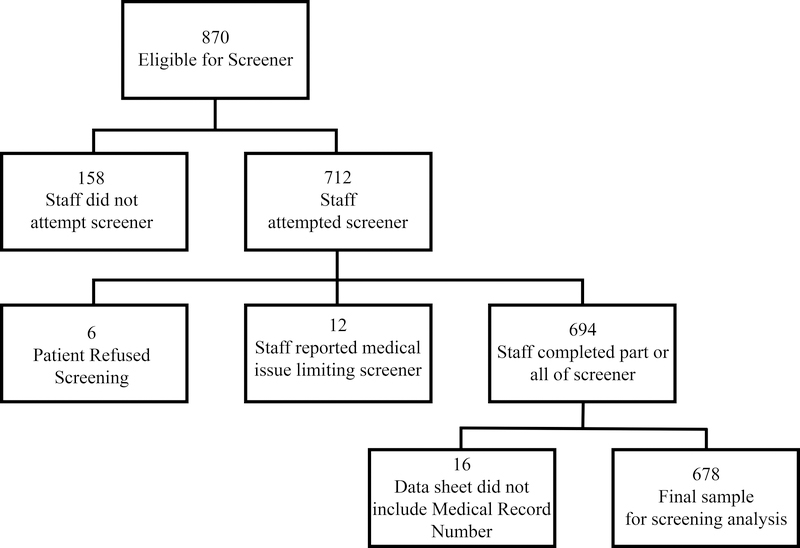
See the Figure and Table 1 to understand missing data and pathway to our final sample. The pilot phase included 276 individuals. The implementation phase was based on a total of 870 adult patients ≥65 years of age who were eligible for screening within the University of Florida Preoperative Anesthesia Clinic (May 2 to July 1, 2016). During the implementation phase, the medical staff approached 712 (82%) of the 870 individuals who qualified for the Frailty–Cognitive Screener. We excluded 18 (2.5%) of these potential participants due to either a medical condition preventing their completion of the screener or refusal to complete the screener. Staff administered the full or partial protocol to 694 patients (97.5%). Of these patients, 16 protocols (2.3%) were missing a label necessary for acquiring demographic and surgery information. Our final implementation phase sample included 678 patients (95%).
Figure 1.
Path to final sample.
Table 1.
Missing Data From the Final Data Set of 678 Patients
| Variable | Missing, n (%) |
|---|---|
| Education | 54 (7.96) |
| Race | 55 (8.11) |
| Charlson comorbiditya | 92 (13.60) |
| Frailtyb | 61 (9.00) |
| 3-word memoryc | 29 (4.28) |
| Surgery type | 92 (13.60) |
| Clock commandd | 0 (0) |
| Clock copye | 0 (0) |
Charlson comorbidity index (range, 0–30; 30 = worse).15
Frailty = frailty categorical cut scores and total score Fried et al.17
3-word = 3-word memory delay number of words recalled, words from Folstein et al.25
Clock command = clock drawing to command condition scored with Montreal Cognitive Assessment clock criteria.27
Clock copy = clock drawing to copy condition scored with Montreal Cognitive Assessment clock criteria.24
Aim 2: Associations Among Age, Education, Frailty, and Comorbidity With the Clock and 3-Word Memory Score
See Table 2 for the relationship among demographics, frailty, and cognitive metrics. Based on the bivariate analyses, the clock command, clock copy, 3-word score, and a derived composite for the Mini-Cog were significantly associated with age, frailty, and education. As expected, age was negatively associated with all performance measures. Education was positively associated with all performance measures; higher cognitive reserve (as indicated by more years of education) was associated with higher performance on cognitive metrics. We also found that frailty was negatively associated with cognitive metrics such that higher frailty was associated with lower cognitive performance. We did not find associations between performance measures and comorbidity.
Table 2.
Spearman Correlation Coefficients and P Value Among Demographics, Frailty, and Cognitive Metrics
| Clock Commanda | Clock Copyb | 3-Word Memoryc | Mini-Cog Derived Totald | |
|---|---|---|---|---|
| Age | −0.20 | −0.20 | −0.13 | −0.23 |
| <.0001 | <.0001 | .0008 | <.0001 | |
| Education | 0.16 | 0.15 | 0.17 | 0.14 |
| <.0001 | .0003 | <.0001 | .0004 | |
| Charlsone comorbidity | 0.01 | 0.03 | −0.01 | 0.00 |
| .76 | .5034 | .8645 | .9464 | |
| Frailtyf | −0.18 | −0.15 | −0.17 | −0.24 |
| <.0001 | .0002 | <.0001 | <.0001 |
Clock command = clock drawing to command condition scored with Montreal Cognitive Assessment clock criteria.27
Clock copy = clock drawing to copy condition scored with Montreal Cognitive Assessment clock criteria.27
3-word = 3-word memory delay number of words recalled, words from Folstein et al.25
Mini-Cog–derived score = derived Mini-Cog score from command condition clock scored to Mini-Cog criteria and 3-word memory.28
Charlson comorbidity index (range, 0–30; 30 = worse).27
Frailty = frailty categorical cut scores and total score Fried et al.17
Aim 3: Summary Statistics of Demographics and Protocol Scores for Surgery Sample
Age was significantly different between surgery types (P = .0157), with cardiothoracic surgery the oldest group (mean age, 75.01 ± 7.05 years) and orthopedic the youngest group (72.17 ± 5.75 years) (Table 3). Sex differed significantly between surgery types (P = .0275), with more females in the orthopedic group and males in the cardiothoracic group. Charlson comorbidity index differed significantly between surgical types (P < .001), with cardiology having the highest comorbidity (2.04 ± 2.51) and gastroenterology having the lowest comorbidity (0.04 ± 0.38). Frailty total score was significantly different by surgery type (P = .0438), with cardiothoracic surgery scoring highest (2.03 ± 1.43) and abdominal (1.43 ± 1.45) the lowest. Race, education, clock-drawing test scores, and 3-word scores were not significantly different by surgery type.
Table 3.
Mean and SDs for Preoperative Demographics, Comorbidity, and Cognitive Characteristics for Pilot and Implementation Phases for All Patients and by Surgery Procedure
| Full Implementation, n = 678a |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Pilot (n = 276) Mean ± SD or n (%) | All Mean ± SD or n (%) N = 678 | Abdominal Mean ± SD or n (%) N = 104 | Cardiothoracic Mean ± SD or n (%) N = 112 | Orthopedic Mean ± SD or n (%) N = 123 | Gastroenterology Mean ± SD or n (%) N = 108 | Neurosurgery Mean ± SD or n (%) N = 51 | Other Mean ± SD or n (%) N = 88 | P Value | |
| Age (y) | 73.72 ± 6.20 | 73.51 ± 6.19 | 73.86 ± 6.73 | 75.01 ± 7.05 | 72.17 ± 5.75 | 72.49 ± 5.33 | 74.27 ± 5.42 | 73.24 ± 5.67 | .0157a |
| Sex | .0275a | ||||||||
| Female | 149 (54) | 324 (48) | 47 (45) | 40 (36) | 66 (54) | 52 (48) | 22 (43) | 51 (58) | |
| Male | 127 (46) | 354 (52) | 57 (55) | 72 (64) | 57 (46) | 56 (52) | 29 (57) | 37 (42) | |
| Race | .2467 | ||||||||
| Caucasian | 240 (90) | 545 (88) | 89 (86) | 101 (90) | 96 (85) | 93 (89) | 51 (100) | 76 (88) | |
| African American | 16 (6) | 52 (8) | 9 (9) | 11 (10) | 9 (8) | 10 (10) | 0 (0) | 4 (5) | |
| Other | 12 (4) | 26 (4) | 6 (6) | 0 (0) | 8 (7) | 2 (2) | 0 (0) | 7 (8) | |
| Charlson comorbidityb | 1.04 ± 1.96 | 1.13 ± 2.03 | 1.38 ± 2.03 | 2.04 ± 2.51 | 1.24 ± 2.01 | 0.04 ± 0.38 | 0.40 ± 0.83 | 1.30 ± 2.37 | <.0001a |
| Education (y) | 13.44 ± 2.61 | 13.82 ± 2.94 | 14.43 ± 2.73 | 13.96 ± 2.96 | 14.40 ± 2.75 | 13.51 ± 2.69 | 14.04 ± 2.44 | 13.59 ± 3.04 | .0981 |
| Frailtyc | .0438a | ||||||||
| Not frail | 61 (32) | 167 (26) | 33 (34) | 20 (21) | 30 (26) | 32 (31) | 14 (30) | 22 (25) | |
| Prefrail | 75 (40) | 274 (43) | 44 (45) | 38 (41) | 60 (50) | 44 (43) | 18 (40) | 39 (48) | |
| Frail | 54 (28) | 198 (31) | 21 (21) | 35 (38) | 29 (24) | 27 (26) | 14 (30) | 22 (25) | |
| Total | 1.49 ± 1.44 | 1.72 ± 1.47 | 1.43 ± 1.45 | 2.03 ± 1.43 | 1.53 ± 1.22 | 1.61 ± 1.54 | 1.81 ± 1.51 | 1.64 ± 1.43 | |
| 3 wordd | .9986 | ||||||||
| 0 | 11 (4) | 22 (3) | 2 (2) | 5 (5) | 3 (2) | 3 (3) | 1 (2) | 3 (3) | |
| 1 | 19 (7) | 65 (10) | 12 (12) | 9 (8) | 14 (12) | 11 (10) | 5 (10) | 8 (10) | |
| 2 | 66 (25) | 130 (20) | 20 (19) | 24 (22) | 23 (19) | 20 (19) | 10 (20) | 17 (19) | |
| 3 | 167 (64) | 432 (67) | 70 (67) | 72 (65) | 81 (67) | 73 (68) | 34 (68) | 60 (67) | |
| Clock commande | .4970 | ||||||||
| 0 | 20 (7) | 37 (5) | 3 (3) | 5 (5) | 7 (6) | 5 (5) | 3 (6) | 4 (5) | |
| 1 | 68 (25) | 155 (23) | 23 (22) | 30 (27) | 19 (15) | 28 (26) | 12 (24) | 18 (20) | |
| 2 | 114 (41) | 284 (42) | 41 (39) | 44 (39) | 55 (45) | 47 (44) | 21 (41) | 39 (44) | |
| 3 | 74 (27) | 202 (30) | 37 (36) | 33 (29) | 42 (34) | 28 (26) | 15 (29) | 27 (31) | |
| copyf | .1656 | ||||||||
| 0 | 8 (3) | 19 (3) | 0 (0) | 4 (4) | 1 (1) | 6 (6) | 1 (2) | 1 (1) | |
| 1 | 36 (13) | 77 (11) | 8 (8) | 15 (13) | 17 (14) | 9 (8) | 4 (8) | 10 (11) | |
| 2 | 116 (42) | 263 (39) | 40 (38) | 46 (41) | 40 (32) | 47 (43) | 22 (43) | 28 (32) | |
| 3 | 116 (42) | 318 (47) | 56 (54) | 47 (42) | 65 (53) | 346 (43) | 24 (47) | 49 (56) | |
| Mini-Cog clockg | .3719 | ||||||||
| Fail (0) | N/A | 297 (44) | 43 (41) | 55 (49) | 45 (37) | 52 (48) | 20 (39) | 39 (45) | |
| Pass (2) | N/A | 381 (56) | 61 (59) | 57 (51) | 78 (63) | 56 (52) | 31 (61) | 49 (55) | |
| Mini-Cog–derived totalg | N/A | 1.42 (3.61) | 1.41 (3.69) | 1.39 (3.50) | 1.32 (3.76) | 1.35 (3.78) | 1.48 (3.57) | 1.44 (3.64) | .7155 |
| Likelihood cognitive impairmenth | N/A | 127 (20) | 19 (18) | 23 (21) | 16 (13) | 24 (22) | 9 (18) | 17 (20) | .7123 |
P values represent comparison between surgery types.
Missing data occurred within the implementation protocol domains. See Table 4 for missing data frequency (eg, we do not know surgery type for 92 of the 678 individuals). See Supplemental Digital Content 2, Document 2, http://links.lww.com/AA/C798; See Table 2 for previous variable legend.
Charlson comorbidity index (range, 0–30; 30 = worse).15
Frailty = frailty categorical cut scores and total score Fried et al.17
3-word = 3-word memory delay number of words recalled, words from Folstein et al.25
Clock command = clock drawing to command condition scored with Montreal Cognitive Assessment clock criteria.27
fClock copy = clock drawing to copy condition scored with Montreal Cognitive Assessment clock criteria.24
Mini-Cog–derived score = derived Mini-Cog score from command condition clock scored to Mini-Cog criteria and 3-word memory.
Likelihood cognitive impairment = likelihood of impairment based on Mini-Cog cut score of ≤2.
Aim 4: Likelihood of Cognitive Impairment in the Full Sample and by Surgery Type
We used 2 separate approaches to assess probability of cognitive impairment (Table 4). First, from the external dementia comparison group, we identified that higher age, lower Montreal Cognitive Assessment command clock score, and lower years of education were associated with a higher likelihood of cognitive impairment. We applied these parameter estimates from the logistic regression to predict the probability of cognitive impairment in the 678 patients who completed at least clock drawing within the full hospital implementation phase. From these estimates, we identified that 19% of the preoperative patients had >90% chance of meeting criteria for minor or major neurocognitive disorder. Second, we applied the Mini-Cog cut scores on the command condition clocks to assess the likelihood of impairment and identified 20% of the total sample met the Mini-Cog cut score. There was no statistical difference in prevalence of cognitive impairment by surgery type.
Table 4.
Logistic Regression Parameters Used to Assess Likelihood of Cognitive Impairment in the Preoperative Patients Using an External Convenience Sample
| Effect | Logistic Regression Model Parameter | Odds Ratio (95% CI) | P |
|---|---|---|---|
| Intercept | −10.42 | … | … |
| Age | 0.26 | 1.29 (1.22–1.37) | <.0001a |
| Sex: female versus male | 0.14 | 1.32 (0.68–2.60) | .4078 |
| Education years | −0.49 | 0.61 (0.54–0.71) | <.0001a |
| Clock commandb | −0.94 | 0.39 (0.25–0.60) | <.0001a |
Indicates significance.
Clock command = clock drawing to command condition scored with Montreal Cognitive Assessment clock criteria.27
Aim 5: Predictive Value of the Preoperative Cognitive Screening Measures on Hospital Length of Stay
Analyses included the subsample with inpatient hospital stays (n = 338) (Table 5). Based on the bivariate analyses, length of stay was significantly associated with preoperative comorbidity and preoperative clock copy scores. There were no significant associations found for education, frailty, clock command, and 3-word recall. When preoperative comorbidity and preoperative clock copy score were included in the multivariable linear regression model, they were both independently associated with length of stay (P =.0006 and .0338, respectively).
Table 5.
Correlation Coefficients (P Value) Among Age, Education, Frailty, Comorbidity, Clock Drawing, 3-Word Memory, and Length of Stay in Hours (n = 338)a
| Variable | Length of Stay |
|---|---|
| Age | 0.11 (0.0523) |
| Education | −0.09 (0.1143) |
| Charlson comorbidity | 0.22 (<0.0001) |
| Frailty | 0.09 (0.1051) |
| Clock command Montreal Cognitive Assessment | −0.08 (0.1369) |
| Clock copy Montreal Cognitive Assessment | −0.12 (0.0230) |
| Recall | 0.06 (0.2671) |
| Mini-Cog command clock | −0.10 (0.0675) |
Sample size restricted to inpatient hospital stays.
DISCUSSION
This study expands on research addressing frailty and cognition for older adults electing surgery with anesthesia. We have 4 important findings. First, it was feasible to implement preoperative frailty and cognitive screening measures. Staff members with no previous training in cognitive assessments (comprised of general clinical nursing and physician staff) successfully administered the screening protocol to 80% of eligible patients over a 2-month period. Second, although we had a meaningful negative association between frailty and the cognitive screeners across all surgery types, we found that our surgery type categories significantly differed in frailty scores but not cognitive/memory scores. These results fit the consensus that frailty is a complex interplay of physical, psychological, and other factors, but also that cognitive impairment and dementia pathology can occur in individuals with little to no frailty.31 For these reasons, it appears crucial to consider both physical and cognitive screening for preoperative planning and risk detection. Third, our 2 separate analytical approaches for calculating cognitive impairment showed that 19%–20% of the preoperative patients had high likelihood of cognitive impairment regardless of planned surgical procedure. If we apply these data toward future screenings in other tertiary centers, then preoperative anesthesia staff can expect 1 out of every 5 presurgery patients to produce profiles indicative of significant cognitive impairment regardless of patients’ planned surgical procedure. Fourth, although our evaluation of postsurgery outcome was limited to length of hospital stay, we confirm preoperative comorbidity severity as a relevant risk factor15 and show that clock drawing to copy as a viable tool for risk prediction. Copy errors may be indicative of more cognitive impairment in the preoperative setting; copy errors are indicative of executive control dysfunction,23,24,26 and research shows that individuals with executive dysfunction have greater levels of postoperative functional difficulty and warrant more caregiver needs.30 Researchers now need to reassess the value of clock drawing to command or copy conditions with larger samples while also controlling for other variances (eg, anesthesia type, surgery).
As with all hospital learning systems, we show the need for protocol modification. The most frequently missed items were frailty, followed by years of education, and then 3-word memory. We were most concerned that staff missed years of education. Individuals with fewer years of formal education are at greater risk for neurocognitive decline, tend to decline at a faster rate than individuals with higher education, and have an increased risk for neurocognitive disorders, such as delirium and dementia.32 Education is one of the most relevant risk factors for assessing neurocognitive risk and is easy to obtain. Still, staff reported discomfort assessing educational levels, particularly if the staff had given the clock drawing or 3-word recall test before asking about education. For this reason, we strongly encourage staff to ask education before administering any of the cognitive screening tools. Regarding frailty, staff members reported intermittent problems with the grip strength device and feeling rushed due to a full workload. Three-word memory was not administered based on staff assumptions that patients would have easily passed the test. Clock drawing was rarely missed reportedly due to the novelty of the test and perceived value. Collectively, these staff responses address the nontraditional aspect of cognitive and memory testing in a medical setting, the need for continued education about their relevance, and the value of continuous process improvement efforts.33 Clinicians also need to consider administration time. Using an estimate of 5 minutes per administration and a typical staff member workload of 2–5 patients >64 years of age per day, the frailty–cognitive screening protocol adds 10–30 minutes of additional administration time per staff member per day. Including the current study, we now have ≥2 studies showing a high rate of impairment in preoperative anesthesia settings. An elegant prospective study conducted by Culley et al9 shows that, of 198 patients who completed a preoperative cognitive assessment, 23% performed below clinical cutoff scores for impairment. Our data support and extend these findings by showing a likelihood of preoperative cognitive impairment in 20% (1 in 5 preoperative patients) regardless of surgery procedure. Clinicians now need to weigh the pros and cons of this additional assessment time against risk prevention.
Patients with dementia experience higher rates of preventable hospital-related complications, including urinary tract infections, pressure ulcers, pneumonia, and delirium, which increase length and cost of hospital stays. Identifying these patients before surgery should lead to improved perioperative monitoring that may lower costly complications and have a positive impact on postoperative family caregiving burden,11 thereby informing precision medicine.34 This is particularly important as we learn which preoperative brain/cognitive profiles interact with anesthesia response35,36 and predict acute37 and long-term changes.38,39 Additional 30 minutes to 2 hours per week per staff, therefore, appears appropriate, so that we can develop additional protocols for minimizing perioperative complication and reducing length of hospital admission. Our findings support preoperative screening programs for early dementia detection.11
We acknowledge limitations. First, our data represent a snapshot of patients visiting the hospital during a specific time of the year. We cannot generalize findings to other times of the year, such as the winter season, when there may have been a greater number of older adults seeking certain surgical procedures in the state of Florida. Second, while we examined likelihood of cognitive impairment, we did not examine type or severity of cognitive impairment. This requires further evaluation. Third, length of stay was our only viable outcome measure from the study cohort. We recognize that length of stay is not a powerful metric and differs by type of surgery. We encourage study replication with different cohorts and more sophisticated outcome variables. Fourth, we used the Fried index17 to assess frailty. There are other measures of frailty that may have provided additional stratification of the degree of frailty, but all require more time or testing than the Fried index. Fifth, we did not examine type of clock-drawing errors,23 a domain of neuropsychological research that could improve our understanding of cognitive phenotypes in a preoperative patient sample. These analyses in combination with digital clock-drawing technologies that include command and copy conditions may prove particularly valuable in large perioperative settings.40 Sixth, we recognize that the value of clock drawing to copy relative to command for patient risk detection needs much more investigation and validation against a more comprehensive data set that includes many other medical and surgical–anesthetic factors. There are also 2 limitations of a statistical nature. We conducted a feasibility study and as such we were concerned with pragmatic and operational aspects of participant acceptability and the logistics of data collection; a sample size justification was not a part of study design (which is appropriate for a feasibility study). However, the data collected in this study will allow for estimation of effect sizes required to adequately power future studies. Also, due to the nature of the study, we did not control for multiple testing, which may inflate the experiment wise type I error rate.
Despite these limitations, the study procedures and data exemplify clinical translational research. Frailty, education, and cognitive screening tools were administered within a complex hospital setting with minimal data loss. Screening provided complementary bits of information. We now need to investigate appropriate rescue pathways for preoperative older adult patients flagged as frail or showing cognitive impairment.
Supplementary Material
KEY POINTS.
Question: Is a preoperative frailty–cognitive protocol feasible in a tertiary care medical center?
Findings: We found that staff administered the protocol to 80% of eligible patients over a 2-month period; surgery type differed by frailty but not cognitive impairment; and clock drawing to copy may be particularly useful for predicting length of hospital stay.
Meaning: Our findings support campaigns to install screening programs into hospitals for early postoperative risk detection.
ACKNOWLEDGMENTS
The authors dedicate this manuscript to the memories of 2 important mentors. First, to J. S. Gravenstein, MD, Department of Anesthesiology, University of Florida, who facilitated research on postoperative cognitive decline and continually pushed excellence in anesthesia research. Second, to Edith Kaplan, PhD, Department of Neurology, Boston University School of Medicine, who remains a guiding light to the field of neuropsychology for her research and training on error analyses and process approach techniques for studying the brain and behavior.
The authors thank the preoperative medical personnel for their willingness to expand their protocol, their continued engagement, and constructive feedback. The authors are particularly grateful to Amy Maclean, ARNP, for her leading the integration of neuropsychology into the preoperative setting. The authors also thank Judith Wishin, Amy Gunnett, Lei Zhang, and Gigi Lipori for their assistance with institutional review board and data management. The authors thank Corey Astrom, ELS, for her editorial expertise and assistance with this manuscript.
Funding: This work was supported by the National Institutes of Health (grant No. R01 NR014181 [to C.C.P.], IIS-1404494 [to C.C.P.], R01 AG055337 [to C.C.P. and P.T.], T32-NS082128 [to S.C.], P50AG047266 [to C.W.G.], and T32-AG049673 to F.A.) and by the I. Heermann Anesthesia Foundation (to C.G. and C.C.P.).
Footnotes
The authors declare no conflicts of interest.
DISCLOSURES
Name: Shawna Amini, MPH.
Contribution: This author helped collect the data, draft the first manuscript, and revise the manuscript.
Name: Samuel Crowley, MS.
Contribution: This author helped interpret the data and write the manuscript.
Name: Loren Hizel, MS.
Contribution: This author helped acquire data, train staff members, and interpret the results.
Name: Franchesca Arias, PhD.
Contribution: This author helped interpret the data and write the manuscript.
Name: David J. Libon, PhD.
Contribution: This author helped collect and interpret the data and revise the manuscript.
Name: Patrick Tighe, MD, MS.
Contribution: This author helped interpret the data and revise the manuscript.
Name: Chris Giordano, MD.
Contribution: This author helped collect the data, revise the manuscript, and secure funding.
Name: Cynthia W. Garvan, PhD.
Contribution: This author helped analyze and interpret the data and revise the manuscript.
Name: F. Kayser Enneking, MD.
Contribution: This author helped conceive the study, collect the data, and revise the manuscript.
Name: Catherine C. Price, PhD.
Contribution: This author helped conceive the study, collect and analyze the data, draft and revise the manuscript, and secure funding.
This manuscript was handled by: Robert Whittington, MD.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesiaanalgesia.org).
Reprints will not be available from the authors.
REFERENCES
- 1.Oresanya LB, Lyons WL, Finlayson E. Preoperative assessment of the older patient: a narrative review. JAMA. 2014;311:2110–2120. [DOI] [PubMed] [Google Scholar]
- 2.Moller JT, Cluitmans P, Rasmussen LS, et al. Long-term post-operative cognitive dysfunction in the elderly ISPOCD1 study. ISPOCD investigators. International Study of Post-Operative Cognitive Dysfunction. Lancet. 1998;351:857–861. [DOI] [PubMed] [Google Scholar]
- 3.Monk TG, Weldon BC, Garvan CW, et al. Predictors of cognitive dysfunction after major noncardiac surgery. Anesthesiology. 2008;108:18–30. [DOI] [PubMed] [Google Scholar]
- 4.Greene NH, Attix DK, Weldon BC, Smith PJ, McDonagh DL, Monk TG. Measures of executive function and depression identify patients at risk for postoperative delirium. Anesthesiology. 2009;110:788–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Price CC, Garvan C, Hizel LP, Lopez MG, Billings FT 4th. Delayed recall and working memory MMSE domains predict delirium following cardiac aurgery. J Alzheimers Dis. 2017;59:1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ginsberg TB, Powell L, Patel A, et al. Frailty phenotype and neuropsychological test performance: a preliminary analysis. J Am Osteopath Assoc. 2017;117:683–687. [DOI] [PubMed] [Google Scholar]
- 7.Crow RS, Lohman MC, Titus AJ, et al. Mortality risk along the frailty spectrum: data from the national health and nutrition examination survey 1999 to 2004. J Am Geriatr Soc. 2018;66:496–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luck T, Then FS, Schroeter ML, et al. Prevalence of DSM-5 mild neurocognitive disorder in dementia-free older adults: results of the population-based LIFE-adult-study. Am J Geriatr Psychiatry. 2017;25:328–339. [DOI] [PubMed] [Google Scholar]
- 9.Culley DJ, Flaherty D, Reddy S, et al. Preoperative cognitive stratification of older elective surgical patients: a cross-sectional study. Anesth Analg. 2016;123:186–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwarze ML, Barnato AE, Rathouz PJ, et al. Development of a list of high-risk operations for patients 65 years and older. JAMA Surg. 2015;150:325–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayden KM, Inouye SK, Cunningham C, et al. Reduce the burden of dementia now. Alzheimers Dement. 2018;14: 845–847. [DOI] [PubMed] [Google Scholar]
- 12.Alvarez-Nebreda ML, Bentov N, Urman RD, et al. Recommendations for preoperative management of frailty from the Society for Perioperative Assessment and Quality Improvement (SPAQI). J Clin Anesth. 2018;47:33–42. [DOI] [PubMed] [Google Scholar]
- 13.Culley DJ, Flaherty D, Fahey MC, et al. Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology. 2017;127:765–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enneking FK, Radhakrishnan NS, Berg K, Patel S, Wishin JM, Vasilopoulos T. Patient-centered anesthesia triage system predicts ASA physical status. Anesth Analg. 2017;124:1957–1962. [DOI] [PubMed] [Google Scholar]
- 15.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 16.Eeles EM, White SV, O’Mahony SM, Bayer AJ, Hubbard RE. The impact of frailty and delirium on mortality in older inpatients. Age Ageing. 2012;41:412–416. [DOI] [PubMed] [Google Scholar]
- 17.Fried LP, Tangen CM, Walston J, et al. ; Cardiovascular Health Study Collaborative Research Group. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–M156. [DOI] [PubMed] [Google Scholar]
- 18.Rubenstein LZ, Stuck AE, Siu AL, Wieland D. Impacts of geriatric evaluation and management programs on defined outcomes: overview of the evidence. J Am Geriatr Soc. 1991;39(9 pt 2):8S–16S; S-18S. [DOI] [PubMed] [Google Scholar]
- 19.Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989;64:651–654. [DOI] [PubMed] [Google Scholar]
- 20.Satz PJN. Brain reserve capacity on symptom onset after brain injury: a formulation and review of evidence for threshold theory. Neuropsychology. 1993;7:273. [Google Scholar]
- 21.Stern Y What is cognitive reserve? Theory and research application of the reserve concept. J Int Neuropsychol Soc. 2002;8:448–460. [PubMed] [Google Scholar]
- 22.Kaplan E The process approach to neuropsychological assessment of psychiatric patients. J Neuropsychiatry Clin Neurosci. 1990;2:72–87. [DOI] [PubMed] [Google Scholar]
- 23.Libon DJ, Malamut BL, Swenson R, Sands LP, Cloud BS. Further analyses of clock drawings among demented and nondemented older subjects. Arch Clin Neuropsychol. 1996;11:193–205. [PubMed] [Google Scholar]
- 24.Price CC, Cunningham H, Coronado N, et al. Clock drawing in the montreal cognitive assessment: recommendations for dementia assessment. Dement Geriatr Cogn Disord. 2011;31:179–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. [DOI] [PubMed] [Google Scholar]
- 26.Cosentino SA, Jefferson AL, Carey M, et al. The clinical diagnosis of vascular dementia: a comparison among four classification systems and a proposal for a new paradigm. Clin Neuropsychol. 2004;18:6–21. [DOI] [PubMed] [Google Scholar]
- 27.Nasreddine ZS, Phillips NA, Bédirian V, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 28.Borson S, Scanlan JM, Chen P, Ganguli MJ JotAGS. The minicog as a screen for dementia: validation in a population‐based sample. 2003;51:1451–1454. [DOI] [PubMed] [Google Scholar]
- 29.Souillard-Mandar W, Davis R, Rudin C, et al. Learning classification models of cognitive conditions from subtle behaviors in the digital clock drawing test. Mach Learn. 2016;102:393–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Price CC, Garvan C, Monk T. Subtype and severity of cognitive impairment in older adults after non-cardiac surgery. Anesthesiology. 2008;108:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wallace LMK, Theou O, Godin J, Andrew MK, Bennett DA, Rockwood K. Investigation of frailty as a moderator of the relationship between neuropathology and dementia in Alzheimer’s disease: a cross-sectional analysis of data from the Rush Memory and Aging Project. Lancet Neurol. 2019;18:177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fotenos AF, Mintun MA, Snyder AZ, Morris JC, Buckner RL. Brain volume decline in aging: evidence for a relation between socioeconomic status, preclinical Alzheimer disease, and reserve. Arch Neurol. 2008;65:113–120. [DOI] [PubMed] [Google Scholar]
- 33.Sherman JB, Chatterjee A, Urman RD et al. Implementation of routine cognitive screening in the preoperative assessment clinic. A A Pract. 2019;12:125–127. [DOI] [PubMed] [Google Scholar]
- 34.Ganguli M, Albanese E, Seshadri S, et al. Population neuroscience: dementia epidemiology serving precision medicine and population health. Alzheimer Dis Assoc Disord. 2018;32:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giattino CM, Gardner JE, Sbahi FM, et al. ; MADCO-PC Investigators. Intraoperative frontal alpha-band power correlates with preoperative neurocognitive function in older adults. Front Syst Neurosci. 2017;11:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price CC, Pereira DB, Andre R, et al. Prospective pilot investigation: presurgical depressive symptom severity and anesthesia response in women undergoing surgery for gynecologic mass removal. Int J Behav Med. 2015;22:521–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang H, Tanner J, Parvataneni H, et al. Impact of total knee arthroplasty with general anesthesia on brain networks: cognitive efficiency and ventricular volume predict functional connectivity decline in older adults. J Alzheimers Dis. 2018;62:319–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Browndyke JN, Berger M, Harshbarger TB, et al. Resting-state functional connectivity and cognition after major cardiac surgery in older adults without preoperative cognitive impairment: preliminary findings. J Am Geriatr Soc. 2017;65:e6–e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Price CC, Tanner JJ, Schmalfuss I, et al. A pilot study evaluating presurgery neuroanatomical biomarkers for postoperative cognitive decline after total knee arthroplasty in older adults. Anesthesiology. 2014;120:601–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hizel LP, Warner ED, Wiggins ME, et al. Clock drawing performance slows for older adults after total knee replacement surgery. Anesth Analg. 2018. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.