Abstract
Maintenance of metal homeostasis is critical to cell survival due to the multitude of cellular processes that depend on one or more metal cofactors. Here, we show that the opportunistic fungal pathogen Candida albicans extensively remodels its metal homeostasis networks to respond to treatment with the antifungal drug fluconazole. Disruption of the ergosterol biosynthetic pathway by fluconazole requires C. albicans adaptation, including increased Cu import and storage, increased retention of Fe, Mn, and Zn, altered utilization of Cu- and Mn-dependent enzymes, mobilization of Fe stores, and increased production of the heme prosthetic group utilized by the enzyme target of fluconazole. The findings offer a new perspective for thinking about fungal response to drug stress that pushes cells out of their metal homeostatic zones, leading them to enact metal-associated adaptation mechanisms to restore homeostasis to survive.
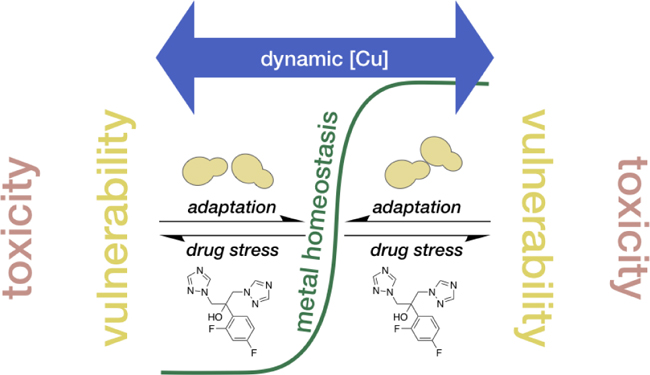
Graphical Abstract
Fluconazole stress pushes Candida albicans outside of metal homeostasis, requiring adaptation. Fungal adaptation to drug stress requires remodeling of metal homeostasis networks, creating vulnerabilities to environmental fluctuations in Cu availability.
Introduction
The crossroads of biological metal trafficking are battlegrounds in the fight between vertebrate hosts and microbial pathogens, as both use a variety of strategies to orchestrate the movement and utilization of d-block biometals to promote their own survival.1 In response to infection, hosts enact mechanisms of nutritional immunity to restrict pathogen access to essential metals like Fe, Mn, Zn and even Cu,2, 3 as well as co-opt metal toxicity to intensify pathogen killing, for example by Cu and Zn.4–6 Successful pathogens, including the prevalent fungal pathogen Candida albicans, have therefore evolved to tune metal transport and utilization based on metal availability, which changes based on location and disease progression.7–12 For example, C. albicans upregulates Fe and Zn import to counter host-imposed restrictions of these metals during early infection stages12, 13 and rebalances Cu efflux versus import as Cu levels change during disseminated infection.11 These transcriptional responses to metal availability are linked to pathogenicity, where relative abundance or scarcity of Mn, Fe, Zn, or Cu signal the need to adapt.14, 15 The ability to adapt to changing nutrient availability becomes especially important when C. albicans faces antifungal drug stress. For example, altering the carbon source of the growth media16 or interfering pharmacologically with phosphate sensing17 alters the susceptibility of C. albicans to a number of antifungal drugs, including amphotericin B, miconazole, and micafungin. In addition, the metal chelator DTPA has been found to increase susceptibility of C. albicans to caspofungin,18 and Fe deprivation is known to sensitize C. albicans to a variety of drugs, including fluconazole.19
Fluconazole is a first line drug against opportunistic fungal infections, like those caused by C. albicans.20 Fluconazole binds and inhibits the heme center of lanosterol 14α-demethylase (Cyp51), which disrupts the biosynthesis of ergosterol, a key component of the fungal cell membrane. Several streams of data suggest that the ability of C. albicans to adapt metal homeostasis mechanisms is important during fluconazole treatment. Analysis of published microarray data for C. albicans reveals that more than 30 genes involved in Cu, Fe, and Mn homeostasis are differentially expressed upon fluconazole treatment.21 In separate studies, fluconazole-resistant C. albicans clinical isolates were found to differentially express genes involved in Fe and Cu homeostasis,22 including repression of the Cu importer Ctr123 and induction of Cu-binding metallothionein Crd2.24 These findings link the ability of C. albicans to regulate metal homeostasis during infection to clinically-relevant drug resistance.
We recently reported that Cu potentiates the activity of fluconazole and other azole antifungals via an unknown mechanism that does not require direct complex formation between the drug molecule and Cu.25 To gain mechanistic insight for the observed Cu potentiation of fluconazole activity, we explored in the present study how fluconazole stress impacts the metallome of C. albicans. We find that C. albicans regulates metal homeostasis to meet the modified cellular metal requirements imposed by fluconazole. Specifically, treatment with fluconazole increases the cell’s need for transition metals and C. albicans meets this need by altering metal transport to raise cellular metal quotas. In addition to increasing the minimal metal requirement, fluconazole stress changes the ways in which C. albicans uses metals in the cell, which is evident from differential expression, activity, and production of metalloenzymes and their cofactors. Small increases in Cu availability in the growth environment dramatically impact cellular Cu status, which is reflected in changes to Cu transport and utilization. These findings suggest that inducing vulnerabilities in metal handling pathways could be leveraged to optimize the efficacy of existing antimicrobial drugs as well as aid in the development of new drugs.
Results
Potentiation of fluconazole activity is Cu specific.
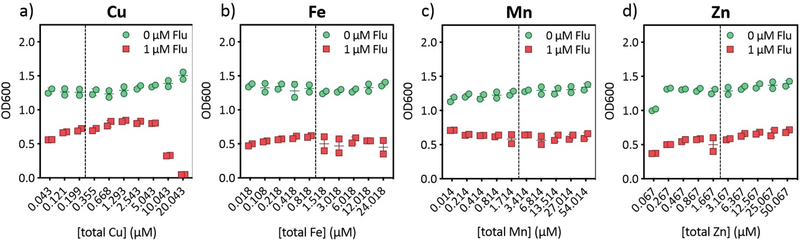
Our previous observations of Cu-potentiated growth inhibition by fluconazole were made in yeast peptone dextrose (YPD), a rich medium of uncontrolled metal content.25 To gain a comprehensive view of the impact of metal availability on fluconazole susceptibility, we performed growth assays in Tris-buffered Synthetic Defined (Tris:SD) medium that was rigorously controlled for Cu, Fe, Mn, and Zn. Media were prepared with each metal individually dropped out, then titrated back to obtain a stepwise gradient from “deficient” to “supplemental” for that particular metal, relative to levels found in commercial SD formulations. As shown in Fig. 1 for the untreated controls, cells sustained 48-h growth in Tris:SD deficient in Cu, Fe, Mn, or Zn, with growth improving slightly as the dropped-out metal was returned, most clearly for Zn (Fig. 1d). In the case of fluconazole treatment, there was no strong dependence on growth inhibition in response to Mn, Zn, or even Fe, whereas supplementation with Cu provided a slight growth recovery from fluconazole up to a point, after which additional Cu caused a sharp decrease in growth (Fig. 1a). The growth response in Fig. 1a mirrors our previous results in YPD, with both Cu depletion and supplementation improving inhibition by fluconazole relative to inhibition under basal Cu levels.25 Admittedly, the effect of Cu depletion in the defined medium is more subtle than that observed in YPD with an extracellular Cu chelator25 and likely reflects a difference in the degree of Cu limitation achieved by omitting Cu from the medium versus chemically sequestering it. We saw a similar trend for Cu in YPD that had been treated with Chelex to deplete metals, although cell growth was significantly impaired for cells cultured in Chelex-treated YPD, even after all metals had been resupplied (Suppl. Fig. 1c–d). For this reason, Chelex-treated YPD was not used for further studies. Of note, fluconazole does not bind Cu in YPD25 or Tris:SD media (Suppl. Fig. 2), so the observed growth phenotype does not arise from Cu(II)-fluconazole complex formation.
Figure 1. Cu availability specifically has a significant impact on growth of fluconazole-treated cells.
48-h growth of fluconazole (Flu)-treated (red squares) and untreated (green dots) C. albicans cells as a function of added Cu (a), Fe (b), Mn (c), and Zn (d) in the respective metal drop-out Tris:SD media. The lowest concentration of each metal represents actual trace levels of the dropped-out metal, analyzed by ICP-MS. The remaining concentration gradients represent theoretical metal concentrations based on what was added on top of trace levels. Concentrations for metal-complete SD media are indicated by dashed vertical lines, with relative deficient and supplemental levels shaded yellow and purple, respectively. Values are reported as mean ± SD. Full metal analyses for each media formulation are reported in Suppl. Tables 7–10.
Fluconazole activates the C. albicans Cu regulon.
The finding that the concentration of Cu in the growth medium uniquely impacts fluconazole efficacy led us to probe the expression of Cu-regulated genes in response to fluconazole under conditions of basal (YPD medium levels, ~0.1–0.3 µM) and supplemental (10 µM) Cu. We chose to test the effects of 10 µM fluconazole because this concentration is sufficient to inhibit 24-h growth but insufficient to prevent 48-h trailing growth in standard growth assays.25 Trailing growth indicates activation of adaptation mechanisms that lead to azole tolerance26 and we believed these to be ideal conditions for studying gene expression. A concentration of 10 µM was also chosen for Cu because it is sufficient to potentiate growth inhibition by fluconazole in 96-well plate assays in YPD but alone is not toxic.25 All biochemical analyses (non-growth assays) in this study were carried out on cells that were treated at the mid-log phase of growth. Treatment with fluconazole at mid-log phase inhibited growth to approximately 50% of the untreated control, and supplementation with Cu had no additional effect on growth under these conditions (Suppl. Fig. 3). These data are in contrast to those obtained in growth assays performed in 96-well plates in which cells are grown from lag phase (OD600 ~0.001). The robust growth of cells in the biochemical assays is favorable because sufficient cell material is obtained. Furthermore, because fluconazole is a fungistatic drug, there are no concerns over cell toxicity, even at high drug concentrations.
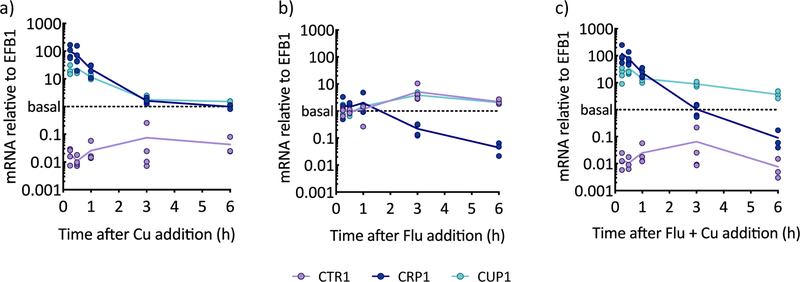
The impact of fluconazole on transcription of the Cu importer CTR1, Cu exporter CRP1, and Cu storage metallothionein CUP1 was monitored by reverse transcription-PCR (qRT-PCR) over 6 h. In as little as 15 min, cells grown in the presence of 10 µM Cu added to YPD (Fig. 2a) strongly up-regulated CRP1 (80-fold) to export Cu as the primary detoxification mechanism and CUP1 (20-fold) to sequester remaining cellular Cu. By 3 h, CRP1 and CUP1 expression returned to baseline, signaling restoration of homeostasis, while CTR1 remained repressed throughout the 6 h period. Thus, the Cu transcriptional profile reveals that, when faced with excess Cu, cells work to restore homeostatic levels, which they accomplish by preventing additional import of Cu, while exporting and sequestering Cu already inside.
Figure 2. Response of Cu regulon to fluconazole under basal and high Cu over time, as monitored by qRT-PCR.
Cu importer CTR1 (purple lines), Cu exporter CRP1 (dark blue lines), and Cu-binding metallothionein CUP1 (light blue lines) mRNA were quantified by qRT-PCR for cells grown to log phase and supplemented with 10 µM CuSO4 (a), 10 µM fluconazole (b), or both (c). mRNA levels were normalized to EFB1. Lines represent the average from n = 3–4 biologically independent samples per timepoint.
In contrast to the rapid response to Cu, the response of the Cu regulon to fluconazole is delayed (Fig. 2b). After 3 h of fluconazole treatment, CTR1 was induced 3-fold, suggesting the cellular requirement for Cu increases upon fluconazole treatment. Induction of metallothionein CUP1 correlated with the timing of CTR1 induction. Interestingly, fluconazole also repressed CRP1, beginning at 3 h and becoming more dramatic by 6 h. These observations suggest a mechanism by which fluconazole treatment causes cells to stockpile Cu through up-regulation of genes responsible for import and storage and repression of the gene responsible for Cu export.
Within the first hour of co-treatment with fluconazole and Cu, the expression of all three genes resembled that of Cu treatment alone (Fig. 2c): CRP1 and CUP1 were highly up-regulated at 15 min (90- and 30-fold, respectively), and CTR1 was repressed. At later timepoints, however, CRP1 became down-regulated while CUP1 expression remained elevated (4- to 9-fold), both results reminiscent of those obtained for cells treated with fluconazole alone. Although Cu supplementation did prevent induction of CTR1, it failed to prevent repression of CRP1. Thus, fluconazole represses CRP1, even when Cu is elevated in the growth medium. Altogether, though there is a Cu detoxification response early, cells transition to a prolonged period of Cu storage, with no attempts to move Cu in or out of the cell. Apparently, Cu is responsible for the initial response and the prolonged repression of CTR1, and fluconazole drives later transcriptional responses, repressing CRP1 and maintaining elevated expression of CUP1.
Deletion of Cu homeostasis genes alters C. albicans’ susceptibility to fluconazole.
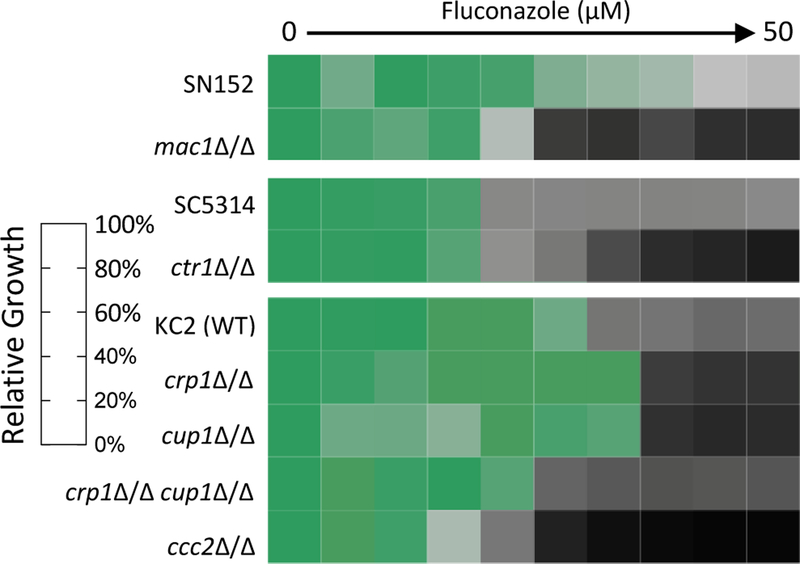
To further address the role of Cu homeostasis in fungal adaptation to fluconazole stress, growth assays were performed for several strains of C. albicans lacking Cu homeostasis genes. In C. albicans, two transcription factors sense Cu availability: MAC1 allows cells to respond to Cu deficiency, and CUP2 to Cu excess.27–30 As shown in Fig. 3, cells lacking MAC1 (mac1Δ/Δ) were more susceptible to fluconazole than isogenic parent strain SN152. Cells lacking MAC1-regulated CTR1 (ctr1Δ/Δ) were also more susceptible to fluconazole compared to parent strain SC5314. These results underscore the need for C. albicans to regulate Cu import during fluconazole stress. Deletion of CUP2 resulted in only a minimal increase in fluconazole susceptibility (Suppl. Fig. 4), suggesting that tight control over Cu export and storage is less important during fluconazole stress. Deletion strains lacking either the plasma membrane Cu exporter CRP1 (crp1Δ/Δ) or the Cu-binding metallothionein CUP1 (cup1Δ/Δ) were overall less susceptible to fluconazole than parent strain KC2 but at higher concentrations appear more susceptible (Fig. 3), indicating that deletion of either component may initially be protective but then becomes detrimental at higher fluconazole concentrations. The strain lacking both the exporter and metallothionein (crp1Δ/Δ cup1Δ/Δ) was slightly more susceptible to fluconazole than WT strain KC2 (Fig. 3). These results suggest a compensatory mechanism in the single deletion strains not possible in the double deletion strain. Indeed, basal expression of CUP1 is known to be enhanced in crp1Δ/Δ cells and vice versa.31 The Cu transporting ATPase Ccc2 transports Cu into the Golgi, where the multicopper oxidase Fet3 is metallated.32 The ccc2Δ/Δ strain was particularly sensitive to fluconazole (Fig. 3), consistent with a previous report that attributed this sensitivity to an inability to metalate Fet3, which is required for high affinity iron uptake.19 Supplementation with 10 µM Cu rescued growth to levels resembling the parent strain (Suppl. Fig. 4), indicating the excess Cu is sufficient to restore Cu to the secretory pathway even in the absence of Ccc2. Notably, Cu’s rescue of fluconazole-treated ccc2Δ/Δ cells is in contrast to Cu’s potentiation of fluconazole activity in wild-type (WT) SC5314, the primary strain used in this study. These opposing effects of Cu on fluconazole efficacy highlight that the impact of Cu supplementation on cellular recovery from drug stress is highly context dependent. We suggest that Cu supplementation rescues ccc2Δ/Δ cells from Cu deficiency, thereby rendering these cells more tolerant of fluconazole. However, fluconazole activity is not potentiated by Cu in KC2, the ccc2Δ/Δ background strain (Suppl. Fig. 4), so differences between the two WT strains may also contribute to the different effects of Cu. Fluconazole susceptibility was not impacted in strains that were individually deleted for cytosolic superoxide dismutase (SOD) enzymes SOD1 or SOD3, and only a moderate decrease in susceptibility was observed for cells in which mitochondrial SOD2 was deleted (Suppl. Fig. 4).
Figure 3. Susceptibility of C. albicans deletion strains to fluconazole.
C. albicans deletion strains were treated with 0–50 µM fluconazole (diluted 2-fold) in YPD media at 30 °C for 48 h then growth was determined by measuring OD600. Data are normalized to the untreated control for each strain. Shown are representative heat maps from n = 2 biologically independent experiments.
Fluconazole increases cellular metal levels.
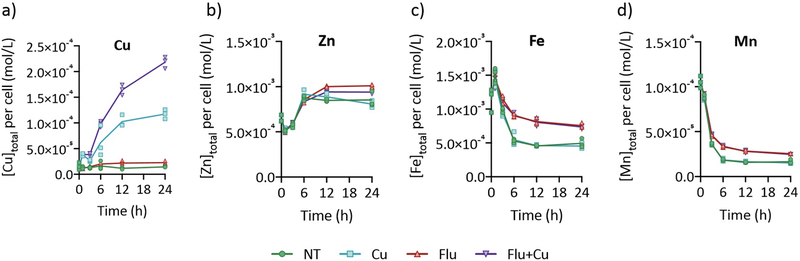
The observation that C. albicans alters the expression of Cu transport genes in response to fluconazole treatment suggested that fluconazole may impact the metal content of these cells. Levels of Cu, Fe, Mn, and Zn were therefore determined over the course of 24 h using inductively coupled plasma-mass spectrometry (ICP-MS). Cu levels remained stable throughout the experiment in untreated cells but were nearly doubled at 12 and 24 h in cells treated with fluconazole (Fig. 4a, Suppl. Fig. 5). This increase in cell-associated Cu is consistent with fluconazole’s induction of Cu importer CTR1 (Fig. 2b). As expected, cells grown in Cu-supplemented YPD exhibited a steady increase in cell-associated Cu over time (Fig. 4a). Strikingly, though, treating Cu-supplemented cells with fluconazole caused them to accumulate significantly more Cu than when fluconazole was not present. Relative to the Cu only control, these cells contained 44%, 63%, 60%, and 88% more Cu at 3, 6, 12, and 24 h, respectively. Considering the difference in Cu levels between fluconazole-treated cells grown in Cu-supplemented versus un-supplemented YPD is even more compelling. By 6 h, fluconazole-treated cells grown with supplemental Cu experienced a 390% increase in Cu relative to cells treated with fluconazole alone (4.9 times more Cu), and reached 900% (10 times) by 24 h. Zn levels increased during the first 6 h for all treatment conditions, then plateaued in untreated cells and declined slightly in Cu-treated cells (Fig. 4b). Fluconazole-treated cells increased in Zn content until 12 h then plateaued, regardless of whether they were Cu-supplemented. Intriguingly, cells treated with fluconazole maintained approximately double the amount of Fe (Fig. 4c) and Mn (Fig. 4d) compared to untreated cells. This phenomenon persisted from 6 to 24 h and was independent of the Cu supplementation status of the growth medium. Notably, the Fe and Mn content decreased between 0 and 6 h under all treatment conditions; however, cells treated with fluconazole decreased by less, resulting in elevated levels of these metals in cells treated with fluconazole.
Figure 4. Metal content of C. albicans cells reported as cellular concentration (mol/L).
Cu levels (a) increased steadily over time in cells treated with Cu (blue line) relative to untreated control cells (green line), in which Cu levels did not change during the course of the experiment. Cells treated with fluconazole alone (red line) exhibited a slight increase in Cu, and treatment with both fluconazole and Cu (purple line) resulted in markedly higher Cu levels than either treatment alone. Zn content (b) was consistent across treatments, except at 12 and 24 h when fluconazole-treated cells +/− Cu maintained slightly elevated levels. Fe (c) and Mn (d) levels were approximately doubled in fluconazole-treated cells +/− Cu. Conditions: [Flu] = 10 µM, [CuSO4] = 10 µM. Cells were grown in YPD media for time indicated in figure legends. Cell-associated metal levels were analyzed by ICP-MS and cellular metal concentrations were calculated as described in Methods. Lines represent the average from n = 3 biologically independent samples per timepoint. Metal content reported as mol metal/mol phosphorus (P) is shown in Suppl. Fig. 6 and average P per sample is plotted in Suppl. Fig. 7.
C. albicans switches to SOD3 during fluconazole treatment.
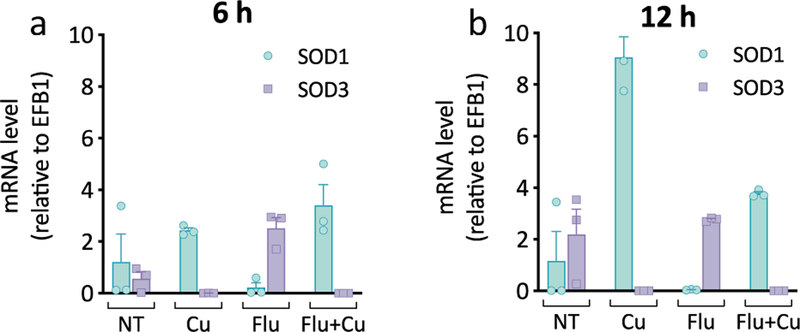
The finding that C. albicans induces CTR1 in response to fluconazole treatment suggests that these cells are experiencing Cu deficiency, a condition known to cause C. albicans to switch from utilizing Cu,Zn-Sod1 to Mn-Sod3.10 Expression of SOD1 and SOD3 was therefore measured by qRT-PCR at 6 h (log phase) and 12 h (stationary phase) as a function of fluconazole, with and without added Cu. As shown in Fig. 5a, untreated cells expressed both SOD1 and SOD3 at 6 h, while only SOD1 expression was detected for cells grown in Cu-supplemented YPD. In contrast, cells treated with fluconazole repressed SOD1 and induced SOD3, illustrating that there is a Cu sparing response during fluconazole stress. Co-supplementation with fluconazole and Cu reversed this effect, with these cells expressing SOD1 exclusively. By 12 h, untreated and fluconazole-treated cells relied more heavily on SOD3 (Fig. 5b), with a higher SOD3:SOD1 ratio at this time point, consistent with previous findings that C. albicans cells switch to Mn-Sod3 as they approach stationary phase.10, 33 Cu-supplemented cells continued to primarily express SOD1, regardless of whether fluconazole was present. These data show that cells reduce Cu utilization by switching to a non-Cu-dependent enzyme during fluconazole treatment. This adaptation can be reversed with Cu supplementation, suggesting that the Cu that hyperaccumulates during co-treatment with Cu and fluconazole is indeed recognized as bioavailable by the cell.
Figure 5. C. albicans switches to Mn-Sod3 during fluconazole treatment.
mRNA levels of SOD1 (blue bars) and SOD3 (purple bars) relative to EFB1 after 6 h (a) and 12 h (b) of growth. Conditions: [Flu] = 10 µM, [CuSO4] = 10 µM in YPD media. Data are reported as mean ± SEM, n = 3 biologically independent samples per group.
Mitochondrial Aconitase is Vulnerable to Cu During Fluconazole Stress.
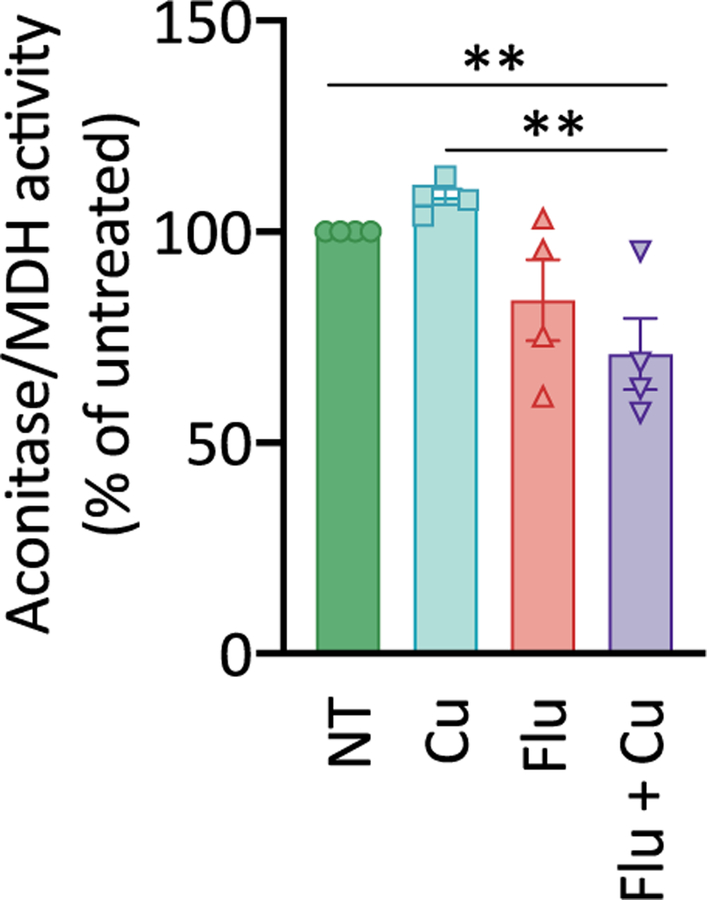
The hallmarks of Cu deficiency observed as a consequence of fluconazole treatment, including induction of CTR1 and switch to Mn-SOD3, suggest that Cu supplementation of fluconazole-treated cells might aid in cell recovery, not prevent it. Yet, supplementation of fluconazole-treated cells with non-toxic levels of Cu reduces growth in both YPD25 and defined Tris:SD media (Fig. 1a). A possible explanation for this paradox is that fluconazole treatment creates vulnerabilities to normally innocuous levels of Cu. One target of cellular Cu toxicity is proteins that contain solvent-exposed iron-sulfur (Fe-S) clusters, which are sensitive to oxidation or mis-metalation by Cu that renders them non-functional.34, 35 While low micromolar levels of Cu alone are not expected to cause damage to these proteins, the predicted changes in the prioritization, allocation, and movement of Fe as the cells work to restore balance after fluconazole hits the Fe-dependent ergosterol biosynthesis pathway21 could leave C. albicans with a heightened vulnerability to excess Cu. Based on this line of reasoning, Fe-S clusters may be more vulnerable to Cu stress during fluconazole treatment. To test this hypothesis, we measured the activity of aconitase, a mitochondrial enzyme of the citric acid cycle containing a [4Fe-4S] cluster. The activity of malate dehydrogenase (MDH) was measured as a control because it is also an enzyme of the citric acid cycle but does not contain an Fe-S cluster. As shown in Fig. 6, co-treatment with fluconazole and Cu resulted in a significant 30% reduction in aconitase activity relative to untreated cells (P <0.006). Although not statistically significant, a modest decrease in activity was also observed for cells treated with fluconazole alone (84%) and a slight increase was observed for Cu-supplemented cells (108%). These results demonstrate that fluconazole stress reduces aconitase activity and this effect is exacerbated by Cu, consistent with fluconazole creating cellular vulnerabilities to Cu.
Figure 6. Aconitase activity is diminished in cells co-treated with Cu and fluconazole.
Activities of aconitase and malate dehydrogenase (MDH) were analyzed for protein extracts obtained from either untreated (green bar), Cu-supplemented (blue bar), Flu-treated (red bar), or Flu-treated/Cu-supplemented (purple bar) exponentially growing cultures. MDH (non-Fe-S enzyme) was used for aconitase normalization. Supplemental Cu does not significantly impact aconitase activity. Fluconazole treatment alone slightly reduces activity relative to untreated control (P <0.076), but co-treatment with Flu and Cu significantly decreases activity relative to the untreated control (P < 0.006). Conditions: [Flu] = 50 µM, [CuSO4] = 10 µM. Cells were grown in yeast nitrogen base (YNB) media for 3 h before analysis. Data are reported as mean ± SEM. n = 4 biologically independent samples per group (2-way repeated-measures ANOVA test).
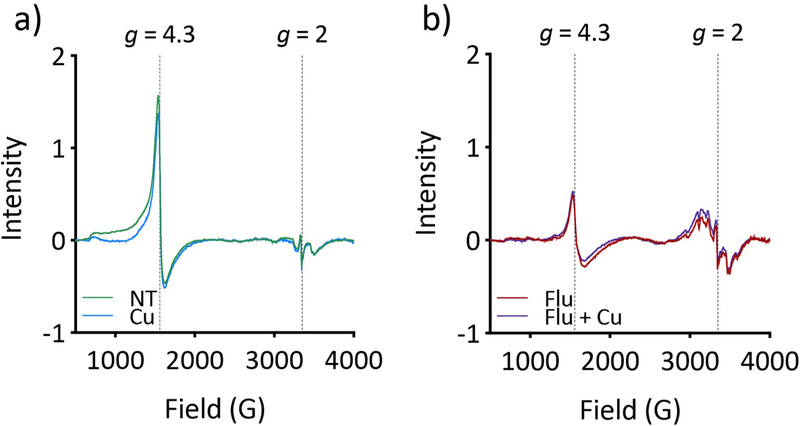
Fluconazole depletes EPR-detectable Fe.
To gather information about the speciation of the metals detected by bulk analysis, whole C. albicans cells were analyzed by electron paramagnetic resonance (EPR). In S. cerevisiae cells, total cellular Fe levels have been shown to correlate with the intensity of the signal at g = 4.3, taken to correspond to the mononuclear high-spin Fe(III) of the “labile” Fe pool, which fluctuates as a function of Fe in the growth medium.36, 37 Given the increase in total Fe in cells treated with fluconazole, it was predicted that the EPR-detectable Fe pool would increase in these cells as well. Instead, fluconazole treatment significantly attenuated the Fe signal at g = 4.3 (Fig. 7b), suggesting that the increased supply of cell-associated Fe is not held in labile pools but instead is incorporated into structures not observable by EPR. As with the bulk Fe measurements, Cu supplementation had no impact— the EPR spectra of cells that were supplemented with Cu alone resembled the spectra of untreated cells (Fig. 7a), and the spectra of Cu-supplemented cells that received fluconazole treatment resembled those of fluconazole-treated cells (Fig. 7b). Signal attenuation by fluconazole occurred as early as 3 h and persisted through 12 h, at which point the signal for untreated cells also diminished as cells reached stationary phase (Suppl. Fig. 8). Increasing the concentration of fluconazole did not further attenuate the signal (Suppl. Fig. 9). Fluconazole-treated cells also exhibit a change in the multiline pattern centered at g = 2, which is characteristic of Mn(II) ions with hyperfine splitting arising from the I = 5/2 Mn nucleus (Fig. 7b). As with the signal at g = 4.3, this signal was unique to fluconazole-treated cells and was not impacted by additional Cu levels in the growth medium.
Figure 7. Fluconazole depletes EPR-detectable Fe and increases EPR-detectable Mn.
(a) Cu supplementation alone (blue trace) does not impact the EPR signal at g = 4.3 relative to the untreated control cells (green trace). (b) Treatment with Flu (red trace) or Flu + Cu (purple trace) substantially attenuates the g = 4.3 signal. The signals in the g = 2 region are taken to be from Mn. Conditions: [Flu] = 10 µM, [CuSO4] = 10 µM. Cells were treated for 6 h in YPD before analysis.
Heme synthesis is involved in fluconazole tolerance.
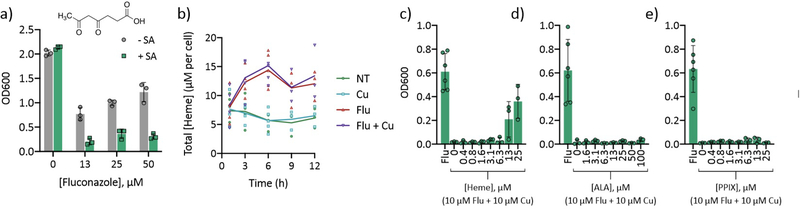
Taken together, the relative elevation in total Fe and decrease in labile Fe as a result of fluconazole treatment suggested that the excess Fe is likely directed toward protein utilization. Heme is one of the most abundant metalloporphyrins in nature and is a cofactor for Cyp51, the target of fluconazole. C. albicans has been shown to upregulate transcription of ERG11, the precursor to the Cyp51 enzyme, within two hours of fluconazole treatment,38, 39 and increased production of Cyp51 would require heme for insertion into the apoprotein.40 To assess whether heme synthesis is important for fluconazole tolerance, growth was determined for cells co-treated with fluconazole and succinylacetone, which inhibits an early step of heme biosynthesis.41, 42 As shown in Fig. 8a, succinylacetone potentiates growth inhibition by fluconazole, consistent with C. albicans requiring heme synthesis to recover from fluconazole treatment. In other words, treatment with succinylacetone seems to interfere with adaptation to fluconazole, suggesting heme synthesis is important for fluconazole tolerance. This potentiation of fluconazole activity by succinylacetone is reminiscent of the potentiation we have observed for Cu.25 Even more intriguing, it has been shown that Cu accumulation can lead to inhibition of heme biosynthesis in the bacterial pathogen Neisseria gonorrhoeae.43 Given the fact that Cu accumulates in cells co-treated with fluconazole and Cu, and because heme biosynthesis is well conserved among prokaryotes and eukaryotes, it seemed possible that this Cu could interfere with heme biosynthesis. Furthermore, the observed reduction in aconitase activity bolsters the idea that Cu accumulation during fluconazole stress can impair metal-dependent processes. If this were the case, supplementation with heme would be expected to rescue growth of cells co-treated with fluconazole and Cu. Indeed, supplementation with heme rescued growth of cells co-treated with fluconazole and Cu in a concentration-dependent manner (Fig. 8c). This rescue was not due to sequestration of fluconazole or Cu via heme binding, as sub-stoichiometric levels of heme still rescued growth (Suppl. Fig. 10). Next, additional growth rescue assays were performed with heme intermediates 5-aminolevulinic acid (ALA) and protoporphyrin IX (PPIX) to determine if inhibition by Cu could be linked to a specific step in the heme biosynthesis pathway. ALA is one of the first intermediates in the pathway, and PPIX is the last intermediate just before ferrochelatase inserts Fe to make heme. Neither ALA nor PPIX rescued growth of cells treated with fluconazole and Cu (Fig. 8d–e), suggesting either that Cu inhibits ferrochelatase, or heme synthesis is not inhibited; rather, the phenotype observed may be the result of a change in heme bioavailability. Both ALA and PPIX supplementation increased growth of cells treated with fluconazole alone (Suppl. Fig. 11), meaning these intermediates are available for cell use and the lack of rescue for co-treated cells is not due to poor uptake. Supplementation of fluconazole-treated cells with heme in the absence of Cu resulted in a concentration-dependent biphasic growth pattern in which growth was improved at all concentrations tested except 3–6 µM (Suppl. Fig. 11). This result could arise from signaling events in which low micromolar levels of supplemental heme reduce flux through the heme biosynthetic pathway but are not sufficient alone to counter fluconazole stress.
Figure 8.
(a) Heme biosynthesis inhibitor succinylacetone potentiates fluconazole activity. 48-h growth of C. albicans cells treated with fluconazole +/− succinylacetone (SA). Conditions: [Flu] = 0–50 µM, [SA] = 1 mM in YPD media. Data are reported as average ± SD. The structure of succinylacetone is inset. (b) Heme levels are highly elevated in fluconazole-treated cells. Heme concentration per cell was determined over the course of 12 h of growth. Conditions: [Flu] = 10 µM, [CuSO4] = 10 µM in YPD media. Lines represent the average from n = 3–5 biologically independent samples per timepoint. (c–e) Supplementation with heme, but not other intermediates, rescues growth of cells co-treated with fluconazole and Cu. 48-h growth of C. albicans treated with fluconazole or fluconazole and Cu +/− heme (c), 5-aminolevulinic acid (ALA, d), and protoporphyrin IX (PPIX, e) at concentrations indicated in figure legends. Data are reported as average ± SD. Conditions: [Flu] = 10 µM, [CuSO4] = 10 µM in YPD medium. For controls, see Suppl. Fig. 10.
The finding that heme supplementation rescues growth of cells treated with fluconazole and Cu supports the hypothesis that Cu accumulation in these cells interferes with either the synthesis or utilization of heme. To determine how heme levels are impacted by these conditions, total cellular heme was measured using a fluorescence-based assay previously described for yeast cells.42 As shown in Fig. 8b, fluconazole-treated cells accumulated significantly higher levels of heme than untreated or Cu-supplemented cells. Indeed, following 3 h of treatment, cellular heme concentrations were nearly two times higher in cells treated with fluconazole, and by 6 h, the levels were three times higher. The timing of the increase in heme correlated with the depletion of labile Fe observed via EPR in which the maximal difference between fluconazole-treated and untreated cells also occurred at 6 h (Suppl. Fig. 8). However, Cu supplementation of fluconazole-treated cells did not prevent this increase in total heme. Taken together, the ability of heme to rescue growth of cells co-treated with fluconazole and Cu and the identical heme levels in fluconazole-treated cells regardless of Cu supplementation suggests that fluconazole and Cu co-treatment does not lead to inhibition of heme biosynthesis, but the transport of this heme may be compromised. Regardless, fluconazole-treated cells contain twice as much total Fe and half as much labile Fe as do untreated or Cu-treated cells, and these changes correlate with two to three times more total heme per cell.
Discussion
A cell’s metal quota, or metallome, is defined as its total metal content, regardless of the metal’s chemical speciation or subcellular distribution.44 A cell achieves metal homeostasis when its quota is between the minimum concentration required to sustain cell function and the maximum concentration at which the cell can buffer that metal. Our data provide a Cu quota of 0.83 ± 0.03 × 106 atoms/cell for untreated C. albicans cells grown in YPD for 24 h (Fig. 4). This value is within an order of magnitude of values reported for S. cerevisiae and Cryptococcus neoformans (1.3 × 106 and 3.7 × 106 atoms Cu/cell, respectively).44, 45 Given the differences in cell volume (95, 50, 13 fL) among C. albicans,46 S. cerevisiae,46, 47 and C. neoformans,44 the atoms of Cu per cell translate to total cellular concentrations of 15, 43, and 473 µM, respectively, implying that C. albicans has the lowest Cu quota out of these three fungal organisms.
Our data also demonstrate that the Cu quota maintained by untreated cells is not sufficient during fluconazole stress, and C. albicans adjusts by increasing Cu to 1.3 ± 0.1 × 106 atoms per cell (or 23 µM) and even further increasing to 12.5 ± 0.4 × 106 atoms per cell (218 µM) if more Cu is available in the growth environment. Cu-supplemented cells accrued only 118 µM in the absence of fluconazole, suggesting that fluconazole drives Cu accumulation. Despite this increase in total cellular Cu, fluconazole treatment leads to a perceived Cu deficiency in C. albicans, as indicated by the induction of CTR1, repression of CRP1, and the switch from Cu-SOD1 to Mn-SOD3 in fluconazole-treated cells. The enactment of Cu acquisition and conservation responses despite an overall increase in cell-associated Cu leads us to conclude that cells experience biological but not chemical Cu deficiency. That is, although fluconazole-treated cells have increased levels of Cu, this Cu appears to be unavailable and/or insufficient to meet the needs of the cell during fluconazole stress. The observed induction of SOD3 is consistent with existing literature reports that fluconazole21 and ketoconazole48 both induce SOD3 expression in C. albicans. The transcription of CTR1, SOD1, and SOD3 are regulated by MAC1, and without MAC1, C. albicans cannot increase cellular Cu via induction of CTR1 or spare existing Cu through repression of SOD1 and induction of SOD3. The increased susceptibility of the mac1Δ/Δ and ctr1Δ/Δ strains to fluconazole further bolsters the conclusion that C. albicans has an increased demand for Cu during fluconazole stress. Interestingly, even under conditions of excess Cu, C. albicans continued to repress Cu exporter CRP1 during fluconazole treatment, indicating that the response to fluconazole overrides the response to Cu. The simultaneous induction of CUP1 likely helps buffer the high levels of Cu that accumulate under these conditions, thereby preventing toxicity. The accrued Cu is sufficient to restore SOD1 expression, meaning that at least some of the Cu that accumulates is bioavailable. However, it is evident from the Cu-potentiated growth reduction of fluconazole-treated cells that the extra Cu is actually detrimental to C. albicans overall. The reduction in aconitase activity following treatment with fluconazole and Cu indicates that aconitase has a heightened vulnerability to Cu during fluconazole stress and represents one of potentially several metal-dependent cell components vulnerable to changes in metal availability as a consequence of drug stress.
Like Cu, the quota for Zn under the same conditions increases over growth time, reaching 4.9 ± 0.3 × 107 atoms/cell (853 µM) at 24 h, significantly higher than that of Cu, and within range of the value reported for S. cerevisiae, 1.5 × 107 atoms/cell (498 µM).49 While levels of Fe and Mn decrease over time, fluconazole treatment causes cells to shed less Fe and Mn compared to no drug treatment, resulting in a net increase relative to untreated cells. Despite the existing literature connecting Fe homeostasis to fluconazole efficacy,19, 50–55 this is the first report identifying that fluconazole treatment increases cellular Fe levels. In fact, at 12 h, fluconazole-treated cells contain 815 µM total Fe compared to 456 µM for untreated cells. Despite this overall increase in Fe content, fluconazole treatment depletes the EPR-detectable Fe signal at g = 4.3, which arises primarily from Fe(III) stored in the vacuole.56 It has been reported for S. cerevisiae that during Fe deficiency, vacuolar Fe is reduced to Fe(II) and exported to the cytosol for cell use, causing a reduction in the g = 4.3 EPR signal.37 Taken together, the elevated levels of total Fe and decreased levels of vacuolar Fe we observe in C. albicans suggest that fluconazole induces biological but not chemical Fe deficiency, similar to what we observe for Cu. These data implicate a model by which C. albicans mobilizes its labile Fe pool to sites of Fe protein synthesis and increases Fe uptake to compensate for this additional need. This model is supported by the increase in heme levels in cells treated with fluconazole. Interestingly, the observation that growth inhibition by fluconazole plus Cu can be overcome by adding heme suggests that the increased heme biosynthesized by the cell is either insufficient to recover from drug stress, or that these conditions impair its trafficking and utilization. The increase in heme is insufficient to fully account for the all of the Fe mobilized from the vacuolar pool. In S. cerevisiae cells grown in low-Fe medium, Fe exists primarily as mitochondrial Fe and nonheme high-spin Fe(II) species located outside of the mitochondria, the function of which is unknown.37 During fluconazole treatment, Fe use appears to be prioritized over Fe storage and Fe is likely redistributed to sites that would not be detectable by whole cell EPR, including Fe-S clusters, low- and high-spin hemes, and nonheme high-spin Fe(II) species.
Conclusions
Our study establishes that drug treatment with fluconazole comprehensively impacts cellular metal requirements of C. albicans and induces metal-specific adaptation strategies by which the cell meets these needs. Paradoxically, the call for increased Cu leads the cell to new vulnerabilities that further delay its growth recovery from drug treatment. The implications of antimicrobial drug treatment on cellular metal homeostasis are likely to vary with the mechanisms and targets of the drugs. Given another drug or stressor, the responses would be expected to differ from those observed for fluconazole. Yet, the requirement for metals is inseparable from so many biological processes that the ability to meet this requirement while under drug stress likely has vast implications for drug treatment outcomes in C. albicans and other pathogens, knowledge that could potentially be exploited to design antifungal compounds that capitalize on metal-related vulnerabilities created during drug stress.57
Methods
Materials and General Methods
Chemicals and solvents were obtained from commercial suppliers and used as received unless otherwise noted. All aqueous solutions were prepared using Milli-Q water unless otherwise noted. Stock solutions were prepared either in DMSO or Milli-Q water. Working stock solutions for each experiment were prepared by serial dilution into growth media. Stock solutions of Mg(II) (1.0 M), Ca(II) (1.0 M), Cu(II) (100 mM), Fe(III) (100 mM), Mn(II) (100 mM), Zn(II) (100 mM), Ni(II) (100 mM), and Co(II) (100 mM) were prepared from MgSO4·7H2O, CaCl2·H2O, CuSO4·5H2O or CuCl2, FeCl3·6H2O, MnCl2·4H2O, ZnSO4·7H2O, NiCl2, and CoCl2. Working solutions of metal salts were prepared for each experiment by serial dilution into growth media from concentrated stock solutions prepared in Milli-Q water.
Yeast Strains and Culture Conditions
Fungal stocks were maintained in 25% glycerol at –80 °C. Unless otherwise noted, experiments were performed with C. albicans clinical isolate SC5314, which was obtained from the American Type Culture Collection (ATCC). The SN152 strain and isogenic mac1Δ/Δ and cup2Δ/Δ strains were obtained from the Fungal Genetics Stock Center.58, 59 The KC2 strain and isogenic crp1Δ/Δ, cup1Δ/Δ, crp1Δ/Δ cup1Δ/Δ, and ccc2Δ/Δ strains as well as the CA-IF100 strain and isogenic sod1Δ/Δ, sod2Δ/Δ, sod3Δ/Δ strains were generously provided by the Culotta Lab of Johns Hopkins University. The ctr1Δ/Δ was generously provided by the Brown Lab of the University of Aberdeen. C. albicans cells were cultured at 30 °C in yeast peptone dextrose (YPD, Gibco) media, unless otherwise indicated. Full strain information is provided in Suppl. Table 12. All experiments were performed in commercial yeast peptone dextrose (YPD) media (Gibco) unless otherwise noted. Analytical metal concentrations for two lots of YPD used in these experiments are provided in Suppl. Tables 5–6.
Growth assays (General)
Prior to all experiments, C. albicans cells were streaked onto YPD agar plates from frozen glycerol stocks and incubated at 30 °C for 24 h. A single colony was used to inoculate growth medium (10 mL), and this culture was incubated overnight (~18 h) at 30 °C with shaking at 200 rpm. The overnight culture was then diluted to an optical density (OD600) of 0.002 with fresh media and this diluted culture was used as the working culture. Compounds to be tested were serially diluted 2-fold in growth medium from DMSO stocks to final concentrations indicated in figure legends, with <1% DMSO and plated in a clear, flat-bottomed 96-well plate. For experiments in which supplemental metals were added, fresh stock solutions were prepared in Milli-Q water and added to appropriate wells at final concentrations indicated in figure legends. The working culture was then aliquoted to the 96-well plate to a final OD600 of 0.001 and a final volume of 200 µL per well. For each experiment, a compound-free positive growth control and a cell-free, negative control were included. Plates were incubated for 48 h at 30 °C, 200 rpm. Plates were covered with air-permeable AeraSeal film (Sigma) to minimize evaporation.
Fungal growth was evaluated by measuring OD600 using a PerkinElmer Victor3 V multilabel plate reader at 0, 24, and 48 h. OD600 values were adjusted by subtracting the 0 h timepoint readings from other timepoint data to remove background medium signal. In cases where the OD was outside of the linear range, cell suspensions were diluted 4-fold in fresh media and rescanned. To calculate actual OD values, the 0 h timepoint reading was subtracted from the diluted readings, and this value was multiplied by four. At least two biological replicates were performed with a minimum of two technical replicates per experiment. For a single experiment, each of the replicate conditions were averaged and the error was calculated as standard deviation (SD). Final 48-h timepoint data is reported by plotting OD600 readings versus treatment conditions.
Metal Drop-Out Growth Assays (Defined Media)
All Tris-buffered Synthetic Defined (Tris:SD) media formulations were prepared from Chelex-treated Milli-Q water with individual addition of media components to allow for rigorous control of metal content. To deplete trace metals from water prior to media preparation, Milli-Q water was treated with Chelex 100 resin 100–200 mesh via batch method (50 g/L, Bio-Rad Laboratories). A concentrated stock of Synthetic Defined media omitted for Cu, Fe, Mn, and Zn (10X SD-) was prepared in the Chelex-treated Milli-Q water by adding glucose and yeast nitrogen base (YNB) ingredients at 10X concentrations. YNB components were added individually to avoid trace metals present in commercial YNB mixtures. To prepare working 1X Tris:SD medium, 10X SD- was diluted into Chelex-treated water (1:10), and Ultra-Pure Tris-HCl (VWR) was added to a final concentration of 50 mM. The pH of the media was adjusted to 6.9 using 1.0 M HCl or 1.0 M NaOH, and this media was filter-sterilized. Finally, CuSO4, FeCl3, MnCl2, and ZnSO4 were added, as appropriate, to create individual dropout medias for each metal. Final metal drop-out formulations were analyzed for metal content (Suppl. Tables 7–10).
Cu Drop-Out Growth Assays (YPD Media)
Cu deplete, Cu basal, and Cu supplemented formulations of YPD were prepared as follows. To deplete all metals, YPD broth (Gibco) was passed through a column of 500 g of Chelex 100 resin 100–200 mesh, which raised the pH of the medium to 11. The pH was adjusted to 6.8 with concentrated HCl, and the Chelex resin was regenerated by flushing with water, HCl, and NaOH as described in the instruction manual, then allowed to dry. The media was passed through the Chelex column a second time and adjusted to pH 6.8 with concentrated HCl. The resulting Chelex-treated YPD was sterile filtered into an acid-washed glass storage bottle. Total levels of Mg, Ca, Cu, Fe, Mn, Zn, Co, and Ni in the Chelex-treated YPD were determined by ICP-MS. Cu deplete YPD was prepared using acid-washed volumetric glassware by resupplying Mg, Ca, Fe, Mn, Zn, Co, and Ni to levels found in commercial, non-Chelex-treated YPD (Suppl. Tables 5–6). A portion of the Cu deplete YPD was set aside for later use, and the remainder was used to prepare Cu basal and Cu supplemented YPD by adding the appropriate amount of Cu using acid-washed volumetric glassware. Analytical metal levels of Chelex-treated YPD, and Cu deplete, Cu basal, and Cu supplemented media formulations prepared from Chelex-treated YPD are reported in Suppl. Tables 1–4.
For growth experiments, an overnight culture of SC5314 in non-Chelex-treated YPD was divided into three aliquots, which were washed twice with either Cu-deplete, Cu-replete, or Cu-supplemented Chelex-treated YPD. Each set of cells was resuspended in the appropriate media, diluted to an OD600 of 0.002, and aliquoted to a 96-well plate. Fluconazole was added to final concentrations ranging from 0–100 µM. Plates were incubated at 30 °C and OD600 recorded at 0, 24, and 48 h. All tests were performed in triplicate for each condition in a single experiment, and two separate experiments were carried out. For a single experiment, each of the three replicate conditions were averaged and the error was calculated as standard deviation (SD).
Preparation of Cells for EPR Spectroscopy
Overnight cultures of C. albicans in YPD medium were inoculated into fresh YPD medium at an OD600 of 0.3 and allowed to grow for 3 h at 30 °C with shaking at 200 rpm. Cultures were then either left untreated or treated with CuSO4 (10 µM), fluconazole (10 µM), or both CuSO4 and fluconazole. At the timepoints indicated in figure legends, 2 × 109 cells were harvested and washed with cold 5 mM EDTA in PBS (1 mL) followed by cold 20 mM Tris-HCl, pH 7.4 (1 mL). The cell pellet was resuspended in 300 µL of 20 mM Tris-HCl, pH 7.4 containing 20% glycerol. The entire suspension was transferred to an EPR tube and cells were flash-frozen immediately and stored at –80 °C until EPR measurements were performed.
UV-Vis and EPR Spectroscopy Measurements
UV-Visible absorption spectra were collected using a Varian Cary 50 UV-Visible spectrophotometer in quartz cuvettes with 1 cm pathlengths. X-band continuous wave (CW) EPR spectroscopy was conducted on a Bruker ESP 300 spectrometer equipped with an Oxford Instruments ESR 910 continuous helium flow cryostat. Typical experimental parameters were at 77 K, 9.37 GHz, 20 mW microwave power, and 5 G modulation amplitude. Spectra were baseline corrected using Bruker Xenon data processing software.
Preparation of Cells for ICP-MS
Overnight cultures of C. albicans in YPD medium were inoculated into fresh YPD medium at an OD600 of 0.3 and allowed to grow for 3 h at 30 °C with shaking at 200 rpm. Cultures were then either left untreated or treated with CuSO4 (10 µM), fluconazole (10 µM), or both CuSO4 and fluconazole. At the timepoints indicated in figure legends, 1 × 108 cells were harvested and placed in 15-mL metal-free centrifuge tubes (VWR, cat. no. 89049–170). Cell suspensions were pelleted at 4,000 x g and washed with 1 mL of 1 mM EDTA prepared in ultrapure water for trace metal analysis (VWR, cat. no. 87003–236), then with 1 mL of ultrapure water for trace metal analysis. Pellets were resuspended in 100 µL of concentrated nitric acid (Trace Metal Grade, Fisher) and heated at 90 °C for 1 h. After digestion, samples were diluted with 1.5 mL of 1% nitric acid.
ICP-MS Measurements
Kim Hutchison (Department of Soil Science, North Carolina State University) performed metal content analysis for the Chelex-treated YPD growth media on a Varian 820 ICP-MS. All other ICP-MS measurements were performed as described below by Martina Ralle in the OHSU Elemental Analysis Core with partial support from the NIH instrumentation grant S10RR025512.
ICP-MS analysis was performed using an Agilent 7700x equipped with an ASX 500 autosampler. The system was operated at a radio frequency power of 1550 W, an argon plasma gas flow rate of 15 L/min, and Ar carrier gas flow rate of 0.9 L/min. Elements were measured in kinetic energy discrimination (KED) mode using He gas (4.3 ml/min). Data were quantified using an 11-point (0, 0.5, 1, 2, 5, 10, 20, 50, 100, 500, 1000 ppb (µg/kg)) for all elements, except Mg and Ca (0, 0.05, 0.1, 0.2, 0.5, 1, 2, 5, 10, 50, 100 ppm (µg /kg)) using a multi-element standard (CEM 2, (VHG-SM70B-100) and single element standards for Mg (Inorganic Ventures, CGMG-1) and Ca (Inorganic Ventures, CGCA-1). For each sample, data were acquired in triplicates and averaged. A coefficient of variance (CoV) was determined from frequent measurements of a sample containing ~10 ppb of all elements except Ca (1 ppm). An internal standard (Sc, Ge, Bi) continuously introduced with the sample was used to correct for detector fluctuations and to monitor plasma stability. Accuracy of the calibration curve was assessed by measuring NIST reference material (water, SRM 1643f) twice during the measurement and found to within +/− 10% for all determined elements.
To obtain cellular metal concentrations (atoms/cell or mol/L/cell), the analytical level of phosphorus (P) determined for each sample by ICP-MS was converted to cell count using a calculated constant for the mol P per cell. The constant, determined to be 1.34 ± 0.07 × 10−14 mol P/cell, was calculated by dividing the analytical mol P for each sample by the theoretical number of cells for each sample (1×108), then averaging these values. This constant represents the average P content per cell and corrects for the variation in the number of cells submitted for analysis. For mol/L/cell, a cell volume of 95 fL was used.46 There was low variability in P content across samples (Suppl. Fig. 7), indicating high precision in the number of cells in each sample submitted for analysis.
RNA isolation and qRT-PCR
Overnight cultures of C. albicans in YPD medium were inoculated into fresh YPD medium at an OD600 of 0.3 and allowed to grow for 3 h at 30 °C with shaking at 200 rpm. Cultures were then either left untreated or treated with CuSO4 (10 µM), fluconazole (10 µM), or both CuSO4 and fluconazole. At the timepoints indicated in the figure legends, cells were harvested and washed once with 1 mL PBS. Cell pellets were flash frozen in liquid nitrogen and stored at −80 °C prior to processing. RNA was isolated from the stored pellets using a Qiagen RNeasy Mini Kit, and DNase was treated with a Turbo DNA-free kit (Roche). Quantitative PCR (qPCR) was performed using specific primers for EFB1, CTR1, CRP1, CUP1, SOD1, and SOD3 (primer sequences are described in Suppl. Table 11) and iQ SYBR green Supermix (Bio-Rad), and amplification and detection were performed using a CFX384 real-time system (Bio-Rad), as previously described.60 Normalized expression levels were determined by calculating the threshold cycle (ΔΔCT) value for each gene in relation to EFB1.
Aconitase enzymatic activity
Overnight cultures of C. albicans in yeast nitrogen base (YNB) medium containing 2% glucose were inoculated into fresh YNB medium at an OD600 of 0.5 and allowed to grow for 2 h at 30 °C with shaking at 200 rpm. Cultures were then either left untreated or treated with CuSO4 (10 µM), fluconazole (50 µM), or both CuSO4 and fluconazole for 3 h. After treatment, cells were washed once with 5 mM EDTA in PBS and twice with PBS. Aconitase activity was measured spectrophotometrically and normalized to malate dehydrogenase (MDH) activity (non-Fe-S enzyme) as described previously.34, 61
Total Heme Quantification
Measurements of total heme were carried out using a fluorimetric assay as described previously.42 The assay is designed to measure the fluorescence of protoporphyrin IX upon release of iron from heme following boiling. Overnight cultures were grown for ~18 h, then diluted to an OD600 of 0.3 and this subculture was grown for an additional 3 h. The resulting culture was aliquoted to sterile Erlenmeyer flasks (50 mL/flask) and either left untreated or treated with CuSO4 (10 µM), fluconazole (10 µM), or both CuSO4 and fluconazole. At the indicated timepoints, 1 × 108 cells were harvested, washed in sterile water, and resuspended in 500 µL of 20 mM oxalic acid. These cell suspensions were stored in the dark at 4 °C overnight (16–24 h). Next, an equal volume (500 µL) of 2 M oxalic acid was added to the 20 mM oxalic acid cell suspensions. Note that 2 M oxalic acid must be heated to ~ 40 °C in a fume hood to promote full solvation. This suspension was mixed well, and the samples were split, with half the cell suspension transferred to a heat block set to 95 °C and heated for 30 min and the other half kept at room temperature (~22 °C) for 30 min. All suspensions were centrifuged on a table-top microcentrifuge at 6000 x g, and the supernatant was collected.
Porphyrin fluorescence (excitation 405 nm, emission 625 nm) of 200 µL of each sample was analyzed on an Edinburgh FS5 Spectrofluorometer with slit widths set to 2 nm. Excitation was provided by a 150 W Xenon lamp and photons were detected by a temperature stabilized Hamamatsu R928P PMT. Spectra are corrected for lamp intensity and spectral throughput including optic, grating, and detector responses as provided by Edinburgh Instruments using calibrated lamps, and automatic order-sorting filters eliminate any second order effects. Each sample for emission measurements was contained in a quartz cuvette with a 5 mm pathlength (325-µL volume; Starna) and analyzed at room temperature (~22 °C). Heme concentrations were calculated from a standard curve prepared by diluting 25–200 µM hemin chloride stock solutions in 0.1 M NaOH into Milli-Q water. To calculate heme concentrations, the fluorescence of the unboiled sample (taken to be the background level of protoporphyrin IX) was subtracted from the fluorescence of the boiled sample (taken to be the free base porphyrin generated upon release of heme iron). The concentration of heme per cell was determined by dividing the moles of heme determined by this fluorescence assay by the number of cells in each measured sample (0.5 × 108), providing moles of heme per cell. This value was then converted to cellular concentration by dividing by the volume of a C. albicans cell, taken to be 95 fL.46
Statistical Analysis
In all figures containing OD600 measurements, error bars represent the standard deviations (SD) of 2–6 technical replicates from a single experiment. In all other figures, error bars represent the standard errors of the means (SEM) of results from a number of biological replicates (n), as indicated in the figure legends. The SEM was used because it provides a measurement of the accuracy of means of results of comparisons between different biological replicates. The statistical test chosen was analysis of variance (ANOVA) for repeated measures. The rationale for using repeated-measures tests is that the experimental samples within a biological replicate are paired by experimental day. Data corresponding to results of statistical tests and the calculated P values are indicated in the figure legends. For those P values calculated with ANOVA which were significant, the Fisher’s LSD test was applied to find differences within the groups and the data were labeled with asterisks corresponding to statistical significance as follows: ****, P = <0.0001; ***, P = 0.0001 to P = <0.001; **, P = 0.001 to P = <0.01; *, P = 0.01 to P = <0.05; ns (not significant), P = >0.05. Nonsignificant values are not indicated.
Supplementary Material
Significance to Metallomics.
Understanding how pathogens respond to drugs is important for improving treatment outcomes of infection. We found that the antifungal drug fluconazole fundamentally influences the metallome of Candida albicans. Furthermore, the amount of copper in the growth environment affects cellular recovery from drug stress. Cells need nutrient metals to thrive, but too much can be damaging. Our findings are important because availability of metals, which is largely regulated by our immune system, varies based on the location and progression of disease. The implications provide a broadly applicable perspective for considering how drug efficacy influences, and is influenced by, mechanisms cells use to manage metals.
Acknowledgments
We are grateful to Prof. Dennis Thiele and Sarela Garcia-Santamarina (Duke University, Durham, NC) for valuable discussions and use of resources in their laboratory. We thank Prof. Amit Reddi and Rebecca Donegan (Georgia Tech, Atlanta, GA) and Prof. Karrera Djoko (Durham University, Durham, UK) for valuable discussions. We are also grateful to Prof. Val Culotta (Johns Hopkins University, Baltimore, MD) for providing several C. albicans deletion strains and for helpful discussions and Prof. Alistair Brown (University of Aberdeen, Aberdeen, UK) for providing the ctr1Δ/Δ strain of C. albicans. This work was supported by the National Institutes of Health (Grant GM084176). E.W.H. acknowledges support from the United States Department of Education GAANN Fellowship (Award Number: P200A150114).
Footnotes
Electronic Supplementary Information (ESI) available: supplementary figures of growth curves, spectra and cellular assays, tables of ICP-MS data, yeast strains and primers.
Conflicts of interest
There are no conflicts to declare.
References
- 1.Weiss G and Carver PL, Role of divalent metals in infectious disease susceptibility and outcome, Clin. Microbiol. Infect, DOI: 10.1016/j.cmi.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 2.Skaar EP and Raffatellu M, Metals in infectious diseases and nutritional immunity, Metallomics, 2015, 7, 926–928. [DOI] [PubMed] [Google Scholar]
- 3.Zygiel EM and Nolan EM, Transition Metal Sequestration by the Host-Defense Protein Calprotectin, Annu. Rev. Biochem, 2018, 87, 621–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ladomersky E and Petris MJ, Copper tolerance and virulence in bacteria, Metallomics, 2015, 7, 957–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djoko KY, Ong C.-l. Y., Walker MJ and McEwan AG, The Role of Copper and Zinc Toxicity in Innate Immune Defense against Bacterial Pathogens, J. Biol. Chem, 2015, 290, 18954–18961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.García-Santamarina S and Thiele DJ, Copper at the Fungal Pathogen-Host Axis, J. Biol. Chem, 2015, 290, 18945–18953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Potrykus J, Stead D, MacCallum DM, Urgast DS, Raab A, van Rooijen N, Feldmann J and Brown AJP, Fungal Iron Availability during Deep Seated Candidiasis Is Defined by a Complex Interplay Involving Systemic and Local Events, PLoS Pathog, 2013, 9, e1003676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen C, Pande K, French SD, Tuch BB and Noble SM, An Iron Homeostasis Regulatory Circuit with Reciprocal Roles in Candida albicans Commensalism and Pathogenesis, Cell Host Microbe, 2011, 10, 118–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Broxton CN and Culotta VC, An Adaptation to Low Copper in Candida albicans Involving SOD Enzymes and the Alternative Oxidase, PLoS One, 2016, 11, e0168400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li CX, Gleason JE, Zhang SX, Bruno VM, Cormack BP and Culotta VC, Candida albicans adapts to host copper during infection by swapping metal cofactors for superoxide dismutase, Proc. Natl. Acad. Sci. USA, 2015, 112, E5336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mackie J, Szabo EK, Urgast DS, Ballou ER, Childers DS, MacCallum DM, Feldmann J and Brown AJP, Host-Imposed Copper Poisoning Impacts Fungal Micronutrient Acquisition during Systemic Candida albicans Infections, PLoS One, 2016, 11, e0158683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu W, Solis NV, Ehrlich RL, Woolford CA, Filler SG and Mitchell AP, Activation and Alliance of Regulatory Pathways in C. albicans during Mammalian Infection, PLoS Biol, 2015, 13, e1002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Crawford AC, Lehtovirta-Morley LE, Alamir O, Niemiec MJ, Alawfi B, Alsarraf M, Skrahina V, Costa ACBP, Anderson A, Yellagunda S, Ballou ER, Hube B, Urban CF and Wilson D, Biphasic zinc compartmentalisation in a human fungal pathogen, PLoS Pathog, 2018, 14, e1007013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crawford A and Wilson D, Essential metals at the host–pathogen interface: nutritional immunity and micronutrient assimilation by human fungal pathogens, FEMS Yeast Res, 2015, 15. [DOI] [PMC free article] [PubMed]
- 15.Ballou ER and Wilson D, The roles of zinc and copper sensing in fungal pathogenesis, Curr. Opin. Microbiol, 2016, 32, 128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ene IV, Heilmann CJ, Sorgo AG, Walker LA, de Koster CG, Munro CA, Klis FM and Brown AJ, Carbon source-induced reprogramming of the cell wall proteome and secretome modulates the adherence and drug resistance of the fungal pathogen Candida albicans, Proteomics, 2012, 12, 3164–3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu N-N, Flanagan PR, Zeng J, Jani NM, Cardenas ME, Moran GP and Köhler JR, Phosphate is the third nutrient monitored by TOR in Candida albicans and provides a target for fungal-specific indirect TOR inhibition, Proc. Natl. Acad. Sci. USA, 2017, 114, 6346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Polvi EJ, Averette AF, Lee SC, Kim T, Bahn Y-S, Veri AO, Robbins N, Heitman J and Cowen LE, Metal Chelation as a Powerful Strategy to Probe Cellular Circuitry Governing Fungal Drug Resistance and Morphogenesis, PLoS Genet, 2016, 12, e1006350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prasad T, Chandra A, Mukhopadhyay CK and Prasad R, Unexpected Link between Iron and Drug Resistance of Candida spp.: Iron Depletion Enhances Membrane Fluidity and Drug Diffusion, Leading to Drug-Susceptible Cells, Antimicrob. Agents Chemother, 2006, 50, 3597–3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown GD, Denning DW and Levitz SM, Tackling Human Fungal Infections, Science, 2012, 336, 647. [DOI] [PubMed] [Google Scholar]
- 21.Vasicek EM, Berkow EL, Flowers SA, Barker KS and Rogers PD, UPC2 Is Universally Essential for Azole Antifungal Resistance in Candida albicans, Eukaryot. Cell, 2014, 13, 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dhamgaye S, Bernard M, Lelandais G, Sismeiro O, Lemoine S, Coppée J-Y, Le Crom S, Prasad R and Devaux F, RNA sequencing revealed novel actors of the acquisition of drug resistance in Candida albicans, BMC Genomics, 2012, 13, 396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu Z, Zhang L-X, Zhang J-D, Cao Y-B, Yu Y-Y, Wang D-J, Ying K, Chen W-S and Jiang Y-Y, cDNA microarray analysis of differential gene expression and regulation in clinically drug-resistant isolates of Candida albicans from bone marrow transplanted patients, Int. J. Med. Microbiol, 2006, 296, 421–434. [DOI] [PubMed] [Google Scholar]
- 24.Rogers PD and Barker KS, Evaluation of differential gene expression in fluconazole-susceptible and-resistant isolates of Candida albicans by cDNA microarray analysis, Antimicrob. Agents Chemother, 2002, 46, 3412–3417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hunsaker EW and Franz KJ, Copper potentiates azole antifungal activity in a way that does not involve complex formation, Dalton Trans, 2019, 48, 9654–9662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosenberg A, Ene IV, Bibi M, Zakin S, Segal ES, Ziv N, Dahan AM, Colombo AL, Bennett RJ and Berman J, Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia, Nat. Commun, 2018, 9, 2470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besold AN, Culbertson EM and Culotta VC, The Yin and Yang of copper during infection, J. Biol. Inorg. Chem, 2016, 21, 137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marvin ME, Williams PH and Cashmore AM, The Candida albicans CTR1 gene encodes a functional copper transporter, Microbiology, 2003, 149, 1461–1474. [DOI] [PubMed] [Google Scholar]
- 29.Marvin ME, Mason RP and Cashmore AM, The CaCTR1 gene is required for high-affinity iron uptake and is transcriptionally controlled by a copper-sensing transactivator encoded by CaMAC1, Microbiology, 2004, 150, 2197–2208. [DOI] [PubMed] [Google Scholar]
- 30.Homann OR, Dea J, Noble SM and Johnson AD, A phenotypic profile of the Candida albicans regulatory network, PLoS Genet, 2009, 5, e1000783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissman Z, Berdicevsky I, Cavari BZ and Kornitzer D, The high copper tolerance of Candida albicans is mediated by a P-type ATPase, Proc. Natl. Acad. Sci. USA, 2000, 97, 3520–3525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eck R, Hundt S, Härtl A, Roemer E and Künkel W, A multicopper oxidase gene from Candida albicans: cloning, characterization and disruption, Microbiology, 1999, 145, 2415–2422. [DOI] [PubMed] [Google Scholar]
- 33.Lamarre C, LeMay J-D, Deslauriers N and Bourbonnais Y, Candida albicans Expresses an Unusual Cytoplasmic Manganese-containing Superoxide Dismutase (SOD3 Gene Product) upon the Entry and during the Stationary Phase, J. Biol. Chem, 2001, 276, 43784–43791. [DOI] [PubMed] [Google Scholar]
- 34.Garcia-Santamarina S, Uzarska MA, Festa RA, Lill R and Thiele DJ, Cryptococcus neoformans Iron-Sulfur Protein Biogenesis Machinery Is a Novel Layer of Protection against Cu Stress, mBio, 2017, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Macomber L and Imlay JA, The iron-sulfur clusters of dehydratases are primary intracellular targets of copper toxicity, Proc. Natl. Acad. Sci. USA, 2009, 106, 8344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srinivasan C, Liba A, Imlay JA, Valentine JS and Gralla EB, Yeast Lacking Superoxide Dismutase(s) Show Elevated Levels of “Free Iron” as Measured by Whole Cell Electron Paramagnetic Resonance, J. Biol. Chem, 2000, 275, 29187–29192. [DOI] [PubMed] [Google Scholar]
- 37.Holmes-Hampton GP, Jhurry ND, McCormick SP and Lindahl PA, Iron Content of Saccharomyces cerevisiae Cells Grown under Iron-Deficient and Iron-Overload Conditions, Biochemistry, 2013, 52, 105–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henry KW, Nickels JT and Edlind TD, Upregulation of ERG Genes in Candida Species by Azoles and Other Sterol Biosynthesis Inhibitors, Antimicrob. Agents Chemother, 2000, 44, 2693–2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Whaley SG, Berkow EL, Rybak JM, Nishimoto AT, Barker KS and Rogers PD, Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species, Front. Microbiol, 2016, 7, 2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Correia MA, Sinclair PR and De Matteis F, Cytochrome P450 Regulation: The Interplay Between its Heme and Apoprotein Moities in Synthesis, Assembly, Repair, and Disposal, Drug Metab. Rev, 2011, 43, 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ebert PS, Hess RA, Frykholm BC and Tschudy DP, Succinylacetone, a potent inhibitor of heme biosynthesis: Effect on cell growth, heme content and δ-aminolevulinic acid dehydratase activity of malignant murine erythroleukemia cells, Biochem. Biophys. Res. Commun, 1979, 88, 1382–1390. [DOI] [PubMed] [Google Scholar]
- 42.Hanna DA, Hu R, Kim H, Martinez-Guzman O, Torres MP and Reddi AR, Heme bioavailability and signaling in response to stress in yeast cells, J. Biol. Chem, 2018, 293, 12378–12393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Djoko KY and McEwan AG, Antimicrobial Action of Copper Is Amplified via Inhibition of Heme Biosynthesis, ACS Chem. Biol, 2013, 8, 2217–2223. [DOI] [PubMed] [Google Scholar]
- 44.Raja MR, Waterman SR, Qiu J, Bleher R, Williamson PR and O’Halloran TV, A copper hyperaccumulation phenotype correlates with pathogenesis in Cryptococcus neoformans, Metallomics, 2013, 5, 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wolford JL, Northwestern Univ, 2006.
- 46.Klis FM, de Koster CG and Brul S, Cell wall-related bionumbers and bioestimates of Saccharomyces cerevisiae and Candida albicans, Eukaryot. Cell, 2014, 13, 2–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adams J and Hansche PE, Population Studies in Microorganisms I. Evolution of Diploidy in Saccharomyces cerevisiae, Genetics, 1974, 76, 327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Synnott JM, Guida A, Mulhern-Haughey S, Higgins DG and Butler G, Regulation of the Hypoxic Response in Candida albicans, Eukaryot. Cell, 2010, 9, 1734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.MacDiarmid CW, Gaither LA and Eide D, Zinc transporters that regulate vacuolar zinc storage in Saccharomyces cerevisiae, EMBO J, 2000, 19, 2845–2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demuyser L, Swinnen E, Fiori A, Herrera-Malaver B, Verstrepen K and Van Dijck P, Mitochondrial Cochaperone Mge1 Is Involved in Regulating Susceptibility to Fluconazole in Saccharomyces cerevisiae and Candida Species, mBio, 2017, 8. [DOI] [PMC free article] [PubMed]
- 51.Fiori A and Van Dijck P, Potent synergistic effect of doxycycline with fluconazole against Candida albicans is mediated by interference with iron homeostasis, Antimicrob. Agents Chemother, 2012, 56, 3785–3796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Savage KA, Parquet M. d. C., Allan DS, Davidson RJ, Holbein BE, Lilly EA and Fidel PL, Iron Restriction to Clinical Isolates of Candida albicans by the Novel Chelator DIBI Inhibits Growth and Increases Sensitivity to Azoles In Vitro and In Vivo in a Murine Model of Experimental Vaginitis, Antimicrob. Agents Chemother, 2018, 62, e02576–02517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ząbek A, Nagaj J, Grabowiecka A, Dworniczek E, Nawrot U, Młynarz P and Jeżowska-Bojczuk M, Activity of fluconazole and its Cu(II) complex towards Candida species, Med. Chem. Res, 2015, 24, 2005–2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peng CA, Gaertner AAE, Henriquez SA, Fang D, Colon-Reyes RJ, Brumaghim JL and Kozubowski L, Fluconazole induces ROS in Cryptococcus neoformans and contributes to DNA damage in vitro, PLoS One, 2018, 13, e0208471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim J, Cho Y-J, Do E, Choi J, Hu G, Cadieux B, Chun J, Lee Y, Kronstad JW and Jung WH, A defect in iron uptake enhances the susceptibility of Cryptococcus neoformans to azole antifungal drugs, Fungal Genet. Biol, 2012, 49, 955–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cockrell AL, Holmes-Hampton GP, McCormick SP, Chakrabarti M and Lindahl PA, Mössbauer and EPR Study of Iron in Vacuoles from Fermenting Saccharomyces cerevisiae, Biochemistry, 2011, 50, 10275–10283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hunsaker EW and Franz KJ, Emerging Opportunities To Manipulate Metal Trafficking for Therapeutic Benefit, Inorg. Chem, 2019, DOI: 10.1021/acs.inorgchem.9b01029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Noble SM and Johnson AD, Strains and strategies for large-scale gene deletion studies of the diploid human fungal pathogen Candida albicans, Eukaryot. Cell, 2005, 4, 298–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCluskey K, Wiest A and Plamann M, The Fungal Genetics Stock Center: a repository for 50 years of fungal genetics research, J. Biosci, 2010, 35, 119–126. [DOI] [PubMed] [Google Scholar]
- 60.Festa RA, Helsel ME, Franz KJ and Thiele DJ, Exploiting Innate Immune Cell Activation of a Copper-Dependent Antimicrobial Agent during Infection, Chem. Biol, 2014, 21, 977–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pierik AJ, Netz DJA and Lill R, Analysis of iron–sulfur protein maturation in eukaryotes, Nat. Protoc, 2009, 4, 753. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.