Abstract
As the largest family of membrane proteins in the human genome, G protein-coupled receptors (GPCRs) constitute the targets of more than one-third of all modern medicinal drugs. In the central nervous system (CNS), widely distributed GPCRs in neuronal and nonneuronal cells mediate numerous essential physiological functions via regulating neurotransmission at the synapses. Whereas their abnormalities in expression and activity are involved in various neuropathological processes. CNS conditions thus remain highly represented among the indications of GPCR-targeted agents. Mounting evidence from a large number of animal studies suggests that GPCRs play important roles in the regulation of neuronal excitability associated with epilepsy, a common CNS disease afflicting approximately 1-2% of the population. Surprisingly, none of the US Food and Drug Administration (FDA)-approved (>30) antiepileptic drugs (AEDs) suppresses seizures through acting on GPCRs. This disparity raises concerns about the translatability of these preclinical findings and the druggability of GPCRs for seizure disorders. The currently available AEDs intervene seizures predominantly through targeting ion channels and have considerable limitations, as they often cause unbearable adverse effects, fail to control seizures in over 30% of patients, and merely provide symptomatic relief. Thus, identifying novel molecular targets for epilepsy is highly desired. Herein, we focus on recent progresses in understanding the comprehensive roles of several GPCR families in seizure generation and development of acquired epilepsy. We also dissect current hurdles hindering translational efforts in developing GPCRs as antiepileptic and/or antiepileptogenic targets and discuss the counteracting strategies that might lead to a potential cure for this debilitating CNS condition.
Keywords: AED, cAMP, epilepsy, epileptogenesis, GPCR, seizure
1. Epilepsy
Epilepsy is one of the most common neurological disorders that afflicts approximately 1-2% of the population globally. The disease is symptomized by epileptic seizures that are defined as unprovoked and spontaneous electrical disturbances in the brain due to abnormally excessive and synchronous activities of a group of brain neurons. The process of transforming a normal brain into one that generates epileptic seizures is known as epileptogenesis. Over the course of decades, we have witnessed the remarkable scientific advances in understanding the pathophysiological processes associated with seizure initiation, escalation and dissemination in the brain, leading to the introduction of the third-generation antiepileptic drugs (AEDs, also often called anticonvulsants) to the drug market (Luszczki, 2009). However, there are still about 30-40% of epilepsy patients who are inadequately treated, as they develop epilepsy forms that are refractory to the current frontline treatments (Ben-Menachem, 2014). Moreover, most antiseizure drugs are well-recognized for their broad neurotoxic adverse effects, the most common of which include, but are not limited to, drowsiness, dizziness, nausea, fatigue, headache, blurred vision, tremor, incoordination, and cognitive impairment in young children (Perucca and Gilliam, 2012).
It is another very unfortunate fact that all current antiseizure medications act merely to suppress seizures in patients who have already been diagnosed with epilepsy (Loscher et al., 2013; Varvel et al., 2015). Notwithstanding, nearly 50 clinical trials for antiepileptogenesis and seizure prevention based on a hypothesis that antiseizure drugs could also be antiepileptogenic, to date there is no therapeutic strategy that has been shown to either prevent the development of epilepsy in patients prior to the first seizure or modify the disease outcome in those diagnosed with epilepsy (Abou-Khalil, 2007; Temkin, 2001, 2009). While some preclinical studies have suggested antiepileptogenic effects from certain AEDs (Blumenfeld et al., 2008; Russo et al., 2010) or potential therapeutic agents (Aronica et al., 2017; Klein et al., 2018; Ravizza and Vezzani, 2018) in animal models of epilepsy, they have not been proven in human studies to date (Kaminski et al., 2014). One seminal conclusion that can be drawn from these monumental efforts at both preclinical and clinical levels is that the biology of epileptogenesis is likely quite different from the biology of the epileptic brain itself. Discovery of epilepsy prevention or modification strategies must ultimately hinge on a well understanding of the signaling pathways that govern the pathogenic process of epileptogenesis following the initial precipitating events, which can help to identify novel molecular targets that can be manipulated by small-molecule agents (Dey et al., 2016).
Epilepsy is a disease with great diversity, as there are more than 15 different seizure types and 30 epilepsy syndromes (Berg et al., 2010). Many Mendelian forms of epilepsy often involve ion channel mutations (Di Cristo et al., 2018). In fact, most of the current AEDs interrupt seizures by directly modulating ligand- or voltage-gated ion channels (Rogawski and Loscher, 2004). However, for the acquired forms of epilepsy we must seek molecular mechanisms that cause the dysfunction of ion channels, which might guide us to identify key epileptogenic mediators that dictate the expression and functional state of ion channel proteins that set the seizure threshold (Varvel et al., 2015). Control of these potential epileptogenic mediators might be the key to interrupting epileptogenesis and could provide prevention and/or modification for acquired epilepsy. Over the past decade, a number of G protein-coupled receptors (GPCRs) have been emerging as candidates for such epileptogenic mediators owing to their essential roles in the regulation of ion channel functions, thereby altering neuronal excitability and setting the seizure threshold. In this review, we highlight our current understanding of GPCRs as potential epileptogenic mediators by focusing on recent preclinical and clinical efforts in seeking novel therapeutic targets to control acute seizures and interrupt acquired epileptogenesis.
2. GPCRs as CNS drug targets
2.1. GPCRs – the most studied drug target family
GPCRs, also known as seven-transmembrane (7-TM) receptors, represent the largest super protein family of receptors that detect extracellular molecules and trigger signal transmission inside of the cell. GPCR signaling transduction occurs through coupling to a number of intracellular proteins, such as heterotrimeric G proteins, β-arrestins and kinases, which then activate different downstream effectors and initiate cascades of molecular, cellular and physiological responses. The heterotrimeric G protein complex consists of a Gα subunit, of which there exist four major types (i.e., Gαs, Gαi/o, Gαq/11, and Gα12/13), coupled to a combination of Gβ and Gγ subunits. In response to the ligand binding, GPCR undergoes conformational change that enables its intracellular portion to function as a guanine nucleotide exchange factor (GEF) to allosterically activate the associated Gα protein by replacing the bound GDP with a GTP. The Gα-GTP then dissociates from Gβγ dimer to further act on intracellular signaling proteins or target functional proteins directly, depending on the type of Gα. GPCR can also mediate pathways that are independent of G protein via recruiting β-arrestins to initiate G protein-independent signaling.
These highly specified membrane receptors are only found in eukaryotes, and there are more than 800 different genes in humans – or approximately 4% of the entire protein-coding genome – that code for GPCRs (Bjarnadottir et al., 2006; Ikeda et al., 2018). Based on their protein sequence and structural similarity, GPCRs are phylogenetically divided into five major classes (GRAFS): Glutamate (15 members to date), Rhodopsin (701 members), Adhesion (24 members), Frizzled/Taste2 (24 members), and Secretin (15 members), with each class displaying distinct ligand binding properties (Bjarnadottir et al., 2006; Stevens et al., 2013). GPCRs are detected in almost all types of tissues and organs, where they are involved in nearly all types of physiological, functional and pathological processes in the body. The ligands that can bind and activate GPCRs include compounds, hormones, odors, pheromones, and neurotransmitters, as they vary in size from small molecules to biological macromolecules, such as nucleic acids, lipids, peptides and proteins, endowing them robust and extensive druggability. As of the year 2018, about 475 drugs targeting 108 unique members of this super receptor family have been approved by the US Food and Drug Administration (FDA). These GPCR-targeted drugs contribute to approximately 34% of all FDA-certified drugs on the market and a global sales volume of over 180 billion US dollars annually (Hauser et al., 2018).
2.2. GPCRs and CNS conditions
In the CNS, more than 90% of non-sensory GPCRs are widely expressed and involved in numerous essential physiological functions including cognition, sensory, mood, appetite, neurogenesis, and synaptic plasticity via regulating neurotransmission at both presynaptic and postsynaptic sites (Gainetdinov et al., 2004; Huang and Thathiah, 2015). Malfunctions in GPCR-regulated neurotransmission contribute to various neuropathological processes such as pain, seizure, anxiety, depression, neurodegeneration, neuroinflammation, etc., and could lead to multiple neurological and psychiatric conditions, making GPCRs appealing molecular targets for these diseases. Indeed, disorders associated with CNS functions remain highly represented among the indications of GPCR-targeted therapeutic agents and account for 124 (~26%) of all FDA-approved GPCR-targeted drugs to date. Among the most prominent targets for the CNS diseases are dopamine, endocannabinoid, opioid, prostanoid, and serotonin receptors. In addition, a myriad of GPCR-targeted agents are currently in clinical studies for CNS indications, demonstrating a sturdy continual interest (Hauser et al., 2017).
Mounting evidence from a large number of animal studies suggests that GPCRs might play some essential roles in the mediation of neuronal excitability via regulating Gαs and Gαi-controlled cAMP signaling as well as Gαq-initiated Ca2+-sensitive pathways, suggesting their appealing therapeutic indications in seizure disorders. In contrast, none of the FDA-approved (>30) current AEDs suppresses epileptic seizures by directly acting on GPCRs. This surprising yet disappointing disparity raises concerns about the translatability of these preclinical findings and the druggability of GPCRs for acute seizures and chronic epilepsy. Among the GPCR families that have been intensively studied for their contributions to the pathophysiological processes related to epileptic seizures are prostanoid, endocannabinoid, adenosine, metabotropic glutamate receptors and several others. Recent progresses in understanding the signaling pathways of these receptors regulating neuronal excitability and setting seizure threshold are summarized below; their potential as novel molecular targets for acute seizures and the development of acquired epilepsy is also discussed.
3. GPCRs in epileptic seizures
3.1. Prostanoid receptors
Prostanoids are a subclass of eicosanoids consisting of prostaglandin D2 (PGD2), PGE2, PGF2α, PGI2 (also known as prostacyclin), and thromboxane A2 (TXA2). These bioactive lipids have been found in nearly all human tissues and mediate numerous physiological and pathological processes including allergy, immunoregulation, inflammation, mitogenesis, muscle contraction, neurotransmission, pain, thrombosis, vasoconstriction, and vasodilatation (Hirata and Narumiya, 2011). Their biosyntheses in the body are comprised of several enzymatic steps and are highly regulated by various physical, chemical and pathophysiological stimuli. During the first step, arachidonic acid, a 20-carbon unsaturated fatty acid, is freed from membrane-bound phospholipids by phospholipase A2 (PLA2) and then converted in a dual enzymatic reaction by cyclooxygenase (COX) to an intermediate molecule prostaglandin H2 (PGH2). Short-lived PGH2 is further rapidly converted to certain prostanoid types by the tissue-specific prostanoid synthases (Qiu et al., 2017; Rojas et al., 2014b; Smyth et al., 2009) (Fig. 1). Prostanoids exert their physiological and pathological functions through acting on a panel of GPCRs. Two currently known GPCRs (DP1 and DP2) are activated by PGD2 and four by PGE2 (EP1, EP2, EP3, and EP4), whereas each of the other three types of prostanoids acts on a single receptor (FP, IP, and TP) (Fig. 1). Hydrophobicity analysis of their protein sequences suggest that they all have a typical GPCR structure, i.e., an extracellular N-terminus, seven membrane-spanning segments, and an intracellular C-terminus, and belong to the rhodopsin-like GPCR receptor family, subfamily A14 (Woodward et al., 2011). Among these nine conventional prostanoid receptor subtypes, EP1, EP2, and FP are the most studied in animal models of epilepsy, yielding extensive evidence that attests to their contributions to epileptic seizures. More studies engaging both pharmacological and genetic strategies are required to clarify whether DP1, EP3, EP4 and TP receptors are also involved in the pathophysiology of seizure disorders (Table 1). To date, no study has been reported about any indication of either DP2 or IP receptor in epileptic seizures.
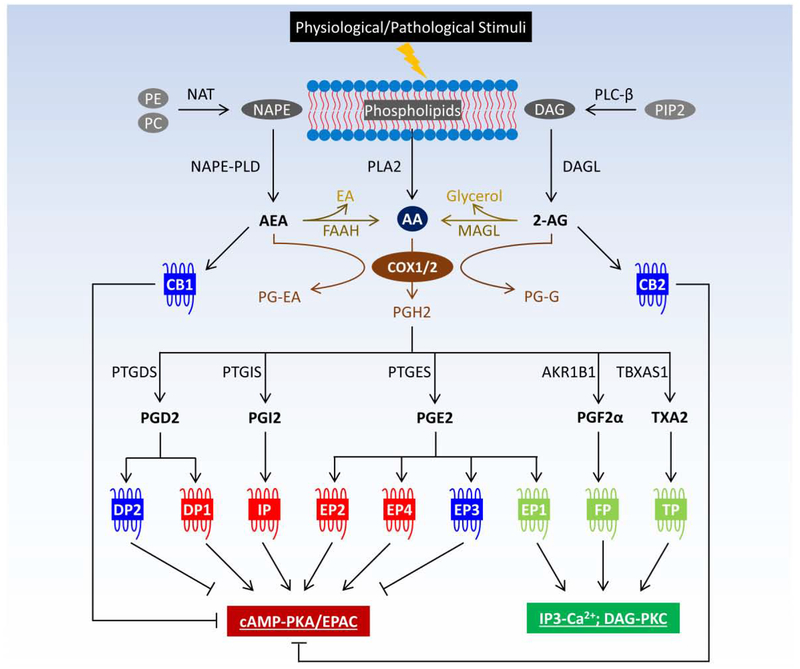
Figure 1.
Crosstalk between prostanoids and endocannabinoids in GPCR signaling. Prostanoids and endocannabinoids are eicosanoids derived from cell membrane-bound phospholipids. Endocannabinoids, such as AEA and 2-AG, can be metabolized to AA, which is the precursor for prostanoid biosynthesis. In addition, COX also metabolizes endocannabinoids to prostaglandin analogs, i.e., PG-EA from AEA and PG-G from 2-AG. Responding to extracellular stimuli, prostanoids and endocannabinoids are rapidly synthesized to mediate wide-ranging physiological and pathological processes via directly acting on a myriad of GPCRs. Abbreviations: 2-AG, 2-arachidonoylglycerol; AA, arachidonic acid; AEA, N-arachidonoylethanolamine; AKR1B1, aldo-keto reductase family 1 member B1 or aldose reductase; cAMP, cyclic adenosine monophosphate; CB, cannabinoid receptor; COX, cyclooxygenase; DAG, diacyl glycerol; DAGL, diacylglycerol lipase; DP, PGD2 receptor; EA, ethanolamine; EP, PGE2 receptor; EPAC, exchange factor directly activated by cAMP; FAAH, fatty acid amide hydrolase; FP, PGF2α receptor; IP, PGI2 receptor; IP3, inositol 1,4,5-triphosphate; MAGL, monoacylglycerol lipase; NAPE, N-arachidonoyl-phosphatidylethanolamine; NAPE-PLD, N-acylphosphatidylethanolamine-specific phospholipase D; NAT, N-acyltransferase; PC, phosphatidylcholine; PE, phosphatidylethanolamine; PG, prostaglandin; PG-EA, PG(D2/E2/F2α/I2)-ethanolamide or prostamide; PG-G, PG(D2/E2/F2α/I2)-glycerol ester; PIP2, phosphatidylinositol 4,5-bisphosphate; PKA, protein kinase A; PKC, protein kinase C; PLA2, phospholipases A2; PLC-β, phospholipase C-β; PTGDS, PGD2 synthase; PTGES, PGE2 synthase; PTGIS, PGI2 synthase; TP, TXA2 receptor; TBXAS1, TXA2 synthase 1; TX, thromboxane.
Table 1.
Prostanoid and cannabinoid receptors in seizure disorders.
| Receptor | G protein | Recent studies and therapeutic outcomes | References |
|---|---|---|---|
| EP1 | Gq/11 | EP1 antagonist SC-19220 (10 nmol, i.c.v.) 15 min before PTZ injection (60 mg/kg, i.p.) reduced PTZ-provoked acute seizures in adult male Wistar rats. | Oliveira et al., 2008 |
| Treatment with EP1 antagonist SC-51089 (10 mg/kg, i.p., b.i.d.) starting 30 min before the first pilocarpine injection interrupted seizure-promoted expression of efflux transporter P-glycoprotein at the blood-brain barrier following lithium-pilocarpine-induced status epilepticus in adult female Wistar rats and restored the anticonvulsant effect of a low dose of phenobarbital (6 mg/kg, i.p.). | Pekcec et al., 2009 | ||
| Pre-Treatment with EP1 antagonist SC-51089 (3-30 mg/kg, i.p.) significantly decreased the seizure severity in adult male NMRI mice after amygdala kindling. | Fischborn et al., 2010 | ||
| Genetic ablation of EP1 receptor increased seizure threshold and showed anti-inflammatory and neuroprotective effects after systemic administration of kainate in male adult C57BL/6 mice. | Rojas et al., 2014 | ||
| Pre-treatment with EP1 antagonist ONO-8713 (10 mg/kg, s.c.) 30 min before PTZ injection (60 mg/kg, i.p.) attenuated, whereas EP1 agonist ONO-DI-004 (10 mg/kg, s.c.) exacerbated PTZ-induced seizures in adult male Swiss mice. | Reschke et al., 2018 | ||
| EP2 | Gs | Treatment with EP2 selective antagonist TG4-155 (5 mg/kg, i.p.) reduced neuronal injury in the hippocampus after pilocarpine (280 mg/kg, i.p.)-induced status epilepticus in male adult C57BL/6 mice. | Jiang et al., 2012 |
| Post-treatment with EP2 antagonist TG6-10-1 (5 mg/kg, i.p.) brought extensive benefits following status epileptics in pilocarpine (280 mg/kg, i.p.)-treated male adult C57BL/6 mice, i.e., reduction in delayed mortality, acceleration of recovery from weight loss and functional impairment, prevention of the blood-brain barrier breakdown, and decrease in neuronal inflammation and injury in the hippocampus. | Jiang et al., 2013; Jiang et al., 2015 | ||
| Post-treatment with TG6-10-1 treatment (5 mg/kg, i.p.) was neuroprotective and accelerated functional recovery after status epilepticus in male adult Sprague Dawley rats treated by DFP (9.5 mg/kg, i.p.). | Rojas et al., 2015 | ||
| Post-treatment with TG6-10-1 (5 mg/kg, i.p.) prevented status epilepticus-induced deficits in the novel object recognition task in male adult Sprague Dawley rats. | Rojas et al., 2016 | ||
| Post-treatment with TG6-10-1 (5 mg/kg, i.p., b.i.d.) reduced prolonged seizure-promoted functional deficits, cytokine induction, reactive gliosis, blood-brain barrier disruption, and hippocampal damage in adult male C57BL/6 mice treated by kainate (30 mg/kg, i.p.). | Jiang et al., 2019 | ||
| EP3 | Gi/o | EP3 antagonist L-826266 (1 nmol, i.c.v.) 15 min before PTZ injection (60 mg/kg, i.p.) increased the latency for clonic seizures induced by PTZ in adult male Wistar rats. | Oliveira et al., 2008 |
| Pre-treatment with EP3 antagonist ONO-AE3-240 (10 mg/kg, s.c.) 30 min before PTZ injection (60 mg/kg, i.p.) attenuated, whereas EP3 agonist ONO-AE-248 (10 mg/kg, s.c.) potentiated PTZ-induced seizures in adult male Swiss mice. | Reschke et al., 2018 | ||
| EP4 | Gs | EP4 antagonist L-161982 (750 pmol, i.c.v.) 15 min before PTZ injection (60 mg/kg, i.p.) increased the latency for PTZ-evoked acute seizures in adult male Wistar rats. | Oliveira et al., 2008 |
| FP | Gq/11 | Intracisternal administration of PGF2α (700 ng/35 g) after pre-treatment with COX inhibitor indomethacin (10 mg/kg, i.p.) prevented seizures and decreased neuronal injury in the hippocampus after systemic injection of kainate for seizure induction in adult male ICR mice; PGF2α alone had no effect on kainate-induced seizures in these animals. | Kim et al., 2008 |
| Intracisternal injection of FP antagonist AL-8810 (10 or 50 ng/35 g) 20 min before kainate injection aggravated seizure activity in adult male ICR mice in a dose-dependent manner. | Kim et al., 2008 | ||
| Intracisternal administration of PGF2α (700 ng) 20 min before kainate injection reduced seizure activity and mortality in immature CD-1 mice. | Chung et al., 2013 | ||
| DP1 | Gs | PGD2 (5 μg, i.c.v.) or an active analog ZK-118.182 (1-100 ng, i.c.v.) 5 min before PTZ injection (60 mg/kg, i.p.) delayed the onset of generalized convulsions and reduced mortality in adult male Wistar rats. | Akarsu et al., 1998 |
| Supression of PGD2 activity by AH-6809 (50 ng, i.c.v.) 20 min before PTZ injection (60 mg/kg, i.p.) or inhibition of PGD2 synthesis by sodium selenite (0.2 μg, i.c.v.) 15 min before PTZ injection (60 mg/kg, i.p.) increased the seizure incidence and intensity in adult male Wistar rats. | Akarsu et al., 1998 | ||
| Genetic ablation of DP1 receptor delayed seizure onset, reduced the duration of generalized tonic-clonic seizures, and decreased number of seizure spikes in adult male C57BL/6 mice treated by PTZ. | Kaushik et al., 2014 | ||
| TP | Gq/11 | TP activation by U-46619 (300 μg/kg, s.c.) 30 min before the injection of PTZ (60 mg/kg; i.p.) increased the latency for PTZ-induced myoclonic jerks and tonic-clonic seizures in adult female C57BL/6 mice. | Freitas et al., 2018 |
| TP antagonist, SQ-29548 (up to 300 μg/kg, i.p.) 30 min before the injection of PTZ (60 mg/kg; i.p.) neither modified PTZ-induced seizures nor blunted the anticonvulsant effect of the agonist U-46619 in adult female C57BL/6 mice. | Freitas et al., 2018 | ||
| CB1 | Gi/o | Adult male C57BL/6 mice lacking CB1 showed decreased seizure threshold in kainate model of temporal lobe epilepsy. | Marsicano et al., 2003 |
| Non-selective CB agonist WIN 55212-2 showed dose-dependent anticonvulsant effects in hippocampal neuronal culture models of status epilepticus and acquired epilepsy; whereas the anticonvulsant action of WIN 55212-2 in these models was specifically blocked by a CB1 antagonist SR141716. | Blair et al., 2006 | ||
| Genetic ablation of CB1 receptor or pre-treatment with the antagonist SR141716 (10 mg/kg) increased seizure severity without altering seizure-promoted cell proliferation and neuronal death after pilocarpine injection in male adult C57BL/6 mice. | Kow et al., 2014 | ||
| Pre-treatment with CB1 agonist CP55940 (0.3 mg/kg, i.p.) did not show any obvious effect on pilocarpine-induced seizures in male adult C57BL/6 mice. | Kow et al., 2014 | ||
| Treatment with CB1 selective inverse agonist AM251 (20 mg/kg, i.p.) 60 min before kainate injection (30 mg/kg, i.p.) shortened the latency to onset of generalized tonic-clonic seizures and increased mortality in adult male and female C57BL/6 mice. | Sugaya et al., 2016 | ||
| CB2 | Gi/o | CB2 inverse agonist AM630 (2 mg/kg, i.p.), when co-administered with non-selective CB receptor agonist WIN 55212-2 (21 mg/kg, i.p.), but not by itself alone, showed considerable antiepileptic effects in the maximal dentate activation model of partial epilepsy in adult male Wistar rats. | Rizzo et al., 2014 |
| Co-treatment with CB2 selective inverse agonist AM630 (2 mg/kg, i.p.) exacerbated the susceptibility of AM251(20 mg/kg, i.p.)-treated adult male and female C57BL/6 mice to kainate (30 mg/kg, i.p.)-induced seizures. | Sugaya et al., 2016 | ||
| Deletion of both CB1 and CB2, but not either CB1 or CB2 alone, showed spontaneous seizures or handling-induced seizures in adult male and female C57BL/6 mice. | Rowley et al., 2017 |
Abbreviations: DFP, diisopropyl fluorophosphate; PTZ, pentylenetetrazol.
3.1.1. EP1 receptor
In rodents and guinea pigs, the EP1 receptor is widely distributed in organs and tissues such as kidney, lung, stomach, as well as several CNS sites (Ricciotti and FitzGerald, 2011); whereas the expression of EP1 in higher species like humans is more restricted to a few organs and tissues including colon, mast cells, myometrium, pulmonary veins, and skin (Woodward et al., 2011). The EP1 receptor is coupled to G protein complex that contains Gαq/11 and Gβ/γ dimer (Fig. 1). Upon PGE2 binding, Gαq is released from the complex to activate phospholipase C (PLC), which in turn hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to inositol trisphosphate (IP3) and diacyl glycerol (DAG) (Jiang et al., 2017; Rojas et al., 2014b). IP3, via binding to IP3 receptors in the endoplasmic reticulum (ER) that function as calcium channels, increases cytosolic Ca2+ levels, thereby regulating Ca2+-sensitive signaling pathways; whereas DAG functions as a second messenger that activates certain isoforms of protein kinase C (PKC) (Fig. 1). The EP1 receptor has long been known for its roles in neurotoxicity caused by NMDA receptor overactivation and Ca2+ dysregulation after cerebral ischemia (Kawano et al., 2006; Zhou et al., 2008), insinuating the potential involvement of the PGE2 receptor in the pathophysiology of prolonged seizures, which are also often associated with substantial excitotoxic damage within the brain. Indeed, centrally-administered EP1 receptor antagonist SC-19220 reduced pentylenetetrazol (PTZ)-provoked acute seizures in Wistar rats (Oliveira et al., 2008). Likewise, systemic administration of another EP1 antagonist ONO-8713 attenuated, whereas EP1 agonist ONO-DI-004 exacerbated, PTZ-induced seizures in Swiss mice (Reschke et al., 2018). Systemic pre-treatment with high dose of EP1 antagonist SC-51089 significantly decreased the seizure severity in NMRI mice after amygdala kindling (Fischborn et al., 2010). Moreover, genetic ablation of EP1 receptor increased seizure threshold and showed marked anti-inflammatory and neuroprotective effects in the hippocampus after systemic administration of kainate in mice (Rojas et al., 2014a). These findings from pharmacological inhibition and genetic inactivation of the EP1 receptor together demonstrate its contribution to increasing the neuronal excitability and lowering the seizure threshold.
Seizures can induce the expression of transporter P-glycoprotein at the blood-brain barrier that might enhance the brain efflux of AEDs, which promotes pharmacoresistance in epilepsy. Among several key signaling pathways that might be involved in seizure-associated transcriptional activation of P-glycoprotein is the COX/PGE2 cascade (Potschka, 2010). COX-2 inhibition by SC-58236, NS-398, indomethacin, or celecoxib attenuated the increase in P-glycoprotein expression in cortex and hippocampus after status epileptics in rats (van Vliet et al., 2010), which was largely detected in brain capillaries (Zibell et al., 2009). Furthermore, the brain penetration of the antiseizure drug phenytoin was markedly increased by chronic COX-2 inhibition in rats that developed spontaneous recurrent seizures (van Vliet et al., 2010). In line, the EP1-selective antagonist SC-51089 interrupted seizure-promoted expression of efflux transporter P-glycoprotein at the blood-brain barrier following pilocarpine-induced status epilepticus in rats and restored an anticonvulsant effect of a low dose of phenobarbital, a conventional AED and P-glycoprotein substrate (Pekcec et al., 2009). These findings together indicate that the EP1 receptor might be a downstream effector of COX-2 cascade in the upregulation of blood-brain barrier transporter P-glycoprotein following prolonged seizures that mediates brain efflux of AEDs, such as phenytoin and phenobarbital. Thus the EP1 antagonism might represent a promising therapeutic strategy to overcome the pharmacoresistance suffered by more than 30% of epilepsy patients (Potschka, 2010), though the EP1 downstream Gq signaling molecules that are directly responsible for the seizure-driven P-glycoprotein expression remain undetermined.
3.1.2. EP2 receptor
The EP2 receptor is coupled to a G protein that contains Gαs and Gβγ complex. Activation of the receptor by PGE2 results in the dissociation of Gs protein heterotrimeric complex into Gαs and Gβγ, which in turn regulate several cellular signaling pathways (Fig. 1). Particularly, Gαs stimulates adenyl cyclase to elevate cellular levels of cAMP thereby activating protein kinase A (PKA), exchange factor directly activated by cAMP (EPAC), and cAMP response element-binding protein (CREB) regulation of gene transcription. The EP2 receptor appears widely distributed in human body and have been primarily detected in bones, CNS, leukocytes, reproductive system, and smooth muscle (Markovic et al., 2017; Woodward et al., 2011), where it mediates many important normal physiological functions, such as immunoregulation, neuronal plasticity, learning and memory. Furthermore, PGE2 signaling via the EP2 receptor has shown some protective effects in injury settings such as allograft rejection, bone fracture, gastrointestinal damage, and kidney failure (Jiang and Dingledine, 2013). However, accumulating evidence from numerous CNS studies suggests that EP2 receptor signaling might exacerbate secondary neuronal toxicity in several models of chronic neuronal inflammation and degeneration (Andreasson, 2010; Jiang and Dingledine, 2013; Kang et al., 2017; Liu et al., 2019). The EP2 receptor-mediated neurotoxicity might be associated with brain innate immunity since under these conditions, EP2 activation diminished the beneficial functions of microglia (Johansson et al., 2013; Johansson et al., 2015), which are the resident brain immune cells (Smolders et al., 2019). Intriguingly, EP2 signaling that initially causes the activation of microglia could facilitate their delayed death later, thereby potentially contributing to the resolution phase of brain inflammation (Fu et al., 2015; Quan et al., 2013). In addition, EP2 receptor expression is highly inducible in neurons by cerebral ischemic injury, and postnatal ablation of the neuronal EP2 receptor in mice is cerebroprotective (Liu et al., 2019). These studies taken together lend support to a translational strategy suppressing the EP2 signaling to reduce inflammation and injury of the brain following excitotoxic insults.
Inspired by the predominant role for the EP2 receptor in the inflammation-associated CNS conditions described above, a series of small-molecular competitive antagonists for the EP2 receptor have been developed for proof-of-concept studies in animal models over the past decade by us and others (af Forselles et al., 2011; Fox et al., 2015; Ganesh et al., 2014a; Ganesh et al., 2013; Ganesh et al., 2014b; Jiang et al., 2012; Qiu et al., 2019). TG4-155, a potent EP2-selective antagonist identified by high-throughput screening (Jiang et al., 2012), diminished the induction of a number of key pro-inflammatory mediators in rat microglia (Quan et al., 2013), and reduced neuronal injury in the hippocampus after pilocarpine-induced status epilepticus in mice (Jiang et al., 2012). TG6-10-1, a second-generation bioavailable EP2 antagonist with improved in vivo half-life and brain-permeability, provided much broader benefits in the same mouse model of epilepsy, namely, reduction in delayed mortality, acceleration of recovery from weight loss and functional impairment, prevention of the blood-brain barrier breakdown, and decrease in neuronal inflammation and injury in the hippocampus (Jiang et al., 2013; Jiang et al., 2015). The beneficial effects from these EP2 antagonists in the mouse pilocarpine model were also mostly demonstrated in diisopropyl fluorophosphate (DFP)-treated rats (Rojas et al., 2015; Rojas et al., 2016), and in the mouse kainate model of status epilepticus (Jiang et al., 2019) , suggesting that they are not model or species-specific findings. Surprisingly, these EP2-targeted compounds had no effect on the progression of convulsive seizures after the administration of pilocarpine, DFP or kainate, as they did not alter the seizure duration or intensity (Jiang et al., 2013; Jiang et al., 2019; Rojas et al., 2016). Therefore, these benefits from EP2 inhibition following prolonged seizures were not caused by a direct anticonvulsant effect, but rather likely resulted from an anti-inflammatory action of the compounds. The fact that pharmacological inhibition of the EP2 receptor recapitulated most benefits of the conditional ablation of COX-2 restricted to forebrain neurons after status epileptics indicates that the EP2 receptor might be a primary culprit of COX-2/PGE2 cascade-mediated detrimental effects in the CNS (Levin et al., 2012; Serrano et al., 2011). However, the question of whether the EP2 receptor activation by PGE2 also plays a role in the development of spontaneous recurrent seizures following status epilepticus in these animal models remains open.
3.1.3. EP3 receptor
The EP3 receptor is widely expressed in the human body, as its mRNA and protein with several splice variants have been detected in the cardiovascular system, CNS, intestinal epithelium, kidney, reproductive system, and urinary bladder, where the receptor has been implicated in a variety of physiological and pathological processes (Woodward et al., 2011). Coupled to Gi protein, EP3 is classified as an inhibitory type of prostanoid receptor owing to its ability, when bound by PGE2, to inhibit the activity of adenyl cyclase, and thereby to lower cytosol cAMP levels and downregulate the activity of cAMP-dependent signaling pathways (Fig. 1). Intrahippocampal injection of kainate in mice and rats induced EP3 receptor expression in hippocampal astrocyte feet along with the elevation of COX-2 and microsomal prostaglandin E synthase-1 (mPGES-1) in the brain microvasculature, suggesting that PGE2 might contribute to neuronal hyperexcitability by regulating glutamate release from astrocytes via activating astrocytic EP3 (Takemiya et al., 2010). Interestingly, central administration of the EP3-selective antagonist L-826266 increased the latency for clonic seizures induced by PTZ in rats (Oliveira et al., 2008). Similarly, systemic administration of EP3 antagonist ONO-AE3-240 attenuated, whereas EP3 agonist ONO-AE-248 potentiated PTZ-induced seizures in Swiss mice (Reschke et al., 2018). It appears that EP3 receptor activation after PTZ treatment in these mice also contributed to the downregulation of Na+/K+-ATPase activity, an enzyme responsible for the homeostatic ionic equilibrium and the resting membrane potential (Reschke et al., 2018). The EP3 receptor may represent a novel molecular target for the development of new antiseizure therapeutics; however, future studies using genetic strategies overexpressing or disrupting the EP3 receptor are required to validate these pharmacological findings.
3.1.4. EP4 receptor
Resembling the EP2 receptor in many respects, EP4 is coupled to Gαs-Gβγ heterotrimeric complex, and upon PGE2 binding to the receptor, dissociates into Gαs and Gβγ that act to regulate cell signaling pathways predominantly in a cAMP/PKA-dependent way (Fig. 1). However, a direct comparison of EP2 and EP4 receptor signaling revealed that the functional coupling to cAMP pathways seems more efficient for the EP2 subtype than for EP4, as EP4 might also mediate other pathways including PI3K/AKT/mTOR, extracellular signal-regulated kinase (ERK), and p38 mitogen-activated protein kinase (MAPK) pathways (Majumder et al., 2016; Woodward et al., 2011). EP4 is widely expressed in the human body, particularly in the brain, dorsal root ganglion, heart, intestine, kidney, thymus, uterus, etc., and has been implicated in a variety of physiological and pathological processes. However, its role in seizure disorders is not well known, except in a study suggesting that intracerebroventricular administration of an EP4 receptor-selective antagonist L-161982 increased the latency for PTZ-evoked acute seizures in Wistar rats (Oliveira et al., 2008). More in-depth studies are needed to validate the role of EP4 receptor in the pathophysiological processes related to epileptic seizures.
3.1.5. FP receptor
As the sole currently known receptor for PGF2α, the FP receptor couples to Gαq-Gβγ complex and is widely distributed in the human body, particularly in the cardiovascular system, CNS, eye, myometrium, and ovarian (Woodward et al., 2011). When bound to PGF2α or other selective agonists, the FP receptor is activated and Gαq is released from the G protein heterotrimeric complex to activate PLC that hydrolyzes PIP2 to IP3 and DAG, which in turn initiate Ca2+-sensitive signaling and activates certain PKC-dependent pathways, respectively (Abramovitz et al., 1994; Woodward et al., 2011) (Fig. 1).
Like other types of prostanoids, PGF2α in the brain can be rapidly and robustly induced by seizures, evidenced in both human patients and animal models of epilepsy (Forstermann et al., 1983). PGF2α levels in the cerebrospinal fluid (CSF) were found to increase nearly 5-fold in children who experienced simple febrile seizures (Tamai et al., 1983). Likewise, environmental stress-induced convulsions in gerbil mice elevated the PGF2α levels in the cortex and hippocampus more than 5-fold within 3 minutes (Seregi et al., 1985). PGF2α levels began to increase in the brain within 10 minutes following systemic injection of kainate in rats and peaked in the hippocampus 30 minutes after the administration of the neurotoxin (Baran et al., 1987). Histological examination of rat brain tissues 30 minutes after kainate injection demonstrated a major presence of PGF2α in hippocampal CA3 neurons, hilar neurons, and granule cells of the dentate gyrus, suggesting the hippocampus is the major source of PGF2α in the brain after kainate-induced seizures (Takei et al., 2012). Because these PGF2α-generating neurons also express the FP receptor, it is likely that they exert PGF2α-mediated functions by activating the FP receptor in an autocrine or paracrine manner following seizures.
Studies on PGF2α in animal seizure models yielded some controversial results. For instance, intracerebroventricular administration of PGF2α promoted both electrically and chemically induced seizures in mice (Climax and Sewell, 1981); whereas intraamygdaloid administration of PGF2α had no effect on seizure activity in an electrically kindled animal model (Croucher et al., 1991). However, intracisternal administration of PGF2α, in the presence of COX inhibitors, completely prevented seizures and decreased neuronal injury in the hippocampus after systemic injection of kainate for seizure induction in ICR mice, though PGF2α alone had no effect on kainate-induced seizures (Kim et al., 2008). In line, AL-8810, a potent and selective FP receptor antagonist, aggravated kainate-induced seizure activity in a dose-dependent manner (Kim et al., 2008; Sharif and Klimko, 2019). Moreover, intracisternal administration of PGF2α in immature mice whose brains have very limited COX-2 expression and induction also reduced kainate-provoked seizure activity and mortality (Chung et al., 2013), suggesting that seizure-induced PGF2α might act as an endogenous anticonvulsant through the FP receptor. Taken together, whether PGF2α plays a pro- or anti-convulsant role in the brain remains elusive; however, the effects of FP receptor activation on seizure activity and neuronal survival are very likely context-dependent, as PGF2α was administered into different brain regions in these studies.
3.1.6. DP1 receptor
As the most abundant lipid metabolite derived from the membrane-released arachidonic acid, PGD2 is synthesized from PGH2 by PGD2 synthase (PGDS), which has two isoforms: hematopoietic PGDS (H-PGDS) and lipocalin PGDS (L-PGDS, or β-trace protein) (Mohri et al., 2003; Post et al., 2018; Urade et al., 2013). PGD2 binds to two receptors, Gαs-coupled DP1 and Gαi-coupled DP2 (Fig. 1), to regulate several physiological and pathological activities, such as inhibition of hair growth, inhibition of platelet aggregation, sleep, smooth muscle contraction and relaxation, vasoconstriction and vasodilation (Garza et al., 2012; Woodward et al., 2011).
Under normal basal conditions, PGD2 level in the brain is relatively low but is quickly and robustly elevated by seizure activities (Forstermann et al., 1983). Intracerebroventricular administration with PGD2 or an active analog ZK-118.182 delayed the onset of generalized convulsions and reduced mortality in rats that were treated by PTZ for seizure induction; whereas inhibition of PGD2 activity or synthesis increased the seizure incidence and intensity (Akarsu et al., 1998). Similarly, genetic ablation of H-PGDS synthase or the DP1 receptor, but not L-PGDS or the DP2 receptor delayed seizure onset, reduced the duration of generalized tonic-clonic seizures, and decreased the number of seizure spikes in mice treated by PTZ. Moreover, the L-PGDS/PGD2/DP1 axis appears to regulate postictal sleep in these animals (Kaushik et al., 2014). Therefore, seizure-induced PGD2 might play a considerable antiepileptic role via activating the Gαs-coupled DP1, but not the Gαi-coupled DP2 receptor. It seems that potentiating PGD2/DP1 signaling in the brain might provide a novel strategy for antiseizure therapeutics. These findings in PTZ seizure model also suggest that targeting specific prostanoid receptors instead of the COX-2 enzyme itself could avoid compromising the PGD2-mediated potential antiseizure effects. However, it would be critical to determine the cellular sources of the DP1 receptor that accounts for its antiepileptic role using cell type-specific ablation of the receptor and to validate these previous findings in other conventional seizure models.
3.1.7. TP receptor
Thromboxane A2 (TXA2) is an essential eicosanoid that is traditionally known for its functions in cardiovascular homeostasis through acting on Gq protein-coupled receptor TP. However, recent studies reveal that TXA2 can be induced by, and play important roles in, some CNS conditions including epileptic seizures. TXA2 levels were found to increase approximately 10-fold in epileptic neocortex specimens from drug-resistant epileptic patients, along with PGE2 and PGI2 that increased about 5-fold compared to their levels in normal tissues (Rumia et al., 2012). Likewise, TXA2 in the brain was elevated by PTZ-induced seizures in mice within 10 minutes after PTZ injection (Kaushik et al., 2014). TP-selective agonist I-BOP impaired synaptic transmission and reduced neuronal excitability in the CA1 pyramidal neurons of rat hippocampal slices (Hsu and Kan, 1996); I-BOP and another TP agonist U-46619 suppressed high-voltage-activated calcium channels in rat hippocampal CA1 pyramidal neurons, decreasing Ca2+ currents and neuronal excitability (Hsu et al., 1996). Therefore, the TP receptor activation might lead to a decrease of neuronal excitability. Indeed, TP activation by U-46619 increased the latency for PTZ-induced myoclonic jerks and tonic-clonic seizures in mice. However, a TP antagonist, SQ-29548, did not modify PTZ-induced seizures, nor did it blunt the anticonvulsant effect of the agonist U-46619 in the same seizure model (Freitas et al., 2018). The reason for these conflicting findings remains elusive but could be related to the selectivity of the pharmacological drugs used in these studies. Hence, it would be important to follow up on these results using genetic inactivation of receptor and other animal seizure models to better understand the role of TXA2/TP signaling in seizure disorders.
3.2. Cannabinoid receptors
The endocannabinoid system is a biological system that consists of endocannabinoids, cannabinoid receptors, transporters, and enzymes for endocannabinoid synthesis and metabolism. Endocannabinoids are endogenous bioactive lipid metabolites derived from membrane-bound phospholipids, and the two most prominent types of endocannabinoid ligands are 2-arachidonoylglycerol (2-AG) and anandamide (also known as N-arachidonoylethanolamine, or AEA). Both AEA and 2-AG are synthesized from membrane lipid precursors by multiple biosynthetic pathways upon the physiological and pathological stimuli (Fig. 1). As the first identified and the most frequently studied endocannabinoid to date, AEA in brain neurons is primarily produced via the hydrolytic cleavage of a cell membrane-bound N-arachidonoyl-phosphatidylethanolamine (NAPE) by N-acyl phosphatidylethanolamine-specific phospholipase D (NAPE-PLD) in a single-step reaction (Di Marzo et al., 1994). The phospholipid precursor NAPE can be formed by transferring arachidonic acid (AA) from phosphatidylcholine (PC) to phosphatidylethanolamine (PE), a reaction catalyzed by N-acyltransferase (NAT). 2-AG, on the other hand, is synthesized via a two-step process in neurons. First, PIP2 in the cell membrane is hydrolyzed by phospholipase C-β (PLC-β) to create DAG. The DAG is then further hydrolyzed to 2-AG by diacylglycerol lipase (DAGL) (Murataeva et al., 2014). To date, two primary membrane GPCRs for endocannabinoids have been identified – cannabinoid receptor type 1 (CB1) and cannabinoid receptor type 2 (CB2), which are preferably bound and activated by AEA and 2-AG, respectively. The actions of endocannabinoids are terminated through hydrolysis by fatty acid amide hydrolase (FAAH) and monoacylglycerol lipase (MAGL). Specifically, AEA is metabolized into AA and ethanolamine (EA) by FAAH; 2-AG is mainly hydrolyzed into AA and glycerol by MAGL (Maccarrone et al., 2015) (Fig. 1). In addition, COX can metabolize endocannabinoids to prostaglandin analogs, namely, PG(D2/E2/F2α/I2)-ethanolamide (PG-EA) from AEA and PG(D2/E2/F2α/I2)-glycerol ester (PG-G) from 2-AG (Fig. 1). PGE2-EA can activate all EP1-EP4 receptors with lower binding affinity and potency than PGE2, whereas PGF2α-EA is a ligand of FP receptor, but their functions remain unclear (Alhouayek and Muccioli, 2014).
Both CB receptors are coupled to Gαi/o and inhibit the activity of adenylyl cyclase (Fig. 1), thereby mediating a variety of physiological and pathological processes in nearly all organs and systems of the human body. In the CNS, endocannabinoids via CB receptors play important roles in emotion, learning and memory, motility, neurogenesis, neuroinflammation, neuroplasticity, neuroprotection, nociception, stress, sleeping, and addiction (Aizpurua-Olaizola et al., 2017; Chiurchiu et al., 2018). The broad contributions of endocannabinoid signaling to so many important physiological and pathological processes in the body make the CB receptors as promising potential targets for many conditions in the periphery as well as the CNS (Aizpurua-Olaizola et al., 2017; Maccarrone et al., 2015). For instance, both CB1 and CB2 receptors can reduce neuronal activity and excitability of the brain, thereby generating tremendous interests and efforts in modulating these two inhibitory GPCRs for potential anticonvulsant therapies (Table 1) (Blair et al., 2015).
3.2.1. CB1 receptor
As the primary molecular target of endocannabinoid ligand AEA, the CB1 receptor is predominantly found in nervous systems, particularly in the brain, where the CB1 receptor is considered the most abundant GPCR. Of note, the CB1 receptor is expressed presynaptically at both GABAergic and glutamatergic interneurons of the hippocampus and, when activated, acts as a neuromodulator to inhibit the release of GABA and glutamate, thereby regulating the balance between the inhibitory and excitatory neurotransmission (Chiarlone et al., 2014; Chiodi et al., 2012). Mice lacking CB1 displayed hypoactivity, hypoalgesia, and increased mortality (Zimmer et al., 1999), and showed decreased seizure threshold in kainate model of temporal lobe epilepsy (TLE) (Marsicano et al., 2003). WIN 55212-2, a non-selective CB agonist showed dose-dependent anticonvulsant effects in hippocampal neuronal culture models of status epilepticus and acquired epilepsy; whereas the anticonvulsant action of WIN 55212-2 in these models was specifically blocked by a CB1 receptor-selective antagonist SR141716 (Blair et al., 2006). Moreover, genetic ablation of the CB1 receptor or pre-treatment with the CB1-selective antagonist SR141716 increased seizure severity without altering seizure-promoted cell proliferation and neuronal death after pilocarpine injection in mice; however, pre-treatment with CB1 agonist CP55940 did not show any noticeable effect on pilocarpine-induced seizures (Kow et al., 2014). Further, pre-treatment with the CB1 selective inverse agonist AM251 reduced the latency to onset of generalized tonic-clonic seizures and increased mortality in systemic kainate-treated mice (Sugaya et al., 2016). It appears that the majority of these studies on the CB1 receptor in various in vitro and in vivo models indicate that CB1 receptor activation causes anticonvulsant effects, while inhibiting CB1 might aggravate convulsive seizures.
3.2.2. CB2 receptor
Compared to CB1 receptor, the expression of CB2 in the body is more controversial, inasmuch as the CB2 receptor was previously reported to be present solely in organs of the immune system, where it contributes to the regulation of immune responses and mediates the anti-inflammatory effects of cannabis (Buckley et al., 2000). However, it is now well-known that CB2 is also expressed in the CNS with restricted distribution. Of note, the CB2 receptor is strongly expressed by microglial cells, where its function is associated with the regulation of innate immunity and neuroinflammation in the CNS (Maresz et al., 2005; Palazuelos et al., 2009; Zoppi et al., 2014). More recently the CB2 receptor was also detected in neurons and astrocytes responding to brain insults or inflammatory stimuli (Chiurchiu et al., 2018). These findings together with the fact that CB2 is expressed by principal neurons of the hippocampus make the receptor an appealing target for seizure control despite its relatively low density in the CNS (Stempel et al., 2016).
Although CB2-lacking mice have not been reported to show a seizure phenotype, activation of the receptor has been revealed to induce a chronic membrane potential hyperpolarization in hippocampal pyramidal cells that was not associated with the CB1 type receptor (Stempel et al., 2016), and to antagonize epileptic seizures in mice after kainate-induced status epileptics (Sugaya et al., 2016). Intriguingly, CB2 inverse agonist AM630, when co-administered with non-selective CB receptor agonist WIN 55212-2, but not by itself alone, showed considerable antiepileptic effects in a rat model of partial epilepsy (Rizzo et al., 2014). Moreover, about one-third of CB1 and CB2 double-knockout mice, but not the CB1 or CB2 single-knockout mice, showed spontaneous seizures or handling-induced seizures (Rowley et al., 2017), suggesting that a significant portion of CB double-knockout mice developed epilepsy. Therefore, CB1 and CB2 receptors likely work synergistically to regulate neuronal excitability and set the seizure threshold.
3.2.3. COX-2 and CB1/2 signaling
Endocannabinoids and prostaglandins are both derived from cell membrane-bound phospholipids; endocannabinoid metabolites can be the precursors of prostaglandins, and vice versa (Fig. 1), suggesting that these two biolipid signaling systems might interact with each other in the initiation of G protein-dependent signaling. Indeed, CB1 inhibition by selective antagonists SR141716 or AM251 enhanced synaptic responses in CA1 pyramidal neurons upon stimulation of the Schaffer collaterals in a GABA-independent manner, and this effect was largely prevented by the treatment with COX-2 inhibitors meloxicam or NS-398. These interesting findings suggest that COX cascade might regulate the formation of endogenous CB1 ligands that downregulates the excitatory neurotransmission in the hippocampus (Slanina and Schweitzer, 2005). Moreover, COX-2 oxidative metabolites of endocannabinoids promoted hippocampal long-term potentiation (LTP), an effect opposite to that of their precursors 2-AG and AEA (Yang et al., 2008), reinforcing the notion that COX-2 is at the interface of the endocannabinoid and prostanoid systems (Alhouayek and Muccioli, 2014). An interesting pharmacological strategy involving substrate-selective COX-2 inhibition augmented endocannabinoid production without altering the synthesis of prostanoids or other related non-endocannabinoid lipids, indicating the importance of COX cascade in the regulation of CB1/2 signaling (Hermanson et al., 2013). Taken together, COX-2-mediated endocannabinoid oxygenation appears to represent an important mechanism for terminating CB signaling and, thus COX-2 inhibition might provide a strategy to positively modulate the CB1/2 signaling for therapeutic potential (Hermanson et al., 2014). Whether this substrate-selective COX-2 inhibition strategy has any effect on CB1/2-mediated Gi signaling pathways during seizures and therefore alters the neuronal excitability in the epileptic brain remains to be determined.
3.3. Adenosine receptors
Endogenous adenosine has long been known for its role in the regulation of neuronal excitability during convulsive states of the brain for nearly four decades (Dunwiddie, 1980). Since then, considerable research has shed light on the concept that adenosine acts as a powerful endogenous neuroprotectant and anticonvulsant neuromodulator (Boison, 2016). Under normal physiological conditions, a main source of synaptic adenosine is the astrocytic release of ATP that is degraded to adenosine by a cascade of extracellular ectonucleotidase (EN) (Boison, 2008). However, adenosine is primarily synthesized in activated neurons and secreted to suppress excitatory neurotransmission in an autonomic feedback manner during prolonged neuronal activities (Lovatt et al., 2012). Excessive adenosine then is taken up by equilibrative nucleoside transporters (ENTs) to astrocytes where it undergoes metabolic clearance by adenosine kinase (ADK) to AMP (Boison et al., 2010) (Fig. 2). Intrahippocampal administration of adenosine to the site of stimulation was anticonvulsant in rats kindled by hippocampal electrical stimulation (Szybala et al., 2009); intraventricular release of adenosine after kainate-induced status epilepticus in rats prevented mossy fiber sprouting and resulted in a substantial long-lasting reduction in spontaneous recurrent seizures in terms of both seizure frequency and intensity (Williams-Karnesky et al., 2013). Conditional ablation of ADK in the forebrain attenuated seizures that were induced by intraamygdaloid injection of kainate in mice, whereas overexpression of ADK within the hippocampal CA3 region increased the spontaneous seizures in this brain region (Li et al., 2007; Li et al., 2008). In line, transient use of a small-molecule ADK inhibitor 5-iodotubercidin, administered twice daily during the latent phase of epileptogenesis from day 3-8 after the intrahippocampal injection of kainate, markedly decreased the percent time of epileptic mice in seizures (Sandau et al., 2019). These results are significant in that astrocyte-based ADK is highly upregulated in the epileptic hippocampus to decrease the adenosine levels at the synaptic sites, thereby contributing to epileptogenesis (Boison, 2008, 2012; Gouder et al., 2004).
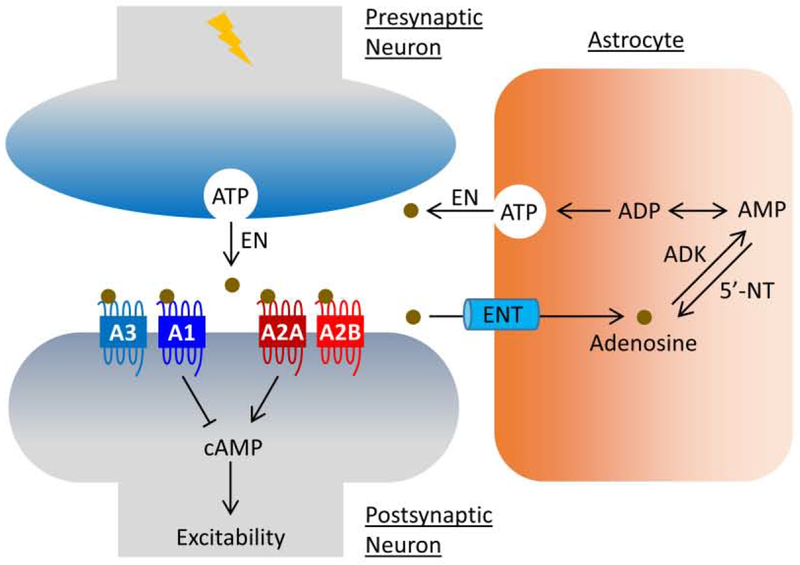
Figure 2.
Adenosine signaling at tripartite synapse. As the primary source of adenosine, ATP is released from astrocytes and neurons via vesicles under normal physiological and pathological conditions, respectively. Upon release, ATP undergoes quick digestion to adenosine by a cascade of EN. Excessive adenosine is then taken up by ENT to astrocytes where it undergoes metabolic clearance by ADK to AMP to complete the balance of the ATP/adenosine conversion cycle. Adenosine regulates neuronal excitability via GPCR-mediated cAMP signaling in the epileptic brain. Abbreviations: 5’-NT, 5′-nucleotidase; A1, adenosine receptor subtype A1; A2A, adenosine receptor subtype A2A; A2B, adenosine receptor subtype A2B; A3, adenosine receptor subtype A3; ADK, adenosine kinase; ADP, adenosine diphosphate; AMP, adenosine monophosphate; ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; EN, extracellular ectonucleotidase; ENT, equilibrative nucleoside transporter.
To date, four adenosine receptor subtypes have been identified in humans and experimental rodents: A1, A2A, A2B and A3, which are a class of purinergic GPCRs and encoded by distinct genes. Adenosine A1 and A3 receptors are associated with Gαi/o to inhibit cAMP biosynthesis; while A2A and A2B receptors are coupled to Gαs to promote cAMP-mediated signaling. Adenosine has been reported to modulate the neuronal excitability in the brain engaging all these four GPCRs, as it is conceivable that any shift in the ratio of stimulatory adenosine receptors to inhibitory adenosine receptors directly influences excitability of the brain and thus the seizure threshold (Boison, 2012, 2016). Though inhibition of ADK or ENTs can be used to increase adenosine levels at synaptic sites and thus represents an emerging strategy to suppress epileptic seizures, preclinical efforts have been made to explore the potential of these GPCRs – particularly A1 and A2A receptors – as antiepileptic and antiepileptogenic targets (Table 2) (Weltha et al., 2018).
Table 2.
Adenosine receptors in seizure disorders.
| Receptor | G protein | Recent studies and therapeutic outcomes | References |
|---|---|---|---|
| A1 | Gi/o | Deletion of A1 receptor exacerbated the neuronal loss, convulsions and subsequent mortality in adult male and female C57BL/6 mice after unilateral intrahippocampal injection of kainate (1 nmol). | Fedele et al., 2006 |
| The proconvulsive effect of A1 receptor ablation was aggravated by experimental traumatic brain injury in adult male and female C57BL/6 mice. | Kochanek et al., 2006 | ||
| Adenosine A1 receptor deficiency mice (adult male C57BL/6) developed spontaneous seizures with homozygous knockout mice showing higher frequency and longer duration of electrographic seizures than their heterozygous cohorts. | Li et al., 2007 | ||
| A2A | Gs | Focal bilateral microinjection of A2A receptor agonist CGS-21680 (up to 3 μg/side) increased, whereas A2A antagonist sCh-58261 (up to 20 μg/side) decreased, the number and duration of the unprovoked spike-wave discharges in young and adult male WAG/Rij rats in a dose-dependent manner. | D’Alimonte et al., 2009 |
| Ablation of A2A receptor decreased intensity and frequency of seizures induced by PTZ or pilocarpine in adult CD1 mice. | El Yacoubi et al., 2009 | ||
| A2A selective agonist CPCA (2 mg/kg, i.p.) decreased the mortality and lowered the seizure threshold in a hyperthermia-induced seizure model in young male Lewis rats. | Fukuda et al., 2011 | ||
| A2B | Gs | Delivery of nonselective adenosine receptor agonist NECA (0.1 mM) into the striatum of adult BALB/c mice via reverse microdialysis increased IL-6, and this cytokine elevation was blocked by A2B receptor antagonist MRS-1706 (0.01 mM). | Vazquez et al., 2008 |
| A3 | Gi/o | Treatment with selective A3 adenosine receptor agonist IB-MEC (100 μg/kg, i.p.) prior to NMDA or PTZ-induced seizures in adult male C57BL/6 mice decreased the percentage of animals with convulsions, delayed seizure onset, reduced neurological impairment, and increased animal survival. | Von Lubitz et al., 1995 |
Abbreviations: IL-6, interleukin 6; NECA, 5′-N-ethylcarboxamidoadenosine; NMDA, N-methyl-D-aspartic acid; PTZ, pentylenetetrazol.
3.3.1. Adenosine A1 receptor
Accumulating evidence from clinical and preclinical studies suggest that the Gi-coupled A1 adenosine receptor might exert an endogenous anticonvulsant effect via downregulating the postsynaptic cAMP signaling (Fig. 2). The A1 receptor expression was found lower in temporal cortex tissues surgically resected from patients with complex partial seizures than those in the normal control post-mortem tissues. This result suggests that the decreased expression of A1 receptor might reduce the Gi-mediated inhibitory function and thereby lower the threshold of epileptic seizures (Glass et al., 1996). Moreover, a study on 206 patients with severe traumatic brain injury (TBI) identified certain gene variants of adenosine A1 receptor associated with post-traumatic seizures, indicating that malfunctioning adenosine A1 receptor might contribute to the post-traumatic epileptogenesis (Wagner et al., 2010). The density of adenosine A1 receptor has also been shown to be decreased in the hippocampal nerve terminal membranes of rats following hippocampal kindling, leading to the deficiency in adenosine neuromodulation and failure of endogenous anticonvulsant mechanisms (Rebola et al., 2003). Adenosine A1 receptor knockout mice have been found to develop spontaneous seizures with homozygous knockout mice showing higher frequency and longer duration of electrographic seizures than their heterozygous cohorts (Li et al., 2007). The proconvulsive effect of adenosine A1 receptor ablation was aggravated by experimental traumatic brain injury, as well as the intrahippocampal injection of kainate, leading to lethal status epilepticus (Fedele et al., 2006; Kochanek et al., 2006). These studies on human patients and experimental animals together suggest that dysregulation of adenosine signaling via the inhibitory A1 receptor is involved in the pathophysiology of epilepsy, and thus the A1 receptor activation is required to prevent seizure intensification and dissemination within the brain (Fig. 2).
3.3.2. Adenosine A2A receptor
The stimulatory A2A adenosine receptor is coupled to Gαs, and its activation by neuron-derived adenosine leads to elevated levels of cytosol cAMP and thereby neuronal hyperexcitability (Fig. 2). The expression of the A2A receptor was increased in the cerebral areas of Wistar Albino Glaxo/Rijswijk rats experiencing absence seizures, but not in their pre-symptomatic cohorts. Moreover, treatment with A2A receptor agonist CGS-21680 increased, whereas A2A antagonist SCH-58261 decreased, the number and duration of the unprovoked spike-wave discharges in these epileptic rats in a dose-dependent manner (D’Alimonte et al., 2009). Compared to the wildtype control cohorts, mice lacking the adenosine A2A receptor showed significant reduced intensity and frequency of seizures induced by the administration of PTZ or pilocarpine (El Yacoubi et al., 2009), suggesting that the A2A knockout mice exhibited partial resistance to limbic seizures. In line, intracerebroventricular administration of another selective A2A antagonist ZM-241385 partially decreased seizure duration in rats after amygdala kindling (Li et al., 2012).
Early-life hyperthermic seizures in rats initially caused a short-term decrease of the adenosine A2A receptor density and 5’-nucleotidase activity in cerebral cortex within two days after seizure induction (Leon-Navarro et al., 2015), but later led to a long-lasting increase in A2A receptor expression, 5’-nucleotidase activity and A2A-mediated adenylate cyclase activity in the cortex when the animals reached to adulthood (Crespo et al., 2018). The activation of the A2A receptor by a selective agonist CPCA decreased the mortality and lowered the seizure threshold in a hyperthermia-induced seizure model in young rats (Fukuda et al., 2011). Thus, the adenosine A2A receptor signaling might aggravate febrile seizures and contribute to the pathogenesis of sudden unexpected death in epilepsy (SUDEP) in children. Taken together, it appears that neuronal hyperexcitability following precipitating insults likely causes the enhanced synaptic adenosine A2A receptor activation, which could exacerbate the aberration of normal circuitry leading to the chronic progression of epileptic seizures.
3.3.3. Adenosine A2B and A3 receptors
Compared to adenosine A1 and A2A receptors that are well studied in neuronal excitability, not much has been uncovered about the role of Gαs-coupled A2B and Gαi-coupled A3 receptors in the seizure generation or development of chronic epilepsy. Using the reverse microdialysis to deliver 5’-N-ethylcarboxamidoadenosine (NECA) – an adenosine analog as nonselective adenosine receptor agonist – into the striatum of freely moving mice, it was shown that the concentration of cytokine IL-6 in the perfusate was rapidly and robustly increased, whereas the NECA-induced IL-6 was selectively blocked by A2B receptor antagonist MRS-1706 (Vazquez et al., 2008). Given that IL-6, as a prototypical inflammatory cytokine, has been widely implicated in hyperexcitability of epileptic brains (Patel et al., 2017; Vezzani and Viviani, 2015), these findings suggest that adenosine stimulates IL-6 release likely by activating the A2B receptor and thereby facilitates epileptogenesis. Systemic administration of a selective A3 adenosine receptor agonist IB-MECA prior to NMDA or PTZ-induced seizures in mice decreased the percentage of mice with convulsions, delayed seizure onset, reduced neurological impairment, and increased animal survival (Von Lubitz et al., 1995). Though A2B and A3 receptors regulate cAMP signaling towards opposite directions (i.e., stimulatory vs. inhibitory), it has been shown that both A2B antagonist MRS-1706 and A3 antagonist MRS-1334 decreased the rundown of GABAA currents (Roseti et al., 2008), suggesting the A2B and A3 receptors might modulate the stability of GABA receptors and therefore fine-tune hyperexcitability of the brain. Nonetheless, it would be important to follow up on these pharmacological findings employing genetic strategies of gene deletion or overexpression in order to better understand the roles of adenosine A2B and A3 receptors in the pathophysiological processes related to epileptic seizures.
3.4. Metabotropic glutamate receptors
The metabotropic glutamate receptors (mGluRs) are members of the group C family of GPCRs that are bound and activated by glutamate, the amino acid that functions as the primary excitatory neurotransmitter of the mammalian CNS. There are eight currently known types of mGluRs, namely from mGluR1 to mGluR8, which are classified into three subgroups I, II, and III based on their amino acid sequence homology profiles and pharmacological properties (Nicoletti et al., 2011). The mGluRs, via modulating other receptors, are involved in a variety of functional and pathological processes in the CNS, such as synaptic plasticity, learning, memory, anxiety, depression, neuropathic pain (Collingridge et al., 2017). The mGluRs are typically found in pre- and postsynaptic neurons of synapses in the cerebral cortex, hippocampus, striatum, cerebellum, spinal cord, and many other areas of the CNS, where their activation upon glutamate binding initiates biochemical signaling cascades, leading to the modulation of other associated proteins, e.g., ion channels (Notartomaso et al., 2017; Olivero et al., 2017; Sidorov et al., 2015). This can further result in alterations in the synaptic excitability through modulating the presynaptic release of neurotransmitters, postsynaptic reactions, or astrocytic functions (Johnson et al., 2017; Sheng et al., 2017; Umpierre et al., 2019). With the use of a variety of pharmacological and genetic approaches, the roles of these receptors in many physiological and pathological processes of the brain are unfolding. The majority of results from a large number of preclinical studies attest that activation of group I mGluRs is proconvulsant through lowering the seizure threshold and increasing excitability of the brain; whereas activation of groups II and III mGluRs pre-dominantly leads to anticonvulsant effects (Table 3) (Wong et al., 2005).
Table 3.
Metabotropic glutamate receptors in seizure disorders.
| Receptor | G protein | Recent studies and therapeutic outcomes | References |
|---|---|---|---|
| Group I mGluR1/5 | Gq/11 | Overexpressing mGluR1 increased seizure susceptibility in pilocarpine-treated adult male BL6SJ/F1 hybrid mice. | Pitsch et al., 2007 |
| Treatment with mGluR1 antagonist AIDA (up to 1 μmol/site, i.c.v.) inhibited, while treatment with group I mGluR agonist DHPG (up to 50 nmol/site, i.c.v.) exacerbated, seizures in PTZ-treated young male ICR mice in a dose-dependent manner. | Watanabe et al., 2011 | ||
| Selective mGluR5 negative allosteric modulator CTEP (2 mg/kg, p.o.) decreased, while mGluR5 positive allosteric modulator RO6807794 (0.3 mg/kg, i.p.) increased the hyperactivity and seizures in Tsc2-deficient male and female mice. | Kelly et al., 2018 | ||
| Positive modulation of mGluR5 by VU0360172 (50 mg/kg, i.p.) attenuated seizures induced by TMEV in young male C57BL/6 mice. | Hanak et al., 2019 | ||
| Conditional ablation of mGluR5 in astrocytes decreased the rate of glutamate clearance in the hippocampus during epileptogenesis of adult male and female mice. | Umpierre et al., 2019 | ||
| Group II mGluR2/3 | Gi/o | Activation of mGluR2/3 by selective agonists LY379268 and LY389795 (0.001-10 nmol, i.c.v. or 1-30 mg/kg, i.p.) suppressed the sound-triggered or mGluR1/5 agonist DHPG-induced clonic seizures in young male and female DBA/2 mice a dose-dependent manner. | Moldrich et al., 2001 |
| Activation of mGluR2/3 by LY379268 (0.33 or 1 mg/kg, i.p.) increased, while mGluR2/3 inhibition by LY341495 (0.33, 1 or 5 mg/kg, i.p.) decreased, the occurrence of SWDs in an adult male rat model of absence epilepsy in a dose-dependent manner. | Ngomba et al., 2005 | ||
| Treatment with mGluR2/3 agonist APDC (20 nmol/site, i.c.v.) inhibited, while treatment with group II mGluR antagonist EGLU (up to 200 nmol/site, i.c.v.) exacerbated, seizures in PTZ-treated young male ICR mice in a dose-dependent manner. | Watanabe et al., 2011 | ||
| Group III mGluR4/6/7/8 | Gi/o | Ablation of mGluR7 elevated excitability and increased susceptibility to tonic-clonic seizures induced by PTZ or bicuculline in mice. | Sansig et al., 2001 |
| Genetic deletion of mGluR4 increased the severity of pilocarpine-induced seizure in adult male CD-1 mice but did not alter the spontaneous recurrent seizures. | Pitsch et al., 2007 | ||
| Positive modulation of mGluR4 by allosteric ligand PHCCC (10 mg/kg, s.c.) enhanced absence-like seizures in Wistar Albino Glaxo/Rijswijk rats as well as the PTZ-treated adult male WAG/Rij and ACI mice. | Ngomba et al., 2008 | ||
| Treatment with mGluR4/8 agonist L-AP4 (up to 20 nmol/site, i.c.v.) inhibited, while treatment with group III mGluR antagonist MPPG (up to 50 nmol/site, i.c.v.) exacerbated, seizures in PTZ-treated young male ICR mice in a dose-dependent manner. | Watanabe et al., 2011 | ||
| Negative modulation of mGluR7 by allosteric inhibitor ADX71743 (100 mg/kg, i.p.) enhanced thalamic synaptic transmission and induced absence epileptic seizures and lethargy in male adult mice. | Tassin et al., 2016 |
Abbreviations: PTZ, pentylenetetrazol; SWD, spike and wave discharge; TMEV, Theiler’s murine encephalomyelitis virus; TSC, tuberous sclerosis complex.
3.4.1. Group I mGluRs (mGluR1/5)
As the two currently known members in the group I mGluRs, mGluR1 and mGluR5 are coupled to Gq/11 proteins and, upon activation by glutamate binding, trigger the polyphosphoinositide hydrolysis of PIP2 by phospholipase C to produce IP3 and DAG, leading to Ca2+-sensitive signaling pathways and PKC activation, respectively. These two receptors have been found on the postsynaptic dendrites of neurons in thalamus as well as on GABAergic interneurons in the cerebral cortex (Wong et al., 2005). Astrocytes also show significant expression of mGluR5 receptor (Parri et al., 2010; Umpierre et al., 2019).
Interestingly, the expression of mGluR1, but not mGluR5, was found to increase in the dentate gyrus of hippocampus in rats after electrical kindling or intraperitoneal injection of kainate as well as in patients with TLE (Blumcke et al., 2000). The overexpression of mGluR1 in mice did not alter the acute stages of seizures after pilocarpine application compared to the wildtype control cohorts; however, these mGluR1 transgenic mice showed a consistent increase in seizure frequency during the chronic epileptic phase beginning a few weeks after status epilepticus (Pitsch et al., 2007), suggesting a role for mGluR1 in increasing the susceptibility to spontaneous recurrent seizures during the process of epileptogenesis. In line, intracerebroventricular administration of AIDA, a selective antagonist for mGluR1 showed marked inhibition on PTZ-induced kindled seizures in mice in a dose-dependent manner; whereas this antiseizure effect was largely prevented by co-treatment with mGluR1-selective agonist (RS)-3,5-DHPG (Watanabe et al., 2011).
Inhibition of mGluR5 by a selective negative allosteric modulator CTEP corrected hyperactivity, decreased epileptic seizures, and enhanced de novo synthesis of synaptic proteins in a mouse model of tuberous sclerosis complex (TSC); while mGluR5 activation by allosteric potentiator RO6807794 led to the exacerbation of these epileptic phenotypes (Kelly et al., 2018). However, treatment with another mGuR5-selective positive allosteric modulator VU0360172 attenuated acute seizures and decreased the pro-inflammatory cytokine-producing macrophages and microglia within the brain in the Theiler’s murine encephalomyelitis virus (TMEV)-induced mouse model of TLE (Hanak et al., 2019). The mechanism underlying these seemingly conflicting results remains unclear, but the selectivity of compounds and the different animal models used in these two studies might be contributory factors, as mGluR5 inhibition by MTEP, another highly selective brain-permeable negative allosteric modulator which is an analog of CTEP, did not alter the seizure outcomes in the same virus-induced epilepsy model (Hanak et al., 2019).
The mechanisms whereby mGluR5-mediated Gq signaling regulates acute seizures and the development of epilepsy remain largely elusive, but could involve its functions in astrocytes, as mGluR5 has been found in astrocytes of resected tissues from epilepsy patients as well as in animals with experimental epilepsy. The mGluR5 receptor was thought to play an essential role in the regulation of structural and functional interactions between neurons and astrocytes at tripartite synapses (Panatier and Robitaille, 2016). It was revealed that mGluR5 expression was markedly increased in the mouse kainate model of TLE, and the mGluR5 function particularly persisted in astrocytes to regulate glutamate uptake throughout the entire course of epileptogenesis following status epilepticus induced by kainate. Meanwhile, animals with only transient mGluR5 expression in astrocytes after kainate-induced status epilepticus did not develop epilepsy (Umpierre et al., 2019), suggesting that astrocytic mGluR5 is an essential component of excitatory signaling regulation in the hippocampus during the process of acquired epileptogenesis.
3.4.2. Group II mGluRs (mGluR2/3)
There are two members in the group II mGluRs: mGluR2 and mGluR3, which are coupled to Gi/o to suppress the production of cAMP through inhibiting the activity of adenylyl cyclase. Groups II mGluRs have been found predominantly on the presynaptic terminal, where they could inhibit the presynaptic glutamate release, as well as in astrocytes of cortex and thalamus, where they might regulate the expression of the excitatory amino acid transporter 1/2 (EAAT1/2) (Aronica et al., 2003; Ngomba and van Luijtelaar, 2018). It was widely reported that mGluR2/3 expression was substantially decreased in hippocampal CA1 and CA3 regions of mice and rats after pilocarpine-induced status epilepticus (Pacheco Otalora et al., 2006; Tang et al., 2004), and in patients with mesial TLE (Tang et al., 2004). A pharmacological study described that both central and systemic administration of two mGluR2/3-selective agonists LY379268 and LY389795 suppressed the sound-triggered or mGluR1/5 agonist DHPG-induced clonic seizures in DBA/2 mice in a dose and time dependent manner (Moldrich et al., 2001). Furthermore, these two mGluR2/3 agonists substantially reduced the spike and wave discharge (SWD) duration in lethargic (lh/lh) mouse model of absence seizures, as well as the electrically induced seizure score and after-discharge duration (ADD) in amygdala-kindled rats (Moldrich et al., 2001). Similarly, mGluR2/3-selective antagonist EGLU has been described to aggravate seizures in PTZ-induced kindled mice and antagonize the antiseizure effect from inhibiting the group I mGluRs (Watanabe et al., 2011). On the contrary, activation of mGluR2/3 by agonist LY379268 increased the numbers of SWD in symptomatic WAG/Rij rats also in a dose-dependent manner, while treatment with mGluR2/3-selective antagonist LY341495 decreased the occurrence of SWD in this rat model of absence epilepsy (Ngomba et al., 2005). It is unknown whether these contradicting results were caused by the experimental differences in selectivity of ligands, pharmacological doses, animal species and models that were used in these studies. Nonetheless, future efforts in developing new ligands that would show preference to mGluR2 or mGluR3 together with strategies of gene deletion or overexpression will help to uncover the specific roles for each of these two Gi-coupled mGluR subtypes in epileptic seizures.
3.4.3. Group III mGluRs (mGluR4/6/7/8)
There are four currently known members in the group III mGluRs: mGlu4, mGlu6, mGlu7 and mGlu8, which like group II mGluRs, are also coupled to Gi/o proteins to downregulate cAMP signaling when activated by glutamate. The mGluR6 is specifically expressed in the retina, where it regulates the responses of bipolar cells to light (Tian and Kammermeier, 2006), while all other group III mGluRs are mainly found in the cortico-basal ganglia-thalamo-cortical loop. The mGluR4 is predominantly expressed on glutamatergic terminals in the thalamic reticular nucleus (TRN) as well as the ventrobasal complex (VB), whereas mGluR7 and mGluR8 are also found on TRN neurons (Alexander and Godwin, 2006). The functions of group III mGluRs have been widely studied in the generation of absence seizures, a type of generalized nonconvulsive seizure that often involves the thalamus (Ngomba and van Luijtelaar, 2018).
Mice lacking mGluR4 showed marked resistance to absence seizures induced by low doses of GABAA receptor antagonists, such as PTZ, picrotoxin or bicuculline. Furthermore, the GABAA receptor antagonist-induced absence seizures were completely blocked by mGluR4 antagonist CPPG while exacerbated by the group I mGluRs agonist L-AP4, both of which were administered bilaterally into the TRN (Snead et al., 2000). In line, mGluR4 activation by systemic injection of an allosteric potentiator PHCCC enhanced absence-like seizures in Wistar Albino Glaxo/Rijswijk rats as well as the PTZ-treated mice (Ngomba et al., 2008), suggesting that mGluR4 activation facilitates the seizure generation in absence epilepsy. However, the ablation of mGluR4 in mice did not inhibit tonic-clonic seizures triggered by high doses of GABAA receptor antagonists, strychnine, or electroshock (Snead et al., 2000). On the contrary, mGluR4 knockout mice showed a significant increase of severity in acute seizures induced by pilocarpine injection and later had enhanced neuronal loss in the hippocampus during the chronic epileptic phase, though they did not display any increase in frequency of spontaneous recurrent seizures (Pitsch et al., 2007). Interestingly, a downregulation of mGluR4 expression and function also has been described in hippocampal CA3 region in rats after pilocarpine-induced status epilepticus (Dammann et al., 2018). These studies together suggest that the activation of mGluR4 might differentially regulate nonconvulsive and convulsive seizures, i.e., facilitation on absence seizures, but inhibition on tonic-clonic seizures.
Mice lacking mGluR7 showed hyperexcitability and increased susceptibility to tonic-clonic seizures induced by PTZ or bicuculline (Sansig et al., 2001), demonstrating an important role for mGluR7 in the regulation of neuronal excitability induced by inhibiting GABA functions. Disrupting the interaction between mGluR7 and its PDZ-interacting protein, protein interacting with C kinase 1 (PICK1), by targeted mutation of the receptor or a cell-permeant dominant-negative peptide, led to behavioral symptoms and EEG discharges in both mice and rats, which are characteristic of absence epilepsy (Bertaso et al., 2008). Therefore, impaired interaction between mGluR7 and PICK1 might contribute to some form of epileptogenesis. Inhibiting mGluR7 with a selective brain-penetrant negative allosteric modulator ADX71743 enhanced thalamic synaptic transmission and induced absence epileptic seizures and lethargy, which were exacerbated by disrupting the interaction between the receptor and the PDZ protein PICK1 (Tassin et al., 2016).
The function of mGluR8 was found downregulated at the presynaptic terminals of the lateral perforant pathway but increased at the at Schaffer collateral-CA1 synapses in rats with spontaneous recurrent seizures after pilocarpine-induced status epilepticus (Dammann et al., 2018; Kral et al., 2003). These findings suggest that the development of epileptic seizures might be associated with the spatiotemporal alterations of mGluR8 expression that leads to the declined autoregulation of glutamate release. L-AP4, a selective agonist for mGluR4/8 has been demonstrated to inhibit seizure activities in PTZ-induced kindled mice. The antiseizure effect of mGluR4/8 activation was further enhanced by co-treatment with either group I mGluRs antagonist AIDA or group II mGluRs agonist APDC, but was completely blocked by MPPG, a group III mGluRs-selective antagonist (Watanabe et al., 2011). These interesting findings together suggest that Gq/11-coupled group I mGluRs are overall proconvulsant, while the activation of Gi/o-coupled groups II and III mGluRs causes anticonvulsant effects (Table 3).
3.5. Other GPCRs
In addition to the four major families of membrane receptors elaborated above, there are also a few other GPCRs that have been well investigated for their potential roles in regulating neuronal excitability and setting seizure threshold. They draw less attention compared to the well-recognized prostanoid, endocannabinoid, adenosine, and metabotropic glutamate receptors; however, their potential as novel antiepileptic and/or antiepileptogenic targets should not be neglected. We are only able to select the following three groups of GPCRs to discuss further due to the limited space and scope of this review (Table 4).
Table 4.
Histamine, GABAB, and galanin receptors in seizure disorders.
| Receptor | G protein | Recent studies and therapeutic outcomes | References |
|---|---|---|---|
| H1 | Gq/11 | Genetic ablation of H1 receptor or pre-treatment with H1-selective antagonist triprolidine (10 mg/kg, i.p.) increased the susceptibility of immature C57Bl/6J mice to kainate-induced seizures. | Kukko-Lukjanov et al., 2010 |
| H3 | Gi/o | H3 receptor selective antagonist pitolisant (20 mg/kg, i.p.) reduced both the frequency and duration of spike-and-wave discharges in genetic absence epilepsy rat from Strasbourg (GAERS). | Kasteleijn-Nolst Trenité et al., 2013 |
| Pitolisant (100 mg/kg, i.p.) completely suppressed EEG epileptiform activity and seizures in the maximal electroshock test in mice, a model for primary and secondarily generalized tonic-clonic seizures. | Kasteleijn-Nolst Trenité et al., 2013 | ||
| Pitolisant (10 or 20 mg/kg, i.p.) decreased the number and cumulated duration of hippocampal discharges in mice treated by kainate. | Kasteleijn-Nolst Trenité et al., 2013 | ||
| H3 receptor selective antagonist pitolisant (20, 40, and 60 mg) showed dose-dependent beneficial effects in patients with photosensitive epilepsy. | Kasteleijn-Nolst Trenité et al., 2013 | ||
| Pre-treatment with an H3 receptor-targeted compound with a hydantoin moiety (10 mg/kg, i.p.) was anticonvulsant in electroshock-induced and PTZ-kindled convulsions in adult male Wistar rats. | Sadek et al., 2014 | ||
| GABAB | Gi/o | GABAB activation by baclofen (< 60 mg/day) was anticonvulsant in a human study involving 12 patients. | Terrence et al., 1983 |
| Allosteric potentiation of GABAB by small-molecule modulators GS39783 (10-100 mg/kg, i.p.), rac-BHFF (30-300 mg/kg, i.p.), CMPPE (10-100 mg/kg, i.p.), A-1295120 (3-30 mg/kg, i.p.), and A-1474713 (10-100 mg/kg, i.p.) before seizure induction showed anticonvulsant effects in the DBA/2J mouse audiogenic seizure model in a dose-dependent manner. These beneficial effects were largely blocked by the GABAB antagonist SCH50911 (50 mg/kg, i.p.). | Brown et al., 2016 | ||
| GalR1 | Gi/o | Genetic ablation of GalR1 increased the vulnerability of male C57BL/6 mice to seizures induced by lithium-pilocarpine or electrical stimulation of the hippocampus. | Mazarati et al., 2004 |
| GalR1 receptor deletion did not affect either the severity or duration of seizures induced by kainate injection in adult male C57BL/6 mice. | Mazarati et al., 2004; Schauwecker, 2010 | ||
| GalR1 receptor activation by galanin analog NAX 5055 (4 mg/kg, i.p.) suppressed audiogenic seizures in male and female Frings mice in a time-dependent manner. | White et al., 2009 | ||
| NAX 5055 (i.p.) was highly potent against acute psychomotor seizures induced by 6 Hz corneal stimulation in adult male albino CF-1 mice with low ED50 values: 0.7 mg/kg for 22 mA, 0.8 mg/kg for 32 mA, and 2.9 mg/kg for 44 mA stimulations. | White et al., 2009 | ||
| NAX 5055 (6 mg/kg, i.p.) showed marked anticonvulsant effects against the fully expressed secondarily generalized seizures in corneal kindling model of partial seizures in adult male albino CF-1 mice. | White et al., 2009 | ||
| NAX 5055 even at a very high dose (20 mg/kg, i.p.) was not active in the mouse traditional maximal electroshock model and was minimally active in the subcutaneous PTZ seizure model in adult male albino CF-1 mice. | White et al., 2009 | ||
| NAX 5055 (0.5-4 mg/kg, i.p.) had no effect in a multiple-hit model of symptomatic infantile spasms of male Sprague-Dawley rats. | Jequier Gygax et al., 2014 | ||
| GalR2 | Gq/11 | GalR2-preferring analog NAX 810-2 (0.2-3 mg/kg, i.v.) showed antiepileptic effects in both adult male albino CF-1 mouse corneal kindling and 6 Hz seizure models. | Metcalf et al., 2017 |
| Genetic deletion of GalR2 increased both the frequency and duration of seizures after intrahippocampal administration of kainate in adult male C57BL/6 mice but had no effect on PTZ-induced seizures. | Drexel et al., 2018 | ||
| GalR3 | Gi/o | Genetic ablation of GalR3 did not affect either the seizure frequency or duration in mouse intrahippocampal kainate model or systemic PTZ seizure model in adult male C57BL/6 mice. | Drexel et al., 2018 |
Abbreviations: GABA: γ-aminobutyric acid; PTZ, pentylenetetrazol.
3.5.1. Histamine receptors
Histamine is a biogenic amine that is derived from the decarboxylation of amino acid histidine and exerts its physiological and pathological functions via four currently known GPCRs: H1, H2, H3 and H4 receptors (Seifert et al., 2013). Owing to the extensive activities of histamine as a local mediator and neurotransmitter, the histaminergic system is thought to be involved in the pathogenesis of diverse human diseases including epilepsy, as early evidence suggests that elevated histamine might suppress convulsive seizures and confer a neuroprotective role (Bhowmik et al., 2012). Among the four histamine receptors, Gq/11-coupled H1 receptor is widely expressed in the CNS as well as many peripheral tissues and contributes to nuclear factor κB (NF-κB)-mediated inflammatory processes. Antihistamines are used as anti-allergy and anti-inflammatory drugs by mainly acting on the H1 receptor (Canonica and Blaiss, 2011). Genetic deletion of H1 receptor or treatment with selective antagonist triprolidine in immature mice increased the susceptibility of animals to kainate-induced seizures, demonstrated by augmented seizure severity and neuronal damage (Kukko-Lukjanov et al., 2010).
The Gi/o-coupled H3 receptor is primarily expressed in the CNS and functions as presynaptic autoreceptor to control the release of histamine and several other neurotransmitters, such as acetylcholine, dopamine, GABA, norepinephrine and serotonin, and thus plays important roles in homeostatic and cognitive processes. The H3 receptor also modulates several intracellular signaling pathways that are associated with epileptic seizures and neurotoxicity, thereby representing an attractive molecular target in the effort of searching for new antiepileptic and/or antiepileptogenic strategies. Though controversial, the pharmacological potential of H3 receptor selective antagonists and inverse agonists continues to receive considerable attention because of their prospective anticonvulsive properties, as an increasing body of evidence from animal studies demonstrates their effectiveness in seizure treatment (Table 4). Pitolisant – a highly selective antagonist for H3 receptor – showed extensive anticonvulsant effects in several preclinical seizure models, such as the mouse kainate model of temporal lobe seizures, the rat absence seizure model, and the mouse maximal electroshock model (Kasteleijn-Nolst Trenite et al., 2013). In a small phase II clinical trial, pitolisant showed dose-dependent beneficial effects in patients with photosensitive epilepsy, a rare type of epilepsy in which seizures are triggered by photic stimuli and resistant to current first-line AEDs (Kasteleijn-Nolst Trenite et al., 2013). Inspired by this promising outcome, a series of novel multiple-target ligands were designed by incorporating various antiepileptic structural motifs to the pharmacophore of pitolisant. These novel compounds displayed high affinities to H3 receptor, and one compound with hydantoin moiety that is present in phenytoin showed moderate anticonvulsant activities in both electroshock-induced and PTZ-kindled convulsions in Wistar rats (Sadek et al., 2014). However, whether the antiepileptic effect of this compound should be attributed to its actions on H3 receptors by the pharmacophore of pitolisant or voltage gated sodium channels by its hydantoin moiety remains elusive (Khanfar et al., 2016).
3.5.2. GABAB receptor
GABAB receptor is a metabotropic transmembrane receptor that is activated by inhibitory neurotransmitter GABA and linked via Gi/o protein to potassium channels. There are two subunits of the GABAB receptor, GABAB1 and GABAB2, which appear to assemble to form a heteromeric dimer in a 1:1 stoichiometry through their intracellular C-terminal domains, and both subtypes are essential for the heterodimer receptor to be fully functional (Pin et al., 2004). As an inhibitory type of receptor, GABAB activation can stimulate the opening of potassium channels to reduce the frequency of action potentials, as well as the release of GABA and other neurotransmitters including glutamate. It also modulates the activity of calcium channels and inhibits adenylyl cyclase via Gi/o protein. The GABAB receptor is highly expressed throughout the mammalian CNS and has been implicated in multiple neurological and psychiatric conditions, including both convulsive and nonconvulsive seizures (Han et al., 2012; Joshi et al., 2016). Baclofen, a lipophilic analog of GABA, is a highly selective agonist for GABAB receptor and binds to its orthosteric site. Baclofen has been used clinically to treat muscle spasms for decades and remains the only marketed drug specifically acting on the GABAB receptor. Its anticonvulsant effect in human epilepsy was first reported nearly four decades ago (Terrence et al., 1983); however, its untoward muscle relaxant and sedative effects, as well as its rapid development of tolerance and short duration of action due to its relatively short in vivo half-life, potentially limit its clinical application. To overcome these pharmacodynamic and pharmacokinetic shortcomings, a number of positive allosteric modulators have been developed by several groups (Pin et al., 2004) and tested in diverse animal seizure models (Table 4). In a recent study, several positive allosteric modulators for GABAB receptor including GS39783, rac-BHFF, CMPPE, A-1295120, and A-1474713 showed robust anticonvulsant effects in the DBA/2J mouse audiogenic seizure model in a dose-dependent manner. However, the antiepileptic effects of these compounds were completely prevented by pre-treatment of the mice with GABAB antagonist SCH50911, suggestive of a GABAB receptor-mediated mechanism. Compared to baclofen, these novel allosteric potentiators showed decreased motor side-effects during the acclimation period of the test (Brown et al., 2016). However, more intense and robust studies are required to validate their antiepileptic and potentially antiepileptogenic effects in classical chemoconvulsant models of epilepsy.
3.5.3. Galanin receptors
Galanin is a neuropeptide that is widely expressed in the mammalian CNS and involved in the modulation and inhibition of action potentials in neurons by acting on three GPCR subtypes: GalR1, GalR2, and GalR3. Several lines of evidence from animal studies using both genetic and pharmacological approaches reveal that galanin, via these three GPCRs, is a potent and effective modulator of neuronal excitability in the hippocampus. Galanin receptors, particularly GalR1 and GalR2, have been explored as potential targets for new antiepileptic therapeutics in multiple animal seizure models (Table 4). Genetic ablation of Gi/o-coupled GalR1 increased the vulnerability of mice to seizures induced by systemic treatment of Li-pilocarpine or electrical stimulation of the perforant path of the hippocampus (Mazarati et al., 2004). However, the deletion of GalR1 did not affect the severity and duration of seizures induced by kainate injection in mice (Mazarati et al., 2004; Schauwecker, 2010). These seemingly conflicting results from different seizure models might be explained by the well-recognized resistance of the C57BL/6 mice used in these studies to kainate-induced seizures and neuronal injury (McKhann et al., 2003). In another recent study, the deletion of Gq/11-coupled GalR2, but not the Gi/o-coupled GalR3, increased the frequency and duration of seizures after intrahippocampal administration of kainate in mice, although the genetic ablation of the galanin receptors had no effect on PTZ-induced seizures (Drexel et al., 2018). Systemic administration of NAX 5055, a bioavailable brain-permeable analog of galanin with binding preference to GalR1, showed profound anticonvulsant activities in several mouse models, including corneal kindling model, audiogenic seizure model, and the 6 Hz model of pharmacoresistant epilepsy (White et al., 2009). However, NAX 5055 was not active in the mouse traditional maximal electroshock model and only showed minimal anticonvulsant activity in the mouse subcutaneous PTZ seizure model even at the highest tested dose (White et al., 2009). In addition, NAX 5055 did not show any benefit in the rat multiple-hit model of symptomatic infantile spasms (Jequier Gygax et al., 2014). NAX 810-2, a GalR2-preferring galanin analog, also showed dose-dependent antiepileptic effects in both mouse corneal kindling and 6 Hz seizure models (Metcalf et al., 2017). These studies together suggest that GalR1 and GalR2 should be explored further for their potential as novel antiepileptic and/or antiepileptogenic targets.
4. Concluding remarks and outlook
4.1. Challenges
GPCRs have gained new momentum in CNS indications, as demonstrated by the scientific impetus in GPCR neurobiology and neuropharmacology, leading to many emerging new drug targets for various conditions associated with the brain. As such, an increasing body of evidence from a large number of preclinical and clinical studies points to indispensable roles for GPCRs in the pathophysiological processes related to acute seizures and potentially the development of acquired forms of epilepsy. Yet, there is a lack of drug that interrupts epileptic seizures via directly acting on GPCRs to date. Moreover, there is no currently known clinical trial to evaluate the therapeutic potential of targeting GPCRs for acquired epilepsy (ClinicalTrials.gov). As the most recently introduced antiseizure medication, cannabidiol (Epidiolex®) was approved by the US FDA as a treatment for two severe forms of epilepsy, i.e., Dravet syndrome and Lennox-Gastaut syndrome, in young patients who are two years of age or older (FDA, 2018). Cannabidiol as an adjuvant treatment can dramatically decrease seizure frequency in childhood-onset drug-resistant epilepsy, but like any other AEDs, it does not lead to becoming seizure-free and treatment-related adversity still remains a considerable concern (O’Connell et al., 2017; Stockings et al., 2018). The exact mechanism of action whereby cannabidiol executes anticonvulsant effects remains unknown and does not seem to be through interaction with CB receptors, and a multimodal action engaging more than 10 potential molecular targets identified has been proposed (Chen et al., 2019). Cannabidivarin (GWP42006), another non-psychoactive analog of cannabidiol that is believed to target the orphan GPCR 55 (GPR55) – a likely cannabinoid receptor (Ryberg et al., 2007) – has recently failed to outperform placebo in a phase 2 clinical trial for focal seizures (https://clinicaltrials.gov/show/NCT02365610; https://clinicaltrials.gov/show/NCT02369471).
The reasons why there is still a lack of GPCR-targeted drug for epilepsy treatment, despite tremendous preclinical and clinical efforts, remain unknown. However, there might be multiple contributory factors and the nature of GPCRs per se certainly could help to explain this disappointing fact. GPCRs in the brain are often expressed by both neuronal and nonneuronal cells, in which they might mediate different, in some cases even opposite, functions due to the distinct cellular contents. For instance, prostaglandin receptor EP2 in neurons mediates a number of important normal physiological functions and also can confer early neuroprotective effects in some injury settings (Andreasson, 2010; Jiang and Dingledine, 2013; Serrano et al., 2011), but the activation microglial EP2 contributes to chronic inflammation in the brain, leading to detrimental effects on neuronal cells (Johansson et al., 2013; Johansson et al., 2015). Conventional agonists or antagonists of the receptor might provide some beneficial effects through acting on EP2 in one cell type, but also can cause undesirable effects via its counterpart in another cell type. Because neurons and microglia may respond to precipitating injuries in different time-courses, it is not uncommon that GPCR-targeted agents, when administered with different treatment regimens, may lead to quite different outcomes even in the same animal model (Du et al., 2016; Jiang et al., 2015). In addition, unlike most ion channels that, when activated, allow certain ions to pass through, resulting in more specific cellular responses, activation of a GPCR usually initiates several downstream signaling pathways, thereby leading to multiple consequences, which can be desirable or undesirable. Developing GPCR-targeted therapeutic must ultimately hinge on a well understanding of the GPCR downstream signaling pathways (Eichel and von Zastrow, 2018), as the downstream molecular effectors could provide alternative targets with more specificity than the receptors themselves.
4.2. Opportunities
Our current understanding of the contribution of GPCRs to epileptic seizures was profoundly based on pharmacological studies in animal seizure models, which largely rely on the selectivity and pharmacokinetic properties of drugs used in those studies. Genetic approaches of gene deletion or overexpression with cell type-specificity have been increasingly used recently to validate the roles for GPCRs in neurons and glial cells of epileptic brains (Stempel et al., 2016; Stoppel et al., 2017; Sugaya et al., 2016; Umpierre et al., 2019), as their functions may depend on the cellular contents that determine the downstream signaling pathways and responding components. A combination of genetic and pharmacological strategies is encouraged to fully understand their complex roles and assess the feasibility of pharmacologically targeting these receptors for epilepsy. As tools and techniques for studying GPCRs grow, so too will our understanding of their physiological and pathological functions in the brain, which can guide translational efforts in developing next-generation antiepileptic and/or antiepileptogenic therapeutics.
To date, the majority of preclinical studies indicate that the activation of Gq/11-associated EP1, FP, and mGluR1/5 receptors and Gs-coupled EP2, A2A and A2B receptors is overall proconvulsive in animal models of epilepsy. Conversely, Gi/o-linked CB1, CB2, A1, A3, mGluR2/3, mGluR4/7/8, GABAB, and GlaR1 act as endogenous anticonvulsants. More in-depth studies are needed to clarify the roles of other GPCRs such as DP1/2, EP3/4, IP, TP, mGluR6, H1, H3, and GalR2 in epileptic seizures due to conflicting results or lack of definitive evidence from previous studies (Tables 1–4). Nonetheless, it appears that GPCRs might regulate neuronal excitability through both Gs/Gi-controlled cAMP and Gq/11-dependent Ca2+ pathways that control the expression and function of various voltage-gated or ligand-gated ion channels including sodium channels, ionotropic glutamate receptors, potassium channels, etc., which work together to control the excitability of the brain and set the seizure threshold (Aizpurua-Olaizola et al., 2017; Collingridge et al., 2017; Weltha et al., 2018). However, the molecular mechanisms whereby GPCRs regulate these ion channels remain largely unknown. Elucidating the downstream effectors of G proteins might provide alternative strategies to targeting the receptors or G proteins themselves for acquired epilepsy with more specificity.
4.3. Downstream effectors and biased ligands
As the second messenger for stimulatory GPCRs, cAMP regulates various important biological processes under both normal and disease conditions. The effects of cAMP within the brain were historically believed to be mediated by either PKA or cyclic nucleotide-regulated ion channels. During the past two decades, PKA-independent cAMP action that is mediated by EPAC, a family of guanine nucleotide exchange factors (GEFs) called cAMP-GEFs, became widely recognized for their roles in a variety of physiological and pathological processes (de Rooij et al., 1998; Kawasaki et al., 1998). In the CNS, the cAMP/EPAC signaling has been reported to regulate glutamate transmitter release in the central neurons, as well as hippocampal long-term potentiation (LTP) (Yang et al., 2012). Interestingly, upon cAMP binding, EPAC can block ATP-sensitive potassium channels (KATP), leading to Ca2+-dependent glutamate release. Genetic ablation of EPAC proteins increases the open probability of KATP channels in the dentate granule neurons and decreases glutamate release, thereby reducing the vulnerability of epileptic seizures in mice following kainate treatment (Zhao et al., 2013). Therefore, the Gs downstream EPAC might represent an alternative molecular target for acute seizures and potentially acquired epileptogenesis.
GPCRs can also mediate G protein-independent signaling through coupling to β-arrestin proteins, which were originally discovered for their roles in desensitization and internalization of the GPCRs (Ferguson et al., 1996; Goodman et al., 1996). Moreover, the recruiting β-arrestins to GPCRs appears to trigger conformational changes that initiate interaction with the endocytic machinery, thereby linking the trafficking and signaling events. β-arrestin-mediated signaling has been found in nearly all GPCR families including those are discussed in this review (Blume et al., 2017; Eng et al., 2016; Jiang and Dingledine, 2013; Mundell and Kelly, 2011). For instance, β-arrestin-1/2 is involved in EP2 receptor-mediated cytokine production in neuroinflammatory conditions (Chu et al., 2015). The interaction between β-arrestin-1 and CB1 receptor during their endocytic trafficking was also reported recently (Delgado-Peraza et al., 2016). β-arrestin-2 couples mGLuR5 to mediate neuronal protein synthesis in the hippocampus, which is independent of Gq/11-mediated signaling pathways (Stoppel et al., 2017). Moreover, β-arrestin-2, but not β-arrestin-1, regulates synaptic plasticity that is mediated by mGluR1 in CA3 pyramidal neurons and mGluR5 in CA1 via Src kinase and MAPK/ERK pathways (Eng et al., 2016). However, whether β-arrestin signaling contributes to GPCRs-mediated pathophysiological processes related to epilepsy remains to be determined. It is expected that pharmacological manipulation of GPCRs with emerging biased ligands targeting β-arrestin-dependent signal transduction could potentially help answer this question (Reiter et al., 2012).
4.4. Allosteric modulators
Marked progresses in developing allosteric modulators for GPCRs during the past decade have led to appealing therapeutic strategies for various CNS conditions including certain forms of epilepsy (Conn et al., 2014; Nickols and Conn, 2014). Though conventional orthosteric agonists and antagonists have traditionally been pursued to target GPCRs, allosteric modulators provide multiple mechanistic advantages owing to their potential capability of providing temporal and spatial context-dependence and distinguishing among closely associated receptor subtypes that are activated by common natural ligands, such as biolipids, neurotransmitters, and nucleosides (Conn et al., 2014; Foster and Conn, 2017; Lane et al., 2013; Nickols and Conn, 2014). Therefore, allosteric modulators could potentially avoid the adverse effects associated with orthosteric agonism or antagonism of the targeted GPCRs. Interestingly, cannabidiol has been identified as a non-competitive negative allosteric modulator (NAM) for CB1 receptor (Laprairie et al., 2015), which might help to explain why it does not appear to have any psychotropic effects such as those caused by Δ9-tetrahydrocannabinol (THC) in marijuana, although the exact mechanism of action for its antiepileptic effects has not been determined. We recently reported positive allosteric modulators (PAMs) for the EP2 receptor that can augment cAMP signaling only in the presence of its natural agonist – PGE2 and do not affect other PGE2 receptor subtypes or GPCRs in other families. These small-molecule PAMs have proven to be valuable tools to elucidate the roles of EP2 receptor following neuronal insults (Jiang et al., 2010; Jiang et al., 2018). Remarkably, allosteric modulators have been developed for all three mGluR groups owing to their essential roles in multiple psychiatric and neurological disorders (Conn et al., 2009). Currently using these valuable chemical tools in the epilepsy research field is mostly limited to absence epilepsy, as mGluRs are known for their regulation of SWD, the electrographic hallmark of absence seizures (Duveau et al. ,2019; Ngomba and van Luijtelaar, 2018). In the future it is important to test them in convulsive seizure models to fully explore their potential to be developed as antiepileptic and/or antiepileptogenic therapies.
4.5. Where do we go from here?
One of the major concerns with GPCR-based therapies is the receptor desensitization, referring to acute and prolonged decreases in response to continuous or repeated stimulation. Desensitization is considered a strategy of mammalian cells to avoid unrestricted growth or toxicity caused by overstimulation. During the short-term desensitization, GPCR remains at the cell membrane, but becomes refractory to repeated stimuli, as β-arrestins uncouple the receptor and G protein. Long-term desensitization occurs when the GPCR is phosphorylated by GPCR kinases (GPCRKs), followed by the degradation in lysosomes, and later the receptor would be restored by protein synthesis and processing, i.e., resensitization (Kelly et al., 2008; Rajagopal and Shenoy, 2018). Desensitization disables the receptor from functioning despite the continuous presence of stimuli, and thus compromises the efficacy of therapeutic agents. As such, desensitization might represent a common molecular mechanism whereby some GPCR-targeted therapies engaging traditional ligands (e.g., baclofen for GABAB receptor) fail to achieve desired antiepileptic or antiepileptogenic effects, which often require extended treatment. In this regard, allosteric modulators and biased ligands might have less propensity for developing tolerance caused by receptor desensitization than conventional agonists or antagonists (Gjoni and Urwyler, 2008; Lin et al., 2018; Slivicki et al., 2018). These nontraditional ligands do not occupy the orthosteric binding site; rather, they stabilize receptor conformation preferentially modulating one specific pathway, and thereby allow more targeted intervention of cellular function and treatment of disease (Gjoni and Urwyler, 2008; Hitchinson et al., 2018; Michel and Charlton, 2018). Owing to these benefits and other advantages discussed earlier, there has been an increasing number of biased ligands along with allosteric modulators targeting GPCRs in clinical trials over the past decade, particularly in the CNS disease indications (Hauser et al., 2017). Future efforts should be encouraged to focus on these nontraditional agonists or antagonists for the next-generation antiepileptic and potential antiepileptogenic strategies targeting GPCRs. Remarkably, we have already witnessed the emerging attention to their potential uses in controlling certain types of epileptic seizures in a number of recent preclinical studies (Brown et al., 2016; Hanak et al., 2019; Kelly et al., 2018; Tassin et al., 2016).
Highlights.
GPCRs are involved in regulating neuronal excitability and setting seizure threshold
Targeting various groups of GPCRs has not yet been translated to clinical use to date
GPCR downstream effectors may provide antiepileptic and/or antiepileptogenic targets
Allosteric or biased agents of GPCRs have potential as novel antiseizure therapeutics
Acknowledgements
This work was supported by the National Institutes of Health (NIH)/National Institute of Neurological Disorders and Stroke (NINDS) grants R00NS082379 (J.J.), R01NS100947 (J.J.), and R21NS109687 (J.J.). We sincerely apologize for not being able to discuss and cite all papers that are relevant to topics in this review due to the space limitation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abou-Khalil BW, 2007. Comparative monotherapy trials and the clinical treatment of epilepsy. Epilepsy currents 7, 127–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abramovitz M, Boie Y, Nguyen T, Rushmore TH, Bayne MA, Metters KM, Slipetz DM, Grygorczyk R, 1994. Cloning and expression of a cDNA for the human prostanoid FP receptor. J Biol Chem 269, 2632–2636. [PubMed] [Google Scholar]
- af Forselles KJ, Root J, Clarke T, Davey D, Aughton K, Dack K, Pullen N, 2011. In vitro and in vivo characterization of PF-04418948, a novel, potent and selective prostaglandin EP(2) receptor antagonist. British journal of pharmacology 164, 1847–1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizpurua-Olaizola O, Elezgarai I, Rico-Barrio I, Zarandona I, Etxebarria N, Usobiaga A, 2017. Targeting the endocannabinoid system: future therapeutic strategies. Drug discovery today 22, 105–110. [DOI] [PubMed] [Google Scholar]
- Akarsu ES, Mamuk S, Comert A, 1998. Inhibition of pentylenetetrazol-induced seizures in rats by prostaglandin D2. Epilepsy Res 30, 63–68. [DOI] [PubMed] [Google Scholar]
- Alexander GM, Godwin DW, 2006. Metabotropic glutamate receptors as a strategic target for the treatment of epilepsy. Epilepsy Res 71, 1–22. [DOI] [PubMed] [Google Scholar]
- Alhouayek M, Muccioli GG, 2014. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci 35, 284–292. [DOI] [PubMed] [Google Scholar]
- Andreasson K, 2010. Emerging roles of PGE2 receptors in models of neurological disease. Prostaglandins Other Lipid Mediat 91, 104–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Bauer S, Bozzi Y, Caleo M, Dingledine R, Gorter JA, Henshall DC, Kaufer D, Koh S, Loscher W, Louboutin JP, Mishto M, Norwood BA, Palma E, Poulter MO, Terrone G, Vezzani A, Kaminski RM, 2017. Neuroinflammatory targets and treatments for epilepsy validated in experimental models. Epilepsia 58 Suppl 3, 27–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aronica E, Gorter JA, Ijlst-Keizers H, Rozemuller AJ, Yankaya B, Leenstra S, Troost D, 2003. Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. The European journal of neuroscience 17, 2106–2118. [DOI] [PubMed] [Google Scholar]
- Baran H, Heldt R, Hertting G, 1987. Increased prostaglandin formation in rat brain following systemic application of kainic acid. Brain research 404, 107–112. [DOI] [PubMed] [Google Scholar]
- Ben-Menachem E, 2014. Medical management of refractory epilepsy--practical treatment with novel antiepileptic drugs. Epilepsia 55 Suppl 1, 3–8. [DOI] [PubMed] [Google Scholar]
- Berg AT, Berkovic SF, Brodie MJ, Buchhalter J, Cross JH, van Emde Boas W, Engel J, French J, Glauser TA, Mathern GW, Moshe SL, Nordli D, Plouin P, Scheffer IE, 2010. Revised terminology and concepts for organization of seizures and epilepsies: report of the ILAE Commission on Classification and Terminology, 2005-2009. Epilepsia 51, 676–685. [DOI] [PubMed] [Google Scholar]
- Bertaso F, Zhang C, Scheschonka A, de Bock F, Fontanaud P, Marin P, Huganir RL, Betz H, Bockaert J, Fagni L, Lerner-Natoli M, 2008. PICK1 uncoupling from mGluR7a causes absence-like seizures. Nature neuroscience 11, 940–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmik M, Khanam R, Vohora D, 2012. Histamine H3 receptor antagonists in relation to epilepsy and neurodegeneration: a systemic consideration of recent progress and perspectives. British journal of pharmacology 167, 1398–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjarnadottir TK, Gloriam DE, Hellstrand SH, Kristiansson H, Fredriksson R, Schioth HB, 2006. Comprehensive repertoire and phylogenetic analysis of the G protein-coupled receptors in human and mouse. Genomics 88, 263–273. [DOI] [PubMed] [Google Scholar]
- Blair RE, Deshpande LS, DeLorenzo RJ 2015. Endocannabinoids and epilepsy In: Cannabinoids in Neurologic and Mental Disease. pp. 125–172. Ed. Fattore L. Academic Press: San Diego. [Google Scholar]
- Blair RE, Deshpande LS, Sombati S, Falenski KW, Martin BR, DeLorenzo RJ, 2006. Activation of the cannabinoid type-1 receptor mediates the anticonvulsant properties of cannabinoids in the hippocampal neuronal culture models of acquired epilepsy and status epilepticus. J Pharmacol Exp Ther 317, 1072–1078. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Becker AJ, Klein C, Scheiwe C, Lie AA, Beck H, Waha A, Friedl MG, Kuhn R, Emson P, Elger C, Wiestler OD, 2000. Temporal lobe epilepsy associated up-regulation of metabotropic glutamate receptors: correlated changes in mGluR1 mRNA and protein expression in experimental animals and human patients. Journal of neuropathology and experimental neurology 59, 1–10. [DOI] [PubMed] [Google Scholar]
- Blume LC, Patten T, Eldeeb K, Leone-Kabler S, Ilyasov AA, Keegan BM, O’Neal JE, Bass CE, Hantgan RR, Lowther WT, Selley DE, Howlett AL, 2017. Cannabinoid Receptor Interacting Protein 1a Competition with beta-Arrestin for CB1 Receptor Binding Sites. Molecular pharmacology 91, 75–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld H, Klein JP, Schridde U, Vestal M, Rice T, Khera DS, Bashyal C, Giblin K, Paul-Laughinghouse C, Wang F, Phadke A, Mission J, Agarwal RK, Englot DJ, Motelow J, Nersesyan H, Waxman SG, Levin AR, 2008. Early treatment suppresses the development of spike-wave epilepsy in a rat model. Epilepsia 49, 400–409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, 2008. The adenosine kinase hypothesis of epileptogenesis. Prog Neurobiol 84, 249–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, 2012. Adenosine dysfunction in epilepsy. Glia 60, 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, 2016. Adenosinergic signaling in epilepsy. Neuropharmacology 104, 131–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D, Chen JF, Fredholm BB, 2010. Adenosine signaling and function in glial cells. Cell death and differentiation 17, 1071–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JW, Moeller A, Schmidt M, Turner SC, Nimmrich V, Ma J, Rueter LE, van der Kam E, Zhang M, 2016. Anticonvulsant effects of structurally diverse GABA(B) positive allosteric modulators in the DBA/2J audiogenic seizure test: Comparison to baclofen and utility as a pharmacodynamic screening model. Neuropharmacology 101, 358–369. [DOI] [PubMed] [Google Scholar]
- Buckley NE, McCoy KL, Mezey E, Bonner T, Zimmer A, Felder CC, Glass M, Zimmer A, 2000. Immunomodulation by cannabinoids is absent in mice deficient for the cannabinoid CB(2) receptor. European journal of pharmacology 396, 141–149. [DOI] [PubMed] [Google Scholar]
- Canonica GW, Blaiss M, 2011. Antihistaminic, anti-inflammatory, and antiallergic properties of the nonsedating second-generation antihistamine desloratadine: a review of the evidence. The World Allergy Organization journal 4, 47–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JW, Borgelt LM, Blackmer AB, 2019. Cannabidiol: A New Hope for Patients With Dravet or Lennox-Gastaut Syndromes. The Annals of pharmacotherapy 53, 603–611. [DOI] [PubMed] [Google Scholar]
- Chiarlone A, Bellocchio L, Blazquez C, Resel E, Soria-Gomez E, Cannich A, Ferrero JJ, Sagredo O, Benito C, Romero J, Sanchez-Prieto J, Lutz B, Fernandez-Ruiz J, Galve-Roperh I, Guzman M, 2014. A restricted population of CB1 cannabinoid receptors with neuroprotective activity. Proc Natl Acad Sci U S A 111, 8257–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiodi V, Uchigashima M, Beggiato S, Ferrante A, Armida M, Martire A, Potenza RL, Ferraro L, Tanganelli S, Watanabe M, Domenici MR, Popoli P, 2012. Unbalance of CB1 receptors expressed in GABAergic and glutamatergic neurons in a transgenic mouse model of Huntington’s disease. Neurobiol Dis 45, 983–991. [DOI] [PubMed] [Google Scholar]
- Chiurchiu V, van der Stelt M, Centonze D, Maccarrone M, 2018. The endocannabinoid system and its therapeutic exploitation in multiple sclerosis: Clues for other neuroinflammatory diseases. Prog Neurobiol 160, 82–100. [DOI] [PubMed] [Google Scholar]
- Chu CH, Chen SH, Wang Q, Langenbach R, Li H, Zeldin D, Chen SL, Wang S, Gao H, Lu RB, Hong JS, 2015. PGE2 Inhibits IL-10 Production via EP2-Mediated beta-Arrestin Signaling in Neuroinflammatory Condition. Molecular neurobiology 52, 587–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JI, Kim AY, Lee SH, Baik EJ, 2013. Seizure susceptibility in immature brain due to lack of COX-2-induced PGF2alpha. Experimental neurology 249, 95–103. [DOI] [PubMed] [Google Scholar]
- Climax J, Sewell RD, 1981. Modification of convulsive behaviour and body temperature in mice by intracerebroventricular administration of prostaglandins, arachidonic acid and the soluble acetylsalicylic acid salt lysine acetylsalicylate. Archives internationales de pharmacodynamie et de therapie 250, 254–265. [PubMed] [Google Scholar]
- Collingridge GL, Manahan-Vaughan D, Nicoletti F, Schoepp DD, 2017. Metabotropic glutamate receptors, 5 years on. Neuropharmacology 115, 1–3. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Christopoulos A, Lindsley CW, 2009. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nature reviews. Drug discovery 8, 41–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conn PJ, Lindsley CW, Meiler J, Niswender CM, 2014. Opportunities and challenges in the discovery of allosteric modulators of GPCRs for treating CNS disorders. Nature reviews. Drug discovery 13, 692–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespo M, Leon-Navarro DA, Martin M, 2018. Early-life hyperthermic seizures upregulate adenosine A2A receptors in the cortex and promote depressive-like behavior in adult rats. Epilepsy & behavior: E&B 86, 173–178. [DOI] [PubMed] [Google Scholar]
- Croucher MJ, Marriott DR, Bradford HF, Wilkin GP, 1991. Lack of effect of focally administered prostaglandins on electrically kindled seizure activity. Prostaglandins 42, 29–38. [DOI] [PubMed] [Google Scholar]
- D’Alimonte I, D’Auro M, Citraro R, Biagioni F, Jiang S, Nargi E, Buccella S, Di Iorio P, Giuliani P, Ballerini P, Caciagli F, Russo E, De Sarro G, Ciccarelli R, 2009. Altered distribution and function of A2A adenosine receptors in the brain of WAG/Rij rats with genetic absence epilepsy, before and after appearance of the disease. The European journal of neuroscience 30, 1023–1035. [DOI] [PubMed] [Google Scholar]
- Dammann F, Kirschstein T, Guli X, Muller S, Porath K, Rohde M, Tokay T, Kohling R, 2018. Bidirectional shift of group III metabotropic glutamate receptor-mediated synaptic depression in the epileptic hippocampus. Epilepsy Res 139, 157–163. [DOI] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL, 1998. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature 396, 474–477. [DOI] [PubMed] [Google Scholar]
- Delgado-Peraza F, Ahn KH, Nogueras-Ortiz C, Mungrue IN, Mackie K, Kendall DA, Yudowski GA, 2016. Mechanisms of Biased beta-Arrestin-Mediated Signaling Downstream from the Cannabinoid 1 Receptor. Molecular pharmacology 89, 618–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dey A, Kang X, Qiu J, Du Y, Jiang J, 2016. Anti-Inflammatory Small Molecules To Treat Seizures and Epilepsy: From Bench to Bedside. Trends Pharmacol Sci 37, 463–484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Cristo G, Awad PN, Hamidi S, Avoli M, 2018. KCC2, epileptiform synchronization, and epileptic disorders. Prog Neurobiol 162, 1–16. [DOI] [PubMed] [Google Scholar]
- Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz JC, Piomelli D, 1994. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 372, 686–691. [DOI] [PubMed] [Google Scholar]
- Drexel M, Locker F, Kofler B, Sperk G, 2018. Effects of galanin receptor 2 and receptor 3 knockout in mouse models of acute seizures. Epilepsia 59, e166–e171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Kemper T, Qiu J, Jiang J, 2016. Defining the therapeutic time window for suppressing the inflammatory prostaglandin E2 signaling after status epilepticus. Expert review of neurotherapeutics 16, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunwiddie TV, 1980. Endogenously released adenosine regulates excitability in the in vitro hippocampus. Epilepsia 21, 541–548. [DOI] [PubMed] [Google Scholar]
- Duveau V, Buhl DL, Evrard A, Ruggiero C, Mande-Niedergang B, Roucard C, Gurrell R, 2019. Pronounced antiepileptic activity of the subtype-selective GABAA -positive allosteric modulator PF-06372865 in the GaERS absence epilepsy model. CNS neuroscience & therapeutics 25, 255–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichel K, von Zastrow M, 2018. Subcellular Organization of GPCR Signaling. Trends Pharmacol Sci 39, 200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yacoubi M, Ledent C, Parmentier M, Costentin J, Vaugeois JM, 2009. Adenosine A2A receptor deficient mice are partially resistant to limbic seizures. Naunyn-Schmiedeberg’s archives of pharmacology 380, 223–232. [DOI] [PubMed] [Google Scholar]
- Eng AG, Kelver DA, Hedrick TP, Swanson GT, 2016. Transduction of group I mGluR-mediated synaptic plasticity by beta-arrestin2 signalling. Nature communications 7, 13571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FDA, FDA Approves First Drug Comprised of an Active Ingredient Derived From Marijuana to Treat Rare, Severe Forms of Epilepsy, June 26, 2018, (https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms).
- Fedele DE, Li T, Lan JQ, Fredholm BB, Boison D, 2006. Adenosine A1 receptors are crucial in keeping an epileptic focus localized. Experimental neurology 200, 184–190. [DOI] [PubMed] [Google Scholar]
- Ferguson SS, Downey WE 3rd, Colapietro AM, Barak LS, Menard L, Caron MG, 1996. Role of beta-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science (New York, N.Y.) 271, 363–366. [DOI] [PubMed] [Google Scholar]
- Fischborn SV, Soerensen J, Potschka H, 2010. Targeting the prostaglandin E2 EP1 receptor and cyclooxygenase-2 in the amygdala kindling model in mice. Epilepsy Res 91, 57–65. [DOI] [PubMed] [Google Scholar]
- Forstermann U, Heldt R, Hertting G, 1983. Increase in brain prostaglandins during convulsions is due to increased neuronal activity and not to hypoxia. Archives internationales de pharmacodynamie et de therapie 263, 180–188. [PubMed] [Google Scholar]
- Foster DJ, Conn PJ, 2017. Allosteric Modulation of GPCRs: New Insights and Potential Utility for Treatment of Schizophrenia and Other CNS Disorders. Neuron 94, 431–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox BM, Beck HP, Roveto PM, Kayser F, Cheng Q, Dou H, Williamson T, Treanor J, Liu H, Jin L, Xu G, Ma J, Wang S, Olson SH, 2015. A selective prostaglandin E2 receptor subtype 2 (EP2) antagonist increases the macrophage-mediated clearance of amyloid-beta plaques. J Med Chem 58, 5256–5273. [DOI] [PubMed] [Google Scholar]
- Freitas ML, Mello FK, Souza TL, Grauncke ACB, Fighera MR, Royes LFF, Furian AF, Oliveira MS, 2018. Anticonvulsant-like effect of thromboxane receptor agonist U-46619 against pentylenetetrazol-induced seizures. Epilepsy Res 146, 137–143. [DOI] [PubMed] [Google Scholar]
- Fu Y, Yang MS, Jiang J, Ganesh T, Joe E, Dingledine R, 2015. EP2 Receptor Signaling Regulates Microglia Death. Molecular pharmacology 88, 161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Suzuki Y, Hino H, Morimoto T, Ishii E, 2011. Activation of central adenosine A(2A) receptors lowers the seizure threshold of hyperthermia-induced seizure in childhood rats. Seizure 20, 156–159. [DOI] [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG, 2004. Desensitization of G protein-coupled receptors and neuronal functions. Annual review of neuroscience 27, 107–144. [DOI] [PubMed] [Google Scholar]
- Ganesh T, Jiang J, Dingledine R, 2014a. Development of second generation EP2 antagonists with high selectivity. Eur J Med Chem 82, 521–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh T, Jiang J, Shashidharamurthy R, Dingledine R, 2013. Discovery and characterization of carbamothioylacrylamides as EP2 selective antagonists. ACS Med Chem Lett 4, 616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganesh T, Jiang J, Yang MS, Dingledine R, 2014b. Lead optimization studies of cinnamic amide EP2 antagonists. J Med Chem 57, 4173–4184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garza LA, Liu Y, Yang Z, Alagesan B, Lawson JA, Norberg SM, Loy DE, Zhao T, Blatt HB, Stanton DC, Carrasco L, Ahluwalia G, Fischer SM, FitzGerald GA, Cotsarelis G, 2012. Prostaglandin D2 inhibits hair growth and is elevated in bald scalp of men with androgenetic alopecia. Science translational medicine 4, 126ra134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gjoni T, Urwyler S, 2008. Receptor activation involving positive allosteric modulation, unlike full agonism, does not result in GABAB receptor desensitization. Neuropharmacology 55, 1293–1299. [DOI] [PubMed] [Google Scholar]
- Glass M, Faull RL, Bullock JY, Jansen K, Mee EW, Walker EB, Synek BJ, Dragunow M, 1996. Loss of A1 adenosine receptors in human temporal lobe epilepsy. Brain research 710, 56–68. [DOI] [PubMed] [Google Scholar]
- Goodman OB Jr., Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL, 1996. Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor. Nature 383, 447–450. [DOI] [PubMed] [Google Scholar]
- Gouder N, Scheurer L, Fritschy JM, Boison D, 2004. Overexpression of adenosine kinase in epileptic hippocampus contributes to epileptogenesis. J Neurosci 24, 692–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han HA, Cortez MA, Snead OC III. 2012. GABAB Receptor and Absence Epilepsy In: Jasper’s Basic Mechanisms of the Epilepsies. Eds. Noebels th, J.L., Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV. National Center for Biotechnology Information (US) [PubMed] [Google Scholar]
- Rogawski Michael A, Delgado-Escueta Antonio V, Noebels Jeffrey L, Avoli Massimo and Olsen Richard W.: Bethesda (MD). [Google Scholar]
- Hanak TJ, Libbey JE, Doty DJ, Sim JT, DePaula-Silva AB, Fujinami RS, 2019. Positive modulation of mGluR5 attenuates seizures and reduces TNF-alpha(+) macrophages and microglia in the brain in a murine model of virus-induced temporal lobe epilepsy. Experimental neurology 311, 194–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AS, Attwood MM, Rask-Andersen M, Schioth HB, Gloriam DE, 2017. Trends in GPCR drug discovery: new agents, targets and indications. Nature reviews. Drug discovery 16, 829–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser AS, Chavali S, Masuho I, Jahn LJ, Martemyanov KA, Gloriam DE, Babu MM, 2018. Pharmacogenomics of GPCR Drug Targets. Cell 172, 41–54 e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson DJ, Gamble-George JC, Marnett LJ, Patel S, 2014. Substrate-selective COX-2 inhibition as a novel strategy for therapeutic endocannabinoid augmentation. Trends Pharmacol Sci 35, 358–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermanson DJ, Hartley ND, Gamble-George J, Brown N, Shonesy BC, Kingsley PJ, Colbran RJ, Reese J, Marnett LJ, Patel S, 2013. Substrate-selective COX-2 inhibition decreases anxiety via endocannabinoid activation. Nature neuroscience 16, 1291–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata T, Narumiya S, 2011. Prostanoid receptors. Chemical reviews 111, 6209–6230. [DOI] [PubMed] [Google Scholar]
- Hitchinson B, Eby JM, Gao X, Guite-Vinet F, Ziarek JJ, Abdelkarim H, Lee Y, Okamoto Y, Shikano S, Majetschak M, Heveker N, Volkman BF, Tarasova NI, Gaponenko V, 2018. Biased antagonism of CXCR4 avoids antagonist tolerance. Science signaling 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu KS, Huang CC, Kan WM, Gean PW, 1996. TXA2 agonists inhibit high-voltage-activated calcium channels in rat hippocampal CA1 neurons. The American journal of physiology 271, C1269–1277. [DOI] [PubMed] [Google Scholar]
- Hsu KS, Kan WM, 1996. Thromboxane A2 agonist modulation of excitatory synaptic transmission in the rat hippocampal slice. British journal of pharmacology 118, 2220–2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Thathiah A, 2015. Regulation of neuronal communication by G protein-coupled receptors. FEbS letters 589, 1607–1619. [DOI] [PubMed] [Google Scholar]
- Ikeda M, Sugihara M, Suwa M, 2018. SEVENS: a database for comprehensive GPCR genes obtained from genomes: -Update to 68 eukaryotes. Biophysics and physicobiology 15, 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jequier Gygax M, Klein BD, White HS, Kim M, Galanopoulou AS, 2014. Efficacy and tolerability of the galanin analog NAX 5055 in the multiple-hit rat model of symptomatic infantile spasms. Epilepsy Res 108, 98–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Dingledine R, 2013. Prostaglandin receptor EP2 in the crosshairs of anti-inflammation, anti-cancer, and neuroprotection. Trends Pharmacol Sci 34, 413–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Ganesh T, Du Y, Quan Y, Serrano G, Qui M, Speigel I, Rojas A, Lelutiu N, Dingledine R, 2012. Small molecule antagonist reveals seizure-induced mediation of neuronal injury by prostaglandin E2 receptor subtype EP2. Proc Natl Acad Sci U S A 109, 3149–3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Ganesh T, Du Y, Thepchatri P, Rojas A, Lewis I, Kurtkaya S, Li L, Qui M, Serrano G, Shaw R, Sun A, Dingledine R, 2010. Neuroprotection by selective allosteric potentiators of the EP2 prostaglandin receptor. Proc Natl Acad Sci U S A 107, 2307–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Qiu J, Li Q, Shi Z, 2017. Prostaglandin E2 Signaling: Alternative Target for Glioblastoma? Trends in cancer 3, 75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Quan Y, Ganesh T, Pouliot WA, Dudek FE, Dingledine R, 2013. Inhibition of the prostaglandin receptor EP2 following status epilepticus reduces delayed mortality and brain inflammation. Proc Natl Acad Sci U S A 110, 3591–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Van TM, Ganesh T, Dingledine R, 2018. Discovery of 2-Piperidinyl Phenyl Benzamides and Trisubstituted Pyrimidines as Positive Allosteric Modulators of the Prostaglandin Receptor EP2. ACS Chem Neurosci 9, 699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Yang MS, Quan Y, Gueorguieva P, Ganesh T, Dingledine R, 2015. Therapeutic window for cyclooxygenase-2 related anti-inflammatory therapy after status epilepticus. Neurobiol Dis 76, 126–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang J, Yu Y, Kinjo ER, Du Y, Nguyen HP, Dingledine R, 2019. Suppressing pro-inflammatory prostaglandin signaling attenuates excitotoxicity-associated neuronal inflammation and injury. Neuropharmacology 149, 149–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JU, Pradhan S, Lokteva LA, Woodling NS, Ko N, Brown HD, Wang Q, Loh C, Cekanaviciute E, Buckwalter M, Manning-Bog AB, Andreasson KI, 2013. Suppression of inflammation with conditional deletion of the prostaglandin E2 EP2 receptor in macrophages and brain microglia. J Neurosci 33, 16016–16032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson JU, Woodling NS, Wang Q, Panchal M, Liang X, Trueba-Saiz A, Brown HD, Mhatre SD, Loui T, Andreasson KI, 2015. Prostaglandin signaling suppresses beneficial microglial function in Alzheimer’s disease models. The Journal of clinical investigation 125, 350–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KA, Mateo Y, Lovinger DM, 2017. Metabotropic glutamate receptor 2 inhibits thalamically-driven glutamate and dopamine release in the dorsal striatum. Neuropharmacology 117, 114–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi K, Cortez MA, Snead OC 2016. Targeting the GABAB Receptor for the Treatment of Epilepsy In: GABAB Receptor, pp. 175–195. Ed. Colombo G. Springer International Publishing: Cham. [Google Scholar]
- Kaminski RM, Rogawski MA, Klitgaard H, 2014. The potential of antiseizure drugs and agents that act on novel molecular targets as antiepileptogenic treatments. Neurotherapeutics : the journal of the American Society for Experimental NeuroTherapeutics 11, 385–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang X, Qiu J, Li Q, Bell KA, Du Y, Jung DW, Lee JY, Hao J, Jiang J, 2017. Cyclooxygenase-2 contributes to oxidopamine-mediated neuronal inflammation and injury via the prostaglandin E2 receptor EP2 subtype. Scientific reports 7, 9459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasteleijn-Nolst Trenite D, Parain D, Genton P, Masnou P, Schwartz JC, Hirsch E, 2013, Efficacy of the histamine 3 receptor (H3R) antagonist pitolisant (formerly known as tiprolisant; BF2.649) in epilepsy: dose-dependent effects in the human photosensitivity model. Epilepsy & behavior: E&B 28, 66–70. [DOI] [PubMed] [Google Scholar]
- Kaushik MK, Aritake K, Kamauchi S, Hayaishi O, Huang ZL, Lazarus M, Urade Y, 2014, Prostaglandin D(2) is crucial for seizure suppression and postictal sleep. Experimental neurology 253, 82–90. [DOI] [PubMed] [Google Scholar]
- Kawano T, Anrather J, Zhou P, Park L, Wang G, Frys KA, Kunz A, Cho S, Orio M, ladecola C, 2006. Prostaglandin E2 EP1 receptors: downstream effectors of COX-2 neurotoxicity. Nature medicine 12, 225–229. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM, 1998. A family of cAMP-binding proteins that directly activate Rap1. Science (New York, N.Y.) 282, 2275–2279. [DOI] [PubMed] [Google Scholar]
- Kelly E, Bailey CP, Henderson G, 2008. Agonist-selective mechanisms of GPCR desensitization. British journal of pharmacology 153 Suppl 1, S379–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly E, Schaeffer SM, Dhamne SC, Lipton JO, Lindemann L, Honer M, Jaeschke G, Super CE, Lammers SH, Modi ME, Silverman JL, Dreier JR, Kwiatkowski DJ, Rotenberg A, Sahin M, 2018. mGluR5 Modulation of Behavioral and Epileptic Phenotypes in a Mouse Model of Tuberous Sclerosis Complex. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology 43, 1457–1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanfar MA, Affini A, Lutsenko K, Nikolic K, Butini S, Stark H, 2016. Multiple Targeting Approaches on Histamine H3 Receptor Antagonists. Frontiers in neuroscience 10, 201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Chung JI, Lee SH, Jung YS, Moon CH, Baik EJ, 2008. Involvement of endogenous prostaglandin F2alpha on kainic acid-induced seizure activity through FP receptor: the mechanism of proconvulsant effects of COX-2 inhibitors. Brain research 1193, 153–161. [DOI] [PubMed] [Google Scholar]
- Klein P, Dingledine R, Aronica E, Bernard C, Blumcke I, Boison D, Brodie MJ, Brooks-Kayal AR, Engel J Jr., Forcelli PA, Hirsch LJ, Kaminski RM, Klitgaard H, Kobow K, Lowenstein DH, Pearl PL, Pitkanen A, Puhakka N, Rogawski MA, Schmidt D, Sillanpaa M, Sloviter RS, Steinhauser C, Vezzani A, Walker MC, Loscher W, 2018. Commonalities in epileptogenic processes from different acute brain insults: Do they translate? Epilepsia 59, 37–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kochanek PM, Vagni VA, Janesko KL, Washington CB, Crumrine PK, Garman RH, Jenkins LW, Clark RS, Homanics GE, Dixon CE, Schnermann J, Jackson EK, 2006. Adenosine A1 receptor knockout mice develop lethal status epilepticus after experimental traumatic brain injury. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism 26, 565–575. [DOI] [PubMed] [Google Scholar]
- Kow RL, Jiang K, Naydenov AV, Le JH, Stella N, Nathanson NM, 2014. Modulation of pilocarpine-induced seizures by cannabinoid receptor 1. PloS one 9, e95922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kral T, Erdmann E, Sochivko D, Clusmann H, Schramm J, Dietrich D, 2003. Down-regulation of mGluR8 in pilocarpine epileptic rats. Synapse 47, 278–284. [DOI] [PubMed] [Google Scholar]
- Kukko-Lukjanov TK, Lintunen M, Jalava N, Lauren HB, Lopez-Picon FR, Michelsen KA, Panula P, Holopainen IE, 2010. Involvement of histamine 1 receptor in seizure susceptibility and neuroprotection in immature mice. Epilepsy Res 90, 8–15. [DOI] [PubMed] [Google Scholar]
- Lane JR, Abdul-Ridha A, Canals M, 2013. Regulation of G protein-coupled receptors by allosteric ligands. ACS Chem Neurosci 4, 527–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM, 2015. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. British journal of pharmacology 172, 4790–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Navarro DA, Albasanz JL, Martin M, 2015. Hyperthermia-induced seizures alter adenosine A1 and A2A receptors and 5′-nucleotidase activity in rat cerebral cortex. Journal of neurochemistry 134, 395–404. [DOI] [PubMed] [Google Scholar]
- Levin JR, Serrano G, Dingledine R, 2012. Reduction in delayed mortality and subtle improvement in retrograde memory performance in pilocarpine-treated mice with conditional neuronal deletion of cyclooxygenase-2 gene. Epilepsia 53, 1411–1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Quan Lan J, Fredholm BB, Simon RP, Boison D, 2007. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron glia biology 3, 353–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D, 2008, Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. The Journal of clinical investigation 118, 571–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Kang H, Liu X, Liu Z, Shu K, Chen X, Zhu S, 2012. Effect of adenosine A2A receptor antagonist ZM241385 on amygdala-kindled seizures and progression of amygdala kindling. Journal of Huazhong University of Science and Technology. Medical sciences = Hua zhong ke ji da xue xue bao. Yi xue Ying De wen ban = Huazhong keji daxue xuebao. Yixue Yingdewen ban 32, 257–264. [DOI] [PubMed] [Google Scholar]
- Lin X, Dhopeshwarkar AS, Huibregtse M, Mackie K, Hohmann AG, 2018. Slowly Signaling G Protein-Biased CB2 Cannabinoid Receptor Agonist LY2828360 Suppresses Neuropathic Pain with Sustained Efficacy and Attenuates Morphine Tolerance and Dependence. Molecular pharmacology 93, 49–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liang X, Wang Q, Wilson EN, Lam R, Wang J, Kong W, Tsai C, Pan T, Larkin PB, Shamloo M, Andreasson KI, 2019. PGE2 signaling via the neuronal EP2 receptor increases injury in a model of cerebral ischemia. Proc Natl Acad Sci U S A 116, 10019–10024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Klitgaard H, Twyman RE, Schmidt D, 2013. New avenues for anti-epileptic drug discovery and development. Nature reviews. Drug discovery 12, 757–776. [DOI] [PubMed] [Google Scholar]
- Lovatt D, Xu Q, Liu W, Takano T, Smith NA, Schnermann J, Tieu K, Nedergaard M, 2012. Neuronal adenosine release, and not astrocytic ATP release, mediates feedback inhibition of excitatory activity. Proc Natl Acad Sci U S A 109, 6265–6270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luszczki JJ, 2009. Third-generation antiepileptic drugs: mechanisms of action, pharmacokinetics and interactions. Pharmacol Rep 61, 197–216. [DOI] [PubMed] [Google Scholar]
- Maccarrone M, Bab I, Biro T, Cabral GA, Dey SK, Di Marzo V, Konje JC, Kunos G, Mechoulam R, Pacher P, Sharkey KA, Zimmer A, 2015. Endocannabinoid signaling at the periphery: 50 years after THC. Trends Pharmacol Sci 36, 277–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumder M, Xin X, Liu L, Tutunea-Fatan E, Rodriguez-Torres M, Vincent K, Postovit L,M, Hess D, Lala PK, 2016. COX-2 Induces Breast Cancer Stem Cells via EP4/PI3K/AKT/NOTCH/WNT Axis. Stem cells (Dayton, Ohio) 34, 2290–2305. [DOI] [PubMed] [Google Scholar]
- Maresz K, Carrier EJ, Ponomarev ED, Hillard CJ, Dittel BN, 2005. Modulation of the cannabinoid CB2 receptor in microglial cells in response to inflammatory stimuli. Journal of neurochemistry 95, 437–445. [DOI] [PubMed] [Google Scholar]
- Markovic T, Jakopin Z, Dolenc MS, Mlinaric-Rascan I, 2017. Structural features of subtype-selective EP receptor modulators. Drug discovery today 22, 57–71. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Goodenough S, Monory K, Hermann H, Eder M, Cannich A, Azad SC, Cascio MG, Gutierrez SO, van der Stelt M, Lopez-Rodriguez ML, Casanova E, Schutz G, Zieglgansberger W, Di Marzo V, Behl C, Lutz B, 2003. CB1 cannabinoid receptors and on-demand defense against excitotoxicity. Science (New York, N.Y.) 302, 84–88. [DOI] [PubMed] [Google Scholar]
- Mazarati A, Lu X, Shinmei S, Badie-Mahdavi H, Bartfai T, 2004. Patterns of seizures, hippocampal injury and neurogenesis in three models of status epilepticus in galanin receptor type 1 (GalR1) knockout mice. Neuroscience 128, 431–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann GM 2nd, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA, 2003. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience 122, 551–561. [DOI] [PubMed] [Google Scholar]
- Metcalf CS, Klein BD, McDougle DR, Zhang L, Kaufmann D, Bulaj G, White HS, 2017. Preclinical evaluation of intravenous NAX 810-2, a novel GalR2-preferring analog, for anticonvulsant efficacy and pharmacokinetics. Epilepsia 58, 239–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel MC, Charlton SJ, 2018. Biased Agonism in Drug Discovery-Is It Too Soon to Choose a Path? Molecular pharmacology 93, 259–265. [DOI] [PubMed] [Google Scholar]
- Mohri I, Eguchi N, Suzuki K, Urade Y, Taniike M, 2003. Hematopoietic prostaglandin D synthase is expressed in microglia in the developing postnatal mouse brain. Glia 42, 263–274. [DOI] [PubMed] [Google Scholar]
- Moldrich RX, Jeffrey M, Talebi A, Beart PM, Chapman AG, Meldrum BS, 2001. Anti-epileptic activity of group II metabotropic glutamate receptor agonists (--)-2-oxa-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY379268) and (--)-2-thia-4-aminobicyclo[3.1.0]hexane-4,6-dicarboxylate (LY389795). Neuropharmacology 41, 8–18. [DOI] [PubMed] [Google Scholar]
- Mundell S, Kelly E, 2011. Adenosine receptor desensitization and trafficking. Biochimica et biophysica acta 1808, 1319–1328. [DOI] [PubMed] [Google Scholar]
- Murataeva N, Straiker A, Mackie K, 2014. Parsing the players: 2-arachidonoylglycerol synthesis and degradation in the CNS. British journal of pharmacology 171, 1379–1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngomba RT, Biagioni F, Casciato S, Willems-van Bree E, Battaglia G, Bruno V, Nicoletti F, van Luijtelaar EL, 2005. The preferential mGlu2/3 receptor antagonist, LY341495, reduces the frequency of spike-wave discharges in the WAG/Rij rat model of absence epilepsy. Neuropharmacology 49 Suppl 1, 89–103. [DOI] [PubMed] [Google Scholar]
- Ngomba RT, Ferraguti F, Badura A, Citraro R, Santolini I, Battaglia G, Bruno V, De Sarro G, Simonyi A, van Luijtelaar G, Nicoletti F, 2008. Positive allosteric modulation of metabotropic glutamate 4 (mGlu4) receptors enhances spontaneous and evoked absence seizures. Neuropharmacology 54, 344–354. [DOI] [PubMed] [Google Scholar]
- Ngomba RT, van Luijtelaar G, 2018. Metabotropic glutamate receptors as drug targets for the treatment of absence epilepsy. Current opinion in pharmacology 38, 43–50. [DOI] [PubMed] [Google Scholar]
- Nickols HH, Conn PJ, 2014. Development of allosteric modulators of GPCRs for treatment of CNS disorders. Neurobiol Dis 61, 55–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoletti F, Bockaert J, Collingridge GL, Conn PJ, Ferraguti F, Schoepp DD, Wroblewski JT, Pin JP, 2011. Metabotropic glutamate receptors: from the workbench to the bedside. Neuropharmacology 60, 1017–1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Notartomaso S, Mascio G, Scarselli P, Martinello K, Fucile S, Gradini R, Bruno V, Battaglia G, Nicoletti F, 2017. Expression of the K(+)/Cl(−) cotransporter, KCC2, in cerebellar Purkinje cells is regulated by group-I metabotropic glutamate receptors. Neuropharmacology 115, 51–59. [DOI] [PubMed] [Google Scholar]
- O’Connell BK, Gloss D, Devinsky O, 2017. Cannabinoids in treatment-resistant epilepsy: A review. Epilepsy & behavior: E&B 70, 341–348. [DOI] [PubMed] [Google Scholar]
- Oliveira MS, Furian AF, Rambo LM, Ribeiro LR, Royes LF, Ferreira J, Calixto JB, Mello CF, 2008. Modulation of pentylenetetrazol-induced seizures by prostaglandin E2 receptors. Neuroscience 152, 1110–1118. [DOI] [PubMed] [Google Scholar]
- Olivero G, Bonfiglio T, Vergassola M, Usai C, Riozzi B, Battaglia G, Nicoletti F, Pittaluga A, 2017. Immuno-pharmacological characterization of group II metabotropic glutamate receptors controlling glutamate exocytosis in mouse cortex and spinal cord. British journal of pharmacology 174, 4785–4796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco Otalora LF, Couoh J, Shigamoto R, Zarei MM, Garrido Sanabria ER, 2006. Abnormal mGluR2/3 expression in the perforant path termination zones and mossy fibers of chronically epileptic rats. Brain research 1098, 170–185. [DOI] [PubMed] [Google Scholar]
- Palazuelos J, Aguado T, Pazos MR, Julien B, Carrasco C, Resel E, Sagredo O, Benito C, Romero J, Azcoitia I, Fernandez-Ruiz J, Guzman M, Galve-Roperh I, 2009, Microglial CB2 cannabinoid receptors are neuroprotective in Huntington’s disease excitotoxicity. Brain : a journal of neurology 132, 3152–3164. [DOI] [PubMed] [Google Scholar]
- Panatier A, Robitaille R, 2016. Astrocytic mGluR5 and the tripartite synapse. Neuroscience 323, 29–34. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V, 2010. Sensory and cortical activation of distinct glial cell subtypes in the somatosensory thalamus of young rats. The European journal of neuroscience 32, 29–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel DC, Wilcox KS, Metcalf CS, 2017. Novel Targets for Developing Antiseizure and, Potentially, Antiepileptogenic Drugs. Epilepsy currents 17, 293–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcec A, Unkruer B, Schlichtiger J, Soerensen J, Hartz AM, Bauer B, van Vliet EA, Gorter JA, Potschka H, 2009. Targeting prostaglandin E2 EP1 receptors prevents seizure-associated P-glycoprotein up-regulation. J Pharmacol Exp Ther 330, 939–947. [DOI] [PubMed] [Google Scholar]
- Perucca P, Gilliam FG, 2012. Adverse effects of antiepileptic drugs. Lancet Neurol 11, 792–802. [DOI] [PubMed] [Google Scholar]
- Pin JP, Kniazeff J, Binet V, Liu J, Maurel D, Galvez T, Duthey B, Havlickova M, Blahos J, Prezeau L, Rondard P, 2004. Activation mechanism of the heterodimeric GABA(B) receptor. Biochemical pharmacology 68, 1565–1572. [DOI] [PubMed] [Google Scholar]
- Pitsch J, Schoch S, Gueler N, Flor PJ, van der Putten H, Becker AJ, 2007. Functional role of mGluR1 and mGluR4 in pilocarpine-induced temporal lobe epilepsy. Neurobiol Dis 26, 623–633. [DOI] [PubMed] [Google Scholar]
- Post JM, Loch S, Lerner R, Remmers F, Lomazzo E, Lutz B, Bindila L, 2018. Antiepileptogenic Effect of Subchronic Palmitoylethanolamide Treatment in a Mouse Model of Acute Epilepsy. Frontiers in molecular neuroscience 11, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potschka H, 2010. Modulating P-glycoprotein regulation: future perspectives for pharmacoresistant epilepsies? Epilepsia 51, 1333–1347. [DOI] [PubMed] [Google Scholar]
- Qiu J, Li Q, Bell KA, Yao X, Du Y, Zhang E, Yu JJ, Yu Y, Shi Z, Jiang J, 2019. Small-molecule inhibition of prostaglandin E receptor 2 impairs cyclooxygenase-associated malignant glioma growth. British journal of pharmacology 176, 1680–1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu J, Shi Z, Jiang J, 2017. Cyclooxygenase-2 in glioblastoma multiforme. Drug discovery today 22, 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quan Y, Jiang J, Dingledine R, 2013. EP2 receptor signaling pathways regulate classical activation of microglia. J Biol Chem 288, 9293–9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopal S, Shenoy SK, 2018. GPCR desensitization: Acute and prolonged phases. Cellular signalling 41, 9–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravizza T, Vezzani A, 2018. Pharmacological targeting of brain inflammation in epilepsy: Therapeutic perspectives from experimental and clinical studies. Epilepsia open 3, 133–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebola N, Coelho JE, Costenla AR, Lopes LV, Parada A, Oliveira CR, Soares-da-Silva P, de Mendonca A, Cunha RA, 2003. Decrease of adenosine A1 receptor density and of adenosine neuromodulation in the hippocampus of kindled rats. The European journal of neuroscience 18, 820–828. [DOI] [PubMed] [Google Scholar]
- Reiter E, Ahn S, Shukla AK, Lefkowitz RJ, 2012. Molecular mechanism of beta-arrestin-biased agonism at seven-transmembrane receptors. Annu Rev Pharmacol Toxicol 52, 179–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reschke CR, Poersch AB, Masson CJ, Jesse AC, Marafiga JR, Lenz QF, Oliveira MS, Henshall DC, Mello CF, 2018. Systemic delivery of selective EP1 and EP3 receptor antagonists attenuates pentylenetetrazole-induced seizures in mice. International journal of physiology, pathophysiology and pharmacology 10, 47–59. [PMC free article] [PubMed] [Google Scholar]
- Ricciotti E, FitzGerald GA, 2011. Prostaglandins and inflammation. Arteriosclerosis, thrombosis, and vascular biology 31, 986–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo V, Carletti F, Gambino G, Schiera G, Cannizzaro C, Ferraro G, Sardo P, 2014. Role of CB2 receptors and cGMP pathway on the cannabinoid-dependent antiepileptic effects in an in vivo model of partial epilepsy. Epilepsy Res 108, 1711–1718. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Loscher W, 2004. The neurobiology of antiepileptic drugs. Nature reviews. Neuroscience 5, 553–564. [DOI] [PubMed] [Google Scholar]
- Rojas A, Ganesh T, Lelutiu N, Gueorguieva P, Dingledine R, 2015. Inhibition of the prostaglandin EP2 receptor is neuroprotective and accelerates functional recovery in a rat model of organophosphorus induced status epilepticus. Neuropharmacology 93, 15–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Ganesh T, Manji Z, O’Neill T, Dingledine R, 2016. Inhibition of the prostaglandin E2 receptor EP2 prevents status epilepticus-induced deficits in the novel object recognition task in rats. Neuropharmacology 110, 419–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Gueorguieva P, Lelutiu N, Quan Y, Shaw R, Dingledine R, 2014a. The prostaglandin EP1 receptor potentiates kainate receptor activation via a protein kinase C pathway and exacerbates status epilepticus. Neurobiol Dis 70, 74–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas A, Jiang J, Ganesh T, Yang MS, Lelutiu N, Gueorguieva P, Dingledine R, 2014b. Cyclooxygenase-2 in epilepsy. Epilepsia 55, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roseti C, Martinello K, Fucile S, Piccari V, Mascia A, Di Gennaro G, Quarato PP, Manfredi M, Esposito V, Cantore G, Arcella A, Simonato M, Fredholm BB, Limatola C, Miledi R, Eusebi F, 2008. Adenosine receptor antagonists alter the stability of human epileptic GaBAA receptors. Proc Natl Acad Sci U S A 105, 15118–15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley S, Sun X, Lima IV, Tavenier A, de Oliveira ACP, Dey SK, Danzer SC, 2017. Cannabinoid receptor 1/2 double-knockout mice develop epilepsy. Epilepsia 58, e162–e166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumia J, Marmol F, Sanchez J, Carreno M, Bargallo N, Boget T, Pintor L, Setoain X, Bailles E, Donaire A, Ferrer E, Puig-Parellada P, 2012. Eicosanoid levels in the neocortex of drug-resistant epileptic patients submitted to epilepsy surgery. Epilepsy Res 99, 127–131. [DOI] [PubMed] [Google Scholar]
- Russo E, Citraro R, Scicchitano F, De Fazio S, Di Paola ED, Constanti A, De Sarro G, 2010. Comparison of the antiepileptogenic effects of an early long-term treatment with ethosuximide or levetiracetam in a genetic animal model of absence epilepsy. Epilepsia 51, 1560–1569. [DOI] [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, Elebring T, Nilsson K, Drmota T, Greasley PJ, 2007. The orphan receptor GPR55 is a novel cannabinoid receptor. British journal of pharmacology 152, 1092–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadek B, Schwed JS, Subramanian D, Weizel L, Walter M, Adem A, Stark H, 2014. Non-imidazole histamine H3 receptor ligands incorporating antiepileptic moieties. Eur J Med Chem 77, 269–279. [DOI] [PubMed] [Google Scholar]
- Sandau US, Yahya M, Bigej R, Friedman JL, Saleumvong B, Boison D, 2019. Transient use of a systemic adenosine kinase inhibitor attenuates epilepsy development in mice. Epilepsia 60, 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sansig G, Bushell TJ, Clarke VR, Rozov A, Burnashev N, Portet C, Gasparini F, Schmutz M, Klebs K, Shigemoto R, Flor PJ, Kuhn R, Knoepfel T, Schroeder M, Hampson DR, Collett VJ, Zhang C, Duvoisin RM, Collingridge GL, van Der Putten H, 2001. Increased seizure susceptibility in mice lacking metabotropic glutamate receptor 7. J Neurosci 21, 8734–8745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schauwecker PE, 2010. Galanin receptor 1 deletion exacerbates hippocampal neuronal loss after systemic kainate administration in mice. PloS one 5, e15657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert R, Strasser A, Schneider EH, Neumann D, Dove S, Buschauer A, 2013. Molecular and cellular analysis of human histamine receptor subtypes. Trends Pharmacol Sci 34, 33–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seregi A, Forstermann U, Heldt R, Hertting G, 1985. The formation and regional distribution of prostaglandins D2 and F2 alpha in the brain of spontaneously convulsing gerbils. Brain research 337, 171–174. [DOI] [PubMed] [Google Scholar]
- Serrano GE, Lelutiu N, Rojas A, Cochi S, Shaw R, Makinson CD, Wang D, FitzGerald GA, Dingledine R, 2011. Ablation of cyclooxygenase-2 in forebrain neurons is neuroprotective and dampens brain inflammation after status epilepticus. J Neurosci 31, 14850–14860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharif NA, Klimko PG, 2019. Prostaglandin FP receptor antagonists: discovery, pharmacological characterization and therapeutic utility. British journal of pharmacology 176, 1059–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng N, Yang J, Silm K, Edwards RH, Nicoll RA, 2017. A slow excitatory postsynaptic current mediated by a novel metabotropic glutamate receptor in CA1 pyramidal neurons. Neuropharmacology 115, 4–9. [DOI] [PubMed] [Google Scholar]
- Sidorov MS, Kaplan ES, Osterweil EK, Lindemann L, Bear MF, 2015. Metabotropic glutamate receptor signaling is required for NMDA receptor-dependent ocular dominance plasticity and LTD in visual cortex. Proc Natl Acad Sci U S A 112, 12852–12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slanina KA, Schweitzer P, 2005. Inhibition of cyclooxygenase-2 elicits a CB1-mediated decrease of excitatory transmission in rat CA1 hippocampus. Neuropharmacology 49, 653–659. [DOI] [PubMed] [Google Scholar]
- Slivicki RA, Xu Z, Kulkarni PM, Pertwee RG, Mackie K, Thakur GA, Hohmann AG, 2018. Positive Allosteric Modulation of Cannabinoid Receptor Type 1 Suppresses Pathological Pain Without Producing Tolerance or Dependence. Biological psychiatry 84, 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smolders SM, Kessels S, Vangansewinkel T, Rigo JM, Legendre P, Brone B, 2019. Microglia: Brain cells on the move. Prog Neurobiol 178, 101612. [DOI] [PubMed] [Google Scholar]
- Smyth EM, Grosser T, Wang M, Yu Y, FitzGerald GA, 2009. Prostanoids in health and disease. J Lipid Res 50 Suppl, S423–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snead OC 3rd, Banerjee PK, Burnham M, Hampson D, 2000. Modulation of absence seizures by the GABA(A) receptor: a critical rolefor metabotropic glutamate receptor 4 (mGluR4). J Neurosci 20, 6218–6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stempel AV, Stumpf A, Zhang HY, Ozdogan T, Pannasch U, Theis AK, Otte DM, Wojtalla A, Racz I, Ponomarenko A, Xi ZX, Zimmer A, Schmitz D, 2016. Cannabinoid Type 2 Receptors Mediate a Cell Type-Specific Plasticity in the Hippocampus. Neuron 90, 795–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens RC, Cherezov V, Katritch V, Abagyan R, Kuhn P, Rosen H, Wuthrich K, 2013. The GPCR Network: a large-scale collaboration to determine human GPCR structure and function. Nature reviews. Drug discovery 12, 25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, Herkes GK, Farrell M, Degenhardt L, 2018. Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence. Journal of neurology, neurosurgery, and psychiatry 89, 741–753. [DOI] [PubMed] [Google Scholar]
- Stoppel LJ, Auerbach BD, Senter RK, Preza AR, Lefkowitz RJ, Bear MF, 2017. beta-Arrestin2 Couples Metabotropic Glutamate Receptor 5 to Neuronal Protein Synthesis and Is a Potential Target to Treat Fragile X. Cell reports 18, 2807–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya Y, Yamazaki M, Uchigashima M, Kobayashi K, Watanabe M, Sakimura K, Kano M, 2016. Crucial Roles of the Endocannabinoid 2-Arachidonoylglycerol in the Suppression of Epileptic Seizures. Cell reports 16, 1405–1415. [DOI] [PubMed] [Google Scholar]
- Szybala C, Pritchard EM, Lusardi TA, Li T, Wilz A, Kaplan DL, Boison D, 2009. Antiepileptic effects of silk-polymer based adenosine release in kindled rats. Experimental neurology 219, 126–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei S, Hasegawa-Ishii S, Uekawa A, Chiba Y, Umegaki H, Hosokawa M, Woodward DF, Watanabe K, Shimada A, 2012. Immunohistochemical demonstration of increased prostaglandin F(2)alpha levels in the rat hippocampus following kainic acid-induced seizures. Neuroscience 218, 295–304. [DOI] [PubMed] [Google Scholar]
- Takemiya T, Matsumura K, Sugiura H, Maehara M, Yasuda S, Uematsu S, Akira S, Yamagata K, 2010. Endothelial microsomal prostaglandin E synthase-1 exacerbates neuronal loss induced by kainate. Journal of neuroscience research 88, 381–390. [DOI] [PubMed] [Google Scholar]
- Tamai I, Takei T, Maekawa K, Ohta H, 1983. Prostaglandin F2 alpha concentrations in the cerebrospinal fluid of children with febrile convulsions, epilepsy and meningitis. Brain & development 5, 357–362. [DOI] [PubMed] [Google Scholar]
- Tang FR, Chia SC, Chen PM, Gao H, Lee WL, Yeo TS, Burgunder JM, Probst A, Sim MK, Ling EA, 2004. Metabotropic glutamate receptor 2/3 in the hippocampus of patients with mesial temporal lobe epilepsy, and of rats and mice after pilocarpine-induced status epilepticus. Epilepsy Res 59, 167–180. [DOI] [PubMed] [Google Scholar]
- Tassin V, Girard B, Chotte A, Fontanaud P, Rigault D, Kalinichev M, Perroy J, Acher F, Fagni L, Bertaso F, 2016. Phasic and Tonic mGlu7 Receptor Activity Modulates the Thalamocortical Network. Front Neural Circuits 10, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temkin NR, 2001. Antiepileptogenesis and seizure prevention trials with antiepileptic drugs: meta-analysis of controlled trials. Epilepsia 42, 515–524. [DOI] [PubMed] [Google Scholar]
- Temkin NR, 2009. Preventing and treating posttraumatic seizures: the human experience. Epilepsia 50 Suppl 2, 10–13. [DOI] [PubMed] [Google Scholar]
- Terrence CF, Fromm GH, Roussan MS, 1983. Baclofen. Its effect on seizure frequency. Arch Neurol 40, 28–29. [DOI] [PubMed] [Google Scholar]
- Tian L, Kammermeier PJ, 2006. G protein coupling profile of mGluR6 and expression of G alpha proteins in retinal ON bipolar cells. Visual neuroscience 23, 909–916. [DOI] [PubMed] [Google Scholar]
- Umpierre AD, West PJ, White JA, Wilcox KS, 2019. Conditional Knock-out of mGluR5 from Astrocytes during Epilepsy Development Impairs High-Frequency Glutamate Uptake. J Neurosci 39, 727–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urade Y, Eguchi N, Hayaishi O 2013. Lipocalin-type prostaglandin D synthase as an enzymic lipocalin In: Madame Curie Bioscience Database [Internet]. Landes Bioscience. [Google Scholar]
- van Vliet EA, Zibell G, Pekcec A, Schlichtiger J, Edelbroek PM, Holtman L, Aronica E, Gorter JA, Potschka H, 2010. COX-2 inhibition controls P-glycoprotein expression and promotes brain delivery of phenytoin in chronic epileptic rats. Neuropharmacology 58, 404–412. [DOI] [PubMed] [Google Scholar]
- Varvel NH, Jiang J, Dingledine R, 2015. Candidate drug targets for prevention or modification of epilepsy. Annu Rev Pharmacol Toxicol 55, 229–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez JF, Clement HW, Sommer O, Schulz E, van Calker D, 2008. Local stimulation of the adenosine A2B receptors induces an increased release of IL-6 in mouse striatum: an in vivo microdialysis study. Journal of neurochemistry 105, 904–909. [DOI] [PubMed] [Google Scholar]
- Vezzani A, Viviani B, 2015. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 96, 70–82. [DOI] [PubMed] [Google Scholar]
- Von Lubitz DK, Carter MF, Deutsch SI, Lin RC, Mastropaolo J, Meshulam Y, Jacobson KA, 1995. The effects of adenosine A3 receptor stimulation on seizures in mice. European journal of pharmacology 275, 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner AK, Miller MA, Scanlon J, Ren D, Kochanek PM, Conley YP, 2010. Adenosine A1 receptor gene variants associated with post-traumatic seizures after severe TBI. Epilepsy Res 90, 259–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Kaida Y, Fukuhara S, Takechi K, Uehara T, Kamei C, 2011. Participation of metabotropic glutamate receptors in pentetrazol-induced kindled seizure. Epilepsia 52, 140–150. [DOI] [PubMed] [Google Scholar]
- Weltha L, Reemmer J, Boison D The Role of Adenosine in Epilepsy, Brain. Res. Bull. 151, 2019, 46–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White HS, Scholl EA, Klein BD, Flynn SP, Pruess TH, Green BR, Zhang L, Bulaj G, 2009. Developing novel antiepileptic drugs: characterization of NAX 5055, a systemically-active galanin analog, in epilepsy models. Neurotherapeutics: the journal of the American Society for Experimental NeuroTherapeutics 6, 372–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams-Karnesky RL, Sandau US, Lusardi TA, Lytle NK, Farrell JM, Pritchard EM, Kaplan DL, Boison D, 2013. Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. The Journal of clinical investigation 123, 3552–3563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong RK, Bianchi R, Chuang SC, Merlin LR, 2005. Group I mGluR-induced epileptogenesis: distinct and overlapping roles of mGluR1 and mGluR5 and implications for antiepileptic drug design. Epilepsy currents 5, 63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward DF, Jones RL, Narumiya S, 2011. International Union of Basic and Clinical Pharmacology. LXXXIII: classification of prostanoid receptors, updating 15 years of progress. Pharmacological reviews 63, 471–538. [DOI] [PubMed] [Google Scholar]
- Yang H, Zhang J, Andreasson K, Chen C, 2008. COX-2 oxidative metabolism of endocannabinoids augments hippocampal synaptic plasticity. Molecular and cellular neurosciences 37, 682–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Shu X, Liu D, Shang Y, Wu Y, Pei L, Xu X, Tian Q, Zhang J, Qian K, Wang YX, Petralia RS, Tu W, Zhu LQ, Wang JZ, Lu Y, 2012. EPAC null mutation impairs learning and social interactions via aberrant regulation of miR-124 and Zif268 translation. Neuron 73, 774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao K, Wen R, Wang X, Pei L, Yang Y, Shang Y, Bazan N, Zhu LQ, Tian Q, Lu Y, 2013. EPAC inhibition of SUR1 receptor increases glutamate release and seizure vulnerability. J Neurosci 33, 8861–8865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Qian L, Chou T, Iadecola C, 2008. Neuroprotection by PGE2 receptor EP1 inhibition involves the PTEN/AKT pathway. Neurobiol Dis 29, 543–551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zibell G, Unkruer B, Pekcec A, Hartz AM, Bauer B, Miller DS, Potschka H, 2009. Prevention of seizure-induced up-regulation of endothelial P-glycoprotein by COX-2 inhibition. Neuropharmacology 56, 849–855. [DOI] [PubMed] [Google Scholar]
- Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI, 1999. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A 96, 5780–5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoppi S, Madrigal JL, Caso JR, Garcia-Gutierrez MS, Manzanares J, Leza JC, Garcia-Bueno B, 2014. Regulatory role of the cannabinoid CB2 receptor in stress-induced neuroinflammation in mice. British journal of pharmacology 171, 2814–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]