Abstract
Background
Zein-based carriers are a promising delivery system for biomedical applications. However, few studies involve systematic investigation on their in vivo biocompatibility and immunogenicity.
Purpose
The objective of this study was to identify the immunogenicity, type of immune response, biocompatibility and systemic recall immune response of zein nanoparticles administrated via different routes in mice.
Animals and methods
Female Balb/c mice were selected as the animal model in this paper. The effect of particle size, dose and inoculation routes on immunogenicity were systematically explored. The mice were challenged at week 50 via intramuscular and subcutaneous routes to investigate the systemic recall immune responses of zein nanoparticles. Hematoxylin and eosin staining was performed to investigate the biocompatibility of zein nanoparticles at injection sites.
Results
The administration of zein particles by parenteral routes led to a long-term systemic immune response. Particle size did not affect zein-specific IgG antibody titers. IgG antibody titers and inflammatory cell infiltration at the injection sites resulting from intramuscular zein particle injection were significantly higher than those from subcutaneous injection of the same dose. For intramuscular inoculation, dose-dependent IgG antibody titers were observed after the third inoculation, while no significant difference was found via the subcutaneous route. For both routes, IgG titer showed a time-dependent decrease at all dose levels from week 5 onward, and finally plateaued at week 28. The IgG subtype assay showed a predominant Th2-type immune response for both administration routes. Challenge with zein nanoparticles at week 50 led to a significant increase in specific IgG titer at all dose levels, indicating systemic recall immune responses. Interestingly, IgG antibody levels in the subcutaneous groups showed a delayed decrease compared to those of the intramuscular injection groups at all dose levels.
Conclusion
This study indicated that immunogenicity may be one of the key challenges of using zein nanoparticles as carriers via parenteral administration. Further investigation is needed to illustrate zein immunogenicity in other forms, and the possible effect of systemic recall immune response on in vivo pharmacokinetic characteristics.
Keywords: zein, protein carrier, drug delivery, immune response, intramuscular injection, subcutaneous injection, parenteral administration
Introduction
Zein is a plant-based prolamine isolated from maize. Owing to its biodegradability, safety, and moisture resistance, zein was approved by the US FDA as a generally-recognized-as-safe (GRAS) excipient in 1985. Currently, the clinical application of zein as a GRAS ingredient is mainly limited to tablet and pellet coatings for oral administration. However, over the past few decades, zein-based carrier systems have received increasing attention from experts in the biomedical field. Zein can be fabricated into films, nano-fibers, 3D scaffolds, nano/micro-particles, hydrogels, in situ gels, and drug-eluting implants.1–4 The potential application of these fabrications in drug delivery has been investigated by many independent research groups.5–9 Many reports reveal that zein-based carrier systems exhibit definite advantages including the controlled release of bioactive agents, protection of drugs from degradation, improved cellular uptake and oral bioavailability, and even tissue-targeted delivery.1–4,10 For example, ivermectin treatment delivered in a zein microparticle-based tablet showed significantly improved oral bioavailability (132.56%) compared to that of a commercial tablet.11 Owing to improved resistance against elimination and degradation, hollow zein nanoparticles of less than 100 nm displayed passive liver-targeting after intravenous administration, while citric acid-crosslinked nanoparticles preferentially accumulated in the kidneys.12 Despite these advantages, the translation of zein-based carrier systems from the lab bench to the bedside requires further study. Two key points that must be addressed are biocompatibility and immunogenicity, as they are required for biomedical applications (especially via parenteral administration routes).
Currently, zein-based carrier system biocompatibility evaluation has mainly involved in vitro cellular assays and a few that were extended to in vivo studies. Cell-based assays reveal that zein films, fibers, and scaffolds with/without cross-linking treatment are compatible with mice skin fibroblast cells (L929), mouse embryonic fibroblast cells (NIH 3T3), rat bone mesenchymal stem cells, primary human dermal fibroblasts, human umbilical vein endothelial cells, human hepatoma cells (BEL-7402), human liver cells (HL-7702), and human bone marrow stroma cells in terms of cell adhesion, extensibility, and proliferation.13–22 In addition, previous reports also confirm that zein nano/micro-particles show excellent compatibility with Caco-2 and Madin Darby canine kidney cells.5,23 In vivo studies using zein as an implant material show that zein rods and scaffolds are gradually degraded after implantation in rats and rabbits, and that the resultant zein fractions are completely absorbed by body.24–26 Further histological analysis reveal that only laminar and ordered collagen remain at the implant sites after complete biodegradation.26 These reports suggest that zein may have excellent compatibility at both cellular and tissue levels.
Despite the wide application of zein in tissue engineering and the parenteral delivery of bioactive agents, few studies include an immunogenicity evaluation of zein-based carrier systems.27,28 Many publications mainly focus on the food allergy aspect of a maize-based diet and related celiac disease.29–33 A study by Hurtado-Lopez and Murdan found that zein microparticles are immunogenic in mice after two intramuscular injections with a twelve-week interval.27 However, in an issued patent, the immunogenicity of zein nanoparticles in mice after subcutaneous injection is particle size-dependent.28 The above studies suggest that zein-based carrier immunogenicity is complicated, and may be affected by various factors such as form, size, dose, and administration route. No further information on immune response type and long-term immunological memory have been reported in these publications.
In this study, the immunogenicity of zein nanoparticles of different sizes and dosages was investigated long term in mice via both intramuscular and subcutaneous routes. The purpose was to illustrate the following: (a) the effect of zein particle size on immunogenicity; (b) the effect of zein nanoparticle administration route and dose on immune response; (c) the immune response type; and (d) the possible systemic recall immune response that may result from a challenge injection eleven months after the third injection. This study provides fundamental information on the effect of size and dosage on zein particle immunogenicity in the two most commonly used parenteral administration routes; this information illustrates the feasibility of utilizing zein as biomaterial from an immunogenicity standpoint.
Materials and Methods
Materials
Zein and mouse monoclonal antibody isotyping reagents (ISO2-1KT) were purchased from Sigma-Aldrich Corp. (St. Louis, MO, USA). D-Mannitol, hematoxylin, eosin, bovine serum albumin (BSA), pentobarbital sodium, horseradish peroxidase-conjugated goat anti-mouse IgG, and alkaline phosphatase-conjugated rabbit anti-goat IgG (H+L) (IA-0091) were provided by Beijing Dingguo Changsheng Biotechnology Corp. (Beijing, China). Deionized water was used throughout the study. All other chemicals were of analytical reagent grade or purer.
Zein Nanoparticle Preparation
Zein nanoparticles were fabricated using a previously developed modified anti-solvent precipitation method.34 Briefly, 50 mg zein powder was dissolved in 5 mL 80% ethanol. Deionized water (15 mL) containing 5% (w/v) D-mannitol was added to the aqueous zein-ethanol solution and continuously stirred. The different injection rates of deionized water were maintained by a peristaltic pump (Longer Precision Pump Corporation, Baoding, China) to achieve a size-controlled preparation of zein nanoparticles. The solution was constantly stirred at 1200 rpm for 3 h to ensure complete ethanol evaporation. The resultant suspension was lyophilized in the presence of mannitol, and the resultant nanoparticles were sealed and stored in a desiccator at 25°C until use.
Zein Nanoparticle Characterization
The particle size and polydispersity of zein nanoparticles re-dispersed in pH 4 deionized water were determined using a Zetasizer Nano ZS (Malvern Instruments, Malvern, UK). The instrument was equipped with a helium–neon laser (633 nm) and the dynamic light scattering intensity was collected at 173°. In addition, zeta potentials were measured with the same instrument according to the electrophoretic mobility of nanoparticles in 1 mM sodium chloride aqueous medium.
Administration and Sera Sample Collection
To determine the effect of particle size on zein carrier immunogenicity, 30 female BALB/c mice (6–8 weeks old) with body weights of approximately 20 g were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China). The mice were housed under a 12 hr light/dark cycle, and were allowed food and water ad libitum. The mice were divided into five groups of six mice each. Each group was inoculated by intramuscular injection with 600 µg of zein particles in one of four particle sizes (250, 450, 750, or 850 nm). Injections were delivered into the left legs at week 0, 1, and 3, respectively. The negative control group followed the same inoculation schedule and was injected with the same volume of the solvent used to disperse the zein nanoparticles.
To determine the effect of administration dose and route on zein particle immunogenicity, 48 female BALB/c mice (6–8 weeks old) with body weights of approximately 20 g were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China) and randomly divided into eight groups of six mice each. The mice were housed under a 12 hr light/dark cycle, and were allowed food and water ad libitum. Three groups were inoculated by intramuscular injection of 290 nm zein nanoparticles (200, 600, or 800 µg/mouse) into the left legs at weeks 0, 1, and 3, respectively; the negative control group followed the same inoculation schedule and was given the same volume of the solvent used to disperse the zein nanoparticles. The other three groups were inoculated with 290 nm zein nanoparticles (200, 600, or 800 µg/mouse) by subcutaneous injection to the back at weeks 0, 1, and 3, respectively; the negative control followed the same inoculation schedule and was given the same volume of the solvent used to disperse the zein nanoparticles. To illustrate the possible systemic recall immune response, all six treatment groups were challenged with zein nanoparticles of the same previously administered doses 50 weeks after the first inoculation. The negative controls were challenged with solvent.
Blood samples were collected from the tail vein at 1, 2, or 4-week intervals for 70 weeks. Blood samples were centrifuged at 3000 rpm for 5 min to obtain sera. The resultant sera were stored at −20 ºC until use. All animal experiments were performed in accordance with the guidelines of the Animal Use Committee of Jilin University for the use and care of laboratory animals, and all experimental procedures were reviewed and approved by the Animal Welfare and Research Ethics Committee at Jilin University.
Hematoxylin and Eosin Staining
To determine inflammatory response at the injection site, 15 female BALB/c mice (6–8 weeks old) with body weights of approximately 20 g were purchased from Liaoning Changsheng Biotechnology Co., Ltd. (Liaoning, China). The mice were housed under a 12 hr light/dark cycle, and were allowed food and water ad libitum. Mice were randomly divided into two groups of six mice that were inoculated with 800 µg of 311 nm zein particles by subcutaneous and intramuscular injection, respectively. A total volume of 50 µL was injected into the left thigh of each mouse at day 0. Another three untreated female BALB/c mice were used as the control group. At days 1, 3, and 7 after inoculation, mice were sacrificed and histological analyses were performed by surgical excision of the injection site muscle and skin. All obtained tissues were treated and stained by hematoxylin and eosin according to published protocols.35 Images were captured using a Nikon Digital Sight DS-SMC camera (Nikon Instruments Inc., New York, NY, USA) and analyzed with Nis-Elements version 4.20 software.
Total Specific IgG Antibody Determination
Levels of total anti-zein IgG in mouse sera were determined by ELISA using zein protein as the coating antigen. Appropriate amount of zein was dissolved in 60% ethanol, and the resulting solution was further diluted using pH 9.6 bicarbonate buffer to a final zein concentration of 5 µg/mL. Each well of the 96-well plate was coated with 100 µL of zein solution and incubated at 4 ºC overnight. After washing three times with phosphate-buffered saline (PBS) containing 0.05% Tween 20, each well on the plate was blocked with 100 µL PBS containing 1% (w/v) BSA and incubated at 37 ºC for 2 h. The blocked plate was washed an additional three times with PBS containing 0.05% Tween 20 to remove the free BSA. Sera diluted with pH 7.4 PBS to different concentrations were then added to the wells and incubated at 37 ºC for 1 h. After washing three times with PBS containing 0.05% Tween 20, the plate was incubated with 1:10,000 diluted horseradish peroxidase-conjugated goat anti-mouse IgG with pH 7.4 PBS at 37 ºC for 1 h. The resultant plate was washed three times with PBS containing 0.05% Tween 20, then incubated with 50 µL 3,3ʹ,5,5ʹ-tetramethylbenzidine substrate in the dark for 30 min. The reaction was stopped by adding 50 µL of 2 M H2SO4, and the plate was measured at 450 nm using an iMark microplate reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA).
IgG Subtype Assay
Sera IgG subtype was determined by ELISA using zein protein as the coating antigen as described above. Briefly, sera were diluted to 1:100 using PBS containing 1% (w/v) BSA, then added to the blocked plate. After incubation at 37 ºC for 1 h, the plate was washed three times with PBS containing 0.05% Tween 20. The 1:1000 diluted goat anti-mouse IgG1, IgG2a, and IgG2b capture antibodies were added to the washed plate, followed by a 1 h incubation at room temperature. After washing three times with PBS containing 0.05% Tween 20, 100 µL of 1:5000 diluted alkaline phosphatase-conjugated rabbit anti-goat IgG (H+L) was added into each well on the plate and incubated for 1 h at room temperature. After washing five times with PBS, 100 µL of 1 mg/mL p-nitrophenyl phosphate substrate was added into each well and reacted for 30 min in the dark. The reaction was stopped by adding 100 µL of 0.2 M NaOH, and the plate was measured at 415 nm using an iMark microplate reader (Bio-Rad Laboratories, Inc.).
Statistical Analysis
All data are presented as the mean ± SD. Statistical analysis was conducted using the OriginPro version 8.5 software (OriginLab Corporation, Northampton, MA, USA). Groups were analyzed using a two-tailed unpaired Student’s t-test. p Values <0.05 were considered significant.
Results
Zein Nanoparticle Preparation and Characterization
Zein nanoparticles were successfully fabricated by a modified phase separation method as previously described.34 The size, size distribution, and zeta potential of the resultant zein nanoparticles are shown in Table 1. All zein nanoparticles showed a single peak in intensity distribution at the desired sizes, and were positively charged with a 35–57 mV zeta potential. In all cases, the polydispersity indices were below 0.3, indicating that zein nanoparticles approached a monodispersed system. Lyophilization and rehydration did not significantly affect particle properties in the presence of mannitol (Figure S1). In all inoculations, freshly lyophilized zein particles were weighted to obtain an accurate dose for administration, and used within 3 days to avoid the possible aggregation and changed particle properties of zein nanoparticles that could result from long-term storage.
Table 1.
Properties of Zein Nanoparticles Used for Immunogenicity and Inflammatory Response Investigation
| Size (nm) | PDI | Zeta Potential (mV) | |
|---|---|---|---|
| Section I: The effect of particle size on zein particle immunogenicity | |||
| First inoculation | |||
| Group 1 | 241.4±8.47 | 0.126±0.022 | 39.2±0.12 |
| Group 2 | 447.3±16.06 | 0.121±0.018 | 44.5±0.20 |
| Group 3 | 716.9±5.25 | 0.189±0.017 | 49.6±1.06 |
| Group 4 | 879.2±12.04 | 0.226±0.074 | 51.1±1.52 |
| Second inoculation | |||
| Group 1 | 250.2±5.17 | 0.139±0.027 | 38.9±0.87 |
| Group 2 | 439.9±6.07 | 0.150±0.025 | 44.9±1.76 |
| Group 3 | 763.8±7.84 | 0.212±0.025 | 49.1±1.16 |
| Group 4 | 838.2±14.66 | 0.268±0.067 | 51.0±0.70 |
| Third inoculation | |||
| Group 1 | 246.7±2.50 | 0.148±0.020 | 41.4±0.25 |
| Group 2 | 434.6±1.79 | 0.202±0.021 | 52.6±0.56 |
| Group 3 | 721.9±15.05 | 0.175±0.014 | 57.2±0.95 |
| Group 4 | 832.9±15.83 | 0.264±0.009 | 57.4±0.78 |
| Section II: The effect of administration dose and route on zein particle immunogenicity | |||
| First injection | 304.0±4.97 | 0.222±0.03 | 42.3±0.96 |
| Second injection | 287.6±2.03 | 0.172±0.02 | 55.1±1.35 |
| Third injection | 289.5±6.22 | 0.265±0.01 | 49.5±1.50 |
| Fourth injection | 287.1±4.98 | 0.206±0.05 | 35.3±0.87 |
| Section III: Zein particle inoculation-induced inflammatory response | |||
| i.m. and s.c. | 335.4±6.85 | 0.175±0.017 | 39.1±0.55 |
Abbreviations: PDI, polydispersity index; i.m., intramuscular injection; s.c., subcutaneous injection.
The Effect of Particle Size on Immunogenicity and Body Weight
To determine the effect of particle size on the immunogenicity of zein carriers, mice were inoculated by intramuscular injection with 600 µg of 250, 450, 750, or 850 nm zein particles to the leg at weeks 0, 1, and 3, respectively. Zein particles with size larger than 850 nm were not tested owing to their high polydispersity index and poor stability after freeze-drying and reconstitution. The 600 µg dose was chosen because it is close to the medium zein nanoparticle dose used as a drug delivery carrier in mice.
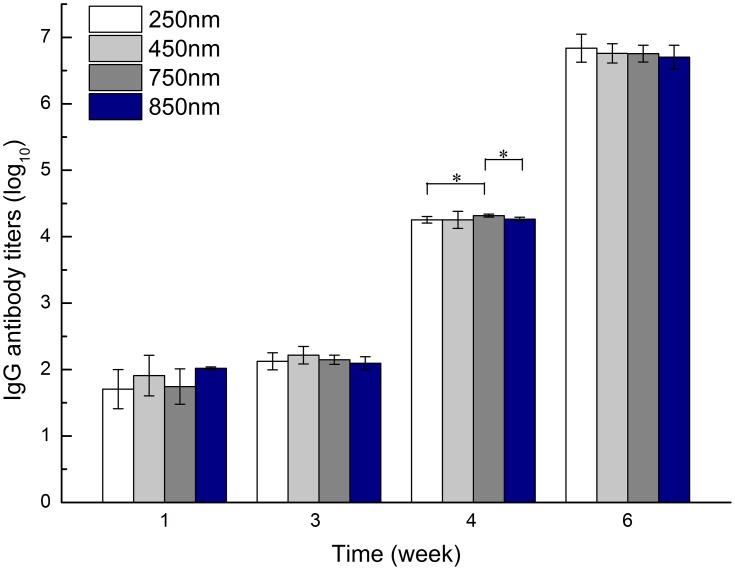
Zein-specific IgG antibody titer results are shown in Figure 1. For 250 nm zein particles, only limited IgG antibody was detected 1 week after the primary injection. The second injection at week 1 led to a slight increase in antibody titer. However, after the third challenge at week 3, zein-specific IgG antibody significantly increased at week 4 and peaked at week 6. Similar trends were observed in all groups inoculated with 450, 750, and 850 nm zein particles. Unexpectedly, no statistically significant difference in zein-specific antibody titer was found between the 250, 450, 750, and 850 nm groups at any sampling time point, except for the 750 nm group at week 4 that showed a higher antibody titer than those of 250 (p = 0.038) and 850 nm (p = 0.011). These findings suggested there was no notable size-dependent effect on zein immunogenicity after each intramuscular injection.
Figure 1.
Zein-specific IgG titers in the sera of mice intramuscularly inoculated with the same dose (600 µg) of 250, 450, 750, or 850 nm zein particles at weeks 0, 1, and 3. The sera of all six mice in each group were separately evaluated three times by ELISA. Results are expressed as means of antibody titers calculated from six mice per group. *p-value <0.05 between two different groups.
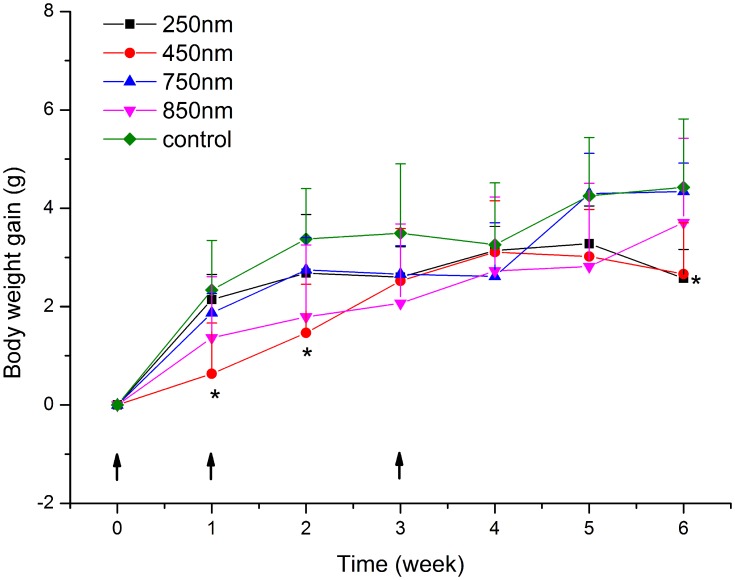
In addition to antibody titers, we also monitored mouse body weight changes. As shown in Figure 2, all groups displayed a gradual weight gain over time. Although the control group showed slightly higher body weight than the other groups at some time points, the only statistically significant differences were seen between the 250 nm group and the control at week 6 (p = 0.042), and the 450 nm group and the control at weeks 1, 2, and 6 (p = 0.024, 0.016, and 0.032, respectively). These results suggest a slightly negative influence of zein particle administration on mouse body weight increase.
Figure 2.
Body weight of mice intramuscularly inoculated with the same dose (600 µg) of 250, 450, 750, or 850 nm zein particles at weeks 0, 1, and 3. The control group followed the same inoculation scheme using the same volume of solvent instead of zein particles. All p-value calculations are based on the t-test of the two-sample equal variance hypothesis. *p < 0.05 compared to control.
The Effect of Dose on Immunogenicity
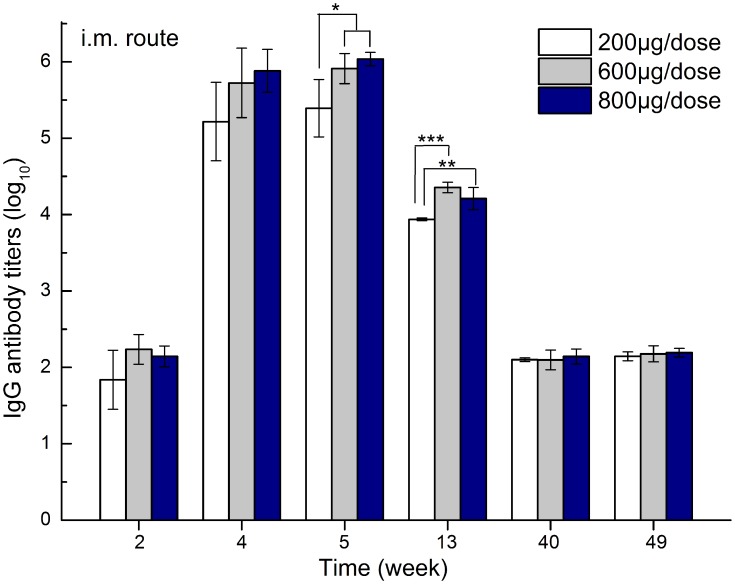
To investigate the influence of dose on zein carrier immunogenicity, 290 nm zein nanoparticles were chosen as a representative sample and intramuscularly injected into mice at 200, 600, and 800 µg doses. Doses below 200 µg were not considered, as they may not be enough to load sufficient therapeutics when zein particles are used as drug delivery carriers. The zein-specific antibody titer results are shown in Figure 3. For all groups, the second challenge at week 1 led to zein-specific antibody production, and the IgG antibody level peaked at week 5 after the third inoculation. As expected, antibody titers gradually decreased over time, and finally reached at a plateau similar to that of the second challenge by week 28 (data not shown). Moreover, the 600 and 800 µg groups showed significantly higher antibody titers than those of the 200 µg group at weeks 5 and 13, suggesting a dose-dependent effect of zein particles on immunogenicity. Higher doses would provide more chances for zein nanoparticles to be presented to T cells by dendritic cells and macrophages, and therefore, promote the differentiation of B cells into antibody-secreting plasma cells more efficiently.
Figure 3.
Zein-specific IgG titers in the sera of mice intramuscularly inoculated with either 200, 600, or 800 µg of 290 nm zein particles at weeks 0, 1, and 3. The sera of all six mice in each group were separately evaluated three times by ELISA. Results are expressed as means of antibody titers calculated from six mice per group. All p-value calculations are based on the t-test of the two-sample equal variance hypothesis. *, **, and *** indicates p < 0.05, p < 0.01, and p < 0.001, respectively.
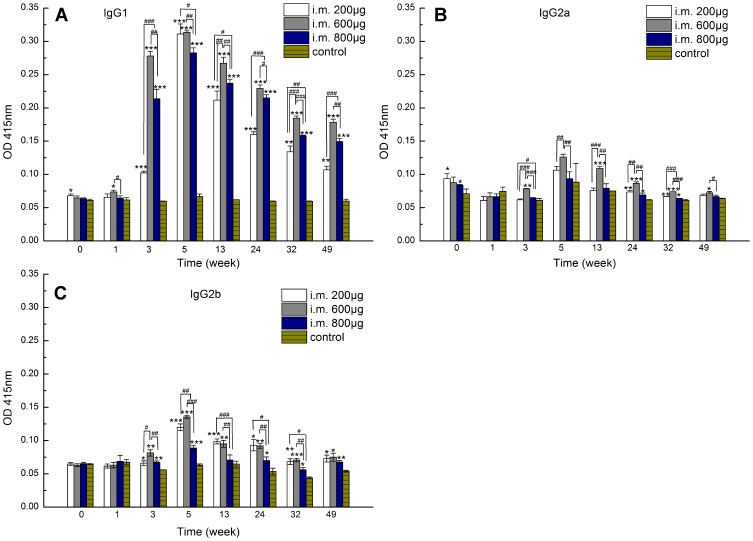
Further investigation into IgG antibody subclasses revealed that, in each group, the IgG1 OD value was remarkably higher than those of the control groups from week 3 onward, whereas IgG2a and IgG2b OD values remained at comparative or slightly higher levels compared to the corresponding control groups (Figure 4A–C). These results suggested that different doses of zein nanoparticles led to a predominantly Th2-type immune response.36 Interestingly, the 600 µg group displayed higher IgG1 antibody OD values after three inoculations than the 200 and 800 µg groups at all sampling time points except week 5. Further study is needed to illustrate the possible reason.
Figure 4.
Zein-specific IgG1 (A), IgG2a (B), and IgG2b (C) OD values at 415 nm in the sera of mice intramuscularly inoculated with either 200, 600, or 800 µg of 290 nm zein particles at weeks 0, 1, and 3. The control group followed the same inoculation scheme using the same volume of solvent instead of zein particles. The sera of all six mice in each group were combined to form a serum pool and separately evaluated three times by ELISA. All p-value calculations are based on the t-test of the two-sample equal variance hypothesis. *, **, and *** indicates p < 0.05, p < 0.01, and p < 0.001, respectively, compared to the control at each time point. #, ##, and ### indicates p < 0.05, p < 0.01, and p < 0.001, respectively.
The Effect of Administration Route on Immunogenicity and Inflammatory Response
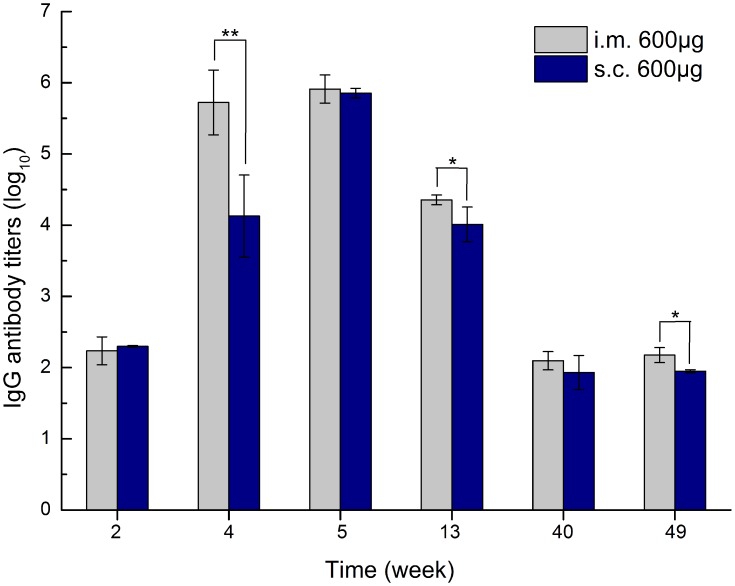
To illustrate the effect of administration routes on zein immunogenicity, we compared zein-specific antibody titers from 600 µg of 290 nm zein particles inoculated via intramuscular and subcutaneous injections, respectively. Each mouse was injected at weeks 0, 1, and 3. The zein-specific IgG antibody titers at different sampling time points are shown in Figure 5. Three intramuscular injections resulted in higher IgG antibody titers at all sampling time points compared to those of subcutaneous inoculation, and a statistically significant difference was found at weeks 4, 13, and 49 (p = 0.002, 0.016, and 0.036, respectively). Further antibody subtype analysis revealed that subcutaneous injection induced the same IgG1-dominated immune response as intramuscular inoculation. The above results indicated that the intramuscular injection of zein particles induces a stronger immune response than subcutaneous injection, while administration routes have no influence on the type of immune response.
Figure 5.
Zein-specific IgG titers in the sera of mice inoculated via intramuscular and subcutaneous routes with 600 µg of 290 nm zein particles at weeks 0, 1, and 3, respectively. The sera of all six mice in each group were separately evaluated three times by ELISA. Results are expressed as means of antibody titers calculated from six mice per group. All p-value calculations are based on the t-test of the two-sample equal variance hypothesis. *p < 0.05, and **p < 0.01.
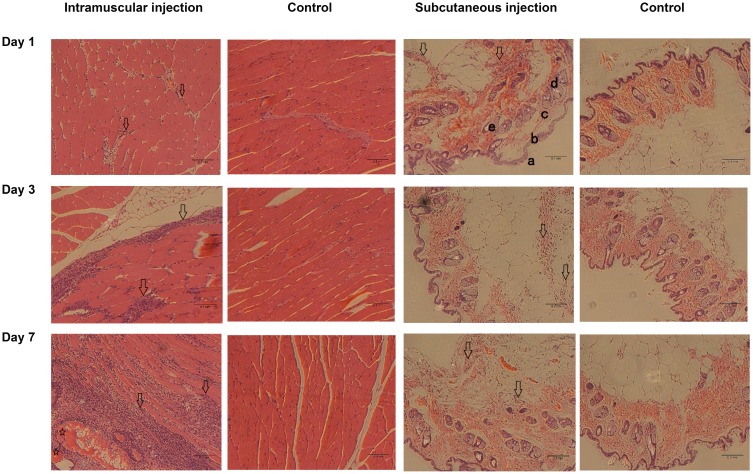
In addition to immunogenicity, we examined whether an inflammatory response occurred at the injection sites at 1, 3, and 7 days after inoculation. Hematoxylin and eosin-stained sections of muscle and skin from the injection sites are shown in Figure 6. Mild-to-moderate inflammatory cell infiltration was found in the intramuscular injection group at days 1 and 3 as evidenced by the increasing amount of blue (nuclear) staining compared to those of the control group. At day 7, damaged muscle fibers and additional inflammatory cells were observed. In contrast, only mild inflammatory cell infiltration was found in the dermis during the 7-day investigation after inoculation. The superiority of intramuscular injection over subcutaneous injection in inducing antibody production may be due to differing inflammatory responses at the injection sites. Inflammatory response has been reported to improve the presentation efficiency of foreign proteins by dendritic cells and macrophages, and therefore, may lead to enhanced antibody titer.37
Figure 6.
Hematoxylin and eosin-stained sections of muscle and skin from injection sites at days 1, 3, and 7 after inoculation. Mice were inoculated by injecting 800 µg of 311 nm zein particles into the left leg at day 0 via intramuscular or subcutaneous routes. Inflammatory cell infiltration (hollow arrows) and damaged muscle fiber (hollow stars) were located in the muscle tissues and dermis. a, stratum; b, epidermis; c, dermis; d, sebaceous glands; e, hair follicles containing hair fibers (bright color).
Systemic Recall Immune Response via Intramuscular and Subcutaneous Routes
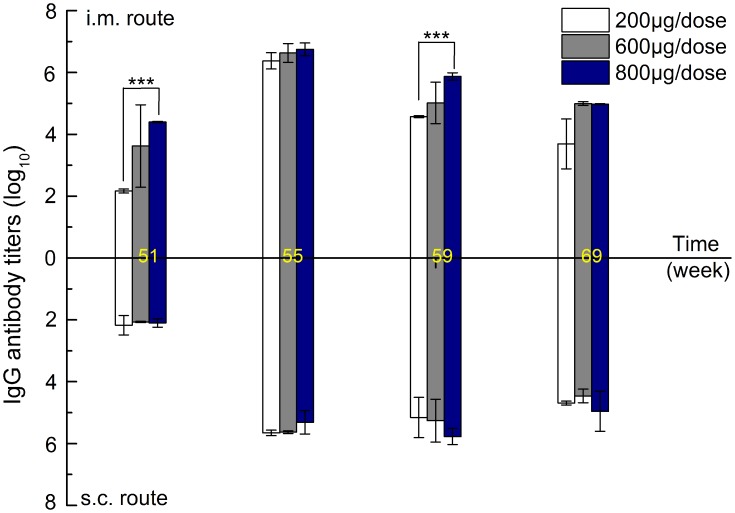
Considering the possible repeated administration of drug-loaded zein particles, systemic recall immune responses over a long interval were investigated by challenging mice at week 50 after three inoculations at week 0, 1, and 3 for each dose group. As shown in Figure 7, in the case of intramuscular injection, zein-specific IgG antibody increased dramatically and peaked at week 55 for each dose level. The peak antibody titer values for each dose level at week 55 were significantly higher than the peak values after three inoculations (Figure S2). The above results suggest that the first three intramuscular injections of zein nanoparticles induced the production of long-lived memory B cells. As a result, a strong systemic recall immune response occurred when the mice were challenged again. Moreover, high dose levels produced higher IgG antibody titers, indicating a dose-dependent influence of zein nanoparticles on memory immune response. As expected, challenge via subcutaneous injection also resulted in a similar, but weaker, recall immune response, as evidenced by the lower antibody titer at week 55 at the same dose. However, the resultant IgG antibody titer showed a delayed decrease over time compared to that of intramuscular injection. In addition, no dose-dependent influence was observed between dose levels. The differences might be due to the varied degradation and antigen-presenting processes that occur at different injection sites. Further investigation is required to illustrate this mechanism.
Figure 7.
Zein-specific IgG titers in the sera of mice inoculated via intramuscular and subcutaneous routes with 200, 600, or 800 µg of 290 nm zein particles at weeks 0, 1, 3, and 50. The sera of all six mice in each group were separately evaluated three times by ELISA. Results are expressed as means of antibody titers calculated from six mice per group. All p-value calculations are based on the t-test of the two-sample equal variance hypothesis. ***p < 0.001.
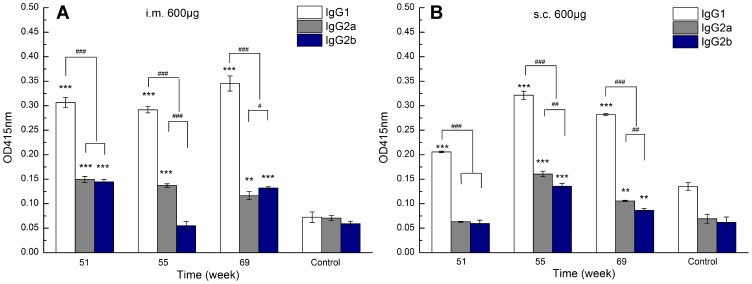
In order to reveal the possible effect of recall immune response on the type of immune response, an antibody subtype assay was also performed. As shown in Figure 8, at the 600 μg representative dose, IgG1 displayed significantly higher OD values than those of IgG2a and IgG2b for both intramuscular and subcutaneous routes. In addition, IgG2a OD values for the intramuscular route that resulted from the fourth injection were higher than those at week 5 (Figure S3). The above results suggest that the fourth challenge does not influence the type of immune response, but lends a slight bias towards a Th1 immune response.
Figure 8.
Zein-specific IgG1, IgG2a, and IgG2b OD values at 415 nm in the sera of mice inoculated via intramuscular (A) and subcutaneous (B) routes with 600 µg of 290 nm zein particles at weeks 0, 1, 3, and 50. The sera of all six mice in each group were combined to form a serum pool and separately evaluated three times by ELISA. All p-value calculations are based on the t-test of the two-sample equal variance hypothesis. ** and *** indicates p < 0.01 and p < 0.001, respectively, compared to the control. #, ##, and ### indicates p < 0.05, p < 0.01, and p < 0.001, respectively.
Discussion
Particle size usually has a complicated influence on the immune response because particles with varied size may interact with immune cells and tissues in different ways.38 For example, polystyrene particles of 20 nm can reach the local lymph node independently of dendritic cells within 2 h after administration into mouse footpad, while 0.5–1 µm particles can only be drained into local lymph node via dendritic cell-mediated infiltration 8 h after injection.39 In another study, ovalbumin-conjugated nanoparticles of 230 nm are more effectively internalized by both macrophage and dendritic cells and have more effective infiltration into lymph node than the 708 nm nanoparticles after subcutaneous injections, and therefore, induce stronger humoral and cellular immune responses.40 In the case of zein nanoparticles, very limited information is available to the effect of particle size on immune response. A previous study reports that 100–400 nm zein nanoparticles were almost non-immunogenic after subcutaneous administration in mice, and therefore, could be used as parenteral drug delivery carriers.28 This is contrary to that of our finding. The disagreement may be explained by the lower dose of zein particles (100 µg/mouse) used in the previous study. In addition, the subcutaneous inoculation route may also contribute to lower immunogenicity. The speculation is confirmed by the inferior immune response resulted from the subcutaneous injections compared with that of intramuscular injections (Figure 5). Besides, two injections at a 2-week interval in the previous study may also explain the negligible immune response as only the third injection induced a significantly higher antibody titer than those of the first two injections (Figure 1 and Figure 5). The size-independent immunogenicity in this study may be due to the strong interactions between zein particles and local tissue that result from their intrinsic hydrophobic characteristics following charge neutralization, and therefore, result in rapid aggregation at the injection sites. This speculation is supported by the phenomenon of rapid particle aggregation observed when we attempted to investigate zein nanoparticle–macrophage interactions in vitro.
Both intramuscular and subcutaneous injection of zein nanoparticles induced a Th2-biased immune response as evidenced by the higher IgG1 OD values than those of IgG2a (Figure 4). Re-challenge with zein nanoparticles at a long-term interval (more than 11 months) did not change the type of immune response but caused a slight bias towards a Th1 immune response, supported by the slightly higher IgG2a OD values for both administration routes (Figure 8). Th2-associated IgG1 is known to fix complement by an alternative pathway, and can lead to accelerated foreign substance clearance via macrophage-mediated phagocytosis.36 Thus, repeated injections of zein nanoparticles might promote the immune system to remove zein carriers more rapidly, and therefore, result in a shorter half-life and variable pharmacokinetic profile of loaded cargo in vivo. In addition, IgG1 can bind to receptors on mast cells and basophils to cause hypersensitivity reactions, which are usually associated with local and systemic side effects.36,41 The infiltration of inflammatory cells at injection sites has confirmed the above speculation (Figure 6). The inflammatory response may explain the lower body weight gain of mice inoculated with zein nanoparticles than those of the control group, although size-dependent effect is not found in this study (Figure 2). The possible accelerated clearance of zein nanoparticle resulted from repeated injections may be similar to those of polyethylene glycol (PEG)-modified liposomes. PEG-modified liposome is known to induce an accelerated blood clearance phenomenon and complement activation-related pseudoallergy when used as parenteral drug delivery carriers repeatedly.42 This is related to the production of antibodies toward PEG.43 It should be noted that, among these antibodies, anti-PEG IgM which is mainly responsible for the accelerated blood clearance and pseudoallergy response, is produced by non-memory B cells without T-cell involvement.44 In other words, the above undesired phenomena can be avoided with repeat injections when time interval is long enough (several weeks).45,46 However, in the case of zein nanoparticles, the immune response resulted by repeated injections involves long-lived memory B cells and can be recalled even after 11 months by challenge (Figure 7). Considering the strong and long-standing immunogenicity of zein nanoparticles, care must be taken when evaluating the in vivo behavior of zein carriers and loaded cargo delivered via parenteral administration routes.
Conclusions
In this study, the immunogenicity of different sizes and doses of zein nanoparticles was investigated in mice via both intramuscular and subcutaneous routes. There was no notable size-dependent effect on zein immunogenicity after three intramuscular injections. However, a dose-dependent effect of zein particles on immunogenicity was found for the intramuscular route. Both intramuscular and subcutaneous zein particle injections induced a Th2-biased immune response, and intramuscular injections led to a higher humoral immune response compared to subcutaneous injections. The immune response is long-lasting, highly specific, and repeated challenge caused rapid and strong systemic recall immune responses via both intramuscular and subcutaneous routes. This study raises a concern about the feasibility of zein nanoparticles as drug delivery carriers for parenteral administration in terms of immunogenicity. Low dose or surface modification of zein particles may be required to reduce or eliminate their immunogenicity when used as delivery carriers via parenteral routes. In addition, further study is needed to illustrate the possible effects of memory immune response on the in vivo pharmacokinetic properties of zein particles, such as distribution, metabolism, and excretion.
Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (grant no. 101832013). This work is partly supported by a scholarship from the China Scholarship Council (CSC NO. 201906175182).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Control Release. 2014;189:108–122. doi: 10.1016/j.jconrel.2014.06.036 [DOI] [PubMed] [Google Scholar]
- 2.Zhang Y, Cui L, Che X, et al. Zein-based films and their usage for controlled delivery: origin, classes and current landscape. J Control Release. 2015;206:206–219. doi: 10.1016/j.jconrel.2015.03.030 [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Cui L, Li F, et al. Design, fabrication and biomedical applications of zein-based nano/micro-carrier systems. Int J Pharm. 2016;513(1–2):191–210. doi: 10.1016/j.ijpharm.2016.09.023 [DOI] [PubMed] [Google Scholar]
- 4.Zhang Y, Cui LL, Chen Y, et al. Zein-based nanofiber for drug delivery: classes and current application. Curr Pharm Des. 2015;21(22):3199–3207. doi: 10.2174/1381612821666150531170448 [DOI] [PubMed] [Google Scholar]
- 5.Zou T, Gu L. TPGS emulsified zein nanoparticles enhanced oral bioavailability of daidzin: in vitro characteristics and in vivo performance. Mol Pharm. 2013;10(5):2062–2070. [DOI] [PubMed] [Google Scholar]
- 6.Dong F, Dong X, Zhou L, et al. Doxorubicin-loaded biodegradable self-assembly zein nanoparticle and its anti-cancer effect: preparation, in vitro evaluation, and cellular uptake. Colloids Surf B Biointerfaces. 2016;140:324–331. doi: 10.1016/j.colsurfb.2015.12.048 [DOI] [PubMed] [Google Scholar]
- 7.Hashem FM, Al-Sawahli MM, Nasr M, Ahmed OA. Optimized zein nanospheres for improved oral bioavailability of atorvastatin. Int J Nanomedicine. 2015;10:4059–4069. doi: 10.2147/IJN.S83906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee S, Alwahab NS, Moazzam ZM. Zein-based oral drug delivery system targeting activated macrophages. Int J Pharm. 2013;454(1):388–393. doi: 10.1016/j.ijpharm.2013.07.026 [DOI] [PubMed] [Google Scholar]
- 9.Lai LF, Guo HX. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int J Pharm. 2011;404(1–2):317–323. doi: 10.1016/j.ijpharm.2010.11.025 [DOI] [PubMed] [Google Scholar]
- 10.Berardi A, Bisharat L, AlKhatib HS, Cespi M. Zein as a pharmaceutical excipient in oral solid dosage forms: state of the art and future perspectives. AAPS PharmSciTech. 2018;19(5):2009–2022. doi: 10.1208/s12249-018-1035-y [DOI] [PubMed] [Google Scholar]
- 11.Gong SJ, Sun SX, Sun QS, Wang JY, Liu XM, Liu GY. Tablets based on compressed zein microspheres for sustained oral administration: design, pharmacokinetics, and clinical study. J Biomater Appl. 2011;26(2):195–208. doi: 10.1177/0885328210363504 [DOI] [PubMed] [Google Scholar]
- 12.Xu H, Shen L, Xu L, Yang Y. Controlled delivery of hollow corn protein nanoparticles via non-toxic crosslinking: in vivo and drug loading study. Biomed Microdevices. 2015;17(1):8. doi: 10.1007/s10544-014-9926-5 [DOI] [PubMed] [Google Scholar]
- 13.Gong S, Wang H, Sun Q, Xue ST, Wang JY. Mechanical properties and in vitro biocompatibility of porous zein scaffolds. Biomaterials. 2006;27(20):3793–3799. doi: 10.1016/j.biomaterials.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 14.Wang HJ, Lin ZX, Liu XM, Sheng SY, Wang JY. Heparin-loaded zein microsphere film and hemocompatibility. J Control Release. 2005;105(1–2):120–131. doi: 10.1016/j.jconrel.2005.03.014 [DOI] [PubMed] [Google Scholar]
- 15.Lin L, Perets A, Har-el YE, et al. Alimentary ‘green’ proteins as electrospun scaffolds for skin regenerative engineering. J Tissue Eng Regen Med. 2013;7(12):994–1008. doi: 10.1002/term.1493 [DOI] [PubMed] [Google Scholar]
- 16.Lin J, Li C, Zhao Y, Hu J, Zhang LM. Co-electrospun nanofibrous membranes of collagen and zein for wound healing. ACS Appl Mater Interfaces. 2012;4(2):1050–1057. doi: 10.1021/am201669z [DOI] [PubMed] [Google Scholar]
- 17.Dong J, Sun Q, Wang JY. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials. 2004;25(19):4691–4697. doi: 10.1016/j.biomaterials.2003.10.084 [DOI] [PubMed] [Google Scholar]
- 18.Sun QS, Dong J, Lin ZX, Yang B, Wang JY. Comparison of cytocompatibility of zein film with other biomaterials and its degradability in vitro. Biopolymers. 2005;78(5):268–274. doi: 10.1002/bip.20298 [DOI] [PubMed] [Google Scholar]
- 19.Wang HJ, Fu JX, Wang JY. Effect of water vapor on the surface characteristics and cell compatibility of zein films. Colloids Surf B Biointerfaces. 2009;69(1):109–115. doi: 10.1016/j.colsurfb.2008.11.015 [DOI] [PubMed] [Google Scholar]
- 20.Wang Q, Xian W, Li S, Liu C, Padua GW. Topography and biocompatibility of patterned hydrophobic/hydrophilic zein layers. Acta Biomater. 2008;4(4):844–851. doi: 10.1016/j.actbio.2008.01.017 [DOI] [PubMed] [Google Scholar]
- 21.Jiang Q, Reddy N, Yang Y. Cytocompatible cross-linking of electrospun zein fibers for the development of water-stable tissue engineering scaffolds. Acta Biomater. 2010;6(10):4042–4051. doi: 10.1016/j.actbio.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 22.Qu ZH, Wang HJ, Tang TT, Zhang XL, Wang JY, Dai KR. Evaluation of the zein/inorganics composite on biocompatibility and osteoblastic differentiation. Acta Biomater. 2008;4(5):1360–1368. doi: 10.1016/j.actbio.2008.03.006 [DOI] [PubMed] [Google Scholar]
- 23.Luo Y, Teng Z, Wang TT, Wang Q. Cellular uptake and transport of zein nanoparticles: effects of sodium caseinate. J Agric Food Chem. 2013;61(31):7621–7629. doi: 10.1021/jf402198r [DOI] [PubMed] [Google Scholar]
- 24.Lin T, Lu C, Zhu L, Lu T. The biodegradation of zein in vitro and in vivo and its application in implants. AAPS PharmSciTech. 2011;12(1):172–176. doi: 10.1208/s12249-010-9565-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tu J, Wang H, Li H, Dai K, Wang J, Zhang X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials. 2009;30(26):4369–4376. doi: 10.1016/j.biomaterials.2009.04.054 [DOI] [PubMed] [Google Scholar]
- 26.Wang HJ, Gong SJ, Lin ZX, et al. In vivo biocompatibility and mechanical properties of porous zein scaffolds. Biomaterials. 2007;28(27):3952–3964. doi: 10.1016/j.biomaterials.2007.05.017 [DOI] [PubMed] [Google Scholar]
- 27.Hurtado-Lopez P, Murdan S. An investigation into the adjuvanticity and immunogenicity of zein microspheres being researched as drug and vaccine carriers. J Pharm Pharmacol. 2006;58(6):769–774. doi: 10.1211/jpp.58.6.0007 [DOI] [PubMed] [Google Scholar]
- 28.Perumal OP, Podaralla SK, Kaushik RS, Inventors; South Dakota State University, assignee. Method of forming non-immunogenic hydrophobic protein nanoparticles and uses therefor. 2009.
- 29.Johnson RB, Labrooy JT, Skerritt JH. Antibody responses reveal differences in oral tolerance to wheat and maize grain protein fractions. Clin Exp Immunol. 1990;79(1):135–140. doi: 10.1111/j.1365-2249.1990.tb05140.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pasini G, Simonato B, Curioni A, et al. IgE-mediated allergy to corn: a 50 kDa protein, belonging to the reduced soluble proteins, is a major allergen. Allergy. 2002;57(2):98–106. doi: 10.1034/j.1398-9995.2002.1o3413.x [DOI] [PubMed] [Google Scholar]
- 31.Lee S-H, Benmoussa M, Sathe SK, Roux KH, Teuber SS, Hamaker BR. A 50 kDa maize γ-zein has marked cross-reactivity with the almond major protein. J Agric Food Chem. 2005;53(20):7965–7970. doi: 10.1021/jf0479618 [DOI] [PubMed] [Google Scholar]
- 32.Cabrera-Chavez F, Rouzaud-Sandez O, Sotelo-Cruz N, Calderon de la Barca AM. Bovine milk caseins and transglutaminase-treated cereal prolamins are differentially recognized by IgA of celiac disease patients according to their age. J Agric Food Chem. 2009;57(9):3754–3759. doi: 10.1021/jf802596g [DOI] [PubMed] [Google Scholar]
- 33.Krishnan HB, Jang S, Kim WS, Kerley MS, Oliver MJ, Trick HN. Biofortification of soybean meal: immunological properties of the 27 kDa gamma-zein. J Agric Food Chem. 2011;59(4):1223–1228. doi: 10.1021/jf103613s [DOI] [PubMed] [Google Scholar]
- 34.Li F, Chen Y, Liu SB, et al. Size-controlled fabrication of zein nano/microparticles by modified anti-solvent precipitation with/without sodium caseinate. Int J Nanomedicine. 2017;12:8197–8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fischer AH, Jacobson KA, Rose J, Zeller R. Hematoxylin and eosin staining of tissue and cell sections. CSH Protoc. 2008;2008:pdb prot4986. doi: 10.1101/pdb.prot4939 [DOI] [PubMed] [Google Scholar]
- 36.Mosmann TR, Coffman RL. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045 [DOI] [PubMed] [Google Scholar]
- 37.Vega-Ramos J, Roquilly A, Asehnoune K, Villadangos JA. Modulation of dendritic cell antigen presentation by pathogens, tissue damage and secondary inflammatory signals. Curr Opin Pharmacol. 2014;17:64–70. doi: 10.1016/j.coph.2014.07.013 [DOI] [PubMed] [Google Scholar]
- 38.Benne N, van Duijn J, Kuiper J, Jiskoot W, Slutter B. Orchestrating immune responses: how size, shape and rigidity affect the immunogenicity of particulate vaccines. J Control Release. 2016;234:124–134. doi: 10.1016/j.jconrel.2016.05.033 [DOI] [PubMed] [Google Scholar]
- 39.Manolova V, Flace A, Bauer M, Schwarz K, Saudan P, Bachmann MF. Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol. 2008;38(5):1404–1413. doi: 10.1002/(ISSN)1521-4141 [DOI] [PubMed] [Google Scholar]
- 40.Li X, Sloat BR, Yanasarn N, Cui Z. Relationship between the size of nanoparticles and their adjuvant activity: data from a study with an improved experimental design. Eur J Pharm Biopharm. 2011;78(1):107–116. doi: 10.1016/j.ejpb.2010.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vlkova M, Rohousova I, Hostomska J, et al. Kinetics of antibody response in BALB/c and C57BL/6 mice bitten by Phlebotomus papatasi. PLoS Negl Trop Dis. 2012;6(7):e1719. doi: 10.1371/journal.pntd.0001719 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 42.Mohamed M, Abu Lila AS, Shimizu T, et al. PEGylated liposomes: immunological responses. Sci Technol Adv Mater. 2019;20(1):710–724. doi: 10.1080/14686996.2019.1627174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ishida T, Ichihara M, Wang X, et al. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J Control Release. 2006;112(1):15–25. doi: 10.1016/j.jconrel.2006.01.005 [DOI] [PubMed] [Google Scholar]
- 44.Semple SC, Harasym TO, Clow KA, Ansell SM, Klimuk SK, Hope MJ. Immunogenicity and rapid blood clearance of liposomes containing polyethylene glycol-lipid conjugates and nucleic Acid. J Pharmacol Exp Ther. 2005;312(3):1020–1026. doi: 10.1124/jpet.104.078113 [DOI] [PubMed] [Google Scholar]
- 45.Wang F, Ye X, Wu Y, et al. Time interval of two injections and first-dose dependent of accelerated blood clearance phenomenon induced by PEGylated liposomal gambogenic acid: the contribution of PEG-specific IgM. J Pharm Sci. 2019;108(1):641–651. doi: 10.1016/j.xphs.2018.10.027 [DOI] [PubMed] [Google Scholar]
- 46.Li C, Zhao X, Wang Y, et al. Prolongation of time interval between doses could eliminate accelerated blood clearance phenomenon induced by pegylated liposomal topotecan. Int J Pharm. 2013;443(1–2):17–25. doi: 10.1016/j.ijpharm.2013.01.012 [DOI] [PubMed] [Google Scholar]