Since its description nearly 130 years ago, hundreds of studies have deepened our understanding of coccidioidomycosis, also known as valley fever (VF), and provided useful diagnostic tests and treatments for the disease caused by the dimorphic fungi Coccidioides spp. In general, most of the literature has addressed well-established infections and has described patients who have experienced major complications.
KEYWORDS: early coccidioidomycosis, early events in valley fever, Coccidioides research, coccidioidomycosis
SUMMARY
Since its description nearly 130 years ago, hundreds of studies have deepened our understanding of coccidioidomycosis, also known as valley fever (VF), and provided useful diagnostic tests and treatments for the disease caused by the dimorphic fungi Coccidioides spp. In general, most of the literature has addressed well-established infections and has described patients who have experienced major complications. In contrast, little attention has been given to the earliest consequences of the pathogen-host interaction and its implications for disease manifestation, progression, and resolution. The purpose of this review is to highlight published studies on early coccidioidomycosis, identify gaps in our knowledge, and suggest new or former research areas that might be or remain fertile ground for insight into the early stages of this invasive fungal disease.
INTRODUCTION
Coccidioides spp. are dimorphic fungi that cause coccidioidomycosis, a disease that was first described by Alejandro Posadas in 1892 (1). Their life cycle progresses from a mycelial (saprobic/soil) phase to a spherule/endospore (parasitic/host) phase (2, 3). The two known species are Coccidioides immitis and Coccidioides posadasii. C. immitis is found predominantly in California, and its range extends to Baja California, Arizona, and parts of Utah and eastern Washington state. C. posadasii is found predominantly in Arizona, and its range extends to Utah, New Mexico, Texas, Mexico, and parts of Central and South America (4, 5). It has long been known that dry, dusty environmental conditions and soil disruption promote the release of arthroconidia into the air, so nearly all infections begin in the lungs (6, 7). Extrapolating from in vitro studies, by 24 h after inhalation, arthroconidia have already begun to transform into large spherules (8, 9). From approximately 24 until 120 to 132 h after inhalation of arthroconidia, spherules continue to grow, septate, develop endospores, rupture, and release the endospores, which can repeat the process (10–12).
When first inhaled, arthroconidia encounter ciliated, goblet, and club cells in the conducting zone (oronasopharynx to the terminal bronchioles) and type I and II alveolar cells (pneumocytes) in the respiratory zone (respiratory bronchioles and alveolar ducts/sacs), along with myeloid-derived cells, such as alveolar macrophages. Arthroconidia are met with mechanical and physical barriers, such as the beating action of cilia, mucus, and tight junctions between cells (13). Humoral factors, such as complement, could be part of the initial host interactions. Histopathological changes are well documented in the murine model, but early histological changes in the human lung are not well understood (14). Pathogen-related factors that aid in inhalation, adhesion, invasion, and survival in the host have been studied, but there are many areas still to be investigated (15). The host response to the pathogen is equally complex and merits further investigation. The innate immune system includes cell types such as polymorphonuclear neutrophils (PMNs), macrophages, dendritic cells (DCs), eosinophils, natural killer (NK) cells, innate lymphoid cells (ILCs), invariant natural killer T (iNKT) cells, γδ T cells, and epithelial cells. Noncellular substances include antimicrobial peptides/molecules, such as lysozyme, lactoferrin, protease inhibitors, human β-defensins, and cathelicidins, and oxidants, such as nitric oxide (NO) and hydrogen peroxide (H2O2) (13, 16, 17). In other infections, innate responses are important in early pathogen recognition, pathogen clearance and/or inhibition, and cooperation with the adaptive immune system to further host defense. However, the exact role of each cell type and noncellular substance in coccidioidomycosis has yet to be determined.
Approximately 60% of persons who inhale Coccidioides arthroconidia develop infections that are subclinical, with either mild symptoms or none at all (18). Most of the remainder experience a respiratory syndrome of community-acquired pneumonia (CAP), including a nonproductive cough, chest pain, and dyspnea (19, 20). Global signs and symptoms of fever, night sweats, fatigue, and weight loss are frequent (21). Smith summarized other symptoms, such as an influenza-like illness followed by skin lesions associated with arthralgia and conjunctivitis, with the synonymous terms San Joaquin valley fever (VF), desert fever, and desert rheumatism and suggested these symptoms predict a more benign course (22). Eventually, despite often protracted morbidity, most patients resolve their illnesses regardless of whether they are treated with antifungal drugs, occasionally with a residual asymptomatic pulmonary nodule or thin-walled cavity evident on chest imaging (23). A few develop either fibrocavitary pulmonary lesions or hematogenous spread beyond the chest, and these complications usually need long-term medical management. Those with self-limited courses of their first infection, and possibly even those with complicated courses, rarely if ever develop illness from a second exposure to Coccidioides (22, 24).
This wide variety of disease manifestations might be due to differences among strains of Coccidioides, but to date there is no or very little evidence in support of this possibility. Perhaps other coexisting conditions, such as concurrent infections, the enteric microbiome, or nutritional factors, are involved, but again, there is very little (if any) evidence directly linking these variables to the spectrum of coccidioidal illness. A 2013 study demonstrated that vitamin D does not play a significant role in host susceptibility to coccidioidomycosis, but investigation of other nutritional factors may be of value (25). It is clear that profound cellular immunosuppression and some gene mutations may predispose patients to disseminated infection (26, 27). Although immunogenetic variations might explain the spectrum of disease manifestation, differences in early innate responses could also be a contributing factor in both disease presentation and course.
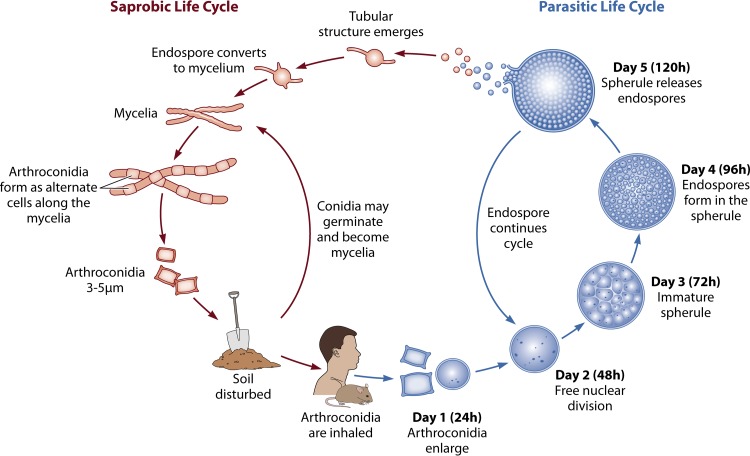
LIFE CYCLE: EARLY EVENTS SURROUNDING THE TRANSITION FROM SAPROBIC TO INVASIVE GROWTH
As a dimorphic fungus, Coccidioides alternates between saprobic (mycelium) and infectious (spherule) phases. Under environmental conditions, the fungus can cycle through the mycelial phase without infecting a mammalian host (11, 28) (Fig. 1). As Mead and others wrote in 2018, “the molecular mechanisms that initiate the morphological switch from a saprobic to a parasitic phase are not understood” (29). The obstacles to understanding these mechanisms include (i) applying in vitro models to in vivo processes, (ii) limitations of applying animal models (primarily murine) to the human disease process (14), (iii) difficulties in studying the early infective process in humans (30), and (iv) the risk of attempting to ascribe biological processes studied in other fungi to Coccidioides.
FIG 1.
Life cycle of Coccidioides. Coccidioides spp. alternate between saprobic (mycelia) (left) and parasitic (spherules) (right). The saprobic cycle is found in the environment and produces infectious arthroconidia. They may become airborne and be inhaled by the host or may return to the environment to continue the saprobic life cycle. (Adapted from reference 11, published under a Creative Commons license.)
Recent studies on the structure, morphogenesis, and immunogenicity of arthroconidia are lacking. Once inhaled, the morphogenesis from small barrel-shaped cells to larger immature spherules begins in just 8 to 24 h (28). Pioneering work by Converse and others (31–35) has advanced in vitro studies of the parasitic phase of Coccidioides by utilizing temperature (approximately 37°C), CO2 tension (10 to 20%), and surfactant-like agents (e.g., Tamol SN [Rohm and Haas, Philadelphia, PA]) to model conditions in the host lung. In vitro modeling has been essential, as the rapid arthroconidial changes and inability to visualize arthroconidia in murine tissue samples after intranasal inoculation limit the assessment of this critical period. Early studies of Coccidioides structure focused on the lipid, carbohydrate, and protein compositions of the various fungal forms. The mechanisms that regulate these changes in chemical composition and their biological significance from one form to another remain undefined. In 1977, Wheat and colleagues described fungal structure by analyzing and comparing the cell wall compositions of arthrospores, mycelia, and spherule walls, noting that arthrospores retain 1.5 times more protein than mycelium walls and 3 times more protein than spherule walls. They also measured the lipid and carbohydrate composition of the cell walls. Differences in lipid content and composition were observed between arthrospores, mycelia, and spherules. The smallest amount of lipid was found in mycelia (36). Taking this work further in 1985, Cole and Sun summarized their efforts to analyze the arthroconidial outer and inner cell walls and suggested there are antigenic differences between arthroconidia and spherules based on their different chemical compositions. Their chapter in Fungal Dimorphism (10) remains the definitive description of “conidiogenesis” (development of arthroconidia from mycelia, illustrating the alternating production of viable arthroconidia between degenerating cells in the branching mycelium). Interestingly, arthroconidia do not cleanly break off from the hyphae but retain a portion of the degenerate hyphal cell wall through inhalation of the arthroconidia by the host (10). In 1983, Drutz and Huppert suggested this retained hyphal outer wall layer (HOWL) served some level of antiphagocytic action (15). Subsequent research has not investigated this idea. In 1987, Cole et al. and Bayley et al. further demonstrated that arthroconidia have a hydrophobic outer layer, similar to the hydrophobins described for many aerially dispersed fungal spores, that contains immunosuppressive compounds that are easily stripped away, revealing a water-soluble fraction with T cell-stimulating antigens (37, 38). As a side note, this soluble wall fraction antigen was demonstrated in the mycelial phase by Ward et al. in 1975 (39) and in the spherule phase by Cox et al. in 1984 (40). It is now known as antigen 2/proline-rich antigen (Ag2/PRA) and has been extensively studied in immune responses and as a vaccine candidate (41–43). The initiation of parasitic-cell differentiation involves two unique events, formation of uninucleate cells and the coalescence of multiple cytoplastic vacuoles into a large central vacuole as arthroconidia grow isotropically into round cells and young spherules (44).
Unlike the study of arthroconidia, interest in spherule composition did not seem to wane as much in the 1990s and 2000s. A great deal of knowledge about spherule outer and inner wall structure is derived from early electron microscopy studies (10, 15, 28, 45). The spherule phase can vary temporally and is generally considered to be from 24 through 120 h after inhalation of arthroconidia. Inhaled arthroconidia are multinucleate (dikaryons are the most common) but within 8 h are uninucleate, and at 24 h, the developing round cells (early spherules) are multinucleate again. The question, proposed by Huppert et al. in 1982, of whether the transition of multinucleate to uninucleate cells is the result of nuclear fusion or degeneration remains unanswered today (28). However, it is clear that during the first 48 h, the spherules undergo repeated mitosis with little cellular enlargement (46). It is thought that mitosis ceases at 48 h, right before spherule septation begins (10). At about 48 h, the immature spherules are about 5 μm in diameter, contain multiple nuclei, and have already begun the segmentation process (28). Septation continues for the next 48 to 72 h and produces a mature spherule with as many as 200 to 300 endospores by approximately 96 to 120 h postinhalation (10, 12, 28). Mature Coccidioides spherules may vary from 60 to more than 100 μm in diameter (12). Spherules have higher lipid and glucosamine concentrations than arthroconidia and mycelia (36). The importance of this difference in composition is not known. Hector and Pappagianis utilized different enzymes to degrade the spherule cell wall and suggested the outer half of the cell wall is largely α-(1-3)-glucan and the inner half is a matrix of chitin and β-(1-3)-glucan. Interspersed between the two halves is a mannan-protein complex that they proposed holds the halves together and anchors the wall to the membrane (47). In 1986, Frey and Drutz described an extracellular glycoprotein matrix that surrounds young and mature spherules, but not arthroconidia and endospores (48). They proposed that the matrix helps spherules resist attacks by PMNs. These proposals have not been further investigated. At that time, it was believed PMNs did not attack spherules, but a study by Lee et al. suggests otherwise (49). Resolving these differences could be an interesting avenue of research. In a similar vein, the spherule outer wall glycoprotein (SOWgp), which is expressed only in the parasitic phase, is thought to act as an adhesin to mammalian extracellular matrix proteins, such as laminin, fibronectin, and collagen type IV, and thereby aids attachment of arthroconidia and spherules to host endothelial tissue (50). Interestingly, a metalloproteinase (Mep1) secreted during endosporulation digests the immunodominant SOWgp and limits host recognition of endospores during the phase of development when these fungal cells are most vulnerable to phagocytosis by the host (51). In the early 2000s, fungal wall components, such as β-glucans, took on new interest as the concept of pathogen-associated molecular patterns (PAMPs) and their interactions with host pattern recognition receptors (PRRs) became more widespread. In 2005, Viriyakosol et al. published a prescient article introducing Charles Janeway’s theory recognizing nonself and self in coccidioidomycosis research (52). The examination of pathogen “pattern” and host “receptor” remains a mainstay of Coccidioides research to this day. PAMPs and PRRs are discussed further below.
The next morphological stage in the Coccidioides life cycle is the endospore, on which there has been scant research since the 1980s. Segmentation of a spherule into primary, then secondary, and finally tertiary segmentation planes is the cue for development of endospores (about 72 h) (10). Measuring about 2 to 4 μm in diameter, endospores are released from a ruptured spherule at approximately 120 to 132 h and during this process are held in clusters by fibrils that eventually give way and release the endospores as round, uninucleate cells (10, 12, 28). The fibrils are derived from the spherule inner wall and released in large 10-μm packets, which are thought to make the endospores less “digestible” for phagocytes. As mentioned in the article, there are no direct studies of this interaction (15). The endospores, as shown for arthroconidia, were eventually phagocytized by either alveolar macrophages (obtained from rhesus macaques) or murine peritoneal macrophages, but in either case were not killed, as demonstrated by Beaman et al. The inability of macrophages to kill endospores was suggested to be partly due to inhibition of fusion between phagosomes containing fungal spores and the lysosomes within the macrophages (9, 53). Other than Hector’s speculation that endospores maintain α-(1-3)-glucan from septating spherules, there are no summaries of the chemical composition of endospores, as there are for mycelia, arthroconidia, and spherules (10, 36, 47). A 2014 article briefly mentions that the endospore cell wall contains chitin, β-(1-3)-glucan, 3-0-methyl mannan, and mannans (54). Garcia-Sherman et al. detected fungal surface amyloids on endospores and spherules that bind to the host serum amyloid protein (SAP) component. This process aids in fungal attachment and forms a biofilm that may diminish the host inflammatory response by protease inhibition (55).
RESPIRATORY TRACT COMPONENTS
The primary route of Coccidioides infection is through inhalation of arthroconidia. Wiesner and Klein provide a succinct overview of the role lung epithelial cells play in fungal immunity. They highlight three pathways for Pneumocystis, Aspergillus, and Cryptococcus. In their concluding remarks, they outline the concept of fungal attachment, penetration, and avoidance (56). This is the essence of the challenge associated with learning more about early events in coccidioidomycosis.
The airway tract consists of two zones: the conducting zone (oronasopharynx through the terminal bronchioles) and the respiratory zone (respiratory bronchioles and alveoli). Far from being a static, passive tube, the conducting airway consists of a variety of cells; the most prominent are ciliated, goblet, basal, and club cells (formerly known as Clara cells), which, besides sweeping out pathogens or producing mucus, initiate early innate immune responses (13). The importance of mucociliary function, apicolateral junctional complexes, and antimicrobial products released by the conducting airway cells is further illustrated and elucidated by Whitsett and Alenghat (57). The role of ciliary cells in coccidioidomycosis has not been examined. There is increasing evidence that secreted mucins, such as MUC5AC and MUC53, besides helping form the mucus protective barriers and rafts, are involved in pulmonary innate immunity (57). There are no studies on the roles of these mucus products in coccidioidomycosis. Club cells become more numerous as the airway transitions to the terminal bronchioles. Aside from a brief mention of club cells’ role in innate defense by Awasthi et al., there are no further studies of their role in coccidioidomycosis (58). The respiratory zone and innate immune system cells were illustrated by Hussell and Bell, especially in relation to alveolar macrophages (30). In the alveoli, the goblet and club cells seen in the respiratory bronchioles give way to type I and II alveolar cells. The elongate type I alveolar cells are primarily responsible for gas exchange, and the more cuboidal type II alveolar cells serve as immune responders (16, 57). Type II alveolar cells express many PRRs, and among them are collectins, which are soluble secreted PRRs, such as the pulmonary surfactant protein A (SP-A) and SP-D (59). Researchers seem split on the question of whether inhaled arthroconidia are able to reach the alveoli or only reach the terminal bronchioles. The barrel-shaped arthroconidia are generally considered to be 3 to 5 μm in width and length. At these dimensions, it could be assumed that arthroconidia are most impactful at the terminal and respiratory bronchioles (60).
HISTOPATHOLOGY OF EARLY COCCIDIOIDES INFECTION
The fate of Coccidioides immediately following inhalation of arthroconidia is poorly understood because of the current inability to study early postinfection events in humans. Researchers have relied on information gathered from both in vitro studies and animal infection models for insight into inhaled arthroconidium, spherule, and endospore morphogenesis and early host immune response. In spite of potential limitations, in vitro studies, such as those using peripheral blood mononuclear cells (PBMCs) and bronchoalveolar lavage fluid (BALF), have provided valuable information about the early events in coccidioidomycosis. For example, an early study by Deresinski et al. using PBMCs demonstrated that PBMCs from both immune and nonimmune subjects could avidly phagocytize killed endospores (61). In a more recent study, Nesbit et al. utilized both cells from BALF and PBMCs of patients with pulmonary coccidioidomycosis and demonstrated specific cellular immune responses, including interleukin 17 (IL-17) expression (62).
While there have been studies utilizing monkeys and rabbits, mice are the best developed and most easily used laboratory model of coccidioidomycosis (63–65). The advantages of the murine model include their small size and ease of handling at animal biosafety level 3 (ABSL3). Another advantage is that, although they vary in their susceptibility to Coccidioides spp. based upon strain genetics, all mice are susceptible and can be infected intranasally to a level at which they develop progressive pneumonia, dissemination, and death within a few weeks. Because the entire lung weighs ∼0.25 g in a normal mouse, the total lung is conducive to extensive study of early events by histopathology. A disadvantage of using a mouse model to mimic human response is their rapid progression to death in 14 to 28 days postchallenge. This may interfere with the assessment of early immune system responses that lead to disease control in humans. In addition, unlike humans, a low inoculum intranasally leads to rapid death (14). There are no studies correlating the inoculum size with disease severity in humans. From epidemiological studies of point source outbreaks, such as archeological excavations, where high inoculum exposures occur, there are much higher rates of symptomatic pulmonary infections. Radiographic findings for such patients often exhibit infiltrate in multiple lobes of both lungs. Although disseminated infections have resulted from such exposures, the frequency is low, and it does not appear that high-inoculum infections are more likely to develop chronic complications. On the other hand, most coccidioidal infections do not occur in clusters, suggesting that point source infections are the exception (66). When pneumonia is present, it is usually unilateral (67, 68). Also, it is surprisingly common that patients with disseminated coccidioidomycosis (DCM) have minor or no evident pulmonary disease (69). This suggests that most, if not nearly all, ambient coccidioidal infections are the result of very small inocula.
Insight into the early pathogen-host interaction and response have been made via sequential assessment of murine lung histopathology by Shubitz and colleagues (70, 71). In a 2011 study, different mouse strains were challenged intranasally with varying numbers of C. posadasii (strain Silveira), arthroconidia. At 24 to 48 h, no spherules were detected, even utilizing a Coccidioides-specific immunohistochemical stain (71). This stain is a polyclonal goat antibody to the ubiquitous Coccidioides wall antigen Ag2/PRA, which was discussed previously (70). The lack of in situ information on the early events in the first 48 h may be due to arthroconidia being small, few in number, and intracellular (72).
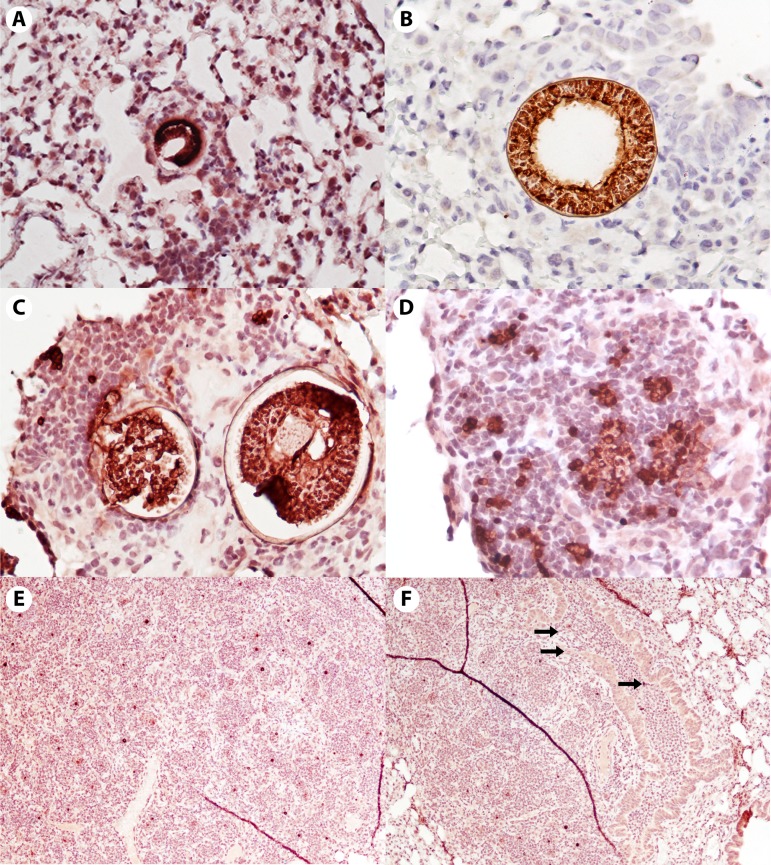
By approximately 72 h, immature spherules can be visualized with the Coccidioides-specific immunohistochemical stain (Fig. 2A). Around 72 to 96 h, the spherules, though visible, have provoked only a minimal inflammatory response. The response consists primarily of a few monocytes and an occasional PMN. PMNs are notably absent even when a layer or two of macrophages and occasional lymphocytes is seen (Fig. 2A and B). While the first-generation spherules remain unruptured, the Coccidioides infection appears to be relatively invisible to the host immune system. Data to confirm this conclusion are lacking, as is information about the potential signaling between pathogen and host. These histopathological observations are supported by a 2018 vaccine study that measured cytokines in the lungs (73). Cytokines were measured in lung cell supernatants from vaccinated and unvaccinated mice on days 1, 2, 4, and 6 following infection with virulent arthroconidia. A mouse Illumina 31-plex panel measured cytokines on 24-h supernatants from the lung cell cultures. In this assay, unvaccinated mice exhibited no increases in IL-4, gamma interferon (IFN-γ), macrophage inflammatory protein 1a (MIP1a), MIP1b, macrophage colony-stimulating factor (MCSF), monokine-induced by IFN-γ (MIG), or tumor necrosis factor alpha (TNF-α) prior to day 4, while the vaccinated mice had early increases in these cytokines, as previously described (73). Causally or coincidentally, spherule rupture starts around 96 h and is nearly completed by 120 h, with dispersing endospores and a dramatic influx of inflammatory cells, primarily PMNs and some macrophages (Fig. 2C and D). Once the spherules rupture, releasing endospores, PMNs and macrophages rapidly and robustly infiltrate the tissue and surround the endospores, so much so that the lesions become visible to the naked eye (∼1 to 2 mm in diameter). It has been reported that endospores are chemotactic for PMNs, and the histopathology is consistent (49, 71). However, PMNs have a limited ability to kill endospores (15). By 144 h (day 6), dense islands of pyogranulomatous inflammation are present, and the first generation of endospores begins to enlarge into spherules (Fig. 2E). PMNs (as well as rare eosinophils and basophils) pack terminal airways adjacent to the original spherules and fill the alveolar spaces where the growing endospores have dispersed (70). Suppurative infiltrates are seen in both the tissue and terminal bronchioles (Fig. 2F).
FIG 2.
Histopathology of early murine lung infection. The panels span 72 to 144 h postinfection with C. posadasii strain Silveira. Tissues were immunohistochemically stained with a polyclonal goat antibody against the Coccidioides cell wall antigen, Ag2/PRA, with hematoxylin counterstain. Note that spherules and endospores appear dark red to brown. (A and B) Unruptured first-generation spherules with very few surrounding immune cells at approximately 72 h (A) and approximately 96 h (B). Virtually all are macrophages. (Magnification, ×200 [A] and ×400 [B].) (C and D) There is an enormous influx of PMNs, and also macrophages, once the endospores are released (approximately 120 h). Note the lack of inflammatory cells around the unruptured spherule in panel C. (Magnification, ×200 [C] and ×400 [D].) (E and F) Ongoing recruitment of inflammatory cells to the area and dispersal of endospores, now enlarging back into early spherules (approximately 144 h). In panel F, the top two arrows point to a damaged airway filled with inflammatory cells; the third arrow points to endospores within the damaged airway. (Magnification, ×40 [E and F].)
PATHOGEN-RELATED COMPONENTS
Since the year 2000, Coccidioides research has involved examination of PAMPs, signal transduction pathways, gene expression, and gene expression products that aid in fungal production of transcription factors, adhesion, and immune system evasion. The production of cell wall components, adhesins, reactive oxygen species (ROS), and biofilms and the expression of virulence factors are areas of interest.
An overview of PAMPs in Coccidioides has been published (74). Noting that the fungal cell wall is a complex structure comprised of mannan, glucans, and chitin, the author illustrates the interaction of mannan with the PRR and mannose receptor (MR) on macrophages or dendritic cells, as well as that of β-glucan with the Dectin-1 receptor. The article further notes that fungal PAMPs that interact with Toll-like receptor 2 (TLR2) and TLR4, as well as SP-A and SP-D, are not known (74). Erwig and Gow noted that fungal α-mannans interact with the host Dectin-2 receptor, but Viryakosol et al. suggested Dectin-2 and MR are not essential for murine resistance to Coccidioides (54, 75).
In 2000, Lengeler et al. published an article describing signaling pathways in various fungi with an emphasis on Saccharomyces cerevisiae as a paradigm for signaling in other organisms, with additional insights provided by Runyanga et al. in 2017 (76, 77). Cyclic AMP-protein kinase A (cAMP-PKA) and mitogen-activated protein kinase (MAPK) are thought to be major signaling pathways and are postulated to be involved in regulation of phase transition in endemic dimorphic fungi. Studies of these pathways have been done in S. cerevisiae and Neurospora crassa and in fungal pathogens, such as Candida albicans and Cryptococcus neoformans (78). There are no specific studies of signal transduction pathways in Coccidioides, and this might be an area of future research.
Early genetic studies in Coccidioides cloned the chitinase genes (cts1 and cts2) that were surmised to be responsible for the cell wall hydrolase chitinase. These genes are involved in spherule growth and endospore release by remodeling chitin (44, 79). A tool for Coccidioides genetic transformation is Agrobacterium tumefaciens, a Gram-negative bacterium that had previously been utilized in plants and filamentous fungi to transfer hygromycin resistance via a transfer DNA (T-DNA) vector plasmid to arthroconidia (80). This is a simple and effective method widely used in fungal-gene manipulations. The availability of a nearly complete genome sequence has furthered the understanding of Coccidioides (https://www.broadinstitute.org/scientific-community/science/projects/fungal-genome-initiative/coccidioides-genomes). Additional chitinase and β-1,3-glucan synthase genes have been described (12, 81, 82). In 2006, Johannesson et al. greatly expanded the understanding of Coccidioides phase-specific gene expression by combining gene expression data with predicted protein localization and gene binding sites, thus identifying a list of putative C. posadasii antigens (83). In addition to primary sequence differences between C. posadasii and C. immitis, Delgado et al. in their transcriptome-sequencing (RNA-seq) analysis showed differential gene expression in the two C. posadasii isolates examined, C735 and Silveira (84). In 2006, Rappleye and Goldman reviewed virulence genes in four dimorphic fungi, Histoplasma, Blastomyces, Paracoccidioides, and Coccidioides. They reviewed the six virulence factors then known and proposed potential candidate virulence factors. They also reviewed pulmonary defenses, such as complement, immunoglobulins, defensins, and collectins. When the article was published, the authors anticipated the completion of genomic sequencing for pathogenic fungi, such as Coccidioides, would provide additional candidate virulence factors (85). With the completion of the genomic sequences of Coccidioides, the identification of additional virulence factors is a potential area for future research.
A 2012 study using next-generation sequencing (NGS) assessed gene expression and identified genes differentially expressed between the saprobic and parasitic growth phases of both C. immitis and C. posadasii. The authors suggest there are genes with unknown functions that may contribute to fungal growth and virulence (86). Viriyakosol and colleagues complemented this study in 2013, investigating differential gene expression in day 2 mycelia and day 8 spherules. By examining a number of gene products and comparing their functions with those of homologous genes in other fungi, they were able to speculate about the functions of either up- or downregulated genes in the various coccidioidal growth phases (87). Similar studies would be valuable in understanding early events in Coccidioides infection. An intriguing prospect for Coccidioides gene expression was suggested by Lewis et al. when they noted that the last common ancestor of both C. immitis and C. posadasii underwent gene expansion for proteases, keratinases, membrane biology, and toxin production (11). Perhaps these gene products could be targets for further studies. Gene disruption remains an important strategy in vaccine development. Narra and colleagues’ recent development of a protective, avirulent strain of Coccidioides by deletion of the chloroplast protein synthesis gene (cps1) is an example of how a virulence factor in one pathogen, Cochliobolus heterostrophus (a maize pathogen), can lead to discovery of analogous genes in other pathogenic fungi. Interestingly, the exact function of the CPS1 gene product in Coccidioides is unknown in spite of extensive study of the mutant strain as a vaccine candidate (88). This underscores the necessity of understanding other fungal pathogenic determinants, as they may be applicable to Coccidioides.
The increasing sophistication of proteomic techniques has enhanced rather than diminished the work of earlier researchers in coccidioidomycosis. In 2016, Grys et al. generated a proteome of nearly 1,400 proteins found in spherulin (a lysate of spherule cells). They hypothesized that fungal proteins and their glycosylation patterns could be different from those of mammals and could be used as bases for identification of fungal elements in the host tissue. They focused on two lectins, Griffonia simplificonia lectin II (GSL-II) and succinylated wheat germ agglutinin (sWGA), that bind to chitin, which is composed of repeating units of N-acetylglucosamine (GlcNAc) (89). The more recent study recalls investigations performed in the 1980s and 1990s demonstrating inhibition of fungal GlcNAc incorporation by PMNs and PBMCs (90–92). Similarly, a general review of urease, a virulence factor in fungal pathogens, published in 2014 might prompt further study of the enzyme (93). Initial studies of urease in Coccidioides were reported in the early 2000s (94, 95). These studies reported host tissue damage by the release of ammonia from spherules via enzymatically active urease. In 2013, the findings of the earlier urease studies were reaffirmed, as disruption of the urease (URE) and uredoglycolate hydrolase (Ugh) genes in the fungus demonstrated less virulence (96). The role of urease and Ugh in early coccidioidomycosis may merit further investigation. Many studies and articles since the 1980s give glimpses into early Coccidioides dimorphism from arthroconidia through spherules to endospores. These individual studies detail a wide range of subjects, including enzymes associated with fungal cell wall remodeling, host conditions that trigger arthroconidium transformation, and various cell wall components that show potential as virulence factors or vaccine candidates. A challenge moving forward will be to tie these individual studies into a comprehensive understanding of early Coccidioides-host interaction.
HOST-RELATED RESPONSE
Schenten and Medzhitov published a comprehensive overview of the control of the adaptive immune response by the innate immune system. They concluded, “however it is becoming increasingly clear that additional layers of control may exist that determine the choice of effector class, the magnitude and duration of the immune response. How these regulatory mechanisms operate in the context of infections is an exciting area for future investigations” (97). This is specifically applicable to understanding the host response in coccidioidomycosis. When reviewing individual components, such as PRRs, signaling transduction pathways, etc., it is important to be mindful of the complex interactions between them.
In many fungal infections, early recognition and control of the pathogen is dependent on its recognition by host PRRs (98). PRRs can be secreted or bound to the cell wall. Secreted PRRs can act as opsonins and activate complement. Bound receptors are involved in both pathogen uptake and processing, as well as T cell stimulation, such as the MR on phagocytes. They can act as inducers of antimicrobial peptides, cytokines, and proinflammatory chemokines (97).
In coccidioidomycosis, the most studied cell wall-bound PRRs are the TLRs and the C-type lectin receptors (CLRs) (54, 87, 99–101). In 2004, Awasthi and Magee demonstrated significant arthroconidium-induced upregulation of TLR2 and TLR4 gene expression, along with IL-12, in bone-marrow-derived DCs in a resistant mouse strain (DBA/2) compared to a susceptible strain (BALB/c). They suggested the DC activation status could be responsible for the differences in C. posadasii susceptibility (102). Viriyakosol et al. in 2005 studied the roles of TLR2 and TLR4 in Coccidioides infections. Stimulation of mouse peritoneal macrophages with either live Coccidioides or formalin-killed spherules (FKS) produced the proinflammatory cytokines TNF-α, MIP-2, IL-6, and IL-12. TLR2−/− macrophages failed to produce these cytokines in response to either live Coccidioides or FKS (52). TLR4−/− macrophages and macrophages from TLR4-deficient C3H/HeJ mice produced levels of cytokines similar to those in wild-type (WT) controls. In vivo, following infection, TLR2−/− mice showed susceptibility to Coccidioides similar to that of WT controls. Interestingly, Awasthi demonstrated that TLR4-deficient C3H/HeJ mice had pulmonary burdens similar to those of functional C3H/HeJ controls but found a 10-fold reduction in spleen dissemination, indicating a role for TLR4 responses in increased Coccidioides dissemination (103). The authors’ concluding suggestions that multiple PRRs determine the final outcome of Coccidioides infection and that more studies need to be done remain valid.
Of the CLRs, Dectin-1 expression has the most importance in Coccidioides infection (52). Mice deficient in Dectin-1, which recognizes the β-1,3-glucan on fungal cell walls, produced lower levels of T helper 17 (Th17) cytokines and had increased lung fungal burdens compared to WT mice (100, 104). Dectin-2 binds fungal α-mannan (105). Dectin-2-deficient macrophages produce fewer cytokines than their WT controls but show no differences in fungal burden. Macrophage-inducible C-type lectin (Mincle), which binds mannose-like structure, is able to bind to Coccidioides spherules, but reporter assays using a lacZ reporter in Mincle-expressing cells showed no activation, indicating the Mincle receptor is not involved in Coccidioides infection (54, 100).
There are extensive studies focusing on Aspergillus and Candida suggesting several common pathways with Coccidioides, but there are many unexplored areas. For example, the role of IL-17 is well established in Aspergillus infection but remains poorly understood in Coccidioides infection (106, 107). The best understood Coccidioides signal transduction pathways are the Card 9 and MyD88 pathways in antigen-presenting cells (APCs), such as macrophages and DCs (54, 75, 108–110). The pathway involved in the signal transduction and activator of transcription 1 (STAT-1) gene mutation that leads to impairment of the IFN-γ/IL-12 receptor and increases susceptibility to DCM is not well known (111). Similarly, while not essential to Coccidioides immunity, the signal transduction pathways involved in the PRRs Dectin-2, MR, and SP-A/SP-D are not known (54, 74).
The study of host genes and their expression in response to Coccidioides infection has its roots in a 1999 study by Fierer et al. that was the first to map coccidioidomycosis susceptibility to loci on murine chromosomes 4 and 6 (112). ROS and NO are produced by mammalian cells (particularly phagocytes) against several microbial pathogens (113, 114). Studies have also examined the roles of ROS and NO in host defense against Coccidioides. These studies suggested they have limited roles in protective immunity against coccidioidomycosis but may contribute to immune modulation (110, 115–117). Future research into the early pathogen-host interaction of coccidioidomycosis may shed light on this subject. Gonzalez reviewed previous studies on the role of arginase in Coccidioides and succinctly described how arginase upregulation may decrease the level of NO production, allowing fungal survival (74). Lewis et al. found two proteins differentially expressed in BALF from mice on day 5 postinfection. Two other gene expression products are the proteins aminopeptidase N and annexin A5, which reduce inflammation and degranulation/apoptosis, respectively (118). A 2017 article by Van Dyken et al. demonstrated that the absence of one of two mammalian chitinases, acidic mammalian chitinase (AMCase), secreted from lung epithelial cells, such as club and type II alveolar cells, can lead to an accumulation of chitin. The AMCase-deficient mice in the study were more prone to develop interstitial lung diseases, such as spontaneous pulmonary fibrosis (119). Deficiency of this enzyme or chitotriosidase, the other hydrolase mentioned in the article, has not been investigated in pulmonary coccidioidomycosis.
Perhaps no subject in the study of Coccidioides has been of more interest over the last 30 years than the signaling cytokines between immune cells. Slagle, Cox, and Kuruganti were the first to demonstrate that C. immitis activates TNF-α, a cytokine that can activate PMNs, augment NK cells, promote T cell and B cell proliferation, and modulate endothelial cell surface antigens, thus ushering in studies of the interaction between innate and adaptive immune responses to Coccidioides infection. They suggested the idea of cytokine production being “rapidly upregulated and downregulated,” which appears to promote a protective response to Coccidioides infection (120). A 1995 study by Magee and Cox elucidated early cytokine interaction with Th1 and Th2 cells in C. immitis infection (121). Published well before the appreciation of multiple Th cell subsets, their work in coccidioidomycosis tied the “protective effects” of the Th1 pathway and the “nonprotective effects” of the Th2 pathways. Studies and review articles by Ampel et al. and Lewis et al. in 2015 provide an overview of the current known main aspects of murine and human cytokine production (11, 122).
IMMUNE RESPONSE
It is becoming clear that the immune response is not easily divided temporally or into distinct processes of an early innate response and later adaptive response. Understanding the complex interaction between the innate and adaptive immune systems remains a formidable task in Coccidioides research.
Innate Immunity
Muñoz-Hernández and colleagues have reported that innate immunity protects healthy individuals from Coccidioides infection in 70% of cases (123). This percentage is an intriguing contention; however, the exact derivation has not been elucidated and may merit further investigation. To deepen the complexity, recent studies expanded the cells generally considered part of the innate response in the lungs, such as PMNs, macrophages, DCs, eosinophils, and NK cells, to include ILCs, iNKT cells, γδ T cells, and epithelial cells (16, 17). Soluble components of the innate immune system include complement, collectins, and antimicrobial peptides, such as defensins.
PMNs are among the first cell responders and are the most studied component of the innate immune system’s response to Coccidioides. A previous study of early PMN migration and the importance of complement (124) was reinforced by Lee et al. in 2015 (49). PMNs phagocytize arthroconidia and endospores but kill less than 20 to 30% (15). They are even less efficient at killing spherules (125). This paradox has not yet been resolved. An intriguing concept is that PMNs can promote the conversion of arthroconidia into spherules; however, the histopathology in the early days of a mouse infection showed few to no PMNs immediately surrounding the developing spherule (71, 91). In 2007, Rubin-Bejerano et al. reported that the minor fungal cell wall component β-1,6-glucan is more stimulating to PMNs than the abundant β-1,3-glucan (126). This has not been studied in Coccidioides, and in fact, only one study mentions β-1,6-glucan as a component of the Coccidioides cell wall (54). Any relevance of this cell wall component to Coccidioides infection is unknown. A 2015 study reported that depletion of neutrophils (PMNs) did not increase mortality in mice infected with Coccidioides, but they appeared to be required for vaccine immunity to develop (99). The study points to the potential inflammatory and anti-inflammatory abilities of neutrophils (PMNs) in Coccidioides infection and underscores the need to better understand the intricacy of the relationship between the innate and adaptive immune systems.
Monocytes are precursors to tissue macrophages and DCs; however, in many Coccidioides studies, PBMCs are the focus and include macrophage and DC precursors, as well as B cells, T cells, and NK cells. Studies in the 1990s in which PBMCs were separated showed that fractions that were predominantly monocytes were capable of killing arthroconidia (92, 127). Beaman and associates isolated macrophages and demonstrated that unstimulated macrophages were not able to kill arthroconidia and endospores but that the addition of previously activated T lymphocytes improved macrophage killing (9, 128). These studies were another step forward in understanding innate and adaptive immune system cooperation in Coccidioides clearance. The above-mentioned 1991 study hypothesized that “at the time of infection and before the development of specific immunity, peripheral blood monocytes, polymorphonuclear leukocytes, and perhaps natural killer cells limit the survival of C. immitis. Subsequently, specific cellular immunity develops against those fungi which have survived. Severe coccidioidomycosis would then occur when both the nonspecific defenses and the specific cellular immune responses are overcome” (92). In the respiratory tract, macrophages are found in the interstitial space and in the alveoli, where they are “tethered” to type II alveolar cells (30). The exact mechanism of their interaction with arthroconidia or spherules remains unknown.
In 2012, Roy and Klein extensively explored and summarized the role of DCs in antifungal immunity. They described DCs’ pivotal position between the innate and adaptive immune systems by driving and fine-tuning the Th response to clear the fungus with minimal host damage (129). In Coccidioides, it has been shown that DCs can ingest killed spherules and stimulate T lymphocytes via CLR and TLR receptors (102, 108, 130). DCs have been proposed as adjuvants for a Coccidioides vaccine or for therapeutic use in patients with DCM to reset an effective cell-mediated immune response, but these proposals require further study, as preliminary results in mice were ambiguous (131, 132).
Dickson and Gifford long ago noted that patients’ eosinophil counts were highest when erythema nodosum (EN) first appeared (6). Later articles suggested that eosinophilia and eosinophilic microabscesses correlate with progressive disease and poor prognosis (133, 134). Beyond their role as a clinical marker, eosinophils’ function in Coccidioides infection is unknown.
NK cells are lymphocytes that are considered components of the innate immune system and that induce apoptosis and are essential to early responses to viruses and tumor cells, and their role in fungal infections has generated renewed interest (135). Recent studies suggest NK cells, long considered innate immune cells, can demonstrate qualities of acquired immunity, such as memory (136). Petkus and Baum suggested NK cells are involved in the early resolution of coccidioidal lung infection (137). It was later speculated that NK cell production of IFN-γ indirectly reduces the Coccidioides fungal burden (138). There are no recent studies of NK cell significance in combating early Coccidioides infection.
More recently identified innate immune cells include ILCs, iNKT cells, and γδ T cells. Now categorized into individual groups of cells, ILCs are believed to be involved in immunity and tissue repair (139, 140). Little is known about ILCs’ role in antifungal immunity in the lung, and there are no studies of ILCs, iNKT cells, or γδ T cells in the host response to Coccidioides infection (17, 141).
Complement is considered a key feature of the innate immune system that has variable pathways leading to recruitment of inflammatory cells, opsonization, and cell membrane perforation. The role of complement has long been suggested in early Coccidioides infection with respect to PMN response (90, 124, 142). Lower CH50 (the CH50 measures the total hemolytic activity of a test sample and is the reciprocal of the dilution of serum complement needed to lyse 50% of a standardized suspension of sheep erythrocytes coated with antierythrocyte antibody) levels have been associated with DCM (142). A more recent study emphasizes the importance of complement in the short-range redirection of recruited immune cells from host to pathogen (49). In spite of this, complement involvement in the control of early Coccidioides infection remains largely unknown.
Other soluble innate immune system components include the collectins, defensins, and antimicrobial peptides (59, 143, 144). Only one study has investigated the role of the collectins SP-A and SP-D in coccidioidomycosis. The authors speculated that SP-A and SP-D reduction could lead to disease progression and fungal dissemination (58). The “target” for SP-A, and SP-D has not been defined (145). Mannose-binding lectin (MBL), a soluble collectin that is synthesized in the liver and activates complement, was found to be low in patients with active coccidioidomycosis (146). Other defensins include neutrophil peptide 1 (NP-1) and NP-2, which were demonstrated by Segal et al. in rabbit granulocytes and have antimicrobial action against arthroconidia. They anticipated a search for peptides analogous to NP-1 and NP-2 in humans with and without coccidioidal infection (147). This search remains to be completed. The study was included in De Lucca and Walsh’s 1999 review of various hosts’ antimicrobial peptide production in defense against pathogenic fungi. The authors noted that antifungal peptides are classified by their modes of action as either acting to lyse fungal cells or interfering with cell wall synthesis or biosynthesis of essential cellular components, such as glucan or chitin. In the mammalian host, they reviewed a number of defensins, including NP-1 to NP-5 and human neutrophil peptide 1 (HNP-1) to HNP-3, along with other antifungal peptides (143). Ordonez et al. recently expanded this review with an update on the current knowledge of soluble innate effector molecules in lung defense against fungi (144). In 1974, Collins and Pappagianis demonstrated the antimicrobial peptide lysozyme’s role in spherule development inhibition (148). There are no recent studies of collectins, defensins, or antimicrobial products in Coccidioides infection.
Adaptive Immunity
Successful host immune response to Coccidioides infection is cell mediated, and increased susceptibility is due to a defective cell-mediated immune (CMI) response (149). The genesis of this understanding has its roots in Smith’s standardization of the mycelium-derived coccidioidin skin test in the 1930s (150, 151). The test and its successor, which is spherule derived, detect delayed-type hypersensitivity (DTH) reaction and demonstrate T cell activation (152). Zweiman and colleagues reported that coccidioidin-induced lymphocytes from Coccidioides-immune subjects proliferated at a higher rate than those from nonimmune subjects (153). Since that time, there have been numerous studies involving the T cell response to Coccidioides. The question posed by Stevens in 1995, when he asked whether coccidioidomycosis progression was the result of deficient CMI response secondary to antigen overload, suppressor (regulatory) cells, immune complexes (ICs), or fungal immune-suppressive substances, remains relevant (154). The discovery of PRRs in ciliated, club, goblet, and type I/II alveolar cells, along with those on alveolar macrophages and DCs, begs the question of just how early the adaptive immune system is engaged in response to Coccidioides infection and underscores how much there is to learn about its interaction with the innate immune system.
Studies of the adaptive immune system in Coccidioides infection have focused mainly on T cells. Catanzaro et al. asked an important question in 1975, inquiring whether T cell dysregulation in Coccidioides infection is a matter of an intrinsic lymphocyte defect or is induced by the fungus itself (155). More recent studies have focused on gene mutation in patients susceptible to DCM, suggesting the first part of this question, but the latter part may merit further investigation (26, 27, 111, 156). The importance of T cells became clear by the 1970s (53, 157, 158). Studies over the last 40 years have demonstrated the importance of Th cells in the host response to fungal infections while expanding the known Th subsets to include Th1, Th2, Th17, regulatory T (Treg), T follicular helper (Tfh), Th9, Th22, and Th25 cells (108, 109). The response of the first three subsets to APC presentation of Coccidioides antigen is well documented and was reviewed by Teixeira and Barker (5). Nonetheless, the roles of other Th subsets in the host response to coccidioidomycosis and the complex interplay of all known (and unknown) subsets require further study.
While the critical importance of T cells in successful host response to Coccidioides is well documented, the role of B cells in a successful host response is much less established. Casadevall reviewed this question in a number of fungal infections with the premise that antibody (B cell) immunity in fungal infections is a controversial subject (159). This controversy still remains in coccidioidal research. An early study of B cells in Coccidioides documented an unsuccessful attempt to transfer passive immunity between immune and nonimmune mice (160). A recent unpublished study by Shubitz and Frelinger showed similar results. Ibrahim and Pappagianis demonstrated a correlation between high serum antibody titers and more severe disease, which is evidence of the lack of a protective role for antibodies (161). There is conjecture in a 2004 article that monoclonal antibodies with protective effects might be found by utilizing monoclonal antibody technology, as was done for C. neoformans (42). Despite previous evidence to the contrary, investigators have reported that B cells are required for a protective vaccine response in mice (162, 163). A recent study abrogating B cells with anti-CD20 antibody during immunization was not able to corroborate these findings. In the study, mice treated with anti-CD20 developed the same immunity as normal C57BL/6 mice (L. F. Shubitz and J. A. Frelinger, unpublished data). There is some evidence that B cells may play a role in host control of coccidioidomycosis, but the bulk of current knowledge does not support this, and therefore further research is warranted.
There are conflicting data on the role of ICs (soluble Coccidioides antigen bound to antibody). Yoshinoya and colleagues found ICs in a significant number of patients with active coccidioidomycosis and proposed that they depressed T cell-mediated DTH responses, inhibited antibody-dependent cell-mediated cytotoxicity, and suppressed the chemotactic response of PMNs (164). These findings were later challenged in a 1987 study demonstrating that ICs did not suppress lymphocyte transformation. They advocated further investigations, but none have been pursued (165).
CLINICAL ASPECTS OF COCCIDIOIDOMYCOSIS
Brown and colleagues in 2013 summarized the history, ecology, geographic range, and risk factors of coccidioidomycosis and concluded that early diagnosis and treatment could lead to improved outcomes, reduced (patient) anxiety, and fewer procedures/treatments (166). Their article and others have identified the “traditional” at-risk populations for progressive coccidioidomycosis, including people with African ethnicity, Filipinos, pregnant women, and the immunocompromised (24, 166, 167). For the foreseeable future, awareness of these broader groups will remain important and will likely be a touchstone as researchers discover specific gene mutations and their products that could identify at-risk individuals for more serious forms of progressive disease (27). Efforts and success in this regard could lead to earlier interventions, targeted treatments, and fewer disease complications.
Symptoms of pulmonary coccidioidomycosis are thought to appear from 1 to 3 weeks (average, 2 weeks) after exposure (22). Researchers at California military bases during World War II meticulously compiled a comprehensive list of early coccidioidomycosis symptoms from the large influx of young, generally healthy, and previously unexposed males. They noted, in descending order, the percentages with fever, chest pain, cough, malaise, anorexia, headache, pharyngitis, chills, joint manifestations, EN, conjunctivitis, erythema multiforme (EM), urticaria, and hemoptysis as presenting symptoms (168). Later studies, each with a smaller but more diverse population, reported similar symptoms and percentages, which supports the continued relevance of the 1945 study (169, 170). The 1996 study, which focused on the symptoms chest pain, EN, fever, cough, and rash, along with chest X-ray findings, skin test results, and laboratory values, highlighted the “crossover” of coccidioidomycosis with other respiratory illnesses and, more importantly, estimated that one- to two-thirds of standard coccidioidal serologic tests may miss the diagnosis (169). It should be noted that this study utilized unconcentrated sera, but current protocols generally use concentrated sera in immunodiffusion tests to improve the diagnostic yield (171, 172).
Historically, as with other diseases, a proper coccidioidomycosis diagnosis has relied upon patient history; symptoms; basic laboratory tests, such as complete blood count (CBC); erythrocyte sedimentation rate (ESR); eosinophil count; and chest X-ray, but these all lack specificity. Obtaining a diagnosis with fungal culture or histopathology, while definitive, can be labor-intensive, invasive, and expensive. A recent article has advocated for the return of skin testing (a positive test for the cell-mediated DTH response proves patient immunity) with the second-generation spherule extract Spherusol. The authors suggested wider use could aid in clinical response to treatment; be of value in prevalence studies; and identify high-risk occupational exposures, as well as those who would not need vaccination (152). A recent study reported potential limitations of skin testing, including the possibility that it might not always identify past controlled infections and has lower positive rates than previously reported (173, 174). Nonetheless, a 2018 study demonstrated the successful use of large-scale skin testing to stratify inmate risk in a coccidioidomycosis prevention program within the California prison system (175).
Serologic testing remains the mainstay of coccidioidomycosis diagnosis. Pappagianis and Zimmer authored a comprehensive overview of the history and utility of serological testing that remains a reference (176). The need to improve the availability, ease of use, rapidity, reliability, and cost of coccidioidomycosis testing has sparked development of lateral-flow assays (LFAs), which show promise as easy to use point-of-care tests that will speed diagnosis and lessen antibiotic overuse in coccidioidomycosis (177). The contrary view could argue that earlier confirmation of VF may not appreciably change clinician antibiotic-prescribing habits or might lead to increased and unnecessary use of antifungals. At such time as these tests become available, studies of this may be valuable. Resolving such questions is facilitated by utilizing the expanding ability of electronic medical records (EMRs) to mine data and perform analytics to ascertain if new tests, such as the LFA, or clinical interventions actually have the desired effects of improved antibiotic stewardship, providing better patient outcomes, or lowering health care costs (178–180).
Another relatively new coccidioidomycosis diagnostic test is real-time PCR (RT-PCR), which has the advantage of a 4-h turnaround time and high sensitivity and specificity for specimens obtained from BALF or bronchial wash (BW) fluid. The most notable disadvantage for use of PCR is lower sensitivity and specificity in tissue specimens other than BALF and BW fluid (181–183).
Treatment decisions for coccidioidomycosis can be challenging due to variable presentations and prognoses. Noting that 60% of those who acquire Coccidioides infection are asymptomatic and the other 40% may have a pulmonary syndrome, recent treatment recommendations advise observation in otherwise healthy or recovering patients and reserve potential antifungal treatment for those with severe disease or persistent symptoms or the immunocompromised (20). The Infectious Diseases Society of America (IDSA) guidelines and recent summary guide are excellent resources for treatment options (184, 185). Ironically, in an effort to diagnose coccidioidomycosis earlier, and in spite of diagnostic advancements, experts have returned to an earlier era, placing renewed emphasis on patient history, symptoms, and examination. They are asking clinicians to remember the mnemonic C-O-C-C-I and consider the diagnosis, order the appropriate tests, check for risk factors, check for complications, and initiate management (21).
Another important consideration for treatment decisions is the recognition of potential resistant Coccidioides isolates, which could alter the clinician’s choice of the triazole medication class. This class of antifungal medications includes the most commonly used, fluconazole, along with itraconazole, posaconazole, and voriconazole (186). This concern, coupled with the protracted treatment course usually utilized for those with progressive coccidioidomycosis, highlights the need to develop improved treatments, such as that offered by a chitin synthase inhibitor, nikkomycin Z, or a new class of medications, the Gwt1 inhibitors, such as APX001 (fosmanogepix) and its active compound, APX001A (manogepix) (187, 188). Other agents under investigation include olorofim, an orotomide analog investigated for central nervous system coccidioidomycosis, and fungal CYP51 inhibitors, such as oteseconazole, studied in canine respiratory coccidioidomycosis (189, 190). In spite of the substantial financial hurdle to developing treatment for an “orphan disease” such as coccidioidomycosis, the coupling of advancements recognizing at-risk individuals with development of a potentially shorter-course curative medication could more than compensate for the medication’s development costs (27).
CONCLUDING REMARKS
There are challenges to making advancements in understanding early pathogen-host events in coccidioidomycosis. Fortunately, there are strategies and solutions to overcome these obstacles. As mentioned above, understanding of early events in coccidioidomycosis is limited by the inability to study the early pathogen-host interaction. This is complicated by the hazardous nature of the fungus, requiring an ABSL3 facility for handling fungal cultures. A strategy to meet this challenge would include ongoing development or discovery of less virulent Coccidioides strains and the development of new technologies or computer modeling that could provide insight into early pathogen-host interaction in a safe and efficient manner. Another challenge in understanding early coccidioidomycosis is how and why nearly 60% of those exposed have few symptoms and develop lifelong immunity. This requires further means to investigate the innate and adaptive immune systems and their complex interaction in response to the fungus. Greater collaborative efforts among clinicians, research groups, and industry would be a way forward.
The lack of coccidioidomycosis awareness, even among health care providers in areas of endemicity, is an ongoing issue leading to unnecessary health care utilization and cost. An example of a simple solution to this problem is the subject of a recent study utilizing EMR that demonstrates potential cost savings and improved antibiotic stewardship with earlier coccidioidomycosis diagnosis (178). As is the case with other diseases, coccidioidomycosis is considered an “orphan disease” and, as such, oftentimes faces funding challenges. One solution is the development of simpler and cost-effective studies, such as an ongoing project examining efforts to raise VF awareness among primary health care providers in an area where Coccidioides is endemic that will assess if such interventions improve patient outcomes and lower costs (F. M. Donovan and J. N. Galgiani, unpublished data). Another challenge shared by pharmaceutical companies and research groups is the use and success of biologics, immune-suppressive agents, and chemotherapy medications. In areas where coccidioidomycosis is endemic, there is justified concern about and evidence of increased susceptibility of previously healthy patients treated with these medications, along with potential reactivation of dormant disease or waning immunity in an aging population. A solution would be the development of tests that are simpler and more consistent than the current skin test that could identify or monitor Coccidioides immunity and thereby help guide therapy and lessen complications from these valuable medications. Probably the most significant research challenge going forward is recognizing Coccidioides as a unique fungal entity that is on the top of the invasive fungal pyramid. Its complex life cycle, structure, and early interaction with the host pose a formidable research problem. Strategies to improve understanding will require integration of bioinformatics, genetics, and proteomics, along with the training of a new generation of research scientists who are well versed in these disciplines and inspired by the previous generation’s experience and enthusiasm.
ACKNOWLEDGMENTS
We report no conflict of interest.
This work received no specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Biographies

Fariba M. Donovan, M.D., Ph.D., as both a practicing physician and a research scientist, has long cultivated a particular interest in medical mycology. Her research focuses on the identification of virulence factors and the interaction of several fungi with the human host. She has conducted studies in Coccidioides with the goal of helping in the earlier diagnosis of valley fever to improve patient outcomes, lower costs, and heighten antibiotic stewardship. Additionally, she is developing strategies to study the host innate immune response to Coccidioides with a focus on the early events in coccidioidomycosis.

Lisa Shubitz, D.V.M., is a research scientist at the Valley Fever Center for Excellence. Her research focus includes developing a vaccine for valley fever and studying the epidemiology of the disease in canines, the ecological distribution of the fungus in southern Arizona, and interactions between the host (both animal and human) and the fungus that causes valley fever, using animal models.

Daniel Powell, Ph.D., received his Ph.D. from the University of Baltimore, Baltimore, MD, in 2011 working on immune responses to lipopolysaccharide mutants. Since then, he has conducted research at the University of Arizona. He is a cellular immunologist with interests in vaccine development, as well as early host responses in lung infections.

Marc Orbach, Ph.D., is a professor of plant pathology in the College of Agriculture and Life Sciences. His focus is based on studying the ecological niche of the fungus that causes valley fever and the analysis of global gene expression in Coccidioides posadasii during both saprobic and parasitic growth. In his analysis, he has been working with the Serial Analysis of Gene Expression (SAGE). His research interests also include the molecular genetics of fungal pathogenicity in animals and plants.

Jeffrey Frelinger, Ph.D., and the members of his laboratory have been interested for some years in immune responses to the lung pathogens influenza virus and Francisella tularensis. They have begun collaborations on the role of T cell responses in Coccidioides infections and the development of an effective vaccine.

John N. Galgiani, M.D., has been working with valley fever (coccidioidomycosis) for the last four decades. As director of the Valley Fever Center for Excellence, his passion is research in the treatment of valley fever. This involves studies to improve the detection of the fungus in the environment, to increase the sensitivity of diagnostic tests for patients, and to develop a vaccine to prevent the disease in both humans and animals.
REFERENCES
- 1.Posadas A. 1892. Un nuevo caso de micosis fungoidea con psorospermias. An Circ Med Argent 15:585–597. [Google Scholar]
- 2.Ophuls W, Moffitt HC. 1900. A new pathogenic mould. (Formerly described as a protozoon: Coccidioides immitis pyogenes.) Preliminary report. Philadelphia Med J 5:1471–1472. [Google Scholar]
- 3.Ophuls W. 1905. Further observations on a pathogenic mold formerly described as a protozoan (Coccidioides immitis, Coccidioides pyogenes). J Exp Med 6:443–486. doi: 10.1084/jem.6.4-6.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Teixeira MM, Barker BM. 2016. Use of population genetics to assess the ecology, evolution, and population structure of Coccidioides. Emerg Infect Dis 22:1022–1030. doi: 10.3201/eid2206.151565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teixeria MM, Barker B. 2017. Coccidioides and coccidioidomycosis, p 255–280. In Mora-Montes H, Lopes-Bezerra L (ed), Current progress in medical mycology. Springer, Cham, Switzerland. doi: 10.1007/978-3-319-64113-3_8. [DOI] [Google Scholar]
- 6.Dickson EC, Gifford MA. 1938. Coccidioides infection (coccidioidomycosis). II. The primary type of infection. Arch Intern Med 62:853–871. doi: 10.1001/archinte.1938.00180160132011. [DOI] [Google Scholar]
- 7.Smith CE, Beard RR. 1946. Effect of season and dust control on coccidioidomycosis. JAMA 132:833–838. doi: 10.1001/jama.1946.02870490011003. [DOI] [PubMed] [Google Scholar]
- 8.Baker O, Braude AI. 1956. A study of stimuli leading to the prodution of spherules in coccidioidomycosis. J Lab Clin Med 47:169–181. [PubMed] [Google Scholar]
- 9.Beaman L, Holmberg CA. 1980. In vitro response of alveolar macrophages to infection with Coccidioides immitis. Infect Immun 28:594–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole GT, Sun SH. 1985. Arthroconidium-spherule-endospore transformation in Coccidioides immitis, p 281–333. In Szaniszlo PJ. (ed), Fungal dimorphism. Plenum Publishing Corp, New York, NY. [Google Scholar]
- 11.Lewis ER, Bowers JR, Barker BM. 2015. Dust devil: the life and times of the fungus that causes valley fever. PLoS Pathog 11:e1004762. doi: 10.1371/journal.ppat.1004762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cole GT, Hung CY. 2001. The parasitic cell wall of Coccidioides immitis. Med Mycol 39:31–40. doi: 10.1080/744118874. [DOI] [PubMed] [Google Scholar]
- 13.Ganesan S, Comstock AT, Sajjan US. 2013. Barrier function of airway tract epithelium. Tissue Barriers 1:e24997. doi: 10.4161/tisb.24997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ampel NM, Hoover SE. 2015. Pathogenesis of coccidioidomycosis. Curr Fungal Infect Rep 9:253–258. doi: 10.1007/s12281-015-0242-1. [DOI] [Google Scholar]
- 15.Drutz DJ, Huppert M. 1983. Coccidioidomycosis: factors affecting the host-parasite interaction. J Infect Dis 147:372–390. doi: 10.1093/infdis/147.3.372. [DOI] [PubMed] [Google Scholar]
- 16.Werner JL, Steele C. 2014. Innate receptors and cellular defense against pulmonary infections. J Immunol 193:3842–3850. doi: 10.4049/jimmunol.1400978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen K, Kolls JK. 2013. T cell-mediated host immune defenses in the lung. Annu Rev Immunol 31:605–633. doi: 10.1146/annurev-immunol-032712-100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith CE, Beard RR, Whiting EG, Rosenberger HG. 1946. Varieties of coccidioidal infection in relation to the epidemiology and control of the disease. Am J Public Health Nations Health 36:1394–1402. doi: 10.2105/ajph.36.12.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Valdivia L, Nix D, Wright M, Lindberg E, Fagan T, Lieberman D, Stoffer T, Ampel NM, Galgiani JN. 2006. Coccidioidomycosis as a common cause of community-acquired pneumonia. Emerg Infect Dis 12:958–962. doi: 10.3201/eid1206.060028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ampel NM. 2015. The treatment of coccidioidomycosis. Rev Inst Med Trop Sao Paulo 57(Suppl 19):51–56. doi: 10.1590/S0036-46652015000700010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galgiani JN, Thompson GR III, Board of Valley Fever Alliance of Arizona Clinicians. 2016. Valley Fever (coccidioidomycosis) tutorial for primary care professionals. Valley Fever Center for Excellence, The University of Arizona, Tucson, AZ: http://www.vfce.arizona.edu/sites/vfce/files/tutorial_for_primary_care_professionals.pdf. [Google Scholar]
- 22.Smith CE. 1940. Epidemiology of acute coccidioidomycosis with erythema nodosum. Am J Public Health Nations Health 30:600–611. doi: 10.2105/ajph.30.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fiese MJ, Cheu S, Sorensen RH. 1955. Mycelial forms of Coccidioides immitis in sputum and tissues of the human host. Ann Intern Med 43:255–270. [DOI] [PubMed] [Google Scholar]
- 24.Drutz DJ, Catanzaro A. 1978. Coccidioidomycosis. Part II Am Rev Respir Dis 117:727–771. doi: 10.1164/arrd.1978.117.4.727. [DOI] [PubMed] [Google Scholar]
- 25.Thompson GR III, Bays D, Taylor SL, Cohen SH, Pappagianis D. 2013. Association between serum 25-hydroxyvitamin D level and type of coccidioidal infection. Med Mycol 51:319–323. doi: 10.3109/13693786.2012.690536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vinh DC, Masannat F, Dzioba RB, Galgiani JN, Holland SM. 2009. Refractory disseminated coccidioidomycosis and mycobacteriosis in interferon-gamma receptor 1 deficiency. Clin Infect Dis 49:e62–e65. doi: 10.1086/605532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Odio CD, Marciano BE, Galgiani JN, Holland SM. 2017. Risk factors for disseminated coccidioidomycosis, United States. Emerg Infect Dis 23:4. doi: 10.3201/eid2302.160505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huppert M, Sun SH, Harrison JL. 1982. Morphogenesis throughout saprobic and parasitic cycles of Coccidioides immitis. Mycopathologia 78:107–122. doi: 10.1007/bf00442634. [DOI] [PubMed] [Google Scholar]
- 29.Mead HL, Teixeira MM, Galgiani JN, Barker BM. 2018. Characterizing in vitro spherule morphogenesis of multiple strains of both species of Coccidioides. Med Mycol 57:478–488. doi: 10.1093/mmy/myy049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hussell T, Bell TJ. 2014. Alveolar macrophages: plasticity in a tissue-specific context. Nat Rev Immunol 14:81–93. doi: 10.1038/nri3600. [DOI] [PubMed] [Google Scholar]
- 31.Converse JL. 1955. Growth of spherules of Coccidioides immitis in a chemically defined liquid medium (22144). Proc Soc Exp Biol Med 90:709–711. doi: 10.3181/00379727-90-22144. [DOI] [PubMed] [Google Scholar]
- 32.Converse JL. 1957. Effect of surface active agents on endosporulation of Coccidioides immitis in a chemically defined medium. J Bacteriol 74:106–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lones GW, Peacock CL. 1960. Studies of the growth and metabolism of Coccidioides immitis. Ann N Y Acad Sci 89:102–108. doi: 10.1111/j.1749-6632.1960.tb20134.x. [DOI] [PubMed] [Google Scholar]
- 34.Lones GW, Peacock CL. 1960. Role of carbon dioxide in the dimorphism of Coccidioides immitis. J Bacteriol 79:308–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Breslau AM, Kubota MY. 1964. Continuous in vitro cultivation of spherules of Coccidioides immitis. J Bacteriol 87:468–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wheat RW, Tritschler C, Conant NF, Lowe EP. 1977. Comparison of Coccidioides immitis arthrospore, mycelium, and spherule cell walls, and influence of growth medium on mycelial cell wall composition. Infect Immun 17:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cole GT, Kirkland TN, Sun SH. 1987. An immunoreactive, water-soluble conidial wall fraction of Coccidioides immitis. Infect Immun 55:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bayry J, Aimanianda V, Guijarro JI, Sunde M, Latge JP. 2012. Hydrophobins—unique fungal proteins. PLoS Pathog 8:e1002700. doi: 10.1371/journal.ppat.1002700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ward ER Jr, Cox RA, Schmitt JA Jr, Huppert M, Sun SH. 1975. Delayed-type hypersensitivity responses to a cell wall fraction of the mycelial phase of Coccidioides immitis. Infect Immun 12:1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cox RA, Huppert M, Starr P, Britt LA. 1984. Reactivity of alkali-soluble, water-soluble cell wall antigen of Coccidioides immitis with anti-Coccidioides immunoglobulin M precipitin antibody. Infect Immun 43:502–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kirkland TN, Zhu S, Kruse D, Hsu L, Seshan KR, Cole GT. 1991. Coccidioides immitis fractions which are antigenic for immune T lymphocytes. Infect Immun 59:3952–3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox RA, Magee DM. 2004. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev 17:804–839. doi: 10.1128/CMR.17.4.804-839.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Awasthi S, Vilekar P, Conkleton A, Rahman N. 2019. Dendritic cell-based immunization induces Coccidioides Ag2/PRA-specific immune response. Vaccine 37:1685–1691. doi: 10.1016/j.vaccine.2019.01.034. [DOI] [PubMed] [Google Scholar]
- 44.Cole GT, Pshko EJ, Seshan KR. 1995. Possible roles of wall hydrolases in the morphogenesis of Coccidioides immitis. Can J Bot 73:132–S1141. doi: 10.1139/b95-369. [DOI] [Google Scholar]
- 45.Breslau AM, Hensley TJ, Erickson JO. 1961. Electron microscopy of cultured spherules of Coccidioides immitis. J Biophys Biochem Cytol 9:627–637. doi: 10.1083/jcb.9.3.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li L, Schmelz M, Kellner EM, Galgiani JN, Orbach MJ. 2007. Nuclear labeling of Coccidioides posadasii with green fluorescent protein. Ann N Y Acad Sci 1111:198–207. doi: 10.1196/annals.1406.014. [DOI] [PubMed] [Google Scholar]
- 47.Hector RF, Pappagianis D. 1982. Enzymatic degradation of the walls of spherules of Coccidioides immitis. Exp Mycol 6:136–152. doi: 10.1016/0147-5975(82)90088-3. [DOI] [Google Scholar]
- 48.Frey CL, Drutz DJ. 1986. Influence of fungal surface components on the interaction of Coccidioides immitis with polymorphonuclear neutrophils. J Infect Dis 153:933–943. doi: 10.1093/infdis/153.5.933. [DOI] [PubMed] [Google Scholar]
- 49.Lee CY, Thompson GR, Hastey CJ, Hodge GC, Lunetta JM, Pappagianis D, Heinrich V. 2015. Coccidioides endospores and spherules draw strong chemotactic, adhesive, and phagocytic responses by individual human neutrophils. PLoS One 10:e0129522. doi: 10.1371/journal.pone.0129522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hung CY, Yu JJ, Seshan KR, Reichard U, Cole GT. 2002. A parasitic phase-specific adhesin of Coccidioides immitis contributes to the virulence of this respiratory fungal pathogen. Infect Immun 70:3443–3456. doi: 10.1128/iai.70.7.3443-3456.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hung CY, Seshan KR, Yu JJ, Schaller R, Xue J, Basrur V, Gardner MJ, Cole GT. 2005. A metalloproteinase of Coccidioides posadasii contributes to evasion of host detection. Infect Immun 73:6689–6703. doi: 10.1128/IAI.73.10.6689-6703.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Viriyakosol S, Fierer J, Brown GD, Kirkland TN. 2005. Innate immunity to the pathogenic fungus Coccidioides posadasii is dependent on Toll-like receptor 2 and Dectin-1. Infect Immun 73:1553–1560. doi: 10.1128/IAI.73.3.1553-1560.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beaman L, Benjamini E, Pappagianis D. 1983. Activation of macrophages by lymphokines: enhancement of phagosome-lysosome fusion and killing of Coccidioides immitis. Infect Immun 39:1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Viriyakosol S, Jimenez MDP, Saijo S, Fierer J. 2014. Neither dectin-2 nor the mannose receptor is required for resistance to Coccidioides immitis in mice. Infect Immun 82:1147–1156. doi: 10.1128/IAI.01355-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garcia-Sherman MC, Lundberg T, Sobonya RE, Lipke PN, Klotz SA. 2015. A unique biofilm in human deep mycoses: fungal amyloid is bound by host serum amyloid P component. NPJ Biofilms Microbiomes 1:15009. doi: 10.1038/npjbiofilms.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiesner DL, Klein BS. 2017. Lung epithelium: barrier immunity to inhaled fungi and driver of fungal-associated allergic asthma. Curr Opin Microbiol 40:8–13. doi: 10.1016/j.mib.2017.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitsett JA, Alenghat T. 2015. Respiratory epithelial cells orchestrate pulmonary innate immunity. Nat Immunol 16:27–35. doi: 10.1038/ni.3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Awasthi S, Magee DM, Coalson JJ. 2004. Coccidioides posadasii infection alters the expression of pulmonary surfactant proteins (SP)-A and SP-D. Respir Res 5:28. doi: 10.1186/1465-9921-5-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Leiva-Juarez MM, Kolls JK, Evans SE. 2018. Lung epithelial cells: therapeutically inducible effectors of antimicrobial defense. Mucosal Immunol 11:21–34. doi: 10.1038/mi.2017.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Austwick PKC. 1980. The pathogenic aspects of the use of fungi: the need for risk analysis and registration of fungi. Ecol Bull 31:91–102. [Google Scholar]
- 61.Deresinski SC, Levine HB, Stevens DA. 1978. Coccidioides immitis endospores: phagocytosis by human cells. Mycopathologia 64:179–181. doi: 10.1007/bf00576371. [DOI] [PubMed] [Google Scholar]
- 62.Nesbit LA, Knox KS, Nguyen CT, Roesch J, Wheat LJ, Johnson SM, Pappagianis D, Chavez S, Ampel NM. 2013. Immunological characterization of bronchoalveolar lavage fluid in patients with acute pulmonary coccidioidomycosis. J Infect Dis 208:857–863. doi: 10.1093/infdis/jit246. [DOI] [PubMed] [Google Scholar]
- 63.Levine HB, Miller RL, Smith CE. 1962. Influence of vaccination on respiratory coccidioidal disease in cynomolgus monkeys. J Immunol 89:242–251. [PubMed] [Google Scholar]
- 64.Williams PL, Sobel RA, Sorensen KN, Clemons KV, Shuer LM, Royaltey SS, Yao Y, Pappagianis D, Lutz JE, Reed C, River ME, Lee BC, Bhatti SU, Stevens DA. 1998. A model of coccidioidal meningoencephalitis and cerebrospinal vasculitis in the rabbit. J Infect Dis 178:1217–1221. doi: 10.1086/515689. [DOI] [PubMed] [Google Scholar]
- 65.Clemons KV, Capilla J, Stevens DA. 2007. Experimental animal models of coccidioidomycosis. Ann N Y Acad Sci 1111:208–224. doi: 10.1196/annals.1406.029. [DOI] [PubMed] [Google Scholar]
- 66.Werner SB, Pappagianis D, Heindl I, Mickel A. 1972. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med 286:507–512. doi: 10.1056/NEJM197203092861003. [DOI] [PubMed] [Google Scholar]
- 67.Jamison HW, Carter RA. 1947. The roentgen findings in early coccidioidomycosis. Radiology 48:323–332. doi: 10.1148/48.4.323. [DOI] [PubMed] [Google Scholar]
- 68.Jude CM, Nayak NB, Patel MK, Deshmukh M, Batra P. 2014. Pulmonary coccidioidomycosis: pictorial review of chest radiographic and CT findings. Radiographics 34:912–925. doi: 10.1148/rg.344130134. [DOI] [PubMed] [Google Scholar]
- 69.Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR. 2004. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine 83:149–175. doi: 10.1097/01.md.0000126762.91040.fd. [DOI] [PubMed] [Google Scholar]
- 70.Shubitz LF, Dial SM, Perrill R, Casement R, Galgiani JN. 2008. Vaccine-induced cellular immune responses differ from innate responses in susceptible and resistant strains of mice infected with Coccidioides posadasii. Infect Immun 76:5553–5564. doi: 10.1128/IAI.00885-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shubitz LP, Lewis ML, Dial SM, Galgiani J. 2011. Early post-infection detection of Coccidioides in intranasally infected mice, p 40. In Proceedings of the 55th Annual Coccidioidomycosis Study Group Meeting.
- 72.Sun SH, Cole GT, Drutz DJ, Harrison JL. 1986. Electron-microscopic observations of the Coccidioides immitis parasitic cycle in vivo. J Med Vet Mycol 24:183–192. doi: 10.1080/02681218680000281. [DOI] [PubMed] [Google Scholar]
- 73.Shubitz LF, Powell DA, Trinh HT, Lewis ML, Orbach MJ, Frelinger JA, Galgiani JN. 2018. Viable spores of Coccidioides posadasii Deltacps1 are required for vaccination and provide long lasting immunity. Vaccine 36:3375–3380. doi: 10.1016/j.vaccine.2018.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gonzalez A. 2013. Innate immune response to the dimorphic fungal pathogen Coccidioides: molecular and cellular mechanisms. J Clin Cell Immunol S13:1–6. doi: 10.4172/2155-9899.S13-001. [DOI] [Google Scholar]
- 75.Erwig LP, Gow NA. 2016. Interactions of fungal pathogens with phagocytes. Nat Rev Microbiol 14:163–176. doi: 10.1038/nrmicro.2015.21. [DOI] [PubMed] [Google Scholar]
- 76.Lengeler KB, Davidson RC, D'Souza C, Harashima T, Shen W-C, Wang P, Pan X, Waugh M, Heitman J. 2000. Signal transduction cascades regulating fungal development and virulence. Microbiol Mol Biol Rev 64:746–785. doi: 10.1128/MMBR.64.4.746-785.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Runyanga TJ, Chen Y, Sun F. 2017. Cell communication and fungal virulence: a review. Res Rev Res J Biol 5:8–16. [Google Scholar]
- 78.Gauthier G, Klein BS. 2008. Insights into fungal morphogenesis and immune evasion: fungal conidia, when situated in mammalian lungs, may switch from mold to pathogenic yeasts or spore-forming spherules. Microbe Wash DC 3:416–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pishko EJ, Kirkland TN, Cole GT. 1995. Isolation and characterization of two chitinase-encoding genes (cts1, cts2) from the fungus Coccidioides immitis. Gene 167:173–177. doi: 10.1016/0378-1119(95)00654-0. [DOI] [PubMed] [Google Scholar]
- 80.Abuodeh RO, Orbach MJ, Mandel MA, Das A, Galgiani JN. 2000. Genetic transformation of Coccidioides immitis facilitated by Agrobacterium tumefaciens. J Infect Dis 181:2106–2110. doi: 10.1086/315525. [DOI] [PubMed] [Google Scholar]
- 81.Mandel MA, Galgiani JN, Kroken S, Orbach MJ. 2006. Coccidioides posadasii contains single chitin synthase genes corresponding to classes I to VII. Fungal Genet Biol 43:775–788. doi: 10.1016/j.fgb.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 82.Kellner EM, Orsborn KI, Siegel EM, Mandel MA, Orbach MJ, Galgiani JN. 2005. Coccidioides posadasii contains a single 1,3-{beta}-glucan synthase gene that appears to be essential for growth. Eukaryot Cell 4:111–120. doi: 10.1128/EC.4.1.111-120.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Johannesson H, Kasuga T, Schaller RA, Good B, Gardner MJ, Townsend JP, Cole GT, Taylor JW. 2006. Phase-specific gene expression underlying morphological adaptations of the dimorphic human pathogenic fungus, Coccidioides posadasii. Fungal Genet Biol 43:545–559. doi: 10.1016/j.fgb.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 84.Delgado N, Hung CY, Tarcha E, Gardner MJ, Cole GT. 2004. Profiling gene expression in Coccidioides posadasii. Med Mycol 42:59–71. doi: 10.1080/1369378031000156890. [DOI] [PubMed] [Google Scholar]
- 85.Rappleye CA, Goldman WE. 2006. Defining virulence genes in the dimorphic fungi. Annu Rev Microbiol 60:281–303. doi: 10.1146/annurev.micro.59.030804.121055. [DOI] [PubMed] [Google Scholar]
- 86.Whiston E, Zhang Wise H, Sharpton TJ, Jui G, Cole GT, Taylor JW. 2012. Comparative transcriptomics of the saprobic and parasitic growth phases in Coccidioides spp. PLoS One 7:e41034. doi: 10.1371/journal.pone.0041034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Viriyakosol S, Singhania A, Fierer J, Goldberg J, Kirkland TN, Woelk CH. 2013. Gene expression in human fungal pathogen Coccidioides immitis changes as arthroconidia differentiate into spherules and mature. BMC Microbiol 13:121. doi: 10.1186/1471-2180-13-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Narra HP, Shubitz LF, Mandel MA, Trinh HT, Griffin K, Buntzman AS, Frelinger JA, Galgiani JN, Orbach MJ. 2016. A Coccidioides posadasii CPS1 deletion mutant is avirulent and protects mice from lethal infection. Infect Immun 84:3007–3016. doi: 10.1128/IAI.00633-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grys TE, Kaushal S, Chowdhury Y, Dasari S, Mitchell NM, Magee DM, Blair JE, Colby TV, Lake DF. 2016. Total and lectin-binding proteome of spherulin from Coccidioides posadasii. J Proteome Res 15:3463–3472. doi: 10.1021/acs.jproteome.5b01054. [DOI] [PubMed] [Google Scholar]
- 90.Galgiani JN, Payne CM, Jones JF. 1984. Human polymorphonuclear-leukocyte inhibition of incorporation of chitin precursors into mycelia of Coccidioides immitis. J Infect Dis 149:404–412. doi: 10.1093/infdis/149.3.404. [DOI] [PubMed] [Google Scholar]
- 91.Galgiani JN. 1986. Inhibition of different phases of Coccidioides immitis by human neutrophils or hydrogen peroxide. J Infect Dis 153:217–222. doi: 10.1093/infdis/153.2.217. [DOI] [PubMed] [Google Scholar]
- 92.Ampel NM, Galgiani JN. 1991. Interaction of human peripheral blood mononuclear cells with Coccidioides immitis arthroconidia. Cell Immunol 133:253–262. doi: 10.1016/0008-8749(91)90195-h. [DOI] [PubMed] [Google Scholar]
- 93.Rutherford JC. 2014. The emerging role of urease as a general microbial virulence factor. PLoS Pathog 10:e1004062. doi: 10.1371/journal.ppat.1004062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li K, Yu JJ, Hung CY, Lehmann PF, Cole GT. 2001. Recombinant urease and urease DNA of Coccidioides immitis elicit an immunoprotective response against coccidioidomycosis in mice. Infect Immun 69:2878–2887. doi: 10.1128/IAI.69.5.2878-2887.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mirbod-Donovan F, Schaller R, Hung CY, Xue J, Reichard U, Cole GT. 2006. Urease produced by Coccidioides posadasii contributes to the virulence of this respiratory pathogen. Infect Immun 74:504–515. doi: 10.1128/IAI.74.1.504-515.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wise HZ, Hung CY, Whiston E, Taylor JW, Cole GT. 2013. Extracellular ammonia at sites of pulmonary infection with Coccidioides posadasii contributes to severity of the respiratory disease. Microb Pathog 59-60:19–28. doi: 10.1016/j.micpath.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Schenten D, Medzhitov R. 2011. The control of adaptive immune responses by the innate immune system. Adv Immunol 109:87–124. doi: 10.1016/B978-0-12-387664-5.00003-0. [DOI] [PubMed] [Google Scholar]
- 98.Plato A, Hardison SE, Brown GD. 2015. Pattern recognition receptors in antifungal immunity. Semin Immunopathol 37:97–106. doi: 10.1007/s00281-014-0462-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hung C-Y, Jiménez-Alzate MDP, Gonzalez A, Wüthrich M, Klein BS, Cole GT. 2014. Interleukin-1 receptor but not Toll-like receptor 2 is essential for MyD88-dependent Th17 immunity to Coccidioides infection. Infect Immun 82:2106–2114. doi: 10.1128/IAI.01579-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Wang H, LeBert V, Hung CY, Galles K, Saijo S, Lin X, Cole GT, Klein BS, Wuthrich M. 2014. C-type lectin receptors differentially induce th17 cells and vaccine immunity to the endemic mycosis of North America. J Immunol 192:1107–1119. doi: 10.4049/jimmunol.1302314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.del Pilar Jimenez AM, Viriyakosol S, Walls L, Datta SK, Kirkland T, Heinsbroek SE, Brown G, Fierer J. 2008. Susceptibility to Coccidioides species in C57BL/6 mice is associated with expression of a truncated splice variant of Dectin-1 (Clec7a). Genes Immun 9:338–348. doi: 10.1038/gene.2008.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Awasthi S, Magee DM. 2004. Differences in expression of cell surface co-stimulatory molecules, Toll-like receptor genes and secretion of IL-12 by bone marrow-derived dendritic cells from susceptible and resistant mouse strains in response to Coccidioides posadasii. Cell Immunol 231:49–55. doi: 10.1016/j.cellimm.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 103.Awasthi S. 2010. Susceptibility of TLR4-defective C3H/HeJ mice to Coccidioides posadasii infection. Med Mycol 48:470–475. doi: 10.3109/13693780903226019. [DOI] [PubMed] [Google Scholar]
- 104.Viriyakosol S, Jimenez MDP, Gurney MA, Ashbaugh ME, Fierer J. 2013. Dectin-1 is required for resistance to coccidioidomycosis in mice. mBio 4:e00597. doi: 10.1128/mBio.00597-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kerscher B, Willment JA, Brown GD. 2013. The Dectin-2 family of C-type lectin-like receptors: an update. Int Immunol 25:271–277. doi: 10.1093/intimm/dxt006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Murdock BJ, Falkowski NR, Shreiner AB, Sadighi Akha AA, McDonald RA, White ES, Toews GB, Huffnagle GB. 2012. Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia. Infect Immun 80:1424–1436. doi: 10.1128/IAI.05529-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Malacco N, Rachid MA, Gurgel I, Moura TR, Sucupira PHF, de Sousa LP, de Souza DDG, Russo RC, Teixeira MM, Soriani FM. 2018. Eosinophil-associated innate IL-17 response promotes Aspergillus fumigatus lung pathology. Front Cell Infect Microbiol 8:453. doi: 10.3389/fcimb.2018.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Castro-Lopez N, Hung CY. 2017. Immune response to coccidioidomycosis and the development of a vaccine. Microorganisms 5:13. doi: 10.3390/microorganisms5010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Geijtenbeek TB, Gringhuis SI. 2009. Signalling through C-type lectin receptors: shaping immune responses. Nat Rev Immunol 9:465–479. doi: 10.1038/nri2569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hung CY, Castro-Lopez N, Cole GT. 2016. Card9- and MyD88-mediated gamma interferon and nitric oxide production is essential for resistance to subcutaneous Coccidioides posadasii infection. Infect Immun 84:1166–1175. doi: 10.1128/IAI.01066-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sampaio EP, Hsu AP, Pechacek J, Bax HI, Dias DL, Paulson ML, Chandrasekaran P, Rosen LB, Carvalho DS, Ding L, Vinh DC, Browne SK, Datta S, Milner JD, Kuhns DB, Long Priel DA, Sadat MA, Shiloh M, De Marco B, Alvares M, Gillman JW, Ramarathnam V, de la Morena M, Bezrodnik L, Moreira I, Uzel G, Johnson D, Spalding C, Zerbe CS, Wiley H, Greenberg DE, Hoover SE, Rosenzweig SD, Galgiani JN, Holland SM. 2013. Signal transducer and activator of transcription 1 (STAT1) gain-of-function mutations and disseminated coccidioidomycosis and histoplasmosis. J Allergy Clin Immunol 131:1624–1634. doi: 10.1016/j.jaci.2013.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Fierer J, Walls L, Wright F, Kirkland TN. 1999. Genes influencing resistance to Coccidioides immitis and the interleukin-10 response map to chromosomes 4 and 6 in mice. Infect Immun 67:2916–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nathan C, Shiloh MU. 2000. Reactive oxygen and nitrogen intermediates in the relationship between mammalian hosts and microbial pathogens. Proc Natl Acad Sci U S A 97:8841–8848. doi: 10.1073/pnas.97.16.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bogdan C. 2001. Nitric oxide and the immune response. Nat Immunol 2:907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 115.Margolis DA, Viriyakosol S, Fierer J, Kirkland TN. 2011. The role of reactive oxygen intermediates in experimental coccidioidomycois in mice. BMC Microbiol 11:71. doi: 10.1186/1471-2180-11-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gonzalez A, Hung CY, Cole GT. 2011. Coccidioides releases a soluble factor that suppresses nitric oxide production by murine primary macrophages. Microb Pathog 50:100–108. doi: 10.1016/j.micpath.2010.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gonzalez A, Hung CY, Cole GT. 2011. Nitric oxide synthase activity has limited influence on the control of Coccidioides infection in mice. Microb Pathog 51:161–168. doi: 10.1016/j.micpath.2011.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Lewis ER, David VR, Doyle AL, Rajabi K, Kiefer JA, Pirrotte P, Barker BM. 2015. Differences in host innate responses among Coccidioides isolates in a murine model of pulmonary coccidioidomycosis. Eukaryot Cell 14:1043–1053. doi: 10.1128/EC.00122-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Van Dyken SJ, Liang HE, Naikawadi RP, Woodruff PG, Wolters PJ, Erle DJ, Locksley RM. 2017. Spontaneous chitin accumulation in airways and age-related fibrotic lung disease. Cell 169:497–509.e13. doi: 10.1016/j.cell.2017.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Slagle DC, Cox RA, Kuruganti U. 1989. Induction of tumor necrosis factor alpha by spherules of Coccidioides immitis. Infect Immun 57:1916–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Magee DM, Cox RA. 1995. Roles of gamma interferon and interleukin-4 in genetically determined resistance to Coccidioides immitis. Infect Immun 63:3514–3519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ampel NM, Nesbit LA, Nguyen CT, Chavez S, Knox KS, Johnson SM, Pappagianis D. 2015. Cytokine profiles from antigen-stimulated whole-blood samples among patients with pulmonary or nonmeningeal disseminated coccidioidomycosis. Clin Vaccine Immunol 22:917–922. doi: 10.1128/CVI.00280-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Muñoz-Hernández B, Palma-Cortés G, Manjarrez ME. 2012. Innovation of the parasitic cycle of Coccidioides spp,p 29–52. In Shah MM. (ed), Parasitology. IntechOpen, London, United Kingdom: http://www.intechopen.com/books/parasitology/innovation-of-the-parasitic-cycle-of-coccidioides-spp-. [Google Scholar]
- 124.Galgiani JN, Isenberg RA, Stevens DA. 1978. Chemotaxigenic activity of extracts from the mycelial and spherule phases of Coccidioides immitis for human polymorphonuclear leukocytes. Infect Immun 21:862–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Galgiani JN. 1995. Differences in oxidant release by human polymorphonuclear leukocytes produced by stimulation with different phases of Coccidioides immitis. J Infect Dis 172:199–203. doi: 10.1093/infdis/172.1.199. [DOI] [PubMed] [Google Scholar]
- 126.Rubin-Bejerano I, Abeijon C, Magnelli P, Grisafi P, Fink GR. 2007. Phagocytosis by human neutrophils is stimulated by a unique fungal cell wall component. Cell Host Microbe 2:55–67. doi: 10.1016/j.chom.2007.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Ampel NM, Bejarano GC, Galgiani JN. 1992. Killing of Coccidioides immitis by human peripheral blood mononuclear cells. Infect Immun 60:4200–4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Beaman L, Benjamini E, Pappagianis D. 1981. Role of lymphocytes in macrophage-induced killing of Coccidioides immitis in vitro. Infect Immun 34:347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Roy RM, Klein BS. 2012. Dendritic cells in antifungal immunity and vaccine design. Cell Host Microbe 11:436–446. doi: 10.1016/j.chom.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Dionne SO, Podany AB, Ruiz YW, Ampel NM, Galgiani JN, Lake DF. 2006. Spherules derived from Coccidioides posadasii promote human dendritic cell maturation and activation. Infect Immun 74:2415–2422. doi: 10.1128/IAI.74.4.2415-2422.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Awasthi S. 2007. Dendritic cell-based vaccine against coccidioides infection. Ann N Y Acad Sci 1111:269–274. doi: 10.1196/annals.1406.013. [DOI] [PubMed] [Google Scholar]
- 132.Ampel NM. 2007. The complex immunology of human coccidioidomycosis. Ann N Y Acad Sci 1111:245–258. doi: 10.1196/annals.1406.032. [DOI] [PubMed] [Google Scholar]
- 133.Echols RM, Palmer DL, Long GW. 1982. Tissue eosinophilia in human coccidioidomycosis. Rev Infect Dis 4:656–664. doi: 10.1093/clinids/4.3.656. [DOI] [PubMed] [Google Scholar]
- 134.Simons CM, Stratton CW, Kim AS. 2011. Peripheral blood eosinophilia as a clue to the diagnosis of an occult Coccidioides infection. Hum Pathol 42:449–453. doi: 10.1016/j.humpath.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 135.Schmidt S, Zimmermann SY, Tramsen L, Koehl U, Lehrnbecher T. 2013. Natural killer cells and antifungal host response. Clin Vaccine Immunol 20:452–458. doi: 10.1128/CVI.00606-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schmidt S, Tramsen L, Lehrnbecher T. 2017. Natural killer cells in antifungal immunity. Front Immunol 8:1623. doi: 10.3389/fimmu.2017.01623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Petkus AF, Baum LL. 1987. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J Immunol 139:3107–3111. [PubMed] [Google Scholar]
- 138.Magee DM, Cox RA. 1996. Interleukin-12 regulation of host defenses against Coccidioides immitis. Infect Immun 64:3609–3613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie AN, Mebius RE, Powrie F, Vivier E. 2013. Innate lymphoid cells—a proposal for uniform nomenclature. Nat Rev Immunol 13:145–149. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 140.Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, Koyasu S, Locksley RM, McKenzie ANJ, Mebius RE, Powrie F, Spits H. 2018. Innate lymphoid cells: 10 years on. Cell 174:1054–1066. doi: 10.1016/j.cell.2018.07.017. [DOI] [PubMed] [Google Scholar]
- 141.Mindt BC, Fritz JH, Duerr CU. 2018. Group 2 innate lymphoid cells in pulmonary immunity and tissue homeostasis. Front Immunol 9:840. doi: 10.3389/fimmu.2018.00840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Galgiani JN, Yam P, Petz LD, Williams PL, Stevens DA. 1980. Complement activation by Coccidioides immitis: in vitro and clinical studies. Infect Immun 28:944–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.De Lucca AJ, Walsh TJ. 1999. Antifungal peptides: novel therapeutic compounds against emerging pathogens. Antimicrob Agents Chemother 43:1–11. doi: 10.1128/AAC.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ordonez SR, Veldhuizen EJA, van Eijk M, Haagsman HP. 2017. Role of soluble innate effector molecules in pulmonary defense against fungal pathogens. Front Microbiol 8:2098. doi: 10.3389/fmicb.2017.02098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nayak A, Dodagatta-Marri E, Tsolaki AG, Kishore U. 2012. An insight into the diverse roles of surfactant proteins, SP-A and SP-D in innate and adaptive immunity. Front Immunol 3:131. doi: 10.3389/fimmu.2012.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Ampel NM, Dionne SO, Giblin A, Podany AB, Galgiani J. 2009. Mannose-binding lectin serum levels are low in persons with clinically active coccidioidomycosis. Mycopathologia 167:173–180. doi: 10.1007/s11046-008-9172-6. [DOI] [PubMed] [Google Scholar]
- 147.Segal GP, Lehrer RI, Selsted ME. 1985. In vitro effect of phagocyte cationic peptides on Coccidioides immitis. J Infect Dis 151:890–894. doi: 10.1093/infdis/151.5.890. [DOI] [PubMed] [Google Scholar]
- 148.Collins MS, Pappagianis D. 1974. Inhibition by lysozyme of growth of the spherule phase of Coccidioides immitis in vitro. Infect Immun 10:616–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cox RA, Baker BS, Stevens DA. 1982. Specificity of immunoglobulin E in coccidioidomycosis and correlation with disease involvement. Infect Immun 37:609–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Smith CE, Whiting EG, Baker EE, Rosenberger HG, Beard RR, Saito MT. 1948. The use of coccidioidin. Am Rev Tuberc 57:330–360. [DOI] [PubMed] [Google Scholar]
- 151.Drutz DJ, Catanzaro A. 1978. Coccidioidomycosis. Part I Am Rev Respir Dis 117:559–585. [DOI] [PubMed] [Google Scholar]
- 152.Wack EE, Ampel NM, Sunenshine RH, Galgiani JN. 2015. The return of delayed-type hypersensitivity skin testing for coccidioidomycosis. Clin Infect Dis 61:787–791. doi: 10.1093/cid/civ388. [DOI] [PubMed] [Google Scholar]
- 153.Zweiman BD, Pappagianis D, Maibach H, Hildreth E. 1969. Coccidioidin delayed hypersensitivity: skin test and in vitro lymphocyte reactivities. J Immunol 102:1284–1289. [PubMed] [Google Scholar]
- 154.Stevens DA. 1995. Current concepts: coccidioidomycosis. N Engl J Med 332:1077–1082. doi: 10.1056/NEJM199504203321607. [DOI] [PubMed] [Google Scholar]
- 155.Catanzaro A, Spitler LE, Moser KM. 1975. Cellular immune response in coccidioidomycosis. Cell Immunol 15:360–371. doi: 10.1016/0008-8749(75)90014-3. [DOI] [PubMed] [Google Scholar]
- 156.Vinh DC, Schwartz B, Hsu AP, Miranda DJ, Valdez PA, Fink D, Lau KP, Long-Priel D, Kuhns DB, Uzel G, Pittaluga S, Hoover S, Galgiani JN, Holland SM. 2011. Interleukin-12 receptor beta1 deficiency predisposing to disseminated coccidioidomycosis. Clin Infect Dis 52:e99–e102. doi: 10.1093/cid/ciq215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Beaman L, Pappagianis D, Benjamini E. 1977. Significance of T cells in resistance to experimental murine coccidioidomycosis. Infect Immun 17:580–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Beaman L, Pappagianis D, Benjamini E. 1979. Mechanisms of resistance to infection with Coccidioides immitis in mice. Infect Immun 23:681–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Casadevall A. 1995. Antibody immunity and invasive fungal infections. Infect Immun 63:4211–4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Kong Y-C, Savage DC, Levine HB. 1966. Enhancement of immune responses in mice by a booster injection of Coccidioides spherules. J Immunol 95:1048–1056. [PubMed] [Google Scholar]
- 161.Ibrahim AB, Pappagianis D. 1973. Experimental induction of anergy to coccidioidin by antigens of Coccidioides immites. Infect Immun 7:786–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Magee DM, Friedberg RL, Woitaske MD, Johnston SA, Cox RA. 2005. Role of B cells in vaccine-induced immunity against coccidioidomycosis. Infect Immun 73:7011–7013. doi: 10.1128/IAI.73.10.7011-7013.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Hung CY, Gonzalez A, Wuthrich M, Klein BS, Cole GT. 2011. Vaccine immunity to coccidioidomycosis occurs by early activation of three signal pathways of T helper cell response (Th1, Th2, and Th17). Infect Immun 79:4511–4522. doi: 10.1128/IAI.05726-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yoshinoya S, Cox RA, Pope RM. 1980. Circulating immune complexes in coccidioidomycosis. Detection and characterization. J Clin Invest 66:655–663. doi: 10.1172/JCI109901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cox RA, Pope RM. 1987. Serum-mediated suppression of lymphocyte transformation responses in coccidioidomycosis. Infect Immun 55:1058–1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Brown J, Benedict K, Park BJ, Thompson GR III. 2013. Coccidioidomycosis: epidemiology. Clin Epidemiol 5:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Stockamp NW, Thompson GR III. 2016. Coccidioidomycosis. Infect Dis Clin North Am 30:229–246. doi: 10.1016/j.idc.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 168.Willett FM, Weiss A. 1945. Coccidioidomycosis in southern California: report of a new endemic area with a review of 100 cases. Ann Intern Med 23:349–375. [Google Scholar]
- 169.Wieden MA, Lundergan LL, Blum J, Delgado KL, Coolbaugh R, Howard R, Peng T, Pugh E, Reis N, Theis J, Galgiani JN. 1996. Detection of coccidioidal antibodies by 33-kDa spherule antigen, Coccidioides EIA, and standard serologic tests in sera from patients evaluated for coccidioidomycosis. J Infect Dis 173:1273–1277. doi: 10.1093/infdis/173.5.1273. [DOI] [PubMed] [Google Scholar]
- 170.Blair JE, Chang YH, Cheng MR, Vaszar LT, Vikram HR, Orenstein R, Kusne S, Ho S, Seville MT, Parish JM. 2014. Characteristics of patients with mild to moderate primary pulmonary coccidioidomycosis. Emerg Infect Dis 20:983–990. doi: 10.3201/eid2006.131842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Pappagianis D. 2001. Serologic studies in coccidioidomycosis. Semin Respir Infect 16:242–250. doi: 10.1053/srin.2001.29315. [DOI] [PubMed] [Google Scholar]
- 172.Blair JE, Coakley B, Santelli AC, Hentz JG, Wengenack NL. 2006. Serologic testing for symptomatic coccidioidomycosis in immunocompetent and immunosuppressed hosts. Mycopathologia 162:317–324. doi: 10.1007/s11046-006-0062-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Mafi NG, Blair J. 2019. A retrospective evaluation of coccidioidomycosis skin testing in patients with pulmonary coccidioidomycosi in an endemic region, poster 14. In Proceedings of the 63rd Annual Coccidioidomycosis Study Group Meeting.
- 174.Johnson R, Kernerman SM, Sawtelle BG, Rastogi SC, Nielsen HS, Ampel NM. 2012. A reformulated spherule-derived coccidioidin (Spherusol) to detect delayed-type hypersensitivity in coccidioidomycosis. Mycopathologia 174:353–358. doi: 10.1007/s11046-012-9555-6. [DOI] [PubMed] [Google Scholar]
- 175.Wheeler C, Lucas KD, Derado G, McCotter O, Tharratt RS, Chiller T, Mohle-Boetani JC. 2018. Risk stratification with coccidioidal skin test to prevent valley fever among inmates, California, 2015. J Correct Health Care 24:342–351. doi: 10.1177/1078345818792679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pappagianis D, Zimmer BL. 1990. Serology of coccidioidomycosis. Clin Microbiol Rev 3:247–268. doi: 10.1128/cmr.3.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Maddox SE. 2017. Rapid detection of anti-Coccidioides antibodies using the sonaTM Coccidioides Ab lateral flow assay, p 36. In Proceedings of the Sixty-First Annual Meeting of the Coccidioidomycosis Study Group. Coccidioidomycosis Study Group, Stanford, CA. [Google Scholar]
- 178.Donovan FM, Wightman P, Zong Y, Gabe L, Majeed A, Ynosencio T, Bedrick EJ, Galgiani JN. 2019. Delays in coccidioidomycosis diagnosis and associated healthcare utilization in Tucson, Arizona. Emerg Infect Dis 25:1745. doi: 10.3201/eid2508.190023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ginn RMR, Bollmann K, Goodsell J, Mendez G, Bradley B, Galgiani JN. 2019. Delays in the diagnosis of coccidioidomycosis and their relationship to healthcare utilization. Emerg Infect Dis. doi: 10.3201/eid2509.190019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Pu J, Donovan F, Galgiani JN. 2019. Informatic profile of primary care practioners’ testing and managing patients with coccidioidomycosis (cm), p 33. In Proceedings of the 63rd Annual Cocci Study Group meeting, April 5–6, Sacramento, California.
- 181.Mitchell M, Dizon D, Libke R, Peterson M, Slater D, Dhillon A. 2015. Development of a real-time PCR assay for identification of Coccidioides immitis by use of the BD Max system. J Clin Microbiol 53:926–929. doi: 10.1128/JCM.02731-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Saubolle MA, Wojack BR, Wertheimer AM, Fuayagem AZ, Young S, Koeneman BA. 2018. Multicenter clinical validation of a cartridge-based real-time PCR system for detection of Coccidioides spp. in lower respiratory specimens. J Clin Microbiol 56:e01277-17. doi: 10.1128/JCM.01277-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Thompson GR III, Sharma S, Bays DJ, Pruitt R, Engelthaler DM, Bowers J, Driebe EM, Davis M, Libke R, Cohen SH, Pappagianis D. 2013. Coccidioidomycosis: adenosine deaminase levels, serologic parameters, culture results, and polymerase chain reaction testing in pleural fluid. Chest 143:776–781. doi: 10.1378/chest.12-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Geertsma F, Hoover SE, Johnson RH, Kusne S, Lisse J, MacDonald JD, Meyerson SL, Raksin PB, Siever J, Stevens DA, Sunenshine R, Theodore N. 2016. 2016 Infectious Diseases Society of America (IDSA) clinical practice guideline for the treatment of coccidioidomycosis. Clin Infect Dis 63:e112-46. doi: 10.1093/cid/ciw360. [DOI] [PubMed] [Google Scholar]
- 185.Donovan FM, Zangeneh TT, Malo J, Galgiani JN. 2017. Top questions in the diagnosis and treatment of coccidioidomycosis. Open Forum Infect Dis 4:ofx197. doi: 10.1093/ofid/ofx197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Thompson GR III, Barker BM, Wiederhold NP. 2017. Large-scale evaluation of in vitro amphotericin B, triazole, and echinocandin activity against Coccidioides species from U.S. institutions. Antimicrob Agents Chemother 61:e02634-16. doi: 10.1128/AAC.02634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ostrosky-Zeichner L, Casadevall A, Galgiani JN, Odds FC, Rex JH. 2010. An insight into the antifungal pipeline: selected new molecules and beyond. Nat Rev Drug Discov 9:719–727. doi: 10.1038/nrd3074. [DOI] [PubMed] [Google Scholar]
- 188.Viriyakosol S, Kapoor M, Okamoto S, Covel J, Soltow QA, Trzoss M, Shaw KJ, Fierer J. 2019. APX001 and other Gwt1 inhibitor prodrugs are effective in experimental Coccidioides immitis pneumonia. Antimicrob Agents Chemother 63:e01715-18. doi: 10.1128/AAC.01715-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Wiederhold NP, Najvar LK, Jaramillo R, Olivo M, Birch M, Law D, Rex JH, Catano G, Patterson TF. 2018. The orotomide olorofim is efficacious in an experimental model of central nervous system coccidioidomycosis. Antimicrob Agents Chemother 62:e00999-18. doi: 10.1128/AAC.00999-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Shubitz LF, Roy ME, Trinh HT, Hoekstra WJ, Schotzinger RJ, Garvey EP. 2017. Efficacy of the investigational antifungal VT-1161 in treating naturally occurring coccidioidomycosis in dogs. Antimicrob Agents Chemother 61:e00111-17. doi: 10.1128/AAC.00111-17. [DOI] [PMC free article] [PubMed] [Google Scholar]