Abstract
Amongst therapeutic radiopharmaceuticals, targeted alpha therapy (TαT) can deliver potent and local radiation selectively to cancer cells as well as the tumor microenvironment and thereby control cancer while minimizing toxicity. In this review, we discuss the history, progress, and future potential of TαT in the treatment of prostate cancer, including dosimetry-individualized treatment planning, combinations with small-molecule therapies, and conjugation to molecules directed against antigens expressed by prostate cancer cells, such as prostate-specific membrane antigen (PSMA) or components of the tumor microenvironment. A clinical proof of concept that TαT is efficacious in treating bone-metastatic castration-resistant prostate cancer has been demonstrated by radium-223 via improved overall survival and long-term safety/tolerability in the phase III ALSYMPCA trial. Dosimetry calculation and pharmacokinetic measurements of TαT provide the potential for optimization and individualized treatment planning for a precision medicine-based cancer management paradigm. The ability to combine TαTs with other agents, including chemotherapy, androgen receptor-targeting agents, DNA repair inhibitors, and immuno-oncology agents, is under investigation. Currently, TαTs that specifically target prostate cancer cells expressing PSMA represents a promising therapeutic approach. Both PSMA-targeted actinium-225 and thorium-227 conjugates are under investigation. The described clinical benefit, safety and tolerability of radium-223 and the recent progress in TαT trial development suggest that TαT occupies an important new role in prostate cancer treatment. Ongoing studies with newer dosimetry methods, PSMA targeting, and novel approaches to combination therapies should expand the utility of TαT in prostate cancer treatment.
Keywords: targeted alpha therapy (TαT), radium-223, prostate cancer, prostate-specific membrane antigen (PSMA)
Key Message
Targeted alpha therapy (TαT) delivers highly potent, selective, and local alpha radiation to cancer cells and the tumor microenvironment. TαT occupies an important place in prostate cancer treatment, and its utility may be expanded to multiple cancer types. This review discusses the history, progress, and potential of TαT in oncology with a focus on prostate cancer.
Introduction
The therapeutic effectiveness of specific radionuclides is determined by the specificity of targeting and the innate characteristics of the particle emitted, including potential range, effective travel distance in tissue, and the magnitude of energy emitted [1]. Radionuclides may be classified by their emission of particles, including alpha and β particles [2]. Beta particles have a low linear energy transfer (LET) (0.2 keV/μm), which results in sparse ionization events, individual DNA lesions, and repairable single-strand DNA breaks. Alpha particles have higher LET (50–230 keV/μm), which results in clusters of double-strand breaks (DSBs) in DNA and increased cytotoxicity relative to β particles. Alpha particles also have a shorter path length in tissue (50–100 μm) compared with β particles (1000–10 000 μm). The short range of alpha particle radiation has the potential to minimize cytotoxic damage in non-targeted cells, potentially enabling specific cancer cell targeting with reduced toxicity to normal cells [3, 4]. This is exemplified by radium-223 dichloride (radium-223, Xofigo®), a first-in-class alpha particle–emitting agent approved for castration-resistant prostate cancer (CRPC) with symptomatic bone metastases [5]. Radium-223 is a calcium mimetic that can preferentially bind to areas of hydroxyapatite deposition, such as bone metastases in prostate cancer (PC) [6–8]. Other bone-seeking, β particle-emitting radionuclides, such as strontium-89 and samarium-153-EDTMP, are indicated for pain relief for skeletal metastases [9–11]. Although these radionuclides provided pain palliation in bone-metastatic CRPC (mCRPC), they have failed to confer survival benefit [10, 12]. In contrast, in addition to reducing bone pain in a subset of patients, radium-223 provided survival benefit when added to best standard of care (SoC) [7, 13, 14]. Recent progress in targeted alpha therapy (TαT) offers the potential to deliver cytotoxic radiation in a highly localized manner [3, 4]. In addition to exploring radium-223 in combination with other cytotoxic agents, androgen receptor (AR)-targeting agents, or immuno-oncology agents, preclinical and clinical studies investigating other alpha particle-emitting radionuclides for use as TαTs (e.g. actinium-225 and thorium-227 conjugates) are ongoing (Table 1). This review will discuss the history, progress, and future potential of TαT for the treatment of PC, including (i) treatment optimization and individualized planning; (ii) combination therapies (e.g. radium-223 with AR-targeting agents, cytotoxic agents, or immuno-oncology agents); (iii) prostate-specific membrane antigen (PSMA) as an emerging therapeutic target; and (iv) next-generation TαTs (e.g. targeted thorium-227 and actinium-225 conjugates).
Table 1.
TαTs in prostate cancer
| TαT | MoA | Half-life | Number of alpha particles emitted | Highlights of ongoing clinical trials or key findings |
|---|---|---|---|---|
| Radium-223 dichloride | Bone-seeking alpha-emitting radionuclide [8] | 11.4 days [15] | 4 [15] | The phase III PEACE III trial on the combination of radium-223 and enzalutamide in mCRPC [16] |
| The phase III DORA trial on the combination of radium-223 and docetaxel in mCRPC [17] | ||||
| Phase I trial on the combination of radium-223 and atezolizumab in mCRPC [18]; phase II trials on pembrolizumab [19] or sipuleucel-T [20] and radium-223 in mCRPC | ||||
| Phase Ib trial of radium-223 and niraparib (PARPi) in CRPC [21]; phase I/II trial of radium-223 and olaparib (PARPi) in mCRPC [22] | ||||
| 213Bi-PSMA-617 | PSMA-targeting small-molecule ligand conjugated with alpha-emitting radionuclide | 45.6 minutes [23] | 1 [23] | Molecular imaging and biochemical responses in a patient with mCRPC [24] |
| 225Ac-PSMA-617 | 10.0 days [25] | 4 [25] | Antitumor activity was observed in patients with mCRPC with xerostomia as dosing-limiting factor and reason of treatment discontinuation [26–28] | |
| 211At-PSMA-pentanedioic acid | 7.2 h [29] | 1 [29] | Significant tumor growth delay and improved survival were seen in preclinical prostate cancer xenograft model and mice bearing prostate cancer micrometastases [30] | |
| 227Th-PSMA-IgG1 | PSMA-targeting mAb conjugated with alpha-emitting radionuclide | 18.7 days [31] | 5 [31] | Preclinical antitumor activity and safety were observed in models of prostate cancer [32, 33]; phase I trial of 227Th-PSMA immune-conjugate in mCRPC [34] |
CRPC, castration-resistant prostate cancer; DSB, double-strand breaks; mAb, monoclonal antibody; mCRPC, metastatic CRPC; MoA, mechanism of action; PARPi, poly (ADP-ribose) polymerase inhibitor; PSA, prostate-specific antigen; PSMA, prostate-specific membrane antigen; TαT, targeted alpha therapy.
What is the proof of concept for TαT?
Mechanism of action of radium-223, the first-in-class TαT in PC
Radium-223 is the most-investigated TαT in clinical settings. Radium-223 is a calcium mimetic that is recruited to newly forming bone and induces an intense, local cytotoxic effect, affecting both cancer cells and the tumor microenvironment [8]. In murine tumor models, radium-223 suppresses tumor-induced osteoblastic activity, tumor growth, and pathological bone formation [8]. In PC xenograft models, radium-223 inhibits abnormal bone metabolic activity by reducing the number of bone-forming osteoblasts and obliterating bone-degrading osteoclasts [8]. In vitro studies suggest that radium-223 may enhance T-cell-mediated lysis of prostate, breast, and lung carcinoma cells through induction of immunogenic cell death [35, 36].
Clinical outcomes of radium-223 treatment
Radium-223 has provided clinical proof of concept that TαT is an effective treatment strategy for PC [5]. In phase III ALSYMPCA trial [37] of patients with bone-mCRPC and no known visceral metastases, radium-223 significantly extended overall survival (OS) [radium-223, 14.9 months; placebo, 11.3 months; hazard ratio, 0.70 (95% CI 0.58–0.83), P < 0.001] [7]. Radium-223 also provided clinically meaningful improvement in health-related quality of life (QoL) and prolonged the time to first symptomatic skeletal event (SSE) and subsequent SSEs [7, 13].
Despite these results, median times to increase in prostate-specific antigen (PSA) levels were similar among treatment arms [7]. While rising PSA has been used as a biomarker for increased tumor growth, it has not been proven to be a reliable biomarker for therapies that do not directly affect the AR pathway [38, 39]. Potential biomarkers for treatment response with radium-223, including alkaline phosphatase (ALP) and lactate dehydrogenase (LDH), are summarized in Table 2. Mutations in DNA repair pathway genes are included, as patients with these alterations may be particularly susceptible to radium-223 treatment [44–46].
Table 2.
Summary of potential biomarkers for treatment response with radium-223
| Potential biomarker | Rationale | Clinical evidence |
|---|---|---|
| Alkaline phosphatase (ALP) | ALP is a nonspecific marker of osteoblast activity [40] | Patients treated with radium-223 experienced significantly prolonged time to increase in tALP and a greater tALP response compared with placebo [7] tALP decline at 12 weeks after radium-223 was initiated correlated with longer OS, but did not meet statistical surrogacy requirements [41] |
| Lactate dehydrogenase (LDH) | LDH is a metabolic enzyme that participates in the glycolysis and gluconeogenesis pathways, important for tumor growth [42, 43] | LDH decline at 12 weeks after radium-223 was initiated correlated with longer OS, but did not meet statistical surrogacy requirements [41] Increased levels of LDH are associated with poor clinical outcomes in prostate cancer and aggressive phenotypes in patients with bone metastases [43] |
| CHEK2 (DNA repair gene) | Radium-223 emits alpha particles, which have high LET, resulting in clusters of DSBs in DNA. Therefore, patients with mCRPC with alterations in their DNA repair pathway genes may be particularly susceptible to radium-223 treatment [44–46] | Mutation corresponds to a higher risk of prostate cancer, but there is no clear familiar association [47] More than one patient who responded to radium-223 was found to have a mutation in this gene [44, 45] |
| BRCA2 (DNA repair gene) | Mutation corresponds to a higher risk of prostate cancer, with a familiar association [48] More than one patient who responded to radium-223 was found to have a mutation in this gene [44, 46] | |
| MRE11A (DNA repair gene) | A patient who responded to radium-223 was found to have a mutation in this gene [44] | |
| CHD1 (DNA repair gene) | Loss of CHD1 in prostate cancer results in increased sensitivity to DNA damaging agents [49] A patient who responded to radium-223 was found to have a mutation in this gene [44] | |
| SPOP (DNA repair gene) | The most commonly mutated gene in primary prostate cancer that serves to modulate DNA DSB repair [50] A patient who responded to radium-223 was found to have a mutation in this gene [44] |
tALP, total alkaline phosphate; OS, overall survival; CRPC, castration-resistant prostate cancer; mCRPC, metastatic CRPC; LET, light energy transfer; DSB, double-strand break.
Combining radium-223 with second-generation AR-targeted therapies
Radium-223 has 6 years of real-world evidence supporting its efficacy and safety in mCRPC [7, 13, 14]. Because abiraterone and enzalutamide have both been shown to improve progression-free survival and OS as a first-line treatment of mCRPC, and neither has overlapping toxicity with radium-223, the combinations of these agents with radium-223 are being investigated [51, 52]. In a retrospective analysis of an international, early access, open-label, single-arm, non-randomized trial including 696 patients with mCRPC, radium-223 used with initial and concurrent treatment with abiraterone or enzalutamide improved survival with no unexpected safety concerns [53]. In the eRADicAte study (NCT02097303), an investigator-initiated, phase II, prospective, single-arm study including 31 patients with mCRPC, radium-223 in combination with abiraterone plus prednisone (initiated <90 days before or <30 days after the first radium-223 treatment) conferred clinically meaningful improvements in QoL and pain, without unexpected adverse toxicities [54]. However, the phase III ERA 223 trial (NCT02043678) investigating radium-223 in concurrent combination with abiraterone plus prednisone/prednisolone raised safety concerns: more fractures (28.6% versus 11.4%, based on central radiological review) were observed in the combination arm versus the arm with abiraterone plus prednisone/prednisolone alone [55, 56]. It should be noted that 79% of the fractures in the combination arm were not at sites of bone metastasis and osteoporotic fractures were most common [57]. The fact that the combination therapy in ERA 223 was administered concomitantly may partially explain the difference in safety profile compared with the ALSYMPCA, eRADicAte, and REASSURE studies. If the combination therapy was instead sequential, this may have allowed the bone to recover from the impact of the normal hormonal agent, thus preventing radium-223 from having a further compounding effect on bone irradiation. Additional analysis of the ERA 223 data showed a lower fracture rate in both study arms in patients who received bone health agents (BHAs) than in those who did not [57], suggesting that the use of BHAs could mitigate the risk of bone fractures for all patients.
The US Food and Drug Administration evaluated the data of ERA 223 and added a warning statement on the use of radium-223 in combination with abiraterone acetate plus prednisone/prednisolone [5]. The Japanese Pharmaceuticals and Medical Devices Agency and Health Canada also issued a similar precaution [58, 59]. However, the European Medicines Agency (EMA) restricted the use of radium-223 to patients who have progressed following two prior systemic treatments for bone-mCRPC or who are ineligible for other treatments. The EMA also issued a contraindication for use in combination with abiraterone acetate plus prednisone/prednisolone [60]. Radium-223 is also being studied in combination with enzalutamide in ongoing phase II (NCT02225704) and phase III trials (NCT02194842) [16, 61]. The initial results of the phase III PEACE III (NCT02194842) trial show that, while fractures increased when radium-223 was added to enzalutamide, this risk was nearly abolished with continuous use of BHAs such as zoledronic acid [62]. After the trial was amended to make BHAs mandatory, no excess fractures were observed in the radium-223 arm [62].
Can we personalize treatment and optimize clinical outcomes of TαT through the addition of dosimetry considerations?
Is TαT dosimetry feasible?
One advantage of TαT compared with other systemic advanced PC modalities is that it enables collection of pharmacokinetic data by imaging in a clinical study setting, which can be used for dosimetry calculations [63]. Radiation dosimetry provides a logical basis for understanding the biological effects of radiation quantities and comparing the effects of different radiation-based treatments [64]. Owing to uptake heterogeneity of alpha particle emitters on a small scale and their potent cytotoxicity, macroscopic dosimetry at the tumor and organ levels is insufficient, and thus microdosimetry that takes into consideration the activity distribution as a function of time at the cellular and subcellular levels should be further developed for more-precise dosimetry calculations [64]. Currently, several small-scale modeling and microdosimetric approaches are being developed, with potential application to clinical imaging and dosimetry [63, 65–67].
Quantification of radium-223 uptake
In vivo dosimetry requires in vivo radium-223 uptake quantification. The low gamma-radiation yield generated through the radium-223 decay pathway allows quantitative imaging of radium-223 therapy and individualized biodistribution studies, although long image acquisition times limit the widespread use of this option [68–70].
Recent evidence has demonstrated a potential correlation between absorbed dose and local lesion response to TαT [69]. A study including nine patients with bone metastases reported that the lesion uptake quantification of radium-223 was significantly correlated with that of technetium-99m-methylene diphosphonate [70]. This introduces the possibility of using conventional radiolabeled diphosphonate scintigraphy to contour bone lesions and define lesion extent in a more reliable manner. Overlapping radium-223 images of the region of interest allow for more accurate radium-223 uptake quantifications for dosimetry and for the assessment of local response of bone metastases to the therapy (i.e. lesion geometric extent and uptake) [70].
Dosimetry and clinical outcomes
The feasibility of TαT dosimetry (e.g. in vivo quantification of radium-223 uptake) [70] might introduce the possibility of clinically investigating the value of personalized treatment and the outcome of radium-223 in relation to the absorbed dose. Recently, in a study including five patients with mCRPC, a correlation between fluorine-18-fluoride (18F-fluoride) and radium-223 uptake was observed [69]. This study, to our knowledge, represents the first observed correlation between absorbed dose and local lesion response of TαT, suggesting that quantitative 18F-fluoride imaging might predict radium-223 uptake and, potentially, treatment response. More robust studies using more advanced imaging techniques are needed to confirm these preliminary findings, explore treatment optimization, and investigate the potential of dosimetry to predict clinical outcomes [69, 70].
In overall survival analyses of the number of radium-223 injections in patients with mCRPC in the US expanded access program, OS was longer in patients receiving five or six injections versus those receiving one to four injections [71]. However, a post hoc analysis of the ALSYMPCA trial showed similar findings with five or six injections of placebo versus one to four injections of placebo [72]. Further study is needed to determine the dosimetry of multiple cycles of radium-223 incorporated in the bone, to assess its impact on biological activity and to investigate the potential of radium-223 dosimetry for treatment optimization and individualized treatment plans [68]. A randomized trial (NCT02023697) compared SSE-free survival (SSE-FS) with different radium-223 regimens in 391 patients with mCRPC and bone metastases: standard activity (55 kBq/kg q4w for 6 cycles), high activity (88 kBq/kg q4w for up to 6 cycles), and extended standard activity (55 kBq/kg q4w for up to 12 cycles) [73]. No statistically significant differences in SSE-FS were observed between the standard activity and either the high or extended standard activity. However, an increased incidence of grade ≥3 treatment-emergent AEs were observed in the latter groups. This result supports the approved radium-223 regimen [73] and also supports the interest of dosimetry calculation to be used to individualize the ‘optimal’ activity regimen, as increased- or extended-activity regimens may not necessarily improve efficacy.
The possibility of evaluating TαT distributions after injection and predicting absorbed radiation doses and exposure within tumor and normal tissues introduces the potential for optimizing treatment to maximize antitumor effect while sparing normal tissues [63].
What is the future of TαT in PC?
Radium-223-based radioimmunoconjugates were challenging to develop due to the difficulties associated with stable chelation and conjugation of radium-223 to biomolecules [74, 75]. However, combination of radium-223 with other treatments and at earlier disease stages may broaden its clinical utility [76]. For example, combination therapy of radium-223 with other antitumor agents [e.g. chemotherapy, AR-targeting agents, DNA damage response (DDR) inhibitors, and immuno-oncology drugs] for PC may be a viable treatment approach. Clinical trials are ongoing to assess these combinations (Tables 1 and 3). Additionally, certain alpha emitters can be targeted to tumors by conjugating them to small molecules (e.g. actinium-225–PSMA-617 and bismuth-213–PSMA-617) [24, 26, 78] or radioimmunotherapy [e.g. thorium-227 conjugated to a monoclonal antibody (mAb)] that bind to specific antigens (e.g. PSMA) on tumor cells [3, 26]. Many preclinical and clinical studies are ongoing to investigate the potential of these therapeutic options, which may expand the utility of TαT in the management of PC.
Table 3.
Clinical trials investigating combination therapies of TαT and immuno-oncology agents or DDR inhibitors
| Agents in combination with TαT | Therapeutic indication | Phase | ClinicalTrials.gov identifier |
|---|---|---|---|
| Immuno-oncology agents in combination with radium-223 | |||
| Atezolizumab (PD-L1 mAb) | mCRPC with disease progression after androgen pathway inhibitor treatment | I | NCT02814669 [77] |
| Atezolizumab (PD-L1 mAb) | Urothelial carcinoma with bone metastases and disease progression after platinum-based chemotherapy | I | NCT03208712 [18] |
| Pembrolizumab (PD-1 receptor mAb) | mCRPC | II | NCT03093428 [19] |
| Sipuleucel-T (autologous cellular immunotherapy Targeting PAP) | Asymptomatic or minimally symptomatic bone-metastatic CRPC | II | NCT02463799 [20] |
| DDR inhibitors in combination with radium-223 | |||
| Niraparib (PARPi) | Bone-metastatic CRPC | Ib | NCT03076203 [21] |
| Olaparib (PARPi) | Bone-metastatic CRPC | I/II | NCT03317392 [22] |
ATRi, ataxia telangiectasia and Rad3-related protein inhibitor; CRPC, castration-resistant prostate cancer; DDR, DNA damage response; PAP, prostatic acid phosphatase; PARPi, poly(ADP-ribose) polymerase inhibitor; mAb, monoclonal antibody; mCRPC, metastatic CRPC; TαT, targeted alpha therapy.
How to develop combination therapies which harness the MoA of TαT?
In addition to combining radium-223 with best SoC (e.g. local external-beam radiation therapy, corticosteroids, antiandrogens, estrogens, estramustine, or ketoconazole) [5], chemotherapy (e.g. docetaxel in the phase III DORA trial [NCT03574571] [17]) and other antiandrogens (e.g. enzalutamide in the phase III PEACE trial [16]), the MoA of radium-223 provides a basis for investigating its potential combination with other therapies, such as immuno-oncology agents and DDR inhibitors.
Combining TαT with immuno-oncology agents
In addition to cytotoxicity caused by the production of difficult-to-repair clusters of DNA DSBs (targeted effects), TαT may also produce off-target effects, such as systemic bystander (abscopal) effects that may be observed at distances from the target [3, 4, 64, 79, 80]. These systemic bystander effects are potentially mediated by the immune response [79] and could be exploited by appropriate treatment combinations. Combining alpha-emitted radiation and immunostimulatory therapies, such as antiprogrammed death receptor-(ligand)1 (PD-1/PD-L1) or anticytotoxic T-lymphocyte-associated protein 4 (CTLA-4) mAbs, has been shown to reduce nonirradiated metastatic tumor size and of metastatic spread [79]. However, randomized trials to confirm these preliminary findings are needed. Radium-223 has also been demonstrated to sensitize breast, prostate, and lung carcinoma cell lines to cytotoxic T-lymphocyte-mediated lysis by the induction of immunogenic cell death [35]. In in vitro cancer model systems, radium-223 can increase expression of major histocompatibility complex-I, induce endoplasmic reticulum stress response in tumor cells, and upregulate the expression of calreticulin and induce its surface translocation. These events ultimately augment tumor sensitivity to T-lymphocyte-mediated lysis [35]. The ability of TαT to induce immunogenic modulation and enhance T-cell-mediated tumor cell lysis across multiple human tumor cell lines suggests wide-ranging applicability and a potential for combination with immuno-oncology agents [35], such as atezolizumab [81], pembrolizumab [82], and sipuleucel-T [83]. Given that TαT may modulate tumor phenotype and enhance T-cell lysis, it has been hypothesized that the immunostimulatory environment created by TαT may potentiate the antitumor activity of these immunotherapies [35]. Given the non-overlapping safety profiles of these therapies and their distinctive MoAs from radium-223, it is potentially advantageous to combine TαT and immuno-oncology agents. Currently, phase I trials examining the combination of atezolizumab and radium-223 are ongoing to investigate its potential in treating mCRPC with disease progression after androgen pathway inhibitor treatment (NCT02814669) [77], and in treating urothelial carcinoma with bone metastases and disease progression after platinum-based chemotherapy (NCT03208712) [18] (Table 3). NCT02814669 includes an adaptive study design with a cohort phase to evaluate the safety and tolerability of the combination with concurrent initiation or a staggered dosing (atezolizumab initiated at the beginning of cycle 2 or 3 of radium-223) before a potential randomization phase [77]. Pembrolizumab in combination with radium-223 is under investigation in a phase II trial in mCRPC (NCT03093428) [19]. Sipuleucel-T combined with radium-223 is being investigated in a phase II trial in men with asymptomatic or minimally symptomatic bone-mCRPC (NCT02463799) [20] (Table 3).
Combining TαT and DDR inhibitors
DDR pathways comprise a network of signaling pathways that maintain genomic stability and cell viability by orchestrating the detection and repair of DNA damage with transient cell cycle arrest [84]. As mentioned above and shown in Table 2, several patients with alterations in DDR pathway genes have been shown to respond to radium-223 treatment [44–46]. Ataxia-telangiectasia mutated (ATM) and ataxia telangiectasia and Rad3-related protein (ATR) kinases are two master regulators of the DDR signaling pathway. ATM is primarily activated by DSBs, whereas ATR responds to a broad spectrum of DNA damage, including DSBs, lesions from interference with DNA replication, and elevated oncogene-induced replication stress [85, 86]. Poly(ADP-ribose) polymerase (PARP) also plays a vital role in DDR and can be activated by a wide range of stimuli (e.g. single-strand breaks, DSBs, and unusual DNA conformations) [87]. Defective PARP functions can lead to genomic instability and cell death, providing a basis for investigating PARP inhibitors (PARPis) in anticancer therapies [87]. It has been hypothesized that combining TαT and ATR inhibitors (ATRis) or PARPis may potentiate synthetic lethality and antitumor effects [76].
TαT and DDR inhibitor combination therapy has been explored in different tumor types [88]. For example, another TαT—thorium-227 conjugate targeting mesothelin, a surface glycoprotein overexpressed in multiple cancer types (e.g. pancreatic, ovarian, and lung cancers and mesothelioma) [89]—has demonstrated synergistic antitumor effects when combined with ATRi or PARPi in preclinical cancer models [88, 90]. These synergistic effects [88, 90] suggest that inhibiting DDR pathways may sensitize cancer cells to TαT and warrant clinical investigation. Currently, multiple clinical studies investigating the potential of combining TαT and DDR inhibitors in patients with mCRPC and bone metastases are ongoing, such as the phase Ib trial of radium-223 and the PARPi niraparib (NCT03076203) [21] and the phase I/II trial of radium-223 and olaparib (NCT03317392) [22] (Table 3).
PSMA—an emerging and promising target for PC therapy
PSMA is a type II transmembrane protein that is expressed at low levels in a variety of tissues (e.g. the prostate, brain, small intestine, salivary glands, and kidneys) and is overexpressed in primary and metastatic PC cells [78, 91–94]. Although 5–10% of men with PC do not express PSMA, and PSMA may not be expressed uniformly within the tumor [95, 96], high levels of expression of PSMA are observed across the spectrum of aggressive and poorly differentiated PC [91–94, 97], making PSMA a promising therapeutic target [98]. PSMA targeting allows the creation of novel treatment options that would be complementary to existing therapies [99] (Figure 1).
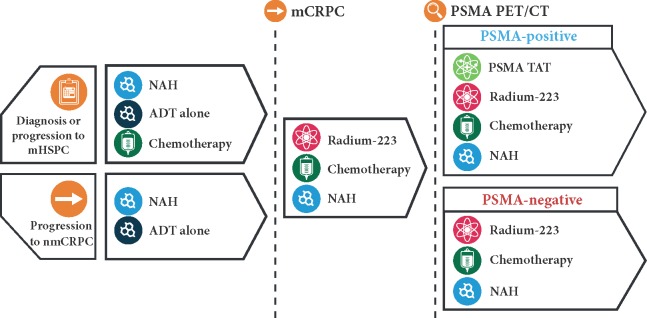
Figure 1.
PSMA TαT is complementary to existing therapies for prostate cancer. Depicted is a suggested clinical sequencing, in which PSMA TαTs would be considered after a failure of a first-line therapy for mCRPC and PSMA expression was confirmed positive. ADT, androgen deprivation therapy; CT, computed tomography; mHSPC, metastatic hormone-sensitive prostate cancer; mCRPC, metastatic castration-resistant prostate cancer; NAH, novel antihormonal; nmCRPC, non-metastatic castration-resistant prostate cancer; PET, positron emission tomography; PSMA, prostate-specific membrane antigen; TAT, targeted alpha therapy.
Investigational agents targeting PSMA can be labeled with different radionuclides, including alpha (e.g. actinium-225 and thorium-227) and β emitters (e.g. lutetium-177 [177Lu] and iodine-131). These agents can be targeted to PSMA via different vehicles, such as antibodies (e.g. thorium-227–labeled anti-PSMA IgG1) and small-molecule ligands (e.g. 225Ac-PSMA-617). While antibodies bind PSMA with high affinity, they have a long serum half-life and greater potential for bone marrow and liver toxicity. Small-molecule ligands have a favorable biodistribution but have risk of off-target toxicity in the kidneys, salivary glands, and lacrimal glands [4, 78, 100]. Other PSMA-targeted therapies under investigation include vaccines and cell therapies.
Among investigational PSMA-targeted therapeutics, β particle-emitting radionuclides targeting PSMA include 177Lu-J591 and 177Lu-PSMA-617. Both agents demonstrated clinical activity in mCRPC [101–108]. A single-arm, single center, phase II study investigating 177Lu-PSMA-617 in mCRPC found that 17 out of 30 patients with mCRPC achieved a ≥50% PSA decline, with a low-toxicity profile and improvement in QoL [101]. A multicenter randomized phase II trial comparing 177Lu-PSMA-617 with cabazitaxel chemotherapy (NCT03392428) is ongoing [109]. The phase III VISION trial investigating PSMA-617 coupled to Lu-177 in mCRPC is also ongoing (NCT03511664) [110]. However, it seems some patients may not respond to 177Lu radioligand therapy, as 9 out of the 30 patients saw no PSA response [111]. Such patients may respond to an alpha particle-emitting radionuclide, such as actinium-225–PSMA-617 (225Ac-PSMA-617) (Figure 2) [26]. Alpha particles are characterized by high LET and short travel distances; therefore, PSMA-targeted alpha therapies may cause potent and local cytotoxicity to PSMA-expressing cells [3, 4]. Alpha-labeled bisphosphonates, such as 225Ac-EDTMP or 225Ac-zolendronate, may also be considered as they accumulate in the bone microenvironment of osteoblastic lesions [112, 113].
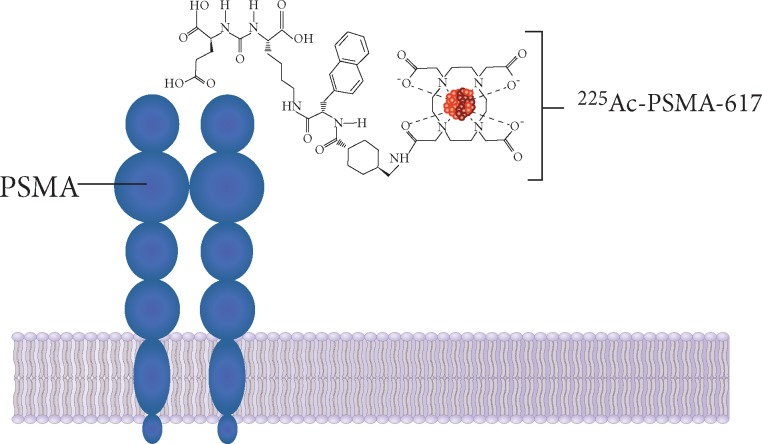
Figure 2.
PSMA as a potential therapeutic target for prostate cancer. PSMA is a transmembrane protein overexpressed in prostate cancer. The extracellular domain of PSMA is internalized after ligand binding, allowing intracellular delivery of conjugated therapeutic agents, such as actinium-225. PSMA, prostate-specific membrane antigen.
What PSMA–TαT agents are under investigation for PC?
PSMA–TαTs under investigation
Investigational TαTs targeting PSMA include bismuth-213–PSMA-617 (213 Bi-PSMA-617) [24], 225Ac-PSMA-617 [95], astatine-211–PSMA–pentanedioic acid (211At-PSMA-pentanedioic acid) [30], and thorium-227-labeled anti-PSMA IgG1 (227Th-PSMA-IgG1) [32]. The first-in-human treatment concept with 213 Bi-PSMA-617 was demonstrated in a patient with mCRPC who had progressed on conventional therapy. Molecular imaging (re-staging with 68Ga-PSMA PET/CT) and biochemical (decline in PSA level from 237 to 43 μg/l) responses were observed in this patient after 11 months [24].
Initial clinical experience with targeted actinium conjugates
225Ac-PSMA-617 was investigated in an observational, single-center study as salvage therapy among patients with mCRPC who had exhausted all approved treatment options and had a PSMA-positive tumor phenotype [26]. In the preliminary report including two patients who received ≥8 prior therapies, 225Ac-PSMA-617 (100 kBq/kg bimonthly) resulted in a PSA decline below the measurable level and complete imaging response in both cases [26]. In the full report including 14 patients, PSA response was observed in 75% of patients, and promising antitumor activity was detected in brain, bone, liver, and pulmonary lesions. However, persistent xerostomia was regularly reported with a dose of 225Ac-PSMA-617 ≥100 kBq/kg per treatment cycle and was considered intolerable when the dose exceeded 150 kBq/kg. All-grade hematological AEs occurred in six patients (42.9%), and xerostomia was observed in eight patients (57.1%). With xerostomia being the dose-limiting factor, 100 kBq/kg was considered the maximum tolerable dose. Based on these findings, 100 kBq/kg 225Ac-PSMA-617 is tolerable and presents promising antitumor activity [27]. Antitumor activity of 225Ac-PSMA-617 was further demonstrated in 40 patients with mCRPC [28]. Although 225Ac-PSMA-617 was generally well tolerated, xerostomia remained the main reason for toxicity-associated treatment discontinuation [28]. Clinical trials with larger patient populations are needed to further investigate the efficacy, safety, and patient selection for 225Ac-PSMA-617.
Preclinical development of targeted astatine and thorium conjugates
211At-PSMA-pentanedioic acid has demonstrated significant tumor growth delay in a PC xenograft model. 211At-PSMA-pentanedioic acid also showed preclinical efficacy by significantly improving survival in mice bearing PC micrometastases. These results expand the potential utility of PSMA–TαTs from macrometastases to micrometastases of PC, including those not visible by traditional imaging techniques [30]. Another PSMA–TαT under preclinical investigation is 227Th-PSMA-IgG1, a targeted thorium conjugate delivering the alpha particle-emitting radionuclide thorium-227 to PC cells through a conjugated mAb (IgG1) specific for PSMA (Figure 3) [32].
Figure 3.
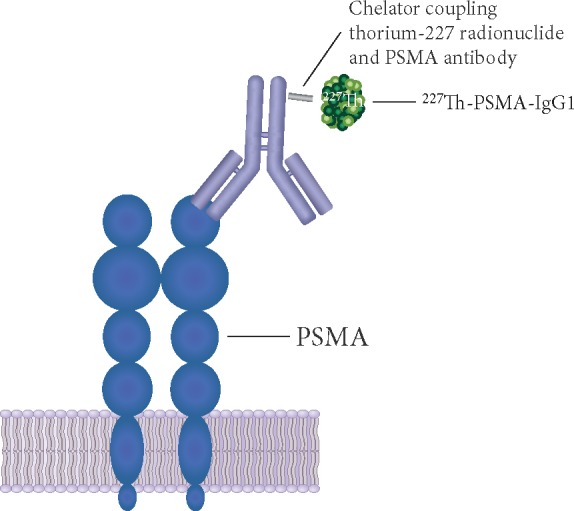
Schematic representation of 227Th-PSMA-IgG1. An N-hydroxysuccinimide-activated 3,2-hydroxypyridinone chelator is coupled to the PSMA antibody and radiolabeled with the thorium-227 radionuclide. PSMA, prostate-specific membrane antigen.
Targeted thorium conjugates are next-generation TαTs that integrate a targeting molecule (e.g. mAb) stably bound to a chelator, and the alpha particle-emitting radioisotope thorium-227 (227Th) [114–116] (Figure 3). Thorium-227 is a promising candidate for TαT for its relatively long half-life (18.7 days) and favorable chemical properties that allow stable chelation, preparation, and administration [31, 116]. In addition, thorium-227 decays via alpha particle emission to radium-223, which subsequently decays to stable lead-207; during this process, five alpha particles are released [31].
Preclinical studies showed that 227Th-PSMA-IgG1 was mainly distributed in tumors, followed by blood, with lower levels of distribution in other tissues (e.g. spleen, kidneys, and liver), indicating highly specific delivery of the alpha therapy to tumors. In vitro studies demonstrated that 227Th-PSMA-IgG1 induced tumor cell death, difficult-to-repair DNA DSBs, and cell cycle arrest in G2/M phase of PC cells. 227Th-PSMA-IgG1 also induced immunogenic cell death of PC cells. These in vitro studies suggest that 227Th-PSMA-IgG1 may cause cytotoxicity through inducing DNA DSBs in PC cells and potentiation of tumor sensitivity to immunogenic lysis [32].
In vivo studies also demonstrated antitumor activity of 227Th-PSMA-IgG1 in several PC xenograft models (LNCaP-luc, C4-2, and LAPC-4) and its tolerability (i.e. no significant body weight loss). Tumor growth inhibition was observed with a single dose of 227Th-PSMA-IgG1 in both PC xenograft models with moderate (22Rv1) and high (MDA-PCa-2b) PSMA expression. In a bone-metastatic PC model, a single dose of 227Th-PSMA-IgG1 reduced tumor burden, abnormal osteoblastic bone growth, and serum PSA levels [32]. Potent dose-dependent antitumor activity of 227Th-PSMA-IgG1 was observed in both hormone-sensitive (ST1273) and enzalutamide-resistant (KuCap-1) patient-derived PC xenograft models [33].
Compelling preclinical evidence [32, 33] supports clinical investigation of 227Th-PSMA-IgG1 for PC, including mCRPC. Further development of other targeted thorium conjugates as precision anticancer modalities to deliver local alpha therapy to other tumor types are ongoing [117–119].
Discussion
Conclusions
TαT has the potential to deliver high-energy alpha radiation selectively to cancer cells and the tumor microenvironment in order to control cancer while minimizing systemic toxicity. The survival benefit and long-term safety profile of radium-223 demonstrated in clinical trials and clinical practice has provided both the proof of concept and real world experience for TαT as a viable therapeutic approach for cancer. Dosimetry calculation and pharmacokinetic measurements of TαT may drive precision medicine-based treatment in a more cost-effective and efficient manner. Potential combination of TαT with chemotherapy, AR-targeting agents, DDR inhibitors, or immuno-oncology drugs may enhance the antitumor activity of each individual therapy through synergistic or complementary interactions, which may expand the clinical utility of TαT in PC. Additionally, PSMA represents a promising therapeutic target for PC. PSMA-targeted thorium and actinium conjugates are under investigation as next-generation, precision anticancer modalities that deliver local alpha therapy to PSMA-expressing cancer cells.
Acknowledgements
Editorial assistance: We thank Yue Liu, PhD (Chameleon Communications International with funding from Bayer) for editorial assistance in the preparation of this manuscript.
Funding
This work was supported by Bayer, Whippany, New Jersey, USA. Bayer provided review for medical accuracy, to ensure the balance of statements, and to confirm that content was correctly referenced during the development of the manuscript. The authors would like to emphasize that this manuscript was written independently and is their own intellectual work. No grant number is applicable.
Disclosure
JEG received honoraria from Bayer Healthcare for scientific advice.
JMO’S received fees from advisory boards and speakers’ bureau from Astellas, Bayer, Janssen, and Sanofi and research funding from Bayer.
GDV received funding from Bayer Healthcare for participation in scientific congresses.
MP participated in the Targeted Alpha Therapy Prostate Working Group as a consultant of Bayer.
VL is an advisory board member and received speaker honoraria from Bayer AG.
WG received speaker fees from Bayer and MSD; consultant fees from Bristol-Myers Squibb, Astellas, Bayer, Sanofi, and Amgen for participation in advisory boards; and research grants from Bayer, Astellas, and Janssen-Cilag.
MH received consultancy fees from Bayer, ITM, and Janssen; grants from Siemens, Eli Lilly, Roche, BMS, Ipsen, ITM, and EZAG; and payment for lectures from Siemens, Bayer Healthcare, and GE Healthcare.
MO received research funding from Astellas and honoraria from Astellas, Sanofi, Janssen, AstraZeneca, and Takeda.
NS received consulting fees from Amgen, Bayer, BMS, Astellas, AstraZeneca, Bayer, Dendreon, Ferring, Janssen, Merck, Pfizer, and Tolmar, and for speakers’ bureau from Janssen, Bayer, and Dendreon.
CP received consulting fees and funding for research activities with Bayer AG and consulting fees from Janssen and AAA Pharmaceutical.
OS has stock and other ownership interests in Eli Lilly, GlaxoSmithKline, and Noria; received consulting fees from Bayer, Bellicum Pharmaceuticals, Johnson & Johnson, Sanofi, AstraZeneca, Dendreon, Endocyte, Constellation Pharmaceuticals, Advanced Accelerator Applications, Pfizer, Bristol-Myers Squibb, Celgene, Bavarian Nordic, OncoGenex, EMD Serono, Astellas Pharma, and Progenics; received research funding from Bayer, Johnson & Johnson, Sanofi, Endocyte, Innocrin Pharma, Merck, and Invitae; provided expert testimony for Sanofi; and received travel/accommodations/expenses support from Bayer, Johnson & Johnson, Sanofi, AstraZeneca, and Progenics.
Contributor Information
Targeted Alpha Therapy Prostate Working Group:
G De Vincentis, W Gerritsen, J E Gschwend, M Hacker, V Lewington, J M O’Sullivan, M Oya, M Pacilio, C Parker, N Shore, and O Sartor
References
- 1. Volkert WA, Goeckeler WF, Ehrhardt GJ, Ketring AR.. Therapeutic radionuclides: production and decay property considerations. J Nucl Med 1991; 32(1): 174–185. [PubMed] [Google Scholar]
- 2. Kassis AI, Adelstein SJ.. Radiobiologic principles in radionuclide therapy. J Nucl Med 2005; 46(Suppl 1): 4S–12S. [PubMed] [Google Scholar]
- 3. Pouget JP, Navarro-Teulon I, Bardies M. et al. Clinical radioimmunotherapy—the role of radiobiology. Nat Rev Clin Oncol 2011; 8(12): 720–734. [DOI] [PubMed] [Google Scholar]
- 4. Dekempeneer Y, Keyaerts M, Krasniqi A. et al. Targeted alpha therapy using short-lived alpha-particles and the promise of nanobodies as targeting vehicle. Expert Opin Biol Ther 2016; 16(8): 1035–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xofigo® [prescribing information]. Bayer HealthCare Pharmaceuticals Inc., Wayne, NJ 2018.
- 6. Henriksen G, Fisher DR, Roeske JC. et al. Targeting of osseous sites with alpha-emitting 223Ra: comparison with the beta-emitter 89Sr in mice. J Nucl Med 2003; 44(2): 252–259. [PubMed] [Google Scholar]
- 7. Parker C, Nilsson S, Heinrich D. et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013; 369(3): 213–223. [DOI] [PubMed] [Google Scholar]
- 8. Suominen MI, Fagerlund KM, Rissanen JP. et al. Radium-223 inhibits osseous prostate cancer growth by dual targeting of cancer cells and bone microenvironment in mouse models. Clin Cancer Res 2017; 23(15): 4335–4346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.METASTRON™ (Strontium-89 Chloride Injection) [Prescribing Information]. GE Healthcare Ltd 2018.
- 10. Sartor O, Reid RH, Hoskin PJ. et al. Samarium-153-lexidronam complex for treatment of painful bone metastases in hormone-refractory prostate cancer. Urology 2004; 63(5): 940–945. [DOI] [PubMed] [Google Scholar]
- 11.Quadramet® [prescribing information]. Lantheus Medical Imaging, Inc. 2018.
- 12. Porter AT, McEwan AJ, Powe JE. et al. Results of a randomized phase-III trial to evaluate the efficacy of strontium-89 adjuvant to local field external beam irradiation in the management of endocrine resistant metastatic prostate cancer. Int J Radiat Oncol Biol Phys 1993; 25(5): 805–813. [DOI] [PubMed] [Google Scholar]
- 13. Sartor O, Coleman R, Nilsson S. et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. Lancet Oncol 2014; 15(7): 738–746. [DOI] [PubMed] [Google Scholar]
- 14. Parker C, Finkelstein SE, Michalski JM. et al. Efficacy and safety of radium-223 dichloride in symptomatic castration-resistant prostate cancer patients with or without baseline opioid use from the Phase 3 ALSYMPCA trial. Eur Urol 2016; 70(5): 875–883. [DOI] [PubMed] [Google Scholar]
- 15. Bruland OS, Nilsson S, Fisher DR, Larsen RH.. High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 2006; 12(20 Pt 2): 6250s–6257s. [DOI] [PubMed] [Google Scholar]
- 16.ClinicalTrials.gov. (NCT02194842) Phase III Radium 223 mCRPC-PEACE III (PEACE III). https://clinicaltrials.gov/ct2/show/NCT02194842 (20 June 2019, date last accessed).
- 17.ClinicalTrials.gov. (NCT03574571) Phase III Trial of Docetaxel vs. Docetaxel and Radium-223 for Metastatic Castration-Resistant Prostate Cancer (mCRPC). https://clinicaltrials.gov/ct2/show/NCT03574571 (31 January 2019, date last accessed).
- 18.ClinicaTrials.gov. (NCT03208712) Radium-223 and Atezolizumab in Patients With Urothelial Carcinoma With Bone Metastases Who Have Had Disease Progression After Platinum-Based Chemotherapy. https://clinicaltrials.gov/ct2/show/NCT03208712 (14 August 2018, date last accessed).
- 19.ClinicalTrials.gov. (NCT03093428) Study Evaluating the Addition of Pembrolizumab to Radium-223 in mCRPC. https://clinicaltrials.gov/ct2/show/NCT03093428 (12 September 2018, date last accessed).
- 20.ClinicalTrials.gov. (NCT02463799) Study of Sipuleucel-T W/or W/O Radium-223 in Men With Asymptomatic or Minimally Symptomatic Bone-MCRPC. https://clinicaltrials.gov/ct2/show/NCT02463799 (14 August 2018, date last accessed).
- 21.ClinicalTrials.gov. (NCT03076203) Phase IB Trial of Radium-223 and Niraparib in Patients With Castrate Resistant Prostate Cancer (NiraRad). https://clinicaltrials.gov/ct2/show/NCT03076203? term=NCT03076203&rank=1 (14 August 2018, date last accessed).
- 22.ClinicalTrials.gov. (NCT03317392) Olaparib and Radium Ra 223: Dichloride in Treating Men With Metastatic Castration-Resistant Prostate Cancer That Has Spread to the Bone. https://clinicaltrials.gov/ct2/show/NCT03317392? term=NCT03317392&rank=1 (12 September 2018, date last accessed).
- 23. Rosenblat TL, McDevitt MR, Mulford DA. et al. Sequential cytarabine and alpha-particle immunotherapy with bismuth-213-lintuzumab (HuM195) for acute myeloid leukemia. Clin Cancer Res 2010; 16(21): 5303–5311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sathekge M, Knoesen O, Meckel M. et al. (213)Bi-PSMA-617 targeted alpha-radionuclide therapy in metastatic castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44(6): 1099–1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brechbiel MW. Targeted alpha-therapy: past, present, future? Dalton Trans 2007; (43): 4918–4928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kratochwil C, Bruchertseifer F, Giesel FL. et al. 225Ac-PSMA-617 for PSMA-targeted alpha-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med 2016; 57(12): 1941–1944. [DOI] [PubMed] [Google Scholar]
- 27. Kratochwil C, Bruchertseifer F, Rathke H. et al. Targeted alpha therapy of mCRPC with 225Actinium-PSMA-617: dosimetry estimate and empirical dose finding. J Nucl Med 2017; 58(10): 1624–1631. [DOI] [PubMed] [Google Scholar]
- 28. Kratochwil C, Bruchertseifer F, Rathke H. et al. Targeted alpha-therapy of metastatic castration-resistant prostate cancer with (225)Ac-PSMA-617: swimmer-plot analysis suggests efficacy regarding duration of tumor control. J Nucl Med 2018; 59(5): 795–802. [DOI] [PubMed] [Google Scholar]
- 29. Guerard F, Gestin JF, Brechbiel MW.. Production of [211At]-astatinated radiopharmaceuticals and applications in targeted alpha-particle therapy. Cancer Biother Radiopharm 2013; 28: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kiess AP, Minn I, Vaidyanathan G. et al. (2S)-2-(3-(1-carboxy-5-(4-211At-astatobenzamido)pentyl)ureido)-pentanedioic acid for PSMA-targeted alpha-particle radiopharmaceutical therapy. J Nucl Med 2016; 57(10): 1569–1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Heyerdahl H, Abbas N, Brevik EM. et al. Fractionated therapy of HER2-expressing breast and ovarian cancer xenografts in mice with targeted alpha emitting 227Th-DOTA-p-benzyl-trastuzumab. PLoS One 2012; 7(8): e42345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hammer S, Larssen A, Ellingsen C. et al. Preclinical pharmacology of the PSMA-targeted thorium-227 conjugate PSMA-TTC: a novel targeted alpha therapeutic for the treatment of prostate cancer [abstract]. Cancer Res 2017; 77(Suppl 13): Abstr 5200. http://cancerres.aacrjournals.org/content/77/13_Supplement/5200 (14 August 2018, date last accessed). [Google Scholar]
- 33. Hammer S, Hagemann UB, Zitzmann-Kolbe S. et al. Preclinical activity of PSMA-TTC, a targeted alpha therapeutic in patient-derived prostate cancer models [abstract]. Cancer Res 2018; 78(Suppl 13): Abstr 844. http://cancerres.aacrjournals.org/content/78/13_Supplement/844 (14 August 2018, date last accessed). [Google Scholar]
- 34.ClinicalTrials.gov. (NCT03076203) Phase IB Trial of Radium-223 and Niraparib in Patients With Castrate Resistant Prostate Cancer (NiraRad). https://clinicaltrials.gov/ct2/show/NCT03076203 (20 June 2019, date last accessed).
- 35. Malamas AS, Gameiro SR, Knudson KM, Hodge JW.. Sublethal exposure to alpha radiation (223Ra dichloride) enhances various carcinomas' sensitivity to lysis by antigen-specific cytotoxic T lymphocytes through calreticulin-mediated immunogenic modulation. Oncotarget 2016; 7(52): 86937–86947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim JW, Shin MS, Kang Y. et al. Immune analysis of radium-223 in patients with metastatic prostate cancer. Clin Genitourin Cancer 2018; 16(2): e469–e476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.ClinicalTrials.gov. (NCT00699751) A Phase III Study of Radium-223 Dichloride in Patients With Symptomatic Hormone Refractory Prostate Cancer With Skeletal Metastases (ALSYMPCA). https://clinicaltrials.gov/ct2/show/NCT00699751? term=NCT00699751&rank=1 (14 August 2018, date last accessed).
- 38. Tai-Lung Cha TT-L, Vogelzang NJ, Huang C-Y. et al. Optimal usage of radium-223 in metastatic castration-resistant prostate cancer. J Formosan Med Assoc 2017; 116: 825–836. [DOI] [PubMed] [Google Scholar]
- 39. Heinrich D, Bektic J, Bergman AM. et al. The contemporary use of radium-223 in metastatic castration-resistant prostate cancer. Clin Genitourin Cancer 2018; 16(1): e223–e231. [DOI] [PubMed] [Google Scholar]
- 40. Cook RJ, Coleman R, Brown J. et al. Markers of bone metabolism and survival in men with hormone-refractory metastatic prostate cancer. Clin Cancer Res 2006; 12(11): 3361–3367. [DOI] [PubMed] [Google Scholar]
- 41. Sartor O, Coleman RE, Nilsson S. et al. An exploratory analysis of alkaline phosphatase, lactate dehydrogenase, and prostate-specific antigen dynamics in the phase 3 ALSYMPCA trial with radium-223. Ann Oncol 2017; 28(5): 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Feng Y, Xiong Y, Qiao T. et al. Lactate dehydrogenase A: a key player in carcinogenesis and potential target in cancer therapy. Cancer Med 2018; 7(12): 6124–6136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Felgueiras J, Silva JV, Fardilha M.. Prostate cancer: the need for biomarkers and new therapeutic targets. J Zhejiang Univ Sci B 2014; 15: 16–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Ramos JD, Mostaghel EA, Pritchard CC, Yu EY.. DNA repair pathway alterations in metastatic castration-resistant prostate cancer responders to radium-223. Clin Genitourin Cancer 2018; 16(2): 106-e110. [DOI] [PubMed] [Google Scholar]
- 45. Isaacsson Velho P, Qazi F, Hassan S. et al. Efficacy of radium-223 in bone-metastatic castration-resistant prostate cancer with and without homologous repair gene defects. Eur Urol 2019; 76(2): 170–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Steinberger AE, Cotogno P, Ledet EM. et al. Exceptional duration of radium-223 in prostate cancer with a BRCA2 mutation. Clin Genitourin Cancer 2017; 15(1): e69–e71. [DOI] [PubMed] [Google Scholar]
- 47. Wang Y, Dai B, Ye D.. CHEK2 mutation and risk of prostate cancer: a systematic review and meta-analysis. Int J Clin Exp Med 2015; 8(9): 15708–15715. [PMC free article] [PubMed] [Google Scholar]
- 48. Alanee SR, Glogowski EA, Schrader KA. et al. Clinical features and management of BRCA1 and BRCA2-associated prostate cancer. Front Biosci (Elite Ed) 2014; 6: 15–30. [DOI] [PubMed] [Google Scholar]
- 49. Shenoy TR, Boysen G, Wang MY. et al. CHD1 loss sensitizes prostate cancer to DNA damaging therapy by promoting error-prone double-strand break repair. Ann Oncol 2017; 28(7): 1495–1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Boysen G, Barbieri CE, Prandi D. et al. SPOP mutation leads to genomic instability in prostate cancer. Elife 2015; 4: e09207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Saad F, Carles J, Gillessen S. et al. Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 2016; 17(9): 1306–1316. [DOI] [PubMed] [Google Scholar]
- 52. Shore N, Tutrone R, Mariados N. et al. eRADicAte: a prospective evaluation combining radium-223 dichloride and abiraterone acetate plus prednisone in castration-resistant prostate cancer patients. Clin Genitourin Cancer 2018; 16(2): 149–154. [DOI] [PubMed] [Google Scholar]
- 53.ClinicalTrials.gov. (NCT02225704) Radium-223 in Combination With Enzalutamide. https://clinicaltrials.gov/ct2/show/NCT02225704 (20 May 2019, date last accessed).
- 54.ClinicalTrials.gov. (NCT02194842) Phase III Radium 223 mCRPC-PEACE III (PEACE III). https://clinicaltrials.gov/ct2/show/NCT02194842 (31 January 2019, date last accessed).
- 55.European Medicines Agency Pharmacovigilance Risk Assessment Committee (PRAC). Assessment Report on Provisional Measures. Procedure Under Article 20 of Regulation (EC) No 726/2004 Resulting From Pharmacovigilance Data for Xofigo (radium-223). http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/002653/WC500246181.pdf (14 August 2018, date last accessed).
- 56.European Medicines Agency. EMA restricts use of prostate cancer medicine Xofigo. http://www.ema.europa.eu/ema/index.jsp? curl=pages/news_and_events/news/2018/07/news_detail_002996.jsp&mid=WC0b01ac058004d5c1 (14 August 2018, date last accessed).
- 57. Smith M, Parker C, Saad F. et al. Addition of radium-223 to abiraterone acetate and prednisone or prednisolone in patients with castration-resistant prostate cancer and bone metastases (ERA 223): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(3): 408–419. [DOI] [PubMed] [Google Scholar]
- 58.Xofigo [Product monograph]. Bayer Inc., Toronto, Canada 2018.
- 59.Xofigo [drug information sheet]. Bayer Yakuhin, Ltd., Osaka, Japan 2018.
- 60.Xofigo [summary of product characteristics]. Bayer Pharma AG, Berlin, Germany 2018.
- 61.ClinicalTrials.gov. (NCT02225704) Radium-223 in Combination With Enzalutamide. https://clinicaltrials.gov/ct2/show/NCT02225704 (20 June 2019, date last accessed).
- 62. Tombal BL, Saad F, McDermott RS. et al. Decreased fracture rate by mandating bone-protecting agents in the EORTC 1333/PEACE III trial comparing enzalutamide and Ra223 versus enzalutamide alone: an interim safety analysis [abstract]. JCO 2019; 37(Suppl); Abstr 5007. http://abstracts.asco.org/239/AbstView_239_260193.html (20 June 2019, date last accessed). [Google Scholar]
- 63. Sgouros G, Hobbs RF.. Dosimetry for radiopharmaceutical therapy. Semin Nucl Med 2014; 44(3): 172–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Sgouros G, Roeske JC, McDevitt MR. et al. MIRD Pamphlet No. 22 (abridged): radiobiology and dosimetry of alpha-particle emitters for targeted radionuclide therapy. J Nucl Med 2010; 51(2): 311–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Akabani G, Kennel SJ, Zalutsky MR.. Microdosimetric analysis of alpha-particle-emitting targeted radiotherapeutics using histological images. J Nucl Med 2003; 44(5): 792–805. [PubMed] [Google Scholar]
- 66. Akabani G, Zalutsky MR.. Microdosimetry of astatine-211 using histological images: application to bone marrow. Radiat Res 1997; 148(6): 599–607. [PubMed] [Google Scholar]
- 67. Chouin N, Bernardeau K, Bardies M. et al. Evidence of extranuclear cell sensitivity to alpha-particle radiation using a microdosimetric model. II. Application of the microdosimetric model to experimental results. Radiat Res 2009; 171(6): 664–673. [DOI] [PubMed] [Google Scholar]
- 68. Flux GD. Imaging and dosimetry for radium-223: the potential for personalized treatment. Br J Radiol 2017; 90: 20160748.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Murray I, Chittenden SJ, Denis-Bacelar AM. et al. The potential of (223)Ra and (18)F-fluoride imaging to predict bone lesion response to treatment with (223)Ra-dichloride in castration-resistant prostate cancer. Eur J Nucl Med Mol Imaging 2017; 44(11): 1832–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Pacilio M, Ventroni G, De Vincentis G. et al. Dosimetry of bone metastases in targeted radionuclide therapy with alpha-emitting (223)Ra-dichloride. Eur J Nucl Med Mol Imaging 2016; 43(1): 21–33. [DOI] [PubMed] [Google Scholar]
- 71. Sartor O, Vogelzang NJ, Sweeney C. et al. Radium-223 safety, efficacy, and concurrent use with abiraterone or enzalutamide: first U.S. experience from an expanded access program. Oncologist 2018; 23(2): 193–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Saad F, Keizman D, O'Sullivan JM. et al. Analysis of overall survival by number of radium-223 injections received in an international expanded access program (iEAP). JCO 2016(Suppl); Abstr 5082. [Google Scholar]
- 73. Sternberg CN, Saad F, Graff JN. et al. A randomized phase 2 study investigating 3 dosing regimens of radium-223 dichloride (Ra-223) in bone metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2018; 36: doi:10.1200/JCO.2018.36.1 [Google Scholar]
- 74. Hagemann UB, Mihaylova D, Uran SR. et al. Targeted alpha therapy using a novel CD70 targeted thorium-227 conjugate in in vitro and in vivo models of renal cell carcinoma. Oncotarget 2017; 8(34): 56311–56326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Henriksen G, Hoff P, Larsen RH.. Evaluation of potential chelating agents for radium. Appl Radiat Isot 2002; 56(5): 667–671. [DOI] [PubMed] [Google Scholar]
- 76. McDevitt MR, Sgouros G, Sofou S.. Targeted and nontargeted alpha-particle therapies. Annu Rev Biomed Eng 2018; 20: 73–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.ClinicalTrials.gov. (NCT02814669) Safety and Tolerability of Atezolizumab (ATZ) in Combination With Radium-223 Dichloride (R-223-D) in Metastatic Castrate-Resistant Prostate Cancer (CRPC) Progressed Following Treatment With an Androgen Pathway Inhibitor. https://clinicaltrials.gov/ct2/show/NCT02814669 (14 August 2018, date last accessed).
- 78. Barrio M, Fendler WP, Czernin J, Herrmann K.. Prostate specific membrane antigen (PSMA) ligands for diagnosis and therapy of prostate cancer. Expert Rev Mol Diagn 2016; 16(11): 1177–1188. [DOI] [PubMed] [Google Scholar]
- 79. Pouget JP, Georgakilas AG, Ravanat JL.. Targeted and off-target (bystander and abscopal)effects of radiation therapy: redox mechanisms and risk/benefit analysis. Antioxid Redox Signal 2018; 29(15): 1447–1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Prise KM, O'Sullivan JM.. Radiation-induced bystander signalling in cancer therapy. Nat Rev Cancer 2009; 9(5): 351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.TECENTRIQTM (atezolizumab) injection [prescribing information]. Genentech, 2018.
- 82.KEYTRUDA® (pembrolizumab) [prescribing information]. Merck & Co., 2018.
- 83.PROVENGE® (sipuleucel-T) [prescribing information]. Dendreon, 2018.
- 84. Weber AM, Ryan AJ.. ATM and ATR as therapeutic targets in cancer. Pharmacol Ther 2015; 149: 124–138. [DOI] [PubMed] [Google Scholar]
- 85. Marechal A, Zou L.. DNA damage sensing by the ATM and ATR kinases. Cold Spring Harb Perspect Biol 2013; 5(9): a012716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Karnitz LM, Zou L.. Molecular pathways: targeting ATR in cancer therapy. Clin Cancer Res 2015; 21(21): 4780–4785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Cseh AM, Fabian Z, Sumegi B, Scorrano L.. Poly(adenosine diphosphate-ribose) polymerase as therapeutic target: lessons learned from its inhibitors. Oncotarget 2017; 8(30): 50221–50239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Wickstroem K, Hagemann UB, Wengner AM. et al. Synergistic effect of MSLN-TTC in combination with DNA damage response inhibitors [abstract]. Cancer Res 2018; 78(Suppl 13): Abstr 849; http://cancerres.aacrjournals.org/content/78/13_Supplement/849 (14 August 2018, date last accessed) [Google Scholar]
- 89. Golfier S, Kopitz C, Kahnert A. et al. Anetumab ravtansine: a novel mesothelin-targeting antibody-drug conjugate cures tumors with heterogeneous target expression favored by bystander effect. Mol Cancer Ther 2014; 13(6): 1537–1548. [DOI] [PubMed] [Google Scholar]
- 90. Wengner AM, Siemeister G, Luecking U. et al. ATR inhibitor BAY 1895344 shows potent anti-tumor efficacy in monotherapy and strong combination potential with the targeted alpha therapy radium-223 dichloride in preclinical tumor models [abstract]. Cancer Res 2017; 77(Suppl 13); Abstr 836. http://cancerres.aacrjournals.org/content/77/13_Supplement/836 (14 August 2018, date last accessed). [Google Scholar]
- 91. Chang SS, Gaudin PB, Reuter VE, Heston WD.. Prostate-specific membrane antigen: present and future applications. Urology 2000; 55(5): 622–629. [DOI] [PubMed] [Google Scholar]
- 92. Wright GL Jr, Haley C, Beckett ML, Schellhammer PF.. Expression of prostate-specific membrane antigen in normal, benign, and malignant prostate tissues. Urol Oncol 1995; 1(1): 18–28. [DOI] [PubMed] [Google Scholar]
- 93. Bander NH, Trabulsi EJ, Kostakoglu L. et al. Targeting metastatic prostate cancer with radiolabeled monoclonal antibody J591 to the extracellular domain of prostate specific membrane antigen. J Urol 2003; 170(5): 1717–1721. [DOI] [PubMed] [Google Scholar]
- 94. Evans JC, Malhotra M, Cryan JF, O'Driscoll CM.. The therapeutic and diagnostic potential of the prostate specific membrane antigen/glutamate carboxypeptidase II (PSMA/GCPII) in cancer and neurological disease. Br J Pharmacol 2016; 173(21): 3041–3079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Mannweiler S, Amersdorfer P, Trajanoski S. et al. Heterogeneity of prostate-specific membrane antigen (PSMA) expression in prostate carcinoma with distant metastasis. Pathol Oncol Res 2009; 15(2): 167–172. [DOI] [PubMed] [Google Scholar]
- 96. Emmett L, Willowson K, Violet J. et al. Lutetium (177) PSMA radionuclide therapy for men with prostate cancer: a review of the current literature and discussion of practical aspects of therapy. J Med Radiat Sci 2017; 64(1): 52–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ross JS, Sheehan CE, Fisher HA. et al. Correlation of primary tumor prostate-specific membrane antigen expression with disease recurrence in prostate cancer. Clin Cancer Res 2003; 9(17): 6357–6362. [PubMed] [Google Scholar]
- 98. Holmes EH. PSMA specific antibodies and their diagnostic and therapeutic use. Expert Opin Investig Drugs 2001; 10: 511–519. [DOI] [PubMed] [Google Scholar]
- 99. Bouchelouche K, Choyke PL, Capala J.. Prostate specific membrane antigen- a target for imaging and therapy with radionuclides. Discov Med 2010; 9(44): 55–61. [PMC free article] [PubMed] [Google Scholar]
- 100. Imai K, Takaoka A.. Comparing antibody and small-molecule therapies for cancer. Nat Rev Cancer 2006; 6(9): 714–727. [DOI] [PubMed] [Google Scholar]
- 101. Hofman MS, Violet J, Hicks RJ, Sandhu S.. [(177)Lu]-PSMA-617 radionuclide therapy in patients with metastatic castration-resistant prostate cancer - Author's reply. Lancet Oncol 2018; 19(8): e373.. [DOI] [PubMed] [Google Scholar]
- 102. Tagawa ST, Milowsky MI, Morris M. et al. Phase II study of Lutetium-177-labeled anti-prostate-specific membrane antigen monoclonal antibody J591 for metastatic castration-resistant prostate cancer. Clin Cancer Res 2013; 19(18): 5182–5191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Ahmadzadehfar H, Rahbar K, Kurpig S. et al. Early side effects and first results of radioligand therapy with (177)Lu-DKFZ-617 PSMA of castrate-resistant metastatic prostate cancer: a two-centre study. EJNMMI Res 2015; 5(1): 114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Ahmadzadehfar H, Eppard E, Kurpig S. et al. Therapeutic response and side effects of repeated radioligand therapy with 177Lu-PSMA-DKFZ-617 of castrate-resistant metastatic prostate cancer. Oncotarget 2016; 7(11): 12477–12488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Rahbar K, Schmidt M, Heinzel A. et al. Response and tolerability of a single dose of 177Lu-PSMA-617 in patients with metastatic castration-resistant prostate cancer: a multicenter retrospective analysis. J Nucl Med 2016; 57(9): 1334–1338. [DOI] [PubMed] [Google Scholar]
- 106. Rahbar K, Ahmadzadehfar H, Kratochwil C. et al. German multicenter study investigating 177Lu-PSMA-617 radioligand therapy in advanced prostate cancer patients. J Nucl Med 2017; 58(1): 85–90. [DOI] [PubMed] [Google Scholar]
- 107. Rahbar K, Bode A, Weckesser M. et al. Radioligand Therapy With 177Lu-PSMA-617 as a novel therapeutic option in patients with metastatic castration-resistant prostate cancer. Clin Nucl Med 2016; 41(7): 522–528. [DOI] [PubMed] [Google Scholar]
- 108. Fendler WP, Reinhardt S, Ilhan H. et al. Preliminary experience with dosimetry, response and patient reported outcome after 177Lu-PSMA-617 therapy for metastatic castration-resistant prostate cancer. Oncotarget 2017; 8(2): 3581–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.ClinicalTrials.gov. (NCT03392428) A Trial of 177Lu-PSMA617 Theranostic Versus Cabazitaxel in Progressive Metastatic Castration Resistant Prostate Cancer (TheraP). https://clinicaltrials.gov/ct2/show/NCT03392428 (20 May 2019, date last accessed).
- 110.ClinicalTrials.gov. (NCT03511664) Study of 177Lu-PSMA-617 In Metastatic Castrate-Resistant Prostate Cancer (VISION). https://clinicaltrials.gov/ct2/show/NCT03511664? term=NCT03511664&rank=1 (12 September 2018, date last accessed).
- 111. Kulkarni HR, Singh A, Schuchardt C. et al. PSMA-based radioligand therapy for metastatic castration-resistant prostate cancer: the Bad Berka experience since 2013. J Nucl Med 2016; 57(Suppl 3): 97S–104S. [DOI] [PubMed] [Google Scholar]
- 112. Beyer GJ, Offord R, Kunzi G. et al. The influence of EDTMP-concentration on the biodistribution of radio-lanthanides and 225-Ac in tumor-bearing mice. The ISOLDE Collaboration. Nucl Med Biol 1997; 24(5): 367–372. [DOI] [PubMed] [Google Scholar]
- 113. Pfannkuchen N, Bausbacher N, Pektor S. et al. In vivo evaluation of [(225)Ac]Ac-DOTA(ZOL) for alpha-therapy of bone metastases. Curr Radiopharm 2018; 11(3): 223–230. [DOI] [PubMed] [Google Scholar]
- 114. Ceder J, Elgqvist J.. Targeting prostate cancer stem cells with alpha-particle therapy. Front Oncol 2016; 6: 273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Dahle J, Borrebaek J, Jonasdottir TJ. et al. Targeted cancer therapy with a novel low-dose rate alpha-emitting radioimmunoconjugate. Blood 2007; 110(6): 2049–2056. [DOI] [PubMed] [Google Scholar]
- 116. Larsen RH, Borrebaek J, Dahle J. et al. Preparation of Th227-labeled radioimmunoconjugates, assessment of serum stability and antigen binding ability. Cancer Biother Radiopharm 2007; 22(3): 431–437. [DOI] [PubMed] [Google Scholar]
- 117.ClinicalTrials.gov. (NCT03724747) Study to Evaluate the Safety, Tolerability, Pharmacokinetics, and Anti-tumor Activity of a Thorium-227 Labeled Antibody-chelator Conjugate, in Patients With Metastatic Castration Resistant Prostate Cancer. https://clinicaltrials.gov/ct2/show/NCT03724747 (23 July 2019, date last accessed).
- 118.ClinicalTrials.gov. (NCT03507452) First-in-human Study of BAY2287411 Injection, a Thorium-227 Labeled Antibody-chelator Conjugate, in Patients With Tumors Known to Express Mesothelin. https://clinicaltrials.gov/ct2/show/NCT03507452 (24 July 2019, date last accessed).
- 119.ClinicalTrials.gov. (NCT02581878) Safety and Tolerability of BAY1862864 Injection in Subjects With Relapsed or Refractory CD22-Positive Non-Hodgkin's Lymphoma. https://clinicaltrials.gov/ct2/show/NCT02581878 (24 July 2019, date last accessed).