Abstract
Background
Extending the duration of adjuvant endocrine therapy reduces the risk of recurrence in a subset of women with early-stage hormone receptor-positive (HR+) breast cancer. Validated predictive biomarkers of endocrine response could significantly improve patient selection for extended therapy. Breast cancer index (BCI) [HOXB13/IL17BR ratio (H/I)] was evaluated for its ability to predict benefit from extended endocrine therapy in patients previously randomized in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial.
Patients and methods
Trans-aTTom is a multi-institutional, prospective–retrospective study in patients with available formalin-fixed paraffin-embedded primary tumor blocks. BCI testing and central determination of estrogen receptor (ER) and progesterone receptor (PR) status by immunohistochemistry were carried out blinded to clinical outcome. Survival endpoints were evaluated using Kaplan–Meier analysis and Cox regression with recurrence-free interval (RFI) as the primary endpoint. Interaction between extended endocrine therapy and BCI (H/I) was assessed using the likelihood ratio test.
Results
Of 583 HR+, N+ patients analyzed, 49% classified as BCI (H/I)-High derived a significant benefit from 10 versus 5 years of tamoxifen treatment [hazard ratio (HR): 0.35; 95% confidence interval (CI) 0.15–0.86; 10.2% absolute risk reduction based on RFI, P = 0.027]. BCI (H/I)-low patients showed no significant benefit from extended endocrine therapy (HR: 1.07; 95% CI 0.69–1.65; −0.2% absolute risk reduction; P = 0.768). Continuous BCI (H/I) levels predicted the magnitude of benefit from extended tamoxifen, whereas centralized ER and PR did not. Interaction between extended tamoxifen treatment and BCI (H/I) was statistically significant (P = 0.012), adjusting for clinicopathological factors.
Conclusion
BCI by high H/I expression was predictive of endocrine response and identified a subset of HR+, N+ patients with significant benefit from 10 versus 5 years of tamoxifen therapy. These data provide further validation, consistent with previous MA.17 data, establishing level 1B evidence for BCI as a predictive biomarker of benefit from extended endocrine therapy.
Trial registration
ISRCTN17222211; NCT00003678.
Keywords: BCI, molecular signature, predictive biomarker, early-stage breast cancer, endocrine benefit
Key Message
Predictive biomarkers are critical to inform the risk–benefit of prolonged endocrine treatment in HR+ breast cancer. Breast cancer index (BCI) predicted endocrine response and outcome from 10 versus 5 years of endocrine therapy in N+ patients treated in the aTTom trial. These data strengthen BCI clinical evidence to inform individual patient selection for extended endocrine treatment.
Introduction
Treatment of HR+ breast cancer with adjuvant antiestrogen therapies has been a mainstay of care for over 40 years. Selection of patients based on estrogen receptor (ER) and/or progesterone receptor (PR) expression marked a pivotal advancement toward modern precision oncology [1]. ER and PR expression is routinely measured in current clinical practice to indicate hormone-responsive disease, and their prognostic effect is well established; however, within the HR+ population they have limited predictive value for selecting patients who derive benefit from antiestrogen treatment [2–5]. To date, predictive biomarkers with robust clinical validation and utility to optimize patient selection and inform prolonged endocrine treatment have been lacking.
Gene expression analyses that provide information on tumor biology have been incorporated into several classifiers with a major impact on patient selection for chemotherapy treatment [6–8]. Since early-stage HR+ breast cancer is associated with a persistent risk of recurrence and death [9], another important decision for patients is whether to extend endocrine therapy to reduce the ongoing risk of late (beyond 5 years of diagnosis) distant recurrence. Multiple trials have demonstrated consistent but modest absolute benefits with continuing endocrine therapy to 10 years in the range of 2%–5% absolute risk reduction in HR+ patients [10–14]. While extending endocrine therapy to 10 years is endorsed by several clinical practice guidelines [15–17], clear guidance on individualized approaches to optimize patient selection for prolonged endocrine regimens remains limited.
The Breast Cancer Index (BCI) is an algorithmic gene expression-based signature comprised of two functional biomarker panels, the molecular grade index (MGI) and the two-gene ratio, HOXB13/IL17BR (H/I), that evaluate tumor proliferation and estrogen signaling, respectively. The BCI test reports both a prognostic as well as a predictive result. Integration of MGI and H/I generates a prognostic BCI score quantifying both the risk of overall (0–10 years) and late (5–10 years) distant recurrence [18–20]. The predictive component of BCI, the H/I ratio, has been shown to predict endocrine response across several different treatment scenarios [18, 20, 21]. In the extended endocrine therapy setting, BCI predicted benefit from an additional 5 years of letrozole after adjuvant tamoxifen in the MA.17 study [18]. The current study was aimed at strengthening the clinical evidence for BCI in the extended endocrine therapy setting through examination of its predictive performance in breast cancer patients treated in the Adjuvant Tamoxifen—To Offer More? (aTTom) trial.
Methods
Study design and patients
The aTTom parent trial is a prospective, phase III trial that included 6956 breast cancer patients who remained disease free after having completed at least 4 years of adjuvant tamoxifen therapy and were randomized to either continue or stop tamoxifen treatment of an additional 5 years [13, 22].
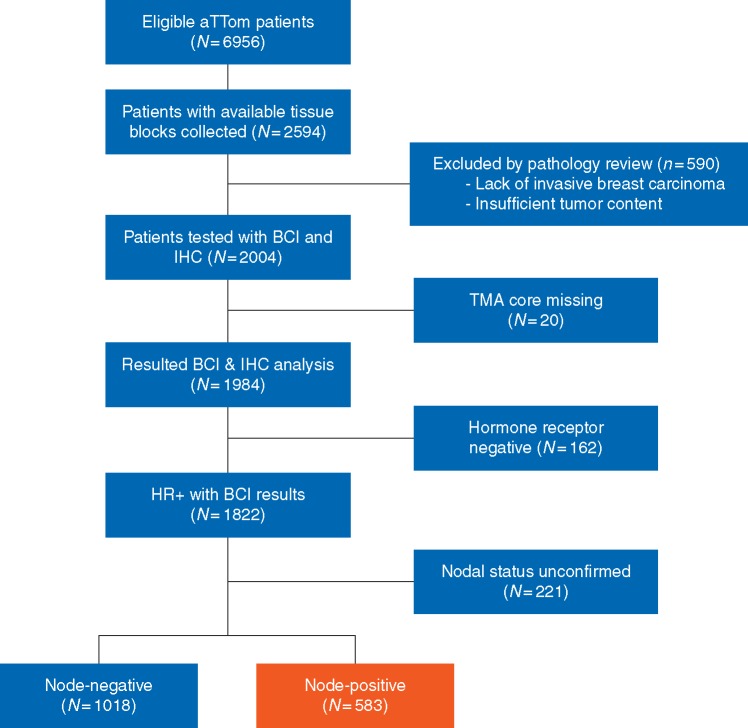
The translational aTTom study, Trans-aTTom, is a multi-institutional, prospective–retrospective study with the objective of validating the predictive performance of BCI in early-stage breast cancer patients in the extended endocrine setting [23]. All patients previously randomized in the aTTom study with available formalin-fixed paraffin-embedded (FFPE) primary resection tumor blocks were eligible. Exclusion criteria included lack of invasive tumor as assessed by histopathology review, insufficient tissue on tissue microarray (TMA) analysis, and insufficient RNA signal (Figure 1).
Figure 1.
Modified REMARK diagram. The diagram shows tumor block collection, specimen processing and molecular testing, leading to a final analyzable cohort of 583 HR+ N+ patients. BCI, Breast Cancer Index; IHC, immunohistochemistry; TMA, tissue microarray; HR+, hormone receptor-positive.
Trans-aTTom was initiated in March 2015 across multiple institutions and laboratories (supplementary Figure S1, available at Annals of Oncology online). The University of Birmingham Cancer Research UK Clinical Trial Unit (CRCTU) was the sponsoring institution and secured ethical and regulatory approvals from the UK Research Ethics Committee (REC, reference 16/EM/0142), Health Research Authority (HRA), Confidentiality Advisory Group (CAG) and from the PPBP in Scotland, and also carried out final biomarker data integration with the aTTom clinical database. Centralized collection and sample processing, construction of TMAs, and tissue sectioning was carried out by the University of Edinburgh Cancer Research Centre (ECRC). Centralized immunohistochemistry (IHC) analysis of HR status was carried out at the Massachusetts General Hospital (MGH). Both BCI and IHC testing were conducted blinded to clinical data and outcome.
Statistical considerations
The primary objective of the study was to determine whether BCI (H/I) status (High versus Low) was predictive of the benefit of 10 versus 5 years of tamoxifen. The secondary objective was to evaluate whether BCI (H/I), as a continuous index, demonstrates a statistically significant treatment to biomarker interaction with extended tamoxifen treatment.
The aTTom parent trial showed a 3.8% absolute benefit in disease-free interval (DFI) with 10 versus 5 years of tamoxifen treatment (HR 0.86; 95% CI 0.77–0.96; P = 0.006) at a median 8.9 years of follow-up [22]. Powering analyses assumed 40% of patients would be classified as BCI (H/I)-High as previously reported [19, 20]. At 80% power, ∼1800 HR+ patients would be required to detect a 9.4% absolute benefit in DFI within the BCI (H/I)-High subset at a 5% significance level. With an estimated attrition rate of 20% due to pathological review, and 10% for HR-negative patients, collection of ∼2500 cases was projected to achieve the minimum powering requirement.
Designed as an endpoint-adaptive trial, Trans-aTTom had two endpoints that were investigated in a pre-specified interim analysis: recurrence-free interval (RFI) that included local, regional and distant recurrences, and DFI that included local, regional, distant recurrences and new breast primaries. Based on the Kim-DeMets power error spending function [24], the nominal two-sided P-value efficacy boundaries for the interim and final analysis were set to 0.0334 and 0.0336, respectively. Interim analysis of 1143 HR+ patients resulted in selection of RFI as the primary endpoint for final analysis. The use of time varying analysis was predetermined based on the parent aTTom trial results and evaluation of the Cox proportional hazards assumption wherein a deviation in proportionality was observed that was attributed to crossing over of the Kaplan–Meier (K–M) survival curves and delayed efficacy of extended tamoxifen (supplementary Figure S2, available at Annals of Oncology online) [22]. Therefore, as pre-specified in the statistical analysis plan (SAP), Fleming–Harrington weighted log rank test and Cox regression analysis using time varying coefficients were utilized [25]. The absolute benefit of extended tamoxifen treatment was represented by the reduction in 17-year (post-randomization at year 5 with 12 years of follow-up) risk of recurrence estimated from K–M analysis. Statistical significance of the interaction between BCI (H/I) and extended tamoxifen treatment was assessed by likelihood ratio tests comparing a full model with an interaction term versus a reduced model without the interaction. All analyses were conducted based on a pre-specified SAP using Stata (version 15.1; https://www.stata.com) and R statistical package (version 3.5.2; http://www.r-project.org).
Unblinding plan
Pre-specified analysis evaluating the effect size and estimated power in the translational cohort (Figure 1; N = 1822) was utilized to inform the unblinding plan. At the time of analysis, powering estimates were <50% for both the overall cohort and the N− subset and >90% for the N+ subset. As such, this initial analysis of Trans-aTTom includes the N+ subset, and collection of additional patients to increase power in the overall cohort is continuing in a blinded manner towards a planned final analysis.
Hormone receptor determination and pathological evaluation
The parent aTTom trial included ∼60% of patients with an unconfirmed HR status; therefore, central determination of ER and PR status by IHC were carried out on all cases. Digital images of H&E stained sections from FFPE tumor blocks were reviewed to confirm the presence of invasive tumor and to select areas for TMA construction [26]. IHC staining of TMAs was carried out following standard protocols using monoclonal antibody clone 6F11 and 16 for ER and PR, respectively (Leica Biosystems). Results were recorded as percentage of IHC-stained cells and adjudicated by two pathologists. Tumors were considered centrally confirmed to be ER or PR expressing when ≥1% of cells showed definitive nuclear staining.
BCI assay
BCI gene expression analysis by RT-PCR was carried out on FFPE primary tumor specimens (Biotheranostics Inc., San Diego, CA) as reported previously [20]. Briefly, macro-dissection was carried out on FFPE sections to enrich tumor content before RNA extraction. Total RNA was reverse transcribed, and the resulting cDNA was pre-amplified by PCR using the PreAmp Master Mix Kit (Thermo Fisher Scientific, Carlsbad, CA) before TaqMan PCR analysis. Calculation of BCI (H/I) was carried out using the prespecified cut-point as described previously [18, 20] and was normalized into a range between 0 and 10.
Results
Archived primary tumor tissue from 2594 patients were retrospectively collected from 53 study sites, representing 37% of the aTTom patient population (Figure 1). The analyzable cohort consisted of 1822 patients with confirmed HR+ status and BCI results, including 1018 node-negative (N0), 583 node-positive (N+) patients and 221 with unconfirmed nodal status (Figure 1). Comparison of clinical variables from the N+ patients in the aTTom trial (N = 2136) versus the Trans-aTTom N+ patients (N = 615) showed no statistically significant differences in the clinicopathological characteristics between the parent and translational cohorts (Table 1); in addition K–M analysis comparing 5 versus 10 year tamoxifen treatment in the N+ subset of aTTom (N = 2136) and Trans-aTTom (N = 615) demonstrated similar patterns of crossover in corresponding survival curves (supplementary Figure S2, available at Annals of Oncology online).
Table 1.
Clinicopathological characteristics for node positive (N+) patients in parent aTTom, Trans-aTTom, and Trans-aTTom HR+ cohorts
| aTToma (n = 2136) | Trans-aTTomb (n = 615) | Trans-aTTom HR+c (n = 583) | P-valued | |
|---|---|---|---|---|
| Age | 0.141 | |||
| <50 | 265 (12) | 97 (16) | 89 (15) | |
| 50–59 | 765 (36) | 208 (34) | 199 (34) | |
| 60–69 | 612 (29) | 163 (27) | 149 (26) | |
| ≥70 | 494 (23) | 147 (24) | 146 (25) | |
| Menopause | 0.059 | |||
| Pre | 70 (3) | 25 (4) | 21 (4) | |
| Post | 1798 (84) | 527 (86) | 503 (86) | |
| Peri | 63 (3) | 23 (4) | 23 (4) | |
| Not known | 205 (10) | 40 (7) | 36 (6) | |
| Tumor size | 0.992 | |||
| T1 | 968 (45) | 275 (45) | 266 (46) | |
| T2 | 903 (42) | 262 (43) | 244 (42) | |
| T3 | 95 (4) | 28 (5) | 25 (4) | |
| Unknown | 170 (8) | 50 (8) | 48 (8) | |
| Histological grade | 0.993 | |||
| Well differentiated – grade I | 313 (15) | 92 (15) | 92 (16) | |
| Moderately differentiated – grade II | 953 (45) | 272 (44) | 267 (46) | |
| Poorly differentiated – grade III | 467 (22) | 133 (22) | 117 (20) | |
| Not known | 403 (19) | 118 (19) | 107 (18) | |
| Surgery type | 0.815 | |||
| Lumpectomy | 1002 (47) | 276 (45) | 265 (46) | |
| Mastectomy | 1129 (53) | 337 (55) | 316 (54) | |
| Not known | 5 (0) | 2 (0) | 2 (0) | |
| Histology | 0.703 | |||
| Ductal | 1473 (69) | 442 (72) | 422 (72) | |
| Lobular | 265 (12) | 73 (12) | 72 (12) | |
| Tubular | 28 (1) | 8 (1) | 8 (1) | |
| Other/mixed | 70 (3) | 17 (3) | 15 (3) | |
| Not known | 300 (14) | 75 (12) | 66 (11) | |
| Locoregional recurrence | 199 (9) | 55 (9) | 54 (9) | 0.839 |
| Distant recurrence | 509 (24) | 151 (25) | 149 (26) | 0.752 |
| New breast primary | 74 (3) | 14 (2) | 14 (2) | 0.179 |
aTTom cohort (n = 2136) includes patients originally unconfirmed for hormone receptor status.
Trans-aTTom cohort (n = 615) included both HR+ and HR-negative patients.
Trans-aTTom HR+ (n = 583) included only HR+ patients.
P-values comparing the aTTom trial and Trans-aTTom cohort were calculated using the Fisher exact test for all variables, except for locoregional recurrence, distant recurrence and new breast primary for which proportional test was used with continuity correction.
N+, node positive; HR+, hormone receptor-positive.
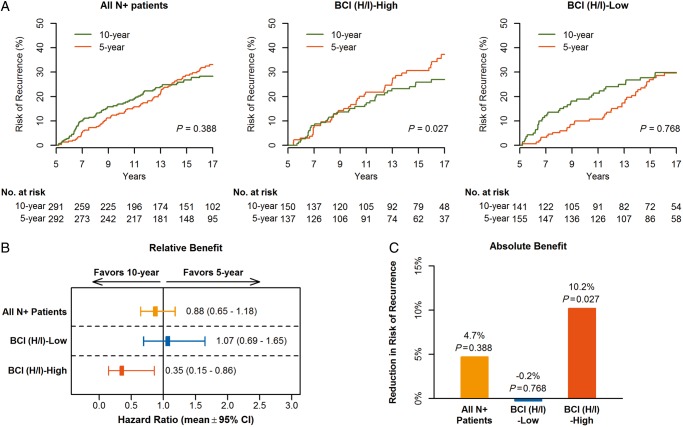
Among the 583 HR+ N+ patients that are the focus of this report, 292 with 92 RFI events comprised the 5-year arm, 291 with 77 RFI events comprised the 10-year arm, 86% were post-menopausal, 42% were T2, 66% had moderately or poorly differentiated tumors, and 54% underwent mastectomy (Table 1). Improved recurrence-free survival was seen in the Trans-aTTom N+ HR+ patients treated with extended tamoxifen; risk of recurrence was 33.1% (95% CI 26.8% to 38.9%) and 28.4% (95% CI 22.6% to 33.7%) in the 5- and 10-year arm, respectively, with a non-significant absolute benefit of 4.7% (P = 0.388) and HR of 0.88 (95% CI 0.65–1.18) (Figure 2 and Table 2; supplementary Figure S2, available at Annals of Oncology online).
Figure 2.
Predictive performance by BCI (H/I) groups based on RFI in HR+ N+ patients (n = 583). Kaplan–Meier analysis (A) of risk of recurrence comparing 10 versus 5 years of tamoxifen in all N+ patients (left), and in BCI (H/I)-High (middle) and BCI (H/I)-Low subset (right), relative benefit as measured by hazard ratios of treatment effect (B) and absolute benefit as measured by the absolute recurrence risk reduction (C). BCI (H/I) indicates Breast Cancer Index HOXB13/IL17BR ratio; CI, confidence interval.
Table 2.
Kaplan–Meier estimates of risk of recurrence for N+ patients treated with 10 versus 5-year of tamoxifen in all patients and BCI(H/I) subsets
| Groups | 5-Year TAM |
10-Year TAM |
|||
|---|---|---|---|---|---|
| No. patients (%) | RFI (%)(95% CI, %) | No. patients (%) | RFI (%)(95% CI, %) | HR (95% CI)a | |
| All N+ patients | 292 (50) | 33.1 (26.8–38.9) | 291 (50) | 28.4 (22.6–33.7) | 0.88 (0.65–1.18) |
| BCI (H/I)-High | 137 (48) | 37.2 (27.1–46.0) | 150 (52) | 27.0 (18.9–34.3) | 0.35 (0.15–0.86) |
| BCI (H/I)-Low | 155 (52) | 29.6 (21.4–37.0) | 141 (48) | 29.8 (21.2–37.4) | 1.07 (0.69–1.65) |
HR was calculated to compare 10-year tamoxifen versus 5-year tamoxifen.
N+, node positive; BCI (H/I), Breast Cancer Index HOXB13/IL17BR ratio; RFI, recurrence-free interval; HR, hazard ratio; TAM, tamoxifen.
A significant benefit from extended tamoxifen was demonstrated in 49% (N = 287) of patients that were classified as BCI (H/I)-High (HR =0.35; 95% CI 0.15–0.86). The risk of recurrence was 27.0% and 37.2% for patients treated with 10- and 5-year tamoxifen, respectively, demonstrating a significant absolute benefit of 10.2% for reduction in the risk of recurrence (P = 0.027) (Figure 2 and Table 2). In contrast, there was no significant benefit from an additional 5 years of tamoxifen in the 51% (N = 296) of patients that were classified as BCI (H/I)-Low (HR =1.07; 95% CI 0.69–1.65). The risk of recurrence was 29.8% and 29.6% for those treated with 10- and 5-year tamoxifen, respectively, showing a non-significant absolute increase in risk of recurrence of 0.2% (P = 0.768). A statistically significant interaction between continuous BCI (H/I) and extended tamoxifen treatment was demonstrated in unadjusted (P = 0.024) and adjusted [including age, tumor size, tumor grade, ER and PR status (P = 0.012)] analyses. Similar findings on the predictive ability of BCI (H/I) were observed evaluating the secondary endpoint of DFI with a significant treatment to biomarker interaction (adjusted P = 0.019) (supplementary Figure S3, available at Annals of Oncology online).
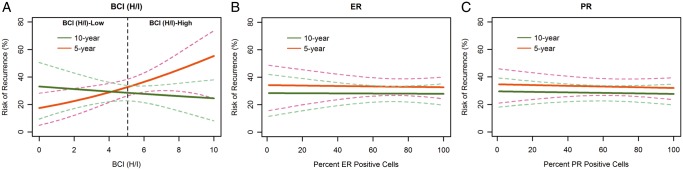
An increased risk of recurrence with rising levels of BCI (H/I) was observed in patients treated with 5-year tamoxifen alongside a decreased risk of recurrence in patients treated with 10-year tamoxifen (Figure 3A, interaction P = 0.024), showing improved outcomes with extended tamoxifen based on BCI (H/I) levels. In contrast, no significant relationship was observed between treatment with extended tamoxifen and the percentage of ER or PR positively stained cells (interaction P = 0.886 and 0.985, respectively; Figure 3B and C).
Figure 3.
Risk of recurrence as a function of continuous BCI (H/I), ER, and PR for patients treated by 10- and 5-year tamoxifen. BCI (H/I), breast cancer index HOXB13/IL17BR ratio; ER, estrogen receptor; PR, progesterone receptor.
Distribution of BCI (H/I) levels across clinical and pathological factors, including age, menopausal status, tumor size, tumor grade and ER/PR positivity did not demonstrate any strong correlations across the factors examined (supplementary Figure S4, available at Annals of Oncology online). A modest positive correlation was seen between BCI (H/I) and tumor grade (supplementary Figure S4D, available at Annals of Oncology online), and a weak negative correlation was seen between BCI (H/I) and ER or PR positivity (supplementary Figure S4E and F, available at Annals of Oncology online).
Discussion
The current study is a prospectively planned, retrospective study of the aTTom trial to examine whether a difference in response from 5 versus 10 years of tamoxifen in patients who were recurrence free after at least 4 years of tamoxifen therapy is dependent on BCI (H/I). This study confirms that BCI status predicted benefit with extended tamoxifen treatment. Patients with BCI (H/I)-High disease derived significant benefit from 10 versus 5 years of tamoxifen treatment, whereas BCI (H/I)-Low patients showed no significant benefit from extended endocrine therapy, despite having positive nodes. In the aTTom trial, N+ patients demonstrated an absolute benefit of 3.6% in RFI with 10 versus 5 years treatment. In the current study, reduction in the absolute risk of late recurrence was 10.2% in N+ patients classified as BCI (H/I)-High (HR = 0.35; 95% CI 0.15–0.86; P = 0.027). Patients with high BCI (H/I) expressing tumors showed a 65% reduction in the relative risk of recurrence when treated with extended endocrine therapy versus stopping treatment at 5 years. In comparison and equally important, patients classified as BCI (H/I)-Low showed no significant benefit from extended endocrine therapy (−0.2% RFI; HR = 1.07; 95% CI 0.69–1.65; P = 0.768).
Whether to prolong endocrine therapy to potentially reduce the risk of late metastatic recurrence is an important decision for patients diagnosed with HR+ breast cancer who remain recurrence free after completing primary adjuvant therapy. HR+ breast cancer is associated with a persistent long-term risk of recurrence [9]. Given the modest benefit and potentially serious adverse effects of extended endocrine therapy beyond 5 years (e.g. endometrial cancer [13], thromboembolic disease [12]), improved approaches to identify patients who are at increased risk of late distant recurrence and who derive benefit from extended endocrine therapy are critical. A recent meta-analysis including >62 000 women with ER+ breast cancer showed the risk of distant recurrence persisted at least 20 years from diagnosis [9]. In this meta-analysis, nodal involvement and larger tumor size were positively correlated with increased risk of late distant recurrence. However, the majority of women who completed 5 years of endocrine therapy remained free of distant recurrence, including those with node-positive tumors, indicating that extended endocrine therapy for all patients with N+ disease results in overtreatment of many. In addition, while nodal status was prognostic for increased risk of late distant recurrence, not all patients with a high estimated risk of recurrence will benefit equally from extended endocrine therapy. In the current study, BCI by low H/I expression identified 51% of N+ patients that did not experience any significant benefit from continuing tamoxifen treatment of an additional 5 years. In addition, BCI (H/I)-Low patients in the 10 years tamoxifen arm initially demonstrated an increased risk of recurrence, suggesting that extended tamoxifen was potentially harmful in these patients (Figure 2A, right panel). However, this effect may be attributed to the crossover observed in the survival curves from both the aTTom and Trans-aTTom N+ cohorts, independent of BCI status (compare supplementary Figure S2A and B, available at Annals of Oncology online). Importantly, results from this study add to the body of evidence that the underlying tumor biology of low BCI (H/I) disease is associated with the lack of a statistically significant endocrine response.
Increasing BCI (H/I) levels, as a continuous linear variable, were directly related to the degree of benefit and reduction in the risk of recurrence following 10 versus 5 years of tamoxifen treatment. In contrast to BCI, no significant relationship was observed between extended endocrine therapy and the percentage of ER or PR positively stained cells in this study. Additionally, distribution of BCI (H/I) levels across a range of clinical and pathological factors including tumor grade, tumor size, age, menopausal status, and ER and PR levels showed no strong relationship across the factors examined (supplementary Figure S4, available at Annals of Oncology online). These data underscore the independent information and increased resolution provided by BCI in addition to standard clinicopathological factors through molecular profiling of primary tumor biology.
The significant association of BCI (H/I) status with patient benefit from endocrine therapy demonstrated in the current study represents the third independent clinical trial validation of BCI as a predictive biomarker of endocrine response. Analysis of BCI in the Stockholm randomized controlled trial (RCT) cohort (N = 600) showed BCI (H/I)-High was predictive of benefit from tamoxifen therapy in the primary adjuvant setting versus placebo (HR = 0.35; 95% CI 0.19–0.65; P = 0.0005), whereas patients classified as BCI (H/I)-Low did not significantly benefit from tamoxifen treatment (HR = 0.67; 95% CI 0.36–1.24; P = 0.204) [20]. Validation of BCI predictive ability in the extended endocrine setting was initially demonstrated in the NCIC-CTG MA.17 RCT cohort (N = 249, 60% N+). Patients categorized as BCI (H/I)-High had a significantly improved outcome with extended letrozole treatment versus placebo: a 67% reduction in risk of recurrence (OR = 0.35; 95% CI 0.16–0.75; P = 0.007), while patients with BCI (H/I)-Low did not have a statistically significant decrease in late recurrence when treated with extended endocrine therapy (OR = 0.68; 95% CI 0.31–1.52; P = 0.35) [18]. Alongside the MA.17 study, data from the current study represent the second prospective–retrospective validation in an RCT of BCI (H/I) as a predictive biomarker in the extended endocrine setting in early-stage HR+ breast cancer and the third prospective–retrospective validation of the predictive value of BCI in randomized controlled trials.
Notably, in all three studies including the current study, a significant treatment by biomarker interaction was demonstrated (supplementary Table S1, available at Annals of Oncology online). BCI predictive activity was significant irrespective of treatment background [selective ER modulator (SERM) versus aromatase inhibitor (AI)] and adjuvant setting (primary versus secondary endocrine treatment). Collectively, these data provide strong evidence that BCI (H/I) has clinical utility across a variety of endocrine treatment backgrounds as a biomarker to select patients with endocrine responsive disease and those who are likely to experience improved outcomes with endocrine therapy.
Clinical practice guidelines for evaluation of tumor biomarkers have recognized both the challenges and value of investigations using archived tumor specimens [16, 27, 28]. In particular, validation of biomarkers in prospective–retrospective studies of archival specimens has served as a gold standard in genomic classification. As described by Simon et al. [23], level 1B classification for clinical utility requires reproducibility in at least two, independent prospective–retrospective studies. This evidentiary framework has also served as the basis for recommended changes in clinical practice and cited in guidelines such as the American Society of Clinical Oncology (ASCO), the European Group on Tumor Markers (EGTM) and the European Society for Medical Oncology (ESMO) [16, 27, 28].
Although several multigene assays provide prognostic information related to the risk of late recurrence, the BCI test is currently the only clinically available multigene classifier with proven ability to predict the likelihood of benefit from extended endocrine therapy. A recently published pan-genomic analysis completed by the TransATAC study group compared the prognostic performance of several genomic classifiers including the BCI prognostic score, 21-gene Recurrence Score (Oncotype Dx), 46-gene ROR score (Prosigna), and the 12-gene EPclin score (EndoPredict) [8]. All classifiers provide prognostic risk of recurrence in the early 0–5 year time period; however, only the BCI score, ROR, and EPclin classifiers demonstrated significant ability to stratify patients for risk of late distant recurrence risk independent of age, tumor size, grade, nodal status, and treatment [8]. However, precision medicine as it relates to extended endocrine therapy will optimally have both a prognostic (risk of late recurrence) as well as a predictive (who will benefit) component to maximize information to advise patient choice.
One of the key limitations of the study is that it reports on a subset of Trans-aTTom patients with node positive disease as block collection is ongoing for the overall cohort. Furthermore, while the current study included post-menopausal women treated solely with tamoxifen, which does not reflect current recommendations that adjuvant endocrine therapy should include an AI [17], BCI (H/I) shows predictive activity in patients treated with either extended AI (MA.17) or extended tamoxifen (this study), suggesting prediction of endocrine response across antiestrogen therapies. Furthermore, tamoxifen monotherapy remains a first-line endocrine treatment of pre-menopausal patients as well as those intolerant to or contraindicated for an AI. An additional treatment option for premenopausal HR+ patients is the addition of ovarian function suppression to tamoxifen or exemestane based on results from the SOFT and TEXT trials [29, 30].
A significant health issue for early-stage HR+ breast cancer is to reduce mortality based on late distant recurrence, and therefore to develop and validate enhanced approaches to select individual patients for extended endocrine therapy since not all patients derive benefit. The current data strengthen the clinical validity of BCI for prediction of endocrine response and its clinical utility in optimizing duration of endocrine therapy.
Supplementary Material
Acknowledgements
We thank the aTTom trialists and the CRCTU office at the University of Birmingham for their oversight. Special thanks to Sarah Thornber, Lucy Doos, Amandip Malhi, Veena Singh, Claire Gaunt, Sarah Bowden, Tristan Harris, Jose Ramirez, Yen Tran, Carrie Cunningham and Monika Sobol for their support of the trial and excellent technical expertise. We are indebted to all the women who participated in the aTTom trial. We also would like to thank the following study pathologists for their important contributions: Fouad Alchami, Nisha Ali, David Bailey, David Barker, Maurizio Brotto, David Butterworth, Pauline Carder, Alison Davies, Rahul Deb, Smita Deshpande, Janet English, David Fish, Sunna Frank, Frances Gallagher, Preethi Gopinath, Alison Green, Lisette Hammond, Andy Hanby, Lesley Hortan, Ehab Husain, Mona Jain, Kamarul Jamil, Vidga Kumaraswamy, Guy Martland, Natalie Meara, Yasmeen Mir, Navid Momtahan, Narenda Mungalsingh, Joseph Murphy, Claire Murray, David Murray, Stephanie Needham, Ashutosh Nerurkar, John O’Dowd, Gary Parves, Demetris Poyiatzis, Elena Provenzano, Colin A Purdie, Lilani Ranasigne, Majid Rashid, Sarah Read-Jones, David Rowlands, Nick Ryley, Luise Seargent, Abeer Shaaban, Balvinder Shoker, Sophia Stanford, Andrew Wagerfield, Malcolm West, Cate Wight, and Fergus Young.
Funding
This work was supported by Biotheranostics, Inc. (no grant number is applicable), Breast Cancer Research Foundation (grant numbers BCRF-17-145, BCRF-18-145) and Ontario Institute for Cancer Research (grant number IA-036).
Disclosure
JMSB receives honoraria from Oncology Education. He is a consultant/advisor for BioNTech AG, Biotheranostics, Inc., Insight Genetics, Pfizer, and RNA Diagnostics and receives travel support from Biotheranostics, Inc. He receives research funding from Genoptix, MammaPrint, NanoString Technologies, Stratifyer GmbH, and ThermoFisher Scientific. He holds patents for Methods and Devices for Predicting Anthracycline Treatment Efficacy and Systems and Devices and Methods for Constructi. DCS is a consultant for Merrimack. DCS, YZ, and CAS are named inventors on a patent to use the HOXB13/IL17BR and Molecular Grade Index assays to predict breast cancer outcome. KT, YZ, CAS, and RS are employees and shareholders of Biotheranostics, Inc. EFB is a consultant for Philips Healthcare and receives research funding from Biotheranostics, Inc. DWR receives honoraria from Genomic Health, Novartis, Pfizer, and Roche and research funding form Biotheranostics, Inc., Celgene and Roche. All remaining authors, IA, TP, and SJP, have declared no conflicts of interest.
References
- 1. Meisel JL, Venur VA, Gnant M, Carey L.. Evolution of targeted therapy in breast cancer: where precision medicine began. Am Soc Clin Oncol Educ Book 2018; (38): 78–86. [DOI] [PubMed] [Google Scholar]
- 2. Bartlett JM, Brookes CL, Robson T. et al. Estrogen receptor and progesterone receptor as predictive biomarkers of response to endocrine therapy: a prospectively powered pathology study in the Tamoxifen and Exemestane Adjuvant Multinational trial. J Clin Oncol 2011; 29(12): 1531–1538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Regan MM, Pagani O, Francis PA. et al. Predictive value and clinical utility of centrally assessed ER, PgR, and Ki-67 to select adjuvant endocrine therapy for premenopausal women with hormone receptor-positive, HER2-negative early breast cancer: TEXT and SOFT trials. Breast Cancer Res Treat 2015; 154(2): 275–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Dowsett M, Allred C, Knox J. et al. Relationship between quantitative estrogen and progesterone receptor expression and human epidermal growth factor receptor 2 (HER-2) status with recurrence in the Arimidex, Tamoxifen, Alone or in Combination trial. J Clin Oncol 2008; 26(7): 1059–1065. [DOI] [PubMed] [Google Scholar]
- 5. Early Breast Cancer Trialists' Collaborative GroupDavies C, Godwin J. et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet 2011; 378: 771–784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sparano JA, Gray RJ, Makower DF. et al. Adjuvant chemotherapy guided by a 21-gene expression assay in breast cancer. N Engl J Med 2018; 379(2): 111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cardoso F, van’t Veer LJ, Bogaerts J. et al. 70-Gene signature as an aid to treatment decisions in early-stage breast cancer. N Engl J Med 2016; 375(8): 717–729. [DOI] [PubMed] [Google Scholar]
- 8. Sestak I, Buus R, Cuzick J. et al. Comparison of the performance of 6 prognostic signatures for estrogen receptor-positive breast cancer: a secondary analysis of a randomized clinical trial. JAMA Oncol 2018; 4(4): 545–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pan H, Gray R, Braybrooke J. et al. 20-Year risks of breast-cancer recurrence after stopping endocrine therapy at 5 years. N Engl J Med 2017; 377(19): 1836–1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Goss PE, Ingle JN, Martino S. et al. Randomized trial of letrozole following tamoxifen as extended adjuvant therapy in receptor-positive breast cancer: updated findings from NCIC CTG MA.17. J Natl Cancer Inst 2005; 97(17): 1262–1271. [DOI] [PubMed] [Google Scholar]
- 11. Mamounas EP, Jeong JH, Wickerham DL. et al. Benefit from exemestane as extended adjuvant therapy after 5 years of adjuvant tamoxifen: intention-to-treat analysis of the National Surgical Adjuvant Breast And Bowel Project B-33 trial. J Clin Oncol 2008; 26(12): 1965–1971. [DOI] [PubMed] [Google Scholar]
- 12. Davies C, Pan H, Godwin J. et al. Long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years after diagnosis of oestrogen receptor-positive breast cancer: ATLAS, a randomised trial. Lancet 2013; 381(9869): 805–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gray GR, Rea D, Handley K. et al. aTTom: long-term effects of continuing adjuvant tamoxifen to 10 years versus stopping at 5 years in 6,953 women with early breast cancer. J Clin Oncol 2013; 31(Suppl 18): 5.23169505 [Google Scholar]
- 14. Mamounas EP, Bandos H, Lembersky BC. et al. Use of letrozole after aromatase inhibitor-based therapy in postmenopausal breast cancer (NRG Oncology/NSABP B-42): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol 2019; 20(1): 88–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gradishar WJ, Anderson BO, Balassanian R. et al. NCCN guidelines insights: breast cancer, Version 1.2017. J Natl Compr Canc Netw 2017; 15(4): 433–451. [DOI] [PubMed] [Google Scholar]
- 16. Senkus E, Kyriakides S, Ohno S. et al. Primary breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2015; 26(Suppl 5): v8–v30. [DOI] [PubMed] [Google Scholar]
- 17. Burstein HJ, Lacchetti C, Anderson H. et al. Adjuvant endocrine therapy for women with hormone receptor-positive breast cancer: ASCO clinical practice guideline focused update. J Clin Oncol 2019; 37(5): 423–438. [DOI] [PubMed] [Google Scholar]
- 18. Sgroi DC, Carney E, Zarrella E. et al. Prediction of late disease recurrence and extended adjuvant letrozole benefit by the HOXB13/IL17BR biomarker. J Natl Cancer Inst 2013; 105(14): 1036–1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sgroi DC, Sestak I, Cuzick J. et al. Prediction of late distant recurrence in patients with oestrogen-receptor-positive breast cancer: a prospective comparison of the breast-cancer index (BCI) assay, 21-gene recurrence score, and IHC4 in the TransATAC study population. Lancet Oncol 2013; 14(11): 1067–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zhang Y, Schnabel CA, Schroeder BE. et al. Breast cancer index identifies early-stage estrogen receptor-positive breast cancer patients at risk for early- and late-distant recurrence. Clin Cancer Res 2013; 19(15): 4196–4205. [DOI] [PubMed] [Google Scholar]
- 21. Sgroi DC, Sestak I, Zhang Y. et al. Evaluation of prognostic and predictive performance of breast cancer index and its components in hormonal receptor-positive breast cancer patients: a TransATAC study. Cancer Res 2012; 72(Suppl); Abstr no. P2-10-15. [Google Scholar]
- 22. Rea DG, Bowden SJ, Handley K. et al. Overall and subgroup findings of the aTTom trial: a randomised comparison of continuing adjuvant tamoxifen to 10 years compared to stopping after 5 years in 6953 women with ER positive or ER untested early breast cancer. Eur J Cancer 2013; 49: S402. [Google Scholar]
- 23. Simon RM, Paik S, Hayes DF.. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Natl Cancer Inst 2009; 101(21): 1446–1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mehta CR, Pocock SJ.. Adaptive increase in sample size when interim results are promising: a practical guide with examples. Statist Med 2011; 30(28): 3267–3284. [DOI] [PubMed] [Google Scholar]
- 25. Cox DR. Regression models and life-tables. J Roy Stat Soc Ser B (Methodological) 1972; 34(2): 187–220. [Google Scholar]
- 26. Loi S, Symmans WF, Bartlett JMS. et al. Proposals for uniform collection of biospecimens from neoadjuvant breast cancer clinical trials: timing and specimen types. Lancet Oncol 2011; 12(12): 1162–1168. [DOI] [PubMed] [Google Scholar]
- 27. Harris LN, Ismaila N, McShane LM. et al. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early-stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 2016; 34(10): 1134–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Duffy MJ, Harbeck N, Nap M. et al. Clinical use of biomarkers in breast cancer: updated guidelines from the European Group on Tumor Markers (EGTM). Eur J Cancer 2017; 75: 284–298. [DOI] [PubMed] [Google Scholar]
- 29. Francis PA, Regan MM, Fleming GF. et al. Adjuvant ovarian suppression in premenopausal breast cancer. N Engl J Med 2015; 372(5): 436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pagani O, Regan MM, Walley BA. et al. Adjuvant exemestane with ovarian suppression in premenopausal breast cancer. N Engl J Med 2014; 371(2): 107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.