Abstract
BACKGROUND
Functional Electrical Stimulation (FES) applied through a foot drop stimulator (FDS) is a rehabilitation intervention that can stimulate the common peroneal nerve to provide dorsiflexion at the correct timing during gait.
OBJECTIVE
To determine if FES applied to the peroneal nerve during walking through a FDS would effectively retrain the electromyographic temporal activation of the tibialis anterior in individuals with stroke.
METHODS
Surface electromyography (EMG) were collected bilaterally from the tibialis anterior (TA) while participants (n = 4) walked with and without the FDS at baseline and 4 weeks. Comparisons were made between stimulation timing and EMG activation timing to produce a burst duration similarity index (BDSI).
RESULTS
At baseline, participants displayed variable temporal activation of the TA. At 4 weeks, TA activation during walking without the FDS more closely resembled the pre-programmed FDS timing demonstrated by an increase in BDSI scores in all participants (P = 0.05).
CONCLUSIONS
Continuous use of FDS during a task specific movement can re-train the neuromuscular system. After 4 weeks of utilization the FDS trained the TA to replicate the programmed temporal activation patterns. These findings begin to establish the FDS as a rehabilitation intervention that may facilitate recovery rather than just compensate for stroke related gait impairments due to foot drop.
Keywords: Stroke, foot drop, hemiplegic gait, EMG, functional electrical stimulation, rehabilitation
1. Introduction
Foot drop (FD) is one of the most common disabling impairments resulting from hemiplegia secondary to stroke (Morone, Fusco, Di Capua, Coiro, & Pratesi, 2012). FD results from weakness and/or lack of voluntary control in the ankle and toe dorsiflexor muscles (Stein et al., 2010). During walking this can cause decreased speed, a disruption in weight acceptance and weight transfer, and an inefficient and unstable gait (Nolan, Savalia, Lequerica, & Elovic, 2009; Nolan & Yarossi, 2011b). The inability to lift the foot during the swing phase of gait causes decreased mobility and disturbances in healthy walking patterns leading to limitations in activities in daily living and long term disability (American Heart Association, 2013). Individuals with post-stroke gait impairments experiencing FD have previously been associated with compensatory strategies such as excessive hip abduction and circumduction which can be further linked to decreased speed, decreased mechanical efficiency, and increased energy expenditure (Perry & Burnfield, 2010). The standard of care for treating FD in chronic stroke has been the application of an ankle foot orthosis (AFO) to compensate for FD throughout the gait cycle. Previous research has shown that AFOs provide an orthotic effect creating an increase in gait speed and functional ambulation (Nolan et al., 2009; Nolan & Yarossi, 2011a). Although the AFO can compensate for foot drop by holding the foot in a neutral position to prevent FD during the swing phase of the gait cycle (Nolan & Yarossi, 2011a), as a rehabilitation intervention it is not targeted to restore muscle function (Stein et al., 2010).
Functional electrical stimulation (FES) used as a targeted rehabilitation intervention may promote motor recovery, especially when applied in a task specific environment (Chae, 2003; Daly & Ruff, 2007; Peckham & Knutson, 2005; Sheffler & Chae, 2007). Motor learning using FES is thought to work through the creation of positive feedback of residual myoelectric activity acting to increase long term potentiation (LTP) of the corticomuscular connection (Chae, 2003; Peckham & Knutson, 2005; Sheffler & Chae, 2007; Stein et al., 2006). Active repetitive movement training in combination with task relevant FES has shown to be perhaps the most promising use of FES for the facilitation of motor recovery (Chae, 2003). Specifically cyclic or electromyography (EMG) based FES activation that acts to promote the movement goal in combination with voluntary effort has been found to produce greater physiological and functional gains than FES alone (Daly & Ruff, 2007).
FES applied to the common peroneal nerve through a foot drop stimulator (FDS) provides targeted FES to the peroneal nerve to promote active dorsiflexion (lift the foot) during the swing phase of gait to sufficiently clear the foot (Everaert et al., 2013; Everaert, Thompson, Chong, & Stein, 2010; Sabut, Lenka, Kumar, & Mahadevappa, 2010). Previous research evaluating the immediate orthotic effect demonstrated small changes in walking speed, improved dorsiflexion angle, and improved temporal-spatial characteristics (Everaert et al., 2013; Knutson & Chae, 2010; Kottink et al., 2007; Sabut et al., 2010; Taylor et al., 1999; Taylor et al., 1999). Research investigating FES in individuals with acute and chronic hemiplegia and FD secondary to stroke indicate that this technology has the potential to restore physiological function and improve community ambulation (Robbins, Houghton, Woodbury, & Brown, 2006; Sabut, Sikdar, Mondal, Kumar, & Mahadevappa, 2010). These results demonstrate the efficacy for FDS utilization in post stroke rehabilitation but they fail to precisely indicate how FDS technology can restore motor function (Everaert et al., 2013; Kesar et al., 2010; Kesar et al., 2009, 2011; Kottink et al., 2004; Stein et al., 2006, 2010; Taylor et al., 1999).
Using the FDS to drive muscle groups in specific activation patterns during walking may improve strength and retrain activation timing (Daly & Ruff, 2007). Changes in activation can occur because the FDS provides task-specific movements to the foot and ankle complex during ambulation which has significant long term benefits for the stroke survivor by helping to restore function. The therapeutic changes in muscular coordination during gait that may result from use of a FDS have not been investigated. In healthy gait, correct activation of the dorsiflexor muscles is characterized by eccentric activation following initial contact, followed by inactivity during single leg stance and finally activation to lift the foot during swing (Perry & Burnfield, 2010). This investigation will assess the retraining effect of using a community based FDS on the electromyographic (EMG) temporal activation of the tibialis anterior (TA) during gait in individuals with hemiplegia and FD resulting from stroke. The purpose of this investigation was to determine if FES applied to the peroneal nerve during dynamic walking through a foot drop stimulator would effectively retrain the EMG temporal activation of the tibialis anterior in individuals with stroke.
2. Methods
2.1. Participants
Individuals with drop foot and hemiplegia secondary to stroke (>3 months) were recruited for participation. Drop foot was determined as inadequate dorsiflexion during the swing phase of gait, resulting in inadequate clearance, defined as −5° plantar flexion. All participants received a commercially available FDS (Walkaide®, Innovative Neurotronics, Inc., Austin, TX, USA) at baseline for use during ambulation as part of the larger multi-site clinical trial. All participants had a positive response to peroneal nerve stimulation testing resulting in adequate dorsiflexion of the ankle. Individuals were able to walk independently for 10 meters without any assistive device and had an initial gait speed <0.8 m/s. All participants were at least 30 days post physical rehabilitation on the lower extremities at time of enrollment and did not receive any lower limb rehabilitation therapy or gait training during the investigation. All procedures performed in this investigation were approved by the Human Subjects Review Board and informed consent was obtained prior to study participation.
2.2. Foot drop stimulator (FDS)
Surface functional electrical stimulation was applied to the peroneal nerve through a commercially available FDS during community ambulation for a period of 4-weeks. Surface FES provides electrically induced muscle activation during the swing phase of gait and initial contact.
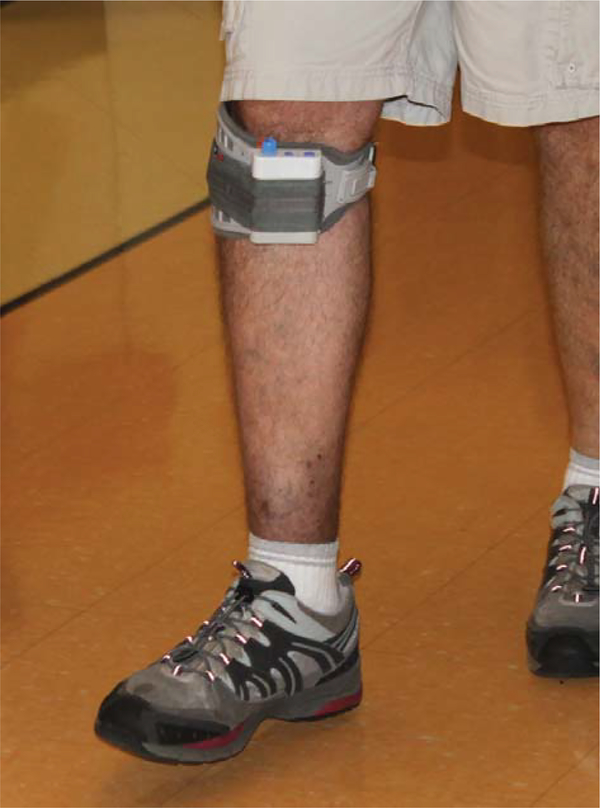
The Walkaide® is a battery operated single-channel, asymmetrical biphasic stimulator with programmable pulse width and frequency that was utilized during walking as a FES orthotic device (Fig. 1). The selected technology is controlled by a tilt sensor and accelerometer to provide electrically induced muscle activation (dorsiflex the foot) at the appropriate time during gait. None of the participants utilized a heel switch to initiate dorsiflexion. The small device (87.9 g, 8.2 cm(H) × 6.1 cm(W) × 2.1 cm(T)) was attached to a molded cuff located just below the knee, secured with a latch, and properly aligned using anatomical landmarks and visual indicators. Two surface electrodes were specifically placed near the head of the fibula, directly over the motor nerve and proximal musculature. Each participant used their own FDS device for daily ambulation in the community and for all walking tests. Each device was custom programmed (stimulus intensity, timing and duration of muscle activation) during the gait cycle by a licensed clinician at baseline.
Fig. 1.
The Walkaide® used for addressing foot drop.
2.3. Procedures
Participants completed 10 walking trials (5 with FDS and 5 without FDS) at a self-selected pace on level ground (4.5 meters) at baseline and following 4 weeks of FDS use. Participants were allowed to stop and rest if necessary and members of the study team provided noncontact guarding during all walking trials for safety. Participants wore shoes for all walking trials and no comparisons were made to a barefoot condition. Surface EMG (MotionLab Systems, Inc. Baton Rouge, LA, USA) were collected bilaterally from the tibialis anterior (TA) at 2520 Hz during all walking tests.
2.4. Data analysis and outcome measures
Demographic information including age, gender and time since stroke was collected and verified with medical records. Data from all assessments are represented as mean (standard deviation).
All data were imported into Matlab (The Mathworks, Inc., Natick, MA, USA) for custom processing and analysis. EMG data were filtered at 20 to 300 Hz, full wave rectified and a root mean square average was applied with a 50 ms time window. The EMG amplitudes were normalized to the maximum voluntary contractions (MVCs). MVCs were done in accordance with SENIAM (Hermens, Freriks, & Merletti, 2000). Previous research has used this alternative methodology in a supine position when the standard technique during standing is not possible (Petersen, Kliim-Due, Farmer, & Nielsen, 2010; Sabut et al., 2010). Baseline EMG was calculated for each muscle as the 500 ms data found to be least active during the gait cycle. The time of onset of EMG for each of the muscles was identified using a computer algorithm that identified the point where the root mean square averaged EMG exceeded the mean of the baseline by 3 standard deviations for a period of 50 ms. The accuracy of EMG onset determined using this method has been shown to be high (Hodges & Bui, 1996). All traces were evaluated visually to verify onset and to ensure onset is not obscured by movement artifact. For each participant, at least 8 gait cycles that represented the normal stimulus response were averaged. The gait data was subdivided into initial double support (IDS), single support (SS), terminal double support (TDS) and swing (SW) based on heel strike and toe off events recorded using 3D Motion Capture System (Vicon Inc, Denver, CO). The data was further normalized to 0–100% of a gait cycle based on two successive heel strikes using Matlab.
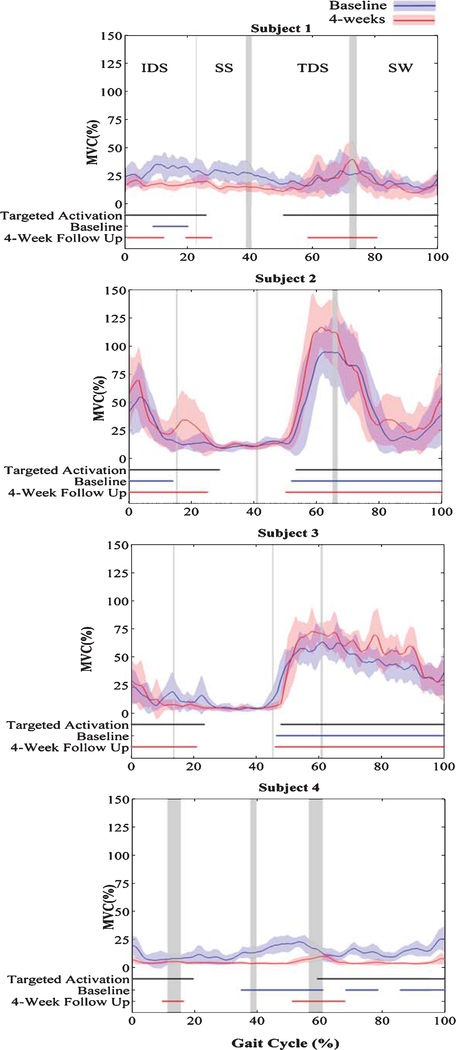
To illustrate a retraining effect on the TA, the temporal activation of the TA with the FDS (Fig. 2a) was used as a reference signal or a “training” signal for TA activation because it represents the corrected activation pattern for the affected TA muscle. This signal also demonstrates the custom timing parameters that were programmed for each participant. The TA was trained through FDS intervention during walking in the community for 4 weeks using these programmed electrical stimulations. Comparisons were made between the training signal and the TA EMG (baseline and 4-week follow up) when stimulation was absent to determine the burst duration similarity index (BDSI). The algorithm for calculating the BDSI from two EMG recordings, Signal 1 (S1) and Signal 2 (S2) are summarized in three steps below.
Fig. 2.
Normalized TA EMG without FDS, targeted activation timing (black) and TA activation timing at baseline (blue) and 4-weeks (red) for four hemiplegic individuals during walking without FDS.
Determine ‘on’ and ‘off’ times for the normalized EMGs S1 and S2 of length N, using commonly utilized 3-standard deviation-threshold onset detection technique (Hodges & Bui, 1996).
- Determine the instances at which S1 and S2 were active (on-timing) as well as inactive (off-timing) simultaneously during a normalized gait (0–100%).
- On-timing – a binary vector of length N with 1 indicating simultaneous activation of S1 and S2 and 0 otherwise.
- Off-timing – a binary vector of length N with 1 indicating simultaneous inactivation of S1 and S2 and 0 otherwise.
The BDSI, as a function of two EMG signals, f(S1, S2), is calculated as,
| (1) |
2.5. Statistical analysis
Paired sample t-tests were performed to determine if there were significant differences in BDSI scores between baseline and 4-week follow-up visits (P ≤ 0.05). BDSI scores allow us to determine if FES applied to the peroneal nerve during dynamic walking using the FDS would effectively retrain the EMG temporal activation of the TA in individuals with stroke.
Secondary analysis investigated if regular use of FDS in a community environment improved the motor unit recruitment of the stimulated muscle of the affected side. Paired sample t-tests were used to determine if there were significant differences in the mean TA EMG amplitudes at the baseline and 4-weeks follow-up visits during each phase of the gait cycle.
3. Results
Four individuals (3 male, 1 female) with right-sided FD and hemiplegia 57.2 ± 29 months post stroke were recruited for participation (age 63.7 ± 8.5 y; height 175.3 ± 8.0 c; mass 100.3 ± 32.3 kg.) All participants used their own FDS custom programmed by the same licensed clinician on their affected limb during ambulation for 4 weeks. The FDS was positioned below the knee, secured with a latch, and properly aligned using anatomical landmarks and visual indicators. None of the subjects reported any problems with the functionality of the device over the 4 week period.
Figure 2 displays the normalized TA EMG activity (% of MVC) averaged over multiple gait cycles at baseline and 4 weeks post FDS utilization. The Targeted Activation is the FDS on and off timing during normalized gait, which remained consistent throughout the investigation (baseline to 4 weeks). This targeted signal was customized for each participant by a licensed clinician during FDS programming. Baseline and 4-Week Follow-Up represent the affected (right) side TA EMG on and off activation timing (during walking without FDS) for 4 participants at baseline and 4 weeks post FDS utilization.
At baseline, participants displayed variable temporal activation of the TA during walking including the inability to activate the TA without the FDS at foot strike (subjects 1, 3, 4), during initial double support (subject 1–4), in preparation for swing (subject 1, 4), and during swing (subject 1, 4). Following 4 weeks of FDS utilization TA activation during walking without the FDS more closely resembled the preprogramed FDS stimulation timing in all subjects (Fig. 2).
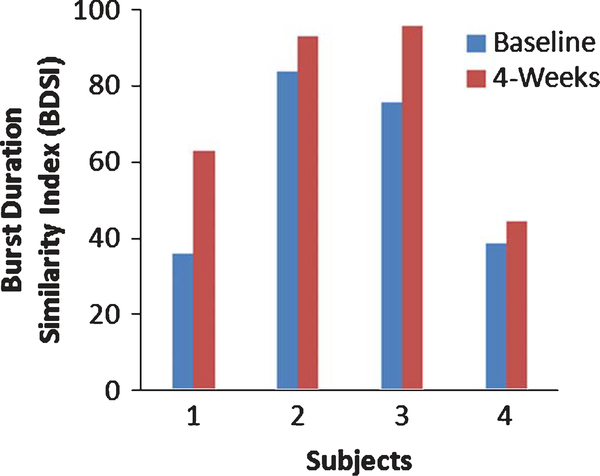
This potential training effect is further demonstrated by improved BDSI scores in all 4 participants after 4 weeks of FDS utilization (Fig. 3). The BDSI score signifcantly increased for all partcipants after 4 weeks of FDS use (Baseline: 58.4(24.7), 4 weeks: 74.1(24.7); t(3) = −3.19, P ≤ 0.05). The BDSI score for subject 1 improved 75.4%, from 35.9 to 63, as TA activation improved at foot strike, during IDS and SW. Subjects 2, 3 and 4 also showed increase in the BDSI scores by 11%, 27% and 15.3%, respectively.
Fig. 3.
BDSI scores for 4 subjets at baseline and 4 weeks after the FDS use.
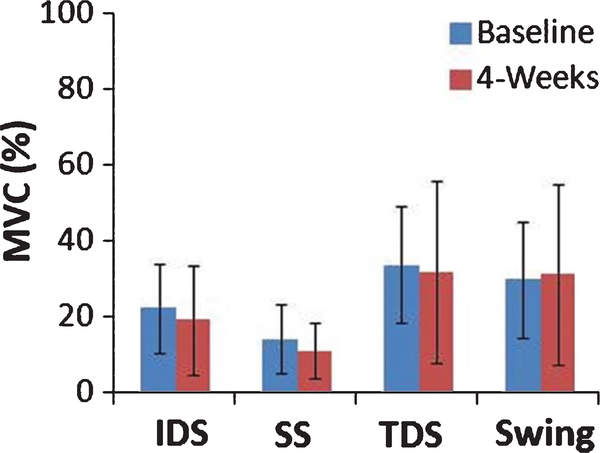
Secondary analysis evaluated the significant differences between mean TA EMG amplitudes at baseline and 4-weeks during each phase of the gait cycle. During all phases of gait (IDS, SS, TDS, SW) the mean TA EMG amplitude shows a trend towards decreasing after 4 weeks of FDS. The mean TA EMG amplitudes during IDS (P = 0.386), SS (P = 0.461), TDS (P = 0.707), and SW (P = 0.773) were not significantly different after 4 weeks of FDS.
4. Discussion
After 4 weeks of utilization, the FDS trained the TA to replicate the ‘targeted’ EMG temporal activation patterns. FDS provides an active way to train the neuromuscular system as an alternative to passive ankle foot orthoses. Previous research suggested that the FDS could improve walking speed, range of motion (ROM) and temporal/spatial gait parameters in individuals with stroke and FD (Everaert et al., 2013; Knutson & Chae, 2010; Kottink et al., 2007; Sabut et al., 2010; Taylor et al., 1999; Taylor et al., 1999). This study extends previous findings by investigating the ability of an FDS device to retrain activation patterns.
Prior to the FDS utilization, individuals with stroke displayed variable temporal activation of the TA including the inability to activate and deactive the TA at the correct timing for efficeint gait. After 4 weeks of FDS use, TA EMGs more closely resembled the pre-programmed activation stimulation timing of the FDS device. These changes were characterized by increases in the length of activation as well as more appropriate activation and cessatation timing (Fig. 2). This provides clear evidence of the ability of the FDS device to train the temporal activation pattern of the TA. The ability to correct EMG activation timing after 4 weeks of FDS use could indicate an adaptation in the impaired neuromuscular system, a direct result of the FES application.
The improvements in the temporal activation of the TA in the current investigation were found in absence of significant changes in the amplitude of activation (Fig. 4). Previous research investigating FDS has mainly focused on EMG amplitude and MVCs. Sabut et al. (2010) found increase in root mean square EMG during maximal voluntary after 12 weeks of FES, and Everhart et al. (2010) also showed a significant increase in MVCs after 3–12 months of FES utilization. These investigations only evaluated the magnitude of activation and not the timing of TA activation during gait. When evaluating the effect of the FDS on gait rehabilitation in individuals with stroke it may be more appropriate to look at correct muscle coordination evidenced by the correct temporal activation of EMG during gait than solely the amplitude of muscular contraction. Furthermore, the current investigation demonstrated that these changes in TA coordination may occur after a comparatively short period of time which provides greater evidence that these changes may be associated with improved motor planning a corticospinal connectivity rather than muscle strengthening.
Fig. 4.
Mean EMG amplitudes during initial double support (IDS), single support (SS), terminal double support (TDS) and swing phase at baseline and 4 weeks across 4 individuals with standard error.
A potential limitation of this investigation is that we did not attempt to evaluate the accuracy of the targeted activation, or improvement in gait patterns. The investigation demonstrated how consistent use of a FDS during a task specific movement such as gait can affect the neuromuscular system. An additional possible limitation of this investigation is the small sample size. The data indicated promising trends with the FDS and future research with a larger sample will allow us to explore the generalizability of these results and the potential utility of a FDS for indivduals with stroke. Research investigating the training effect of a FDS in a larger sample will provide additional information on responders and non-responders of this type of intervention. Future research will continue to evaluate TA training using the FDS and correlate our current findings with anticipated to improvements in gait and functional recovery.
5. Conclusion
The purpose of this investigation was to determine if FES applied to the peroneal nerve during dynamic walking using a FDS would effectively retrain the temporal activation of the TA in individuals with stroke. After 4 weeks of utilization the FDS trained the TA to replicate the ‘training’ EMG temporal activation patterns and showed significantly improved BDSI scores. Although, there was no statistical difference between mean EMG amplitudes at baseline and 4-weeks, these findings begin to establish the FDS as a rehabilitation intervention that may facilitate recovery rather than just compensate for stroke related gait impairments due to FD.
Acknowledgments
The authors would like to thank Arvind Ramanujam, MS and Kathleen Chervin, BS for their valuable help in the data collection for this study.
Footnotes
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- American Heart Association (2013). Heart Disease and Stroke Statistics – 2013 Update: A Report From the American Heart Association. Circulation, 127(1), e6–e245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae J (2003). Neuromuscular electrical stimulation for motor relearning in hemiparesis. Phys Med Rehabil Clin N Am, 14(1 Suppl), S93–109. [DOI] [PubMed] [Google Scholar]
- Daly JJ, & Ruff RL (2007). Construction of efficacious gait and upper limb functional interventions based on brain plasticity evidence and model-based measures for stroke patients. Scientific World Journal, 7, 2031–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everaert DG, Stein RB, Abrams GM, Dromerick AW, Francisco GE, Hafner BJ, Huskey TN, Munin MC, Nolan KJ, & Kufta CV (2013). Effect of a foot-drop stimulator and ankle-foot orthosis on walking performance after stroke: A multicenter randomized controlled trial. Neurorehabil Neural Repair, 27(7), 579–591. [DOI] [PubMed] [Google Scholar]
- Everaert DG, Thompson AK, Chong SL, & Stein RB (2010). Does functional electrical stimulation for foot drop strengthen corticospinal connections? Neurorehabil Neural Repair, 24(2), 168–177. [DOI] [PubMed] [Google Scholar]
- Hermens HJ, Freriks B, & Merletti R (2000). European Recommendations for Surface Electromyography: Results of the Seniam Project (SENIAM). The Netherlands Roessingh Research and Development [Google Scholar]
- Hodges PW, & Bui BH (1996). A comparison of computer-based methods for the determination of onset of muscle contraction using electromyography. Electroencephalography and Clinical Neurophysiology/Electromyography and Motor Control, 101(6), 511–519. [DOI] [PubMed] [Google Scholar]
- Kesar TM, Perumal R, Jancosko A, Reisman DS, Rudolph KS, Higginson JS, & Binder-Macleod SA (2010). Novel patterns of functional electrical stimulation have an immediate effect on dorsiflexor muscle function during gait for people poststroke. Phys Ther, 90(1), 55–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar TM, Perumal R, Reisman DS, Jancosko A, Rudolph KS, Higginson JS, & Binder-Macleod SA (2009). Functional electrical stimulation of ankle plantarflexor and dorsiflexor muscles: Effects on poststroke gait. Stroke, 40(12), 3821–3827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesar TM, Reisman DS, Perumal R, Jancosko AM, Higginson JS, Rudolph KS, & Binder-Macleod SA (2011). Combined effects of fast treadmill walking and functional electrical stimulation on post-stroke gait. Gait Posture, 33(2), 309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson JS, & Chae J (2010). A novel neuromuscular electrical stimulation treatment for recovery of ankle dorsiflexion in chronic hemiplegia: A case series pilot study. Am J Phys Med Rehabil, 89(8), 672–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottink AI, Hermens HJ, Nene AV, Tenniglo MJ, van der Aa HE, Buschman HP, & Ijzerman MJ (2007). A randomized controlled trial of an implantable 2-channel peroneal nerve stimulator on walking speed and activity in poststroke hemiplegia. Arch Phys Med Rehabil, 88(8), 971–978. [DOI] [PubMed] [Google Scholar]
- Kottink AI, Oostendorp LJ, Buurke JH, Nene AV, Hermens HJ, & MJ IJ (2004). The orthotic effect of functional electrical stimulation on the improvement of walking in stroke patients with a dropped foot: A systematic review. Artif Organs, 28(6), 577–586. [DOI] [PubMed] [Google Scholar]
- Morone G, Fusco A, Di Capua P, Coiro P, & Pratesi L (2012). Walking training with foot drop stimulator controlled by a tilt sensor to improve walking outcomes: A randomized controlled pilot study in patients with stroke in subacute phase. Stroke Res Treat, Article ID: 523564, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan KJ, Savalia KK, Lequerica AH, & Elovic EP (2009). Objective Assessment of Functional Ambulation in Adults with Hemiplegia using Ankle Foot Orthotics after Stroke. PM R, 1(6), 524–529. [DOI] [PubMed] [Google Scholar]
- Nolan KJ, & Yarossi M (2011a). Preservation of the First Rocker is Related to Increases in Gait Speed in Individuals with Hemiplegia and AFO. Clinical Biomechanics, 26(6), 655–660. [DOI] [PubMed] [Google Scholar]
- Nolan KJ, & Yarossi M (2011b). Weight Transfer Analysis in Adults with Hemiplegia Using Ankle Foot Orthosis Prosthetics and Orthotics International, 35(1), 45–53. [DOI] [PubMed] [Google Scholar]
- Peckham PH, & Knutson JS (2005). Functional electrical stimulation for neuromuscular applications. Annu Rev Biomed Eng, 7, 327–360. [DOI] [PubMed] [Google Scholar]
- Perry J, & Burnfield JM (2010). Gait Analysis: Normal and Pathological Function, 2nd ed. (2nd ed.). Thorofare, NJ: Slack Incorporated. [Google Scholar]
- Petersen TH, Kliim-Due M, Farmer SF, & Nielsen JB (2010). Childhood development of common drive to a human leg muscle during ankle dorsiflexion and gait. J Physiol, 588(Pt 22), 4387–4400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins SM, Houghton PE, Woodbury MG, & Brown JL (2006). The therapeutic effect of functional and transcutaneous electric stimulation on improving gait speed in stroke patients: A meta-analysis. Arch Phys Med Rehabil, 87(6), 853–859. [DOI] [PubMed] [Google Scholar]
- Sabut SK, Lenka PK, Kumar R, & Mahadevappa M (2010). Effect of functional electrical stimulation on the effort and walking speed, surface electromyography activity, and metabolic responses in stroke subjects. J Electromyogr Kinesiol, 20(6), 1170–1177. [DOI] [PubMed] [Google Scholar]
- Sabut SK, Sikdar C, Mondal R, Kumar R, & Mahadevappa M (2010). Restoration of gait and motor recovery by functional electrical stimulation therapy in persons with stroke. Disabil Rehabil, 32(19), 1594–1603. [DOI] [PubMed] [Google Scholar]
- Sheffler LR, & Chae J (2007). Neuromuscular electrical stimulation in neurorehabilitation. Muscle Nerve, 35(5), 562–590. [DOI] [PubMed] [Google Scholar]
- Stein RB, Chong S, Everaert DG, Rolf R, Thompson AK, Whittaker M, Robertson J, Fung J, Preuss R, Momose K, & Ihashi K (2006). A multicenter trial of a footdrop stimulator controlled by a tilt sensor. Neurorehabil Neural Repair, 20(3), 371–379. [DOI] [PubMed] [Google Scholar]
- Stein RB, Everaert DG, Thompson AK, Chong SL, Whittaker M, Robertson J, & Kuether G (2010). Long-term therapeutic and orthotic effects of a foot drop stimulator on walking performance in progressive and nonprogressive neurological disorders. Neurorehabil Neural Repair, 24(2), 152–167. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Burridge JH, Dunkerley AL, Lamb A, Wood DE, Norton JA, & Swain ID (1999). Patients’ perceptions of the Odstock Dropped Foot Stimulator (ODFS). Clin Rehabil, 13(5), 439–446. [DOI] [PubMed] [Google Scholar]
- Taylor PN, Burridge JH, Dunkerley AL, Wood DE, Norton JA, Singleton C, & Swain ID (1999). Clinical use of the Odstock dropped foot stimulator: Its effect on the speed and effort of walking. Arch Phys Med Rehabil, 80(12), 1577–1583. [DOI] [PubMed] [Google Scholar]