Abstract
There has been an increase in the mortality rate and morbidity of kidney cancer (KC) with kidney renal clear cell carcinoma (KIRC) being the most common subtype of KC. GRAMD1C (GRAM Domain Containing 1C) has not been reported to relate to prognosis and immunotherapy in any cancers. Using bioinformatics methods, we judged the prognostic value of GRAMD1C expression in KIRC and investigated the underlying mechanisms of GRAMD1C affecting the overall survival of KIRC based on data downloaded from The Cancer Genome Atlas (TCGA). The outcome revealed that reduced GRAMD1C expression could be a promising predicting factor of poor prognosis in kidney renal clear cell carcinoma. Meanwhile, GRAMDIC expression was significantly correlated to several tumor-infiltrating immune cells (TIICs), particularly the regulatory T cells (Tregs). Furthermore, GRAMD1C was most significantly associated with the mTOR signaling pathway, RNA degradation, WNT signaling pathway, toll pathway and AKT pathway in KIRC. Thus, GRAMD1C has the potential to become a novel predictor to evaluate prognosis and immune infiltration for KIRC patients.
Keywords: Kidney renal clear cell carcinoma, Biomarkers, Bioinformatics, TCGA, Immune infiltrate, GRAMD1C
Introduction
In all malignancies of adults worldwide, among which around 2% to 3% are afflicted by kidney cancer (KC) (Ferlay et al., 2010; Torre et al., 2015). Meanwhile, the highest incidence amongst all the urinary system tumors is the case of kidney cancer. There has been an increase in the mortality rate and morbidity of KC with kidney renal clear cell carcinoma (KIRC) being the most common subtype of KC (Cohen & McGovern, 2005). Due to the ineffective therapeutic methods available at present and late diagnosis, the survival rate of KIRC is far from ideal. Nevertheless, KIRC is known to be amongst the earliest malignancies that responded to immunotherapy and being most responsive (Escudier, 2012; Atkins, Regan & McDermott, 2004). The treatment of renal tumors over the last two decades has significantly improved with the emergence of the immune checkpoint blockade (ICB), indicating a positive direction in KIRC towards immunotherapy (Song et al., 2017). The prognosis and efficacy of immunotherapy and chemotherapy have been found to be influenced by tumor-infiltrating immune cells like tumor-infiltrating neutrophils and tumor associated macrophages (Zhang et al., 2018; Waniczek et al., 2017). However, there is very little data examining the relationship between the tumor characteristics and immune responses. Hence, for the development of fresh targeted therapies and efficient prognosis of KIRC, the identification of the immune-related biomarkers for examining the progression of the malignancy becomes imperative.
The GRAM domain is an intracellular protein-binding or lipid-binding signaling domain (Jiang, Ramamoorthy & Ramachandran, 2008). The importance of the GRAM domain was indicated by the mutations disrupting its phosphatase activity leading to X-linked myotubular myopathy in case of myotubularin (Doerks et al., 2000). Under tumorigenesis, the other members of the GRAM domain family were also shown to play a part (John et al., 2011). For instance, recently some reports implicated that GRAMD1B was responsible for chemo-resistance of the ovarian cancer patients, while the isolation of this gene resulted in anti-tumor effect in combination with paclitaxel synergistically (Wu et al., 2014). GRAMD1C (GRAM Domain Containing 1C), an uncharacterized protein belonging to the GRAM domain family of proteins, has not been reported to relate to prognosis and immune response in any cancers. In present study, bioinformatic analysis using high throughput RNA-sequencing data from TCGA demonstrated that a reduced expression of GRAMD1C correlated to poor prognosis in KIRC.
Thus, the purpose of our present study was to evaluate the prognostic value of GRAMD1C expression in KIRC based on data downloaded from TCGA and investigated the underlying mechanisms of GRAMD1C affecting the prognosis of KIRC.
In our study, based on the gene expression profiles of KIRC and a comprehensive bioinformatics analysis, we analyzed the association of GRAMD1C with the characteristics of KIRC patients. Then, we investigated the correlation of GRAMD1C with TIICs in KIRC via a widely accepted evaluation algorithm, CIBERSORT. Moreover, we calculated the influence of different subtypes of TIICs on overall survival. The GSEA was performed finally with an intent to gain further breakthroughs in the underlying mechanism of GRAMD1C by identifying the biological pathways involved.
Materials & Methods
Data resources and data preprocessing
Gene expression profile and paired clinical information of KIRC patients, including 539 tumor samples and 72 para-carcinoma samples, were acquired from the Cancer Genome Atlas (TCGA) data portal (https://tcga-data.nci.nih.gov/tcga/), which serves as a public repository for archiving high-throughput microarray experimental data (Liu et al., 2018). The sequencing data of LUAD were generated with Illumina HiSeq_RNA-Seq platforms. In the subsequent processing, the trimmed mean of M values (TMM) normalization method was utilized for normalization of the downloaded data (Robinson, McCarthy & Smyth, 2010). The average expression data were calculated when met duplicate data. The processing process of the study fully satisfies the TCGA publication requirements. All preprocessing processes were realized by R software (version: 3.5.3) and Strawberry Perl. The processed expression data (Dataset S2) and script file for transformation and normalization of gene expression data (Dataset S3) have been uploaded into supplementary file. Cases lacking key clinical information, such as overall survival time, age, histologic grade (8 cases), gender, stage (3 cases), tumor status (T) (2 cases), and distant metastasis (M) (62 cases) were excluded. Then, we had to exclude all lymph node (N) data on account of the better part of KIRC cases downloaded from TCGA with no data on lymph node. Finally, mRNA expression level of 462 patients with kidney renal clear cell carcinoma and corresponding clinical information were reserved and used for further study. Table 1 uncovered KIRC patients’ characteristics were downloaded. Our study cohort, the mean age at diagnosis was 60 years old. Most patients were (n = 302, 65.4%) male, 160 (34.6%) were female. The histologic grade of KIRC in our study included undifferentiated (G4), poor-differentiated (G3), moderately-differentiated (G2) and well-differentiated (G1), separately taking up 1.9%, 42.0%, 41.1%, 14.9%. The tumor status contained 49.1% (n = 227) T1, 13.0% (n = 60) T2, 35.9% (n = 166) T3 and 1.9% (n = 9) T4. 73(15.8%) cases had distant metastasis and 389 (84.2%) had no-distant metastasis. At last contact, 307 (66.5%) subjects were alive, 155 (33.5%) were dead.
Table 1. Kidney renal clear cell carcinoma patient characteristics in TCGA database.
| Clinical characteristics | Total (462) | Percent | |
|---|---|---|---|
| Age at diagnosis (years) | 60(26–90) | ||
| Gender | Female | 160 | 34.6% |
| Male | 302 | 65.4% | |
| Histologic grade | G1 | 9 | 1.9% |
| G2 | 194 | 42.0% | |
| G3 | 190 | 41.1% | |
| G4 | 69 | 14.9% | |
| Stage | Stage I | 221 | 47.8% |
| Stage II | 49 | 10.6% | |
| Stage III | 116 | 25.1% | |
| Stage IV | 76 | 16.5% | |
| Tumor status | T1 | 227 | 49.1% |
| T2 | 60 | 13.0% | |
| T3 | 166 | 35.9% | |
| T4 | 9 | 1.9% | |
| Distant metastasis | Negative (M0) | 389 | 84.2% |
| Positive (M1) | 73 | 15.8% | |
| Vital status | Dead | 155 | 33.5% |
| Alive | 307 | 66.5% |
CIBERSORT estimation
CIBERSORT is a widely used approach (Gentles et al., 2015; Ceccarelli et al., 2016) to characterize the cell composition of complex tissues based on their gene expression profiles, and it is highly consistent with ground truth estimations in many cancers (Newman et al., 2015). After uploading the gene expression data with standard annotation on to the CIBERSORT web portal (http://cibersort.stanford.edu/), the algorithm using the LM22 signature was run (Newman et al., 2015). LM22, the annotated gene signature matrix defining 22 immune cell subtypes, was downloaded from the CIBERSORT web portal (http://cibersort.stanford.edu/) (Ge et al., 2019). Then, CIBERSORT derived a P-value for the deconvolution for each sample using Monte Carlo sampling, providing a measure of confidence in the results (Newman et al., 2015). Instances where the CIBERSORT output was of p < 0.05, it indicated that the inferred fractions of the immune cell populations produced by CIBERSORT were accurate (Anjum et al., 2016), and consequently further analysis with them was considered to be possible. For efficient comparison across the diverse samples, the CIBERSORT output were summarized to the Fig. S1, assisting in the visualization of the immune cell fraction of each sample. Types of immune cells could be sensitively and accurately discerned by CIBERSORT include T cells, B cells, macrophages, natural killer cells, dendritic cells and myeloid subsets. We grouped the samples into high and low GRAMD1C expressions based on median GRAMD1C expression value (1.922) to evaluate the difference of proportion of immune cells between high and low GRAMD1C expression.
Identification of prognostic subtypes of TIICs in KIRC
We tried to identify the prognosis-related immune cell subtypes in KIRC. Based on the immune cell fraction of each sample evaluated by CIBERSORT analysis and clinical information acquired from TCGA database, we performed survival curves using “survival” package. Considering that clinical stage is a crucial factor determining prognosis of KIRCs, boxplots of clinical stage were performed using “ggplot2” package to visualize the association between the proportions of different types of TIICs and clinical stage.
Gene set enrichment analysis
Gene set enrichment analysis (GSEA), a calculation method that could estimate whether a list of previous defined genes shows concordant differences with statistical significance between two biological processes (Subramanian et al., 2005). This study carried out the GSEA to elucidate the significant difference in survival rates observed between the low and high GRAMD1C groups after initially generating a sequential list of all genes according to their correlation to GRAMD1C expressions. For each analysis, the gene set permutations were performed 1000 times. The phenotype label was identified in the level of the GRAMD1C expression. In order to sort out the pathways enriched in each phenotype the Normalized Enrichment Score (NES), the nominal p value was utilized. The absolute value of NES>1.5 and P value < 0.05 were considered with statistical significance.
Statistical analysis
All statistical analyses were performed by R (v.3.5.3). For evaluating the correlation between GRAMD1C expression and the other clinical characteristics (gender, age, histologic grade, clinical stage, tumor status and distant metastasis), we performed χ2 tests. The median GRAMD1C expression value determined the cut-off value of χ2 tests. The COX regression analysis was conducted to identify overall survival-related clinical characteristics in the TCGA patients. To study whether different proportions of tumor-infiltrating immune cells related to different clinical stages and diverse survival outcome, we performed boxplot and survival curve using “survival” and “ggplot2” packages (Wickham, 2011) based on results of CIBERSORT and clinical characteristics acquired from TCGA database. P value lower than 0.05 was considered statistically significant in this study.
Results
Correlation of the GRAMD1C expression with clinical characteristics
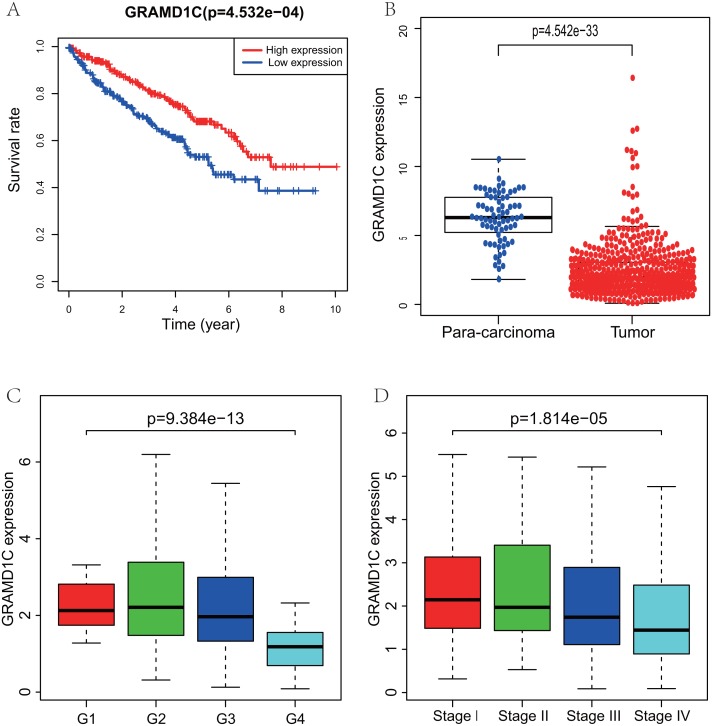
Using R (v.3.5.3), a total of 462 KIRC samples with GRAMD1C expression data and several patient characteristics (overall survival, histologic grade, clinical stage) were analyzed. Survival curve derived by “survival” package and boxplot of different histologic grade and clinical stages derived by “ggplot2” package were performed. Group cutoff value of survival curve was the median GRAMD1C expression (1.922). Figure 1 suggests that decreased GRAMD1C expression is significantly associated with poor overall survival (P < 0.01), advanced clinical stage (P < 0.01) and histologic grade (P < 0.01). In addition, the GRAMD1C expression in tumor tissues is obviously lower than that in para-carcinoma tissues (P-value < 0.01). In addition, χ2 tests (Table 2) reveal the relationship between the GRAMD1C expression and the clinical characteristics. The results demonstrate that the up-regulated of GRAMD1C expression in tumor tissues is significantly related to gender (P < 0.001), age (P = 0.041), histologic grade (P < 0.001), clinical stage (P = 0.001), tumor status (stage T, P = 0.001), distant metastasis (stage M, P = 0.007).
Figure 1. Correlations of GRAMD1C expression with several key clinicopathologic characteristics.
(A) Decreased GRAMD1C expression significantly associates with poor overall survival. (B) The GRAMD1C expression in tumor tissues is obviously lower than in para-carcinoma tissues. (C) Decreased GRAMD1C expression significantly associates with unfavorable histologic grade. (D) Decreased GRAMD1C expression significantly associates with advanced clinical stage.
Table 2. Correlations between the expression of GRAMD1C and clinicopathological characteristics in KIRC.
| Characteristic | n | Low or no expression | High expression | Pearson χ2 | P |
|---|---|---|---|---|---|
| Total | 462 | 231(50.00) | 231(50.00) | ||
| Gender | 13.806 | <0.001* | |||
| Male | 302 | 170(56.30) | 132(43.70) | ||
| Female | 160 | 61(38. 10) | 99(61.90) | ||
| Age | 4.191 | 0.041* | |||
| ≤60 | 230 | 104(45.20) | 126(54.80) | ||
| >60 | 232 | 127(54.70) | 105(45.30) | ||
| Histologic grade | 33.168 | <0.001* | |||
| I& II | 203 | 84(41.40) | 119(58.60) | ||
| III | 190 | 91(47.90) | 99(52.10) | ||
| IV | 69 | 56(81.20) | 13(18.80) | ||
| Clinical stage | 16.064 | 0.001* | |||
| I | 221 | 91(41.20) | 130(58.80) | ||
| II | 49 | 24(49.00) | 25(51.00) | ||
| III | 116 | 67(57.80) | 49(42.20) | ||
| IV | 76 | 49(64.50) | 27(35.50) | ||
| Stage T | 13.098 | 0.001* | |||
| I | 227 | 95(41.90) | 13258.10) | ||
| II | 60 | 31(51.70) | 29(48.30) | ||
| III &IV | 175 | 105(60.00) | 70(40.00) | ||
| Stage M | 7.175 | 0.007* | |||
| 0 | 389 | 184(47.30) | 205(52.70) | ||
| I | 73 | 47(64.40) | 26(35.60) |
Notes.
p < 0.05.
GRAMD1C is an independent predictor of prognosis in KIRC
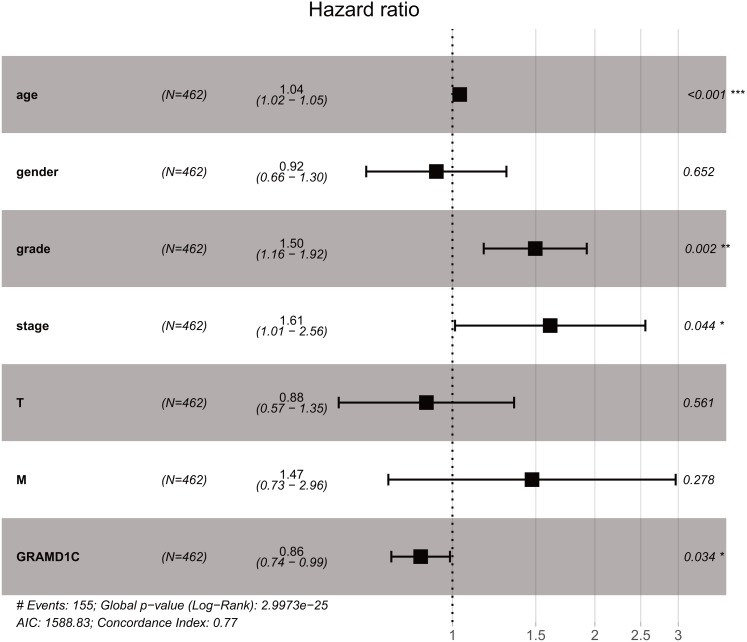
As shown in Table 3, Univariate analysis of correlation using Cox regression reveal that several factors, including age (HR = 1.03, P-value<0.01), histologic grade (HR = 2.38, P-value < 0.01), clinical stage (HR = 1.89, P-value < 0.01), tumor status (HR = 1.92, P-value < 0.01), distant metastasis (HR = 4.45, P-value < 0.01) as well as the GRAMD1C expression (HR = 0.72, P-value < 0.01) have significant correlation with overall survival. At multivariate analysis (Table 3B, Fig. 2), decreased GRAMD1C expression, advanced clinical stage, higher histologic grade and older age are individual predictors for poor prognosis.
Table 3. Results of Cox regression analysis.
(A) Correlations of clinicopathologic characteristics with overall survival in KIRCs using Cox regression. (B) Multivariate survival model after variable selection.
| Clinicopathologic variable | HR | HR.95L | HR.95H | p value | |
|---|---|---|---|---|---|
| (A) | |||||
| Age | 1.031 | 1.017 | 1.045 | 0.000 | |
| Gender | 0.991 | 0.713 | 1.376 | 0.956 | |
| Grade | 2.377 | 1.908 | 2.962 | 0.000 | |
| Stage | 1.893 | 1.646 | 2.177 | 0.000 | |
| Tumor status (T) | 1.920 | 1.611 | 2.287 | 0.000 | |
| Distant metastasis (M) | 4.451 | 3.204 | 6.184 | 0.000 | |
| GRAMD1C | 0.721 | 0.621 | 0.835 | 0.000 | |
| (B) | |||||
| Age | 1.036 | 1.020 | 1.052 | 0.000 | |
| Grade | 1.496 | 1.164 | 1.923 | 0.002 | |
| Stage | 1.609 | 1.012 | 2.557 | 0.044 | |
| GRAMD1C | 0.857 | 0.743 | 0.988 | 0.034 |
Figure 2. The outcome of Cox regression analysis.
Multivariate analysis suggests that decreased GRAMD1C expression, advanced clinical stage and positive distant metastasis are individual predictors for poor prognosis in KIRC.
GRAMD1C expression relates to the proportion of different types of TIICs in KIRC
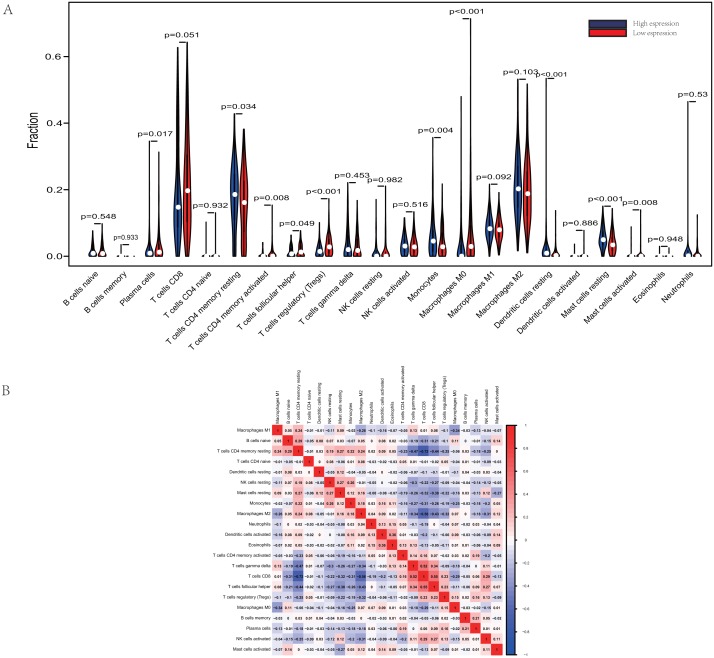
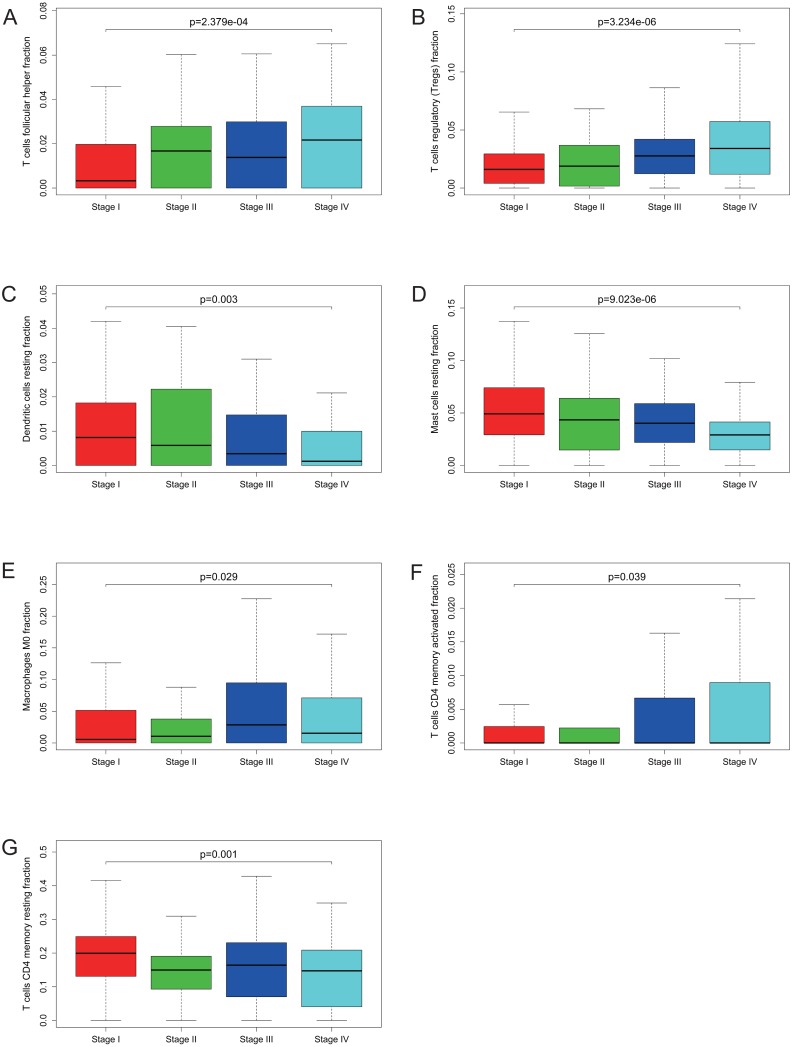
The proportions of TIICs vary substantially across immune subtypes and tumor types. Kidney renal clear cell carcinoma is one of tumors within the top leukocyte fraction and most responsive to immune checkpoint inhibitors (Thorsson et al., 2019). Therefore, we made an effort to find whether GRAMD1C expression has a correlation with immune infiltration level in KIRC. 462 tumor samples were split into 2 parts according to the median GRAMD1C expression (1.922) and a widely accepted computational resource (CIBERSORT) was used to infer the infiltrating level of 22 subtypes of immune cells. Barplot (Fig. S1) summarizes the outcome achieved from 462 KIRC patients. As shown in Fig. 3A and Table 4, plasma cells, T cells CD4 memory resting, T cells CD4 memory activated, T cells follicular helper, T cells regulatory (Tregs), monocytes, macrophages M0, dendritic cells resting, mast cells resting and mast cells activated significantly relate to the GRAMD1C expression. Among them, T cells CD4 memory resting (p = 0.034), monocytes (p = 0.004), dendritic cells resting (p < 0.001) and mast cells resting (p < 0.001) are apparently increased in high expression group relative to low expression group. In contrast, the proportions of plasma cells (p = 0.017), T cells follicular helper (p = 0.049), T cells regulatory (Tregs) (p < 0.001), macrophages M0 (p < 0.001) are lower in high expression group. The outcome intensely suggests that GRAMD1C plays a role in regulating the formation of immune microenviroment in kidney renal clear cell carcinoma, especially impacting on those subtypes of T cells and macrophages. Proportions of different types of immune cells subsets were weakly and then moderately correlated (Fig. 3B). T cells follicular helper and T cells CD8 displayed the strongest positive correlation (Pearson correlation = 0.55), while T cells CD8 and T cells CD4 memory resting showed the strongest negative correlation (Pearson correlation = 0.55).
Figure 3. Evaluation of proportions of TIICs based on CIBERSORT.
(A) The proportions of 22 tumor-infiltrating immune cells in high-GRAMD1C and low-GRAMD1C expression group. (B) Correlation matrix of all 22 immune cell proportions.
Table 4. Relative proportions of 22 types of immune cells in high and low risk score groups.
| Immune cell type | Low GRAMD1C group | High GRAMD1C group | P values |
|---|---|---|---|
| B cells naive | 1.53% ± 1.73% | 1.55% ± 1.88% | 0.548 |
| B cells memory | 0.04% ± 0.29% | 0.01% ± 0.06% | 0.933 |
| Plasma cells | 1.91% ± 3.57% | 2.96% ± 4.67% | 0.017 |
| T cells CD8 | 19.76% ± 14.89% | 21.86% ± 14.38% | 0.051 |
| T cells CD4 naive | 0.04% ± 0.58% | 0.03% ± 0.48% | 0.82 |
| T cells CD4 memory resting | 17.74% ± 9.67% | 15.69% ± 9.77% | 0.034 |
| T cells CD4 memory activated | 0.40% ± 0.89% | 0.82% ± 1.96% | 0.008 |
| T cells follicular helper | 1.33% ± 1.58% | 1.71% ± 1.86% | 0.049 |
| T cells regulatory (Tregs) | 1.86% ± 1.83% | 3.30% ± 2.83% | <0.001 |
| T cells gamma delta | 3.78% ± 4.76% | 3.07% ± 3.64% | 0.453 |
| NK cells resting | 1.22% ± 2.83% | 1.37% ± 3.36% | 0.982 |
| NK cells activated | 3.40% ± 2.67% | 3.30% ± 2.79% | 0.516 |
| Monocytes | 5.90% ± 5.90% | 4.28% ± 4.25% | 0.004 |
| Macrophages M0 | 3.02% ± 6.24% | 6.32% ± 9.29% | <0.001 |
| Macrophages M1 | 8.64% ± 3.78% | 8.00% ± 3.45% | 0.092 |
| Macrophages M2 | 21.16% ± 9.45% | 19.78% ± 9.41% | 0.103 |
| Dendritic cells resting | 1.83% ± 4.22% | 0.82% ± 1.57% | <0.001 |
| Dendritic cells activated | 0.13% ± 0.52% | 0.18% ± 0.85% | 0.886 |
| Mast cells resting | 5.45% ± 3.14% | 3.92% ± 2.96% | <0.001 |
| Mast cells activated | 0.12% ± 0.81% | 0.31% ± 1.42% | 0.0089 |
| Eosinophils | 0.06% ± 0.30% | 0.04% ± 0.24% | 0.948 |
| Neutrophils | 0.72% ± 3.37% | 0.70% ± 1.54% | 0.530 |
Identification of prognostic subtypes of TIICs in KIRC
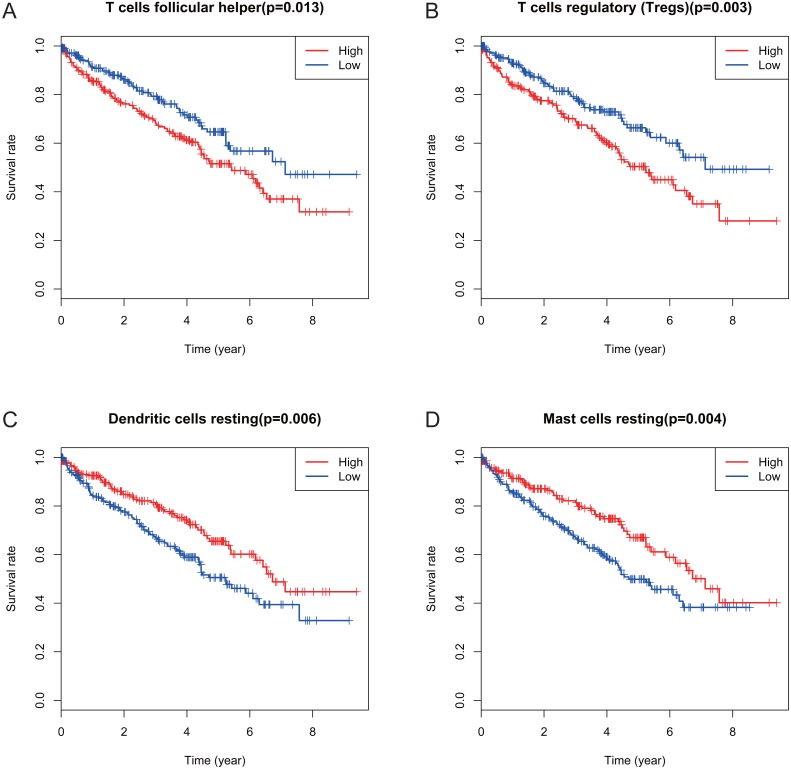
Many previous studies have demonstrated that the proportions of TIICs are reliable predictors of prognosis (Dunn, Dunn & Curry, 2007; Clemente et al., 1996). Thus, we tried to identify the prognostic subtypes of TIICs in KIRC. Based on lymphocyte fraction of each sample evaluated by CIBERSORT and clinical information (overall survival and clinical stage) acquired from TCGA, using R (v.3.5.3), we graphed survival curve and clinical staging boxplot of 22 types of different immune cells. Detailed outcome was shown in Figs. S2 and S3. Selected survival curves and clinical staging boxplots of subtypes of immune cells which relate to GRAMD1C expression (p < 0.05) were exhibited in Figs. 4 and 5. The outcome uncovered that T cells follicular helper (p = 0.013), T cells regulatory (Tregs) (p = 0.003), dendritic cells resting (p = 0.006) and mast cells resting (p = 0) were 4 types of immune cells which were associated with overall survival of KIRC patients. Meanwhile, according to the results of CIBERSORT, these 4 types of immune cells were all related to GRAMD1C expression. B cells memory (p = 0.048), T cells CD8 (p = 0.004), T cells CD4 memory resting (p = 0.001), T cells CD4 memory activated (p = 0.039), T cells follicular helper (p = 0.000), T cells regulatory (Tregs) (p = 0.000), T cells gamma delta (p = 0.032), NK cells resting (p = 0.007), macrophages M0 (p = 0.029), macrophages M2 (p = 0.000), dendritic cells resting (p = 0.003) and mast cells resting (p = 0.000) were correlated with clinical stage of KIRC, thereinto T cells CD4 memory resting, T cells CD4 memory activated, T cells follicular helper, T cells regulatory (Tregs), macrophages M0, mast cells resting and dendritic cells resting are GRAMD1C-related immune cells. In general, T cells follicular helper and T cells regulatory (Tregs) tend to be associated with poor outcome and advanced clinical stage, consistent with proposed role of Tregs as pro-tumourigenic immune suppressors (Shimizu, Yamazaki & Sakaguchi, 1999; Onizuka et al., 1999). In contrast, mast cells resting and dendritic cells resting relate to favourable prognosis and clinical stage.
Figure 4. Correlations between the proportions of TIICs and overall survival.
(A) Decreased proportion of T cells follicular helper is significantly associated with higher overall survival of KIRC (p = 0.013). (B) Decreased proportion of T cells regulatory (Tregs) is significantly associated with higher overall survival of KIRC (P = 0.003). (C) Increased proportion of dendritic cells resting is significantly associated with higher overall survival of KIRC (p = 0.006) (D) Increased proportion of Mast cells resting is significantly associated with higher overall survival of KIRC (p = 0.004).
Figure 5. Correlations between the proportions of TIICs and clinical stage.
T cells follicular helper (A), T cells regulatory (Tregs) (B), dendritic cells resting (C), mast cells resting (D), macrophages M0 (E), T cells CD4 memory activated (F) and T cells CD4 memory resting (G) are significantly correlated with clincial stage in KIRC.
Gene set enrichment analysis
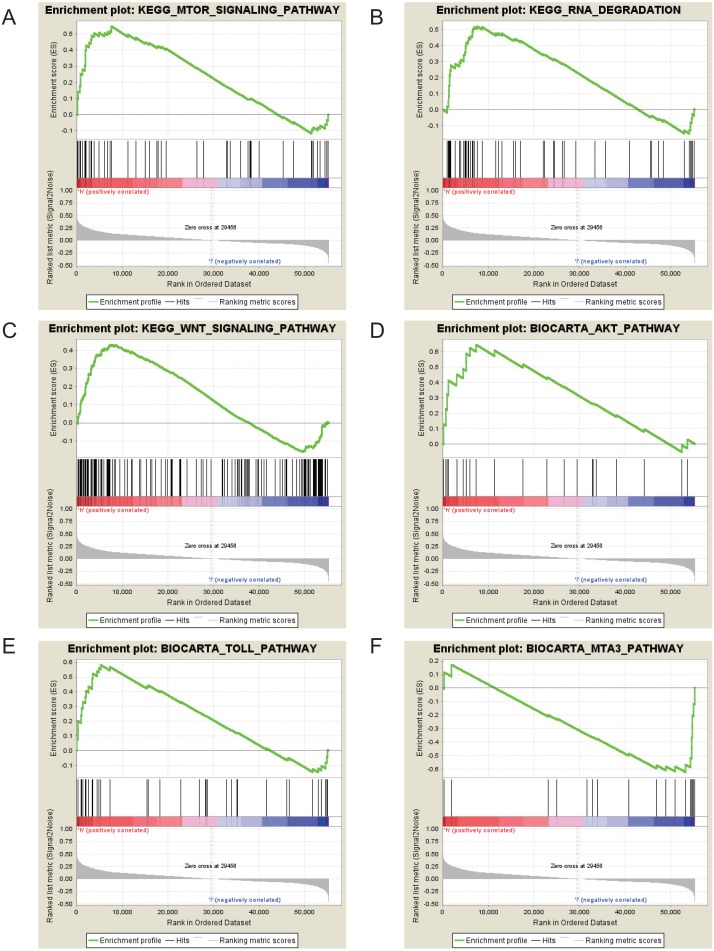
The Gene Set Enrichment Analysis (GSEA) between high and low GRAMD1C expression data sets was conducted to identify the signaling pathways differentially activated in KIRC. In the enrichment analysis of MSigDB Collection (c2.cp.biocarta and c2.cp.kegg), significant differences caused by GRAMD1C were revealed in the GSEA (p-value < 0.05). According to their normalized enrichment scores (NES) we selected the most highly enriched signaling pathways (Fig. 6 and Table 5). The outcome revealed that mTOR signaling pathway, RNA degradation, WNT signaling pathway, toll pathway and AKT pathway were differentially enriched in high GRAMD1C expression phenotype. MTA3 pathway was enriched in low expression phenotype.
Figure 6. Enrichment plots from gene set enrichment analysis (GSEA).
mTOR signaling pathway (A), RNA degradation (B), WNT signaling pathway (C), AKT pathway (D), Toll pathway (E) and MTA3 pathway (F) are differentially enriched in GRAMD1C-related KIRC.
Table 5. Gene sets enrichment.
| MSigDB collection | Gene set name | NES | p-val |
|---|---|---|---|
| c2.cp.kegg.v6.2.symbols.gmt | KEGG_MTOR_SIGNALING_PATHWAY | 1.987 | 0.004 |
| c2.cp.biocarta.v6.2.symbols.gmt | BIOCARTA_AKT_PATHWAY | 1.911 | 0.006 |
| KEGG_RNA_DEGRADATION | 1.823 | 0.025 | |
| BIOCARTA_TOLL_PATHWAY | 1.774 | 0.033 | |
| KEGG_WNT_SIGNALING_PATHWAY | 1.679 | 0.041 | |
| BIOCARTA_MTA3_PATHWAY | −1.846 | 0.004 |
Notes.
- NES
- normalized enrichment score
Gene sets with p-val smaller than 0.05 were considered.
Discussion
Renal clear cell carcinoma (KIRC) is the most common kidney renal cell cancer (Srigley et al., 2013). Despite advances in diagnosis, screening, surgery and drug therapy, the clinical outcome of KIRC remains unsatisfactory. Immune response is closely associated with clinical outcome in KIRC. Tumor- infiltrating immune cells (TIICs) form an ecosystem in the tumor microenvironment to regulate cancer progression and have shown potential prognostic value (Grivennikov, Greten & Karin, 2010). GRAMD1C is a messenger RNA which has not been reported to relate to prognosis and respond to immunotherapy in any cancers. Here, based on mining the gene expression profiles of KIRC and a comprehensive bioinformatics analysis, we found that the expression of GRAMD1C is correlated to leukocyte fraction, especially the regulatory T cell (Tregs) and overall survival. In addition, it is potential to serve as a predictor of prognosis for KIRC patients.
The reduced expression of GRAMD1C in KIRC was established to be associated with advanced clinical pathologic characteristics (high clinical stage, distant metastasis, and bad histological grade), poor prognosis and lower overall survival through the bioinformatic analysis with high throughput RNA-sequencing data from TCGA by this study. The multivariate analysis suggested GRAMD1C is an independent predictor of overall survival. To further investigate the functions of GRAMD1C in KIRC, we performed immune-related analysis using CIBERSORT, which showed that the GRAMD1C expression significantly effects the leukocyte fraction in tumor microenvirenment of KIRC. In addition, based on clinical information acquired from TCGA and CIBERSORT results, we found that several tumor-infiltrating immune cells related to GRAMD1C expression have significant correlation with prognosis of KIRC patients, which is consistent with previous studies (Xu et al., 2013; Wang & Ke, 2011; Overacre-Delgoffe et al., 2017). More interestingly, the GRAMD1C expression has a negative correlation with the regulatory T cell (Tregs) and T cells follicular helper which predicted a poor clinical outcome and advanced clinical stage. Another important aspect of this study was that GRAMD1C expression was correlated with diverse well-known pathways associated with cancer processes and immune responses, such as mTOR signaling pathway, WNT signaling pathway and AKT pathway.
Several previous studies could explain why GRAMD1C expression correlates to the poor prognosis and the proportion of regulatory T cells (Tregs). The activation of the tumor-infiltrating immune cells was affected by the mTOR/AKT pathway as examined by the research in the field of immunology in the last decade. The Akt-mTOR axis has been widely recognized as the critical negative regulator of the regulatory T cell (Tregs) de novo differentiation in the regulatory T cell (Tergs) compartment (Delgoffe et al., 2009; Sokol et al., 2008; Sauer et al., 2008; Liu et al., 2010) with growth in population (Battaglia, Stabilini & Roncarolo, 2005). Interestingly, our present study demonstrated that over expression of GRAMD1C in KIRC was associated with activated Akt/mTOR pathway and reduced proportion of the regulatory T cell (Tregs). Thus, in the writer’s view, GRAMD1C regulates the regulatory T cells by activating mTOR/AKT pathway, but the exact molecular mechanism needs to be further studied. This may partially explain why reduced expression of GRAMD1C relates to poor prognosis in KIRCs. Another important pathway we have discovered associated with GRAMD1C is MTA3 pathway. Researchers have manifested that MTA3 can serve as a regulatory factor of the proteins of p-PARP, BAX, Cleved-Caspase-3 and Bcl-2 to accelerate the cellular apoptosis in NSCLCs (Li et al., 2015). It has also been described as an independent biomarker for unfavorable prognosis in hepatocellular carcinoma (Wang et al., 2017) and uterine non-endometrioid carcinomas (Mylonas & Bruning, 2012). In present study, the results of GSEA established a correlation of low GRAMD1C expression phenotype and intense MTA3 signaling, which was associated with poor prognosis.
The most interesting thing we found about this study is the regulation of GRAMD1C on tumor-infiltrating immune cells, especially the regulatory T cells (Tregs). The Terg cells are implicated in a range of medical conditions like cancer and other autoimmune diseases, but are also known to be the immunosuppressive subset of CD4+ T cells that maintain the immune homeostasis by regulating the numerous facets of immune response (Sakaguchi et al., 1995; Khattri et al., 2003). The various types of effector lymphocytes are suppressed by the Treg cells migrating into the inflammatory sites (Ashutosh et al., 2009; Yeonseok et al., 2011; Koch et al., 2009; Linterman et al., 2011). Treg cells are often found in inflamed tumors harboring large numbers of TH cells and CTLs with regard to cancer (Williams et al., 2017). In tumorigenesis the development of the immune escape mechanisms is a significant factor involving the recruitment and/or induction of the Tergs, programmed cell death 1 ligand 1 (PD-L1), programmed cell death 1 (PD-1), and the immunosuppressive cells (Schreiber, Old & Smyth, 2011). Hence, by depleting or inhibiting these immunosuppressive factors the responses of the anti-tumor immune system could be potentially unleashed. The reinvigorate dysfunctional or ‘exhausted’ cytotoxic CD8+ T cells can be enabled to attack the cancer cells and counter the immunosuppression by anticancer immunotherapy with immune checkpoint inhibitors (ICIs) (Zou, Wolchok & Chen, 2016; Pardoll, 2012); anti-PD-L1 monoclonal antibodies (mAbs), anti-PD-1 and anti-CTLA-4, have shown remarkable clinical efficacy across a range of different cancers, with advanced-stage disease patients (Topalian et al., 2012) (Taneja, 2012). More effective therapies along with immunotherapy combinations, are urgently needed as in majority of the cases the efficacy of the ICIs have been found to be unsatisfactory. Considering the fact that by suppressing the antitumor immunity the Treg cells can promote tumor progression (Shimon et al., 2010; Wing & Sakaguchi, 2010), by targeting the immunosuppressive factors or by manipulating Tregs can be looked upon as a new anticancer treatment methodology with promising results (Onizuka et al., 1999; Shimizu, Yamazaki & Sakaguchi, 1999). Thus, Considering the regulatory effect of GRAMD1C on the infiltration of Tregs, GRAMD1C is likely to play a role in promoting immunologic escape in KIRC. However, the exact biologic process needs to be further demonstrated.
Our study, for the first time, established a correlation of reduced GRAMD1C with poor prognosis of KIRC and higher infiltration level of several types of immune infiltrating immune cells and discovered that GRAMD1C was likely could serve as a new prognostic marker for KIRC patients. Nonetheless, the prediction of protein expression using mRNA was found to be very unreliable as formulated by Guo et al. (2008). The correlation between GRAMD1C protein and the GRAMD1C mRNA expression could not be assessed clearly due to the limitations in the design of this study. The concept of GRAMD1C needs to be explored further.
Conclusions
Our study indicated that reduced GRAMD1C expression correlates with diverse clinical characteristics (gender, age, histologic grade, clinical stage, tumor status and distant metastasis). Reduced GRAMD1C expression is an independent predicting factor of poor prognosis in kidney renal clear cell carcinoma. Meanwhile, GRAMDIC expression significantly correlates with several tumor-infiltrating immune cells, particularly the regulatory T cells (Tregs). Moreover, the mTOR signaling pathway, RNA degradation, WNT signaling pathway, toll pathway and AKT pathway serve as the major pathway affected by GRAMD1C in KIRC. Above all, GRAMD1C is a promising biomarker of prognosis and correlates with immune infiltration in KIRC.
Supplemental Information
The barplot summarizes the outcome achieved from CIBERSORT analysis of 462 KIRC patients. The lengths of columns represnt the relative proportions of immune cells. The colors of columns represent the subtypes of immune cells.
Each survival curve reveals the correlation between the relative proportion of this subtype of immune cells and overall survival in KIRC.
Each histogram reveals the correlation bewteen the relative proportion of immune cellsand clinical stage.
The processed clinical information acquired from TCGA. Cases lacking key clinical information, such as overall survival time, age, histologic grade (8 cases), gender, clinical stage (3 cases), tumor status (T) (2 cases), and distant metastasis (M) (62 cases) were excluded.
The processed gene expression profile of kidney renal clear cell carcinoma tissues and para-carcinoma tissues. The row data was acquired from TCGA database.
The scripts of R software and Strawberry Perl for transformation and normalization of gene expression data.
Acknowledgments
We would like to thank Professor Lihua Shen from the College of Foreign Languages, Nantong University for language polishing.
Funding Statement
This work was supported by Jiangsu Modern Hospital Management Research (GRANT / AWARD NUMBER: JSY-3-2019-053), Technological Innovation and Demonstration of Social Undertakings Projects (GRANT / AWARD NUMBER: MS32017012) and the Training Program of Innovation and Entrepreneurship for College Students in Jiangsu (GRANT / AWARD NUMBER: 201913993001Y, 201913993021H). There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Wenliang Ge, Email: gewl@ntu.edu.cn.
Wei Wang, Email: 1517073033@xlxy.ntu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Haiyan Hao and Ziheng Wang conceived and designed the experiments, performed the experiments, prepared figures and/or tables, approved the final draft.
Shiqi Ren analyzed the data, prepared figures and/or tables, approved the final draft.
Hanyu Shen performed the experiments, prepared figures and/or tables, approved the final draft.
Hua Xian conceived and designed the experiments, authored or reviewed drafts of the paper, approved the final draft.
Wenliang Ge conceived and designed the experiments, analyzed the data, approved the final draft.
Wei Wang conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the paper, approved the final draft.
Data Availability
The following information was supplied regarding data availability:
The raw data was downloaded from the publicly available TCGA database: search term TCGA-KIRC.
References
- Atkins, Regan & McDermott (2004).Atkins MB, Regan M, McDermott D. Update on the role of interleukin 2 and other cytokines in the treatment of patients with stage IV renal carcinoma. Clinical Cancer Research. 2004;10:6342s–6346s. doi: 10.1158/1078-0432.Ccr-040029. [DOI] [PubMed] [Google Scholar]
- Battaglia, Stabilini & Roncarolo (2005).Battaglia M, Stabilini A, Roncarolo MG. Rapamycin selectively expands CD4+CD25+FoxP3+ regulatory T cells. Blood. 2005;105:4743–4748. doi: 10.1182/blood-2004-10-3932. [DOI] [PubMed] [Google Scholar]
- Ceccarelli et al. (2016).Ceccarelli M, Barthel FP, Malta TM, Sabedot TS, Salama SR, Murray BA, Morozova O, Newton Y, Radenbaugh A, Pagnotta SM, Anjum S, Wang J, Manyam G, Zoppoli P, Ling S, Rao AA, Grifford M, Cherniack AD, Zhang H, Poisson L, Carlotti Jr CG, Tirapelli DP, Rao A, Mikkelsen T, Lau CC, Yung WK, Rabadan R, Huse J, Brat DJ, Lehman NL, Barnholtz-Sloan JS, Zheng S, Hess K, Rao G, Meyerson M, Beroukhim R, Cooper L, Akbani R, Wrensch M, Haussler D, Aldape KD, Laird PW, Gutmann DH, Noushmehr H, Iavarone A, Verhaak RG. Molecular profiling reveals biologically discrete subsets and pathways of progression in diffuse glioma. Cell. 2016;164:550–563. doi: 10.1016/j.cell.2015.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen & McGovern (2005).Cohen HT, McGovern FJ. Renal-cell carcinoma. New England Journal of Medicine. 2005;353:2477–2490. doi: 10.1056/NEJMra043172. [DOI] [PubMed] [Google Scholar]
- Delgoffe et al. (2009).Delgoffe GM, Kole TP, Zheng Y, Zarek PE, Matthews KL, Xiao B, Worley PF, Kozma SC, Powell JD. The mTOR kinase differentially regulates effector and regulatory T cell lineage commitment. Immunity. 2009;30:832–844. doi: 10.1016/j.immuni.2009.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doerks et al. (2000).Doerks T, Strauss M, Brendel M, Bork P. GRAM, a novel domain in glucosyltransferases, myotubularins and other putative membrane-associated proteins. Trends in Biochemical Sciences. 2000;25:483–485. doi: 10.1016/s0968-0004(00)01664-9. [DOI] [PubMed] [Google Scholar]
- Dunn, Dunn & Curry (2007).Dunn GP, Dunn IF, Curry WT. Focus on TILs: prognostic significance of tumor infiltrating lymphocytes in human glioma. Cancer Immunity. 2007;7:12. [PMC free article] [PubMed] [Google Scholar]
- Escudier (2012).Escudier B. Emerging immunotherapies for renal cell carcinoma. Annals of Oncology. 2012;23(Suppl 8):viii35–40. doi: 10.1093/annonc/mds261. [DOI] [PubMed] [Google Scholar]
- Ferlay et al. (2010).Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. International Journal of Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- Ge et al. (2019).Ge P, Wang W, Li L, Zhang G, Gao Z, Tang Z, Dang X, Wu Y. Profiles of immune cell infiltration and immune-related genes in the tumor microenvironment of colorectal cancer. Biomedicine and Pharmacotherapy. 2019;118:109228. doi: 10.1016/j.biopha.2019.109228. [DOI] [PubMed] [Google Scholar]
- Gentles et al. (2015).Gentles AJ, Newman AM, Liu CL, Bratman SV, Feng W, Kim D, Nair VS, Xu Y, Khuong A, Hoang CD, Diehn M, West RB, Plevritis SK, Alizadeh AA. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nature Medicine. 2015;21:938–945. doi: 10.1038/nm.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grivennikov, Greten & Karin (2010).Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell. 2010;140:883–899. doi: 10.1016/j.cell.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Ramamoorthy & Ramachandran (2008).Jiang SY, Ramamoorthy R, Ramachandran S. Comparative transcriptional profiling and evolutionary analysis of the GRAM domain family in eukaryotes. Developmental Biology. 2008;314:418–432. doi: 10.1016/j.ydbio.2007.11.031. [DOI] [PubMed] [Google Scholar]
- John et al. (2011).John K, Alla V, Meier C, Putzer BM. GRAMD4 mimics p53 and mediates the apoptotic function of p73 at mitochondria. Cell Death and Differentiation. 2011;18:874–886. doi: 10.1038/cdd.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattri et al. (2003).Khattri R, Cox T, Yasayko SA, Ramsdell F. An essential role for Scurfin in CD4+CD25+ T regulatory cells. Nature Immunology. 2003;4:337–342. doi: 10.1038/ni909. [DOI] [PubMed] [Google Scholar]
- Koch et al. (2009).Koch MA, Tucker-Heard G, Perdue NR, Killebrew JR, Urdahl KB, Campbell DJ. The transcription factor T-bet controls regulatory T cell homeostasis and function during type 1 inflammation. Nature Immunology. 2009;10:595–602. doi: 10.1038/ni.1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li et al. (2015).Li H, Wang Q, Zhang L, Bao H, Zhang H. Regulation mechanism of MTA3 in the apoptosis of NSCLC cells. Zhongguo fei ai za zhi = Chinese Journal of Lung Cancer. 2015;18:610–615. doi: 10.3779/j.issn.1009-3419.2015.10.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linterman et al. (2011).Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nature Medicine. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2010).Liu G, Yang K, Burns S, Shrestha S, Chi H. The S1P(1)-mTOR axis directs the reciprocal differentiation of T(H)1 and T(reg) cells. Nature Immunology. 2010;11:1047–1056. doi: 10.1038/ni.1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu et al. (2018).Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, Kovatich AJ, Benz CC, Levine DA, Lee AV, Omberg L, Wolf DM, Shriver CD, Thorsson V, Hu H. An integrated TCGA pan-cancer clinical data resource to drive high-quality survival outcome analytics. Cell. 2018;173:400–416. doi: 10.1016/j.cell.2018.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mylonas & Bruning (2012).Mylonas I, Bruning A. The metastasis-associated gene MTA3 is an independent prognostic parameter in uterine non-endometrioid carcinomas. Histopathology. 2012;60:665–670. doi: 10.1111/j.1365-2559.2011.04103.x. [DOI] [PubMed] [Google Scholar]
- Newman et al. (2015).Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, Xu Y, Hoang CD, Diehn M, Alizadeh AA. Robust enumeration of cell subsets from tissue expression profiles. Nature Methods. 2015;12:453–457. doi: 10.1038/nmeth.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onizuka et al. (1999).Onizuka S, Tawara I, Shimizu J, Sakaguchi S, Fujita T, Nakayama E. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Research. 1999;59:3128–3133. [PubMed] [Google Scholar]
- Overacre-Delgoffe et al. (2017).Overacre-Delgoffe AE, Chikina M, Dadey RE, Yano H, Brunazzi EA, Shayan G, Horne W, Moskovitz JM, Kolls JK, Sander C, Shuai Y, Normolle DP, Kirkwood JM, Ferris RL, Delgoffe GM, Bruno TC, Workman CJ, Vignali DAA. Interferon-gamma drives treg fragility to promote anti-tumor immunity. Cell. 2017;169:1130–1141. doi: 10.1016/j.cell.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll (2012).Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nature Reviews Cancer. 2012;12:252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, McCarthy & Smyth (2010).Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakaguchi et al. (1995).Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. Journal of Immunology. 1995;155:1151–1164. [PubMed] [Google Scholar]
- Sauer et al. (2008).Sauer S, Bruno L, Hertweck A, Finlay D, Leleu M, Spivakov M, Knight ZA, Cobb BS, Cantrell D, O’Connor E, Shokat KM, Fisher AG, Merkenschlager M. T cell receptor signaling controls Foxp3 expression via PI3K, Akt, and mTOR. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:7797–7802. doi: 10.1073/pnas.0800928105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreiber, Old & Smyth (2011).Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. doi: 10.1126/science.1203486. [DOI] [PubMed] [Google Scholar]
- Shimizu, Yamazaki & Sakaguchi (1999).Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. Journal of Immunology. 1999;163:5211–5218. [PubMed] [Google Scholar]
- Song et al. (2017).Song E, Ma X, An R, Zhang P, Zhang X, Wang B, Shi T, Sun S. Retroperitoneal laparoscopic partial nephrectomy for tumors larger than 7 cm in renal cell carcinoma: initial experience of single-institution. Journal of Laparoendoscopic & Advanced Surgical Techniques. 2017;27(11):1127–1131. doi: 10.1089/lap.2016.0668. [DOI] [PubMed] [Google Scholar]
- Srigley et al. (2013).Srigley JR, Delahunt B, Eble JN, Egevad L, Epstein JI, Grignon D, Hes O, Moch H, Montironi R, Tickoo SK, Zhou M, Argani P. The international society of urological pathology (ISUP) vancouver classification of renal neoplasia. American Journal of Surgical Pathology. 2013;37:1469–1489. doi: 10.1097/PAS.0b013e318299f2d1. [DOI] [PubMed] [Google Scholar]
- Subramanian et al. (2005).Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taneja (2012).Taneja SS. Re: safety and activity of anti-PD-L1 antibody in patients with advanced cancer. Journal of Urology. 2012;188:2148–2149. doi: 10.1016/j.juro.2012.08.169. [DOI] [PubMed] [Google Scholar]
- Thorsson et al. (2019).Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Ou Yang TH, Porta-Pardo E, Gao GF, Plaisier CL, Eddy JA, Ziv E, Culhane AC, Paull EO, Sivakumar IKA, Gentles AJ, Malhotra R, Farshidfar F, Colaprico A, Parker JS, Mose LE, Vo NS, Liu J, Liu Y, Rader J, Dhankani V, Reynolds SM, Bowlby R, Califano A, Cherniack AD, Anastassiou D, Bedognetti D, Mokrab Y, Newman AM, Rao A, Chen K, Krasnitz A, Hu H, Malta TM, Noushmehr H, Pedamallu CS, Bullman S, Ojesina AI, Lamb A, Zhou W, Shen H, Choueiri TK, Weinstein JN, Guinney J, Saltz J, Holt RA, Rabkin CS, Lazar AJ, Serody JS, Demicco EG, Disis ML, Vincent BG, Shmulevich I. The immune landscape of cancer. Immunity. 2019;51:411–412. doi: 10.1016/j.immuni.2019.08.004. [DOI] [PubMed] [Google Scholar]
- Topalian et al. (2012).Topalian SL, Hodi FS, Brahmer JR, Gettinger SN, Smith DC, McDermott DF, Powderly JD, Carvajal RD, Sosman JA, Atkins MB, Leming PD, Spigel DR, Antonia SJ, Horn L, Drake CG, Pardoll DM, Chen L, Sharfman WH, Anders RA, Taube JM, McMiller TL, Xu H, Korman AJ, Jure-Kunkel M, Agrawal S, McDonald D, Kollia GD, Gupta A, Wigginton JM, Sznol M. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. New England Journal of Medicine. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torre et al. (2015).Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA: A Cancer Journal for Clinicians. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Wang et al. (2017).Wang C, Li G, Li J, Li J, Li T, Yu J, Qin C. Overexpression of the metastasis-associated gene MTA3 correlates with tumor progression and poor prognosis in hepatocellular carcinoma. Journal of Gastroenterology and Hepatology. 2017;32:1525–1529. doi: 10.1111/jgh.13680. [DOI] [PubMed] [Google Scholar]
- Wang & Ke (2011).Wang J, Ke XY. The four types of Tregs in malignant lymphomas. Journal of Hematology & Oncology. 2011;4:50. doi: 10.1186/1756-8722-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waniczek et al. (2017).Waniczek D, Lorenc Z, Snietura M, Wesecki M, Kopec A, Muc-Wierzgon M. Tumor-associated macrophages and regulatory T cells infiltration and the clinical outcome in colorectal cancer. Archivum Immunologiae et Therapiae Experimentalis. 2017;65:445–454. doi: 10.1007/s00005-017-0463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham (2011).Wickham HJWIRCS. ggplot2. Wiley Interdisciplinary Reviews: Computational Statistics. 2011;3:180–185. doi: 10.1002/wics.147. [DOI] [Google Scholar]
- Williams et al. (2017).Williams JB, Horton BL, Zheng Y, Duan Y, Powell JD, Gajewski TF. The EGR2 targets LAG-3 and 4-1BB describe and regulate dysfunctional antigen-specific CD8+ T cells in the tumor microenvironment. Journal of Experimetnal Medicine. 2017;214:381–400. doi: 10.1084/jem.20160485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing & Sakaguchi (2010).Wing K, Sakaguchi S. Regulatory T cells exert checks and balances on self tolerance and autoimmunity. Nature Immunology. 2010;11:7–13. doi: 10.1038/ni.1818. [DOI] [PubMed] [Google Scholar]
- Wu et al. (2014).Wu SY, Yang X, Gharpure KM, Hatakeyama H, Egli M, McGuire MH, Nagaraja AS, Miyake TM, Rupaimoole R, Pecot CV, Taylor M, Pradeep S, Sierant M, Rodriguez-Aguayo C, Choi HJ, Previs RA, Armaiz-Pena GN, Huang L, Martinez C, Hassell T, Ivan C, Sehgal V, Singhania R, Han HD, Su C, Kim JH, Dalton HJ, Kovvali C, Keyomarsi K, McMillan NA, Overwijk WW, Liu J, Lee JS, Baggerly KA, Lopez-Berestein G, Ram PT, Nawrot B, Sood AK. 2’-OMe-phosphorodithioate-modified siRNAs show increased loading into the RISC complex and enhanced anti-tumour activity. Nature Communications. 2014;5:3459. doi: 10.1038/ncomms4459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu et al. (2013).Xu W, Liu H, Song J, Fu HX, Qiu L, Zhang BF, Li HZ, Bai J, Zheng JN. The appearance of Tregs in cancer nest is a promising independent risk factor in colon cancer. Journal of Cancer Research and Clinical Oncology. 2013;139:1845–1852. doi: 10.1007/s00432-013-1500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang et al. (2018).Zhang H, Liu H, Shen Z, Lin C, Wang X, Qin J, Qin X, Xu J, Sun Y. Tumor-infiltrating neutrophils is prognostic and predictive for postoperative adjuvant chemotherapy benefit in patients with gastric cancer. Annals of Surgery. 2018;267:311–318. doi: 10.1097/sla.0000000000002058. [DOI] [PubMed] [Google Scholar]
- Zou, Wolchok & Chen (2016).Zou W, Wolchok JD, Chen L. PD-L1 (B7-H1) and PD-1 pathway blockade for cancer therapy: mechanisms, response biomarkers, and combinations. Science Translational Medicine. 2016;8:328rv324. doi: 10.1126/scitranslmed.aad7118. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The barplot summarizes the outcome achieved from CIBERSORT analysis of 462 KIRC patients. The lengths of columns represnt the relative proportions of immune cells. The colors of columns represent the subtypes of immune cells.
Each survival curve reveals the correlation between the relative proportion of this subtype of immune cells and overall survival in KIRC.
Each histogram reveals the correlation bewteen the relative proportion of immune cellsand clinical stage.
The processed clinical information acquired from TCGA. Cases lacking key clinical information, such as overall survival time, age, histologic grade (8 cases), gender, clinical stage (3 cases), tumor status (T) (2 cases), and distant metastasis (M) (62 cases) were excluded.
The processed gene expression profile of kidney renal clear cell carcinoma tissues and para-carcinoma tissues. The row data was acquired from TCGA database.
The scripts of R software and Strawberry Perl for transformation and normalization of gene expression data.
Data Availability Statement
The following information was supplied regarding data availability:
The raw data was downloaded from the publicly available TCGA database: search term TCGA-KIRC.