Abstract
Exposure to cholera is a risk for individuals and groups travelling to endemic areas, and the bacteria can be imported to cholera-free countries by returning travellers. This systematic review of the literature describes the circumstances in which cholera infection can occur in travellers and considers the possible value of the cholera vaccine for prevention in travellers. PubMed and EMBASE were searched for case reports of cholera or diarrhoea among travellers, with date limits of 1 January 1990–30 April 2018. Search results were screened to exclude the following articles: diarrhoea not caused by cholera, cholera in animals, intentional cholera infection in humans, non-English articles and publications on epidemics that did not report clinical details of individual cases and publications of cases pre-dating 1990. Articles were reviewed through descriptive analytic methods and information summarized. We identified 156 cases of cholera imported as a consequence of travel, and these were reviewed for type of traveller, source country, serogroup of cholera, treatment and outcomes. The case reports retrieved in the search did not report consistent levels of detail, making it difficult to synthesize data across reports and draw firm conclusions from the data. This clinical review sheds light on the paucity of actionable published data regarding the risk of cholera in travellers and identifies a number of gaps that should drive additional effort. Further information is needed to better inform evidence-based disease prevention strategies, including vaccination for travellers visiting areas of cholera risk. Modifications to current vaccination recommendations to include or exclude current or additional traveller populations may be considered as additional risk data become available. The protocol for this systematic review is registered with PROSPERO (registration number: 122797).
Keywords: Serogroup O139, Vibrio cholera, Serogroup 01, vibriocidal antibody, Cholera vaccine, Cholera risk
Introduction
Cholera, an acute, secretory diarrhoeal disease caused by toxigenic strains of Gram-negative bacterium Vibrio cholerae (O1 and O139 serogroups), is spread through contaminated food and water.1,2 Improved sanitation and access to safe water have largely eliminated indigenous cholera in high-income countries, but cholera remains a problem in lower income countries, where adequate sanitation and safe water are not widely available and large epidemics can occur. Cholera is endemic in at least 47 countries2,3 but this number is dynamic as affected countries, as listed on the Centers for Disease Control and Prevention (CDC) website, frequently change.1 It is believed that 1.4 billion people are at risk from cholera in endemic countries, with an estimated 1.2 million cases annually.4 Cholera usually manifests itself as diarrhoea, though not usually as ‘cholera gravis’ (i.e. profuse watery diarrhoea that results in death if not rapidly treated).5 The World Health Organization (WHO) has estimated that officially-reported cases represent only 5–10% of the true number of cases.6 Countries with significant endemic seasonal transmission still do not publicly report cases of cholera, while countries with outbreaks continue to report cases and deaths due to ‘acute watery diarrhoea’.4 Considering the insufficient number of surveillance studies, efforts have been made to estimate the cholera disease burden by using modelling approaches.3 Notably, the number of cholera cases in the USA is estimated to be ~33 times higher than those diagnosed7 but this may be primarily of academic interest as the majority of these are mild cases with limited public health import. There have also been recent reports of an underappreciated asymptomatic carrier state, the prevalence of which may be as high as 3–100 asymptomatic individuals for every clinical case.8
Exposure to cholera is a possible risk for individuals and groups traveling to endemic countries (such as tourists, business travellers, those engaging in humanitarian, medical or missionary work, or the military), with the degree of risk varying according to specific areas visited and the duration of stay.1 In addition, cholera can be imported from areas where it is endemic or epidemic to cholera-free countries.2,9 In 2017, the WHO reported 675 cases of imported cholera, with 12 of these being in North America.4 However, it is widely recognized that there may be widespread under-reporting and under-diagnosis of cholera globally, due to economic, social and political disincentives, inadequate investigation or lack of diligence.3,10 Differentiation of cholera from other diarrhoeal diseases on clinical grounds is often difficult9,11; poor laboratory resources and epidemiological surveillance in endemic regions also hinder diagnosis, and within an outbreak setting, not all specimens may be tested.12
Cholera is a rare disease among travellers from non-endemic to endemic areas, with an estimated risk of 0.2 cases per 100 000 European and North American travellers.13 In healthy adults travelling to endemic areas, cholera is effectively treated as, and not distinguished from, other causes of acute watery travellers’ diarrhoea (TD) and is under-reported as a specific cause of illness.9,14,15 Thus, the burden of cholera in travellers is not well understood because most cases are not reported. Effective antibiotic treatment can shorten the duration of illness and reduce shedding of the infectious agent.15,16
Current recommendations for TD, including probable and confirmed cholera, occurring in endemic or outbreak settings are for rehydration therapy and antibiotic treatment (with or without loperamide) for all secretory (watery) cases.14,15,17
The quest for a cholera vaccine dates back to Louis Pasteur’s early work on vaccines in the 1870s and gained substantial attention after the current cholera pandemic began in 1961.5,14 In modern times, the whole-cell killed parenteral vaccines for international travel came into wider use during the years that followed, both on its own and in combination with typhoid vaccine.19 In an effort to control the spread of cholera across international boundaries (and shortly after the success of vaccination in controlling the spread of smallpox), the WHO introduced mandatory cholera vaccination in 1969.20 However, it rapidly became apparent that the parenteral vaccines then in use were not effective at controlling the spread of cholera, and it also became clear that the prevailing serogroups were causing a milder illness than ‘classic’ cholera. In 1973, the World Health Assembly deleted from the International Health Regulations the requirement for presentation of a cholera vaccination certificate,21 and the WHO recommended that its partner countries no longer require cholera vaccination for entry of travellers.
However, given the persistent threat of cholera among resource poor populations of the world, and the growth in global travel to regions far and wide, there has been a renewed focus since the 1990s on developing safer and more effective oral cholera vaccines. Vaccines for cholera are now widely available, including for travellers, though it should be noted that these vaccines have limited effectiveness against the El Tor biotype of V. cholerae. A better understanding of the current epidemiology of cholera is needed to help evaluate the role of vaccination beyond the endemic populations at highest risk. We carried out a systematic review of the literature to provide information on the circumstances in which cholera infection has been reported in travellers and to consider the utility of vaccination for prevention of cholera in travellers.
Methods
Data sources and searches
PubMed and EMBASE were searched using the search terms such as (cholera* OR diarrh* in Title) AND (Imported OR travel* OR touris* OR migrant* OR immigrant* OR migrat* OR immigrat* OR refugee* OR military OR soldier* OR troops OR army OR armies OR war OR forces in All Fields) with date limits of 1 January 1990–30 April 2018. Cases mentioned in reviews but not found in the searches were retrieved by hand.
Apart from abstracts listed in EMBASE, grey literature (e.g. government resources and congress publications) was not included, and duplicate articles were removed.
Study selection
The search results were screened by a non-blinded reviewer to exclude the articles or publications on: diarrhoea not caused by cholera, cases caused by non-O1 or non-O139 serogroups of V. cholerae, cholera in animals, intentional cholera infection in humans (e.g. challenge studies, vaccine studies and microbiological studies), reporting epidemics where clinical details of individual cases are not reported, non-English articles (if there was sufficient information in an English abstract, they were included) and those including cases pre-dating 1990.
Data extraction and quality assessment
Possible articles of interest were retrieved and reviewed, and the accuracy of extracted data was assessed by an independent reviewer. Dual validation was not performed as this was a search for case report data, rather than a quantitative meta-analysis.
Data synthesis and analysis
Description of study type (case-control, cohort case series), year of publication, country of origin, country of destination, duration of travel (mean or median) and population type (business, casual, military, etc.) were reviewed through descriptive analytic methods, and information about the number of cases, serotype and circumstances of the infection were summarized.
For descriptive purposes, cases were grouped according to the following parameters: caused by food but travel-associated, serotype involved, treatment (if reported), duration of illness and illness outcome (complete recovery, death, secondary transmission).
‘Food’ was not included in the search strategy, and therefore, the focus of this review is on travel-associated cholera rather than cases resulting from consumption of imported contaminated food.
The details of this systematic review have been registered on the PROSPERO site, registration number 122797.
Role of the funding source
Independent editorial support was provided by Elements Communications Ltd and was funded by Emergent BioSolutions. Emergent BioSolutions was not involved with the design, analysis, interpretation of data, writing, editing or approval for publication.
Results
Study characteristics
After removal of duplicates, 2974 article titles were screened and the majority of potential papers (n = 2788) were excluded due to their content not relating to cholera. After further reviews of the citations at either the abstract (n = 167) or full publication level (n = 19), 91 case reports were included in this review (Figure 1). In most of the case reports, cholera vaccination information was not provided, or the authors noted that the patient had not received cholera vaccination. Seven review articles were retrieved,22–28 reviewing a total of 499 cases of imported cholera. Five of these articles covered 491 cases in the USA between 1965 and 2011, of which 342 were acquired outside the USA.22,23,25,26,28 One article covered 129 cases imported into France from 1973 to 200524 and a seventh review article reported 28 cases occurring in China from 1995 to 2012, most of which were associated with eating unclean food rather than due to travel.27
Figure 1.
PRISMA diagram showing identification of cases for inclusion
The total number of individual cases of imported cholera specifically identified in this analysis between 1990 and 2018 was 183. Among these cases, 150 were associated with the travel of people rather than the importation of food or other miscellaneous cases.
Cases of cholera imported from one country to another by travel
There were 150 cases of cholera due to serotype O1 or O130 imported as a consequence of travel. These cases are shown stratified by type of traveller in Figure 2. Most of the cases were in tourists (116 cases), followed by military personnel and/or aid workers (25 cases). The remaining reports concerned business travellers, refugees or the patient’s status was unknown. Four of these reports date from the last 5 years and approximately half date from the 1990s.
Figure 2.
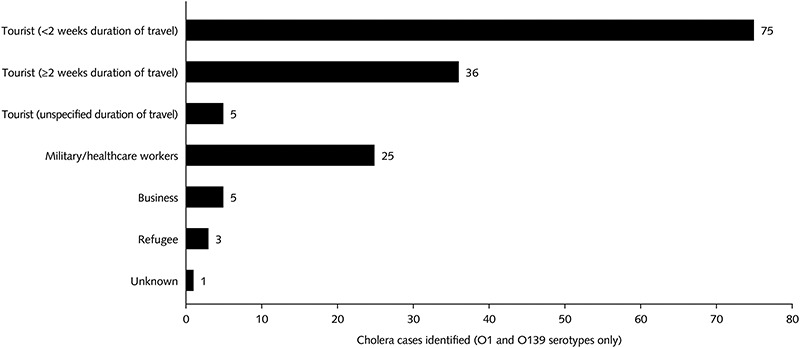
Cholera cases among different types of traveller (O1 and O139 serotype only; n = 150)
The source countries (i.e. the countries where the individual was infected) are shown in Supplementary Table 1 for serotypes O1 and O139. Infections were mostly contracted in countries and regions where cholera is endemic or in affected regions during outbreaks.3 South America and the Caribbean (including Haiti, Dominican Republic, Cuba, Mexico, Ecuador, and Peru) had the highest number of cases reported (n = 91), followed by Asia (including India, Thailand and Indonesia) with 41 cases, and Africa with 8 cases.
The case reports in the literature relating to O1 and O139 mostly concerned patients who were diagnosed in countries where cholera is not endemic, i.e. North America (79 cases). A total of 78 of these cases were imported into and diagnosed in the USA. The imported cases had mainly come from Central and South America. Among the cases diagnosed in Europe and other parts of the world, the majority of cases had originated in Asia or Africa.
Causative serogroups
Among patients where the serogroup was identified, most cases of cholera were caused by either serogroup O1 or O139 (Table 1). Serogroup O1 was found to be responsible in 140 cases, while O139 was found to be responsible in 9 cases. There was one report of dual infection with both O1 and O139.29
Table 1.
Cases caused by O1 or O139 serogroups of Vibrio cholerae and their region of origin
| Serogroup identified | Countries of origin | Number of cases | Type of traveller | Year of diagnosis | References |
|---|---|---|---|---|---|
| South America and the Caribbean (91 cases) | |||||
| Serogroup O1 | Peru | 32 | 31T, 1B | 1991; 1992 | [30,31] |
| Ecuador | 3 | 3VFR | 1991; 1992 | [32–35] | |
| Mexico | 2 | 1T, 1VFR | 1992; 1995 | [32,36] | |
| Haiti | 30 | 1VFR, 21M, 8T | 2010; 2011; 2012 | [37–42] | |
| Haiti/Dominican Republic | 22 | 16VFR, 4M, 2B | 2010 | [28] | |
| Dominican Republic | 1 | 1T | 2011 | [43] | |
| Cuba | 1 | 1T | 2013 | [44 | |
| Asia (41 cases) | |||||
| Bangladesh | 1 | 1T | 1995 | [36] | |
| India | 16 | 14T, 1B | 1995; 2006; 2009; 2010; 2005–2012; 2017 | [36,45–50] | |
| Iraq | 2 | 2T | 2015 | [51] | |
| Pakistan | 4 | 4T | 1992; 1995; 2004 | [36,52,53] | |
| Thailand, Indonesia | 10 | 10T | 1994; 1995 | [29,36,54] | |
| The Philippines | 2 | 1VFR, 1B | 1992; 2015 | [32,35,55] | |
| Turkey | 6 | 6T | 2005 | [56 | |
| Africa (8 cases) | |||||
| Kenya | 5 | 5T | 1995; 1998 | [36,57] | |
| Senegal | 1 | 1T | 2005 | [58] | |
| Tanzania | 2 | 2R | 2005 | [59] | |
| Serogroup O139 | Unknown | 1 | NA | 1994 | [60] |
| India | 6 | 6T | 1993; 1994 | [61,62] | |
| Pakistan | 1 | 1T | 1994 | [63] | |
| Thailand, Indonesia | 2 | 2T | 1994; 1995 | [29,64] | |
Abbreviations: B, business traveller; M, military or healthcare personnel; R, refugee; T, tourist; VFR, visiting friends or relations
Treatment of cases and outcomes
From the case reports, information on patient outcomes was not reported consistently or uniformly, making it difficult to provide summary statements of the consequence and responsiveness of therapy to medical treatments. In general, of the O1 and O139 cholera cases reviewed here, antibiotics were used in conjunction with rehydration. The average duration of illness ranged from 2 to 10 days in those cases in which this information was provided, and most patients recovered (only one death was reported among the cases reviewed here). In many of the cases, the report either did not state that antibiotics were used or, if they were, did not specify which antibiotics were used.
Discussion
In this systematic review of the literature, the total number of individual cases of imported cholera reported between 1990 and 2018 was 183 cases—a remarkably low number, both in absolute terms and by comparison with overall data from endemic areas reported by WHO.4 Of these 183 cases, 150 cases of cholera were imported as a consequence of travel, and these were most often contracted in countries and regions considered high risk and where cholera is endemic, with many associated with travel to countries during outbreaks. For example, a large number of the cases reported during the 1990s were associated with travel to South America during the outbreak that began in Peru during 1991.65 Similarly, a considerable number of cases reported during 2010–2011 involved travel to Haiti and the Dominican Republic during the 2010 cholera outbreak.66 Most of the reports were of cases diagnosed in countries where cholera is not endemic, such as the USA. There were no reports of patients who were infected in one endemic country and diagnosed after travelling to another endemic country.
Most cases occurred in tourists (i.e. not business travellers or missionaries), and a majority of those had been travelling for <2 weeks. This may be because tourists outnumber other travellers to these countries. Most reported cases were caused by serogroup O1 or O139, but in a proportion of cases disease was caused by non-O1 serogroups. The proportion of non-O1 serotypes may be greater than would be expected based on epidemiologic data, which could reflect publication bias towards the reporting of uncommon serotypes.67 Among these cases, there were atypical presentations, and in some of these cases, the patient had underlying disease or comorbidity. However, the case reports are not consistent in terms of the level of detail that they report, making it difficult to draw firm conclusions on whether comorbidities could increase risk of infection or whether atypical presentations are related to the serogroup of the infecting organism.
The cholera cases reviewed here were mostly acquired in parts of South and Southeast Asia, Central and South America, as well as sub-Saharan Africa. The WHO records all cases reported and publishes data on endemic countries,68 highlighting a high risk in certain African countries, as well as Yemen and the continued risk in South Asia. The small number of cases we identified from sub-Saharan Africa may reflect the fact that fewer travellers from non-endemic countries, in whom accurate diagnosis might be possible, tend to visit areas within Africa where there is a high risk of exposure to cholera (for example, areas where there is poor sanitation and overcrowding). Alternatively, these differences may reflect under-diagnosis, under-reporting and/or publication biases. Given the challenges in estimating disease risk and incidence in travellers, future studies utilizing travel-expedient stool collection and testing methods (e.g. filter paper plus PCR) or measurement of vibriocidal antibody seroconversion associated with illness could be considered to improve estimates of traveller disease risk. Large databases such as the National Health and Nutrition Examination Survey (NHANES) or Department of Defense Serum Repository may be amenable to conduct population-based force of infection studies.69,70
Reported cases of cholera in travellers are rare, but since cholera can lead to severe disease, clinicians need to consider this diagnosis in any returning traveller with acute watery diarrhoea. The emergence of rapid point of care diagnostics71 may facilitate detection of cholera in clinics providing post-travel care for returning travellers in non-endemic countries; in areas where cholera is endemic, rapid dipstick testing is often available to test stool samples and make a diagnosis of cholera within 20 minutes.71 However, it should be noted that rapid dipstick testing is not confirmatory and stool culture is required to confirm the diagnosis of cholera for all suspect cases in the USA.72 In countries where cholera is not endemic, medical personnel may not be expecting to see it, and testing may be delayed. In our searches, we noted that several reports mentioned delays in diagnosis.41,53 From a pragmatic standpoint, and based on current treatment guidelines, travellers and healthcare providers should recognize the importance of initiating treatment, including rehydration and empiric antibiotics for patients during or upon return from travel with moderate to severe symptoms of watery diarrhoea that is impacting travel or activities.17,73 Travellers to cholera-endemic areas should be informed of the risk and of the watery diarrhoea presentation of cholera infection and given appropriate counselling and self-treatment options (oral rehydration and antibiotics for moderate to severe diarrhoea) to mitigate morbidity.
Given the effectiveness of current cholera vaccines, we support the CDC and Advisory Committee on Immunization Practices (ACIP) recommendations on the use of cholera vaccine in travellers, where vaccination is recommended for adult travellers from the USA to areas of active cholera transmission.74 To support these recommendations, the CDC provides an online list of countries with active cholera transmission and notes that cholera is mostly spread in limited outbreaks, with travellers rarely at risk.1 ACIP states that persons at higher risk for exposure might include travellers visiting friends and relatives, healthcare personnel, cholera outbreak response workers and persons traveling to or living in a cholera-affected area for extended periods. The primary prevention strategy for cholera recommended by ACIP for all travellers is consistent access to and exclusive use of safe water and food and frequent handwashing. Nonetheless, ACIP notes that travellers to areas of active cholera transmission, which include areas with current or recent endemic or epidemic cholera activity, might be exposed to toxigenic V. cholerae O1 through inadvertent or unexpected means, despite efforts to adhere to prevention measures.74 ACIP recommends that travellers who develop severe diarrhoea should seek prompt medical attention, particularly fluid replacement therapy.74 CVD 103-HgR is recommended for adult travellers (aged 18–64 years) from the USA to an area of active cholera transmission.74 An area of active cholera transmission is defined by ACIP as a province, state or other administrative subdivision within a country with endemic or epidemic cholera caused by toxigenic V. cholerae O1 and includes areas with cholera activity within the last year that are prone to recurrence of cholera epidemics.74
Several European countries, Canada and Australia have further refined these recommendations by advising the use of cholera vaccine for humanitarian aid workers in epidemic situations, individuals with underlying medical conditions such as achlorhydria that increase risk of acquiring gastrointestinal pathogens and travellers to remote areas where there is ongoing cholera transmission and limited access to safe water and medical care.75–78 Extension of these recommendations to other high-risk traveller populations should be considered, as additional risk data become available to guide appropriate review of evidence-based recommendations.
This review highlights the limitations of the current evidence-base surrounding cholera in travellers. We found many inconsistencies in data reporting across the articles we retrieved and in data extracted from retrospective case reports. Bearing in mind that case reports considerably underestimate true case numbers and that effective mitigation strategies are now available, we feel that better surveillance and more consistent reporting of cases would provide quantitative data capable of informing more effective preventive measures among travellers.
Conclusions
Cholera presents a risk to those living in endemic zones and will continue to do so as long as the challenges of poverty, poor infrastructure and conflict remain. Global human development is improving generally,79 but counter-prevailing forces remain a serious problem in the most impoverished parts of the world. Together with factors such as increased human migration from high-risk areas, climate change and inadequate access to clean water, these conspire to extend the risk of cholera to human populations. Whether at a population or an individual level, decisions about vaccine intervention require good data to support them. This clinical review highlights the paucity of actionable information for cholera risk in travellers and identifies a number of gaps that should drive further effort to define the problem.
Supplementary Material
Acknowledgements
The authors would like to thank Tina Patrick of Elements Communications Ltd for conducting the systematic review and screening papers for inclusion following the search strategy and inclusion criteria that were determined by the authors and for drafting the initial version of the paper following an outline created by the authors, which the authors subsequently edited for intellectual content and scope. They would also like to thank Louisa Reed of Elements Communications Ltd, who undertook edits on subsequent drafts of the manuscript on instruction from the authors.
Funding
Independent editorial support was provided by Elements Communications Ltd and was funded by Emergent BioSolutions.
Conflict of interest statement
For this work, all authors received editorial assistance from Elements Communications Ltd, which was funded by Emergent BioSolutions. Outside of this work, Dr Connor reports personal fees from Emergent BioSolutions and Valneva and grants from Aries Pharmaceuticals and BioFire Diagnostics. Dr Dawood and Dr Riddle have nothing to disclose. Dr Hamer reports a speaking honorarium from Emergent BioSolutions and a consultancy fee from Valneva, serving as a consultant for World Aware, and salary and travel support from GeoSentinel, which is funded by a co-operative agreement from CDC.
Author contributions
All authors were involved in the conception and design of the search strategy, data selection, extraction and synthesis. All authors critically reviewed the drafts and approved the final content and are wholly accountable for its content. Emergent BioSolutions played no role in editing the paper and had no input into the conclusions drawn.
References
- 1. Center for Disease Control and Prevention Cholera. 2018. Accessed at https://wwwnc.cdc.gov/travel/diseases/cholera on 30 October 2018.
- 2. Harris JB, LaRocque RC, Qadri F et al. . Cholera. Lancet 2012; 379:2466–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ali M, Nelson AR, Lopez AL, Sack DA. Updated global burden of cholera in endemic countries. PLoS Negl Trop Dis 2015; 9:e0003832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cholera, 2017. Wkly Epidemiol Rec 2018; 93:489–500. [Google Scholar]
- 5. World Health Organization Cholera Fact Sheet. 2019. https://www.who.int/news-room/fact-sheets/detail/cholera (16 May 2019, date last accessed.
- 6. Global Task Force on Cholera Control Ending Cholera. A Global Roadmap to 2030. World Health Organisation, 2017. [Google Scholar]
- 7. Scallan E, Hoekstra RM, Angulo FJ et al. . Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis 2011; 17:7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. King AA, Ionides EL, Pascual M, Bouma MJ. Inapparent infections and cholera dynamics. Nature 2008; 454:877–80. [DOI] [PubMed] [Google Scholar]
- 9. Zuckerman JN, Rombo L, Fisch A. The true burden and risk of cholera: implications for prevention and control. Lancet Infect Dis 2007; 7:521–30. [DOI] [PubMed] [Google Scholar]
- 10. Ali M, Lopez AL, Ae You Y, Eun Kim Y et al. . The global burden of cholera. Bull World Health Organ 2012; 209–18A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Learoyd TP, Gaut RM. Cholera: under diagnosis and differentiation from other diarrhoeal diseases. J Travel Med 2018; 25:S46–51. [DOI] [PubMed] [Google Scholar]
- 12. Kapata N, Sinyange N, Mazaba ML et al. . A Multisectoral emergency response approach to a cholera outbreak in Zambia: October 2017–February 2018. J Infect Dis 2018; 218:S181–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Steffen R, Acar J, Walker E, Zuckerman J. Cholera: assessing the risk to travellers and identifying methods of protection. Travel Med Infect Dis 2003; 1:80–8. [DOI] [PubMed] [Google Scholar]
- 14. World Health Organization Management of the Patient with Cholera. 2015. https://www.who.int/topics/cholera/publications/WHO_CDD_SER_91_15/en/ (31 April 2019, date last accessed).
- 15. Centers for Disease Control and Prevention Managing Travelers’ Diarrhea while Traveling Abroad. 2017. https://www.cdc.gov/features/managing-travelers-diarrhea/index.html (21 May 2019, date last accessed).
- 16. Nelson EJ, Nelson DS, Salam MA, Sack DA. Antibiotics for both moderate and severe cholera. N Engl J Med 2011; 364:5–7. [DOI] [PubMed] [Google Scholar]
- 17. Riddle MS, Connor BA, Beeching NJ et al. . Guidelines for the prevention and treatment of travelers’ diarrhea: a graded expert panel report. J Travel Med 2017; 24:S63–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inaya Hajj Hussein NC, Chams S, El Sayegh S et al. . Vaccines through centuries: major cornerstones of global health. Front Public Health 2015; 3:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Czerkinsky C, Holmgren J. Vaccines against enteric infections for the developing world. Philos Trans R Soc Lond B Biol Sci 2015; 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. World Health Organization Frequently Asked Questions about the International Health Regulations 2005 . https://www.who.int/ihr/about/FAQ2009.pdf (20 May 2019, date last accessed).
- 21. World Health Organization Prevention and Control of Cholera Outbreaks: WHO Policy and Recommendations. 2019. https://www.who.int/cholera/prevention_control/recommendations/en/index6.html (05 February 2019, date last accessed).
- 22. Loharikar A, Newton AE, Stroika S et al. . Cholera in the United States, 2001–2011: a reflection of patterns of global epidemiology and travel. Epidemiol Infect 2015; 143:695–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Steinberg EB, Greene KD, Bopp CA et al. . Cholera in the United States, 1995–2000: trends at the end of the twentieth century. J Infect Dis 2001; 184:799–802. [DOI] [PubMed] [Google Scholar]
- 24. Tarantola A, Ioos S, Rotureau B et al. . Retrospective analysis of the cholera cases imported to France from 1973 to 2005. J Travel Med 2007; 14:209–14. [DOI] [PubMed] [Google Scholar]
- 25. Weber JT, Levine WC, Hopkins DP, Tauxe RV. Cholera in the United States, 1965–1991. Risks at home and abroad. Arch Intern Med 1994; 154:551–6. [PubMed] [Google Scholar]
- 26. Mahon BE, Mintz ED, Greene KD et al. . Reported cholera in the United States, 1992-1994: a reflection of global changes in cholera epidemiology. JAMA 1996; 276:307–12. [PubMed] [Google Scholar]
- 27. Bao Z-Y, Xiao-ming, Xioa-dong Y, Shu-hong D. Epidemiological and clinical characteristics of 28 cases of cholera. Infect Int 2014; 3:35. [Google Scholar]
- 28. Newton AE, Heiman KE, Schmitz A et al. . Cholera in United States associated with epidemic in Hispaniola. Emerg Infect Dis 2011; 17:2166–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sakaue Y, Yoshida H, Iida T et al. . An imported cholera case infected with both O139 synonym Bengal and O1 vibrio cholerae in Japan. Eur J Epidemiol 1995; 11:713–4. [DOI] [PubMed] [Google Scholar]
- 30. Cholera associated with an international airline flight, 1992. MMWR Morb Mortal Wkly Rep 1992; 41:134–5. [PubMed] [Google Scholar]
- 31. From the Centers for Disease Control Importation of cholera from Peru. JAMA 1991; 265:2659. [PubMed] [Google Scholar]
- 32. From the Centers for Disease Control Cholera--international travel, 1992. JAMA 1992; 268:1648–9. [PubMed] [Google Scholar]
- 33. From the Centers for Disease Control Cholera--New Jersey and Florida. JAMA 1991; 265:2658–9. [PubMed] [Google Scholar]
- 34. Cholera--New York, 1991. MMWR Morb Mortal Wkly Rep, 1991; 40:516–8. [PubMed] [Google Scholar]
- 35. Cholera associated with international travel, 1992--United States. Can Commun Dis Rep 1992; 18:166–8. [PubMed] [Google Scholar]
- 36. Cholera imported into England and Wales, 1995. Commun Dis Rep CDR Wkly 1996; 6:55. [PubMed] [Google Scholar]
- 37. Gilmour MW, Martel-Laferriere V, Levesque S et al. . Vibrio cholerae in traveler from Haiti to Canada. Emerg Infect Dis 2011; 17:1124–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Haus-Cheymol R, Theodose R, Quilici ML et al. . A cluster of acute diarrhea suspected to be cholera in French travelers in Haiti, December 2010. J Travel Med 2012; 19:189–91. [DOI] [PubMed] [Google Scholar]
- 39. Update on Cholera --- Haiti, Dominican Republic, and Florida, 2010. MMWR Morb Mortal Wkly Rep 2010; 59:1637–41. [PubMed] [Google Scholar]
- 40. Reyes-Corcho A, Pinsker RW, Sarkar S et al. . Cholera gravis associated with acute renal failure in a traveler from Haiti to the United States. Travel Med Infect Dis 2012; 10:236–9. [DOI] [PubMed] [Google Scholar]
- 41. Sachinwalla EM, Fernandes C, Potula R et al. . A case of cholera imported from Haiti. Clinical Microbiology Newsletter 2015; 37:22–3. [Google Scholar]
- 42. Kanaparthi C, Olaywi M, Anand S. Cholera arrives in Brooklyn! Bariatric surgery may have been a compounding factor. Am J Gastroenterol 2012; 107:S270. [Google Scholar]
- 43. Ryan ET, Madoff LC, Ferraro MJ. Case records of the Massachusetts General Hospital. Case 20-2011. A 30-year-old man with diarrhea after a trip to the Dominican Republic. N Engl J Med 2011; 364:2536–41. [DOI] [PubMed] [Google Scholar]
- 44. Mascarello M, Deiana ML, Maurel C et al. . Cholera with severe renal failure in an Italian tourist returning from Cuba, July 2013. Euro Surveill 2013; 18:20572. [DOI] [PubMed] [Google Scholar]
- 45. Ismail H, Smith AM, Archer BN et al. . Case of imported vibrio cholerae O1 from India to South Africa. J Infect Dev Ctries 2012; 6:897–900. [DOI] [PubMed] [Google Scholar]
- 46. Kuleshov KV, Vodop'ianov SO, Dedkov VG et al. . Travel-associated vibrio cholerae O1 El Tor, Russia. Emerg Infect Dis 2016; 22:2006–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Tarantola A, Vaucel J, Laviolle C et al. . A cluster of vibrio cholerae O1 infections in French travelers to Rajasthan (India), May 2006. J Travel Med 2008; 15:273–7. [DOI] [PubMed] [Google Scholar]
- 48. Neghina R, Neghina AM. A case of imported cholera in Romania in 2009. Infect Dis Clin Pract 2012; 20:148–9. [Google Scholar]
- 49. van Furth AM, Croughs RD, Terpstra L et al. . A boy with cholera from India. Ned Tijdschr Geneeskd 2006; 150:210–3. [PubMed] [Google Scholar]
- 50. Pougnet L, Pougnet R, Voarino A et al. . Cholera in Brest, France. Ann Biol Clin (Paris) 2018; 76:107–10. [DOI] [PubMed] [Google Scholar]
- 51. Mukhopadhyay AK, Al Benwan K, Samanta P et al. . Vibrio cholerae O1 imported from Iraq to Kuwait, 2015. Emerg Infect Dis 2016; 22:1693–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Enzensberger R, Besier S, Baumgartner N, Brade V. Mixed diarrhoeal infection caused by vibrio cholerae and several other enteric pathogens in a 4-year-old child returning to Germany from Pakistan. Scand J Infect Dis 2005; 37:73–5. [DOI] [PubMed] [Google Scholar]
- 53. Gradon JD, Lutwick LI, Chavda R, Levi M. The fortuitous diagnosis of cholera in a two-year-old girl. Diagn Microbiol Infect Dis 1992; 15:161–4. [DOI] [PubMed] [Google Scholar]
- 54. Turk KS, Kovacevic M, Melink A et al. . Imported cholera case serogroup O1 from Thailand. Trop Med Int Health 2011; 16:255. [Google Scholar]
- 55. Slesak G, Fleck R, Jacob D et al. . Imported cholera with acute renal failure after a short business-trip to the Philippines, Germany, October 2015. Euro Surveill 2016; 21. [DOI] [PubMed] [Google Scholar]
- 56. De Schrijver K, Boeckx H, Top G et al. . Cholera among Belgian travellers in Turkey in 2005. Travel Med Infect Dis 2007; 5:236–8. [DOI] [PubMed] [Google Scholar]
- 57. Cholera in British tourists returning from Kenya. Commun Dis Rep CDR Wkly 1998; 8:139, 42. [PubMed] [Google Scholar]
- 58. Ciofi degli Atti M, Finarelli AC, Pompa MG et al. . A case of cholera imported from Senegal to Rimini, Italy, June 2005. Euro Surveill 2005; 10:E050630.6. [DOI] [PubMed] [Google Scholar]
- 59. Chen LF, Woolley IJ, Visvanathan K, Korman TM. Hypovolemic shock and metabolic acidosis in a refugee secondary to O1 serotype vibrio cholerae enteritis. Commun Dis Intell Q Rep 2006; 30:233–5. [PubMed] [Google Scholar]
- 60. Dalsgaard A, Nielsen GL, Echeverria P et al. . The first case of vibrio cholerae 0139 in Denmark. Ugeskr Laeger 1996; 158:5169–71. [PubMed] [Google Scholar]
- 61. Tay L, Goh KT, Lim YS. Vibrio cholerae 0139 'Bengal' in Singapore. J Trop Med Hyg 1994; 97:317–20. [PubMed] [Google Scholar]
- 62. Imported cholera associated with a newly described toxigenic Vibrio cholerae O139 strain--California, 1993. MMWR Morb Mortal Wkly Rep 1993; 42:501–3. [PubMed] [Google Scholar]
- 63. Emeis M, Liesenfeld O, Stephan R et al. . Imported cholera infection caused by a new nonagglutinating cholera agent. Dtsch Med Wochenschr 1994; 119:875–8. [DOI] [PubMed] [Google Scholar]
- 64. Sharma TK, Snyder MB, Eisenberg BM, Cutler AF. Cholera gravis caused by vibrio cholerae O139, a novel, imported pathogen. Clin Infect Dis 1995; 21:241–2. [DOI] [PubMed] [Google Scholar]
- 65. Guthmann JP. Epidemic cholera in Latin America: spread and routes of transmission. J Trop Med Hyg 1995; 98:419–27. [PubMed] [Google Scholar]
- 66. Chin CS, Sorenson J, Harris JB et al. . The origin of the Haitian cholera outbreak strain. N Engl J Med 2011; 364:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lekshmi N, Joseph I, Ramamurthy T, Thomas S. Changing facades of vibrio cholerae: an enigma in the epidemiology of cholera. Indian J Med Res 2018; 147:133–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. World Health Organization Global Health Observatory data - Cholera. 2016. https://www.who.int/gho/epidemic_diseases/cholera/en/ (31 April 2019, date last accessed).
- 69. Hariri S, Dunne EF, Sternberg M et al. . Seroepidemiology of human papillomavirus type 11 in the United States: results from the third National Health and nutrition examination survey, 1991--1994. Sex Transm Dis 2008; 35:298–303. [DOI] [PubMed] [Google Scholar]
- 70. Perdue CL, Cost AA, Rubertone MV et al. . Description and utilization of the United States department of defense serum repository: a review of published studies, 1985-2012. PLoS One 2015; 10:e0114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Ley B, Khatib AM, Thriemer K et al. . Evaluation of a rapid dipstick (crystal VC) for the diagnosis of cholera in Zanzibar and a comparison with previous studies. PLoS One 2012; 7:e36930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. CDC Diagnosis and Detection - Cholera. https://www.cdc.gov/cholera/diagnosis.html (16 October 2019, date last accessed).
- 73. Riddle MS, DuPont HL, Connor BA. ACG clinical guideline: diagnosis, treatment, and prevention of acute diarrheal infections in adults. Am J Gastroenterol 2016; 111:602–22. [DOI] [PubMed] [Google Scholar]
- 74. Wong KK, Burdette E, Mahon BE et al. . Recommendations of the advisory committee on immunization practices for use of cholera vaccine. MMWR Morb Mortal Wkly Rep 2017; 66:482–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Santé publique France Bulletin épidémiologique hebdomadaire. Recommandations sanitaires pour les voyageurs, 2019 (à l’attention des professionnels de santé) 2019. https://www.mesvaccins.net/textes/hcspa20190322_recommasanitaipourlesvoyageur.pdf (19 July 2019, date last accessed)
- 76. Public Health Agency of Canada Canadian Immunization Guide: Part 4 - Active Vaccines . https://www.canada.ca/en/public-health/services/publications/healthy-living/canadian-immunization-guide-part-4-active-vaccines/page-3-cholera-enterotoxigenic-escherichia-coli-travellers-diarrhea-vaccine.html#a6 (19 July 2019, date last accessed).
- 77. Australian Government Department of Health Australian Immunisation Handbook. Vaccine Preventable Diseases. Cholera . https://immunisationhandbook.health.gov.au/vaccine-preventable-diseases/cholera (19 July 2019, date last accessed).
- 78. Public Health England Cholera: The Green Book, Chapter 14. 2013. https://www.gov.uk/government/publications/cholera-the-green-book-chapter-14 (19 July 2019, date last accessed).
- 79. United Nations Development Programme Human Development Reports 2018 . http://hdr.undp.org/en/2018-update (2 February 2019, date last accessed).
- 80. Besser RE, Feikin DR, Eberhart-Phillips JE et al. . Diagnosis and treatment of cholera in the United States. Are we prepared? JAMA 1994; 272:1203–5. [PubMed] [Google Scholar]
- 81. Eberhart-Phillips J, Besser RE, Tormey MP et al. . An outbreak of cholera from food served on an international aircraft. Epidemiol Infect 1996; 116:9–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Kyriacou DN, Newton EJ, Jain A. Imported cholera in a 31-year-old Peruvian female. J Emerg Med 1993; 11:717–21. [DOI] [PubMed] [Google Scholar]
- 83. Roginsky G, Mazulis A, Ecanow JS, Ehrenpreis ED. Mesenteric panniculitis associated with vibrio cholerae infection. ACG Case Rep J 2015; 3:39–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Lin MC, Fang JT, Huang CC. Cholera associated with acute renal failure and rhabdomyolysis: a case report. Changgeng Yi Xue Za Zhi 1996; 19:371–6. [PubMed] [Google Scholar]
- 85. Boyce TG, Mintz ED, Greene KD et al. . Vibrio cholerae O139 Bengal infections among tourists to Southeast Asia: an intercontinental foodborne outbreak. J Infect Dis 1995; 172:1401–4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


