Abstract
Pancreatic cystic lesions (PCLs) are well-known precursors of pancreatic cancer. Their diagnosis can be challenging as their behavior varies from benign to malignant disease. Precise and timely management of malignant pancreatic cysts might prevent transformation to pancreatic cancer. However, the current consensus guidelines, which rely on standard imaging features to predict cyst malignancy potential, are conflicting and unclear. This has led to an increased interest in radiomics, a high-throughput extraction of comprehensible data from standard-of-care images. Radiomics can be used as a diagnostic and prognostic tool in personalized medicine. It utilizes quantitative image analysis to extract features in conjunction with machine learning and artificial intelligence (AI) methods like support vector machines, random forest, and convolutional neural networks for feature selection and classification. Selected features can then serve as imaging biomarkers to predict high-risk PCLs. Radiomics studies conducted heretofore on PCLs have shown promising results. This cost-effective approach would help us to differentiate benign PCLs from malignant ones and potentially guide clinical decision-making leading to better utilization of healthcare resources. In this review, we discuss the process of radiomics, its myriad applications such as diagnosis, prognosis, and prediction of therapy response. We also discuss the outcomes of studies involving radiomic analysis of PCLs and pancreatic cancer, and challenges associated with this novel field along with possible solutions. Although these studies highlight the potential benefit of radiomics in the prevention and optimal treatment of pancreatic cancer, further studies are warranted before incorporating radiomics into the clinical decision support system.
Keywords: Pancreatic cystic lesions, pancreatic cancer, Radiomics, radiomics in pancreatic cancer, machine learning
Introduction
Pancreatic cystic lesions (PCLs) are challenging to manage clinically as their behavior varies from benign to malignant disease. Furthermore, only a few subtypes of these cysts are considered precursor lesions of pancreatic carcinoma [1]. As a result, timely and precise management of high-risk PCLs might prevent their progression to pancreatic cancer and can curtail the resources needed for their life-long surveillance. Additionally, due to the improved quality and ubiquitous use of modern imaging modalities, the number of incidental PCLs has risen substantially in recent times with an estimated prevalence ranging from 2.4 - 13.5% [2, 3]. The incidence of PCLs increases with age, reaching up to 30% by the eighth decade of life [4].
Pancreatic cancer is one of the most lethal cancers, with a 5-year survival rate of 8%, and is the third most common cause of cancer-related deaths in the United States [5]. Given the lower incidence, aggressive behavior, rapid progression, and lack of an accepted biomarker, pancreatic cancer has been considered unsuitable for preventive and early detection screening programs [6]. However, recently published studies have changed this historic view. These groups have proposed that pancreatic cancer follows a stepwise carcinogenic progression through increasing grades of dysplasia of precursor lesions that can take more than a decade to form invasive cancer [7–9]. More recently, this progression model was challenged by Notta et al. who proposed that pancreatic cancer progression is neither gradual nor follows a sequential mutational order to develop invasive cancer [10]. Regardless, there is a significant window of opportunity to intervene and reduce mortality if precursor neoplastic cystic lesions can be appropriately detected at a benign stage [11].
Pancreatic cystic lesions
PCLs are divided into two categories: non-neoplastic and neoplastic cysts. Pancreatic pseudocysts are the principal non-neoplastic cysts, associated primarily with acute interstitial edematous pancreatitis [12]. Neoplastic cysts can be further sub-classified, per WHO classification, as follows: serous cystic neoplasms (SCNs), which include serous cystadenoma and serous cystadenocarcinoma; mucinous cystic neoplasms (MCNs), which include mucinous cystadenoma, mucinous cystic neoplasm with moderate dysplasia and mucinous cystadenocarcinoma; intraductal papillary mucinous neoplasms (IPMNs), which include intraductal papillary mucinous adenoma, intraductal papillary mucinous neoplasm with moderate dysplasia and intraductal papillary mucinous carcinoma; and solid pseudopapillary neoplasms (SPENs), which include solid pseudopapillary neoplasm and solid pseudopapillary carcinoma [13].
Serous cystic neoplasms
SCNs occur predominantly in women in their sixties and their distinctive imaging feature is microcystic or honeycomb appearance with central scar. SCNs can be managed conservatively with serial monitoring, as these lesions show an extremely low incidence of malignancy [14].
Mucinous cystic neoplasms
MCNs, which occur almost exclusively in women, are mucin producing unilocular cysts and show no pancreatic duct communication [15]. The European guidelines advocate surveillance for MCNs < 4 cm in size without mural nodules; however, Fukuoka guidelines recommend surgical resection for all surgically fit patients with MCNs, regardless of the size of the cyst [16, 17]. In comparison, the American College of Gastroenterology (ACG) guidelines also recommend surveillance for MCNs measuring < 4 cm with no concerning symptoms or features [18]. Altogether, these contrasting guidelines, relatively young age of patients, risk of progression to malignancy, and complete amelioration following resection make surgery the most suitable treatment for patients with MCNs. Hence, newer modalities are needed to identify high-risk MCNs for risk stratification and better management of these lesions [15, 19, 20].
Intraductal papillary mucinous neoplasm
IPMNs, recognized in 1996 as a separate entity from MCNs by the WHO, are the most common neoplastic PCLs with an equal incidence in men and women [21]. IPMNs are mucin producing cysts and may involve pancreatic main duct, branch duct or a combination of both [22]. Branch-duct IPMNs (BD-IPMN) carry an average risk of 31.1% for high-grade dysplasia and invasive carcinoma, and 18.5% for invasive IPMN. For main-duct IPMNs (MD-IPMN), the risk for high-grade dysplasia and invasive carcinoma is 61.6% and 43.1% for invasive IPMN [23]. As a result, it is difficult to predict which lesions will progress to malignancy and which can be safely observed. The survival rate for patients with non-invasive/low-grade IPMNs is around 100% following complete resection, whereas it is reduced to almost half for patients with invasive IPMN [24, 25]. In spite of the improvement in postoperative outcomes, pancreatic surgeries have morbidity of ~20% and a mortality rate of ~3% [26]. Therefore, there exists not only a need for identifying high-risk cysts at an early stage, but also for avoiding overtreatment in patients with low-risk lesions who can be observed safely without surgery.
Solid-pseudopapillary neoplasms
SPENs are a rare type of pancreatic cyst and although clearly malignant, can be almost completely treated by surgical resection. The 5-year survival rate is as high as 97% after complete cystic resection [27].
Cystic pancreatic neuroendocrine neoplasms
Cystic PNENs account for only 1-2% of all PCLs and less than 10% of them are cystic, making cystic PNENs very rare. Endoscopic ultrasound-guided fine needle aspirate (EUS-FNA) is often required for their diagnosis. This, in combination with pathognomic imaging features, provides a confirmed diagnosis [28]. Similar to SPEN, surgery is curative and improves survival rates for PNENs [29].
IPMNs and MCNs are the only radiographically identifiable precursors of pancreatic cancer [30, 31]. Consequently, accurate assessment of the malignant potential of these cystic lesions may allow early detection of resectable pancreatic cancers prior to oncogenesis [32]. The latest guidelines propose a practical approach for their management and surveillance, yet the clinical management of these mucinous cystic lesions remains challenging [23]. The variable risk of malignant transformation combined with high risks associated with pancreatic surgery have led to conflicting recommendations for the management of mucinous cystic lesions.
We have tabulated various types of cysts with their characteristic imaging features and cystic fluid contents in Table 1. We have also graphically represented the most common PCLs, showing their pathognomic imaging characteristics in Figure 1.
Table 1 –
Types of pancreatic cystic lesions
| Cyst type | Clinical associations | Imaging and fluid analysis |
|---|---|---|
| NON-NEOPLASTIC | ||
| Pseudocyst | Acute pancreatitis | Well circumscribed, thick walled cysts. May contain fluid alone or with debris. Aspirate: Dark colored fluid, high amylase/lipase, low carcinoembryonic antigen (CEA). |
| NEOPLASTIC | ||
| Serous cystic neoplasm | Three times more common in women. Age - 6th decade | Microcystic or honeycomb appearance. Presence of central calcifications is pathognomic. Aspirate: low CEA, low amylase/lipase. |
| Mucinous cystic neoplasm | Found almost exclusively in women. Age - 5th to 7th decade. | Appear predominantly in the body or tail. Unilocular cyst, may have septations or wall calcification. Do not show communication with pancreatic duct. Aspirate: high CEA, variable amylase. |
| Intraductal papillary mucinous neoplasm | Incidence is same for men and women. Age - 7th decade. | Mucin producing cyst. Involves main or side branches of pancreatic duct. Aspirate: high CEA, high amylase. |
| a) Side branch | Most common incidental PCL. Associated with low risk of malignancy. |
Microcystic with appearance like bunch of grapes. Communicates with pancreatic duct. Dilatation of single or multiple side branches. |
| b) Main duct | Lower incidence than side branch IPMN. Higher risk of malignancy. |
Appears as dilated main pancreatic duct, may be diffuse or segmental. |
| c) Mixed | Rare incidence; malignancy risk similar to main duct IPMN. | Appearance as dilated main and side branch pancreatic ducts. |
| Solid-pseudopapillary neoplasm | Ten times more common in women. Age – 20s. | Single solid and cystic neoplasm, can occur anywhere in the pancreas. Intratumoral hemorrhage may be seen. |
| Cystic pancreatic neuroendocrine neoplasm | Incidence similar for men and women. Age - 5th to 6th decade. May be associated with multiple endocrine neoplasia syndrome. | Characteristic finding - hyper vascular ring. Aspirate: low CEA, low amylase/lipase. |
Fig. 1.

Schematic representation of the characteristic morphological and imaging features of various PCLs. PCLs can be classified into two categories; non-neoplastic and neoplastic cysts. Pancreatic pseudocysts are the principal non-neoplastic cysts, associated primarily with pancreatitis. Neoplastic cysts can be further sub-classified into: Serous cystic neoplasms (SCN), Mucinous cystic neoplasms (MCN), Intraductal papillary mucinous neoplasms (IPMN), and Solid pseudopapillary neoplasms (SPEN). The characteristic imaging features of each sub-type are listed alongside.
EUS-FNA has proved to be of diagnostic importance in the management of PCLs, especially for evaluation of BD-IPMNs without concerning features. It detected 30% more malignancies in IPMNs measuring < 3cm in one study, as compared to high-risk imaging features such as dilatation of the main pancreatic duct and presence of mural nodules [33]. In another study, cystic fluid analysis using EUS-FNA predicted invasive mucinous cyst with 90% sensitivity [34]. However, it is still considered investigational and recommended to be used in centers with expertise in EUS-FNA and cytological interpretation. Moving forward, more data is needed to determine the accuracy of EUS-FNA before adopting it as the standard of care [23].
Currently, only three molecular markers - CEA and CA 19-9 levels and KRAS mutation expression, are used in clinics for diagnosing malignant mucinous cysts. However, due to low diagnostic accuracy, they are only employed in high risk patients. Multiple other proteins (MUC1 and MUC2), microRNA (miR21 and miR155), monoclonal antibody (mAb Das-1 – reactive against colonic epithelial protein), mutated DNA (GNAS, BRAF) and immune markers (neutrophil to lymphocyte ratio), extracted through pancreatic juice and peripheral blood are under consideration as novel biomarkers. These biomarkers for PCLs stratification have been comprehensively reviewed recently by Morris et al. [35]. However, further studies are warranted before employing these biomarkers in clinical practice.
The internationally accepted consensus guidelines, the Fukuoka Criteria, and the European guidelines for identifying malignant PCLs do not give a clear consensus on management of these lesions. This is due to the inability of the current imaging and cytological technologies to accurately distinguish between low-risk and high-risk IPMNs. The failure rate for these guidelines is high, especially in high volume centers [36–38]. Although the guidelines have considerably improved the sensitivity of detecting malignancy, they lack specificity and often incorrectly recommend benign lesions for surgery [39]. Thus, novel markers of malignancy are required to precisely identify low-risk lesions to minimize the morbidity, mortality, and financial burden associated with surgery. Effective biomarkers should discriminate between high-risk cysts requiring curative resection and low-risk cysts that could be closely followed, sparing patients from needless invasive surgeries [40].
Recently Springer et al. developed a comprehensive test, CompCyst, using supervised machine learning multivariate organization of combinatorial alterations (MOCA) algorithm to stratify PCLs malignancy risk. CompCyst combined molecular markers [mutations in 11 common genes, loss of heterozygosity, aneuploidy, and cyst fluid protein markers – CEA, Vascular endothelial growth factor (VEGF)], clinical characteristics (age, sex, abdominal pain, jaundice, weight loss, diabetes, and pancreatitis), and imaging features (size, location and number of cysts, presence of mural nodule, communication with pancreatic duct and size of main pancreatic duct), to form a comprehensive panel. Data from a cohort of 436 patients was used to train the algorithm. Consequently, in a validation cohort of 426 patients, CompCyst achieved an accuracy of 69% vs 56% by conventional tools (based on consensus guidelines), in stratifying PCLs into three groups – require resection, require regular surveillance and no further management required. Although the group attained promising results, a major limitation was the use of invasive techniques to obtain cystic fluid molecular markers [41].
Radiomics has the potential to generate novel noninvasive imaging markers of malignancy [42]. This technique uses machine learning algorithms to derive texture and other features from medical images and combines them with bio-statistical methods to build clinical prediction models [43]. However, studies using radiomics in pancreatic cancer and specifically to predict the risk of malignant progression of IPMNs are limited. Herein, we discuss the studies that have been completed in this field, as well as future implications of this technological advancement.
Radiomics
Medical images store more information than is visible to the trained eyes of physicians. This hidden information, when extracted and analyzed by computational tools, can provide more details about the region of interest than previously observed [44]. Various imaging modalities such as ultrasonography (USG), computed tomography (CT), magnetic resonance imaging (MRI), and positron emission tomography (PET) are ubiquitously used on patients presenting to the oncology department. Most patients will have several imaging sessions throughout the course of their management. The effort to transform these standard-of-care digital images to high-quality mineable data through the extraction of quantitative features has been expanding [45]. This process of high throughput extraction of comprehensible data from the acquired standard of care images has been termed Radiomics [46, 47]. It is the integration of information from radiology images with “omics” data obtained from quantification of molecules present in cells, made possible with the advent of high-throughput arrays and next-generation sequencing (NGS) technologies [48].
Computer-aided diagnosis (CAD) can be considered a stepping-stone in the formulation of radiomics. CAD has an analogous workflow to radiomics but on a less complex level. CAD has been successfully utilized to assist radiologists in diagnosing malignant nodules in breast and lung for over a decade. However, it has only been used to identify these nodules, and radiologists must still differentiate them into benign or malignant based on their imaging features [49–52]. Moreover, radiomics builds on already established image analysis techniques to examine texture, shape, and gray level statistics, combined with Haralick methods [53–56]. In fact, texture analysis of medical images has been used to measure lesion heterogeneity, and exhibited a statistically significant correlation between imaging features and clinical endpoints even before the term “radiomics” was coined [57]. Mir et al. used texture analysis on liver CT images of patients to stratify them into normal, visible malignancy and invisible malignancy [58]. Building upon the popular concept of quantitative image analysis, radiomics research has dramatically grown since the start of this decade, and unraveled its significance in modern patient care.
The fundamental basis of radiomics is that advanced image analysis of radiographic images can produce numerous quantitative imaging biomarkers capable of assigning different clinical phenotypes to the imaged lesions. This premise is supported by the fact that lesions exhibit measurable differences in shape, texture, and grayness in the radiographic images captured [59]. These biomarkers can then be utilized to build descriptive models to provide invaluable predictive, prognostic, and diagnostic information. Eventually, radiomics may help to characterize tumor biology by linking imaging biomarkers with underlying pathology [60].
Process
The workflow of radiomics can be divided into five steps: 1) acquisition of standard-of-care images; 2) lesion segmentation and contouring; 3) feature extraction and selection; 4) model building; and 5) analysis [47] (Figure 2). Image analysis for radiomics is done on the standard-of-care radiographic images, and most of the technologically advanced softwares used in radiomics are open access [61]. Hence, radiomics analyses can be performed without subjecting the patients to more economic and mental stress from additional diagnostic testing. Some researchers include an image pre-processing step to reduce the inherent noise of the images before subjecting them to analysis. However, this can significantly affect the extraction of radiomics features [62]. Accordingly, standard protocols need to be used for image acquisition and processing to minimize confounding variables [63].
Fig. 2.
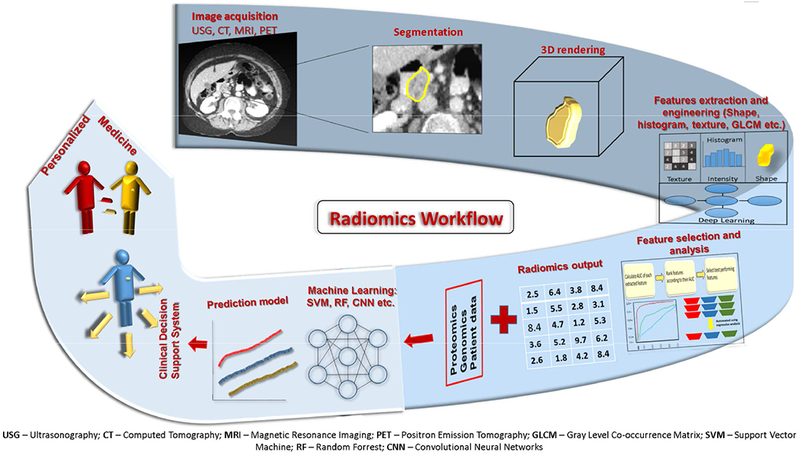
The workflow of Radiomics. The first step in the process is selection of the region of interest through manual or (semi)automated segmentation on acquired/archived standard of care radiographic images. Selecting the region of interest on these series of images forms a 3D rendering of the lesion. Subsequently, radiomics features are extracted from this region of interest. In the next step, statistical analysis is used to determine the most pertinent features from all the extracted features. These judiciously chosen features are then combined with the patient’s clinical data to build a prediction model with the help of machine learning tools. Furthermore, this model is validated against an unknown data set to prove its accuracy. Consequently, the radiomics model can form a part of the clinical decision support system to personalize medical care for the patients in the future.
The second step is identifying the tumor or suspected lesion as the volume of interest and defining its boundaries on images. This is known as lesion segmentation and can be done either manually by an experienced radiologist or a (semi)automated procedure. Segmentations are primarily done in a volumetric way by creating a 3D-rendered model through the selection of all the voxels within the region of interest [64]. Although manual segmentation is accurate, it suffers from high inter-viewer variability, and is labor intensive and the most time-consuming step. Furthermore, a slight variation in segmentation boundaries can give rise to varied results, which makes it impractical for large data sets. On the other hand, features extracted through (semi)automation are more robust and reproducible [65]. For this reason, segmentation should be as automated as possible. The most commonly used automated segmentation methods include region growing (click and grow), level sets, and graph cuts [47, 66]. However, at present, no standards for segmentation have been established.
The next step is the extraction of radiomics features from the region of interest. First-order metrics reflect asymmetry, sharpness, entropy, and uniformity of intensity in the voxels [59]. Texture features are second-order metrics that describe the grayscale variation. These features capture the distribution of intensity among neighboring voxels [53]. Lastly, the higher-order metrics such as Fractal analysis describe repetitive and non-repetitive patterns. Some examples and explanations of these radiomics features are outlined in Table 2. Additionally, an extensive list of radiomics features can be extracted from images as reviewed earlier [67]. Notably, not all features extracted are ultimately used for the final analysis. In addition, the number of features extracted can be much higher than the number of patients, leading to over-fitting [47]. Therefore, highly correlated and redundant features are dropped. This can be done either by manually selecting the germane features or using automated regression analysis [56]. Thus, from hundreds of features extracted, only the best performing, task-specific, robust, and non-redundant features are ultimately used in the formation of a high-quality database [68]. A few of the notable open-source softwares available for radiomics analysis include IBEX [69], CGITA [70], MaZda [71],and 3D Slicer [72].
Table 2 –
Types of radiomics features
| Radiomics Metrics | Examples | Description |
|---|---|---|
| First Order | Histogram (Skewness, Kurtosis) | Describe the distribution of values of individual voxels without concern for spatial relationships. These reduce a region of interest to single values for mean, median, maximum, minimum, as well as the skewness (asymmetry) and kurtosis (flatness) of the intensities on image. |
| Second Order | Gray level co-occurrence matrix, gray level difference matrix etc. | Described as “texture” features; they describe statistical interrelationships between voxels with similar (or dissimilar) contrast values, e.g. uniformity or randomness (entropy) of the intensities on the image, contrast, cluster prominence and shade, inertia etc. |
| Transform Analysis | Fourier, wavelets, Gabor and law methods etc. | These higher-order statistical methods extract repetitive or non-repetitive patterns, magnitude, phase, direction and fractal dimensions. |
| Structural Analysis | Fractal analysis |
Adapted from the reference [137]
The final step in the process is to build an efficient model and test its accuracy. Patients’ clinical characteristics can be integrated with selected radiomics metrics to improve the efficacy of the model [47]. This process is done by a machine learning approach, which starts with defining a set endpoint. It is a form of supervised learning in which the data set is divided into training and validation sets (internal validation) and ideally, there should be enough data for this separation [73]. Some examples of supervised machine learning classifiers include support vector machines [74], random forests [75] and neural networks [76]. The final performance of the model varies according to the choice of the classifier, so only the highest-performing one should be used [77]. The classification algorithm is presented with the training set in conjunction with the ground-truth label (endpoint) [78]. Then, classifiers are trained using multiple cross-validations of training sets to learn the ground truth. In the end, classifiers separate the data with respect to outcome variables. Lastly, classifiers are tested for their accuracy by summary measures such as F1 score, Youden J index, and area under the receiver operating characteristic curve. Each measure has its own advantages and disadvantages, so an appropriate measure should be chosen according to the data set and the desired end result [79]. Ultimately, the predictive clinical performance of the final model should also be tested on a separate data set to achieve external validation. It’s important to realize that variations can result from variation in any of the above-mentioned processes. Thus, standardization is the key to generate a robust and clinically applicable model.
Emerging applications of Radiomics
Radiomics has myriad applications in modern medical care, which are not limited to differentiating between benign and malignant lesions. This section discusses some of the recent studies to highlight the potential utility of this relatively new field in the clinical decision support system.
Diagnosis
Radiomics-based models have been extensively used to distinguish malignant lesions from benign ones in various solid cancers. Bickelhaupt et al. used a kurtosis (a first-order radiomics metric) radiomics model on diffusion-weighted MRI images, while Zhang et al. applied a radiomics approach to sono-elastography images for differentiating benign and malignant breast lesions [80, 81]. Yao et al. built a model based on features extracted from multimodal ultrasound images to distinguish between benign focal liver lesions and hepatocellular carcinoma, with an accuracy of 94% [82]. In another instance, a radiomics model based on texture features (Energy, Entropy, Correlation, Homogeneity, and Inertia) was able to differentiate cancerous peripheral zone prostate tumors from normal peripheral zone tissue in T2 and diffusion-weighted MRI images [83]. To take it a step further, Zhao et al. developed a model to predict lung cancer histological subtypes on multi-phasic contrast-enhanced CT (CECT) images [84].
Risk stratification
Features extracted via radiomics can be utilized for the prognostication of cancer by either predicting survival, response to therapy, or metastatic potential. To predict five-year survival rate, Wu et al. combined clinical features with five statistically selected radiomics metrics (two texture and three wavelet features) extracted from pre-treatment CT images of osteosarcoma patients [85]. Similarly, Braman et al. extracted radiomics features from breast tumors and their surroundings in dynamic contrast-enhanced MRI images to predict response to therapy. The authors inferred that co-occurrence of local anisotropic gradient orientations (CoLIAGe) features were most predictive to identify patients obtaining a complete response to neoadjuvant chemotherapy [86]. In another instance, Liu et al. distinguished thyroid tumors with lymph node metastatic potential from non-metastatic tumors based on 50 selected features and achieved an accuracy of ~70% [87]. Similarly, Coroller et al. selected 35 radiomics features extracted from pretreatment CT images of lung adenocarcinoma to accurately predict their potential for distant metastasis [88]. Radiomics features have also been used to differentiate between early and late stage cancer. Mu et al. classified cervical cancer patients into early and advanced stages, based on texture index derived from GLRLM (gray-level run-length matrix), a second-order radiomics feature extracted from PET images [89]. Using radiomics features to more accurately predict prognosis can remarkably assist physicians to stratify patients, measure patients’ response to therapy in real time and eventually select the appropriate treatment.
Virtual biopsy
Solid cancers show remarkable spatial and temporal heterogeneity. Unfortunately, the whole tumor cannot be entirely characterized by biopsy as it extracts only a small section of tissue at a specific time. Potentially, radiomics can solve this problem since it is capable of extracting features from the whole tumor. As a result, it captures the spatial heterogeneity of the tumor to achieve its optimal characterization, which can subsequently be used to identify the appropriate site for biopsy and guide therapy. It can relieve the necessity of multiple biopsies. Although not in radiomics, this has been done with use of PET/CT images to better guide biopsies in patients with abdominal malignancies [90]. Likewise, radiomics analysis can potentially be done at multiple time points to characterize the temporal heterogeneity of tumors [68].
Radiogenomics
Since the realization that cancer is a genetic disease, physicians and researchers have tried to uncover a noninvasive means to determine the genetic profile of tumors. Medicine has long correlated imaging phenotypes with clinical outcomes, disease aggressiveness, and therapeutic response. With the inception of radiomics, many investigators have been able to correlate extracted imaging features with the expression of a specific gene or genetic profile.[91, 92]. This use of imaging biomarkers as a tool for genetic profiling is known as “radiogenomics”, a term coined by Bai et al. [93], as it highlights the synergism between radiomics and genomics by linking images with gene outcomes [91]. The recent “omics” revolution, along with the increase in sophistication of machine learning technology, and advancements in computation have provided an elegant means of integrating imaging data with systems biology.
The power of radiogenomics is exemplified by its broad application. One such application is elucidating the presence or absence of a specific gene/mutation within a tumor. This has been done in a myriad of cancers, including ER/PR/HER2 status in breast [94], EGFR mutation in lung [95–97], EGFRv3 and IDH1 status in glioblastoma [98, 99], KRAS/NRAS/BRAF mutations in colorectal carcinoma (CRC) [100], β-catenin mutation in hepatocellular carcinoma (HCC) [101], and PBMR1 in clear cell renal cell carcinoma [102]. Interestingly, radiogenomics has also proven capable of determining epigenetic characteristics including RUNX2 methylation status in clear cell renal cell carcinoma [103] and MGMT methylation status in GBM [104]. Consequently, the radiomics analysis was able to distinguish between molecular prognostic markers.
Other exciting radiogenomics uses involve applications that go beyond genetic profiling. Imaging and genetic information can be integrated to improve patient characterization and outcome prediction. One such use involves the correlation of radiogenomics outputs with current clinical recurrence predictors. In a study by Li et al., radiomics signatures were highly correlated to multigene assay recurrence scores of Oncotype DX, MammaPrint, and PAM50 to predict recurrence in breast cancer patients [105]. Along with this, delta radiogenomics (genetic profiling with images collected over time) has been able to accurately predict response to chemical and precision therapies as well as determine genetic changes in response to these interventions [106].
Another important aspect of radiogenomics is the ability to assess the tumor microenvironment (TME). Insight into the TME has emphasized its influence on cancer initiation, progression, and therapeutic response [107, 108]. Recently, radiogenomics markers have been described that provide insight into the hypoxic state of the TME [109], metabolite profile [110], and immune milieu [111]. Radiogenomics TME evaluation, especially immune phenotyping, will prove invaluable in the future for immune therapy selection and treatment evaluation.
Radiogenomics offers a means of noninvasively personalizing medicine in a manner that was heretofore not possible. The use of somatic (subjective elements determined by an observer) and agnostic (objective elements determined by the machine learning algorithm) features in the analyses increases the number of measurable parameters extracted from the imaging data, thereby improving specificity and conferring the ability for personalization. Thus, imaging surrogates could be utilized as a noninvasive means of determining a tumor’s genetic identity, thereby influencing patient-specific decisional support for cancer therapy.
Radiomics in pancreatic cystic lesions
Manual identification of cyst type has an accuracy of only 60-70%, even by highly-trained radiologists [112]. Thus, imaging markers need to be developed for correctly identifying the type and malignant potential of PCLs through radiomics. Unfortunately, radiomics studies for malignancy risk assessment of PCLs, particularly IPMNs, are very limited.
In one of the first studies, Dmitriev et al. presented an algorithm to discriminate between the four most common types of neoplastic pancreatic cysts: IPMN, MCN, SCA, and SPN. They developed an ensemble model combining patient demographic factors with intensity and shape features extracted from the cyst images. Segmentation of the cystic lesions was achieved by a semi-automated graph-based segmentation technique and random forest classifier and convolutional neural networks were used for feature selection. This pioneering study achieved a promising accuracy of ~84% in distinguishing different cyst types [113].
Wei et al. proposed a preoperative radiomics based diagnostic model to differentiate SCNs from other neoplastic PCLs, to avoid overtreatment in these almost benign and indolent cystic lesions. Out of 409 quantitative features derived from 260 patients diagnosed with PCLs, 17 intensity and texture features (predominantly intensity T-range, wavelet intensity T-median, and wavelet neighborhood gray-tone difference matrix (NGTDM) busyness) and 5 guideline-based features (sex, location, moment difference, mean rectangular fitting factor and size) proved to be most statistically significant in identifying SCNs. Support vector machine and LASSO regression machine learning tools contributed towards model building. Their final model provided an accuracy of ~76% in cross-validation cohort and ~83% in an independent validation cohort of 60 patients. Notably, only 31 out of 102 SCN cases, included in this study, were accurately diagnosed before the surgery [114]. Similarly, Yang et al. differentiated 25 patients with MCN from 53 patients with SCN using a preliminary model based on texture features (GLCM, GLRLM, GLZLM, and NGLDM) extracted from CECT images selected via LASSO regression and random forest classifiers. Interestingly, they also evaluated the consistency of texture features extracted from CT images of 2 mm and 5 mm thick slices and found a good correlation among the extracted features, neglected by many previous studies. Although a difference in slice thickness did not affect feature extraction, they proposed using similar slice thickness CT images for radiomics analysis. In the validation group, they achieved an accuracy of 74% in 2 mm slice thickness group and 83% in 5 mm slice thickness group [115]. These studies highlighted the ability of radiomics in reducing misdiagnosis and avoiding overtreatment.
Hanania et al. applied radiomics principles to successfully grade IPMNs, based on their malignancy potential. They extracted 360 radiomics features from pre-surgery CT scans of 53 patients diagnosed with IPMN, who consequently underwent resection. Based on final pathological findings, IPMNs were divided into two groups: cysts with low-grade dysplasia, and cysts with high-grade dysplasia/malignancy. Out of 360 features extracted by quantitative image analysis, 14 features selected using principal component analysis, when evaluated in combination, were 96% accurate in discriminating between the two groups. All of these features were within the domain of GLCM, a second-order radiomics feature. They also analyzed the performance of the Fukuoka guidelines in these resected cystic lesions and found they had a false positive rate of 36%. This study proved that high-grade/invasive IPMNs have distinct radiomics features that can be utilized to stratify patients via noninvasive imaging [116].
Permuth et al. extracted 112 radiomics features from pre-surgical CT images of 37 patients diagnosed with IPMN, which were then resected. They also analyzed miRNA data from this patient cohort along with the extracted radiomics features. They interpreted that 14 (11 texture features and 3 size and shape features) out 112 extracted radiomics features and 5 (miR-200a-3p, miR-1185-5p, miR-33a-5p, miR-574-4p, and miR-664b) out of 800 mi-RNAs selected through logistic regression analyses were able to distinguish low-grade from high-grade/invasive IPMNs. Upon principal component analysis and cross-validation, they found that a combination of miRNA and radiomics features had the best accuracy, ~92%, in differentiating between the two groups [117]. This study again proved the utility of radiomics in accurately predicting the malignancy potential of IPMNs.
Chakraborty et al. utilized radiomics features extracted from pre-surgical CT images, as markers for assessment of malignancy risk of BD-IPMNs. Similar to the previous studies, they categorized their cohort of 103 patients into low-risk and high-risk IPMNs based on final pathological findings after cyst resection. They extracted four new radiographically inspired features (enhanced boundary fraction, enhanced inside fraction, filled largest connected component fraction and average weighted eccentricity), along with intensity and orientation-based texture features from the CT images. By adopting a new approach, they segmented the boundaries of the cystic lesions and the pancreas to extract these novel radiographically inspired features (RiFs). Analysis using random forest classifier and support vector machine tools predicted only 12 texture features showed statistically significant association with malignancy risk. They also included five clinical variables (age at resection, cyst size, presence of solid component, presence of abdominal pain, and gender), which had previously shown association with IPMN risk. On final analysis, the model that combined the newly extracted RiFs with clinical variables provided the best accuracy of ~ 80% to predict the malignancy risk of IPMNs. Notably, the negative predictive value of their model was 94%, highlighting the accuracy of their model [118].
Although only a few number of studies on use of radiomics in risk stratification of PCLs have been published, these studies have demonstrated that radiomics can be utilized to non-invasively discriminate between low-risk and high-risk PCLs before resection. This cost-effective approach would enable us to accurately recommend lifesaving surgery for individuals with malignant cysts and spare those with benign lesions the morbidity, mortality and high costs associated with pancreatic surgeries. Consequently, more studies are warranted to develop these imaging biomarkers which can be used to differentiate between benign and malignant PCLs.
Radiomics in pancreatic cancer
Few studies have employed radiomics in pancreatic cancer research. Those have used radiomics to either predict survival or treatment response in pancreatic cancer patients. Yue et al. first employed radiomics for a pancreatic cancer study. They calculated and identified metabolic texture variations in pre- and post-radiotherapy PET/CT images of pancreatic cancer patients. Based on these features (standardized uptake value max, homogeneity, variance, sum mean and cluster tendency), they were able to stratify patients into low-risk and high-risk groups with longer and shorter overall survival, respectively [119]. Similarly, Chakraborty et al. extracted texture features (angle co-occurrence matrices, which are edge-based features) from pre-treatment CT images to accurately predict the two-year survival of pancreatic cancer patients [120]. Two more groups used radiomics to predict pancreatic cancer aggressiveness and disease-free survival, albeit it in different ways. Cassinotto et al. did so by extracting simple quantitative imaging biomarkers such as attenuation, while Yun et al. extracted second-order radiomics features, like histograms and gray-level co-occurrence matrix (GLCM) from preoperative CT images [121, 122]. More significant results were shown by Cozzi et al., who identified a CT-based radiomics signature, based on GLCM; GLRM, neighborhood gray-level different matrix (NGLDM); and gray-level zone-length matrix (GLZLM), which correlated with overall survival and local tumor control after stereotactic body radiation therapy [123]. Radiomics features have also been used to predict treatment response. Chen et al. analyzed radiation-induced changes in quantitative CT features of pancreatic cancer patients post chemo-radiation therapy. They observed significant changes in these features, which can be used to assess early treatment response and intensify therapy in non/low responders [124]. These findings demonstrate the importance of identifying accurate pre-operative prognostic factors that can improve the selection of first-line therapies for patients with aggressive tumors.
Challenges and possible solutions
Despite its potential value in oncology, radiomics is not free from its challenges and limitations. Some of the technical challenges associated with the individual steps of radiomics have already been discussed. Herein we describe a few of the broader limitations associated with this multi-disciplinary field.
Firstly, being a new field, radiomics lacks standardization. Variation in image acquisition, feature extraction, and statistical analyses is wide across the medical centers performing these studies [47]. These variations can produce artifacts that are not attributable to underlying biological effects, leading to poor reproducibility [125]. For this reason, image acquisition protocols should be standardized across the imaging centers. Additionally, multiple segmentation, serial imaging, and phantom studies should be performed to produce robust, reproducible, and repeatable radiomics metrics [126]. In fact, robust features have proven to be more stable over repeated scans and showed low inter-observer variability [127].
Another limitation of radiomics is the lack of validation. All the radiomics studies in PCLs and most of the studies in other fields did not use a validation dataset [93]. Ideally, as with any biomarker study, validation of results against an external independent dataset is required. For reference, validation against independent cohorts is the standard for verifying any newly identified biomarker [128]. One of the main reasons for lack of validation in radiomics might be the small sample size in the studies. The lack of publicly available shared databases for radiomics metrics hinders progress as well [129]. The Cancer Imaging Archive (TCIA) [130] and Qualitative Imaging Biomarker Alliance (QIBA) [131] are examples of the few of the data sharing platforms.
Overfitting is another challenge researchers have to face while using machine learning, especially in radiomics. The main reason for overfitting is more features are extracted than the number of samples, so they become redundant and irrelevant [132]. The possible solution is a reduction of the number of features by test-retest studies and bootstrapping to select only the robust features capable of providing accurate results in any given data set [65, 127].
Another possible hurdle is extracting inferences from “big data”, i.e. large data sets that are not derived from carefully controlled experiments. The data derived from medical images is in the form of complex constructs that make it difficult to understand [68].
Radiomics Quality Score (RQS) [133], available online, is a recent initiative proposed by Lambin et al. to assess the quality of both past and future radiomics studies. It is an evaluation criterion, consisting of sixteen key components, and each component is assigned a number of points corresponding to its importance. It also serves as reporting guidelines that can be used by researchers and reviewers to ascertain the quality of radiomics studies and verify whether the investigators have followed the best practices and procedures [67]. Following such guidelines will help standardize and advance radiomics into future applications.
Conclusions and future directions
Radiomics embodies the interdisciplinary shared vision of precision medicine. It has shown immense potential as a feasible tool for diagnosis, prognosis, prediction, and assessment of therapeutic responses in cancer patients. Notably, radiomics studies have proven that it is possible to predict the malignancy potential of PCLs non-invasively, which could benefit the prevention and optimal treatment of pancreatic cancer. However, being a relatively new field, further studies are required prior to its usage in the clinical decision support system. Also, serial radiomics studies are warranted to account for temporal heterogeneity within tumors. Furthermore, to better correlate radiomics features with tissue biology, imaging features should be extracted from combined datasets acquired from multiple modalities (e.g. CT and MRI). In conclusion, we envision a future in which imaging biomarkers become standard of care for cancer patients, to be used alongside histopathology.
Highlights -.
Pancreatic cystic lesions are well known radiographically identifiable precursor lesions of pancreatic ductal adenocarcinoma.
Precise and timely management of malignant pancreatic cysts might prevent transformation to pancreatic cancer.
Radiomics can be used as a diagnostic tool to differentiate benign pancreatic cystic lesions from malignant ones and potentially guide clinical decision-making.
We review the outcomes of radiomics studies done in pancreatic cystic lesions and pancreatic cancer, and challenges associated with radiomics along with possible solutions.
Acknowledgements –
We would like to thank Dr. Jessica Mercer for editing this manuscript.
Funding – The authors in this article are supported, in parts, by the following grants from the National Institutes of Health (P01 CA217798, U01 CA200466, U01 CA210240, R01 CA195586, R44DK117472 and R44CA224619).
List of abbreviations
- PCL
pancreatic cystic lesion
- AI
Artificial intelligence
- SCN
serous cystic neoplasm
- MCN
mucinous cystic neoplasm
- IPMN
intraductal papillary mucinous neoplasm
- SPEN
solid pseudopapillary neoplasm
- PNEN
pancreatic neuroendocrine neoplasm
- EUS-FNA
endoscopic ultrasound-guided fine needle aspirate
- CEA
carcinoembryonic antigen
- MOCA
multivariate organization of combinatorial alterations
- VEGF
vascular endothelial growth factor
- ACG
American College of Gastroenterology
- BD-IPMN
Branch-duct IPMN
- MD-IPMN
main-duct IPMN
- USG
ultrasonography
- CT
computed tomography
- MRI
magnetic resonance imaging
- PET
positron emission tomography
- NGS
next-generation sequencing
- CAD
computer-aided diagnosis
- CECT
contrast-enhanced CT
- CoLIAGe
co-occurrence of local anisotropic gradient orientations
- GLRLM
gray-level run-length matrix
- CRC
colorectal carcinoma
- HCC
hepatocellular carcinoma
- TME
tumor microenvironment
- GLCM
gray-level co-occurrence matrix
- NGLDM
neighborhood gray-level different matrix
- GLZLM
gray-level zone-length matrix
- RiF
radiographically inspired feature
- TCIA
The Cancer Imaging Archive
- QIBA
Qualitative Imaging Biomarker Alliance
- RQS
Radiomics Quality Score
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations
Ethics approval and consent to participate – Not applicable
Consent for publication – Not applicable
Availability of data and material – Not applicable
Conflict of interest - Dr. Surinder Batra is a co-founder of Sanguine Diagnostics and Therapeutics. All other authors declare they have no conflict of interest with the information presented in this manuscript.
References:
- 1.Zamboni G, et al. , Precancerous lesions of the pancreas. Best Pract Res Clin Gastroenterol, 2013. 27(2): p. 299–322. [DOI] [PubMed] [Google Scholar]
- 2.European evidence-based guidelines on pancreatic cystic neoplasms. 2018. 67(5): p. 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Oliveira PB, et al. , Prevalence of incidental pancreatic cysts on 3 tesla magnetic resonance. PLoS One, 2015. 10(3): p. e0121317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee KS, et al. , Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am J Gastroenterol, 2010. 105(9): p. 2079–84. [DOI] [PubMed] [Google Scholar]
- 5.Cancer Statistics. [cited 2019 04/09]; Available from: https://cancerstatisticscenter.cancer.org/#!/.
- 6.Del Chiaro M, et al. , Early detection and prevention of pancreatic cancer: is it really possible today? World J Gastroenterol, 2014. 20(34): p. 12118–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maitra A, Kern SE, and Hruban RH, Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol, 2006. 20(2): p. 211–26. [DOI] [PubMed] [Google Scholar]
- 8.Yachida S, et al. , Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature, 2010. 467(7319): p. 1114–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Peters MLB, et al. , Progression to pancreatic ductal adenocarcinoma from pancreatic intraepithelial neoplasia: Results of a simulation model. Pancreatology, 2018. 18(8): p. 928–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Notta F, et al. , A renewed model of pancreatic cancer evolution based on genomic rearrangement patterns. Nature, 2016. 538(7625): p. 378–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shin EJ and Canto MI, Pancreatic cancer screening. Gastroenterol Clin North Am, 2012. 41(1): p. 143–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pan G, et al. , Classification and Management of Pancreatic Pseudocysts. Medicine (Baltimore), 2015. 94(24): p. e960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zamboni G, K G, Hruban RH, et al. , Mucinous cystic neoplasms of the pancreas, in World Health Organization Classification of Tumours. Pathology and Genetics of Tumours of the Digestive System, Aaltonen LA HS, Editor. 2000, IARC Press: Lyon, France: p. 234. [Google Scholar]
- 14.Jais B, et al. , Serous cystic neoplasm of the pancreas: a multinational study of 2622 patients under the auspices of the International Association of Pancreatology and European Pancreatic Club (European Study Group on Cystic Tumors of the Pancreas). Gut, 2016. 65(2): p. 305–12. [DOI] [PubMed] [Google Scholar]
- 15.Zamboni G, et al. , Mucinous cystic tumors of the pancreas: clinicopathological features, prognosis, and relationship to other mucinous cystic tumors. Am J Surg Pathol, 1999. 23(4): p. 410–22. [DOI] [PubMed] [Google Scholar]
- 16.European Study Group on Cystic Tumours of the, P., European evidence-based guidelines on pancreatic cystic neoplasms. Gut, 2018. 67(5): p. 789–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanaka M, et al. , International consensus guidelines 2012 for the management of IPMN and MCN of the pancreas. Pancreatology, 2012. 12(3): p. 183–97. [DOI] [PubMed] [Google Scholar]
- 18.Elta GH, et al. , ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am J Gastroenterol, 2018. 113(4): p. 464–479. [DOI] [PubMed] [Google Scholar]
- 19.Reddy RP, et al. , Pancreatic mucinous cystic neoplasm defined by ovarian stroma: demographics, clinical features, and prevalence of cancer. Clin Gastroenterol Hepatol, 2004. 2(11): p. 1026–31. [DOI] [PubMed] [Google Scholar]
- 20.Sarr MG, et al. , Clinical and pathologic correlation of 84 mucinous cystic neoplasms of the pancreas: can one reliably differentiate benign from malignant (or premalignant) neoplasms? Ann Surg, 2000. 231(2): p. 205–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kloppel G, Solcia E, Longnecker DS, Capella C, Sobin, Leslie H. et al. in collaboration with pathologists in 7 countries, Histological typing of tumours of the exocrine pancreas. 2nd ed. Berlin: : Springer-Verlag, 1996. [Google Scholar]
- 22.Kloppel G, Kosmahl M, and Luttges J, [Intraductal neoplasms of the pancreas: cystic and common]. Pathologe, 2005. 26(1): p. 31–6. [DOI] [PubMed] [Google Scholar]
- 23.Tanaka M, et al. , Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology, 2017. 17(5): p. 738–753. [DOI] [PubMed] [Google Scholar]
- 24.Crippa S, et al. , Mucin-producing neoplasms of the pancreas: an analysis of distinguishing clinical and epidemiologic characteristics. Clin Gastroenterol Hepatol, 2010. 8(2): p. 213–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi H, et al. , Surgical outcomes of noninvasive and minimally invasive intraductal papillary-mucinous neoplasms of the pancreas. Ann Surg Oncol, 2006. 13(7): p. 955–60. [DOI] [PubMed] [Google Scholar]
- 26.Peluso H, et al. , Treatment outcomes, 30-day readmission and healthcare resource utilization after pancreatoduodenectomy for pancreatic malignancies. 2019. 26(5): p. 187–194. [DOI] [PubMed] [Google Scholar]
- 27.Law JK, et al. , A systematic review of solid-pseudopapillary neoplasms: are these rare lesions? Pancreas, 2014. 43(3): p. 331–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cloyd JM and Poultsides GA, Non-functional neuroendocrine tumors of the pancreas: Advances in diagnosis and management. World J Gastroenterol, 2015. 21(32): p. 9512–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hill JS, et al. , Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer, 2009. 115(4): p. 741–51. [DOI] [PubMed] [Google Scholar]
- 30.Adsay NV, Cystic neoplasia of the pancreas: pathology and biology. J Gastrointest Surg, 2008. 12(3): p. 401–4. [DOI] [PubMed] [Google Scholar]
- 31.Matthaei H, et al. , Cystic precursors to invasive pancreatic cancer. Nat Rev Gastroenterol Hepatol, 2011. 8(3): p. 141–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kimura H, et al. , Predictors and Diagnostic Strategies for Early-Stage Pancreatic Ductal Adenocarcinoma: A Retrospective Study. Pancreas, 2015. 44(7): p. 1148–54. [DOI] [PubMed] [Google Scholar]
- 33.Genevay M, et al. , Cytology adds value to imaging studies for risk assessment of malignancy in pancreatic mucinous cysts. Ann Surg, 2011254(6): p. 977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cocieru A, Brandwein S, and Saldinger PF, The role of endoscopic ultrasound and cyst fluid analysis in the initial evaluation and follow-up of incidental pancreatic cystic lesions. HPB (Oxford), 2011. 13(7): p. 459–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moris D, et al. , Updates and Critical Evaluation on Novel Biomarkers for the Malignant Progression of Intraductal Papillary Mucinous Neoplasms of the Pancreas. Anticancer Res, 2017. 37(5): p. 2185–2194. [DOI] [PubMed] [Google Scholar]
- 36.Roch AM, et al. , International Consensus Guidelines parameters for the prediction of malignancy in intraductal papillary mucinous neoplasm are not properly weighted and are not cumulative. HPB (Oxford), 2014. 16(10): p. 929–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Heckler M, et al. , The Sendai and Fukuoka consensus criteria for the management of branch duct IPMN - A meta-analysis on their accuracy. Pancreatology, 2017. 17(2): p. 255–262. [DOI] [PubMed] [Google Scholar]
- 38.Xu MM, et al. , Comparison of the diagnostic accuracy of three current guidelines for the evaluation of asymptomatic pancreatic cystic neoplasms. Medicine (Baltimore),2017. 96(35): p. e7900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goh BK, et al. , Evaluation of the Fukuoka Consensus Guidelines for intraductal papillary mucinous neoplasms of the pancreas: Results from a systematic review of 1,382 surgically resected patients. Surgery, 2015. 158(5): p. 1192–202. [DOI] [PubMed] [Google Scholar]
- 40.Campbell NM, et al. , Imaging patterns of intraductal papillary mucinous neoplasms of the pancreas: an illustrated discussion of the International Consensus Guidelines for the Management of IPMN. Abdom Imaging, 2015. 40(3): p. 663–77. [DOI] [PubMed] [Google Scholar]
- 41.Springer S, et al. , A multimodality test to guide the management of patients with a pancreatic cyst. Sci Transl Med, 2019. 11(501). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Savadjiev P, et al. , Image-based biomarkers for solid tumor quantification. Eur Radiol, 2019. [DOI] [PubMed] [Google Scholar]
- 43.Zhang L, et al. , IBEX: an open infrastructure software platform to facilitate collaborative work in radiomics. Med Phys, 2015. 42(3): p. 1341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Castellano G, et al. , Texture analysis of medical images. Clin Radiol, 2004. 59(12): p. 1061–9. [DOI] [PubMed] [Google Scholar]
- 45.Sanduleanu S, et al. , Tracking tumor biology with radiomics: A systematic review utilizing a radiomics quality score. Radiother Oncol, 2018. 127(3): p. 349–360. [DOI] [PubMed] [Google Scholar]
- 46.Lambin P, et al. , Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer, 2012. 48(4): p. 441–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kumar V, et al. , Radiomics: the process and the challenges. Magn Reson Imaging, 2012. 30(9): p. 1234–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Laura Antonelli MRG, Lucia Maddalena, Mara Sangiovann, Integrating imaging and omics data: A review. Biomedical Signal Processing and Control, 2019: p. 264–280. [Google Scholar]
- 49.van Ginneken B, Schaefer-Prokop CM, and Prokop M, Computer-aided diagnosis: how to move from the laboratory to the clinic. Radiology, 2011261 (3): p. 719–32. [DOI] [PubMed] [Google Scholar]
- 50.McCarville MB, et al. , Distinguishing benign from malignant pulmonary nodules with helical chest CT in children with malignant solid tumors. Radiology, 2006. 239(2): p. 514–20. [DOI] [PubMed] [Google Scholar]
- 51.Drukker K, Sennett CA, and Giger ML, Automated method for improving system performance of computer-aided diagnosis in breast ultrasound. IEEE Trans Med Imaging, 2009. 28(1): p. 122–8. [DOI] [PubMed] [Google Scholar]
- 52.Way TW, et al. , Computer-aided diagnosis of pulmonary nodules on CT scans: segmentation and classification using 3D active contours. Med Phys, 2006. 33(7): p. 2323–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Haralick RM, Shanmugam K, and Dinstein I, Textural Features for Image Classification. IEEE Transactions on Systems, Man, and Cybernetics, 1973. SMC-3(6): p. 610–621. [Google Scholar]
- 54.M.Galloway M, Texture analysis using gray level run lengths. Computer Graphics and Image Processing, 1975. 4(2): p. 172–179. [Google Scholar]
- 55.Amadasun M and King R, Textural features corresponding to textural properties. Vol. 19 1989. 1264–1274. [Google Scholar]
- 56.Parekh V and Jacobs MA, Radiomics: a new application from established techniques. Expert Rev Precis Med Drug Dev, 2016. 1(2): p. 207–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Castiglioni I and Gilardi MC, Radiomics: is it time to compose the puzzle? Clin Transl Imaging, 2018. 6(5): p. 411–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mir AH, Hanmandlu M, and Tandon SN, Texture analysis of CT-images for early detection of liver malignancy. Biomed Sci Instrum, 1995. 31: p. 213–7. [PubMed] [Google Scholar]
- 59.Aerts HJWL, et al. , Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nature Communications, 2014. 5: p. 4006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.European Society of R, White paper on imaging biomarkers. Insights Imaging, 2010. 1(2): p. 42–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nioche C, et al. , LIFEx: A Freeware for Radiomic Feature Calculation in Multimodality Imaging to Accelerate Advances in the Characterization of Tumor Heterogeneity. Cancer Res, 2018. 78(16): p. 4786–4789. [DOI] [PubMed] [Google Scholar]
- 62.Fave X, et al. , Impact of image preprocessing on the volume dependence and prognostic potential of radiomics features in non-small cell lung cancer. Vol. 5 2016. 349–363. [Google Scholar]
- 63.Yip SS and Aerts HJ, Applications and limitations of radiomics. Phys Med Biol, 2016. 61(13): p. R150–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Polan DF, Brady SL, and Kaufman RA, Tissue segmentation of computed tomography images using a Random Forest algorithm: a feasibility study. Phys Med Biol, 2016. 61(17): p. 6553–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Parmar C, et al. , Robust Radiomics feature quantification using semiautomatic volumetric segmentation. PLoS One, 2014. 9(7): p. e102107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Balagurunathan Y, et al. , Reproducibility and Prognosis of Quantitative Features Extracted from CT Images. Transl Oncol, 2014. 7(1): p. 72–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lambin P, et al. , Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol, 2017. 14(12): p. 749–762. [DOI] [PubMed] [Google Scholar]
- 68.Gillies RJ, Kinahan PE, and Hricak H, Radiomics: Images Are More than Pictures, They Are Data. Radiology, 2016. 278(2): p. 563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.IBEX, [cited 2019 04/09]; Available from: http://bit.ly/IBEX_MDAnderson.
- 70.CGITA. [cited 2019 04/09]; Available from: http://code.google.com/p/cgita.
- 71.MaZda. [cited 2019 04/09]; Available from: http://www.eletel.p.lodz.pl/programy/mazda/.
- 72.3d Slicer. [cited 2019 04/09]; Available from: https://www.slicer.org/.
- 73.Mohri M, R.A TA, Foundations of machine learning. 2012: MIT press. [Google Scholar]
- 74.Gao X, et al. , The method and efficacy of support vector machine classifiers based on texture features and multi-resolution histogram from (18)F-FDG PET-CT images for the evaluation of mediastinal lymph nodes in patients with lung cancer. Eur J Radiol, 2015. 84(2): p. 312–7. [DOI] [PubMed] [Google Scholar]
- 75.Hoeben BA, et al. , Systematic analysis of 18F-FDG PET and metabolism, proliferation and hypoxia markers for classification of head and neck tumors. BMC Cancer, 2014. 14: p. 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Qin Z, et al. , How convolutional neural network see the world - A survey of convolutional neural network visualization methods. 2018.
- 77.Parmar C, et al. , Machine Learning methods for Quantitative Radiomic Biomarkers. Sci Rep, 2015. 5: p. 13087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erickson BJ, et al. , Machine Learning for Medical Imaging. Radiographics, 2017. 37(2): p. 505–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.England JR and Cheng PM, Artificial Intelligence for Medical Image Analysis: A Guide for Authors and Reviewers. AJR Am J Roentgenol, 2019. 212(3): p. 513–519. [DOI] [PubMed] [Google Scholar]
- 80.Bickelhaupt S, et al. , Radiomics Based on Adapted Diffusion Kurtosis Imaging Helps to Clarify Most Mammographic Findings Suspicious for Cancer. Radiology, 2018. 287(3): p. 761–770. [DOI] [PubMed] [Google Scholar]
- 81.Zhang Q, et al. , Sonoelastomics for Breast Tumor Classification: A Radiomics Approach with Clustering-Based Feature Selection on Sonoelastography. Ultrasound Med Biol, 2017. 43(5): p. 1058–1069. [DOI] [PubMed] [Google Scholar]
- 82.Yao Z, et al. , Preoperative diagnosis and prediction of hepatocellular carcinoma: Radiomics analysis based on multi-modal ultrasound images. BMC Cancer, 2018. 18(1): p. 1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wibmer A, et al. , Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol, 2015. 25(10): p. 2840–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.E L, et al. , Radiomics for Classifying Histological Subtypes of Lung Cancer Based on Multiphasic Contrast-Enhanced Computed Tomography. J Comput Assist Tomogr, 2019. 43(2): p. 300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wu Y, et al. , Survival Prediction in High-grade Osteosarcoma Using Radiomics of Diagnostic Computed Tomography. EBioMedicine, 2018. 34: p. 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Braman NM, et al. , Intratumoral and peritumoral radiomics for the pretreatment prediction of pathological complete response to neoadjuvant chemotherapy based on breast DCE-MrI. Breast Cancer Res, 2017. 19(1): p. 57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu T, et al. , Prediction of Lymph Node Metastasis in Patients With Papillary Thyroid Carcinoma: A Radiomics Method Based on Preoperative Ultrasound Images. Technol Cancer Res Treat, 2019. 18: p. 1533033819831713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Coroller TP, et al. , CT-based radiomic signature predicts distant metastasis in lung adenocarcinoma. Radiother Oncol, 2015. 114(3): p. 345–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mu W, et al. , Staging of cervical cancer based on tumor heterogeneity characterized by texture features on (18)F-FDG PET images. Phys Med Biol, 2015. 60(13): p. 5123–39. [DOI] [PubMed] [Google Scholar]
- 90.Tatli S, et al. , Abdominal masses sampled at PET/CT-guided percutaneous biopsy: initial experience with registration of prior PET/CT images. Radiology, 2010. 256(1): p. 305–11. [DOI] [PubMed] [Google Scholar]
- 91.Pinker K, et al. , Background, current role, and potential applications of radiogenomics. J Magn Reson Imaging, 2018. 47(3): p. 604–620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jansen RW, et al. , Non-invasive tumor genotyping using radiogenomic biomarkers, a systematic review and oncology-wide pathway analysis. Oncotarget, 2018. 9(28): p. 20134–20155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bai HX, et al. , Imaging genomics in cancer research: limitations and promises. Br J Radiol, 2016. 89(1061): p. 20151030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li H, et al. , Quantitative MRI radiomics in the prediction of molecular classifications of breast cancer subtypes in the TCGA/TCIA data set. NPJ Breast Cancer, 2016. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Gevaert O, et al. , Predictive radiogenomics modeling of EGFR mutation status in lung cancer. Sci Rep, 2017. 7: p. 41674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yang X, et al. , Computed Tomography-Based Radiomics Signature: A Potential Indicator of Epidermal Growth Factor Receptor Mutation in Pulmonary Adenocarcinoma Appearing as a Subsolid Nodule. Oncologist, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Jia TY, et al. , Identifying EGFR mutations in lung adenocarcinoma by noninvasive imaging using radiomics features and random forest modeling. Eur Radiol, 2019. [DOI] [PubMed] [Google Scholar]
- 98.Akbari H, et al. , In vivo evaluation of EGFRvIII mutation in primary glioblastoma patients via complex multiparametric MRI signature. Neuro Oncol, 2018. 20(8): p. 1068–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hong EK, et al. , Radiogenomics correlation between MR imaging features and major genetic profiles in glioblastoma. Eur Radiol, 2018. 28(10): p. 4350–4361. [DOI] [PubMed] [Google Scholar]
- 100.Yang L, et al. , Can CT-based radiomics signature predict KRAS/NRAS/BRAF mutations in colorectal cancer? Eur Radiol, 2018. 28(5): p. 2058–2067. [DOI] [PubMed] [Google Scholar]
- 101.Kitao A, et al. , Hepatocellular Carcinoma with beta-Catenin Mutation: Imaging and Pathologic Characteristics. Radiology, 2015. 275(3): p. 708–17. [DOI] [PubMed] [Google Scholar]
- 102.Kocak B, et al. , Radiogenomics in Clear Cell Renal Cell Carcinoma: Machine Learning-Based High-Dimensional Quantitative CT Texture Analysis in Predicting PBRM1 Mutation Status. AJR Am J Roentgenol, 2019. 212(3): p. W55–W63. [DOI] [PubMed] [Google Scholar]
- 103.Cen D, et al. , Renal cell carcinoma: predicting RUNX3 methylation level and its consequences on survival with CT features. Eur Radiol, 2019. [DOI] [PubMed] [Google Scholar]
- 104.Li ZC, et al. , Multiregional radiomics features from multi parametric MRI for prediction of MGMT methylation status in glioblastoma multiforme: A multicentre study. Eur Radiol, 2018. 28(9): p. 3640–3650. [DOI] [PubMed] [Google Scholar]
- 105.Li H, et al. , MR Imaging Radiomics Signatures for Predicting the Risk of Breast Cancer Recurrence as Given by Research Versions of MammaPrint, Oncotype DX, and PAM50 Gene Assays. Radiology, 2016. 281(2): p. 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jeon SH, et al. , Delta-radiomics signature predicts treatment outcomes after preoperative chemoradiotherapy and surgery in rectal cancer. Radiat Oncol, 2019. 14(1): p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wu J, et al. , Tumor microenvironment as the “regulator” and “target” for gene therapy. J Gene Med, 2019: p. e3088. [DOI] [PubMed] [Google Scholar]
- 108.Fabian A, et al. , Metastasis of pancreatic cancer: An uninflamed liver micromilieu controls cell growth and cancer stem cell properties by oxidative phosphorylation in pancreatic ductal epithelial cells. Cancer Lett, 2019. 453: p. 95–106. [DOI] [PubMed] [Google Scholar]
- 109.Crispin-Ortuzar M, et al. , Predicting hypoxia status using a combination of contrast-enhanced computed tomography and [(18)F]-Fluorodeoxyglucose positron emission tomography radiomics features. Radiother Oncol, 2018. 127(1): p. 36–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Miura T, et al. , Distinct clinicopathological phenotype of hepatocellular carcinoma with ethoxybenzyl-magnetic resonance imaging hyperintensity: association with gene expression signature. Am J Surg, 2015. 210(3): p. 561–9. [DOI] [PubMed] [Google Scholar]
- 111.Cho HR, et al. , Radiogenomics Profiling for Glioblastoma-related Immune Cells Reveals CD49d Expression Correlation with MRI parameters and Prognosis. Sci Rep, 2018. 8(1): p. 16022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Sahani DV, et al. , Prospective evaluation of reader performance on MDCT in characterization of cystic pancreatic lesions and prediction of cyst biologic aggressiveness. AJR Am J Roentgenol, 2011. 197(1): p. W53–61. [DOI] [PubMed] [Google Scholar]
- 113.Dmitriev K, et al. , Classification of Pancreatic Cysts in Computed Tomography Images Using a Random Forest and Convolutional Neural Network Ensemble. Med Image Comput Comput Assist Interv, 2017. 10435: p. 150–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Wei R, et al. , Computer-Aided Diagnosis of Pancreas Serous Cystic Neoplasms: A Radiomics Method on Preoperative MDCT Images. Technol Cancer Res Treat, 2019. 18: p. 1533033818824339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yang J, et al. , Discrimination of Pancreatic Serous Cystadenomas From Mucinous Cystadenomas With CT Textural Features: Based on Machine Learning. Front Oncol, 2018. 9: p. 494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hanania AN, et al. , Quantitative imaging to evaluate malignant potential of IPMNs. Oncotarget, 2016. 7(52): p. 85776–85784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Permuth JB, et al. , Combining radiomic features with a miRNA classifier may improve prediction of malignant pathology for pancreatic intraductal papillary mucinous neoplasms. Oncotarget, 2016. 7(52): p. 85785–85797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Chakraborty J, et al. , CT radiomics to predict high-risk intraductal papillary mucinous neoplasms of the pancreas. Med Phys, 2018. 45(11): p. 5019–5029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Yue Y, et al. , Identifying prognostic intratumor heterogeneity using pre- and post-radiotherapy 18F-FDG PET images for pancreatic cancer patients. J Gastrointest Oncol, 2017. 8(1): p. 127–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chakraborty J, et al. , Preliminary study of tumor heterogeneity in imaging predicts two year survival in pancreatic cancer patients. PLoS One, 2017. 12(12): p. e0188022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cassinotto C, et al. , Resectable pancreatic adenocarcinoma: Role of CT quantitative imaging biomarkers for predicting pathology and patient outcomes. Eur J Radiol, 2017. 90: p. 152–158. [DOI] [PubMed] [Google Scholar]
- 122.Yun G, et al. , Tumor heterogeneity of pancreas head cancer assessed by CT texture analysis: association with survival outcomes alter curative resection. Sci Rep, 2018. 8(1): p. 7226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cozzi L, et al. , Computed tomography based radiomic signature as predictive of survival and local control after stereotactic body radiation therapy in pancreatic carcinoma. PLoS One, 2019. 14(1): p. e0210758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chen X, et al. , Assessment of treatment response during chemoradiation therapy for pancreatic cancer based on quantitative radiomic analysis of daily CTs: An exploratory study. PLoS One, 2017. 12(6): p. e0178961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mayerhoefer ME, et al. , Effects of MRI acquisition parameter variations and protocol heterogeneity on the results of texture analysis and pattern discrimination: an application-oriented study. Med Phys, 2009. 36(4): p. 1236–43. [DOI] [PubMed] [Google Scholar]
- 126.Varghese BA, et al. , Texture Analysis of Imaging: What Radiologists Need to Know. AJR Am J Roentgenol, 2019. 212(3): p. 520–528. [DOI] [PubMed] [Google Scholar]
- 127.Leijenaar RT, et al. , Stability of FDG-PET Radiomics features: an integrated analysis of test-retest and inter-observer variability. Acta Oncol, 2013. 52(7): p. 1391–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Hayes DF, Biomarker validation and testing. Mol Oncol, 2015. 9(5): p. 960–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sala E, et al. , Unravelling tumour heterogeneity using next-generation imaging: radiomics, radiogenomics, and habitat imaging. Clin Radiol, 2017. 72(1): p. 3–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Clark K, et al. , The Cancer Imaging Archive (TCIA): maintaining and operating a public information repository. J Digit Imaging, 2013. 26(6): p. 1045–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.QIBA. [cited 2019 04/09]; Available from: http://rsna.org/QIBA.aspx.
- 132.Neri E, et al. , Radiomics and liquid biopsy in oncology: the holons of systems medicine. Insights Imaging, 2018. 9(6): p. 915–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Radiomics Quality Score. 2017. [cited 2017 04/09]; Available from: http://www.radiomics.world/.
- 134.Brugge WR, et al. , Cystic neoplasms of the pancreas. N Engl J Med, 2004. 351(12): p. 1218–26. [DOI] [PubMed] [Google Scholar]
- 135.rugge WR, et al. , Diagnosis of pancreatic cystic neoplasms: a report of the cooperative pancreatic cyst study. Gastroenterology, 2004. 126(5): p. 1330–6. [DOI] [PubMed] [Google Scholar]
- 136.Cunningham SC, H R., Schulick RD. Pancreas Cyst Worksheet; Johns Hopkins Medical Institutions. [cited 2019 09/17]; Available from: http://pathology.jhu.edu/pancreas/professionals/ipmn.php.
- 137.Rizzo S, et al. , Radiomics: the facts and the challenges of image analysis. Eur Radiol Exp, 2018. 2(1): p. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]


