Abstract
Objectives
Chigger mites are vectors for scrub typhus. This study evaluated the annual fluctuations in chigger mite populations and Orientia tsutsugamushi infections in South Korea.
Methods
During 2006 and 2007, chigger mites were collected monthly from wild rodents in 4 scrub typhus endemic regions of South Korea. The chigger mites were classified based on morphological characteristics, and analyzed using nested PCR for the detection of Orientia tsutsugamushi.
Results
During the surveillance period, the overall trapping rate for wild rodents was 10.8%. In total, 17,457 chigger mites (representing 5 genera and 15 species) were collected, and the average chigger index (representing the number of chigger mites per rodent), was 31.7. The monthly chigger index was consistently high (> 30) in Spring (March to April) and Autumn (October to November). The mite species included Leptotrombidium pallidum (43.5%), L. orientale (18.9%), L. scutellare (18.1%), L. palpale (10.6%), and L. zetum (3.6%). L. scutellare and L. palpale populations, were relatively higher in Autumn. Monthly O. tsutsugamushi infection rates in wild rodents (average: 4.8%) and chigger mites (average: 0.7%) peaked in Spring and Autumn.
Conclusion
The findings demonstrated a bimodal pattern of the incidence of O. tsutsugamushi infections. Higher infection rates were observed in both wild rodents and chigger mites, in Spring and Autumn. However, this did not reflect the unimodal incidence of scrub typhus in Autumn. Further studies are needed to identify factors, such as human behavior and harvesting in Autumn that may explain this discordance.
Keywords: mites, Orientia tsutsugamushi, rodents, scrub typhus
Introduction
Scrub typhus is a notorious, endemic, vector-borne disease that is prevalent in Asian countries, including Japan, Taiwan, China, Thailand, and South Korea [1]. In total, 10,365 cases were reported in South Korea in 2013 which translated to over 20 cases per 100,000 population in South Korea [2]. In contrast, less than 500 cases have been recorded in Japan and Taiwan since 2009 (unpublished data). Scrub typhus is transmitted by chigger mites (larval trombiculid mites) infected with the gram-negative intracellular rickettsial bacterium Orientia tsutsugamushi [3].
In South Korea, O. tsutsugamushi is transmitted by 2 major species of chigger mites, namely, Leptotrombidium pallidum and L. scutellare. L. palpale, L. orientale, L. zetum, Neotrombicula japonica, and Euschoengastia koreaensis also contribute to the transmission of O. tsutsugamushi [4,5]. Mites of the genus Leptotrombidium are known to be the major vectors for scrub typhus in many countries however, the dominant species varies. L. pallidum and L. scutellare are believed to be the major vectors in Japan [6]. L. deliense is a key vector in Taiwan, and L. deliense, L. imphalum, and L. chiangraiensis are primary vectors in Thailand [7]. In China, “Summer” and “Autumn/Winter” types of scrub typhus are transmitted by L. deliense and L. scutellare, respectively [8].
The geographical distribution of chigger mites in South Korea are relatively evenly distributed throughout the Korean peninsula where there are populations of L. pallidum, L. palpale, and L. orientale [9]. L. scutellare is mainly distributed in the western and southern regions, where scrub typhus is prevalent [9].
Annual fluctuations in chigger mite populations suggest that populations of L. scutellare and L. palpale are predominant in the Shandong province in Northern China in Autumn and Winter [10]. In Jeollanam-do, in the southern part of South Korea, L. pallidum and L. scutellare populations are at their highest peaks in November and October (Autumn), respectively. L. pallidum has been reported to be perennial [except in Summer (June to August)], and L. scutellare has only been obtained between September and January [11].
In Japan, L. pallidum and L. scutellare are believed to transmit O. tsutsugamushi Gilliam and Karp genotypes, and Kawasaki and Kuroki genotypes, respectively [6]. In China, L. deliense and L. scutellare are believed to transmit Karp, Gilliam, and Kato, and Gilliam and Kawasaki genotypes, respectively [12].
To improve the current understanding of high incidences of scrub typhus in Autumn, 4 scrub typhus endemic areas of South Korea were surveyed. Annual fluctuations in wild rodent trapping (a measure of rodent activity), the prevalence of O. tsutsugamushi infections in wild rodents, the chigger index (CI), which represents the number of chigger mites per rodent, and infection rates in these mites were examined.
Materials and Methods
1. Localities and periods of surveillance
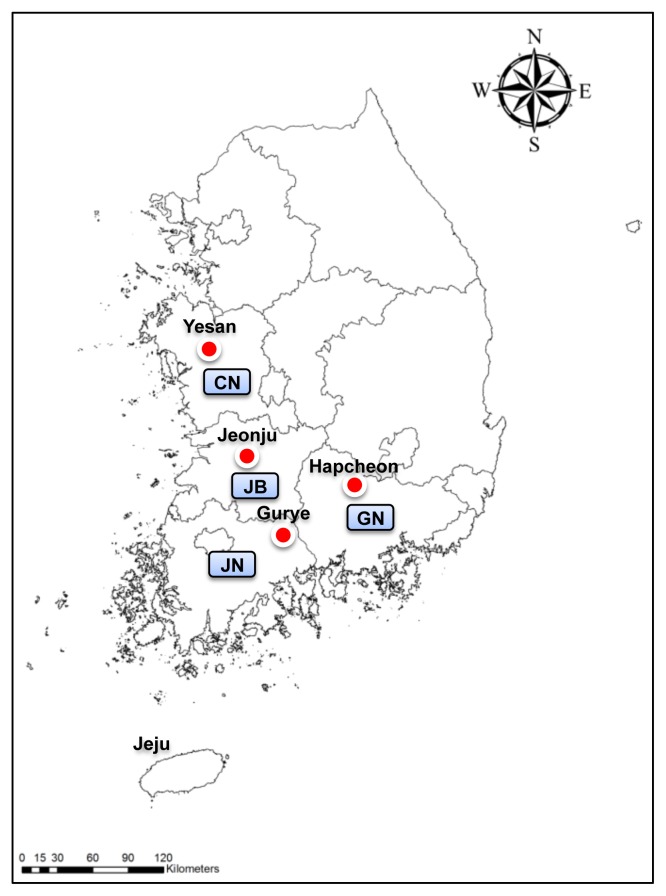
Chigger mites were collected from wild rodents captured from 4 scrub typhus endemic regions, namely, Yesan in Chungcheongnam-do, Jeonju in Jeollabuk-do, Gurye in Jeollanam-do, and Hapcheon in Gyeongsangnam-do, between 2006 and 2007 (Figure 1). To analyze the demographics of the vector species in the context of the seasonal prevalence of scrub typhus, it was essential to monitor monthly population fluctuations of chigger mite species. Therefore, rodents were collected monthly from each of the scrub typhus endemic regions, for 2 years. Data on the collection, including locality, collection year and month, number of traps installed, and number of rodents captured, have been summarized in Tables 1 and 2. The trapping locations included rice fields, crop fields, reservoirs, waterways, hillsides, grass fields, and riversides.
Figure 1.
Collection sites for wild rodents in scrub typhus endemic regions of South Korea: Yesan in Chungcheongnam-do (CN), Jeonju in Jeollabuk-do (JB), Gurye in Jeollanam-do (JN), and Hapcheon in Gyeongsangnam-do (GN).
Table 1.
Coordinates of the rodent collection sites.
| Area | Detailed location | N | E |
|---|---|---|---|
| Yesan | Nojeon-ri, Gwangsi-myeon, Yesan-gun, Chungcheongnam-do | 36° 32′ 2.25″ | 125° 45′ 40.7″ |
| Jeonju | Samcheon-dong, Jeonju-si, Jeollabuk-do | 35° 48′ 17.46″ | 127° 08′ 15.81 |
| Gurye | Samsan-ri, Ganjeon-myeon, Gurye-gun, Jeollanam-do | 35° 9′ 28.79″ | 127° 32′ 49.40″ |
| Hapcheon | Jung-ri, Chogye-myeon, Hapcheon-gun, Gyeongsangnam-do | 35° 33′ 40.75″ | 128° 15′ 15.37″ |
Table 2.
Summary of collection data and O. tsutsugamushi infections in wild rodents and chigger mites.
| No. of scrub typhus patients | No. of installed traps | No. of collected rodents | Rate of rodent trapping (%) | No. of rodents infested by chigger mites | Proportion of rodents infested by chigger mites (%) | No. of collected chigger mites | Chigger Index | No. of Ot positive rodents | Proportion of Ot positive rodents (%) | No. of Ot positive chigger mite pools | No. of tested chigger mites | Minimum infection rate (%) of chigger mites | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | |||||||||||||
| Jan | - | 220 | 25 | 11.4 | 12 | 48.0 | 218 | 8.7 | 1 | 4.0 | - | 50 | - |
| Feb | - | 270 | 36 | 13.3 | 26 | 72.2 | 1,275 | 35.4 | - | - | 3 | 582 | 0.5 |
| Mar | 1 | 300 | 38 | 12.7 | 33 | 86.8 | 2,148 | 56.5 | 5 | 13.2 | 8 | 1,011 | 0.8 |
| Apr | - | 265 | 39 | 14.7 | 27 | 69.2 | 2,929 | 75.1 | 5 | 12.8 | 18 | 1,484 | 1.2 |
| May | - | 315 | 42 | 13.3 | 26 | 61.9 | 691 | 16.5 | 1 | 2.4 | 1 | 278 | 0.4 |
| Jun | - | 450 | 67 | 14.9 | 11 | 16.4 | 34 | 0.5 | 1 | 1.5 | - | - | - |
| Jul | - | 441 | 57 | 12.9 | 1 | 1.8 | 1 | 0.0 | - | - | - | - | - |
| Aug | - | 430 | 53 | 12.3 | 10 | 18.9 | 81 | 1.5 | - | - | - | 18 | - |
| Sep | 1 | 245 | 23 | 9.4 | 6 | 26.1 | 118 | 5.1 | - | - | - | 29 | - |
| Oct | 77 | 490 | 44 | 9.0 | 25 | 56.8 | 1,713 | 38.9 | 1 | 2.3 | 8 | 813 | 1.0 |
| Nov | 141 | 485 | 38 | 7.8 | 27 | 71.1 | 1,773 | 46.7 | 3 | 7.9 | 7 | 832 | 0.8 |
| Dec | 11 | 395 | 44 | 11.1 | 41 | 93.2 | 1,741 | 39.6 | 4 | 9.1 | 5 | 745 | 0.7 |
| Subtotal | 231 | 4,306 | 506 | 11.8 | 245 | 48.4 | 12,722 | 25.1 | 21 | 4.2 | 50 | 5,842 | 0.9 |
| 2007 | |||||||||||||
| Jan | - | 345 | 38 | 11.0 | 28 | 73.7 | 499 | 13.1 | 3 | 7.9 | - | 166 | - |
| Feb | - | 360 | 40 | 11.1 | 28 | 70.0 | 2,139 | 53.5 | 4 | 10.0 | 5 | 1,029 | 0.5 |
| Mar | - | 465 | 52 | 11.2 | 43 | 82.7 | 2,838 | 54.6 | 2 | 3.8 | 6 | 1,373 | 0.4 |
| Apr | - | 435 | 46 | 10.6 | 26 | 56.5 | 1,810 | 39.3 | - | - | 4 | 848 | 0.5 |
| May | - | 510 | 45 | 8.8 | 18 | 40.0 | 460 | 10.2 | 2 | 4.4 | 2 | 169 | 1.2 |
| Jun | 1 | 485 | 47 | 9.7 | 4 | 8.5 | 22 | 0.5 | 1 | 2.1 | - | 9 | - |
| Jul | 1 | 505 | 48 | 9.5 | 1 | 2.1 | 1 | 0.0 | - | - | - | - | - |
| Aug | - | 315 | 30 | 9.5 | 9 | 30.0 | 28 | 0.9 | - | - | - | - | - |
| Sep | 49 | 335 | 26 | 7.8 | 10 | 38.5 | 150 | 5.8 | 1 | 3.8 | 1 | 47 | 2.1 |
| Oct | 136 | 430 | 40 | 9.3 | 38 | 95.0 | 4,517 | 112.9 | 3 | 7.5 | 3 | 2,220 | 0.1 |
| Nov | 3 | 435 | 44 | 10.1 | 38 | 86.4 | 5,798 | 131.8 | - | - | 25 | 2,779 | 0.9 |
| Dec | 190 | 560 | 66 | 11.8 | 54 | 81.8 | 1,577 | 23.9 | - | - | - | 622 | - |
| Subtotal | 421 | 5,180 | 522 | 10.1 | 297 | 56.9 | 19,839 | 38.0 | 16 | 3.1 | 46 | 9,262 | 0.5 |
| Total | 9,486 | 1,028 | 10.8 | 542 | 52.7 | 32,561 | 31.7 | 37 | 3.6 | 96 | 15,104 | 0.6 |
Ot = Orientia tsutsugamushi.
This study included data on the geographical distribution of L. scutellare and scrub typhus incidence in South Korea obtained from Yesan (April–October 2007), Jeonju (April–October 2007), Gurye (April 2006), and Hapcheon (November 2006 and March 2007) which was previously published [9].
2. Collection of wild rodents and chigger mites
Specific permission was not required for the collection of rodents because the sites were not located within national parks or protected areas. The selection of collection sites and the collection of wild rodents were supported by the local Research Institute of Health and Environment centers. The rodent species collected were not endangered or protected in Korea. The animal-handling protocol used in this study was reviewed and approved based on the guidelines for ethical procedures and scientific care of the Institutional Animal Care and Use Committee of the Korea Centers for Disease Control and Prevention (KCDC-046-13-2A).
The collection of wild rodents was performed monthly at all 4 collection sites. At each site, 10 to 15 Sherman folding live traps (3 × 3.5 × 9 inches, BioQuip, USA) baited with a peanut butter-spread biscuit, were set up at 5–7 points, at 3–5-meter intervals, and were collected the next morning. The wild rodents collected were euthanized with compressed carbon dioxide, and subsequently suspended for 24 hours over glass bowls filled with tap water to collect the shed chigger mites. The mites were then recovered from the surface of the water using a fine brush, and stored at 4°C until further examination. Half of the mites collected were used for morphological identification, and the remainder were used for the detection of O. tsutsugamushi infections.
3. Identification of chigger mites
Individual chigger mites were transferred to glass slides and mounted using polyvinyl alcohol mounting medium (BioQuip, Rancho Dominguez, CA, USA). The species of the specimens were identified by stereo-microscopic examination, using morphological keys [13].
4. Detection of O. tsutsugamushi in wild rodents and chigger mites
Deoxyribonucleic acid (DNA) was extracted from the blood of individual wild rodents, and pooled chigger mites (5–30 per rodent), using the G-spin total DNA extraction kit (iNtRON Biotechnology, Korea). The nucleotide sequence (475 bp) of the gene that encodes the 56 kDa antigen of O. tsutsugamushi was detected using a nested polymerase chain reaction (PCR) assay, as described in a previous report [14]. For the first PCR, 5 μL of the template DNA of chigger mites was added to the PCR premix (BIONEER, Korea). This was then incubated at 94°C for 5 minutes, followed by 30 cycles of 94°C for 30 seconds, 60°C for 2 minutes, 72°C for 2 minutes, and finally, 72°C for 10 minutes for amplification. Except for the use of a second pair of PCR primers, the procedure for amplification of 2 μL of the first PCR product, for the second PCR, was the same as described. The second PCR product was analyzed by electrophoresis on a 1.5% agarose gel. The nucleotide sequence of the nested PCR products was analyzed using the BLAST program from the National Center for Biotechnology Information (http://blast.ncbi.nlm.nih.gov) to confirm that the gene originated from the 56 kDa protein of O. tsutsugamushi. The minimum infection rate was calculated as a percent (%) ratio of the number of positive pools/numbers of tested chigger mites.
Results
1. Collection of chigger mites and O. tsutsugamushi infection rates in rodents
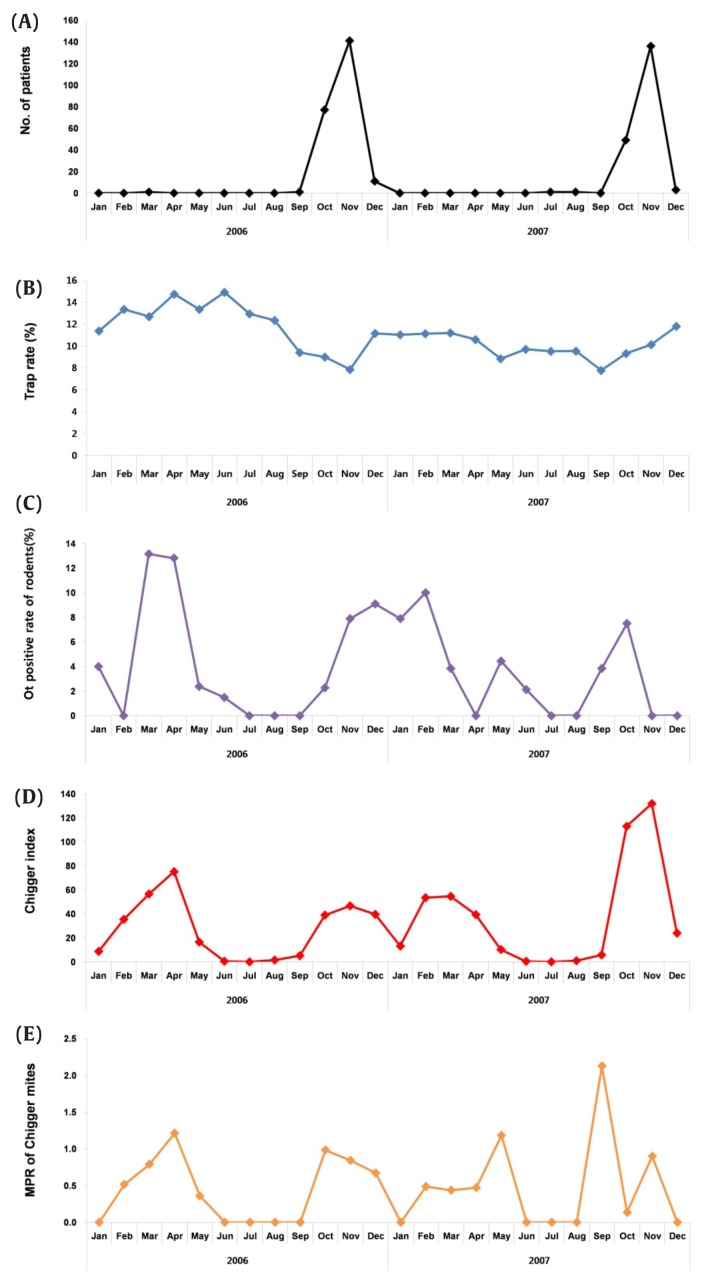
Overall, 9,486 traps were installed, and 1,028 wild rodents were captured from 4 regions of the scrub typhus endemic provinces (Figure 1; Table 1). The overall trapping rate of the study period was 10.8%; the highest (14.9%) and lowest (7.8%) trapping rates were recorded in June 2006, November 2006 and September 2007, respectively. Among the trapped rodents, Apodemus agrarius was the dominant species in all regions, accounting for 97.9% of the collection, followed by Crocidura lasiura and Craseomys regulus at 2.0% and 0.1%, respectively (data not shown). The detection rate of O. tsutsugamushi in wild rodents was 3.6% and infection rates peaked in March (13.2%), April (12.8%), December (9.1%), and November (7.9%) of 2006, and in February (10.0%), January (7.9%), and October (7.5%) of 2007 (Table 2).
2. Chigger indices and O. tsutsugamushi infections in mites
The CI represents the number of chigger mites per rodent. In total, 32,561 mites representing 5 genera and 15 species were collected, with an average CI of 31.7 (Figure 2; Table 2). The CIs in Spring (February, March, and April) and Autumn (October and November) were higher than the average value (Table 2). The predominant chigger mite species identified in this study was Leptotrombidium pallidum (43.5%), followed by L. orientale (18.9%), L. scutellare (18.1%), and L. palpale [10.6% (Table 3; Figure 3)].
Figure 2.
Annual fluctuation in the population of scrub typhus vectors and O. tsutsugamushi infections in these vectors. (A) Scrub typhus incidence in the collection regions in 2006–2007, (B) trapping rate of rodents, (C) O. tsutsugamushi infection rate in rodents, (D) chigger index, and (E) minimum O. tsutsugamushi positivity rate of chigger mites.
Table 3.
Species of chigger mites collected from wild rodents.
| C. ika | E. kor | L. ful | L. gem | L. ori | L. pall | L. palp | L. scu | L. sub | L. zet | N. gar | N. jap | N. kwa | N. tam | W. fra | L. spp | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2006 | |||||||||||||||||
| Jan | - | 1 | - | - | 102 | - | 45 | - | 1 | 19 | - | - | - | - | - | - | 168 |
| Feb | - | 2 | - | - | 168 | 353 | 85 | 1 | 2 | 82 | - | - | - | - | - | - | 693 |
| Mar | 1 | 8 | - | 1 | 458 | 579 | 48 | 5 | 2 | 35 | - | - | - | - | - | - | 1,137 |
| Apr | - | 2 | - | - | 453 | 949 | 9 | 1 | 2 | 29 | - | - | - | - | - | - | 1,445 |
| May | 1 | 3 | - | - | 84 | 303 | 2 | - | 5 | 5 | - | - | - | - | 10 | - | 413 |
| Jun | - | - | - | - | 8 | 22 | - | - | 2 | 2 | - | - | - | - | - | - | 34 |
| Jul | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Aug | - | - | - | - | 29 | 9 | - | - | - | - | 19 | - | - | - | - | 6 | 63 |
| Sep | - | - | - | - | 7 | 48 | - | 16 | - | - | 9 | - | - | - | - | 9 | 89 |
| Oct | 1 | 71 | - | - | 61 | 253 | 9 | 504 | - | - | - | - | 1 | - | - | - | 900 |
| Nov | - | 21 | - | - | 128 | 264 | 147 | 361 | - | 6 | - | 1 | 1 | 12 | - | - | 941 |
| Dec | 1 | 23 | - | - | 293 | 215 | 274 | 107 | 4 | 58 | - | - | - | 21 | - | - | 996 |
| Subtotal | 4 | 131 | - | 1 | 1,792 | 2,995 | 619 | 995 | 18 | 236 | 28 | 1 | 2 | 33 | 10 | 15 | 6,880 |
| 2007 | |||||||||||||||||
| Jan | - | 6 | - | - | 76 | 74 | 137 | 3 | - | 28 | - | - | - | 9 | - | - | 333 |
| Feb | - | 5 | - | - | 219 | 638 | 116 | 16 | 7 | 104 | - | - | - | 5 | - | - | 1,110 |
| Mar | 1 | 5 | - | - | 361 | 934 | 87 | 5 | 4 | 60 | - | - | - | 8 | - | - | 1,465 |
| Apr | - | 3 | - | - | 332 | 582 | 16 | 1 | 5 | 23 | - | - | - | - | - | - | 962 |
| May | 1 | 3 | - | - | 69 | 208 | 1 | - | - | 9 | - | - | - | - | - | - | 291 |
| Jun | - | - | - | - | 3 | 10 | - | - | - | - | - | - | - | - | - | - | 13 |
| Jul | - | - | - | - | 1 | - | - | - | - | - | - | - | - | - | - | - | 1 |
| Aug | 3 | - | 7 | - | 10 | 2 | - | - | 1 | - | 5 | - | - | - | - | - | 28 |
| Sep | - | 11 | 1 | - | 33 | 43 | - | 2 | 1 | - | 12 | - | - | - | - | - | 103 |
| Oct | 2 | 88 | 8 | - | 124 | 817 | 20 | 1,151 | 1 | 11 | 70 | 1 | - | 2 | 2 | - | 2,297 |
| Nov | - | 41 | - | - | 180 | 918 | 613 | 892 | 2 | 116 | 2 | 2 | - | 253 | - | - | 3,019 |
| Dec | - | 10 | - | - | 101 | 372 | 243 | 91 | 2 | 50 | - | - | - | 86 | - | - | 955 |
| Subtotal | 7 | 172 | 16 | - | 1,509 | 4,598 | 1,233 | 2,161 | 23 | 401 | 89 | 3 | - | 363 | 2 | - | 10,577 |
| Total | 11 | 303 | 16 | 1 | 3,301 | 7,593 | 1,852 | 3,156 | 41 | 637 | 117 | 4 | 2 | 396 | 12 | 15 | 17,457 |
| % | 0.1 | 1.7 | 0.1 | 0.0 | 18.9 | 43.5 | 10.6 | 18.1 | 0.2 | 3.6 | 0.7 | 0.0 | 0.0 | 2.3 | 0.1 | 0.1 | 100.0 |
C. ika = Cheladonta ikaoensis; E. kor = Euschoengastia koreaensis; L. ful = Leptotrombidium fuller; L. gem = Leptotrombidium gemiticulum; L. ori = Leptotrombidium orientale; L. pall = Leptotrombidium pallidum; L. palp = Leptotrombidium palpale; L. scu = Leptotrombidium scutellare; L. sub = Leptotrombidium subintermedium; L. zet = Leptotrombidium zetum; N. gar = Neotrombicula gardellai; N. jap = Neotrombicula japonica; N. kwa = Neotrombicula kwangneungensis; N. tam = Neotrombicula tamiyai; W. fra = Walchia fragilis; L. spp. = Leptotrombidium species.
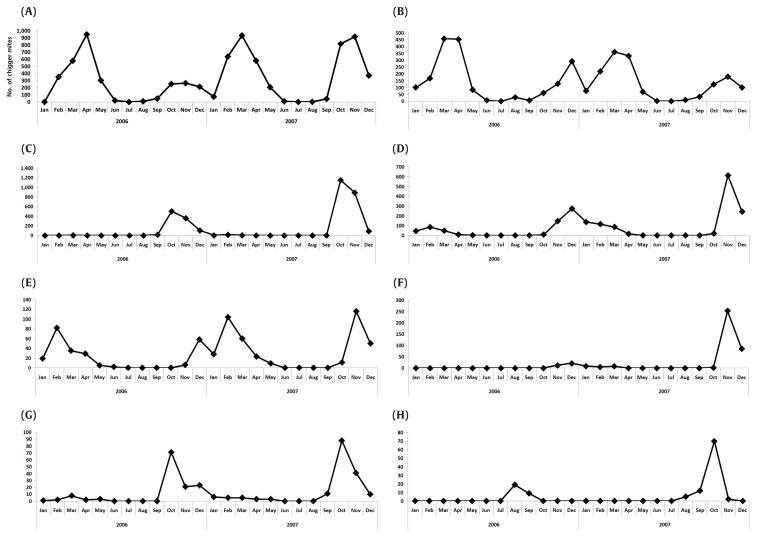
Figure 3.
Annual fluctuations in the populations of the 8 dominant chigger mite species. (A) L. pallidum, (B) L. orientale, (C) L. scutellare, (D) L. palpale, (E) L. zetum, (F) N. tamiyai, (G) E. koreaensis, (H) N. gardellai.
The minimum infection rate in these mites was 0.6%. High infection rate peaks were observed in March (0.8%), April (1.2%), October (1.0%), November (0.8%), and December (0.7%) 2006, and in May (1.2%), September (2.1%), and November (0.9%) 2007. The Boryong genotype of O. tsutsugamushi was predominantly obtained from both positive rodents (59.5%) and chigger mites [72.9% (data not shown)].
In this survey, 8 chigger mite species were analyzed based on their relative abundance, and 3 patterns of incidence were identified (Figure 3). The populations of L. pallidum, L. orientale, and L. zetum demonstrated similar high peaks in Spring and Autumn. Interestingly, L. palpale and E. koreaensis showed low and high peaks in Spring and Autumn, respectively. Additionally, L. scutellare and 2 Neotrombicula species showed no peak in Spring (1-digit collection recorded) and a high peak in Autumn.
Discussion
The egg-laying season for adult trombiculid mites in South Korea is Summer [15]. The population density of chigger mite larvae is therefore very low in Summer, and increases from September. Therefore, in South Korea, the high density of chigger mites may affect the high incidence of scrub typhus in the Autumn (October to November). However, the high CI in Spring does not explain the low incidence of scrub typhus. In terms of agricultural activity, Spring and Autumn are the seeding and harvesting seasons, respectively. This implies that there are fewer grasses or weeds to shelter chigger mites in the Spring. However, there is an abundance of crops and grasses in the Autumn, increasing the probability of contact between humans and vectors, including chigger mites.
In this survey, L. pallidum was determined to be the predominant species, followed by L. orientale and L. scutellare. This finding was similar to that of several previous reports conducted in Korea. In 1995, Ree et al [5] reported that L. pallidum and L. scutellare were predominant in Chungcheongnam-do and Jeollanam-do, respectively. Song et al [16] also reported that L. pallidum (76.3%) was the dominant species, followed by L. scutellare (12.9%). L. scutellare was mainly distributed in the southern parts of Korea, including Jeju Island. The northernmost areas of distribution of L. scutellare included regions where the mean annual temperature was above 10ºC [11]. In another survey, Lee et al [17] reported that L. pallidum was the predominant species collected between October and November 2006 in Chungcheongnam-do (100%), Jeollabuk-do (73.9%), and Jeollanam-do (77.0%). However, in Gyeongsangnam-do, L. scutellare was the predominant species (77.9%). In recent years, Lee et al [11] also surveyed chigger mite populations in Jeollanam-do between November 2006 and October 2007, and reported that L. scutellare (54.0%) was the predominant species, followed by L. pallidum (39.4%), L. orientale (4.4%), L. palpale (1.1%), and Neotrombicula tamiyai (0.6%).
The O. tsutsugamushi infection rates in both rodents and chigger mites were relatively high in Spring and Autumn, with similar patterns of fluctuation of the CI. The predominant genotype in this survey was Boryong. Ree et al [18] also reported that Boryong was the predominant genotype in mice (78.3%) and chigger mite pools (82.9%), and was distributed widely in the Korean peninsula; the Karp genotype was confined to central Korea. They also reported that the Karp genotype was found in areas of L. pallidum distribution, whereas Boryong was collected in areas where both, L. pallidum and L. scutellare, were prevalent. In this current study, pooled chigger mites were used therefore, the O. tsutsugamushi genotypes, based on individual chigger mite species could not be determined. This difference in O. tsutsugamushi genotypes in relation to individual chigger mite species warrants further investigation to improve the understanding of the epidemiological relationship between the vector and pathogen of scrub typhus.
Conclusion
In South Korea, scrub typhus has a unimodal incidence pattern during the epidemic season of Autumn. Conversely, in Taiwan and Japan, a bimodal incidence pattern (Spring and Autumn) has been reported [19]. The findings from this current 2-year survey demonstrated the seasonal relationship between scrub typhus and chigger mites in the endemic regions of South Korea, highlighting the epidemiology of this disease. In this survey, the annual fluctuation in the trapping rate, CI, and infection rates in wild rodents and chigger mites showed a bimodal pattern over the 2-year surveillance period; this differed from the incidence pattern of scrub typhus. However, the numbers of chigger mites collected and their infection rates reflected the incidence pattern of human scrub typhus in Autumn, but not in Spring. Further studies are needed to determine the factors responsible for this inconsistency. In South Korea, the discordance between the prevalence of wild rodents and chigger mites, and the incidence of scrub typhus in Spring may be attributed to human behavior, including agricultural activities (cultivation and harvesting).
Acknowledgments
This study was funded by the Korean Centers for Disease Control and Prevention, under the budget for health promotion (no.: 4800-4851-304).
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to declare.
References
- 1.Chang WH. Current status of tsutsugamushi disease in Korea. J Korean Med Sci. 1995;10(4):227–38. doi: 10.3346/jkms.1995.10.4.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korea Centers for Disease Control and Prevention. The National Notifiable Disease Surveillance System. Cheongju (Korea): Korea Centers for Disease Control and Prevention; 2013. Dec, [Google Scholar]
- 3.Lee YS, Wang PH, Tseng SJ, et al. Epidemiology of scrub typhus in eastern Taiwan, 2000–2004. Jpn J Infect Dis. 2006;59(4):235–8. [PubMed] [Google Scholar]
- 4.Lee HI, Shim SK, Song BG, et al. Detection of Orientia tsutsugamushi, the causative agent of scrub typhus, in a novel mite species, Eushoengastia koreaensis, in Korea. Vector Borne Zoonotic Dis. 2011;11(3):209–14. doi: 10.1089/vbz.2009.0180. [DOI] [PubMed] [Google Scholar]
- 5.Ree HI, Lee IY, Jeon SH, et al. Geographical distribution of vectors and sero-strains of tsutsugamushi disease at mid-south inland of Korea. Korean J Parasitol. 1997;35(3):171–9. doi: 10.3347/kjp.1997.35.3.171. [DOI] [PubMed] [Google Scholar]
- 6.Seto J, Suzuki Y, Otani K, et al. Proposed vector candidate: Leptotrombidium palpale for Shimokoshi type Orientia tsutsugamushi. Microbiol Immunol. 2013;57(2):111–7. doi: 10.1111/1348-0421.12015. [DOI] [PubMed] [Google Scholar]
- 7.Coleman RE, Monkanna T, Linthicum KJ, et al. Occurrence of Orientia tsutsugamushi in small mammals from Thailand. Am J Trop Med Hyg. 2003;69(5):519–24. doi: 10.4269/ajtmh.2003.69.519. [DOI] [PubMed] [Google Scholar]
- 8.Bang HA, Lee MJ, Lee WC. Comparative research on epidemiological aspects of tsutsugamushi disease (scrub typhus) between Korea and Japan. Jpn J Infect Dis. 2008;61(2):148–50. [PubMed] [Google Scholar]
- 9.Roh JY, Song BG, Park WI, et al. Coincidence between geographical distribution of Leptotrombidium scutellare and scrub typhus incidence in South Korea. PLoS One. 2014;9(12):e113193. doi: 10.1371/journal.pone.0113193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang M, Zhao ZT, Yang HL, et al. Molecular epidemiology of Orientia tsutsugamushi in chiggers and ticks from domestic rodents in Shandong, northern China. Parasit Vectors. 2013;6(1):312. doi: 10.1186/1756-3305-6-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH, Lee YS, Lee IY, et al. Monthly occurrence of vectors and reservoir rodents of scrub typhus in an endemic area of Jeollanam-do, Korea. Korean J Parasitol. 2012;50(4):327–31. doi: 10.3347/kjp.2012.50.4.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu YX, Feng D, Suo JJ, et al. Clinical characteristics of the autumn-winter type scrub typhus cases in south of Shandong province, northern China. BMC Infect Dis. 2009;9:82. doi: 10.1186/1471-2334-9-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ree HI. Fauna and key to the chigger mites of Korea (Acarina: Trombiculidae and Leeuwenhoekiidae) Korean J System Zool. 1990;6:57–70. [Google Scholar]
- 14.Furuya Y, Yoshida Y, Katayama T, et al. Serotype-specific amplification of Rickettsia tsutsugamushi DNA by nested polymerase chain reaction. J Clin Microbiol. 1993;31(6):1637–40. doi: 10.1128/jcm.31.6.1637-1640.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kawamura JA, Hiroshi T, Akira T. Tsutsugamushi disease. Tokyo (Japan): Tokyo Press; 1995. [Google Scholar]
- 16.Song HJ, Kim KH, Kim SC, et al. Population density of chigger mites, the vector of tsutsugamushi disease in Chollanam-do, Korea. Korean J Parasitol. 1996;34(1):27–33. doi: 10.3347/kjp.1996.34.1.27. [DOI] [PubMed] [Google Scholar]
- 17.Lee IY, Kim HC, Lee YS, et al. Geographical distribution and relative abundance of vectors of scrub typhus in the Republic of Korea. Korean J Parasitol. 2009;47(4):381–6. doi: 10.3347/kjp.2009.47.4.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ree HI, Kim TE, Lee IY, et al. Determination and geographical distribution of Orientia tsutsugamushi genotypes in Korea by nested polymerase chain reaction. Am J Trop Med Hyg. 2001;65(5):528–34. doi: 10.4269/ajtmh.2001.65.528. [DOI] [PubMed] [Google Scholar]
- 19.Park JH, Kim SJ, Youn SK, et al. Epidemiology of scrub typhus and the eschars patterns in South Korea from 2008 to 2012. Jpn J Infect Dis. 2014;67(6):458–63. doi: 10.7883/yoken.67.458. [DOI] [PubMed] [Google Scholar]