PRACTICAL IMPLICATIONS
Consider the possibility of neural relapse in leprosy whenever there are new neural symptoms in a patient previously treated for leprosy.
Relapsed leprosy refers to situations in which patients who underwent regular treatment with standardized official multidrug therapy regimens, and were discharged because cure was achieved, now present new clinical signs and symptoms of disease activity. Such cases generally occur more than 5 years after cure, although they can occur at any time after treatment.1
Over recent years, the numbers of cases of relapsed leprosy have been increasing. This, together with cases of therapeutic failure, could even be contributing toward selection of mutant strains of Mycobacterium leprae associated with drug resistance. In combination with the emergence of primary resistant multidrug leprosy, this set of circumstances may compromise disease control strategies, thus making this a priority within public health policies.1,2
However, relapsed leprosy in its primary neural form remains underdiagnosed. These cases show clinical evidence of peripheral neuropathy, but with the absence of new skin lesions, and are negative on slit-skin smear bacilloscopy.3
This case series characterizes the epidemiologic, clinical, neurophysiologic, and laboratory aspects of 12 patients with diagnoses of neural relapse of leprosy who were attended at a national reference center in Brazil between 2012 and 2017. Approval for this analysis was granted by the Ethics Committee of the Federal University of Uberlandia.
All these individuals underwent clinical, serologic, molecular, and neurophysiologic evaluations.3,4 Slit-skin smears from 6 sites (both ear lobes, both elbows, and both knees) were examined. Despite the absence of skin lesions, biopsies were taken from the elbow tissue (a cold region with possible intradermal impairment) after evaluation by 2 experienced leprosy specialists. Nerves that underwent biopsy were selected according to the patient's clinical condition and included exclusively sensory nerves that showed electrophysiologic abnormality. During nerve biopsy, skin biopsies were also taken from the overlying area.3,4
The cases of leprosy neural relapse were classified as follows3:
Possible—clinical and/or electroneuromyographic pattern compatible with the diagnosis of neural leprosy, but with negative complementary examinations.
Probable—clinical and/or electroneuromyographic pattern compatible with the diagnosis of neural leprosy, associated with the positivity of some complementary examinations (ELISA antiphenolic glycolipid I [PGL1]; and skin biopsy/slit-skin smear real-time quantitative PCR [qPCR]).
Definitive—clinical and/or electroneuromyographic pattern compatible with the diagnosis of neural leprosy, associated with some abnormality in peripheral nerve biopsy (bacilloscopy and/or qPCR).
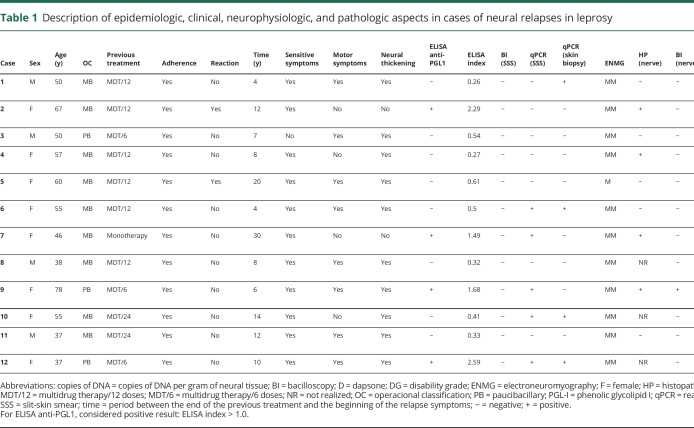
Between 2012 and 2017, 907 leprosy cases were seen. Of these, 9.9% (90/907) were classified as relapsed leprosy, and 12 patients (13.3%, 12/90) had the neural form. These patients were all negative on slit-skin smear bacilloscopy and did not present any new cutaneous lesions compatible with leprosy (table 1). All household contacts of these patients were evaluated, and none presented evidence suggestive of multibacillary leprosy, thus making reinfection unlikely.
Table 1.
Description of epidemiologic, clinical, neurophysiologic, and pathologic aspects in cases of neural relapses in leprosy
Their average age was 52.5 years (±11.9), and 66.7% (8/12) were women. The time between the end of the previous treatment and the relapse diagnosis was 11.3 years (±7.1); 75% (9/12) were classified as multibacillary at the initial diagnosis. All patients reported adherence to the first treatment, and only 16.7% (2/12) presented reactional episodes after discharge. There were no epidemiologic differences between the groups with neural relapse and with other relapsed leprosy.
All patients were symptomatic and presented asymmetrical neural impairment, with the predominance of sensory symptoms (91.7%; 11/12), particularly hypesthesia, paresthesia, and pain, shown by thermal, painful, and/or tactile impairment; 66.6% (8/12) had muscle weakness and/or amyotrophy. Thickening of 1 or more nerves was observed in 83.3% (10/12). All the patients presented insidious evolution, with symptoms lasting more than 3 months, and 33.3% (4/12) presented visible deformities.
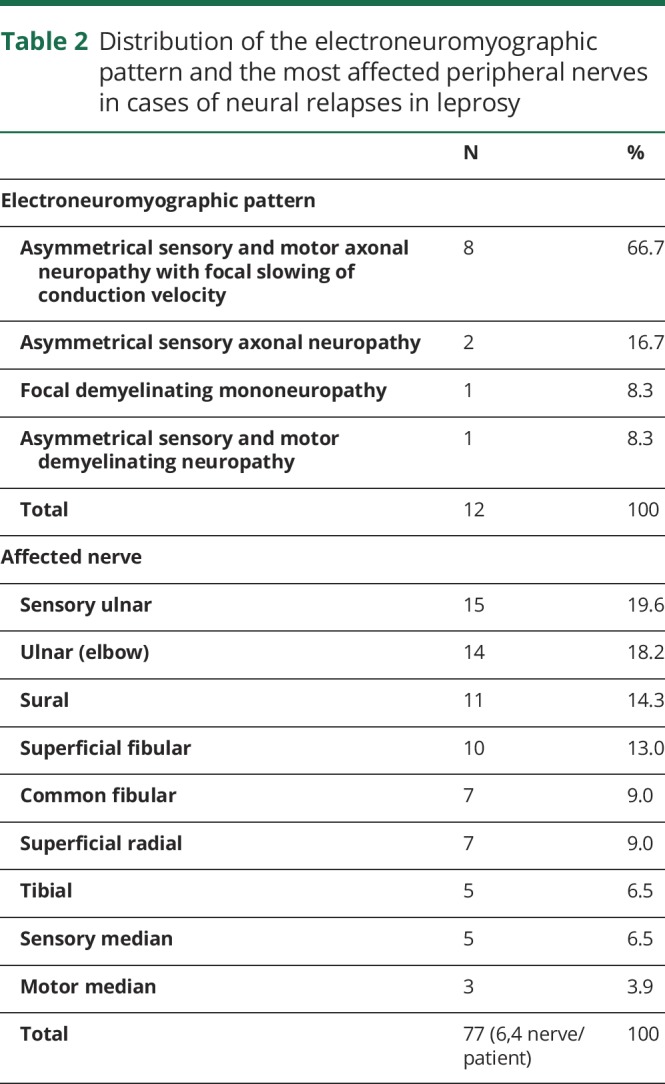
Electroneuromyographic evaluation showed that 8.3% (1/12) only had 1 altered nerve (mononeuropathy), whereas 91.7% (11/12) had 2 or more affected nerves (asymmetrical multiple mononeuropathy) (table 2).
Table 2.
Distribution of the electroneuromyographic pattern and the most affected peripheral nerves in cases of neural relapses in leprosy
The ELISA anti-PGL1 IgM serologic test was positive in 33.3% (4/12). The qPCR DNA M. leprae test on peripheral blood was positive in only 8.3% (1/12) and, on slit-skin smears, was positive in 50.0% (6/12). The slit-skin smear bacilloscopy was negative in all cases.
The electroneuromyography patterns showed that 75.0% (9/12) had at least 1 nerve eligible for biopsy, and 44.4% (4/9) presented some histopathologic alterations suggestive of leprosy, e.g, presence of endoneurial or epineurial infiltrate, fibrosis, perineurial thickening, or endoneurial granuloma. Only 1 case (11.1%; 1/9) presented positive bacilloscopy on a peripheral nerve biopsy. The qPCR test on nerve biopsies was positive in 88.9% (8/9).
Despite the diagnosis of leprosy neural relapse being essentially clinical, according to such results, 8 cases were classified as definitive (cases 1, 2, 4, 5, 6, 7, 9 and 11), 2 cases as probable (cases 10 and 12), and 2 cases as possible (cases 3 and 8). These patients were treated with a mensal single dose of rifampicin, ofloxacin, and minocycline, during 24 months, with the exception of cases 8 and 11 who, because of documented bacterial resistance, were treated with a mensal single dose of minocycline, moxifloxacin, and clarithromycin, also during 24 months.
Discussion
Early diagnosis of suspected leprosy neuropathy, especially in relapsed cases, is very challenging in clinical practice, especially because of the long disease incubation period and difficulty in making differential diagnoses with sequelae and other conditions such as neuropathic pain. Patients report variable insidious symptoms that need to be detailed and evaluated in following up these cases. This context demonstrates that neural relapse is underdiagnosed and causes severe disabilities. Its prevalence is hidden, and this maintains the disease transmission chain.
Considering that leprosy remains a public health problem, development and implementation of new tools for detecting M. leprae and its neural impairments is essential for ensuring early diagnosis and adequate treatment to prevent physical disability and stigma.
Appendix. Authors
Study funding
This study received financial support from Brazilian funding agencies: the Brazilian National Council for Scientific and Technological Development CNPq) and Foundation for Research Support of the State of Minas Gerais (FAPEMIG).
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Cambau E, Saunderson P, Matsuoka M, et al. Antimicrobial resistance in leprosy: results of the first prospective open survey conducted by a WHO surveillance network for the period 2009–15. Clin Microbiol Infect 2018;24:1305–1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Salgado CG, Barreto JG, da Silva MB, et al. Are leprosy case numbers reliable? Lancet Infect Dis 2018;18:135–137. [DOI] [PubMed] [Google Scholar]
- 3.Santos DFD, Mendonça MR, Antunes DE, et al. Revisiting primary neural leprosy: clinical, serological, molecular, and neurophysiological aspects. PLoS Negl Trop Dis 2017;11:e0006086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santos DFD, Mendonça MR, Antunes DE, et al. Molecular, immunological and neurophysiological evaluations for early diagnosis of neural impairment in seropositive leprosy household contacts. PLoS Negl Trop Dis 2018;12:e0006494. [DOI] [PMC free article] [PubMed] [Google Scholar]