PRACTICAL IMPLICATIONS
Consider lacosamide as a well-tolerated alternative to carbamazepine or oxcarbazepine for the treatment of vestibular paroxymia.
According to the current diagnostic criteria, vestibular paroxysmia (VP) is characterized by at least 10 attacks of spontaneous spinning or nonspinning vertigo with a duration of less than 1 minute, stereotyped phenomenology in a particular patient, and response to treatment with carbamazepine (CBZ)/oxcarbazepine (OXC).1 A response to these drugs—which are thought to primarily block the use-dependent fast voltage-gated sodium channels—was reported in several observational studies2,3 and 1 recent randomized controlled trial (RCT).4 However, many patients cannot be treated with CBZ/OXC because of contraindications or their intolerance to the plethora of side effects, which leads to bad compliance and adherence; for instance, in the latter RCT, the dropout rate was 60%.
An alternative could be lacosamide because, on the one hand, its primary mode of action (a blocking of sodium channels) is thought to be similar to CBZ/OXC and, on the other hand, it has fewer contraindications and side effects than CBZ/OXC (see reference 5 for details). Therefore, we evaluated the effects of lacosamide on the frequency, severity, and duration of attacks of vertigo in patients with VP before and during treatment. The study was approved by the local ethics committee.
In a prospective observational case series, 7 patients (3 men, age range 40–78 years; table) who fulfilled the diagnostic criteria for VP (5 who had already responded to CBZ or OXC but did not tolerate these drugs very well) or probable VP1 (2 who received lacosamide as their first treatment) were examined. The patients were asked about the frequency of attacks per month, the severity (“mild, moderate, or severe”), and duration (“seconds, minutes”) before and during treatment when they were treated with a constant daily dosage for at least 3 months. Therapy was started with 50 mg lacosamide twice per day, and then, the dosage was increased depending on the efficacy.
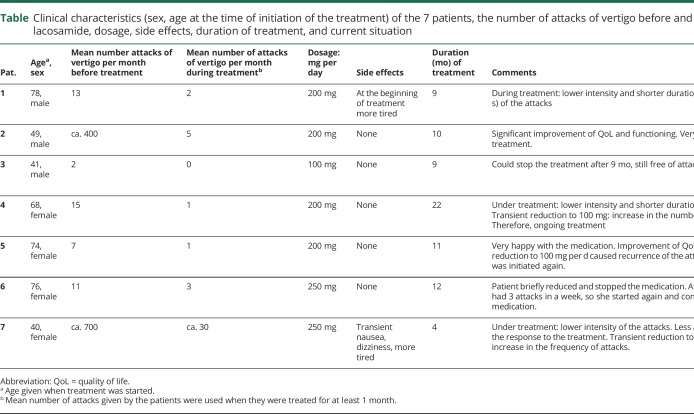
Table.
Clinical characteristics (sex, age at the time of initiation of the treatment) of the 7 patients, the number of attacks of vertigo before and during treatment with lacosamide, dosage, side effects, duration of treatment, and current situation
Before treatment with lacosamide, the mean number of attacks of vertigo per month was 13 (range 2–700—a wide range which is common in VP3) (table). During treatment with lacosamide, the mean number decreased to 3 per month (range 0–30). In 3 patients, the intensity of the attacks was lower (from severe to mild), and in 2 patients, the duration was shorter (only a few seconds). Because of good response, 2 patients transiently reduced their daily dosage to 100 mg per day and 2 patients to no medication, but 3 of them experienced an increase in the frequency of attacks, so they were back to taking the original dosage again. The fourth patient remained free of symptoms for more than 6 months, with no attacks recurred. In August 2018, 3 patients were taking 250 mg and 3 other patients were taking 200 mg of lacosamide per day (table). By August 2018, the duration of treatment was between 4 and 22 months. As known from other studies (see reference 5), lacosamide was well tolerated, except for transient tiredness in 1 patient and transient dizziness with nausea in another patient (table).
As expected from the similar mode of action (“sodium-channel blocker”) of CBZ/OXC and lacosamide, the latter reduces the attacks of vertigo in patients with VP. Lacosamide, however, had very few side effects in all patients, leading to high compliance and adherence. This small observational study with all its methodological shortcomings evident (e.g., a low number of individuals, not placebo-controlled, no dose finding) suggests that lacosamide can be effective in VP and is a well-tolerated alternative to CBZ or OXC. Finally, it could also be considered for the pharmacotherapy of other neurovascular cross-compression syndromes.6
Acknowledgment
The authors thank Katie Göttlinger for helping with copyediting.
Appendix. Authors

Study funding
This work was supported by the German Ministry of Education and Research (BMBF), Grant No. 01EO0901 to the German Center for Vertigo and Balance Disorders (IFBLMU).
Disclosure
M. Strupp is the Joint Chief Editor of the Journal of Neurology, the Editor-in-Chief of Frontiers of Neuro-otology, the Section Editor of F1000, and on the Editorial Board of Neurology; has received speaker honoraria from Abbott, Actelion, Auris Medical, Biogen, Eisai, Grünenthal, GSK, Henning Pharma, Interacoustics, Merck, MSD, Otometrics, Pierre Fabre, TEVA, and UCB; is a shareholder of IntraBio; and acts as a consultant for Abbott, Actelion, Auris Medical, Heel, IntraBio, and Sensorion. N. Böttcher reports no disclosures. C. E. Elger is an Associate Editor of Epilepsy & Behavior; has received speaker honoraria from Desitin, Pfizer, UCB, and Novartis; and receives grants or research support from DFG, BMBF, and Marga und Walter Boll-Stiftung. Funding information and disclosures are provided at the end of the article. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Strupp M, Lopez-Escamez JA, Kim JS, et al. Vestibular paroxysmia: diagnostic criteria. J Vestib Res 2016;26:409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brandt T, Dieterich M. Vestibular paroxysmia: vascular compression of the eighth nerve? Lancet 1994;343:798–799. [DOI] [PubMed] [Google Scholar]
- 3.Hüfner K, Barresi D, Glaser M, et al. Vestibular paroxysmia: diagnostic features and medical treatment. Neurology 2008;71:1006–1014. [DOI] [PubMed] [Google Scholar]
- 4.Bayer O, Brémová T, Strupp M, Hüfner K. A randomized double-blind, placebo-controlled, cross-over trial (vestparoxy) of the treatment of vestibular paroxysmia with oxcarbazepine. J Neurol 2018;265:291–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanner AM, Ashman E, Gloss D, et al. Practice guideline update summary: Efficacy and tolerability of the new antiepileptic drugs I: Treatment of new-onset epilepsy: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 2018;91:74–81. [DOI] [PubMed] [Google Scholar]
- 6.Strupp M, Dieterich M, Brandt T, Feil K. Therapy of vestibular paroxysmia, superior Oblique Myokymia, and Ocular Neuromyotonia. Curr Treat Options Neurol 2016;18:34. [DOI] [PubMed] [Google Scholar]