PRACTICAL IMPLICATIONS
Consider neuronal intranuclear inclusion disease in a patient with high diffusion-weighted imaging signals along the corticomedullary junction and paravermal high T2 signals on brain MRI. A prompt skin biopsy must be performed to assess for the presence of characteristic intranuclear inclusions.
Neuronal intranuclear inclusion disease (NIID) is a rare neurodegenerative disorder characterized by the presence of eosinophilic hyaline intranuclear inclusion bodies within cells in the nervous system, visceral organs, and skin.1–4 It has a variable clinical presentation and can manifest as neuropathy, ataxia, or dementia.1–3,5 Both sporadic and familial cases have been reported, and it can present at any age from infancy to adulthood.1 It can therefore be challenging to diagnose antemortem. However, in recent years, skin biopsy and common MRI characteristics have facilitated its diagnosis.4 There have been several recent reports of NIID, but they have been exclusively identified in the Japanese population.1,3–6 We report the case of NIID in a Chinese-Canadian patient who presented with syncope and a subacute history of cognitive dysfunction.
Case
A 56-year-old Canadian woman of Chinese descent presented to our emergency department with transient loss of consciousness, followed by altered mental status and transcortical motor aphasia, which resolved over 8 hours. She described a 30-year history of episodic loss of consciousness, which occurs once or twice a year. They were typically triggered by positional change and exposure to heat and relieved with lying flat. She had never previously had aphasia after these episodes, which is what prompted her presentation to the hospital. However, it was later revealed that she had sustained a concussion from the fall before presentation. She also described a 2-year history of short-term memory loss and word-finding difficulties. Her mother, who passed away at age 67, had dementia and similar episodes of loss of consciousness and later developed convulsive seizures in the last years of her life.
The score of cognitive testing with the Behavioral Neurology Assessment–Revised was 256 of 330, with an impaired delayed recall and borderline impairment in immediate recall, executive function, and language. Routine bloodwork was within normal limits, and toxicology screen was negative. Her EEG did not reveal epileptiform activity. MRI brain imaging showed symmetrical, bilateral, high signals along the corticomedullary junction of bilateral cerebral hemispheres with frontal lobe predilection on T2-weighted, fluid-attenuated inversion recovery (T2-FLAIR) and diffusion-weighted imaging (DWI) (figure 1, A and B). Infratentorially, there were symmetrical high T2-FLAIR signals in bilateral paravermal regions associated with atrophy of the vermis (figure 1, C and D) and symmetric patchy high T2-FLAIR signals along bilateral middle cerebellar peduncles (figure 1D).
Figure 1. Flair and DWI imaging.
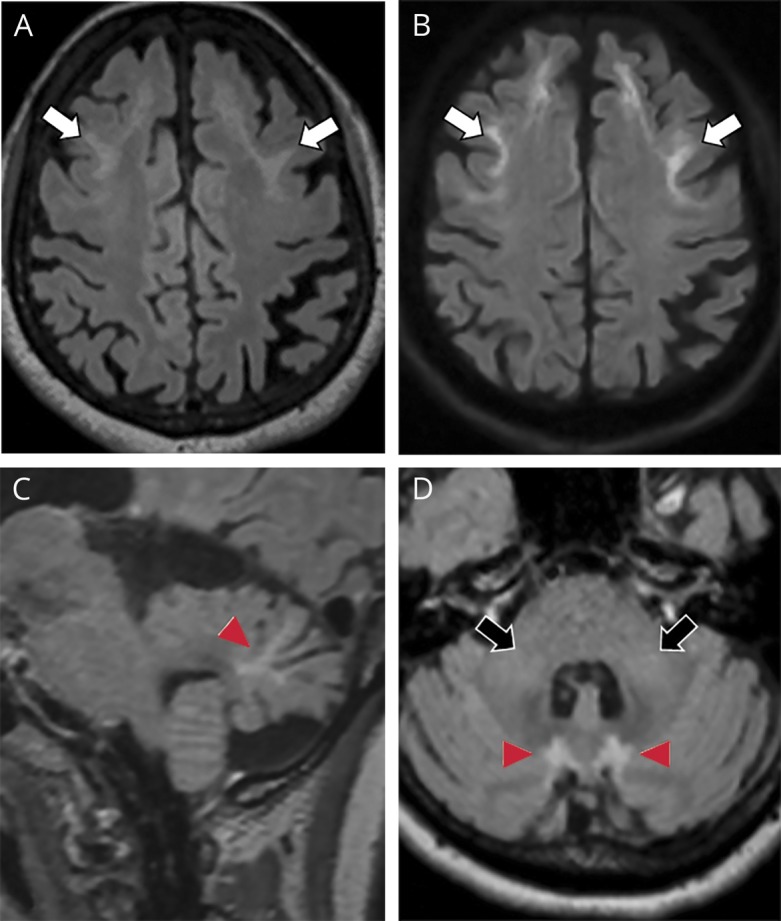
(A) Axial fluid-attenuated inversion recovery (FLAIR) and (B) diffusion-weighted imaging (DWI) of the supratentorial brain show high FLAIR signal with corresponding high DWI signals involving the corticomedullary junction of bilateral frontal lobes (white arrows, A and B). (C) Sagittal FLAIR and (D) axial FLAIR of the posterior fossa show FLAIR hyperintensities within paravermal regions (arrowheads, C and D) and in bilateral cerebellar peduncles (black arrows, D).
She underwent skin biopsy with H&E and immunochemistry staining for anti-p62 which demonstrated numerous eosinophilic intranuclear inclusions in the sweat glands (figure 2), fibroblasts, and adipocytes. Genetic testing for fragile X mental retardation 1 (FMR1) gene premutation ruled out fragile X-associated tremor/ataxia syndrome (FXTAS).
Figure 2. Skin biopsy.
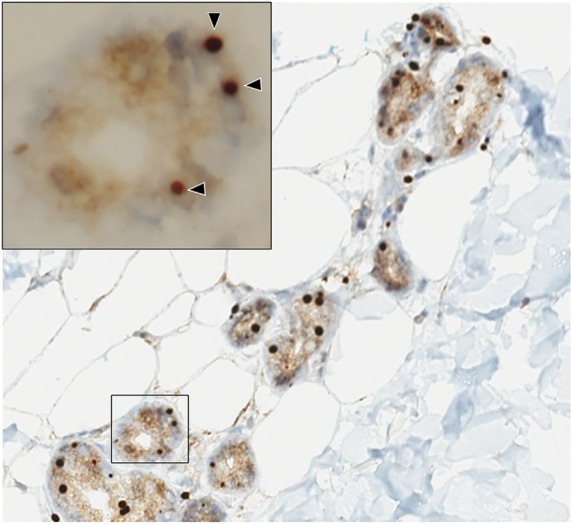
Skin biopsy with anti-p62 immunohistochemistry staining shows multiple intranuclear inclusions within the sweat glands (arrowheads).
Discussion
Adult-onset NIID typically presents at the age of 60–70 years with cognitive dysfunction, parkinsonism, ataxia, autonomic dysfunction, and, rarely, with generalized convulsions and subacute encephalopathy.1,3,5 The reported patient's recurrent loss of consciousness was clinically suggestive of syncope, although conformational autonomic testing could not be performed at our institute. Although the clinical presentation of NIID can be varied, certain MRI features have been commonly reported. High signals along corticomedullary junction on DWI have been described as a pathognomonic MRI finding in NIID.3,5 This may relate to the presence of eosinophilic hyaline inclusions in astrocytes, resulting in damage to the white matter.3,5 The high DWI signals strongly correlate with spongiotic changes proximal to the subcortical U-fibers.3 Other reported features include abnormal T2 signals in the paravermal region and symmetric, high T2 signals along middle cerebellar peduncles.5 Other differentials to consider would be seizure-induced signal changes, toxic leukoencephalopathies, inherited leukodystrophies, and neurodegenerative disease.
Another diagnostic tool is skin biopsy, which reveals intranuclear inclusions that can be labeled with antibodies for ubiquitin, SUMO1, and p62.1,3,4 The presence of intranuclear inclusions, however, may be indistinguishable from the changes seen in FXTAS.1,6,7 Furthermore, recently published studies have reported FXTAS cases can also demonstrate a similar pattern of high DWI signals and paravermal lesions similar to that of NIID.6,7 It is therefore recommended that patients with NIID be tested for FMR1 gene premutation.1,6,7 We proposed the diagnostic flowchart of NIID on figure e-1 (links.lww.com/CPJ/A99).
This case highlights several important points. First, our case and a number of new NIID diagnoses in recent years1,3–5 illustrate that NIID may be more common than we previously thought. Second, we question an ethnic predilection of NIID because the literature predominantly reports its diagnosis in Japanese patients1,3,5,6 and our report identifies it in a Canadian woman of Chinese descent. Further studies will be required to clarify this ethnic association. This case also underscores the importance of MRI and skin biopsy findings in the diagnosis of NIID in patients of Asian descent, especially because of the heterogeneous clinical presentation of the disease. FXTAS must be excluded as an alternative diagnosis.
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
The authors report no disclosures relevant to the manuscript. Full disclosure form information provided by the authors is available with the full text of this article at Neurology.org/cp.
References
- 1.Sone J, Mori K, Inagaki T, et al. Clinicopathological features of adult-onset neuronal intranuclear inclusion disease. Brain 2016;139:3170–3186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munoz-Garcia D, Ludwin SK. Adult-onset neuronal intranuclear hyaline inclusion disease. Neurology 1986;36:785–790. [DOI] [PubMed] [Google Scholar]
- 3.Yokoi S, Yasui K, Hasegawa Y, et al. Pathological background of subcortical hyperintensities on diffusion-weighted images in a case of neuronal intranuclear inclusion disease. Clin Neuropathol 2016;35:375–380. [DOI] [PubMed] [Google Scholar]
- 4.Sone J, Tanaka F, Koike H, et al. Skin biopsy is useful for the antemortem diagnosis of neuronal intranuclear inclusion disease. Neurology 2011;76:1372–1376. [DOI] [PubMed] [Google Scholar]
- 5.Sugiyama A, Sato N, Kimura Y, et al. MR imaging features of the cerebellum in adult-onset neuronal intranuclear inclusion disease: 8 cases. AJNR Am J Neuroradiol 2017;38:2100–2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sugiyama A, Sato N. Reply. AJNR Am J Neuroradiol 2018;39:E102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padilha IG, Nunes RH, Scortegagna FA, et al. MR imaging features of adult-onset neuronal intranuclear inclusion disease may be indistinguishable from fragile X-associated tremor/ataxia syndrome. AJNR Am J Neuroradiol 2018;39:E100–E101. [DOI] [PMC free article] [PubMed] [Google Scholar]


