Abstract
Biomaterial properties such as mechanics, degradation rate, and cell adhesivity affect cell behaviors including spreading, proliferation, and differentiation. To engineer complex tissues, it is often desirable to achieve precise spatial control over these properties. Here, methacrylated alginate (MA-ALG) was used to create hydrogels comprising a single base material with regions of different types and levels of crosslinking and subsequently different material properties. Ionic and ultraviolet light crosslinking mechanisms were combined to create dual-crosslinked hydrogels with significantly increased stiffness and decreased swelling compared to calcium-crosslinked or UV-crosslinked hydrogels. MC3T3 cells showed significantly enhanced proliferation on the surface of dual-crosslinked hydrogels compared with calcium-crosslinked hydrogels. Photomasks were then used to create patterned hydrogels with precise spatial control over regions that were only calcium-crosslinked versus dual-crosslinked. This spatial variation in crosslinking mechanism permitted local regulation of the hydrogel physical properties and alignment of cells seeded on their surface. Photomasks were also used to create hydrogels with patterned presentation of cell adhesion ligands, leading to spatial control over cell attachment and proliferation. This biomaterial system can be useful for providing patterned, instructive cues to guide cell behavior for engineering complex tissues.
Graphical Abstract
INTRODUCTION
During healing and development, cells are exposed to complex microenvironments comprising biochemical and physical signals.1,2 To better understand the effects these signals have on cell behavior, it is important to engineer biomaterial systems where they are regulated in time and space. For example, manipulating mechanical signals can promote changes in cell behavior such as spreading, proliferation, migration, and differentiation.3–7 It is therefore desirable to isolate and vary individual biochemical or physical cues within a single biomaterial without changing its base chemistry to determine the effects of these signals on cells. Changes in crosslinking of biomaterial hydrogels are often used to change their physical properties. This has been used to create hydrogels with different mechanical properties using chemically modified materials including polyethylene glycol (PEG),8 hyaluronic acid (HA),9,10 alginate,11 polydimethylsiloxane (PDMS),12 dextran,13 and a number of others.14
Extending this idea, hydrogel systems have been developed using two different crosslinking mechanisms applied sequentially.15–23 Hydrogels crosslinked by both mechanisms exhibit different physical properties than those that are single-crosslinked. For example, a methacrylated κ-carrageenan system used chemical and physical crosslinking separately and in combination to vary hydrogel mechanical properties including modulus and toughness.17 Dual-crosslinked hydrogel systems with a light-based crosslinking step can easily be adapted for spatial control of material properties, with the goal of controlling and/or studying cell responses to these micro-environmental parameters. In this approach, the hydrogels are uniformly crosslinked throughout (single-crosslinking), and then additional crosslinks are formed with ultraviolet (UV) light in specific spatial regions using a photomask (dual-crosslinking). For example, HA modified with methacrylate groups was chemically crosslinked with butanediol diglycidyl ether or dithiothreitol, and then UV light was used to photo-crosslink remaining methacrylate groups.20,21 The extent of crosslinking influenced the hydrogel swelling and mechanical properties, which affected human mesenchymal stem cell (hMSC) spreading and proliferation in two dimensions (2D) and three dimensions (3D).21,22 Similar cell proliferation results were obtained using human adipose-derived stem cells (hASCs) in methacrylated alginate chemically crosslinked with an 8-arm PEG amine and then additionally UV-crosslinked in controlled regions. This alginate/PEG system was also used to explore the effect of patterned material properties on hASC differentiation, demonstrating the utility of dual-crosslinked hydrogel systems in examining complex cell behaviors.23
Alginate, a natural polysaccharide derived from brown algae, is a powerful material for studying the effects of physical and biochemical signals on cells. Cells do not adhere or spread on hydrogels made of native alginate, but it can be modified with cell adhesion ligands to allow for controlled cell attachment.24,25 Alginate can be ionically crosslinked with divalent cations, usually calcium, that interact with the carboxylic acids on the macromer backbone. Changing the cation concentration can change the crosslinking density and thus the hydrogel mechanical properties.11 Calcium-crosslinking is gentle enough to form hydrogels with encapsulated cells which maintain high viability.26 Our group has previously chemically modified alginate with methacrylate groups that form covalent crosslinks through free radical polymerization in the presence of low level UV light and a photoinitiator.27 Since the methacrylated alginate (MA-ALG) still has remaining free carboxylic acids, it can also interact with calcium ions to form additional crosslinks. Therefore, MA-ALG is a unique material because it allows for both ionic and covalent crosslinking. While the ionic crosslinks degrade due to dissociation of the macromer chains as calcium ions are replaced by monovalent cations such as sodium ions,28,29 the covalent crosslinks degrade via hydrolysis of ester linkages.27 These two fundamentally different crosslinking mechanisms may also result in independent control of hydrogel swelling profiles and/or mechanical properties by varying the relative amount of each crosslink type, allowing for even greater manipulation of biomaterial properties compared to dual-crosslinked systems based on two separate covalent crosslinking mechanisms.
Here, we present a system of dual-crosslinked alginate that is used to locally regulate hydrogel mechanics by creating regions of calcium only crosslinking and dual-crosslinking. The utility of the approach to subsequently regulate adhesion and/or proliferation of cells seeded on the surface is then demonstrated in hydrogels with spatially patterned mechanics and adhesion ligand presentation. To our knowledge, this is the first work that uses both ionic and covalent crosslinking of one base material with simple photomask patterning to achieve spatial control over hydrogel physical and biochemical properties.
RESULTS
Crosslinking Mode Effects on Hydrogel Physical Properties.
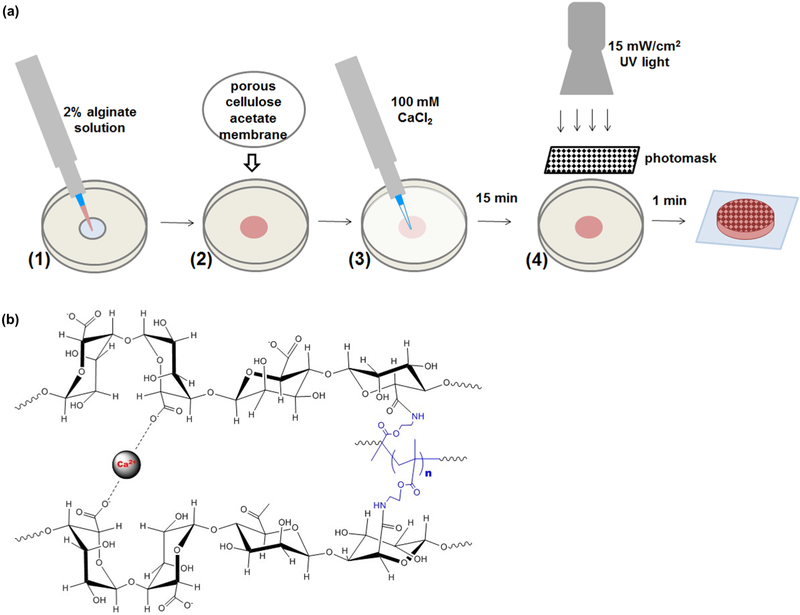
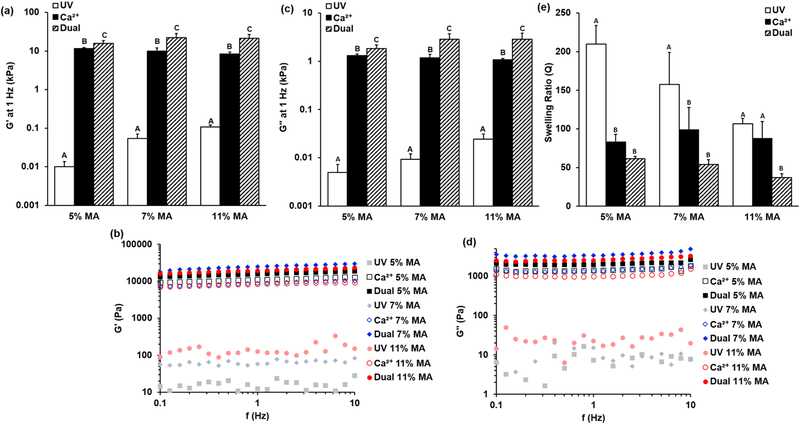
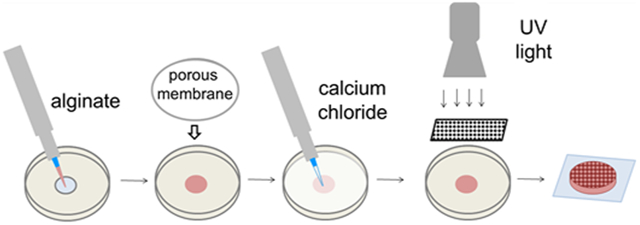
Single and dual crosslinked hydrogels were formed as shown in Figure 1a. Single-crosslinked hydrogels were crosslinked with UV light or calcium alone, and dual-crosslinked hydrogels contained both calcium- and photo-crosslinks as shown in Figure 1b. Dual-crosslinked hydrogels had higher shear storage and loss moduli than those with the same level of methacrylation that were crosslinked with calcium or UV alone (Figure 2a and c). Hydrogels with only UV-crosslinking were by far the least stiff, with storage moduli 2–3 orders of magnitude lower than calcium or dual-crosslinked hydrogels with the same levels of methacrylation. Representative curves show that neither the G′ nor G″ exhibited frequency-dependent behavior over the range examined (Figure 2b and d). The main effect of crosslinking mechanism was significant (p < 0.001), and within each level of methacrylation, storage moduli for hydrogels crosslinked with UV alone, calcium alone, and dual-crosslinking were all statistically different from each other (p < 0.05). The storage moduli of the system ranged from G′ = 0.01–0.11 kPa for UV-crosslinked hydrogels, G′ = 8.3–11.5 kPa for calcium-crosslinked hydrogels, and G′ =15.7–22.3 kPa for dual-crosslinked hydrogels. The loss moduli of the system ranged from G″ = 0.005–0.024 kPa for UV-crosslinked hydrogels, G″ = 1.1–1.3 kPa for calcium-crosslinked hydrogels, and G″ = 1.8–2.9 kPa for dual-crosslinked alginate.
Figure 1.
Dual-crosslinked hydrogel (a) fabrication and (b) chemistry. Calcium-crosslinked hydrogels are prepared using only steps 1–3. Patterned hydrogels required the use of a photomask and UV light in step 4.
Figure 2.
Shear (a) storage (G′) and (c) loss (G″) moduli at 1 Hz. The main effect of crosslinking mechanism was significant at p < 0.001. Representative (b) G′ and (d) G″ moduli curves across a range of frequencies. (e) Swelling of calcium- and dual-crosslinked hydrogels. The main effect of crosslinking mechanism was significant at p < 0.001. Individual comparisons significant at p < 0.05 within methacrylation levels for groups indicated with different letters.
Average swelling correlated inversely with mechanical properties: dual-crosslinked hydrogels swelled less than calcium-crosslinked hydrogels at the same methacrylation level (Figure 2e). Again, the main effect of crosslinking type led to significantly different average swelling (p < 0.001). Swelling differences were significant between calcium- and dual-crosslinked hydrogels for 11% MA (p < 0.05), and between UV-only and dual-crosslinked hydrogels at all methacrylation levels (p < 0.05). Swelling for UV-only crosslinked hydrogels was significantly greater than that of calcium-crosslinked hydrogels for 5% and 7% MA-ALG, likely because the low levels of methacrylation limited the number of UV-crosslinks that could form. Average swelling ratio, Q, ranged from a low of 36.9 for 11% MA, dual-crosslinked alginate, to a high of 210.1 for 5% MA UV-crosslinked hydrogels, with the average for calcium-crosslinked hydrogels ranging from Q = 83.0–98.6.
Crosslinking Mode Effects on Cell Proliferation.
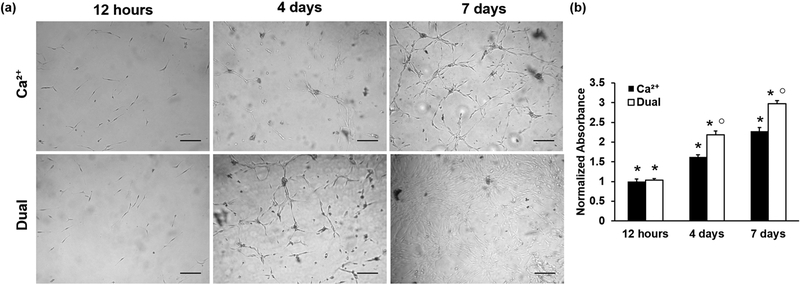
After establishing the effect of crosslinking mode on hydrogel physical properties, it was important to evaluate how these differences in physical properties affect cell behavior. MC3T3 preosteoblast cells could adhere and spread on both calcium- and dual-crosslinked 7% MA hydrogels modified uniformly with the RGD adhesion ligand (Figure 3a). After 12 h of culture, the cells exhibited no visible differences in cell number or initial spreading. However, there were visible qualitative differences in cell proliferation over time. At 4 days, some increase in cell number was observed on calcium-crosslinked hydrogels, but there was marked proliferation on the dual-crosslinked hydrogels. At 7 days, cell proliferation was substantial on the calcium-crosslinked hydrogels, while cells had reached near confluence on the dual-crosslinked hydrogels (Figure 3a). Quantification of metabolic activity by MTS assay, an indirect measure of cell number, confirmed these qualitative findings. While there was no difference in the initial MTS readings indicating the same number of cells adhered to the hydrogels at 12 h, absorbance readings normalized to day zero were 34.3% and 30.5% higher for the dual-crosslinked hydrogels compared to the calcium-crosslinked hydrogels at 4 and 7 days, respectively (Figure 3b). These increases were significant at p < 0.01.
Figure 3.
(a) Photomicrographs of MC3T3-E1 cells seeded on calcium- and dual-crosslinked hydrogels and cultured over 7 days. Top row, Ca2+-crosslinked; bottom row, dual-crosslinked. (Scale bars = 200 μm.) (b) Proliferation of cells seeded on Ca2+ or dual-crosslinked alginate-RGD hydrogels as quantified by MTS assay (*p < 0.01 compared to all other time points for same crosslinking type, ○ p < 0.01 compared to different crosslinking mechanism for the same time point).
Spatial Control over Hydrogel Patterning to Direct Cell Behavior.
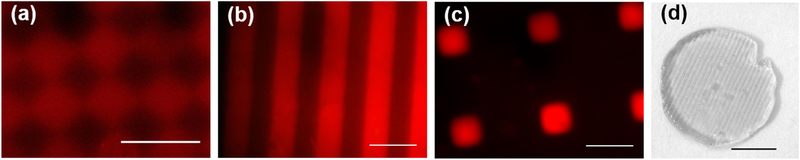
The ability to spatially control the physical properties described above would dramatically increase the utility of the dual-crosslinked alginate. Successful photo-patterning of the hydrogels was verified with methacrylated rhodamine, a fluorescent tag which covalently bonds to the methacrylate groups on MA-ALG during exposure to UV light. After 24 h equilibration in DMEM, the free methacrylated rhodamine diffused out of the hydrogel in areas that were crosslinked by calcium alone. This left only the methacrylated rhodamine that was coupled to the dual-crosslinked regions, and could be detected with a fluorescence microscope. Images of hydrogels made with a variety of photomask geometries demonstrate that patterns were fabricated in a number of shapes with resolution on the order of tens of microns (Figure 4a–c). Micropatterned hydrogels resulted in regional differences in surface swelling after incubation in media for 48 h. These were grossly observed visually as demonstrated with 11% MA alginate (Figure 4d), which had the most dramatic differences in swelling between calcium- and dual-crosslinked hydrogels (Figure 2e). Other levels of methacrylation do not lead to visible patterns of surface swelling, but these patterns may be present on the microscopic scale.
Figure 4.
Fluorescence photomicrographs using methacrylated rhodamine to show micropatterns in 7% MA hydrogels: (a) 100 μm checkerboard, scale bar = 200 μm; (b) 250 μm stripes, scale bar = 500 μm, and (c) 200 μm islands, scale bar = 500 μm. Red indicates coupled methacrylated rhodamine, which demonstrates where UV crosslinking occurred. (d) Patterned surface swelling in 11% MA hydrogel patterned with 250 μm stripes, after 48 h incubation in media at 4 °C.
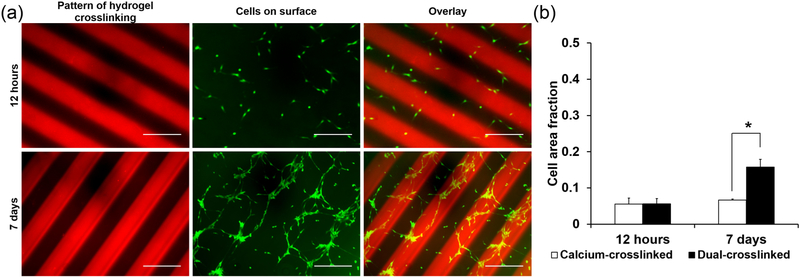
Given that the properties studied in the bulk hydrogels were shown to affect cell behavior, it was hypothesized that spatial control of these material properties can be used to direct cell behavior. When MC3T3 cells were seeded on hydrogels with uniform adhesion ligand presentation patterned with 250 μm stripes of dual-crosslinking, there was no visible pattern in their initial attachment (Figure 5a, top row). After 7 days of culture on the patterned hydrogels, the cells exhibited visible proliferation and formed aligned cell structures on the 250 μm dual-crosslinked stripes (Figure 5a, bottom row). In contrast, cells seeded on single- or dual-crosslinked hydrogels that were not patterned exhibited no visible pattern of alignment after 7 days in culture (Figure 3a). Increased cell coverage of dual crosslinked regions of the hydrogel, which is a function of cell number and cell spreading, was verified quantitatively. There was no statistically significant difference in initial cell area fraction on the calcium- vs dual-crosslinked hydrogels at 12 h (5.5% of the calcium-crosslinked area was covered with adhered cells compared with 5.6% of dual-crosslinked area). After 7 days, however, a significantly greater fraction of the dual-crosslinked area of the hydrogels was covered with cells (15.8% of dual-crosslinked area compared with 6.7% of calcium-crosslinked area, p < 0.01, Figure 5b).
Figure 5.
Cells seeded onto a 250 μm striped micropatterned dual-crosslinked hydrogel. (a) Fluorescence photomicrographs of hydrogels and cells after 12 h (top row), and after 7 days in culture (bottom row). Scale bars = 500 um. (b) Quantification of cell distribution on calcium- and dual-crosslinked regions (* p < 0.01 compared to different crosslinking mechanism at same time point).
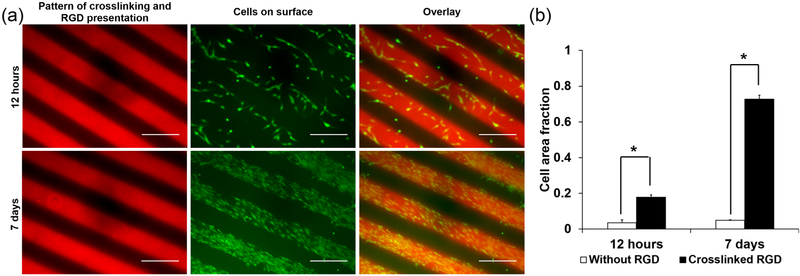
This dual-crosslinking system was then used to achieve control over cell behavior by exploiting its capacity for patterning biomolecule presentation. Here, this was demonstrated using MA-ALG that was not modified with RGD peptides throughout, but instead mixed with acrylated RGD-containing peptides. Much like the methacrylated rhodamine dye, the acrylated RGD can be mixed into the MA-ALG macromer solution prior to crosslinking, and is only immobilized in the dual-crosslinked regions. Overnight incubation of the hydrogels in DMEM allowed the uncrosslinked acrylated RGD in the regions not exposed to UV light to diffuse out of the hydrogel. In contrast to the hydrogels modified uniformly with RGD-containing peptides, which showed no patterns in cell attachment (Figure 5a, top row), on hydrogels with micropatterns of adhesion ligands MC3T3s attached predominantly to peptide-containing regions (Figure 6a, top row). Over 7 days the cells continued to proliferate almost exclusively on these regions (Figure 6a, bottom row). There were statistically significant differences in cell distribution on the RGD-patterned hydrogels after both 12 h, when 3.5% of calcium-crosslinked areas without RGD were covered with cells compared to 17.7% of dual-crosslinked areas with coupled RGD, (p < 0.01), and after 7 days, when 4.9% of hydrogel areas without RGD were covered with cells compared to 72.8% of areas with coupled RGD (p < 0.01, Figure 6b).
Figure 6.
Cells seeded onto micropatterned 250 μm striped dual-crosslinked hydrogels presenting cell adhesion ligands only in regions of dual crosslinking. (a) Fluorescence photomicrographs of hydrogels and cells after 12 h (top row), and after 7 days in culture (bottom row). Scale bars = 500 um. (b) Quantification of cell distribution on calcium- and dual-crosslinked regions (* p < 0.01 compared to different crosslinking mechanism at same time point).
DISCUSSION
In this study, dual ionic- and photo-crosslinking was used to create alginate hydrogels with spatially controlled physical and chemical properties. Before achieving spatial control, it was first important to characterize the bulk properties of single- and dual-crosslinked hydrogels. Mechanical testing showed that for all conditions, the storage moduli were higher than the loss moduli, which indicates crosslinked hydrogel networks that have passed their G′/G″ crossover, or gel points.30 However, dual-crosslinking led to hydrogels with higher shear storage moduli than either calcium- or UV-crosslinking alone. This trend is consistent with other hydrogel systems where increasing the number of crosslinks led to increased stiffness; additional crosslinks add to a material’s ability to resist deformation under applied load.14,18,20 The inverse relationship seen between hydrogel stiffness and swelling follows as expected from Flory–Rehner theory, where increased crosslink density correlates with decreased hydrogel swelling.31 Of note, increased alginate methacrylation led to increased storage moduli and decreased swelling of UV-crosslinked hydrogels, similar to results reported previously,27 and a decreasing trend in the storage moduli of calcium-crosslinked hydrogels. This is likely due to the decreased availability of carboxylic acid groups for ionic-crosslinking in hydrogels with higher methacrylate modification.
The hydrogels presented here comprise a broader storage moduli range than some existing systems. For example, the HA system described by Zawko et al. exhibited G′ < 1 kPa (single-crosslinked regions) up to a maximum G′ = 1.37 kPa (dual-crosslinked regions),20 compared with this alginate system, where the G′ range is from <1 to 22.3 kPa. Because hydrogels are typically viscoelastic, it is difficult to make direct comparisons with systems that report elastic moduli obtained through constant strain rate compression tests. Results on viscoelastic materials are highly dependent on testing parameters including hydrogel geometry and strain rate. However, the shear storage moduli of these hydrogels are in the range of others reported in the literature and shown to support the viability of cells both in and on the hydrogels.14
It is useful to note that ionic and covalent crosslinks lead to hydrogels with unique stress relaxation behaviors. Ionic crosslinking of alginate allows for the breakage and reformation of crosslinks under loading. Covalent crosslinks are fixed and instead force the migration of water through the hydrogel network in response to applied loads.32,33 By regulating the location of ionic-only versus dual-crosslinking, it may be possible to create a material with regional differences in viscoelastic mechanical properties. For example, a patterned hydrogel can be subjected to a relatively uniform compressive load in one direction, such as that from a simple compressive loading bioreactor,34,35 but cells in the differently crosslinked regions will be exposed to different deformations and patterns of fluid flow depending partially on how easily crosslinks in the material of these regions break and reform.
Since photopolymerizable alginate can be easily modified throughout with cell adhesion ligands, such as those containing the RGD peptide sequence, with minimal changes to hydrogel physical properties such as swelling and elastic modulus,25 the system can be used to examine effects of changing hydrogel physical properties while maintaining biochemical properties, such as cell adhesion, constant. The range of mechanical properties achieved in this dual-crosslinked system was wide enough to induce changes in cell proliferation, which is known to be influenced by biomaterial mechanical properties in both 2D and 3D.4,36 MC3T3 cells showed increased proliferation on dual-crosslinked hydrogels, probably due to their higher stiffness. Cells cultured on other cell-adhesive hydrogels have also exhibited increased proliferation on stiffer surfaces.5,37,38 This is likely because cell spreading, which is enhanced on substrates of increased stiffness,42,43 is often necessary for division of adhesion-dependent cells.39 Since both crosslinking mechanisms are gentle, ensuring high viability of encapsulated cells (data not shown), this system will allow for the highly controlled study of the effects of a range of substrate mechanical properties using hydrogels with similar biochemistry, a useful tool in fields like tissue engineering and cancer biology.
Hydrogels with photopatterned physical properties showed no localized differences in cell adhesion, but over time cells formed aligned structures on the surfaces that matched the hydrogel patterns, with the majority of cell area covering the dual-crosslinked regions of the hydrogels. This could be due to spatial differences in gel mechanics leading to preferential proliferation on dual-crosslinked regions and/or gel curvature resulting from differential swelling. Hydrogels with patterned RGD cell adhesion peptide showed striking control over both localized cell adhesion and proliferation. Given the versatile chemistry, other peptide sequences40 and patterns could be used with similar effect. For example, islands of adhesion ligands can be created in defined geometries on the scale of tens of microns, directing adhesion and spreading of cells and/or cell clusters, and potentially affecting proliferation and differentiation.41,42 In a similar manner, other bioactive molecules can be functionalized with acrylate or methacrylate groups and patterned in the dual-crosslinked regions of these hydrogels. For instance, an osteopontin mimicking peptide43 might be used to locally drive osteogenesis, matrix metalloproteinase-degradable peptides44 could permit regional control over where cells degrade hydrogel crosslinks, and methacrylated heparin45 could be used to sequester heparin-binding growth factors in specific regions of dual-crosslinking.
Importantly, this system also differs fundamentally from most existing dual-crosslinked systems in that the two crosslinking mechanisms can be applied in either order: UV light can be applied to an ionically crosslinked hydrogel, or divalent cations in solution can diffuse into a photo-crosslinked hydrogel. This can be used to stiffen regions of hydrogel at different time points without affecting viability of cells seeded on the hydrogel surface or in its interior. Alternately, to soften hydrogels at a desired point in time, calcium-binding molecules, such as EDTA or citrate, could be delivered to local regions of a hydrogel to remove the ionic-crosslinks46 while leaving UV-crosslinked regions unaltered. These system capabilities may be used to yield insight into cellular behavior in response to externally and spatially controlled temporal changes in substrate mechanical properties.47,48
Spatial control of ionic-crosslinking following photo-cross-linking could be accomplished on the macroscopic scale by only permitting access of a divalent cation-containing solution to part of the hydrogel. UV-based calcium release would allow for fine spatial resolution. Applying UV light again to photo-crosslinked alginate hydrogels that have first been rinsed to remove unreacted residual photoinitiator will not result in additional photo-crosslinking. This means that, for example, calcium caged DM-nitrophen, a photolytic molecule that releases calcium ions in response to UV light and is small enough to diffuse into photo-crosslinked hydrogels,49 could be used to ionically crosslink hydrogels only in regions directed by a photomask.50 Given that the differences between the properties of the dual-crosslinked hydrogels and photo-crosslinked hydrogels were even more dramatic than those between the dual-crosslinked and calcium-crosslinked hydrogels that were described in this work, using patterned calcium ion presentation has the potential to create hydrogels with an even wider range of properties.
Advances such as two-photon excitation technology and lasers with digital micromirror devices have been employed in the literature to localize UV light to specific locations in 3D space to allow for even finer control over the presentation of these signals as opposed to the 2D patterning reported.51 These technologies could allow for the study of the effects that precise 3D spatial presentation of different bioactive factors have on cell behavior. Such tools could ultimately be used to generate complex instructive biomaterials to create 3D patterned templates for tissue regeneration.
In summary, this work demonstrates the capacity of combined ionic and photo-crosslinking to control alginate material properties. Dual-crosslinking created hydrogels with significantly increased elastic modulus and significantly decreased swelling compared to UV- or calcium-crosslinking alone. Further, a photomask could control exposure of UV light, creating a spatially patterned biomaterial with micron scale resolution. These differences in hydrogel physical properties affected cell behavior: preosteoblast cells exhibited significantly increased proliferation on dual-crosslinked compared to calcium-crosslinked hydrogels, and formed aligned cell structures on patterned hydrogels. Acrylated cell adhesion peptides could also be spatially patterned using photomasks, directing cell attachment as well as proliferation. Such a system will be useful for studying effects of hydrogel material properties on cells, and for patterning these properties in three-dimensional space to create instructive hydrogels for the regeneration of complex tissues.
EXPERIMENTAL PROCEDURES
Synthesis of Methacrylated Alginate.
MA-ALG was prepared as previously described.27 Briefly, 5 g of 5 Mrad irradiated Protanal LF 20/40 alginate (52 kDa, FMC Biopolymer, Philadelphia, PA) was dissolved at 1% w/v in a pH 6.5 solution of 50 mM 2-morpholinoethanesulfonic acid (MES, Sigma-Aldrich, St. Louis, MO) and 0.5 M NaCl. To prepare alginate with a theoretical methacrylation (MA) of 45% of its monomers, N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (NHS, 1.31 g and EDC, 4.38g, respectively, Sigma-Aldrich) were added to activate the carboxylic acid groups of the alginate, after which 2-aminoethyl methacrylate (AEMA, 1.9 g, Polysciences, Inc., Warrington, PA) was added and allowed to react for 24 h. The molar ratio of NHS:EDC:AEMA was maintained at 1:2:1, varying the amount of AEMA to achieve varying degrees of methacrylation. The product was precipitated in excess acetone, dried under reduced pressure, rehydrated to 1% w/v in ultrapure deionized water (diH2O), and dialyzed against diH2O (MWCO 3500, Spectrum Laboratories, Rancho Dominguez, CA) for 3 days. It was then treated with 5 g/L activated charcoal for 30 min, filtered through a 0.22 μm filter, and lyophilized until dry. The degree of alginate methacrylation was characterized by 1H NMR (Varian Inc., Palo Alto, CA) and integrating the peaks of the methylene protons of the methacrylate groups as previously described.27 The synthesized alginates were labeled by their calculated percent of methacrylated carboxylic acids, for example, 7% MA. For material to be used in cell experiments with uniform adhesion ligand presentation, MA-ALG was further modified for cell adhesion with 15 mg of the amino acid peptide sequence GGGGRGDSP (Gly-Gly-Gly-Gly-Arg-Gly-Asp-Ser-Pro, Mimotopes, Clayton, Victoria, Australia) per gram of alginate using sulfo-NHS (Sigma-Aldrich) and EDC (28.1 mg sulfo-NHS and 49.7 mg EDC per gram of alginate) under reaction conditions as previously described.25,52
Hydrogel Preparation.
For calcium-crosslinked hydrogels, 50-mm-diameter cell culture dishes with 14-mm-diameter holes (MatTek, Ashland, MA) were placed on top of 18 mm square glass coverslips (Fisher Scientific, Fair Lawn, NJ) to create a small well, and a small amount of water between the dish and coverslip created a seal. A 2% alginate solution (225 μL) in Dulbecco’s Modified Eagle Medium (DMEM, Thermo Fisher Scientific, Waltham, MA) with 0.05% Irgacure 2959, a photoinitiator, (Sigma-Aldrich) was deposited into the well, and covered with a 47-mm-diameter, 5 μm pore size cellulose acetate membrane (GE Water, Trevose, PA). The dish was filled with 100 mM calcium chloride (Fisher Scientific) in diH2O for ionic crosslinking. After 15 min, the calcium solution and membrane were removed, and the alginate hydrogel remained on the glass coverslip. For UV-crosslinked hydrogels, the alginate was deposited into the well as described above, but not covered with a membrane, and placed under UV light (Lumen Dynamics, Missisagua, Ontario, Canada) with 320–500 nm filter at 2 mW/cm2 for 60 s. All dual-crosslinked gels were first calcium-crosslinked. Before the hydrogel was removed from the dish, the dish was inverted so the glass coverslip was on top, and exposed to UV light as described for the UV-crosslinked hydrogels. In the case of patterned hydrogels, a custom photomask made from chrome on soda lime glass in either 250-μm-wide stripes or a 200 μm checkerboard pattern (Advance Reproductions Corporation, North Andover, MA) was placed in between the glass coverslip and the UV lamp.
Characterization of Bulk Hydrogel Physical Properties.
The rheological properties of the photo-crosslinked hydrogels were measured using a strain-controlled AR-2000ex rheometer or ARES rheometer (TA Instruments, New Castle, DE, USA) with stainless-steel parallel plate geometry. Samples prepared in the same manner measured on both rheometers showed similar properties. The measurement was performed using a dynamic frequency sweep test in which a sinusoidal shear strain of constant peak amplitude (0.1%) was applied over a 0.1–10 Hz frequency range, with N = 4 samples per condition. Storage modulus (G′) and loss modulus (G″) at 1 Hz were compared for all samples. Swelling ratio was calculated by incubating the hydrogels for 48 h in DMEM, and taking the ratio Q = wet mass/dry mass at that time, with N = 4 samples per condition.
Cell Proliferation.
Prepared hydrogels were transferred to nonadhesive 24-well cell culture plates (Corning Inc., Corning, NY) and rinsed overnight in 1 mL DMEM at 4 °C. Cell proliferation experiments used MC3T3-E1 preosteoblast cells (passage 24, ATCC CRL 2593, Manassas, VA) seeded at 5000 cells per cm2. Cells were seeded onto the surface of the hydrogels, cultured in 0.5 mL of Minimum Essential Medium Alpha (αMEM, Thermo Fisher Scientific) with 10% fetal bovine serum (FBS, Fisher Scientific) changed every other day, and observed at 12 h, 4 days, and 7 days. Cells were photographed on a TMS Microscope (Nikon, Tokyo, Japan) using a Nikon CoolPix camera. At these time points, cells on the hydrogels were incubated for 1 h with phanazine methosulfate (PMS) and 3-(4,5-Dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, (MTS) (Promega, Madison, WI), which can be reduced in the presence of metabolically active cells.53 The assay was performed per manufacturer’s guidelines, and the absorption of resulting formazan product was measured at 490 nm using a VersaMax plate reader (Molecular Devices, Sunnyvale, CA), with N = 4 samples per condition at each time point. Absorbance readings were normalized to the calcium-cross-linked hydrogels at the 12 h time point, allowing a relative measure of cell number changes over time.
Visualization of Micropatterning.
To visualize pattern formation, 7% MA micropatterned dual-crosslinked alginate gels were prepared as described above using a variety of photomasks, but 0.01% w/v of methacrylated rhodamine was added to the alginate solution before crosslinking. After 24 h incubation in media at 4 °C to remove the uncrosslinked rhodamine, hydrogels were imaged for red fluorescence using an Eclipse TE300 fluorescence microscope (Nikon) with a Retiga-SRV camera (QImaging, Burnaby, BC, Canada). To visualize patterns in surface swelling, micropatterned, dual-crosslinked 11% MA alginate gels were prepared as described above with a 250 μm stripe photomask, allowed to incubate in media for 48 h as in the swelling experiments, and photographed with a Nikon Coolpix camera. MC3T3 cells were then seeded on these patterned hydrogels as described above, again at 5000 cells per cm2. After 12 hours or after 7 days, hydrogels were incubated with 30 μg/mL fluorescein diacetate (Sigma-Aldrich) for 2 min at room temperature and imaged for both red and green fluorescence as described above. At each time point there were N = 4 hydrogels per condition, with representative images reported. Hydrogels were not returned to culture after imaging. Using these images, quantification of cell coverage on calcium-crosslinked and dual-crosslinked areas of the hydrogels was performed using ImageJ software (U.S. National Institutes of Health, Bethesda, Maryland, USA). Images of both the cells and patterns were thresholded, and the total pattern areas and cell areas on each part of the pattern were calculated using the Analyze Particles tool. From this, the cell area fraction on each part of the substrates was determined. Images from N = 4 hydrogels from each time point were examined, with one image per hydrogel.
Visualization of Hydrogels with Micropatterned Adhesion Ligand Presentation.
Acrylated RGD was prepared as previously described,54 using GGGGRGDSP peptide (Mimotopes), and added to a final concentration of 1 mg/mL to a solution of 7% MA alginate that was not already modified with peptide. The material was calcium-crosslinked, UV-crosslinked through a 250 μm striped photomask, and incubated overnight in 1 mL of DMEM at 4 °C to allow uncrosslinked acrylated RGD to diffuse out of the hydrogel. MC3T3 cells were seeded on the surface of the hydrogels, and at 12 h or 7 days cells were incubated with fluorescein diacetate and imaged as described earlier. There were N = 4 hydrogels per condition, with representative images reported. Hydrogels were not returned to culture after imaging. Quantification of preferential cell adhesion and proliferation on the RGD-modified regions was performed using these images as described in the previous paragraph.
Statistical Analysis.
All quantitative data is expressed as mean ± standard deviation. Statistical analysis was performed with one- or two-way analysis of variance (ANOVA) with Tukey honestly significant difference post hoc tests using Minitab software (Minitab Inc., State College, PA). A value of p < 0.05 was considered statistically significant.
ACKNOWLEDGMENTS
The authors thank Michael Hill for providing the acrylated RGD and Oju Jeon for creating the crosslinking chemistry schematic, and gratefully acknowledge funding support from the National Institutes of Health (R01AR063194, R21AR061265, and R56DE022376; EA) and NDSEG and NSF Graduate Fellowships (JES).
Footnotes
The authors declare no competing financial interest.
REFERENCES
- (1).Kleinman HK, Philp D, and Hoffman MP (2003) Role of the extracellular matrix in morphogenesis. Curr. Opin. Biotechnol 14, 526–532. [DOI] [PubMed] [Google Scholar]
- (2).Lutolf MP, and Hubbell JA (2005) Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat. Biotechnol 23, 47–55. [DOI] [PubMed] [Google Scholar]
- (3).Pelham RJ Jr., and Wang Y (1997) Cell locomotion and focal adhesions are regulated by substrate flexibility. Proc. Natl. Acad. Sci. U. S. A 94, 13661–13665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Haugh MG, Murphy CM, McKiernan RC, Altenbuchner C, and O’Brien FJ (2011) Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng, Part A 17, 1201–1208. [DOI] [PubMed] [Google Scholar]
- (5).Saha K, Keung AJ, Irwin EF, Li Y, Little L, Schaffer DV, and Healy KE (2008) Substrate modulus directs neural stem cell behavior. Biophys. J 95, 4426–4438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (6).Peyton SR, and Putnam AJ (2005) Extracellular matrix rigidity governs smooth muscle cell motility in a biphasic fashion. J. Cell. Physiol 204, 198–209. [DOI] [PubMed] [Google Scholar]
- (7).Alsberg E, von Recum HA, and Mahoney MJ (2006) Environmental cues to guide stem cell fate decision for tissue engineering applications. Expert Opin. Biol. Ther 6, 847–866. [DOI] [PubMed] [Google Scholar]
- (8).Bryant SJ, and Anseth KS (2002) Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed Mater. Res 59, 63–72. [DOI] [PubMed] [Google Scholar]
- (9).Baier Leach J, Bivens KA, Patrick CW Jr., and Schmidt CE (2003) Photocrosslinked hyaluronic acid hydrogels: natural, biodegradable tissue engineering scaffolds. Biotechnol. Bioeng 82, 578–589. [DOI] [PubMed] [Google Scholar]
- (10).Park YD, Tirelli N, and Hubbell JA (2003) Photopolymerized hyaluronic acid-based hydrogels and interpenetrating networks. Biomaterials 24, 893–900. [DOI] [PubMed] [Google Scholar]
- (11).Genes NG, Rowley JA, Mooney DJ, and Bonassar LJ (2004) Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch. Biochem. Biophys 422, 161–167. [DOI] [PubMed] [Google Scholar]
- (12).Mata A, Fleischman AJ, and Roy S (2005) Characterization of polydimethylsiloxane (PDMS) properties for biomedical micro/nanosystems. Biomed. Microdevices 7, 281–293. [DOI] [PubMed] [Google Scholar]
- (13).Zhang X, Wu D, and Chu CC (2004) Synthesis and characterization of partially biodegradable, temperature and pH sensitive Dex-MA/PNIPAAm hydrogels. Biomaterials 25, 4719–4730. [DOI] [PubMed] [Google Scholar]
- (14).Nemir S, and West JL (2010) Synthetic materials in the study of cell response to substrate rigidity. Ann. Biomed. Eng 38, 2–20. [DOI] [PubMed] [Google Scholar]
- (15).Cha C, Kim SR, Jin YS, and Kong H (2012) Tuning structural durability of yeast-encapsulating alginate gel beads with interpenetrating networks for sustained bioethanol production. Biotechnol. Bioeng 109, 63–73. [DOI] [PubMed] [Google Scholar]
- (16).Tan H, Li H, Rubin JP, and Marra KG (2011) Controlled gelation and degradation rates of injectable hyaluronic acid-based hydrogels through a double crosslinking strategy. J. Tissue Eng. Regen. Med 5, 790–797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Mihaila SM, Gaharwar AK, Reis RL, Marques AP, Gomes ME, and Khademhosseini A (2013) Photocrosslinkable kappa-carrageenan hydrogels for tissue engineering applications. Adv. Heathcare Mater 2, 895–907. [DOI] [PubMed] [Google Scholar]
- (18).Jeon O, Samorezov JE, and Alsberg E (2014) Single and dual crosslinked oxidized methacrylated alginate/PEG hydrogels for bioadhesive applications. Acta Biomater 10, 47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (19).Nemir S, Hayenga HN, and West JL (2010) PEGDA hydrogels with patterned elasticity: Novel tools for the study of cell response to substrate rigidity. Biotechnol. Bioeng 105, 636–644. [DOI] [PubMed] [Google Scholar]
- (20).Zawko SA, Suri S, Truong Q, and Schmidt CE (2009) Photopatterned anisotropic swelling of dual-crosslinked hyaluronic acid hydrogels. Acta Biomater 5, 14–22. [DOI] [PubMed] [Google Scholar]
- (21).Marklein RA, and Burdick JA (2010) Spatially controlled hydrogel mechanics to modulate stem cell interactions. Soft Matter 6, 136–143. [Google Scholar]
- (22).Khetan S, and Burdick JA (2010) Patterning network structure to spatially control cellular remodeling and stem cell fate within 3-dimensional hydrogels. Biomaterials 31, 8228–8234. [DOI] [PubMed] [Google Scholar]
- (23).Jeon O, and Alsberg E (2013) Regulation of stem cell fate in a three-dimensional micropatterned dual-crosslinked hydrogel system. Adv. Funct. Mater 23, 4765–4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (24).Rowley JA, Madlambayan G, and Mooney DJ (1999) Alginate hydrogels as synthetic extracellular matrix materials. Biomaterials 20, 45–53. [DOI] [PubMed] [Google Scholar]
- (25).Jeon O, Powell C, Ahmed SM, and Alsberg E (2010) Biodegradable, photocrosslinked alginate hydrogels with independently tailorable physical properties and cell adhesivity. Tissue Eng, Part A 16, 2915–2925. [DOI] [PubMed] [Google Scholar]
- (26).Smidsrod O, and Skjak-Braek G (1990) Alginate as immobilization matrix for cells. Trends Biotechnol 8, 71–78. [DOI] [PubMed] [Google Scholar]
- (27).Jeon O, Bouhadir KH, Mansour JM, and Alsberg E (2009) Photocrosslinked alginate hydrogels with tunable biodegradation rates and mechanical properties. Biomaterials 30, 2724–2734. [DOI] [PubMed] [Google Scholar]
- (28).Kong HJ, Alsberg E, Kaigler D, Lee KY, and Mooney DJ (2004) Controlling degradation of hydrogels via the size of crosslinked junctions. Adv. Mater 16, 1917–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Bajpai SK, and Sharma S (2004) Investigation of swelling/degradation behaviour of alginate beads crosslinked with Ca2+ and Ba2+ ions. React. Funct. Polym 59, 129–140. [Google Scholar]
- (30).Winter H (1987) Can the gel point of a crosslinking polymer be detected by the G′–G ″crossover? Polym. Eng. Sci 27, 1698–1702. [Google Scholar]
- (31).Peppas N, Huang Y, Torres-Lugo M, Ward J, and Zhang J (2000) Physicochemical foundations and structural design of hydrogels in medicine and biology. Annu. Rev. Biomed. Eng 2, 9–29. [DOI] [PubMed] [Google Scholar]
- (32).Zhao X, Huebsch N, Mooney DJ, and Suo Z (2010) Stress-relaxation behavior in gels with ionic and covalent crosslinks. J. Appl. Phys 107, 63509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Rouillard AD, Tsui Y, Polacheck WJ, Lee JY, Bonassar LJ, and Kirby BJ (2010) Control of the electromechanical properties of alginate hydrogels via ionic and covalent crosslinking and microparticle doping. Biomacromolecules 11, 2184–2189. [DOI] [PubMed] [Google Scholar]
- (34).Thorpe SD, Buckley CT, Vinardell T, O’Brien FJ, Campbell VA, and Kelly DJ (2008) Dynamic compression can inhibit chondrogenesis of mesenchymal stem cells. Biochem. Biophys. Res. Commun 377, 458–462. [DOI] [PubMed] [Google Scholar]
- (35).Terraciano V, Hwang N, Moroni L, Park HB, Zhang Z, Mizrahi J, Seliktar D, and Elisseeff J (2007) Differential response of adult and embryonic mesenchymal progenitor cells to mechanical compression in hydrogels. Stem Cells 25, 2730–2738. [DOI] [PubMed] [Google Scholar]
- (36).Lutolf MP, Gilbert PM, and Blau HM (2009) Designing materials to direct stem-cell fate. Nature 462, 433–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (37).Georges PC, and Janmey PA (2005) Cell type-specific response to growth on soft materials. J. Appl. Physiol 98, 1547–1553. [DOI] [PubMed] [Google Scholar]
- (38).Wang HB, Dembo M, and Wang YL (2000) Substrate flexibility regulates growth and apoptosis of normal but not transformed cells. Am. J. Physiol. Cell Physiol 279, C1345–1350. [DOI] [PubMed] [Google Scholar]
- (39).Schwartz MA, and Assoian RK (2001) Integrins and cell proliferation regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci 114, 2553–2560. [DOI] [PubMed] [Google Scholar]
- (40).Weber LM, Hayda KN, Haskins K, and Anseth KS (2007) The effects of cell–matrix interactions on encapsulated β-cell function within hydrogels functionalized with matrix-derived adhesive peptides. Biomaterials 28, 3004–3011. [DOI] [PubMed] [Google Scholar]
- (41).Chen CS, Mrksich M, Huang S, Whitesides GM, and Ingber DE (1997) Geometric control of cell life and death. Science 276, 1425–1428. [DOI] [PubMed] [Google Scholar]
- (42).McBeath R, Pirone DM, Nelson CM, Bhadriraju K, and Chen CS (2004) Cell shape, cytoskeletal tension, and rhoA regulate stem cell lineage commitment. Dev. Cell 6, 483–495. [DOI] [PubMed] [Google Scholar]
- (43).Shin H, Zygourakis K, Farach-Carson MC, Yaszemski MJ, and Mikos AG (2004) Attachment, proliferation, and migration of marrow stromal osteoblasts cultured on biomimetic hydrogels modified with an osteopontin-derived peptide. Biomaterials 25, 895–906. [DOI] [PubMed] [Google Scholar]
- (44).Kim S, and Healy KE (2003) Synthesis and characterization of injectable poly(N-isopropylacrylamide-co-acrylic acid) hydrogels with proteolytically degradable crosslinks. Biomacromolecules 4, 1214–1223. [DOI] [PubMed] [Google Scholar]
- (45).Jeon O, Powell C, Solorio LD, Krebs MD, and Alsberg E (2011) Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J. Controlled Release 154, 258–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Shilpa A, Agrawal S, and Ray AR (2003) Controlled delivery of drugs from alginate matrix. J. Macromol. Sci., Polym. Rev 43, 187–221. [Google Scholar]
- (47).Guvendiren M, and Burdick JA (2012) Stiffening hydrogels to probe short-and long-term cellular responses to dynamic mechanics. Nat. Commun 3, 792. [DOI] [PubMed] [Google Scholar]
- (48).Kloxin AM, Benton JA, and Anseth KS (2010) In situ elasticity modulation with dynamic substrates to direct cell phenotype. Biomaterials 31, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (49).Ellis-Davies GC (2007) Caged compounds: photorelease technology for control of cellular chemistry and physiology. Nat. Methods 4, 619–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (50).Chueh B. h., Zheng Y, Torisawa Y. s., Hsiao AY, Ge C, Hsiong S, Huebsch N, Franceschi R, Mooney DJ, and Takayama S (2010) Patterning alginate hydrogels using light-directed release of caged calcium in a microfluidic device. Biomed. Microdevices 12, 145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Schade R, Weiss T, Berg A, Schnabelrauch M, and Liefeith K (2010) Two-photon techniques in tissue engineering. Int. J. Artif. Organs 33, 219–227. [PubMed] [Google Scholar]
- (52).Alsberg E, Anderson K, Albeiruti A, Franceschi R, and Mooney D (2001) Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res 80, 2025–2029. [DOI] [PubMed] [Google Scholar]
- (53).Cory AH, Owen TC, Barltrop JA, and Cory JG (1991) Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun 3, 207–212. [DOI] [PubMed] [Google Scholar]
- (54).Jeon O, and Alsberg E (2013) Photofunctionalization of alginate hydrogels to promote adhesion and proliferation of human mesenchymal stem cells. Tissue Eng, Part A 19, 1424–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]