Abstract
Radiotherapy is a treatment choice for local control of breast cancer, particularly after the removal of tumor tissues by surgery. However, intrinsic radioresistance of cancer cells limits therapeutic efficacy. Here, we determined in breast cancer cells the potential radiosensitizing activity of SM-164, a small molecule compound, that mimics the activity of SMAC, a mitochondrial protein released during apoptosis to activate caspases by inhibiting cellular inhibitor of apoptosis proteins, cIAP-1, and XIAP. We found that SM-164 at nanomolar concentrations promoted degradation of cIAP-1, disrupted the inhibitory binding of XIAP to active caspase-9, and sensitized breast cancer cells to radiation with a sensitization enhancement ratio (SER) of 1.7–1.8. In one line of breast cancer cells resistant to SM-164 as a single agent, SM-164 radiosensitization was mediated by intrinsic apoptosis pathway through activation of caspases-9 and −3. In a line of breast cancer cells sensitive to SM-164 as a single agent, SM-164 radiosensitization was mediated by both extrinsic and intrinsic apoptosis pathways through activation of caspases-9, −8, and −3. Consistently, blockage of caspase activation, through siRNA knockdown or treatment with a pan-caspase inhibitor z-VAD-fmk, inhibited apoptosis and abrogated SM-164 radiosensitization. Our study demonstrates that IAPs are valid radiosensitizing targets in breast cancer cells and SM-164 could be further developed as a novel class of radiosensitizers for the treatment of radioresistant breast cancer.
Keywords: Apoptosis, Caspase activation, XIAP, cIAP-1 degradation, Radiosensitization
Introduction
Breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death among women, which accounts for 23% of the total cancer cases and 14% of the cancer deaths [1]. Although early detection contributes to the decrease of the mortality rates, the high incidence of breast cancer requires continued use of different treatment modalities, including combinations of surgery, radiotherapy, and chemotherapy. Radiotherapy plays a crucial role in achieving the local control of the disease, particularly after conservative surgery [2]. However, development of radioresistance in breast cancer cells limits the curable potential and contributes to local recurrences [3]. Thus, development of therapeutic agents that sensitize breast cancer to radiation will be extremely important for the improvement of therapeutic outcome.
Increasing lines of evidence suggest that radioresistance of breast cancer is associated with overexpression of epidermal growth factor receptors (e.g. HERS), insulin-like growth factor-1 receptor (IGF-1R), endothelial growth factor (VEGF), activation of PI3K pathway, and defects in DNA damage response pathways. Furthermore, the status of p53, BRCA, and BCL2 family members also determines radiation responsiveness (for reviews, see [3–6]). However, it remains elusive if inhibitor of apoptosis proteins (IAPs), a well-known family of anti-apoptotic proteins, plays a role in conferring breast cancer cells to radioresistance and if targeting IAPs would induce radiosensitization.
IAP family proteins consist of at least seven members, including XIAP, cIAP-1, cIAP-2, NAIP, Survivin, Livin/ML-IAP, and Bruce, which are characterized by containing one or several BIR (baculoviral IAP repeat) domains required for suppression of apoptosis. Some family members also have a RING finger domain at the C-terminus that is required for their E3 ubiquitin ligase activity [7]. The main function of IAPs is to suppress apoptosis through binding to and thus inhibiting active caspases 3/7 and 9. The most potent inhibitor of caspases among IAP family members is XIAP with sub-nanomole inhibitory constants. In XIAP, the third BIR domain (BIR3) potently inhibits the activity of the active caspase 9, whereas the linker region between BIR1 and BIR2, together with the BIR2 domain, selectively targets the active caspases 3 and 7 [8].
Second mitochondria-derived activator of caspase (SMAC) is a mitochondrial protein that is released to the cytoplasm on induction of apoptosis [9, 10]. In its dimeric form, SMAC through its AVPI tetrapeptide binding motif interacts with both BIR2 and BIR3 domains of multiple IAPs to release the binding of IAPs to both initiator (caspase 9) and effector (caspase 3/7) caspases [11, 12]. SMAC or a synthetic SMAC peptide with AVPI in its N-terminus is sufficient to activate caspase-3 [11, 13] and sensitizes cancer cells to apoptosis induced by chemotherapeutic agents [14] and radiation [15]. Indeed, several SMAC mimetic small molecules have been discovered, which acting as a new class of anticancer drugs, effectively overcome apoptosis resistance in different types of cancer cells with high levels of IAPs and sensitize cancer cells to current chemotherapeutic agents in vitro and in vivo [16–26]. Although overexpression of SMAC protein itself enhanced radiation-induced apoptosis in several lines of cancer cells, including neuroblastoma, glioblastoma, and pancreatic carcinoma [15], it remains elusive whether SMAC mimetics can act as a radiosensitizing agent.
SM-164 is a water-soluble and cell-permeable bivalent SMAC mimetic compound that binds to both the BIR2 and BIR3 domains of XIAP with a Ki of 0.56 nM. SM-164 also binds to cIAP-1 and cIAP-2 proteins with Ki of 0.31 and 1.1 nM, respectively [17, 26, 28]. We have recently shown that SM-164 acts as a novel class of radiosensitizers that confers a subset of otherwise radioresistant head and neck squamous carcinoma cells (HNSCC) to radiation [27]. In this study, we investigated if SM-164 acts as a radiosensitizer in breast cancer cells, which has not been previously investigated. Our data showed that SM-164 acts as a potent radiosensitizing agent with mechanism involving caspase activation and apoptosis induction.
Materials and methods
Compound
SM-164, a water-soluble and cell-permeable SMAC mimetic compound, binds to both the BIR2 and BIR3 domains of XIAP with a Ki of 0.56 nM. SM-164 also binds to cIAP-1 and cIAP-2 proteins with Ki of 0.31 and 1.1 nM, respectively [17, 26, 28]. The synthesis of this compound was performed as described previously [17, 26].
Cell culture
Breast cancer lines, including SK-BR-3, MDA-MB-468, MDA-MB-453, MCF-7, ZR-75–1, T-47D, along with immortalized breast epithelial MCF10A, were obtained from ATCC and were grown in DMEM (Gibco) with 10% fetal bovine serum. MCF 10A cells were cultured in Cell Culture Basal Media for mammary epithelial cells (MEBM, Biowhittaker Technologies, Inc).
Radiation exposure and clonogenic assay
Cells were seeded in 60-mm dishes at proper cell densities in duplicate and exposed to different doses of radiation (Philips RT250, Kimtron Medical) after 24-h pretreatment with SM-164, followed by incubation at 37°C for 6–11 days. Survival curves were fitted using the linear-quadratic equation, and the mean inactivation dose was calculated [29]. Irradiations were performed in the Experimental Irradiation Core of the University of Michigan Cancer Center.
Western blot analysis
The assay was performed as described [24]. The sources of antibodies used are as follows: cIAP-1 (a gift from Dr. Silke at La Trobe University, Australia) [30], XIAP (BD Pharmingen, CA), cIAP-2, PARP and Caspase-3 (Cell Signaling, MA), Caspase-9 (Santa Cruz Biotechnology, CA), Caspase-8 (Calbiochem, NJ), and β-Actin (Sigma, MO). Beads-conjugated mouse anti-HA (Sigma, MO), Rabbit anti-HA (Sigma, MO), and Rat anti-HA (Roche, CA).
ATPlite growth assay and IC50 determination
Cell were seeded in 96-well plates in triplicate and treated the next day with SM-164 in various doses ranging from 0.03 to 10 μM for 24 h. The viability of the cells was then measured using one-step ATPlite cell proliferation assay (Perkin Elmer) [24].
DNA fragmentation assay
Cells were seeded in 100-mm dishes at 2.0 × 106 (MDAMB-468) or 0.5 × 106 (SK-BR-3) cells per dish, and pretreated next day with DMSO (control) or SM-164 (200 nM) for 6 h before being exposed to radiation (6 Gy). The cells were harvested for 24 h (MDA-MB-468) or 48 h (SK-BR-3) post-radiation by scraping, pelleted and lysed in 600 μl of lysis buffer (5 mM Tris–HCl, pH 8, 20 mM EDTA, and 0.5% Triton X-100). The fragmented DNAs in the supernatant after 14,000 rpm centrifugation were extracted with PCI (phenol/chloroform/isopropanol) (Fisher) and precipitated with ethanol, followed by electrophoresis in a 1.8% agarose gel [24].
siRNA silencing of caspases-3, −8, and −9
The siRNA oligonucleotides for silencing caspase-3 (5′-AAUGACAUCUCGGUCUGGUAC-3′), caspase-8 (5′-GCCCAAACUUCACAGCAUU-3′), caspase-9 (5′-CGGUGAAAGGGAUUUAUAA-3′), and scrambled control siRNA (siCONT: 5′-ATTGTATGCGATCGCAGACTT-3′) were from Dharmacon (USA). Cells were transfected with siRNA using Lipofectamine 2000 and split 48 h late. One portion was used for clonogenic assay or DNA fragmentation and the other portion for Western blotting [31].
FACS (fluorescence-activated cell sorting) analysis
Cells were treated with SM-164 or exposed to radiation alone or in combination. Cells were harvested at 24 or 48 h post-radiation and analyzed by a flow cytometry [24].
Caspase activation assay
The activity of caspase-3, −9, or −8 was analyzed using a fluorogenic caspase assay with Z-DEVD-AFC, Ac-LEHDAFC (Calbiochem), or Ac-IETD-AFC (BD Pharmingen) as a substrate, respectively [27]. The results are expressed as fold changes compared with the control.
Immunoprecipitation
SK-BR3 cells were transiently transfected with pEBB-HA-XIAP. About 48 h later, cells were pretreated with SM164 for 2 h, followed by radiation. Cells were lysed 24 h post-radiation in a lysis buffer containing freshly added protease inhibitor tablet [24]. XIAP was pulled down using beads-conjugated anti-HA antibody, and bound active caspase-9 was detected by Western blotting using anti-caspase-9 antibody.
Statistical analysis
ANOVA were used with SPSS (Statistical Product and Service Solution) software for statistical comparisons involving multiple groups, followed by SNK post hoc test to determine significance of each two group (P < 0.05). Paired or unpaired two-tailed Student t test was performed in comparison between two groups, using SAS software.
Results
Expression of family members of IAPs among breast cancer cell lines
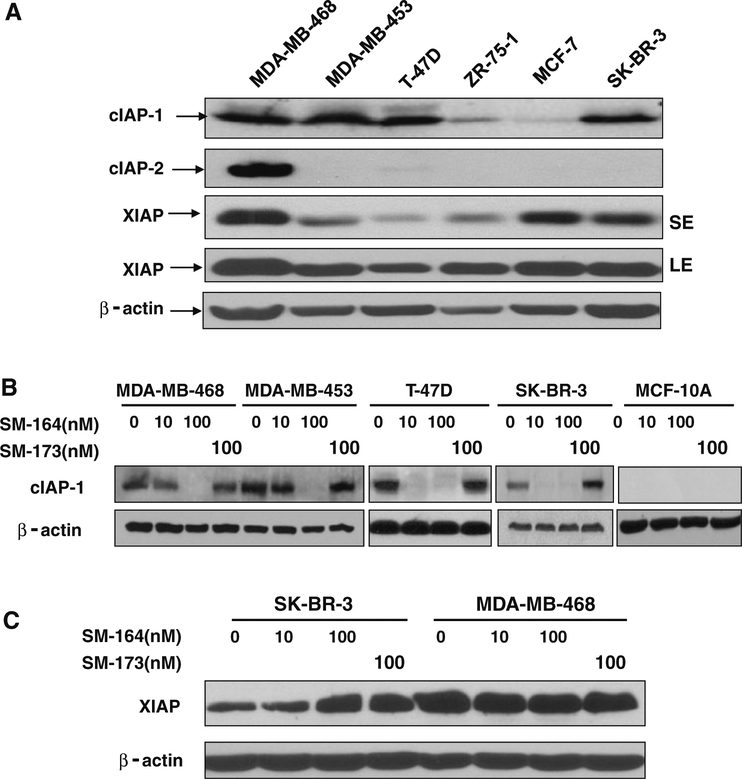
To determine if IAPs are potential radiosensitizing targets for breast cancer cells, we measured the levels of three most common family members, cIAP-1, cIAP-2, and XIAP, in six breast cancer cell lines by Western blotting. As shown in Fig. 1a, the levels of cIAP-1 varied among different lines with a high expression in four lines and low expression in other two. While cIAP-2 expression was only detectable in MDA-MB-468 cells, XIAP expression varies with three lines expressing relatively higher levels than other three. Among all six lines tested, MDA-MB468 was the only line having a high expression of all three family members, whereas SK-BR3 line had a high expression of both cIAP-1 and XIAP.
Fig. 1.
Expression of IAPs in breast cancer cells and cIAP-1 degradation by SM-164. a The levels of IAPs in breast cancer lines: Subconfluent cells were lysed and subjected to Western blotting analysis using antibodies against cIAP-1, cIAP-2 and XIAP. b and c Dose-dependent degradation of cIAP-1, but not XIAP: Cells were treated with SM-164, along with its inactive analogue, SM-173 at indicated concentrations for 1 h. Cells were then harvested and lysed for Western blotting with indicated Abs (SE short exposure, LE longer exposure)
SM-164 was shown to be a potent inducer of cIAP-1 degradation in HNSCC cells [27]. We next determined the sensitivity of four breast cancer lines (MDA-MB-468, MDA-MB-453, T47D, and SK-BR-3) with high expression of cIAP-1 to SM-164-mediated degradation. As shown in Fig. 1b, treatment of cells with SM-164 at 10 (in case of T-47D and SK-BR-3) or 100 nM (in case of MDA-MB-468 and MDA-MB-453) for 1 h caused complete elimination of cIAP1, whereas SM-173, a structural inactive analogue [26], was completely inactive at 100 nM. SM-164 had no effects on XIAP levels (Fig 1c and data not shown). We also included MCF10A, an immortalized breast epithelial cells [32] as a normal control, and found that cIAP-1 was not expressed in this line. The results indicate that SM-164 induces rapid degradation of cIAP-1 at low nanomolar concentrations in breast cancer cells.
Sensitivity of breast cancer cells to SM-164 as a single agent or in combination with radiation
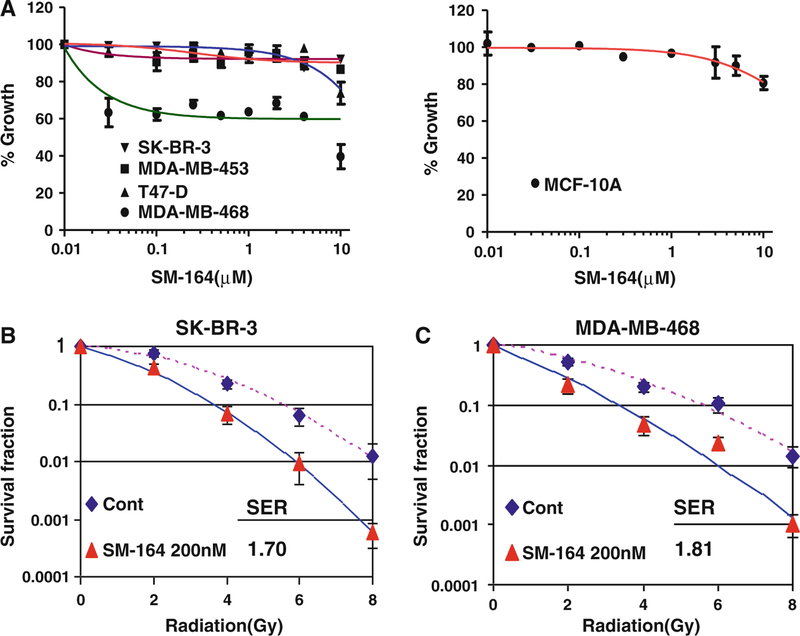
We next determined the sensitivity of these four breast cancer lines, along with MCF10A cells, to SM-164 at various drug concentrations up to 10 μM for 24 h treatment, using an ATP-lite cell proliferation assay. As shown in Fig. 2a, three lines (SK-BR3, MDA-MB-453, and T47D), along with MCF10A cells, are resistant to SM-164 as a single agent with only 10–15% growth inhibition at 10 μM SM-164. In contrast, MDA-MB-468 cells are relatively sensitive to SM-164 with 60% growth inhibition after exposure to 10 μM SM-164 for 24 h. Thus, most breast cancer cell lines, along with one immortalized line, are resistant to SM-164 as a single agent. Furthermore, it appears that cellular sensitivity to SM-164 as a single agent is not determined by the levels of cIAP-1, given the fact cIAP-1 was degraded by SM-164 in all tested breast cancer cells regardless of their drug sensitivity.
Fig. 2.
SM-164 radiosensitizes breast cancer cells. a Sensitivity of breast cancer lines to SM-164: Four lines of breast cancer cells (left panel) and MCF10A cells (right panel) were seeded in 96-well plate in triplicate and treated with various concentrations of SM-164 for 24 h. Cells were then lysed and subjected to ATP-lite assay for cell viability. Shown is mean ± SD (n = 3). b and c Radiosensitization by SM-164 in SK-BR-3 cells (b) and MDA-MB-468 cells (c): Cells were seeded in 60-mm dishes and 6 h later treated with SM-164 (200 nM) for 24 h, followed by exposure to radiation at incremental doses up to 8 Gy. SER was calculated as the ratio of the mean inactivation dose under untreated control conditions divided by the mean inactivation dose with SM-164 treatment. Shown is mean ± SEM from three independent experiments, each runs in duplicate
We then chose one resistant line (SK-BR3) and one sensitive line (MDA-MB-468) to determine potential radiosensitization activity of SM-164, given the fact that both lines had (1) high levels of cIAP-1 and XIAP, and (2) formed countable colonies in standard clonogenic assay. MCF10A line was not included because of its inability to form the colonies from single cell. Cells were treated with SM-164 alone at 200 nM, which is sufficient to degrade cIAP-1, or in combination with different doses of radiation. As shown in Fig. 2b and c, SM-164 induced a remarkable radiosensitzation with a SER (sensitization enhancement ratio) of 1.7 and 1.8 in both SM-164-resistant SK-BR3 and SM-164-sensitive MDA-MB-468 cells, respectively.
Radiosensitization by SM-164 is associated with enhanced apoptosis
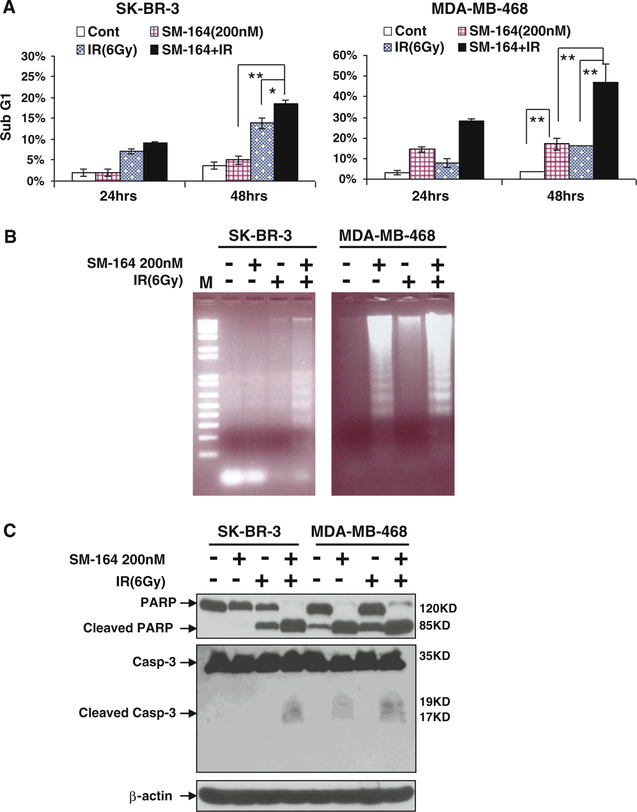
To determine the nature of SM-164 radiosensitization, we treated SK-BR-3 and MDA-MB-468 cells with SM-164 (200 nM) and radiation (6 Gy), alone and in combination, for 24 and 48 h, followed by FACS analysis to detect sub-G1 apoptotic population. In SM-164-resistant SK-BR-3 cells, SM-164 treatment had little effect on apoptotic sub-G1 population, whereas radiation exposure alone or in combination with SM-164 caused a moderate increase in apoptotic cells at 24 h, but a substantially increase at 48 h. The difference between groups of combinational and radiation alone treatment was statistically significant (P < 0.05) (Fig. 3a, left panel). In contrast, in sensitive MDA-MB-468 cells, single agent treatment with SM-164, but not with radiation for 24 h, caused a significant induction of apoptosis (P < 0.01), which was further enhanced when SM-164 was combined with radiation (P < 0.01). At 48 h, combinational treatment had a synergetic effect on apoptosis induction (P < 0.01) (Fig. 3a, right panel).
Fig. 3.
SM-164 induces apoptosis in breast cancer cells alone or in combination with radiation. a FACS profiling: Cells were treated with SM-164 or radiation (6 Gy) alone or in combination (SM-164 pretreatment for 6 h, followed by radiation exposure). Cells were then harvested and analyzed by FACS profiling for apoptosis (sub-G1 population) at 24 or 48 h post-radiation, respectively. Shown is mean ± SEM from three independent experiments (*P < 0.05; **P < 0.01). b Induction of DNA fragmentation: Cells were treated as described above. Both detached and attached cells were harvested by scraping at 24 h (MDA-MB-468) or 48 h (SK-BR-3) post-radiation, respectively, for DNA fragmentation assay. A 1-kb plus size marker (M) is shown. c Cleavage of PARP and Caspase-3: Cells were treated as described above and harvested at 24 h (MDA-MB-468) or 48 h (SK-BR-3) post-radiation for Western blotting analysis using antibodies against PARP and caspase-3 with β-Actin as a loading control
To further confirm that SM-164 radiosensitization was associated with apoptosis induction, we measured three well-established biochemical markers of apoptosis. First, the assay for DNA fragmentation, the hall-marker of apoptosis, revealed that in SM-164-resistant SK-BR3 cells, the 180-bp DNA ladders were only obviously seen in SM-164/radiation combinational treatment group, whereas in SM-164-sensitive MDA-MB-468 cells, single treatment with SM-164, but not with radiation, caused the substantial DNA fragmentation, which was further enhanced by radiation (Fig. 3b). Consistently, other two biochemical assays of apoptosis, PARP cleavage and caspase-3 activation/cleavage, also demonstrated a maximal induction of cleaved PARP and caspase-3 by combinational treatment in both lines (Fig. 3c). These results strongly suggested that SM-164 radiosensitization is associated with enhanced apoptosis with potential involvement of caspase activation.
SM-164 radiosensitization is associated with caspase activation in breast cancer cells
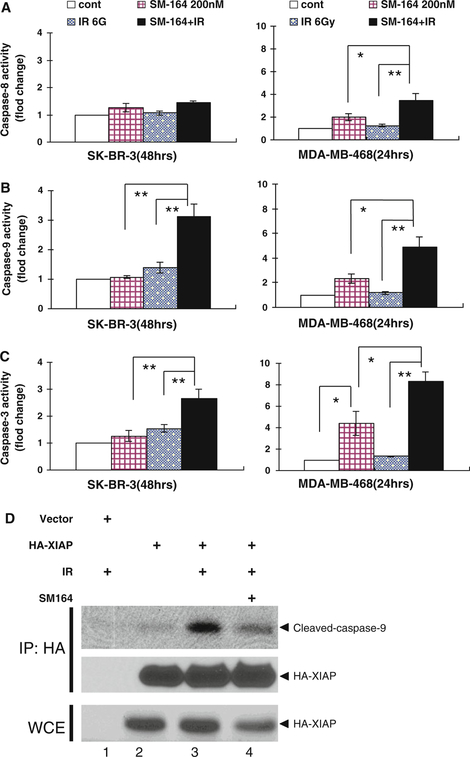
Because the mechanism of SM-164 action is to promote cIAP-1 degradation and to disrupt XIAP binding to active caspases [26], and we have observed caspase-3 activation on combinational treatment (Fig. 3c), we reasoned that SM-164 radiosensitization would be associated with caspase activation in breast cancer cells. We, therefore, determined a direct involvement of caspase activation in SM-164 radiosensitization. Cells were treated with SM-164, radiation alone or in combination and activity of caspase-3, −8, and −9 was measured. As shown in Fig. 4a–c, in resistant SK-BR-3 cells, single treatment with SM-164 or radiation caused a minor, if any, activation of caspase-9 and −3, whereas combinational treatment significantly activated both caspase-9 and −3 up to 3-fold (P < 0.01). Neither single nor combinational treatment has any measurable effect on caspase-8 activation. In contract, in sensitive MDA-MB-468 cells, single treatment with SM-164, but not with radiation, caused a 2-, 2.3-, and 4.4-fold activation of caspases-8, −9, and −3, respectively. Significantly, combinational treatment caused a significant increase of active caspases with an up to 4-, 5-, and 8-fold activation of caspases-8, −9, and −3, respectively (P < 0.05 or P < 0.01). Taken together, these results suggest that SM-164 radiosensitization in SK-BR-3 cells is mediated by intrinsic mitochondrial apoptosis pathway, whereas in MDA-MB-468 cells, it is mediated by both extrinsic death receptor and intrinsic mitochondrial apoptosis pathways.
Fig. 4.
SM-164 activates caspases alone or in combination with radiation (a–c) Cells were treated with SM-164 or radiation (6 Gy) alone or in combination (SM-164 pretreatment for 6 h, followed by radiation exposure). Cells were then harvested and analyzed by ELISA-based caspase activity assay for caspase-8 (a), −9 (b) and −3(c) at 24 h (MDA-MB-468) or 48 h (SK-BR-3) post-radiation, respectively. Shown are mean ± SEM from three independent experiments (*P < 0.05; **P < 0.01). d SM-164 disrupts the binding between XIAP and active caspase-9: SK-BR-3 cells were transiently transfected with HA-XIAP, followed by SM-164 (200 nM) pretreatment for 2 h and then radiation (6 Gy). Cells were harvested 24 h post-radiation. Cell lysates (~2 mg protein) were immunoprecipitated with beads-conjugated HA-antibody and bound active caspase-9 was detected by Western blotting using anti-caspase-9 antibody. Whole cell extract (WCE) was subjected to direct Western blotting using anti-HA antibody
SM-164 abrogates the binding between XIAP and active caspase-9
Because mitochondrial apoptosis pathway is involved in SM-164 radiosensitization, we reasoned that this pathway is likely activated through SM-164 disruption of XIAP-caspase-9 binding, as previously shown in a cell-free biochemical assay [26]. To test this hypothesis, we transiently transfected HA-tagged XIAP into SK-BR-3 cells, followed by treatment with SM-164 or radiation, alone, or in combination. Ectopically expressed XIAP was then pulled down by immunoprecipitation with beads-conjugated HA antibody and XIAP-bound active caspase-9 was detected by anti-capase-9 antibody. As shown in Fig. 4d, a basal very low level of active caspase-9 was found to bind to XIAP (lane 2). The amount of XIAP-bound active caspase-9 was significantly increased on radiation exposure (lane 3), which was remarkably inhibited by SM-164 (lane 4). Thus, SM-164 radiosensitization in SK-BR-3 cells is mediated by releasing XIAP from its binding to active caspase-9, leading to subsequent caspase-3 activation and apoptosis induction.
Blockage of caspase activation largely abrogated SM-164 enhancement of apoptosis and radiosensitization
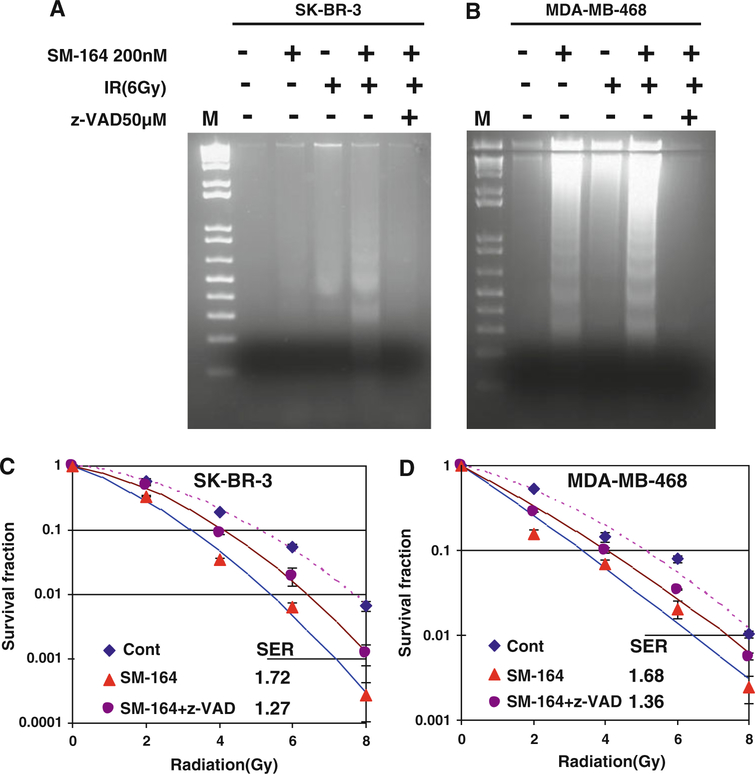
Finally, we tested if caspase activation, which leads to apoptosis induction, is the main cause for SM-164 radiosensitization. We used two approaches to inactivate caspases by (a) z-VAD-fmk, a pan caspase inhibitor and (b) siRNA silencing/knockdown. As shown in Fig. 5a and b, z-VAD-fmk treatment completely blocked apoptotic DNA fragmentation induced by combination of SM-164 and radiation in both SK-BR3 and MDA-MB-468 cells. Consequently, z-VAD-fmk largely abrogated SM-164 radiosensitization with SER reduction from 1.72 to 1.27 in SK-BR3 cells and 1.68–1.36 in MDA-MB-468 cells, both are statistically significant (P < 0.05) (Fig. 5c, d).
Fig. 5.
Pan-caspase inhibitor, z-VAD inhibits apoptosis and SM-164 radiosensitization: a z-VAD inhibits DNA fragmentation induced by SM-164 and radiation: Cells were treated with SM-164 or radiation, alone or in combination (6 h SM-164 treatment before radiation) in the absence or presence of z-VAD-fmk (50 μM, 2 h before SM-164 treatment and 8 h before radiation). Both detached and attached cells were harvested by scraping at 24 h (MDA-MB-468) and 48 h (SKBR-3) post-radiation for DNA fragmentation. b z-VAD largely abrogates SM-164 radiosensitization: Cells were treated with SM-164 for 24 h or in combination with z-VAD-fmk (30 μM, added 2 h before SM-164 treatment), followed by exposure to different doses of radiation and standard clonogenic survival assay. Shown are mean ± SEM from two independent experiments, each runs in duplicate
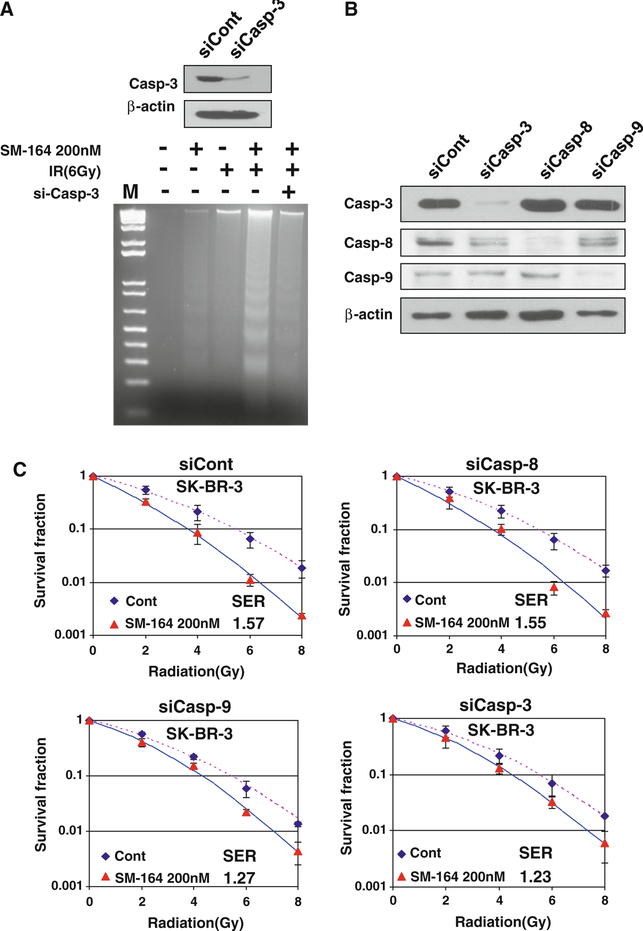
We next used siRNA silencing/knockdown approach in SK-BR3 cells and found that caspase-3 knockdown completely inhibited apoptotic DNA fragmentation (Fig. 6a). Consistently, siRNA knockdown of caspase-9 and caspase-3 (Fig. 6b) significantly abrogated SM-164 radiosensitization with SER reduction from 1.57 to 1.27 or 1.23, respectively, (P < 0.05) (Fig. 6c), whereas caspase-8 knockdown (Fig. 6b) had no effect with SER change from 1.57 to 1.55, as expected (Fig. 6c). Taken together, these results indicated that SM-164 radiosensitization is causally related to activation of caspases and induction of apoptosis.
Fig. 6.
siRNA silencing of caspases inhibits apoptosis and SM-164 radiosensitization: a Caspase-3 silencing inhibits DNA fragmentation induced by SM-164 and radiation: SK-BR-3 cells were transfected with siRNA oligonucleotide targeting caspase-3, along with scrambled control siRNA (50 nM). Forty-eight hours post-transfection, one portion of cells was subjected to Western blotting analysis (top) and the second portion was used for DNA fragmentation. b, c siRNA silencing of caspases largely abrogates SM-164 radiosensitization: SK-BR-3 cells were transfected with siRNA oligonucleotides, targeting caspase-3, −8 or −9, along with scrambled control. Forty-eight hours post-transfection, one portion of cells was subjected to Western blotting (b) and the other portion was subjected for standard clonogenic assay (c) after exposure to various dose of radiation in combination with SM-164 (200 nM). Shown is mean ± SEM from two independent experiments, each runs in duplicate
Discussion
Discovery of mechanism-based anticancer cancer drugs relies on thorough validation of potential cancer targets. Targeting apoptosis signaling pathways have attracted much attention in past decades with few promising agents advanced to the phase I/II clinical trials [33–35]. One appealing target among the targets of apoptosis pathways is the IAPs family, which binds to both an initiator caspase (caspase-9) and effector caspases (caspase-3 and −7) to block caspase-dependent apoptosis. Thus, targeting these negative blockers of caspases triggers apoptosis and sensitizes cancer cells to conventional chemo and radiation therapies [36].
Our previous study revealed that most breast cancer cell lines are resistant to a SMAC mimetic compound [16] as a single agent, which sensitized sensitive cells much more effectively than resistant cells to cell killing induced by TRAIL or etoposide [24]. In this study, we showed that most breast cancer cell lines are also resistant to SM-164, a potent inhibitor of IAPs [26], as a single agent, but SM-164 acts as a radiosensitizing agent equally effective in both sensitive MD-MBA-468 cells and resistant SK-BR-3 cells, suggesting that SM-164 is a potent radiosensitizer in breast cancer cells. It is well established that in vertebrate cells, apoptosis typically proceeds through one of two signaling cascades termed the intrinsic mitochondrial and extrinsic death receptor pathways, both of which converge on activating the executioner caspases, caspase-3, and caspase-7 [37, 38]. Our study revealed mechanistically that cellular sensitivity to SM-164 as a single agent is attributable to its intrinsic sensitivity to caspase-8 activation, followed by activation of extrinsic apoptosis pathway when cIAP is degraded by SM-164. On the other hand, SM-164 radiosensitization in resistant cells is mainly mediated by activation of intrinsic apoptosis pathway because of disruption of XIAP-casapse-9 binding, leading to full activation of caspase-9.
We elucidated that the underlying mechanism of SM-164 radiosensitization is mainly through activation of caspases and induction of apoptosis. This conclusion was strongly supported by the functional rescue experiments in which blockage of apoptosis through inactivation of caspases using pharmaceutical (z-VAD) or molecular biology (siRNA) approaches largely abrogates SM-164 radiosensitization. Thus, induction of apoptosis through caspase activation plays a key role in radiosensitization by SM-164 in breast cancer cells. These results are consistent with our recent finding of SM-164 radiosensitization in head and neck squamous carcinoma cells, which is dependent on intrinsic sensitivity to caspase activation and apoptosis induction [27].
In summary, we report here that SM-164 is a potent and novel class of radiosensitizers for breast cancer cells, regardless their sensitivity to SM-164 as a single agent. SM-164 radiosensitization is mainly mediated by caspase activation through removal of negative blockers cIAP-1 (through degradation) and XIAP (through disrupting its inhibitory binding to active caspase-9), leading to induction of apoptosis. Our study suggests that breast cancer patients might benefit from SM-164-radiation combinational therapy and provides the proof-of-concept for future development of SM-164 or other SMAC mimetic compounds as a novel class of radiosensitizing drugs against breast cancer cells.
Acknowledgments
We would like to thank Dr. John Silke at La Trobe University, Australia for anti-cIAP-1 antibody and Dr. Colin Duckett at University of Michigan for HA-XIAP plasmid. This work is supported by the National Cancer Institute (NCI) grants (CA111554, CA118762, and CA156744) to Yi Sun and the Nanchang University Scholarship Fund in China to Dong Yang. Dr. Shaomeng Wang is associated with Ascenta as a consultant and stock holder. He also receives funding from Ascenta
Contributor Information
Shaomeng Wang, Department of Internal Medicine, University of Michigan, 4424B Medical Science I, 1301 Catherine Street, Ann Arbor, MI 48109-5637, USA.
Gongxian Wang, Department of Urology, the First Affiliated Hospital of Nanchang University, 17 Yongwaizhen Road, Nanchang 330006, China.
Yi Sun, Division of Radiation and Cancer Biology, Department of Radiation Oncology, University of Michigan, 4424B Medical Science I, 1301 Catherine Street, Ann Arbor, MI 48109-5637, USA.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D (2011) Global cancer statistics. CA Cancer J Clin 61:69–90 [DOI] [PubMed] [Google Scholar]
- 2.Malmstrom P, Holmberg L, Anderson H, Mattsson J, Jonsson PE, Tennvall-Nittby L, Balldin G, Loven L, Svensson JH, Ingvar C, Moller T, Holmberg E, Wallgren A (2003) Breast conservation surgery, with and without radiotherapy, in women with lymph node-negative breast cancer: a randomised clinical trial in a population with access to public mammography screening. Eur J Cancer 39:1690–1697 [DOI] [PubMed] [Google Scholar]
- 3.Jameel JK, Rao VS, Cawkwell L, Drew PJ (2004) Radioresistance in carcinoma of the breast. Breast 13:452–460 [DOI] [PubMed] [Google Scholar]
- 4.Gee JM, Nicholson RI (2003) Expanding the therapeutic repertoire of epidermal growth factor receptor blockade: radiosensitization. Breast Cancer Res 5:126–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jorgensen TJ (2009) Enhancing radiosensitivity: targeting the DNA repair pathways. Cancer Biol Ther 8:665–670 [DOI] [PubMed] [Google Scholar]
- 6.Sartor CI (2000) Biological modifiers as potential radiosensitizers: targeting the epidermal growth factor receptor family. Semin Oncol 27:15–20 Discussion: 92–100 [PubMed] [Google Scholar]
- 7.Deveraux QL, Reed JC (1999) IAP family proteins–suppressors of apoptosis. Genes Dev 13:239–252 [DOI] [PubMed] [Google Scholar]
- 8.Shi Y (2004) Caspase activation, inhibition, and reactivation: a mechanistic view. Protein Sci 13:1979–1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33–42 [DOI] [PubMed] [Google Scholar]
- 10.Verhagen AM, Ekert PG, Pakusch M, Silke J, Connolly LM, Reid GE, Moritz RL, Simpson RJ, Vaux DL (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43–53 [DOI] [PubMed] [Google Scholar]
- 11.Chai J, Du C, Wu JW, Kyin S, Wang X, Shi Y (2000) Structural and biochemical basis of apoptotic activation by Smac/DIABLO. Nature 406:855–862 [DOI] [PubMed] [Google Scholar]
- 12.Hu S, Yang X (2003) Cellular inhibitor of apoptosis 1 and 2 are ubiquitin ligases for the apoptosis inducer Smac/DIABLO. J Biol Chem 278:10055–10060 [DOI] [PubMed] [Google Scholar]
- 13.Wu G, Chai J, Suber TL, Wu JW, Du C, Wang X, Shi Y (2000) Structural basis of IAP recognition by Smac/DIABLO. Nature 408:1008–1012 [DOI] [PubMed] [Google Scholar]
- 14.Arnt CR, Chiorean MV, Heldebrant MP, Gores GJ, Kaufmann SH (2002) Synthetic Smac/DIABLO peptides enhance the effects of chemotherapeutic agents by binding XIAP and cIAP1 in situ. J Biol Chem 277:44236–44243 [DOI] [PubMed] [Google Scholar]
- 15.Giagkousiklidis S, Vogler M, Westhoff MA, Kasperczyk H, Debatin KM, Fulda S (2005) Sensitization for gamma-irradiation-induced apoptosis by second mitochondria-derived activator of caspase. Cancer Res 65:10502–10513 [DOI] [PubMed] [Google Scholar]
- 16.Li L, Thomas RM, Suzuki H, De Brabander JK, Wang X, Harran PG (2004) A small molecule Smac mimic potentiates TRAIL-and TNFalpha-mediated cell death. Science 305:1471–1474 [DOI] [PubMed] [Google Scholar]
- 17.Sun H, Nikolovska-Coleska Z, Lu J, Meagher JL, Yang CY, Qiu S, Tomita Y, Ueda Y, Jiang S, Krajewski K, Roller PP, Stuckey JA, Wang S (2007) Design, synthesis, and characterization of a potent, nonpeptide, cell-permeable, bivalent Smac mimetic that concurrently targets both the BIR2 and BIR3 domains in XIAP. J Am Chem Soc 129:15279–15294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zobel K, Wang L, Varfolomeev E, Franklin MC, Elliott LO, Wallweber HJ, Okawa DC, Flygare JA, Vucic D, Fairbrother WJ, Deshayes K (2006) Design, synthesis, and biological activity of a potent Smac mimetic that sensitizes cancer cells to apoptosis by antagonizing IAPs. ACS Chem Biol 1:525–533 [DOI] [PubMed] [Google Scholar]
- 19.Chauhan D, Neri P, Velankar M, Podar K, Hideshima T, Fulciniti M, Tassone P, Raje N, Mitsiades C, Mitsiades N, Richardson P, Zawel L, Tran M, Munshi N, Anderson KC (2007) Targeting mitochondrial factor Smac/DIABLO as therapy for multiple myeloma (MM). Blood 109:1220–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glover CJ, Hite K, DeLosh R, Scudiero DA, Fivash MJ, Smith LR, Fisher RJ, Wu JW, Shi Y, Kipp RA, McLendon GL, Sausville EA, Shoemaker RH (2003) A high-throughput screen for identification of molecular mimics of Smac/DIABLO utilizing a fluorescence polarization assay. Anal Biochem 320:157–169 [DOI] [PubMed] [Google Scholar]
- 21.Mizukawa K, Kawamura A, Sasayama T, Tanaka K, Kamei M, Sasaki M, Kohmura E (2006) Synthetic Smac peptide enhances the effect of etoposide-induced apoptosis in human glioblastoma cell lines. J Neurooncol 77(3):247–255 [DOI] [PubMed] [Google Scholar]
- 22.Wilkinson JC, Wilkinson AS, Scott FL, Csomos RA, Salvesen GS, Duckett CS (2004) Neutralization of Smac/Diablo by inhibitors of apoptosis (IAPs). A caspase-independent mechanism for apoptotic inhibition. J Biol Chem 279:51082–51090 [DOI] [PubMed] [Google Scholar]
- 23.Wu TY, Wagner KW, Bursulaya B, Schultz PG, Deveraux QL (2003) Development and characterization of nonpeptidic small molecule inhibitors of the XIAP/caspase-3 interaction. Chem Biol 10:759–767 [DOI] [PubMed] [Google Scholar]
- 24.Bockbrader KM, Tan M, Sun Y (2005) A small molecule Smac-mimic compound induces apoptosis and sensitizes TRAIL- and etoposide-induced apoptosis in breast cancer cells. Oncogene 24:7381–7388 [DOI] [PubMed] [Google Scholar]
- 25.Chen DJ, Huerta S (2009) Smac mimetics as new cancer therapeutics. Anticancer Drugs 20:646–658 [DOI] [PubMed] [Google Scholar]
- 26.Lu J, Bai L, Sun H, Nikolovska-Coleska Z, McEachern D, Qiu S, Miller RS, Yi H, Shangary S, Sun Y, Meagher JL, Stuckey JA, Wang S (2008) SM-164: a novel, bivalent Smac mimetic that induces apoptosis and tumor regression by concurrent removal of the blockade of cIAP-1/2 and XIAP. Cancer Res 68:9384–9393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yang J, McEachern D, Li W, Davis MA, Li H, Morgan MA, Bai L, Sebolt JT, Sun H, Lawrence TS, Wang S, Sun Y (2011) Radiosensitization of head and neck squamous cell carcinoma by a SMAC-mimetic compound, SM-164, requires activation of caspases. Mol Cancer Ther 10:658–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nikolovska-Coleska Z, Wang R, Fang X, Pan H, Tomita Y, Li P, Roller PP, Krajewski K, Saito NG, Stuckey JA, Wang S (2004) Development and optimization of a binding assay for the XIAP BIR3 domain using fluorescence polarization. Anal Biochem 332:261–273 [DOI] [PubMed] [Google Scholar]
- 29.Fertil B, Dertinger H, Courdi A, Malaise EP (1984) Mean inactivation dose: a useful concept for intercomparison of human cell survival curves. Radiat Res 99:73–84 [PubMed] [Google Scholar]
- 30.Vince JE, Wong WW, Khan N, Feltham R, Chau D, Ahmed AU, Benetatos CA, Chunduru SK, Condon SM, McKinlay M, Brink R, Leverkus M, Tergaonkar V, Schneider P, Callus BA, Koentgen F, Vaux DL, Silke J (2007) IAP antagonists target cIAP1 to induce TNFalpha-dependent apoptosis. Cell 131:682–693 [DOI] [PubMed] [Google Scholar]
- 31.Zheng M, Morgan-Lappe SE, Yang J, Bockbrader KM, Pamarthy D, Thomas D, Fesik SW, Sun Y (2008) Growth inhibition and radiosensitization of glioblastoma and lung cancer cells by small interfering RNA silencing of tumor necrosis factor receptor-associated factor 2. Cancer Res 68:7570–7578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Swaroop M, Sun Y (2003) Mdm2 ligase dead mutants did not act in a dominant negative manner to re-activate p53, but promoted tumor cell growth. Anticancer Res 23:3167–3174 [PubMed] [Google Scholar]
- 33.Fesik SW (2005) Promoting apoptosis as a strategy for cancer drug discovery. Nat Rev Cancer 5:876–885 [DOI] [PubMed] [Google Scholar]
- 34.Schlotter CM, Vogt U, Allgayer H, Brandt B (2008) Molecular targeted therapies for breast cancer treatment. Breast Cancer Res 10:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Han SW, Roman J (2010) Targeting apoptotic signaling pathways in human lung cancer. Curr Cancer Drug Targets 10:566–574 [DOI] [PubMed] [Google Scholar]
- 36.Straub CS (2011) Targeting IAPs as an approach to anti-cancer therapy. Curr Top Med Chem 11:291–316 [DOI] [PubMed] [Google Scholar]
- 37.Hengartner MO (2000) The biochemistry of apoptosis. Nature 407:770–776 [DOI] [PubMed] [Google Scholar]
- 38.Tait SW, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11:621–632 [DOI] [PubMed] [Google Scholar]