Abstract
Purpose
Hyperuricemia is an important potential pathogenic factor for hypertension, cardiovascular disease and stroke. The current study aimed to investigate the prevalence of hyperuricemia and its relationship to lifestyle characteristics and dietary habits in centenarians and near-centenarians.
Patients and methods
In total, 966 centenarians and 788 near-centenarians were included. Community-based surveys were conducted to collect information about lifestyle. Blood examinations were performed using enzymatic assays. T-tests and χ2 tests were used to investigate significant indicators of hyperuricemia, and multivariate logistic regression was used to analyze the related risk factors. A comprehensive analysis of nineteen modifiable factors, including lifestyle characteristics, dietary habits, general characteristics and blood test indexes, was conducted.
Results
The prevalence of hyperuricemia was 29.02%. The percentage of men, waist circumference (WC), waist-hip ratio, estimated glomerular filtration rate (eGFR), levels of total protein (TP), alanine aminotransferase, aspartate aminotransferase, triglycerides, high-density lipoprotein cholesterol, serum homocysteine, serum uric acid, serum urea and serum creatinine, passive smoking, alcohol consumption, snoring, preference for fried flavors, and meat, seafood and vegetable consumption were significantly different between the hyperuricemia group and the normouricemia group (p<0.05). Multivariate logistic regression analysis showed that WC (OR=1.020), eGFR (OR=0.960), TP level (OR=1.038), serum urea level (OR=1.154), passive smoking (OR=2.589), snoring (OR=2.003), meat consumption (OR=2.506), seafood consumption (OR=1.422) and vegetable consumption (OR=0.521) were significantly associated with the risk of hyperuricemia (p<0.05).
Conclusion
Low eGFR and vegetable consumption, high WC, TP, and serum urea levels, passive smoking, snoring, and high meat and seafood consumption were independent risk factors for hyperuricemia. It is recommended that people at high risk for hyperuricemia should actively limit their intake of fried food, alcohol and purine-rich food, increase their intake of fresh vegetables, actively treat sleep apnea syndrome, avoid passive smoking, maintain a healthy WC and seek to improve their kidney and liver function.
Keywords: centenarians, hyperuricemia, lifestyle, dietary, risk factors
Introduction
Hyperuricemia is a disease in which purine metabolism is abnormal for various reasons, resulting in an increase in uric acid concentration in the blood.1 With improvements in living standards and changes in dietary habits, the prevalence of hyperuricemia has increased annually.2 The 2007–2008 National Health and Nutrition Examination Survey (NHANES) found that the overall prevalence of hyperuricemia in the US population was 21.5%, an increase of 2.4% compared to the 1988–1994 NHANES-III results.3 Though hyperuricemia in the normal population does not cause clinically obvious symptoms, it is generally considered a precursor of gout and a potential pathogenic factor in metabolic syndrome, hypertension, diabetes, cardiovascular diseases and stroke.4,5 Therefore, it is important to identify patients who are at high risk of hyperuricemia in a timely manner and treat them, preventing subsequent related diseases and the consequential increased financial burden. Further analysis showed that the prevalence of hyperuricemia gradually increased with increasing age. The prevalence rate was 27.7% in the 60-year-old age group, 31.5% in the 70-year-old age group, and 36.8% in the 80-year-old age group.3 Thus, exploring the prevalence of hyperuricemia and associated risk factors in late life is very important, especially in centenarians.
Centenarians are a widely accepted model of successful aging and represent an extreme of human life expectancy, because they have reached the limit of the human life span. The study of centenarians has allowed us to identify and focus on some of the most interesting biological problems of aging and longevity. However, there have been no reports on the prevalence of hyperuricemia in Chinese centenarians. The China Hainan Centenarian Cohort Study (CHCCS) was conducted in Hainan Province, which has the highest density of centenarians in China. Given that only a few modifiable factors associated with hyperuricemia have been identified, this study conducted a comprehensive analysis of nineteen modifiable factors, including lifestyle characteristics, dietary habits, general characteristics and blood test indexes. Our study aimed to investigate the prevalence of hyperuricemia and identify the related risk factors to enable us to implement appropriate preventive and treatment measures for at-risk individuals.
Participants And Methods
Study Subjects
We used data from the CHCCS, which was one of the largest centenarian health interdisciplinary studies that was designed to investigate centenarians’ physical and mental health statuses and their social conditions.6 The researchers obtained ethical approval of the study protocol from the Ethics Committee of Hainan Branch of Chinese PLA General Hospital (serial no.: 301HNLL-2016-01). Participants received an extensive description of the study and signed an informed participation consent form that included permission to perform analyses on the biological specimens that were collected and stored. For those unable to fully consent because of cognitive or physical problems, surrogate consent was obtained from a close relative.
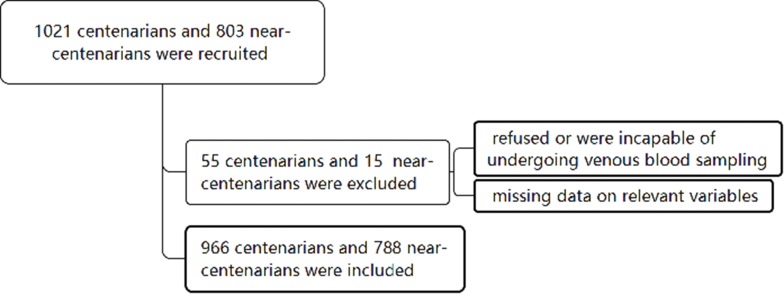
There were 1824 participants enrolled in the CHCCS, and 1754 of these had basic information and blood biochemical index data available. The details of how the participants were recruited and proceeded to medical tests are shown in Figure 1.
Figure 1.
Diagram of the study population.
Experimental Procedures
A community-based survey was conducted to collect demographic information (gender, age and ethnicity). Questionnaires were administered and blood samples were obtained from each participant. The ethnicities of the participants were classified as either Han or non-Han. Health-related variables such as standing height, weight, heart rate, systolic blood pressure (SBP), diastolic blood pressure (DBP), waist circumference (WC), and hip circumference were measured as described in He et al.6 Blood samples were obtained after the patients had fasted for 12 hrs and rested for at least 15 mins and were stored separately in refrigerated containers until they were analyzed on the same day. Blood biochemical examinations and blood routine examinations were performed using enzymatic assays (Roche Products Ltd., Basel, Switzerland) on a fully automatic biochemical autoanalyzer (Cobas 8000; Roche Products Ltd, Basel, Switzerland).
Smoking habits were categorized as current, former, and never. Passive smoke exposure, defined as living or working with smokers and therefore unintentionally inhaling smoke and various toxic substances, was categorized as yes (at least once per week for more than 1 year) or no.7 The consumption of alcohol, meat, seafood, vegetables and fruits were categorized into two groups, namely frequent (>3 times/week) and occasional/never (≤3 times/week). Late bedtimes were dichotomized as yes (used to going to sleep after 11 o’clock at night) or no. Information on whether the patient snores was provided by relatives (yes or no). Personal preferences were also recorded, including drinking habits (tea, coffee and other), taste preferences (salt, sweet, fried, spicy), a regular diet and noon break (yes and no) and eating habits (half full or full).
We defined hyperuricemia as serum uric acid (SUA) >416 μmol/L (7 mg/dL) for men and >357 μmol/L (6 mg/dL) for women.3 The estimated glomerular filtration rate (eGFR) was calculated according to the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation.8
Statistical Analysis
Normally distributed data were expressed as the mean ±standard deviation and were compared using unpaired Student’s t-tests. Nonnormally distributed data were expressed as medians with the corresponding 25th and 75th percentiles (interquartile range) and compared using Mann–Whitney U-tests. The χ2 test or Fisher’s exact test was used to compare categorical variables. Multivariate logistic regression analyses were used to determine the independent factors associated with hyperuricemia. P values less than 0.05 were considered statistically significant. The statistical analyses were performed using SPSS software (version 19.0 SPSS, Chicago IL, USA).
Results
Prevalence Of Hyperuricemia And General Characteristics Of Participants
The prevalence of hyperuricemia was 28.26% (32.57% in males and 27.31% in females) in centenarians and 29.95% (34.18% in males and 27.12% in females) in near-centenarians (Table 1). The overall prevalence of hyperuricemia was 29.02% (33.60% in males and 27.24% in females).
Table 1.
Participant Recruitment In CHCCS
| Centenarians | Near-Centenarians | Total | |
|---|---|---|---|
| Age inclusion criteria | Over 100 years old | 80~100 years old | —– |
| Recruited | 1021 | 803 | 1824 |
| Men: N (%) | 182 (17.83%) | 326 (40.60%) | 508 (27.85%) |
| Women: N (%) | 839 (82.17%) | 477 (59.40%) | 1316 (72.15%) |
| Both basic information and blood biochemical indexes are available | 966 | 788 | 1754 |
| Hyperuricemia (%) | 273 (28.26%) | 236 (29.95%) | 509 (29.02%) |
| Normouricemia (%) | 693 (71.74%) | 552 (70.05%) | 1245 (70.98%) |
| Men: N (%) | 175 | 316 | 491 |
| Hyperuricemia (%) | 57 (32.57%) | 108 (34.18%) | 165 (33.60%) |
| Normouricemia (%) | 118 (67.43%) | 208 (65.82%) | 326 (66.40%) |
| Women: N (%) | 791 | 472 | 1263 |
| Hyperuricemia (%) | 216 (27.31%) | 128 (27.12%) | 344 (27.24%) |
| Normouricemia (%) | 575 (72.69%) | 344 (72.88%) | 919 (72.76%) |
Abbreviation: CHCCS, The China Hainan Centenarian Cohort Study.
As shown in Table 2, most of the participants (88.6%) were of Han ethnicity. The percentage of men (p=0.008), WC (p<0.001) and waist-hip ratio (WHR) (p=0.010) were greater in the hyperuricemia group than in the normouricemia group. The eGFR was lower in the hyperuricemia group than in the normouricemia group (p<0.001). However, age, ethnicity, heart rate, body mass index (BMI), SBP, and DBP were not significantly different between the two groups (p=0.438, 0.324, 0.236, 0.134, 0.346 and 0.231, respectively).
Table 2.
Baseline Characteristics Of Participants By Serum Uric Acid Levels
| Total (n=1754) | Hyperuricemia (n=509) | Normouricemia (n=1245) | P-value | |
|---|---|---|---|---|
| Age (years) | 100 (85, 102) | 100 (85, 102) | 100 (85, 102) | 0.438 |
| Men (%) | 491 (28.0) | 165 (32.4) | 326 (26.2) | 0.008** |
| Ethnic Han (%) | 1554 (88.6) | 445 (87.4) | 1109 (89.1) | 0.324 |
| Heart rate (bpm) | 76 (70, 85) | 76 (69, 84) | 76 (70, 86) | 0.236 |
| BMI (kg/m2) | 18.2 (15.9, 20.3) | 18.4 (16.3, 20.7) | 18.1 (15.9, 20.3) | 0.134 |
| WC (cm) | 76.0 (70.0, 83.0) | 78.0 (72.3, 85.0) | 76.0 (70.0, 82.0) | <0.001*** |
| Waist-hip ratio | 0.88 (0.84, 0.93) | 0.89 (0.85, 0.93) | 0.88 (0.84, 0.92) | 0.010* |
| SBP (mmHg) | 148 (132, 168) | 149 (133, 170) | 148 (132, 167) | 0.346 |
| DBP (mmHg) | 76 (69, 85) | 76 (68, 84) | 76 (69, 85) | 0.231 |
| eGFR (mL/min/1.73m2) | 59.8 (47.6, 71.5) | 49.2±16.6 | 64.2 (52.9, 73.7) | <0.001*** |
Notes: *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: BMI, body mass index; WC, Waist circumference; SBP, Systolic blood pressure; DBP, Diastolic blood pressure; eGFR, estimated glomerular filtration rate.
Analyses Of Hyperuricemia With Blood Test Indexes
In the blood test results (Table 3), we found that the levels of total protein (TP) (p=0.001), alanine aminotransferase (ALT) (p=0.001), aspartate aminotransferase (AST) (p=0.048), triglycerides (p<0.001), serum homocysteine (p<0.001), SUA (p<0.001), serum urea (p<0.001) and serum creatinine (Scr) (p<0.001) were greater in the hyperuricemia group than in the normouricemia group. The high-density lipoprotein cholesterol (HDL-C) level was lower in the hyperuricemia group than in the normouricemia group (p<0.001). However, the levels of red blood cells, mean corpuscular volume, hemoglobin, albumin, fasting plasma glucose, total cholesterol and low-density lipoprotein cholesterol (LDL-C) levels were not significantly different between the two groups (p=0.828, 0.994, 0.655, 0.078, 0.707, 0.592 and 0.158, respectively).
Table 3.
Blood Test Indexes Of Participants By Serum Uric Acid Levels
| Total(n=1754) | Hyperuricemia (n=509) | Normouricemia (n=1245) | P-value | |
|---|---|---|---|---|
| RBC (10^12/L) | 4.2 (3.8, 4.6) | 4.2 (3.7, 4.7) | 4.2 (3.8, 4.5) | 0.828 |
| MCV (fl) | 120.0 (109.0, 131.0) | 119.0 (106.0, 133.0) | 120.0 (110.0, 130.0) | 0.994 |
| Hemoglobin (g/L) | 92.9 (87.8, 96.5) | 92.8 (88.0, 96.6) | 92.9 (87.6, 96.4) | 0.655 |
| Total protein (g/dL) | 70.3 (66.2, 74.2) | 70.9 (67.0, 75.0) | 70.0 (65.7, 73.9) | 0.001** |
| Albumin (g/dL) | 40.5 (37.5, 42.9) | 40.8 (37.8, 43.1) | 40.4 (37.4, 42.8) | 0.078 |
| FPG (mmol/L) | 4.6 (4.0, 5.5) | 4.7 (3.9, 5.7) | 4.6 (4.0, 5.5) | 0.707 |
| ALT (U/L) | 10.6 (8.1, 14.2) | 11.0 (8.3, 15.6) | 10.4 (8.0, 13.7) | 0.001** |
| AST (U/L) | 21.3 (18.3, 25.3) | 22.0 (18.5, 26.0) | 21.1 (18.3, 25.0) | 0.048* |
| TC (mmol/L) | 4.7 (4.2, 5.5) | 4.7 (4.2, 5.6) | 4.7 (4.2, 5.4) | 0.592 |
| TG (mmol/L) | 1.1 (0.8, 1.4) | 1.2 (0.9, 1.6) | 1.0 (0.8, 1.4) | <0.001*** |
| HDL-C (mmol/L) | 1.4 (1.1, 1.7) | 1.3 (1.1, 1.6) | 1.4 (1.2, 1.7) | <0.001*** |
| LDL-C (mmol/L) | 2.8 (2.3, 3.4) | 2.9 (2.4, 3.5) | 2.8 (2.3, 3.4) | 0.158 |
| Serum HCY (µmol/L) | 23.1 (18.5, 28.5) | 25.9 (21.3, 32.1) | 22.1 (17.8, 27.2) | <0.001*** |
| SUA (µmol/L) | 326.0 (266.0, 393.0) | 439.0 (395.5, 484.0) | 394.0 (247.0, 333.0) | <0.001*** |
| Serum urea (mmol/L) | 5.7 (4.5, 7.0) | 6.6 (5.2, 8.4) | 5.3 (4.3, 6.5) | <0.001*** |
| Scr (µmol/L) | 79.0 (65.0, 98.0) | 96.0 (78.0, 118.0) | 74.0 (62.0, 90.0) | <0.001*** |
Notes: *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: RBC, red blood cells; MCV, Mean corpuscular volume; FPG, Fasting plasma glucose; ALT, Alanine aminotransferase; AST, Aspartate aminotransferase; TC, Total cholesterol; TG, Triglycerides; HDL-C, High-density lipoprotein cholesterol; LDL-C, Low-density lipoprotein cholesterol; HCY, homocysteine; SUA, Serum uric acid; Scr, Serum creatinine.
Analyses Of Hyperuricemia With Lifestyle Characteristics And Dietary Habits
According to Table 4, we found that exposure to passive smoking (p=0.049), alcohol consumption (p<0.001), snoring (p<0.001), fried flavor preference (p=0.018), meat consumption (p=0.006), seafood consumption (p<0.001) and vegetable consumption (p=0.006) were significantly different between the hyperuricemia group and the normouricemia group. However, there were no significant differences between the two groups in terms of active smoking, staying up late, regular noon break, drinking habits, regular diet, eating habits, salty flavor preference, sweet flavor preference, spicy flavor preference and fruit consumption (all p>0.05).
Table 4.
Lifestyle Characteristics And Dietary Habits Of The Participants By Serum Uric Acid Levels
| Total (n=1754) | Hyperuricemia (n=509) | Normouricemia (n=1245) | P-value | |
|---|---|---|---|---|
| Active smoking (%) | 0.092 | |||
| Current | 2.5 | 3.6 | 2.1 | |
| Former | 7.9 | 6.6 | 8.5 | |
| Never | 89.5 | 89.8 | 89.5 | |
| Passive smoking (%) | 0.049* | |||
| Yes | 27.3 | 30.5 | 25.8 | |
| No | 72.7 | 69.5 | 74.2 | |
| Alcohol drinking (%) | <0.001*** | |||
| Frequent | 13.2 | 18.5 | 11.1 | |
| Occasional/never | 86.8 | 81.5 | 88.9 | |
| Stay up late (%) | 0.076 | |||
| Yes | 19.4 | 16.8 | 20.5 | |
| No | 80.6 | 83.2 | 79.5 | |
| Snoring (%) | <0.001*** | |||
| Yes | 22.4 | 31.3 | 18.8 | |
| No | 77.6 | 68.7 | 81.2 | |
| Regular noon break (%) | 0.626 | |||
| Yes | 83.0 | 82.3 | 83.3 | |
| No | 17.0 | 17.7 | 16.7 | |
| Drinking habits (%) | 0.126 | |||
| Tea | 1.8 | 2.6 | 1.5 | |
| Coffee | 1.4 | 0.8 | 1.7 | |
| Other | 96.7 | 96.6 | 96.8 | |
| Regular diet (%) | 0.342 | |||
| Yes | 94.7 | 93.9 | 95.0 | |
| No | 5.3 | 6.1 | 5.0 | |
| Eating habits (%) | 0.375 | |||
| Half full | 63.2 | 61.6 | 63.8 | |
| Full | 36.8 | 38.4 | 36.2 | |
| Salt flavor preference (%) | 0.516 | |||
| Yes | 13.6 | 14.5 | 13.3 | |
| No | 86.4 | 85.5 | 86.7 | |
| Sweet flavor preference (%) | 0.134 | |||
| Yes | 61.0 | 63.8 | 59.9 | |
| No | 39.0 | 36.2 | 40.1 | |
| Fried flavor preference (%) | 0.018* | |||
| Yes | 2.7 | 4.2 | 2.1 | |
| No | 97.3 | 95.8 | 97.9 | |
| Spicy flavor preference (%) | 0.419 | |||
| Yes | 2.9 | 3.4 | 2.7 | |
| No | 97.1 | 96.6 | 97.3 | |
| Meat consumption (%) | 0.006** | |||
| Frequent | 59.1 | 64.2 | 57.1 | |
| Occasional/never | 40.9 | 35.8 | 42.9 | |
| Seafood consumption (%) | <0.001*** | |||
| Frequent | 60.0 | 69.0 | 56.3 | |
| Occasional/never | 40.0 | 31.0 | 43.7 | |
| Vegetable consumption (%) | 0.006** | |||
| Frequent | 90.4 | 87.3 | 91.6 | |
| Occasional/never | 9.6 | 12.7 | 8.4 | |
| Fruit consumption (%) | 0.080 | |||
| Frequent | 39.3 | 36.1 | 40.6 | |
| Occasional/never | 60.7 | 63.9 | 59.4 |
Notes: *P<0.05, **P<0.01, ***P<0.001.
Multivariate Logistic Regression Analyses
We performed multivariate logistic regression and adjusted for all related factors including the percentage of men, WC, WHR, eGFR, TP, ALT, AST, triglyceride, HDL-C, serum homocysteine, serum urea, and Scr levels, exposure to passive smoking, alcohol consumption, snoring, fried flavor preference, meat consumption, seafood consumption and vegetable consumption (Table 5). Multivariate logistic regression analysis showed that WC (OR=1.020, 95% CI: 1.006–1.035), TP level (OR=1.038, CI: 1.014–1.062), serum urea level (OR=1.154, 95% CI: 1.079–1.234), passive smoking (OR=2.589, 95% CI: 1.841–3.642), snoring (OR=2.003, 95% CI: 1.339–2.995), meat consumption (OR=2.506, 95% CI: 1.746–3.598), and seafood consumption (OR=1.422, 95% CI: 1.052–1.922) were independent risk factors for hyperuricemia, while a high eGFR (OR=0.960, 95% CI: 0.940–0.981) and increased vegetable consumption (OR=0.521, 95% CI: 0.319–0.850) were protective factors against hyperuricemia.
Table 5.
Significant Factors Affecting Hyperuricemia Identified By Logistic Regression Analysis
| Variables | β | SE | Wald | OR | 95% CI | P-value |
|---|---|---|---|---|---|---|
| WC (cm) | 0.02 | 0.007 | 7.319 | 1.020 | 1.006–1.035 | 0.007** |
| eGFR (mL/min/1.73m2) | −0.041 | 0.011 | 14.234 | 0.960 | 0.940–0.981 | <0.001*** |
| Total protein (g/dL) | 0.037 | 0.012 | 9.938 | 1.038 | 1.014–1.062 | 0.002** |
| Serum urea (mmol/L) | 0.143 | 0.034 | 17.599 | 1.154 | 1.079–1.234 | <0.001*** |
| Passive smoking | 0.951 | 0.174 | 29.878 | 2.589 | 1.841–3.642 | <0.001*** |
| Snoring | 0.695 | 0.205 | 11.444 | 2.003 | 1.339–2.995 | 0.001** |
| Meat consumption | 0.919 | 0.184 | 24.819 | 2.506 | 1.746–3.598 | <0.001*** |
| Seafood consumption | 0.352 | 0.154 | 5.256 | 1.422 | 1.052–1.922 | 0.022* |
| Vegetable consumption | −0.653 | 0.25 | 6.801 | 0.521 | 0.319–0.850 | 0.009** |
Notes: *P<0.05, **P<0.01, ***P<0.001.
Abbreviations: WC, Waist circumference; eGFR, estimated glomerular filtration rate; β, logistic regression coefficient; SE, standard error; OR, odds ratio; CI, confidence interval.
Discussion
In recent years, the incidence of hyperuricemia has increased worldwide, making it a global public health issue.2 Our study is the first to report the prevalence of hyperuricemia in a population-based sample of centenarians in China. Furthermore, this study provided associations between the prevalence of hyperuricemia and lifestyle characteristics as well as dietary habits in this population.
According to our study, the prevalence rate of hyperuricemia was as high as 29.02%, indicating that hyperuricemia is highly prevalent in Chinese centenarians and near-centenarians. It has been reported that the prevalence of hyperuricemia in adults in China is 8.4%,9,10 and the prevalence of hyperuricemia in elderly individuals in China is 16.7%11 while in the US, it is 21.5%.3 The differences in the prevalence may be due to sex, age, race, culture and dietary habits. In addition, we found that men (33.60%) had a higher prevalence of hyperuricemia than women (27.24%) in our study. Similarly, a previous study found that the SUA level was higher in men than in women.11 Different levels of sex hormones in men and women may be the possible mechanism for this difference.11 Therefore, elderly individuals, especially women, should pay attention to the occurrence of hyperuricemia.
Hyperuricemia is caused by increased uric acid production or reduced uric acid discharge. Renal functional decline is an important cause of hyperuricemia due to reduced uric acid clearance.12 We found that the indicators of decreased renal function including eGFR, serum homocysteine, serum urea and Scr were significantly different between the hyperuricemia group and the normouricemia group. In addition, a low eGFR and a high serum urea level were independent risk factors for hyperuricemia. This suggests that people with low renal function should pay attention to monitoring SUA levels and preventing hyperuricemia.
A previous study revealed that hyperuricemia is closely related to disorders of lipid metabolism and dietary factors.13 We found that the WC, WHR and triglyceride level were greater but the HDL-C level was lower in the hyperuricemia group than in the normouricemia group. The logistic regression analyses showed that WC was an independent risk factor for hyperuricemia. Our study showed that hyperuricemia is associated with abnormal lipid metabolism and central obesity. The possible mechanism is that abnormal lipid metabolism may affect the afferent arteries and efferent arterioles, resulting in their stenosis or even occlusion and leading to a reduction in uric acid removal by the kidneys.14,15 This decreased SUA clearance in the body leads to a decrease in lipoprotein esterase activity, which in turn significantly reduces the decomposition of triacylglycerol, eventually leading to elevated levels of triacylglycerol and lipid metabolism disorders.16
On the other hand, we found that the proportions of people with a fried flavor preference and dietary consumption of meat and seafood were greater in the hyperuricemia group than the normouricemia group. This phenomenon may be caused by the excessive intake of energy and lipids, leading to obesity and dyslipidemia, which can increase the risk of hyperuricemia.17 In addition, we found that meat and seafood consumption were independent risk factors for hyperuricemia; people who ate meat and seafood more than 3 times per week were 2.5 times and 1.4 times respectively, more likely to have hyperuricemia than people who ate these foods less than 3 times per week. The possible reason is that these are purine-rich foods, and excessive intake of purine-rich foods causes an increase in SUA levels.18,19 Some reports have revealed that high levels of SUA could cause increased oxidative stress in the body, which leads to damage to hepatocytes and is closely related to non-alcoholic fatty liver disease, even individuals with SUA levels in the normal range.20 When a large amount of purine-rich food is consumed, xanthine oxidase activity is enhanced, resulting in increases in the synthesis and concentration of SUA in hepatocytes and increased hepatocyte membrane permeability, which then causes elevations of serum ALT, AST and TP levels.16,21 In contrast, low vegetable consumption was an independent risk factor for hyperuricemia. People who ate vegetables less than 3 times per week were approximately 1.9 times more likely to have hyperuricemia than people ate vegetables more than 3 times per week. The possible mechanism may be that vegetables are low-fat and alkaline foods. Increasing the intake of vegetables in the diet can alkalinize the urine, increasing the solubility of uric acid and facilitating uric acid excretion.4
We found that the proportion of people with exposure to passive smoking was greater in the hyperuricemia group than in the normouricemia group; however, the proportion of active smokers was not different between the two groups. Similarly, some studies reported that passive smoking was a risk factor for hyperuricemia.22,23 In contrast, some previous studies showed that cigarette smoking was negatively associated with the SUA level and gout.24 However, that relationship existed in men but not in women.24 The reason for this inconsistency may be due to the different gender ratios of the study populations. The percentages of women in those studies were very small, while in our study, it was as high as 72.0%. Our results showed that passive smoking was an independent risk factor for hyperuricemia. People with exposure to passive smoking at least once per week for more than 1 year were approximately 2.6 times more likely to have hyperuricemia than people without this exposure. We found that the proportion of people who consumed alcohol was greater in hyperuricemia group than in the normouricemia group. The possible mechanism may be that alcohol intake increases the body’s lactic acid production, which has a competitive inhibitory effect on uric acid excretion. At the same time, ethanol can increase the rate of sputum synthesis in humans and increase urate production.25
Few studies have focused on the relationship between sleep habits and SUA levels. We conducted a comprehensive analysis on the relationship of hyperuricemia and sleep habits. We found that the proportion of people who snored was greater in the hyperuricemia group than in the normouricemia group and that snoring was an independent risk factor for hyperuricemia. People who snored were approximately 2.0 times more likely to have hyperuricemia than the people who did not snore. However, staying up late and taking regular noon break were not significantly different between the two groups. Snoring is a common symptom of sleep apnea syndrome in the elderly.26 The possible mechanism of this interesting phenomenon may be that poor quality sleep leads to further fatigue and energy loss, resulting in the accumulation of metabolic products including uric acid.26 The related mechanism remains unclear and needs to be further studied.
The prevention strategies for high-risk subjects should be based on individual circumstances, and the above risk factors should be monitored and modified. It is recommended that people at high risk for hyperuricemia should adjust their lifestyles and diets. For example, they should actively limit their intake of fried food, alcohol and purine-rich food such as meat and seafood. In addition, they should increase their intake of fresh vegetables, actively treat sleep apnea syndrome, avoid passive smoking, maintain a healthy WC and seek to improve their kidney and liver function.
Our study had some strengths. First, we reported for the first time the incidence of hyperuricemia in a large population of Chinese centenarians and explored the related risk factors. Second, a considerable number of potential confounding lifestyle and dietary factors were adjusted for to improve the reliability of the multivariable model. The limitations of this study are mainly reflected in the following aspects. First, this was a cross-sectional study and could not determine causality. Thus, further prospective studies are needed to verify the causal relationships between lifestyle characteristics and dietary factors and the prevalence of hyperuricemia. Second, the study individuals were all Chinese, so the results may not be generalizable to patients with other ethnic backgrounds.
Conclusion
In conclusion, the prevalence rate of hyperuricemia was as high as 29.39% and was highly prevalent in Chinese centenarians and near-centenarians. Low eGFR and vegetable consumption, high WC, TP, and serum urea levels, passive smoking, snoring, and high meat and seafood consumption were independent risk factors for hyperuricemia. Our results provided a scientific basis for the prevention, early diagnosis and treatment of hyperuricemia. It is recommended that people at high risk for hyperuricemia should actively limit their intake of fried food, alcohol and purine-rich food, increase their intake of fresh vegetables, actively treat sleep apnea syndrome, avoid passive smoking, maintain a healthy WC and seek to improve their kidney and liver function.
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (No. 2016YFC1305500), the Key Research and Development Program of Hainan (Nos. ZDYF2016135, ZDYF2017095), the National Key R&D Program of China (2018YFA0108800), and the National Natural Science Foundation of China (Nos. 61971441, 61671479, U1604284, 81670663).
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Lu X, Li X, Zhao Y, et al. Contemporary epidemiology of gout and hyperuricemia in community elderly in Beijing. Int J Rheum Dis. 2014;17(4):400–407. doi: 10.1111/apl.2014.17.issue-4 [DOI] [PubMed] [Google Scholar]
- 2.Cicero AFG, Fogacci F, Giovannini M, et al. Serum uric acid predicts incident metabolic syndrome in the elderly in an analysis of the Brisighella heart study. Sci Rep. 2018;8(1):11529. doi: 10.1038/s41598-018-29955-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu Y, Pandya BJ, Choi HK. Prevalence of gout and hyperuricemia in the US general population: the National Health and Nutrition Examination Survey 2007–2008. Arthritis Rheum. 2011;63(10):3136–3141. doi: 10.1002/art.30520 [DOI] [PubMed] [Google Scholar]
- 4.Guasch-Ferre M, Bullo M, Babio N, et al. Mediterranean diet and risk of hyperuricemia in elderly participants at high cardiovascular risk. J Gerontol A Biol Sci Med Sci. 2013;68(10):1263–1270. doi: 10.1093/gerona/glt028 [DOI] [PubMed] [Google Scholar]
- 5.Fu S, Yao Y, Zhao Y, et al. Relationships of hyperhomocysteinemia and hyperuricemia with metabolic syndrome and renal function in chinese centenarians. Front Endocrinol (Lausanne). 2018;9:502. doi: 10.3389/fendo.2018.00502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He Y, Zhao Y, Yao Y, et al. Cohort profile: the China Hainan Centenarian Cohort Study (CHCCS). Int J Epidemiol. 2018. [DOI] [PubMed] [Google Scholar]
- 7.Liu Y, Dai M, Bi Y, et al. Active smoking, passive smoking, and risk of nonalcoholic fatty liver disease (NAFLD): a population-based study in China. J Epidemiol. 2013;23(2):115–121. doi: 10.2188/jea.JE20120067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu H, Zhang XM, Wang YL, et al. Prevalence of hyperuricemia among Chinese adults: a national cross-sectional survey using multistage, stratified sampling. J Nephrol. 2014;27(6):653–658. doi: 10.1007/s40620-014-0082-z [DOI] [PubMed] [Google Scholar]
- 10.Zhang L, Wang F, Wang L, et al. Prevalence of chronic kidney disease in China: a cross-sectional survey. Lancet. 2012;379(9818):815–822. doi: 10.1016/S0140-6736(12)60033-6 [DOI] [PubMed] [Google Scholar]
- 11.Liu M, He Y, Jiang B, et al. Association between serum uric acid level and metabolic syndrome and its sex difference in a Chinese community elderly population. Int J Endocrinol. 2014;2014:754678. doi: 10.1155/2014/754678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuo CM, Chien WH, Shen HC, et al. Clinical epidemiology of reduced kidney function among elderly male fishing and agricultural population in Taipei, Taiwan. Biomed Res Int. 2013;2013:214128. doi: 10.1155/2013/214128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villegas R, Xiang YB, Elasy T, et al. Purine-rich foods, protein intake, and the prevalence of hyperuricemia: the Shanghai Men’s Health Study. Nutr Metab Cardiovasc Dis. 2012;22(5):409–416. doi: 10.1016/j.numecd.2010.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karalis DG. Intensive lowering of low-density lipoprotein cholesterol levels for primary prevention of coronary artery disease. Mayo Clin Proc. 2009;84(4):345–352. doi: 10.1016/S0025-6196(11)60544-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Assmann G, Cullen P, Erbey J, et al. Plasma sitosterol elevations are associated with an increased incidence of coronary events in men: results of a nested case-control analysis of the Prospective Cardiovascular Munster (PROCAM) study. Nutr Metab Cardiovasc Dis. 2006;16(1):13–21. doi: 10.1016/j.numecd.2005.04.001 [DOI] [PubMed] [Google Scholar]
- 16.Zhang X, Lu Q, Zhang Z, et al. Value of three-dimensional speckle tracking echocardiography to assess left ventricular function in hyperuricemia patients. Clin Rheumatol. 2018;37(9):2539–2545. doi: 10.1007/s10067-018-4132-0 [DOI] [PubMed] [Google Scholar]
- 17.Kedar E, Simkin PA. A perspective on diet and gout. Adv Chronic Kidney Dis. 2012;19(6):392–397. doi: 10.1053/j.ackd.2012.07.011 [DOI] [PubMed] [Google Scholar]
- 18.Choi HK, Atkinson K, Karlson EW, et al. Purine-rich foods, dairy and protein intake, and the risk of gout in men. N Engl J Med. 2004;350(11):1093–1103. doi: 10.1056/NEJMoa035700 [DOI] [PubMed] [Google Scholar]
- 19.Choi HK, Liu S, Curhan G. Intake of purine-rich foods, protein, and dairy products and relationship to serum levels of uric acid: the Third National Health and Nutrition Examination Survey. Arthritis Rheum. 2005;52(1):283–289. doi: 10.1002/(ISSN)1529-0131 [DOI] [PubMed] [Google Scholar]
- 20.Hwang IC, Suh SY, Suh AR, et al. The relationship between normal serum uric acid and nonalcoholic fatty liver disease. J Korean Med Sci. 2011;26(3):386–391. doi: 10.3346/jkms.2011.26.3.386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, Que S, Zhou L, et al. Dose-response relationship of serum uric acid with metabolic syndrome and non-alcoholic fatty liver disease incidence: a meta-analysis of prospective studies. Sci Rep. 2015;5:14325. doi: 10.1038/srep14325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu S, Yang H, Guo X, et al. Prevalence of hyperuricemia and its correlates in rural Northeast Chinese population: from lifestyle risk factors to metabolic comorbidities. Clin Rheumatol. 2016;35(5):1207–1215. doi: 10.1007/s10067-015-3051-6 [DOI] [PubMed] [Google Scholar]
- 23.Li X, Song P, Li J, et al. Relationship between hyperuricemia and dietary risk factors in Chinese adults: a cross-sectional study. Rheumatol Int. 2015;35(12):2079–2089. doi: 10.1007/s00296-015-3315-0 [DOI] [PubMed] [Google Scholar]
- 24.Yang T, Zhang Y, Wei J, et al. Relationship between cigarette smoking and hyperuricemia in middle-aged and elderly population: a cross-sectional study. Rheumatol Int. 2017;37(1):131–136. doi: 10.1007/s00296-016-3574-4 [DOI] [PubMed] [Google Scholar]
- 25.Eastmond CJ, Garton M, Robins S, et al. The effects of alcoholic beverages on urate metabolism in gout sufferers. Br J Rheumatol. 1995;34(8):756–759. doi: 10.1093/rheumatology/34.8.756 [DOI] [PubMed] [Google Scholar]
- 26.Kanbay A, Inonu H, Solak Y, et al. Uric acid as a potential mediator of cardiovascular morbidity in obstructive sleep apnea syndrome. Eur J Intern Med. 2014;25(5):471–476. doi: 10.1016/j.ejim.2014.04.005 [DOI] [PubMed] [Google Scholar]