Abstract
Background:
Newborns in neonatal intensive care units (NICUs) are in contact with a variety of medical products whose production might include synthetic chemicals with hormonal activity.
Objectives:
Our aim was to assess the content of bisphenol A (BPA) and parabens (PBs) and the hormone-like activities of a subset of medical products commonly used in NICUs in prolonged intimate contact with NICU newborns.
Methods:
Fifty-two NICU items were analyzed, determining the concentrations of BPA and PBs [methyl- (MeP), ethyl- (EtP), propyl- (PrP), and butylparaben (BuP)] and using the E-Screen and PALM-luciferase assays to measure the in vitro (anti-)estrogenic and (anti-)androgenic activity, respectively, of the extracts. Items found to have elevated BPA/PB content or hormone-like activities were further extracted using leaching methodologies.
Results:
BPA was found in three-fifths and PBs in four-fifths of tested NICU items, and and of extracts evidenced estrogenic and anti-androgenic activity, respectively. The highest BPA content was found in the three-way stopcock (), followed by patterned transparent film dressing, gastro-duodenal feeding tubes, sterile gloves, single-lumen umbilical catheters, and intravenous (IV) infusion extension sets (concentrations ranged from 100 to BPA). A total PB concentration () was observed in several items, including light therapy protection glasses, patterned transparent film dressing, winged IV catheters, IV infusion extension sets, and textile tape. The highest estrogenic activity [ estradiol equivalent ()] was found in small dummy nipples, three-way stopcocks, and patterned transparent film dressing and the highest anti-androgenic activity [ procymidone equivalent units per gram ()] in small dummy nipples and three-way stopcocks.
Discussion:
According to these findings, neonates might be exposed to multiple sources of BPA and PBs in NICUs via inhalation, dermal, oral, and IV/parenteral routes. There is a need to address the future health implications for these extremely vulnerable patients and to adopt precautionary preventive measures as a matter of urgency. https://doi.org/10.1289/EHP5564
Introduction
Bisphenol A (BPA) is a high-production volume chemical commonly used in the manufacturing of epoxy resins and polycarbonate plastics and as an additive in many other plastics, for example, polyvinyl chloride (PVC) (Gimeno et al. 2015). Parabens (PBs) are a family of alkyl esters of p-hydroxybenzoic acid [methylparaben (MeP), ethylparaben (EtP), propylparaben (PrP), and butylparaben (BuP) congeners]. They are widely included in personal care products and pharmaceuticals as antimicrobial preservatives and as an additive in plastics for food packaging and beverages (Darbre and Harvey 2008). Over the past few decades, it has been shown that BPA and PBs demonstrate estrogen-like effects, and these compounds have therefore been described as endocrine disrupting chemicals (EDCs) (Boberg et al. 2010; Darbre and Harvey 2008; Perez et al. 1998). Moreover, numerous studies have associated BPA and PB exposure with metabolic disorders in adults and with impaired neurodevelopment and disrupted sexual maturation in children (Giulivo et al. 2016). Particular concerns have been raised about early life EDC exposure because of its potential to cause adverse health consequences throughout life. Furthermore, the pharmacokinetics of xenobiotics, which differ between neonates and older children/adults, can also be affected by underlying medical conditions (Nellis et al. 2015). Thus, it has been suggested that exposure to EDCs such as BPA and PBs during susceptible periods of child development (e.g., perinatal period) may be associated with various adverse effects in children, including alterations in behavior and executive function (Ghassabian et al. 2018; Jiang et al. 2019), accelerated pubertal timing (Berger et al. 2018; Harley et al. 2019), or respiratory disease (Agier et al. 2019; Buckley et al. 2018).
Very-low-birth-weight (VLBW) newborns () and low-birth-weight (LBW) newborns () often require a complex care environment in a neonatal intensive care unit (NICU) to simulate in utero conditions until the proper development of their immature skin and gastrointestinal, immune, nervous, or respiratory systems (Harrison and Goodman 2015). LBW infants are also more likely to require medical appliances in intimate contact with them, including a) bags of intravenous (IV) fluids and total parenteral nutrition; b) nasogastric and enteral feeding tubes, respiratory masks/endotracheal tubes, and venous catheters; and c) cardiopulmonary bypass circuits, among others. Many of these medical devices are made of polycarbonate or PVC plastics, in which residual nonpolymerized BPA can remain after the polymerization process and may leach from the product (Gimeno et al. 2015). BPA may also leach after hydrolysis of the polymer under certain conditions (EU 2010; Mercea 2009), and its release is greater with longer contact time, higher temperature, and elevated pH (as in hydroxide aqueous solutions) (Geens et al. 2011, 2012). To date, only two studies have addressed the exposure of NICU neonates (Calafat et al. 2009; Duty et al. 2013). One of these (Calafat et al. 2009) found that urinary BPA concentrations were 3.42- to 8.75-fold higher when the intensity of medical device utilization was medium or high versus low. The other study (Duty et al. 2013) observed 16- to 32-fold higher BPA concentrations in NICU infants than in infants from the general population. Concerning PBs, Calafat et al. (2009) also found higher urinary MeP levels among newborns with a greater use of medical devices, although potential sources of exposure were not identified. In addition, infants are also potentially exposed to BPA and PB exposures from personal care products, clothing, and other baby products, including socks, diaper-changing mats, and baby mattresses (Asimakopoulos et al. 2016; Freire et al. 2019; Xue et al. 2017). Thus, NICU-admitted infants can be inadvertently exposed to BPA and PBs via dermal, ingestion, inhalation, IV, and parenteral routes. Furthermore, the combined exposure of NICU infants to other EDCs besides BPA and BPs, such as phthalates, should be taken in consideration. Previous studies have reported the exposure of VLBW and LBW newborns to phthalates in NICUs (Calafat et al. 2004; Green et al. 2005; Stroustrup et al. 2018b; Weuve et al. 2006), suggesting that it impacts neurobehavioral performance (Stroustrup et al. 2018a).
In response to increasing concerns about EDC exposure in the hospital environment, the European Commission published Opinions on the risk of oral, subcutaneous, and IV exposure to BPA (SCENIHR 2015) and phthalates (SCENIHR 2016) from medical devices made of materials that may potentially leach these chemicals. They described a particular risk of systemic BPA availability after nonoral exposures, as in the case of neonates in NICUs, infants undergoing prolonged medical procedures, and patients receiving dialysis (SCENIHR 2015). The highest daily exposure was reported to be for neonates in NICUs, at body weight (BW), whereas the daily exposure for adult dialysis patients was BW, and SCENIHR (2015) called for urgent research on the composition and release of BPA from medical devices. However, no attention has been paid to the presence of PBs in the NICU environment. The present study is part of a wider project that aims to assess the potential adverse health impact on neonates in a NICU of exposure to EDCs from their medical care, diet, and environment. The general aim of this first study was to identify potential sources of exposure to EDCs in neonates admitted to NICUs. Therefore, we assessed the content of BPA and PBs in an extensive array of a) plastic medical devices, b) textiles, and c) semisolid/liquid products (including ointments and nutritional supplements) that are commonly used in NICUs and are in intimate contact with newborns, and we measured both the estrogenic and anti-estrogenic activities and the androgenic and anti-androgenic activities of extracts from these products.
Material and Methods
Sample Collection
In June 2018, we collected a total of 52 unused items usually employed in the NICU of the Virgen de las Nieves Hospital, in Granada (Spain). This convenience sample was selected by three pediatricians of the unit (J.A.H., M.P.C., and L.S.), who together compiled a list of all items used in the unit that were in intimate contact with neonates. It included 25 plastic medical devices, 18 textiles, and 9 semisolid/liquid products, as detailed in Table 1, which also displays the information on their composition reported by the manufacturers on the packaging or on their website. We report the extraction method, concentrations of BPA and PBs (MeP, EtP, PrP, BuP, and total paraben compounds (), and the average length of time in contact with the neonate for each item (estimated by the pediatricians). The country of manufacture was in Europe for 37 items, the United States for 8, Asia for 6, and Australia for 1. Of the 25 plastic items, only 8 described the raw material used (polyhexahydrotriazine in 1, polyethylene in 1, PVC in 4, polyurethane in 1, and cotton/polyamide/polyurethane in 1). With regard to additives, the presence or absence of latex was indicated in 17 items (16 items were declared latex-free) and the presence or absence of di-(2-ethylhexyl)-phthalate (DEHP) in 7 items, including 4 declared as DEHP-free.
Table 1.
NICU item detail information.
| Item no. | Description | Route of exposure | Countrya | Contact time with neonatesb | Material | Extraction method | Raw materialc | Details |
|---|---|---|---|---|---|---|---|---|
| 1 | Feeding syringe I | Oral | France | Hours | Plastic | Own methodology | NF | — |
| 2 | Feeding syringe II | Oral | France | Hours | Plastic | Own methodology | NF | — |
| 3 | Gastro-duodenal feeding tube | Oral | France | Week | Plastic | Genay et al. 2011 | NF | — |
| 4 | Extension tube for feeding syringe | Oral | France | Days | Plastic | Genay et al. 2011 | PVC | — |
| 5 | Feeding sampling straw | Oral | France | Minutes | Plastic | Genay et al. 2011 | NF | — |
| 6 | Small dummy | Oral | Germany | Week | Plastic | Own methodology | NF | Latex |
| 7 | Large dummy | Oral | Germany | Week | Plastic | Own methodology | NF | — |
| 8 | Human milk fortifier | Oral | Switzerland | Minutes | Semisolid/liquid | Wang and Zhou 2013 | NF | — |
| 9 | Pulse oximeter adhesive sensor I | Dermal | USA | Days | Plastic/textile | Own methodology | NF | Latex-free |
| 10 | Pulse oximeter adhesive sensor II | Dermal | China | Days | Textile | Own methodology | NF | Latex-free |
| 11 | ECG electrode | Dermal | Malaysia | Days | Textile | Xue et al. 2017 | NF | Latex-free |
| 12 | Light therapy protection glasses | Dermal | USA | Days | Textile | Xue et al. 2017 | Cotton/PA/PUR | — |
| 13 | Occlusive skin wrap | Dermal | New Zealand | Minutes | Plastic | Genay et al. 2011 | PE | — |
| 14 | Sterile gloves | Dermal | Malasia | Minutes | Plastic | Genay et al. 2011 | NF | Latex-free |
| 15 | Latex gloves | Dermal | Malasia | Minutes | Plastic | Genay et al. 2011 | NF | — |
| 16 | Patterned transparent film dressing | Dermal | USA | Weeks | Textile | Xue et al. 2017 | NF | Latex-free |
| 17 | White hypoallergenic paper tape | Dermal | Spain | Weeks | Textile | Xue et al. 2017 | NF | — |
| 18 | Textile tape | Dermal | France | Weeks | Textile | Xue et al. 2017 | NF | — |
| 19 | Surgical tape | Dermal | Germany | Weeks | Textile | Xue et al. 2017 | NF | — |
| 20 | Self-adhesive dressing pad | Dermal | Spain | Weeks | Textile | Xue et al. 2017 | NF | Latex-free |
| 21 | Wound dressing transparent with paper frame | Dermal | China | Weeks | Textile | Xue et al. 2017 | NF | Latex-free |
| 22 | Transparent adhesive film dressing | Dermal | England | Weeks | Textile | Xue et al. 2017 | NF | — |
| 23 | Hydrocolloid transparent dressing | Dermal | Denmark | Weeks | Textile | Xue et al. 2017 | NF | — |
| 24 | White cohesive bandage | Dermal | Spain | Weeks | Textile | Xue et al. 2017 | NF | Latex-free |
| 25 | Infant flow LP headgear | Dermal | Mexico | Weeks | Textile | Xue et al. 2017 | NF | Latex-free, DEHP-free |
| 26 | Sterile non-woven swabs | Dermal | Spain | Minutes | Textile | Xue et al. 2017 | NF | Latex-free |
| 27 | Nonsterile non-woven swabs | Dermal | Spain | Minutes | Textile | Xue et al. 2017 | NF | Hypoalergenic, transpirant |
| 28 | Absorbent bed underpad | Dermal | Portugal | Days | Textile | Xue et al. 2017 | NF | — |
| 29 | XS-sized diaper | Dermal | Germany | Hours | Textile | Xue et al. 2017 | NF | — |
| 30 | S-sized diaper | Dermal | Germany | Hours | Textile | Xue et al. 2017 | NF | — |
| 31 | Chlorhexidine | Dermal | Spain | Minutes | Semisolid/liquid | Wang and Zhou 2013 | NF | clorhexidine/ solution |
| 32 | Hand sanitizer | Dermal | Germany | Minutes | Semisolid/liquid | Zhang et al. 2005 | NF | 2-propanol, 1-propanol, mecetronium ethylsulfate each of solution |
| 33 | Talcum and zinc oxide cream | Dermal | Spain | Minutes | Semisolid/liquid | Wang and Zhou 2013 | NF | Lanoline, dimethicone, glycerin, rose essence |
| 34 | Proteolytic enzyme cream | Dermal | United Kingdom | Minutes | Semisolid/liquid | Wang and Zhou 2013 | NF | Collagenase A, liquid paraffin, solid paraffin |
| 35 | Winged IV catheter (transparent section) | IV/parenteral | Belgium | Days | Plastic | Own methodology | NF | — |
| 36 | Winged IV catheter | IV/parenteral | Germany | Week | Plastic | Genay et al. 2011 | NF | — |
| 37 | Single-lumen umbilical vein catheter | IV/parenteral | France | Week | Plastic | Genay et al. 2011 | PVC | DEHP-free |
| 38 | Double-lumen umbilical vein catheter | IV/parenteral | France | Week | Plastic | Genay et al. 2011 | PUR | — |
| 39 | Extension set for IV infusion system | IV/parenteral | United Kingdom | Week | Plastic | Genay et al. 2011 | PVC | Latex-free, DEHP-free |
| 40 | Extension set for IV infusion system (light resistant) | IV/parenteral | Italy | Week | Plastic | Genay et al. 2011 | PVC | Latex-free, DEHP-free |
| 41 | Three-way stopcock | IV/parenteral | Israel | Week | Plastic | Own methodology | NF | — |
| 42 | Disinfecting cap for needle-free connectors | IV/parenteral | USA | Week | Plastic | Own methodology | NF | — |
| 43 | Hypodermic injection needle | IV/parenteral | USA | Week | Plastic | Own methodology | NF | — |
| 44 | Syringe | IV/parenteral | Spain | Day | Plastic | Own methodology | NF | — |
| 45 | Caffeine perfusion | IV/parenteral | Italy | Minutes | Semisolid/liquid | Sanchez-Prado et al. 2011 | NF | — |
| 46 | Water for injection solvent for parenteral use | IV/parenteral | Spain | Minutes | Semisolid/liquid | Sanchez-Prado et al. 2011 | NF | — |
| 47 | 0.9% NaCl solution for IV flush (syringe) | IV/parenteral | Germany | Minutes | Semisolid/liquid | Sanchez-Prado et al. 2011 | NF | — |
| 48 | 0.9% NaCl solution for IV (ampoule) | IV/parenteral | Spain | Minutes | Semisolid/liquid | Sanchez-Prado et al. 2011 | NF | — |
| 49 | Endotracheal tube | Respiratory | Malaysia | Weeks | Plastic | Genay et al. 2011 | PHT | Latex-free, DEHP |
| 50 | Closed suction system | Respiratory | Mexico | Weeks | Plastic | Genay et al. 2011 | NF | Latex-free, DEHP |
| 51 | Nasal cannula | Respiratory | Lithuania | Weeks | Plastic | Genay et al. 2011 | NF | Latex-free, DEHP |
| 52 | Nasal prong | Respiratory | USA | Weeks | Plastic | Genay et al. 2011 | NF | Latex-free, DEHP-free |
Note: —, not available; DEHP, di-(2-ethylhexyl)-phthalate; ECG, electrocardiograph; IV, intravenous; LP, low pressure; NaCl, sodium chloride; NF, not found; NICU, neonatal intensive care unit; PA, polyamide; PE, polyethylene; PHT, polyhexahydrotriazine; PUR, polyurethane; PVC, polyvinyl chloride; S, small; XS, extra small.
Country in which the item was manufactured.
Average time that the item is in contact with newborns, based on a survey of pediatricians.
cInformation obtained from packaging or manufacturer’s website.
Chemicals and Reagents
All reagents were analytical grade unless otherwise specified. BPA, MeP, EtP, PrP, BuP, labeled deuterium BPA (BPA-d16), and labeled EtP ring 13C6 (EtP-13C6) were purchased from Sigma-Aldrich. Solvents for extraction procedures—ethyl acetate, tetrahydrofuran, and dichloromethane—were purchased from Merck, and methanol (MeOH) and acetone were supplied by Sigma-Aldrich. Liquid chromatography/mass spectrometry (LC-MS)–grade acetonitrile, water, and ammonia (25%) were purchased from Sigma-Aldrich. Water () was purified using an in-house Milli-Q® system (Millipore).
For chemical analyses, stock standard solutions () of each compound were prepared in acetonitrile and stored at 4°C in the dark. The solutions remained stable for at least 2 months. Working standards were prepared immediately before use by dilution with pure acetonitrile.
For in vitro cell assays, reference standards for (), methyltrienolone (R1881), ICI 182780 (henceforth, ICI), procymidone, puromycin, geneticin (G418), luciferin (sodium salt), sulforhodamine B (SRB), and trichloroacetic acid (TCA) were obtained from Sigma-Aldrich. Stock solutions () of , R1881, procymidone, and ICI were prepared in ethanol, and successive dilutions were performed in culture medium. Stock solutions were kept at , and dilution series were freshly prepared before each experiment. The culture medium and fetal bovine serum (FBS) were supplied by Gibco (Invitrogen) and all cell culture plastics by Falcon (VWR International Eurolab).
Instrumentation and Ultra-High-Performance Liquid Chromatography–Mass Spectrometry Conditions
Analyses were performed by ultra-high-performance liquid chromatography coupled to mass spectrometry (UHPLC-MS/MS), using an ACQUITY UPLC™ H-Class (Waters) consisting of ACQUITY UPLC™ binary solvent manager and ACQUITY UPLC™ sample manager. A Xevo TQS tandem quadrupole MS (Waters) equipped with an orthogonal Z-spray™ electrospray ionization (ESI) source was used for BPA and PBs detection. Chromatographic separation of compounds was performed using an ACQUITY UPLC BEH™ C18 ( internal diameter, particle size) from Waters. The gradient mobile phase consisted of 0.025% vol/vol ammonia aqueous solution (Solvent A) and 0.025% vol/vol ammonia in acetonitrile (Solvent B). Gradient conditions were as follows: , 60% Solvent B; , Solvent B; , 100% Solvent B and back to 60% for . Flow rate was . The injection volume was . The column temperature was maintained at 40°C. The MS was operated in negative ESI mode, using optimized MS/MS parameters as defined in a previous study (Vela-Soria et al. 2014).
Sample Extraction, Treatment, and LC-MS Conditions
Samples were extracted from the selected items using different methodologies according to the nature of the material. The extraction procedure for each type of material is described below.
Plastic medical devices.
BPA and PBs from hard plastic materials in medical devices were studied as follows: of sample was accurately weighed and placed in a glass vial; of acetone was then added and left for , followed by sonication for 30 min. Samples were then centrifuged at 20°C for 10 min at , and the organic phase was taken, filtered through a polytetrafluoroethylene (PTFE) filter, and dried under a nitrogen stream. The residue was dissolved with acetonitrile (containing of BPA-d16 and of EP-13C6)/Milli-Q® water 1:1 vol/vol, and centrifuged at 4°C for 10 min at for cleaning. BPA and PBs from soft plastic materials were studied using a slight modification of the method described by Genay et al. (2011). Briefly, of each sample was accurately weighed and placed in a glass vial, and tetrahydrofuran was added and left for 30 min. Next, of the solution was poured into another glass vial and of MeOH was added. After mixing, was taken and dried under a nitrogen stream. The residue was then dissolved with acetonitrile (containing of BPA-d16 and of EtP-13C6)/Milli-Q® water 1:1 vol/vol and centrifuged at 4°C for 10 min at for cleaning, followed by the injection of into the LC system.
When high BPA/PB content or elevated estrogenic/anti-androgenic activity was detected in plastic components, the concentration of released BPA and PBs was then studied under soft extraction conditions. In brief, plastic components were fully immersed in a 0.9% sodium chloride (NaCl) aqueous solution (250, 500, or with pH of 7.5) for 20 d at 37°C, followed by analysis of the solutions to determine the presence of BPA and PBs. BPA and PBs were extracted from the NaCl solutions by solid-phase extraction as described elsewhere (Real et al. 2015). Briefly, Isolute® C18 cartridges were conditioned with MeOH and Milli-Q® water, and NaCl solutions were then loaded at , followed by drying for and elution with of MeOH. The eluent was dried under a nitrogen stream and the residue dissolved with acetonitrile (containing BPA-d16 and EP-13C6)/Milli-Q® water, 1:1 vol/vol. was injected into the LC system.
Textile products.
Extraction of BPA and PBs from textile products was performed as previously described (Xue et al. 2017) with some modifications. Briefly, of each textile was accurately weighed, cut, placed in glass centrifuge tubes, and spiked with of an isotope-labeled surrogate mixture solution ( BPA-d16 and EP-13C6 in acetonitrile), whereas of a mixture of acetone and dichloromethane (1:4 vol/vol) was used for the extraction. After sonication for 20 min and centrifugation at for 5 min, the solvent was collected, filtered through a nylon filter, and transferred to another glass tube. The solvent was evaporated to dryness under a gentle stream of nitrogen and the residue dissolved with of acetonitrile for injection into the LC system.
Liquid/semisolid products.
Extraction of BPA and PBs from ointments was performed as previously described (Wang and Zhou 2013) with some modifications. Briefly, of each ointment was accurately weighed and placed in glass centrifuge tubes, followed by heating at 80°C for 2 min to homogenize and spiking with of the isotope-labeled surrogate mixture solution ( of BPA-d16 and of EP-13C6, in acetonitrile). Next, of MeOH was added, followed by shaking for 2 min and sonication in an ultrasonic bath for 20 min. After centrifugation at for 10 min, the solvent was collected and filtered through a PTFE filter. The filtered solvent was evaporated to dryness under a gentle stream of nitrogen and the residue dissolved with of acetonitrile/Milli-Q® water, 1:1 vol/vol. Next, the extract was placed in a Eppendorf® tube and centrifuged at for 10 min at 4°C. The extract was placed in a chromatographic vial for injection into the LC system.
BPA and PBs were extracted from NaCl (in plastic and glass ampoules), IV caffeine, and sterile water using a modification of the extraction method described by Sanchez-Prado et al. (2011). Briefly, of each sample was taken, placed in a glass centrifuge tube, and spiked with of the isotope-labeled surrogate mixture solution ( BPA-d16 and EP-13C6 in acetonitrile), followed by the addition of ethyl acetate, shaking for 2 min, and sonication in an ultrasonic bath for 20 min. After centrifugation at for 10 min, the solvent was collected and filtered through a PTFE filter. The filtered solvent was evaporated to dryness under a gentle nitrogen stream, and the residue was dissolved with of acetonitrile/Milli-Q® water, 1:1 vol/vol, with being injected into the LC system.
BPA and PBs were extracted from hand sanitizer liquid using a slight modification of the extraction procedure described by Zhang et al. (2005). Briefly, of each sample was taken, placed in a glass centrifuge tube, and spiked with of the isotope-labeled surrogate mixture solution ( BPA-d16 and EP-13C6 in acetonitrile), followed by the addition of MeOH, shaking for 2 min, and sonication in an ultrasonic bath for 10 min. A further MeOH was then added, and the solution was filtered through a PTFE filter. Next, was taken, placed in a chromatographic vial, and of Milli-Q® water was added, with being injected into the LC system.
Quality Assurance and Quality Control in Chemical Analyses
Matrix-matched calibration was performed for the analysis of textiles and ointments. Samples used as blanks were previously analyzed to confirm that the compounds of interest were absent or below the limit of detection (LOD). Standard addition calibration was used for the samples of the liquids, hard plastics, and soft plastics.
LODs and limits of quantification (LOQs) were determined on the basis of the lowest point of the calibration standard with a signal-to-noise (S/N) ratio of and , respectively. LODs for textiles were for BPA, for MeP and BuP, and for EtP and PrP. LODs for ointments were for BPA, for MeP and BuP, and for EtP and PrP. LODs for the standard addition calibration were for BPA and for PBs.
Samples were analyzed in duplicate. Extraction was carried out in batches of 15, with each batch containing 12 samples as well as the following 3 quality control samples: a procedural blank (no sample) to test for interference or laboratory contamination and two spiked blank samples ( of BPA and of PBs). Samples were frozen after extraction until injection into the LC system. No BPA or PBs were detected in any procedural blank. Recoveries for all target compounds in the quality-control spiked samples ranged from 86% to 104%, and the coefficient of variation (CV) was below 20% in all cases.
Hormone-Like Activity Assessment
The E-Screen bioassay and PALM luciferase assay were performed as previously described (Molina-Molina et al. 2014, 2019) with some modifications.
MCF-7 and PALM cell lines: culture conditions.
MCF7 BUS human breast cancer cells (Soule et al. 1973) were a gift from C. Sonnenschein (Tufts University, Boston, MA). PALM human prostate cancer cells, from a human androgen-dependent stable transfected cancer line (Térouanne et al. 2000), were provided by P. Balaguer (DR2 at INSERM U896, Montpellier, France). Both cell lines were cultured as previously described (Molina-Molina et al. 2014). In brief, MCF-7 cells were cultured in DMEM (Gibco, Invitrogen) with phenol red supplemented with 10% FBS (seeding medium), whereas PALM cells were cultured in Ham’s F12 medium supplemented with 10% FBS, G418, and puromycin (seeding medium). Given the hormonal activity of phenol red and FBS, experiments were performed in a test culture medium of phenol red-free DMEM (Gibco, Invitrogen) supplemented with 10% dextran-coated charcoal-FBS (10% DCC-FBS) for MCF-7 cells and Ham’s F12 medium supplemented with 6% DCC-FBS and 1% antibiotic for PALM cells, in a 5% carbon dioxide humidified atmosphere at 37°C.
E-Screen.
Briefly, MCF-7 cells were trypsinized and plated in 96-well culture plates at initial concentrations of cells per well. One day later, the seeding medium was removed and replaced with test culture medium. For agonistic assays, dry extracts of the samples were resuspended in of test culture medium, vigorously shaken, left at rest for 30 min, and then filtered through a filter (PALL® Acrodisc® Syringe Filters with Supor® Membrane, 13 mm) and tested ( added per well) on MCF-7 cells at dilutions of 1:1 to 1:10. A dose–response curve () for estradiol () and a negative control of cells treated solely with hormone-free medium (test culture medium) and a solvent control (0.1% ethanol in test culture medium) were included in each experiment. The bioassay was ended on Day 6 (late exponential phase) by removing the media from wells, fixing the cells with TCA (10% wt/vol at 4°C, 30 min), and staining them with SRB [0.4% wt/vol in acetic acid (1% vol/vol), at room temperature, 30 min]. Finally, the bound dye was solubilized using tris(hydroxymethyl)aminomethane (, at room temperature, 30 min, pH 10.4) and the absorbance read at . This method relies on the ability of SRB to bind stoichiometrically to cell membrane proteins under mild acidic conditions and to be removed under basic conditions. The amount of bound dye can therefore serve as a proxy for cell number, which can then be extrapolated to measure cell proliferation. Next, the ratio of SRB-stained cells between treated cells and hormone-free control cells (negative controls) was calculated for each concentration. Tests were done in triplicate, and results were expressed as proliferative effect (PE) [MCF-7 cell proliferation (fold over control)]. The antagonistic activities of sample extracts were determined by co-incubation with the agonist at . Because the PE only provides information on the effect of the extract in the E-Screen bioassay, this was transformed into equivalent () or antiestrogen (ICI 182780) equivalent (ICIeq) units related to of sample by reading from dose–response curves of or ICI (see Figure S1A,B). In this manner, the PE of each extract was referred to the maximal PE obtained with or ICI and transformed into or ICIeq. and ICIeq values for each sample extract were calculated by using the concentration that obtained the greatest induction or inhibition of cell proliferation, respectively. and ICIeq values were corrected for the dilution factor and reported as w or ICIeq/g of the original NICU sample.
PALM assay.
PALM cells were seeded at a density of cells per well in 96-well white opaque tissue culture plates in test culture medium. Dry extracts of the samples were serially diluted (as described above for the E-Screen bioassay), and per well was added at after seeding. Serial dilutions of the synthetic human androgen receptor agonist methyltrienolone-R1881 () and the test culture medium alone were included on each plate with the test samples as positive and negative controls, respectively. PALM cells were incubated for at 37°C, and the medium was then removed and replaced by test culture medium containing luciferin (Sigma-Aldrich). Next, the 96-well plate was introduced into a luminometer for to measure luminescence from intact living cells.
Human androgen receptor (hAR)-agonistic activities were tested at 1:1 to 1:10 dilutions of the samples, performing tests in quadruplicate for each dilution. Values were normalized to the readings with R1881 alone (), and this was taken as the maximum response or maximal luciferase activity (100%). Negative controls (test culture medium alone) were used to define the minimum response (10%). The antagonistic activity of extracts was determined by co-incubation with R1881 agonist (). Results were expressed as a percentage of maximal luciferase activity. Finally, the luciferase activity in each sample extract was expressed as percentage of the maximal luciferase activity obtained with R1881 or procymidone (Proc) and transformed into R1881 or procymidone equivalent units (R1881eq or Proceq, respectively) by reading from dose–response curves for R1881 or procymidone (standard serial dilutions) included on each plate (see Figure S1C,D). R1881eq and Proceq values were calculated from the concentration that obtained the greatest induction or inhibition of luciferase activity, respectively. R1881eq and Proceq values obtained were corrected for the dilution factor and reported as and of the original sample.
Statistical Data Analysis
Mean concentrations and coefficients of variation (CVs) of BPA, MeP, EtP, PrP, BuP, and were calculated, as well as the estrogenic and anti-androgenic activities from two separate extractions. Results for EDC content and hormone-like activities were summarized according to the main route of exposure. Nevertheless, it should be taken into account that the EDC content of some NICU items (e.g., BPA or PBs) may plausibly reach internal body compartments via multiple routes; for instance, the endotracheal tube might be both a respiratory and dermal route of exposure to hormone-like chemicals. Spearman correlation analysis was performed to examine the relationship between log-transformed concentrations of BPA and PBs. The relative weight of each chemical in relation to the total EDC concentration was calculated for each exposure route. Therefore, we divided the concentrations of each compound by the sum of BPA and PBs found in each item, followed by calculation of the mean relative weight of each compound in the items related to each exposure route, and the results were expressed as percentages. Statistical significance was set at . SPSS software (version 23.0; IBM) was used for the statistical analyses.
Results
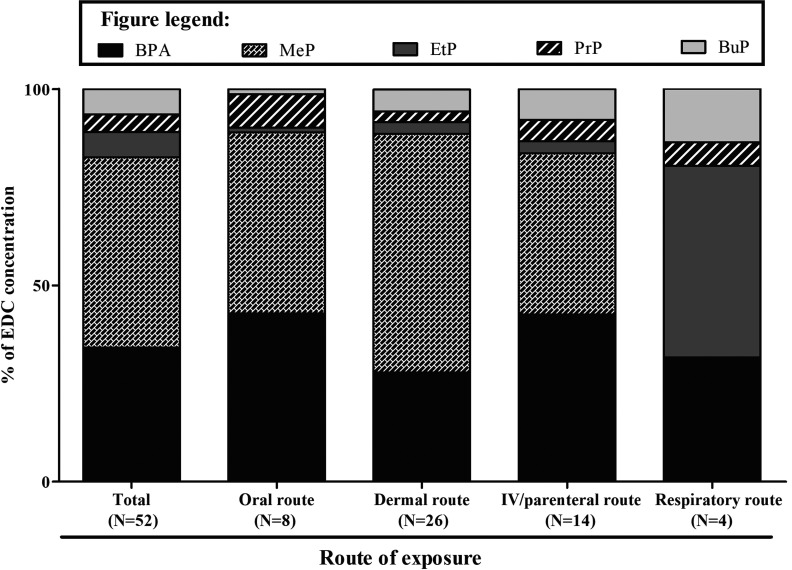
Data on the BPA and PBs content and (anti-)estrogen and (anti-)androgenic activities of extracts are exhibited in Tables 2–5 according to the exposure route (oral, dermal, IV/parenteral, or inhalation). Figure 1 depicts the mean relative concentration of each compound in items in contact with newborns via the same exposure route. As shown, BPA concentrations represented of the total concentration of the studied EDCs via all routes of exposure, whereas MeP represented of total BPA and PB content via all routes except for inhalation. In addition, BPA levels were significantly and positively correlated with in the samples (rho coefficient 0.411, ). Estrogenic and anti-androgenic activities were detected in 13 (25%) and 5 (10%) of the 52 tested samples, respectively (Tables 2–5). With the exception of the winged IV catheter, all NICU items that exhibited estrogenic activity contained BPA at detectable levels. No anti-estrogenic or androgenic activity was observed in any sample. Estrogenic activity was observed in two of five DEHP-free items (40.0%) and in one of three DEHP-containing items (33.3%).
Table 2.
Concentrations of BPA and PBs and hormone-like activities in NICU items related to oral routes of exposure ().
| Item no. | Description | EDC content (ng/g) | Hormone-like activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPA | MeP | EtP | PrP | BuP | E-Screen | PALM assay | ||||||||
| Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | eq/g (pM)a | (mM)b | |||
| 1A | Feeding syringe I | — | — | — | — | — | — | ND | ND | |||||
| 1B | Feeding syringe I (piston) | — | — | — | — | — | — | ND | ND | |||||
| 2A | Feeding syringe II | — | — | — | — | — | — | ND | ND | |||||
| 2B | Feeding syringe II (piston) | — | — | — | — | — | — | ND | ND | |||||
| 3 | Gastro-duodenal feeding tube | 301.1 | 13.2 | 64.8 | 15.7 | 5.5 | 6.7 | 3.5 | 18.1 | — | 73.8 | 193.9 | ND | |
| 4 | Extension tube for feeding syringe | 11.9 | 6.7 | 17.4 | 2.1 | — | — | — | 17.4 | ND | ND | |||
| 5 | Feeding sampling straw | 107.7 | 15.3 | 5.0 | 12.9 | — | 17.4 | 2.1 | 2.4 | 7.4 | 24.8 | ND | ND | |
| 6A | Small dummy (nipple) | 6.5 | 12.3 | 63.7 | 5.4 | 5.3 | 11.5 | 20.4 | 5.4 | 1.4 | 10.6 | 90.8 | 2,403.8 | 9,788.0 |
| 6B | Small dummy (hard section) | — | — | — | — | — | — | ND | ND | |||||
| 7A | Large dummy (nipple) | 7.8 | 8.6 | 24.2 | 14.8 | — | 6.3 | 11.5 | 1.7 | 16.6 | 32.2 | ND | ND | |
| 7B | Large dummy (hard section) | 3.6 | 5.6 | 9.4 | 4.1 | — | — | — | 9.4 | ND | ND | |||
| 8 | Human milk fortifier | — | — | — | — | — | — | ND | ND | |||||
| NICU items [ (%)] | 5 (62.5%) | 5 (62.5%) | 2 (25.0%) | 4 (50.0%) | 3 (37.5%) | 6 (75.0%) | 2 (25.0%) | 1 (12.5%) | ||||||
Note: Values below the limit of detection are represented by the symbol < followed by the limit of detection. —, not applicable; BPA, bisphenol A; BuP, butyl-paraben; CV, coefficient of variance; , estradiol; EDC, endocrine disrupting chemical; EtP, ethyl-paraben; MeP, methyl-paraben; ND, not detected; NICU, neonatal intensive care unit; PrP, propyl-paraben; , total concentrations of parabens.
Concentrations equivalent to per gram.
Concentrations equivalent to procymidone per gram.
Table 5.
Concentrations of BPA and PBs and hormone-like activities in NICU items related to respiratory routes of exposure ().
| Item no. | Description | EDC content (ng/g) | Hormone-like activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPA | MeP | EtP | PrP | BuP | E-Screen | PALM assay | ||||||||
| Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | eq/g (pM)a | (mM)b | |||
| 49 | Endotracheal tube | 95.4 | 16.6 | — | 58.6 | 14.0 | 5.6 | 11.1 | 7.9 | 10.2 | 72.1 | 148.1 | ND | |
| 50 | Closed suction system | — | — | 8.4 | 7.3 | — | — | 8.4 | ND | ND | ||||
| 51 | Nasal cannula | — | — | 76.2 | 3.6 | 18.1 | 9.8 | 22.3 | 19.3 | 116.6 | ND | ND | ||
| 52 | Nasal prong | 33.9 | 18.4 | — | — | 3.4 | 12.3 | 15.6 | 7.6 | 19.0 | ND | ND | ||
| NICU items [ (%)] | 2 (50.0%) | 0 (0.0%) | 3 (75.0%) | 3 (75.0%) | 6 (75.0%) | 4 (100%) | 1 (25.0%) | 0 (0.0%) | ||||||
Note: Values below the limit of detection are represented by the symbol < followed by the limit of detection. —, not applicable; BPA, bisphenol A; BuP, butyl-paraben; CV, coefficient of variance; , estradiol; EDC, endocrine disrupting chemical; EtP, ethyl-paraben; MeP, methyl-paraben; ND, not detected; NICU, neonatal intensive care unit; PB, paraben; PrP, propyl-paraben; , total concentrations of parabens.
Concentrations equivalent to per gram.
Concentrations equivalent to procymidone per gram.
Figure 1.
Distribution profiles of the BPA and paraben content found in NICU items according to the main route of exposure. Bars represent the mean relative concentration of each compound in items in contact with neonates via the same exposure route.
Oral Route of Exposure
BPA was found in five (62.5%) of the eight oral-use items at concentrations ranging up to (Table 2). The highest BPA concentration was recorded for the gastro-duodenal feeding tube (), followed by the feeding sampling straw (). PBs were detected in five (62.5%) of the oral-use items, with the highest concentrations observed in the small dummy nipple () and gastro-duodenal feeding tube (). The most frequently detected PB congener was MeP, with the highest concentrations observed in the small dummy nipple () and gastro-duodenal feeding tube (), which were also positive for estrogenicity ( and , respectively). The highest antiandrogen activity was recorded for the small dummy nipple ( ). The large dummy leached detectable levels of BPA () and the small dummy exhibited anti-androgenic activity ( ) (see Table S1).
Dermal Route of Exposure
Table 3 displays the BPA and PB content and hormonal activity of the NICU items in dermal contact with neonates. BPA was detected in 17 (65.4%) of the 26 items at concentrations ranging from 0.90 to . The highest BPA concentration was in patterned transparent film dressing (), followed by sterile gloves () and the hard section of the pulse oximeter adhesive sensor I ().
Table 3.
Concentrations of BPA and PBs and hormone-like activities in NICU items related to dermal routes of exposure ().
| Item no. | Description | EDC content (ng/g) | Hormone-like activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPA | MeP | EtP | PrP | BuP | E-Screen | PALM assay | ||||||||
| Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | eq/g (pM)a | (mM)b | |||
| 9A | Pulse oximeter adhesive sensor I (hard section) | 73.6 | 13.5 | 81.9 | 15.7 | — | 11.5 | 17.1 | — | 93.4 | 1,400.0 | ND | ||
| 9B | Pulse oximeter adhesive sensor I (adhesive section) | 4.1 | 8.4 | 8.3 | 12.3 | — | — | — | 8.3 | 346.2 | ND | |||
| 10 | Pulse oximeter adhesive sensor II | 2.7 | 5.1 | 12.7 | 16.8 | 0.8 | 5.1 | — | — | 13.5 | ND | ND | ||
| 11 | ECG electrode | 33.1 | 9.0 | — | 0.3 | 1.1 | — | — | 0.3 | 126.2 | ND | |||
| 12 | Light therapy protection glasses | 4.6 | 9.0 | 480.7 | 4.1 | 4.2 | 12.3 | — | — | 484.9 | 197.0 | ND | ||
| 13 | Occlusive skin wrap | 67.0 | 10.2 | 4.3 | 8.4 | — | — | 9.2 | 12.3 | 13.5 | ND | ND | ||
| 14 | Sterile gloves | 140.5 | 12.3 | 19.4 | 14.0 | — | 14.3 | 12.8 | — | 33.5 | ND | 1.8 | ||
| 15 | Nonsterile gloves | 17.8 | 10.6 | 32.4 | 2.1 | — | 13.0 | 4.4 | — | 45.4 | ND | 2.4 | ||
| 16 | Patterned transparent film dressing | 688.1 | 11.2 | 208.0 | 4.8 | 11.7 | 6.1 | 1.4 | 12.3 | — | 221.1 | 2,107.4 | 1.0 | |
| 17 | White hypoallergenic paper tape | 1.4 | 0.9 | 13.4 | 6.2 | 2.1 | 11.1 | — | — | 15.5 | ND | ND | ||
| 18 | Textile tape | 6.4 | 8.0 | 108.0 | 12.7 | 3.4 | 11.7 | — | 1.6 | 13.4 | 113.0 | 171.4 | ND | |
| 19 | Surgical tape | 0.9 | 13.4 | 7.5 | 1.4 | — | — | — | 7.5 | ND | ND | |||
| 20 | Self-adhesive dressing pad | 1.2 | 8.0 | 79.1 | 13.0 | 32.8 | 13.0 | — | — | 111.9 | ND | ND | ||
| 21 | Transparent wound dressing with paper frame | 4.3 | 13.9 | 40.5 | 15.0 | 7.1 | 2.2 | — | — | 47.6 | 144.0 | ND | ||
| 22 | Transparent adhesive film dressing | 1.1 | 16.6 | 6.1 | 5.4 | — | — | — | 6.1 | ND | ND | |||
| 23 | Hydrocolloid transparent dressing | 26.7 | 13.1 | 1.5 | 5.4 | — | — | — | 1.5 | ND | ND | |||
| 24 | White cohesive bandage | 2.7 | 5.9 | 5.4 | 11.5 | 0.9 | 3.6 | — | — | 6.3 | ND | ND | ||
| 25 | Infant flow LP headgear | — | 4.3 | 6.7 | — | 1.7 | 10.4 | — | 6.0 | ND | ND | |||
| 26 | Sterile non-woven swabs | — | 1.1 | 3.6 | — | — | — | 1.1 | ND | ND | ||||
| 27 | Nonsterile non-woven swabs | — | 3.8 | 8.0 | — | — | — | 3.8 | ND | ND | ||||
| 28 | Absorbent bed underpad | — | 0.3 | 0.1 | — | — | — | 0.3 | ND | ND | ||||
| 29 | XS-sized diaper | — | — | — | — | — | — | ND | ND | |||||
| 30 | S-sized diaper | — | 1.1 | 10.6 | — | — | — | 1.1 | ND | ND | ||||
| 31 | Chlorhexidine | 1.1 | 11.3 | 3.2 | 16.4 | — | — | — | 3.2 | ND | ND | |||
| 32 | Hand sanitizer | — | — | — | — | — | — | ND | ND | |||||
| 33 | Talcum and zinc oxide cream | — | 2.6 | 2.1 | — | 0.2 | 10.1 | 1.1 | 6.1 | 3.9 | ND | ND | ||
| 34 | Proteolytic enzyme cream | — | 1.9 | 13.4 | — | — | 10.7 | 12.1 | 12.6 | ND | ND | |||
| NICU items [ (%)] | 17 (65.4%) | 23 (88.5%) | 9 (34.6%) | 6 (23.1%) | 4 (15.4%) | 24 (92.3%) | 7 (26.9%) | 3 (11.5%) | ||||||
Note: Values below the limit of detection are represented by the symbol < followed by the limit of detection. —, not applicable; BPA, bisphenol A; BuP, butyl-paraben; CV, coefficient of variance; , estradiol; EDC, endocrine disrupting chemical; ECG, electrocardiograph; EtP, ethyl-paraben; LP, low pressure; MeP, methyl-paraben; ND, not detected; NICU, neonatal intensive care unit; PrP, propyl-paraben; S, small; XS, extra small; , total concentrations of parabens.
Concentrations equivalent to per gram.
Concentrations equivalent to procymidone per gram.
Detectable concentrations of at least one PB congener were found in 24 (92.3%) of the 26 items, and values ranged from 0.30 to . The most frequently detected PB was MeP (88.5%), which showed the highest concentration among PBs [], followed by EtP (). The highest MeP concentration was found in light therapy protection glasses (), followed by patterned transparent film dressing (), textile tape (), the hard section of the pulse oximeter adhesive sensor I (), and self-adhesive dressing pads ().
As in the case of BPA and PB concentrations, the highest estrogenic activity was found in patterned transparent film dressing, followed by hard and adhesive sections of the pulse oximeter adhesive sensor I, light therapy protection glasses, textile tape, transparent wound dressing with paper frame, and electrocardiograph (ECG) electrodes. Anti-androgenic activity was detected by the PALM assay in nonsterile and sterile gloves and in patterned transparent film dressing, with values ranging from 1.0 to .
Intravenous and Parenteral Routes of Exposure
Table 4 displays the BPA and PB content and hormonal activity of the NICU items related to the IV/parenteral exposure route. BPA was detected in 7 (50.0%) of the 14 items. The highest BPA concentration (among all 52 items in the study) was found in the three-way stopcock (clear section) (), followed by the single-lumen umbilical vein catheter (), IV infusion system extension set (), and double-lumen umbilical vein catheter (). PBs were detected in 11 items (78.6%), with the highest concentrations being ( PrP) for the winged IV catheter (Item No. 36) and ( MeP) for the IV infusion system extension set. Detectable levels of PBs were also observed in some liquid solutions, including 0.9% NaCl (in commercial syringe or ampoule preparations)
Table 4.
Concentrations of BPA and PBs and hormone-like activities in NICU items related to IV/parenteral routes of exposure ().
| Item no. | Description | EDC content (ng/g) | Hormone-like activity | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BPA | MeP | EtP | PrP | BuP | E-Screen | PALM assay | ||||||||
| Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | Mean | CV (%) | eq/g (pM)a | (mM)b | |||
| 35A | Winged IV catheter (disinfecting cap for needle) | — | 21.5 | 14.0 | — | — | — | 21.5 | ND | ND | ||||
| 35B | Winged IV catheter (colored section) | — | — | — | — | — | — | ND | ND | |||||
| 36 | Winged IV catheter | — | 43.8 | 8.5 | 7.0 | 10.6 | 96.9 | 14.8 | 1.8 | 1.5 | 149.5 | 300.0 | ND | |
| 37 | Single-lumen umbilical vein catheter | 130.4 | 13.1 | — | 10.9 | 3.6 | — | — | 10.9 | 281.0 | ND | |||
| 38 | Double-lumen umbilical vein catheter | 49.0 | 5.9 | — | — | — | — | — | ND | ND | ||||
| 39 | Extension set for IV infusion system | 112.7 | 4.8 | 106.4 | 5.4 | — | 19.4 | 9.3 | — | 125.8 | 192.2 | ND | ||
| 40 | Extension set for IV infusion system (light resistant) | 29.8 | 20.2 | — | — | — | — | — | ND | ND | ||||
| 41A | Three-way stopcock (clear section) | 7,052.7 | 18.2 | 10.0 | 13.1 | 1.2 | 5.9 | — | — | 11.2 | 489.8 | 5.4 | ||
| 41B | Three-way stopcock (cap) | — | — | — | — | — | — | ND | ND | |||||
| 42 | Disinfecting cap for needle | 5.2 | 14.0 | 17.6 | 2.8 | — | — | — | 17.6 | ND | ND | |||
| 43 | Hypodermic injection needle (plastic protector) | 6.7 | 11.4 | 12.2 | 15.7 | — | — | — | 12.2 | ND | ND | |||
| 44A | Syringe | — | 4.7 | 7.3 | — | — | — | 4.7 | ND | ND | ||||
| 44B | Syringe (plunger) | — | — | — | — | — | — | ND | ND | |||||
| 45 | Caffeine perfusion infant | — | — | — | — | 0.8 | 15.7 | 0.8 | ND | ND | ||||
| 46 | Water for injection solvent for parenteral use | — | — | — | — | — | — | ND | ND | |||||
| 47 | 0.9% NaCl solution for IV flush (syringe) | — | 5.7 | 6.7 | 2.6 | 10.2 | — | 0.8 | 9.3 | 9.1 | ND | ND | ||
| 48 | 0.9% NaCl solution for IV flush (ampoule) | — | 3.2 | 6.1 | — | — | — | 3.2 | ND | ND | ||||
| NICU items [ (%)] | 7 (50.0%) | 9 (62.3%) | 4 (28.6%) | 2 (14.3%) | 3 (21.4%) | 11 (78.6%) | 4 (28.6%) | 1 (7.1%) | ||||||
Note: Values below the limit of detection are represented by the symbol < followed by the limit of detection. —, not applicable; BPA, bisphenol A; BuP, butyl-paraben; CV, coefficient of variance; , estradiol; EDC, endocrine disrupting chemical; EtP, ethyl-paraben; IV, intravenous; MeP, methyl-paraben; NaCl, sodium chloride; ND, not detected; NICU, neonatal intensive care unit; PB, paraben; PrP, propyl-paraben; , total concentrations of parabens.
Concentrations equivalent to per gram.
Concentrations equivalent to procymidone per gram.
In addition to the presence of BPA or PBs, estrogen-like activity was detected in the clear section of the three-way stopcock ( ), the winged IV catheter (Item No. 36) ( ), the single-lumen umbilical vein catheter ( ), and the IV infusion system extension set ( ). Anti-androgenic activity was observed in the clear section of the three-way stopcock (). Both the three-way stopcock and the single-lumen umbilical vein catheter leached detectable levels of BPA (4.7 and , respectively) and exhibited estrogenic activity (18.3 and , respectively) (see Table S1).
Inhalation Exposure Route
Table 5 displays the BPA and PB content and hormonal activity of the four NICU items related to the inhalation route. BPA was detected in two of these, at a concentration of in the endotracheal tube and in the nasal prong. At least two PB congeners were detected in each item, with ranging up to (nasal cannula). The PB congener with the highest concentration was BuP in the nasal prong and EtP in the remaining three items. The endotracheal tube, which contained BPA and , also showed estrogenic activity ( ). No anti-androgenic activity was observed in any of these items. Endotracheal tube samples were found to leach EtP () and PrP (), and the extracts exhibited estrogenic activity ( ) (see Table S1).
Discussion
This study reports, to our knowledge for the first time, the presence of BPA and PBs in materials in contact with newborns in NICUs. It also presents the first evidence that the contents of NICU materials exert hormonal activities. Thus, BPA was detected in 59.6% of the 52 NICU items tested and PBs in 86.5%, and estrogenic activity was observed in 26.9% of item extracts and anti-androgenic activity in 9.6%. These findings indicate that various NICU materials may act as potential sources of exposure to BPA and PBs for the extremely vulnerable neonates admitted to this type of unit.
Diet is the main route of BPA human exposure in the general population. Although we did not assess nutritional samples (breast milk or formula) in the present investigation, a previous study revealed that nutritional intake might not be a crucial contributor to urinary BPA concentrations in NICU newborns (Duty et al. 2013). With regard to the oral intake of PBs, only limited evidence has been published on the PB content of medication administered in NICUs (Nellis et al. 2015). Interestingly, we found that some medical devices related to the nutrition system have detectable levels of BPA and PBs and show estrogenic and anti-androgenic activities. In particular, we observed a high concentration of BPA and PBs in the gastro-duodenal feeding tube and of BPA in the feeding sampling straw. Higher urinary BPA concentrations were previously reported in NICU neonates who required feeding-related medical devices (e.g., nasogastric tube) in comparison with those who did not (Duty et al. 2013). Estrogenic activity was also shown by two of the items related to oral exposure. The highest estrogenic and anti-androgenic activities in any studied item were observed in extracts from the small dummy used in the NICU, likely due not only to the BPA and PB content but also to nontested EDCs such as DEHP, whose presence is suggested by the plasticity of the material. In this context, authors using a water and MeOH migration technique found that baby teethers leached BPA and PBs (Asimakopoulos et al. 2016; Potouridis et al. 2016).
As in the case of adult patients, the skin of hospitalized neonates is usually in direct contact with dressings, tapes, bandages, electrodes, and wraps, among others. The epidermis of preterm neonates is especially fragile due to incomplete maturation of the skin barrier, exacerbating their susceptibility to chemical irritation and local or systemic infections (Eichenfield and Hardaway 1999; Oranges et al. 2015). Moreover, the incomplete development of the stratum corneum can increase the permeability of neonatal skin to topical agents (Oranges et al. 2015). In the present study, some of the NICU items were identified as putative sources of dermal exposure to BPA and PBs and included patterned transparent film dressing, textile tapes, and light therapy protection glasses, whose extracts also evidenced estrogenic or anti-androgenic activity, as did extracts from pulse oximeter adhesive sensor I, ECG electrodes, and sterile and nonsterile gloves. The range of BPA concentrations in these dermal-contact items (range ) is similar to that reported in infant clothing () (Xue et al. 2017) and lower than that observed in infant socks () (Freire et al. 2019; Xue et al. 2017). However, the dermal exposure of neonates to these EDCs from NICU items is likely to be higher, given the aforementioned immaturity of their skin.
Several IV/parenteral tubing items contained BPA and PBs (e.g., three-way stopcocks, umbilical vein and winged catheters, IV infusion system extension sets), and their extracts also showed hormonal (estrogenic and/or anti-androgenic) activity. In this regard, detectable amounts of BPA were reported to leach from hemodialyzers (Haishima et al. 2001; Murakami et al. 2007), and plasma BPA levels were lower in hemodialysis patients using polynephron (BPA-free) versus conventional polysulfone dialyzer membranes (Mas et al. 2018). Likewise, BPA concentrations were increased after a single hemodialysis session in patients with diabetes (Turgut et al. 2016) or uremia (Shintani 2001), and urinary BPA levels were significantly higher in patients using BPA-free dialyzers in comparison with BPA-containing dialyzers (Bosch-Panadero et al. 2016). Similar studies have reported increased serum DEHP levels in hemodialysis patients (Wahl et al. 2004). We also found that BPA was leached by the IV/parenteral tubing and accessories, and the eluted extracts exhibited estrogenic activity.
With regard to the inhalation exposure route, high BPA and PBs concentrations () were observed in endotracheal tubes and nasal cannulas. In this line, Duty et al. (2013) found significantly higher median BPA urinary concentrations in newborns who required a nasal cannula ( vs. ) or continuous positive airway pressure ( vs. ) in comparison with those who did not. The extract of endotracheal tube assayed in the present study exhibited estrogenic activity, a novel finding. Endotracheal tubes, nasal cannulas, and nasal prongs were made of plasticized PVC, and it is known that products made of plasticized PVC, polycarbonate and epoxy resins may contain BPA (López-Cervantes and Paseiro-Losada 2003; Sun et al. 2001). Chiellini et al. (2011) reported detectable levels of DEHP in PVC endotracheal tubes.
Our findings reveal the widespread presence of hormonally active chemicals in medical items used in NICUs and related to inhalation, oral, dermal, and IV/parenteral routes. Given the extreme vulnerability of these neonates, these findings are of major concern. In adults, the toxicokinetics of BPA and PB vary widely according to the exposure route (Søeborg et al. 2014), whereas the metabolism of BPA and PB in neonates appears to be similar for all routes of exposure (Taylor et al. 2008). For instance, first-pass metabolism considerably reduces the bioavailability of free BPA and PBs after oral exposure in adults (Shin et al. 2019; Søeborg et al. 2014), the capacity for phase II metabolism is slowly maturing in neonates and infants ( months of age), increasing the bioavailability of free BPA and PBs (Mulla et al. 2015; Nachman et al. 2014), which might be even more pronounced in VLBW and LBW pre-term neonates. In contrast, BPA and PBs are systemically bioavailable after dermal or IV exposure, even in adults (SCENIHR 2015; Søeborg et al. 2014). Furthermore, in the case of preterm neonates, the absorption fraction of EDCs after dermal exposure is likely to be higher than the range estimated for adults (), given their increased skin permeability (SCENIHR 2015) and higher skin surface area-to-body weight and weight-to-intake ratios (Guzeilan et al. 1992). Alongside the immaturity of their xenobiotic metabolism capacity, this means that the bioavailability of EDCs may be substantively greater in neonates than in older children or adults for the same exposure doses.
The European Union (EU) has banned the use of BPA in baby bottles (EU 2011) and in materials in contact with foods intended for infants and young children (EU 2018). The EU has also set a maximum BPA migration limit of in toys designed for use by children under 3 years of age or designed to be placed in the mouth (EU 2017). With regard to PBs, the sole restriction in EU legislation is that personal care products for children under 3 years of age should carry a label warning against their use in the diaper area (EU 2014). The evidence presented in the present study indicates the need to regulate the presence of BPA and PBs in materials commonly used in hospitals, especially in NICUs. In this regard, SCENIHR (2015) recommended the selection of medical devices that do not leach EDCs when possible. However, current EU regulations on the prohibition of BPA and PBs do not include hospital materials in intimate contact (oral and nonoral) with extremely vulnerable VLBW and LBW neonates admitted to NICUs.
The EDC concentrations observed in plastic NICU items should not be considered as leaching levels, although the leaching of plastic items with high BPA/PB content or estrogenic/anti-androgenic activity was further examined by using softer extraction methods that may be closer to physiological temperature and pH conditions. Likewise, concentrations in textile and liquid/semisolid products should not be interpreted as absorbed concentrations, and the variability in skin permeability (SCENIHR 2015) prevents estimation of the daily dermal exposure dose. Another study limitation was the failure to measure other potential EDCs such as phthalates, although the presence or absence of phthalates was indicated on the label of some items. We highlight that no item indicated the presence or absence of BPA or PBs. However, we cannot rule out the presence in these extracts of other hormonally active compounds, including phthalates as well as hitherto unidentified compounds with hormonal potency, or the presence of mixtures of various compounds. Furthermore, the possible effect of chemical mixtures, including additive, synergistic, or antagonistic interactions, cannot be predicted from individual chemical contents. Of special interest in this regard was the winged IV catheter (NICU Item 36) in the IV/parenteral route of exposure, in which no BPA or PB was detected but a high level of estrogenic activity was recorded. It should be borne in mind that this study was designed to explore the presence of these compounds in a range of NICU medical devices but not to provide exhaustive analyses of all materials used in NICUs or to compare products among different suppliers. Finally, the results cannot be generalized to NICUs worldwide, given potential differences in hospital protocols and procedures. However, the findings of our exploratory investigation have revealed the widespread presence of hormonally active chemicals in several NICU items, indicating the need for careful examination by competent authorities of all materials and devices in contact with NICU neonates, assessing not only their EDC content and leaching rates but also their biological activity.
Neonates in NICUs may be potentially exposed to hormonally active chemicals from multiple sources. There is a need for further research to verify these findings, to support the development of preventive measures, and to ensure that health care professionals are aware of the EDC content/leaching of materials and devices. It is also crucial to include data on the utilization of medical devices in clinical–epidemiological studies on adverse effects in NICU neonates.
Conclusions
We contribute the first report, to our best knowledge, of direct measurements of the BPA/PB content and hormone-like activity of medical devices and products widely used in NICUs and in prolonged intimate contact with newborns. Our findings suggest that NICU newborns may be exposed to BPA and PBs via inhalation, oral, dermal, and IV/parenteral routes, with the possibility that other hospitalized infants may be similarly exposed. There is an urgent need to investigate the potential short-, mid- and long-term implications of our findings for the health of these highly vulnerable neonates.
Supplementary Material
Acknowledgments
We gratefully acknowledge editorial assistance from R. Davies. This research was funded in part by grants from the European Union Commission (The European Human Biomonitoring Initiative H2020-EJP-HBM4EU), the Spanish Ministry of Economy and Competitiveness, Institute of Health Carlos III - FEDER (PI16/01820, PI16/01812, PI16/01858, PI17/01743, and PI17/01526), the Andalusia Regional Government (PI-0538-2017), and the Spanish Consortium for Research on Epidemiology and Public Health (CIBERESP). The authors are also grateful to the Carlos III Institute of Health (ISCIII) for the predoctoral research contract (FI17/00316) granted to L.M.I.-D., the postdoctoral research contract granted to C.F. (Miguel Servet-FEDER fund MS16/00085), and the José María Segovia de Arana contract granted to N.O. (INT18/00060). This paper is part of the PhD thesis developed by L.M.I.-D. in the context of the “Clinical Medicine and Public Health Program” of the University of Granada.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5564).
These authors equally contributed to this work.
These authors equally contributed to this work.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Agier L, Basagaña X, Maitre L, Granum B, Bird PK, Casas M, et al. 2019. Early-life exposome and lung function in children in Europe: an analysis of data from the longitudinal, population-based HELIX cohort. Lancet Planet Health 3(2):e81–e92, PMID: 30737192, 10.1016/S2542-5196(19)30010-5. [DOI] [PubMed] [Google Scholar]
- Asimakopoulos AG, Elangovan M, Kannan K. 2016. Migration of parabens, bisphenols, benzophenone-type UV filters, triclosan, and triclocarban from teethers and its implications for infant exposure. Environ Sci Technol 50(24):13539–13547, PMID: 27993041, 10.1021/acs.est.6b04128. [DOI] [PubMed] [Google Scholar]
- Berger K, Eskenazi B, Kogut K, Parra K, Lustig RH, Greenspan LC, et al. 2018. Association of prenatal urinary concentrations of phthalates and bisphenol A and pubertal timing in boys and girls. Environ Health Perspect 126(9):97004, PMID: 30203993, 10.1289/EHP3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boberg J, Taxvig C, Christiansen S, Hass U. 2010. Possible endocrine disrupting effects of parabens and their metabolites. Reprod Toxicol 30(2):301–312, PMID: 20381602, 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- Bosch-Panadero E, Mas S, Sanchez-Ospina D, Camarero V, Perez-Gomez MV, Saez-Calero I, et al. 2016. The choice of hemodialysis membrane affects bisphenol a levels in blood. J Am Soc Nephrol 27(5):1566–1574, PMID: 26432902, 10.1681/ASN.2015030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley JP, Quirós-Alcalá L, Teitelbaum SL, Calafat AM, Wolff MS, Engel SM. 2018. Associations of prenatal environmental phenol and phthalate biomarkers with respiratory and allergic diseases among children aged 6 and 7 years. Environ Int 115:79–88, PMID: 29550712, 10.1016/j.envint.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calafat AM, Needham LL, Silva MJ, Lambert G. 2004. Exposure to di-(2-ethylhexyl) phthalate among premature neonates in a neonatal intensive care unit. Pediatrics 113(5):e429–e434, PMID: 15121985, 10.1542/peds.113.5.e429. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, et al. 2009. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect 117(4):639–644, PMID: 19440505, 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiellini F, Ferri M, Latini G. 2011. Physical-chemical assessment of di-(2-ethylhexyl)-phthalate leakage from poly(vinyl chloride) endotracheal tubes after application in high risk newborns. Int J Pharm 409(1–2):57–61, PMID: 21356303, 10.1016/j.ijpharm.2011.02.024. [DOI] [PubMed] [Google Scholar]
- Darbre PD, Harvey PW. 2008. Paraben esters: review of recent studies of endocrine toxicity, absorption, esterase and human exposure, and discussion of potential human health risks. J Appl Toxicol 28(5):561–578, PMID: 18484575, 10.1002/jat.1358. [DOI] [PubMed] [Google Scholar]
- Duty SM, Mendonca K, Hauser R, Calafat AM, Ye X, Meeker JD, et al. 2013. Potential sources of bisphenol A in the neonatal intensive care unit. Pediatrics 131(3):483–489, PMID: 23420909, 10.1542/peds.2012-1380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eichenfield LF, Hardaway CA. 1999. Neonatal dermatology. Curr Opin Pediatr 11(5):471–474, PMID: 10555601, 10.1097/00008480-199910000-00017. [DOI] [PubMed] [Google Scholar]
- EU (European Union). 2010. European Union risk Assessment Report: Human Health Addendum of April 2008—4,4ʹ-isopropylidenediphenol (bisphenol-A)—Part 2 Human Health. EUR 24589 EN. Ispra, Italy: European Commission Joint Research Centre, Institute for Health and Consumer Protection; https://publications.jrc.ec.europa.eu/repository/bitstream/JRC59988/lbna24589enn.pdf [accessed 4 November 2019]. [Google Scholar]
- EU. 2011. Commission Directive 2011/8/EU of 28 January 2011 amending Directive 2002/72/EC as regards the restriction of use of bisphenol A in plastic infant feeding bottles. OJ L 26:11–14. [Google Scholar]
- EU. 2014. Commission Regulation (EU) no 1004/2014 of 18 September 2014 amending Annex V to Regulation (EC) no 1223/2009 of the European Parliament and of the Council on cosmetic products. OJ L 282:5–8. [Google Scholar]
- EU. 2017. Commission Directive (EU) 2017/898 of 24 May 2017 amending, for the purpose of adopting specific limit values for chemicals used in toys, Appendix C to Annex II to Directive 2009/48/EC of the European Parliament and of the Council on the safety of toys, as regards bisphenol A. OJ L 138:128–130. [Google Scholar]
- EU. 2018. Commission Regulation (EU) 2018/213 of 12 February 2018 on the use of bisphenol A in varnishes and coatings intended to come into contact with food and amending Regulation (EU) no 10/2011 as regards the use of that substance in plastic food contact materials. OJ L 41:6–12. [Google Scholar]
- Freire C, Molina-Molina J-M, Iribarne-Durán LM, Jiménez-Díaz I, Vela-Soria F, Mustieles V, et al. 2019. Concentrations of bisphenol A and parabens in socks for infants and young children in Spain and their hormone-like activities. Environ Int 127:592–600, PMID: 30986741, 10.1016/j.envint.2019.04.013. [DOI] [PubMed] [Google Scholar]
- Geens T, Aerts D, Berthot C, Bourguignon JP, Goeyens L, Lecomte P, et al. 2012. A review of dietary and non-dietary exposure to bisphenol-A. Food Chem Toxicol 50(10):3725–3740, PMID: 22889897, 10.1016/j.fct.2012.07.059. [DOI] [PubMed] [Google Scholar]
- Geens T, Goeyens L, Covaci A. 2011. Are potential sources for human exposure to bisphenol-A overlooked? Int J Hyg Environ Health 214(5):339–347, PMID: 21570349, 10.1016/j.ijheh.2011.04.005. [DOI] [PubMed] [Google Scholar]
- Genay S, Luciani C, Décaudin B, Kambia N, Dine T, Azaroual N, et al. 2011. Experimental study on infusion devices containing polyvinyl chloride: to what extent are they di(2-ethylhexyl)phthalate-free? Int J Pharm 412(1–2):47–51, PMID: 21497186, 10.1016/j.ijpharm.2011.03.060. [DOI] [PubMed] [Google Scholar]
- Ghassabian A, Bell EM, Ma WL, Sundaram R, Kannan K, Buck Louis GM, et al. 2018. Concentrations of perfluoroalkyl substances and bisphenol A in newborn dried blood spots and the association with child behavior. Environ Pollut 243(Pt B):1629–1636, PMID: 30296759, 10.1016/j.envpol.2018.09.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimeno P, Spinau C, Lassu N, Maggio AF, Brenier C, Lempereur L. 2015. Identification and quantification of bisphenol A and bisphenol B in polyvinylchloride and polycarbonate medical devices by gas chromatography with mass spectrometry. J Sep Sci 38(21):3727–3734, PMID: 26332920, 10.1002/jssc.201500552. [DOI] [PubMed] [Google Scholar]
- Giulivo M, Lopez de Alda M, Capri E, Barceló D. 2016. Human exposure to endocrine disrupting compounds: their role in reproductive systems, metabolic syndrome and breast cancer. A review. Environ Res 151:251–264, PMID: 27504873, 10.1016/j.envres.2016.07.011. [DOI] [PubMed] [Google Scholar]
- Green R, Hauser R, Calafat AM, Weuve J, Schettler T, Ringer S, et al. 2005. Use of di(2-ethylhexyl) phthalate-containing medical products and urinary levels of mono(2-ethylhexyl) phthalate in neonatal intensive care unit infants. Environ Health Perspect 113(9):1222–1225, PMID: 16140631, 10.1289/ehp.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzeilan PS, Henry CJ, Olin SS. 1992. Similarities and Differences between Children and Adults: Implications for Risk Assessment. Washington, DC: ILSI Press. [Google Scholar]
- Haishima Y, Hayashi Y, Yagami T, Nakamura A. 2001. Elution of bisphenol-a from hemodialyzers consisting of polycarbonate and polysulfone resins. J Biomed Mater Res 58(2):209–215, PMID: 11241341, . [DOI] [PubMed] [Google Scholar]
- Harley KG, Berger KP, Kogut K, Parra K, Lustig RH, Greenspan LC, et al. 2019. Association of phthalates, parabens and phenols found in personal care products with pubertal timing in girls and boys. Human Reprod 34(1):109–117, PMID: 30517665, 10.1093/humrep/dey337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison W, Goodman D. 2015. Epidemiologic trends in neonatal intensive care, 2007–2012. JAMA Pediatr 169(9):855–862, PMID: 26214387, 10.1001/jamapediatrics.2015.1305. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Zhao H, Xia W, Li Y, Liu H, Hao K, et al. 2019. Prenatal exposure to benzophenones, parabens and triclosan and neurocognitive development at 2 years. Environ Int 126:413–421, PMID: 30831476, 10.1016/j.envint.2019.01.023. [DOI] [PubMed] [Google Scholar]
- López-Cervantes J, Paseiro-Losada P. 2003. Determination of bisphenol A in, and its migration from, PVC stretch film used for food packaging. Food Addit Contam 20(6):596–606, PMID: 12881134, 10.1080/0265203031000109495. [DOI] [PubMed] [Google Scholar]
- Mas S, Bosch-Panadero E, Abaigar P, Camarero V, Mahillo I, Civantos E, et al. 2018. Influence of dialysis membrane composition on plasma bisphenol a levels during online hemodiafiltration. PLoS One 13(3):e0193288, PMID: 29529055, 10.1371/journal.pone.0193288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercea P. 2009. Physicochemical processes involved in migration of bisphenol A from polycarbonate. J Appl Polym Sci 112(2):579–593, 10.1002/app.29421. [DOI] [Google Scholar]
- Molina-Molina JM, Jiménez-Díaz I, Fernández MF, Rodriguez-Carrillo A, Peinado FM, Mustieles V, et al. 2019. Determination of bisphenol A and bisphenol S concentrations and assessment of estrogen- and anti-androgen-like activities in thermal paper receipts from Brazil, France, and Spain. Environ Res 170:406–415, PMID: 30623888, 10.1016/j.envres.2018.12.046. [DOI] [PubMed] [Google Scholar]
- Molina-Molina JM, Real M, Jimenez-Diaz I, Belhassen H, Hedhili A, Torné P, et al. 2014. Assessment of estrogenic and anti-androgenic activities of the mycotoxin zearalenone and its metabolites using in vitro receptor-specific bioassays. Food Chem Toxicol 74:233–239, PMID: 25455890, 10.1016/j.fct.2014.10.008. [DOI] [PubMed] [Google Scholar]
- Mulla H, Yakkundi S, McElnay J, Lutsar I, Metsvaht T, Varendi H, et al. 2015. An observational study of blood concentrations and kinetics of methyl- and propyl-parabens in neonates. Pharm Res 32(3):1084–1093, PMID: 25236342, 10.1007/s11095-014-1520-2. [DOI] [PubMed] [Google Scholar]
- Murakami K, Ohashi A, Hori H, Hibiya M, Shoji Y, Kunisaki M, et al. 2007. Accumulation of bisphenol A in hemodialysis patients. Blood Purif 25(3):290–294, PMID: 17622711, 10.1159/000104869. [DOI] [PubMed] [Google Scholar]
- Nachman RM, Hartle JC, Lees PS, Groopman JD. 2014. Early life metabolism of bisphenol A: a systematic review of the literature. Curr Environ Health Rep 1(1):90–100, PMID: 25838989, 10.1007/s40572-013-0003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nellis G, Metsvaht T, Varendi H, Toompere K, Lass J, Mesek I, et al. 2015. Potentially harmful excipients in neonatal medicines: a pan-European observational study. Arch Dis Child 100(7):694–699, PMID: 25854872, 10.1136/archdischild-2014-307793. [DOI] [PubMed] [Google Scholar]
- Oranges T, Dini V, Romanelli M. 2015. Skin physiology of the neonate and infant: clinical implications. Adv Wound Care (New Rochelle) 4(10):587–595, PMID: 26487977, 10.1089/wound.2015.0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez P, Pulgar R, Olea-Serrano F, Villalobos M, Rivas A, Metzler M, et al. 1998. The estrogenicity of bisphenol A-related diphenylalkanes with various substituents at the central carbon and the hydroxy groups. Environ Health Perspect 106(3):167–174, PMID: 9449681, 10.1289/ehp.98106167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potouridis T, Berger E, Püttmann W. 2016. Analysis of alkyl esters of p-hydroxybenzoic acid (parabens) in baby teethers via gas chromatography-quadrupole mass spectrometry (GC-QMS) using a stable isotope dilution assay (SIDA). Anal Methods 8(17):3466–3474, 10.1039/C6AY00261G. [DOI] [Google Scholar]
- Real M, Molina-Molina JM, Jiménez-Díaz I, Arrebola JP, Sáenz JM, Fernández MF, et al. 2015. Screening of hormone-like activities in bottled waters available in Southern Spain using receptor-specific bioassays. Environ Int 74:125–135, PMID: 25454229, 10.1016/j.envint.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Sanchez-Prado L, Llompart M, Lamas JP, Garcia-Jares C, Lores M. 2011. Multicomponent analytical methodology to control phthalates, synthetic musks, fragrance allergens and preservatives in perfumes. Talanta 85(1):370–379, PMID: 21645712, 10.1016/j.talanta.2011.03.079. [DOI] [PubMed] [Google Scholar]
- SCENIHR (Scientific Committee on Emerging and Newly Identified Health Risks). 2015. Opinion on the Safety of the Use of Bisphenol A in Medical Devices. Luxembourg: European Commission. [Google Scholar]
- SCENIHR. 2016. Opinion on the Safety of Medical Devices Containing DEHP-Plasticized PVC or Other Plasticizers on Neonates and Other Groups Possibly at Risk (2015 Update). Luxembourg: European Commission. [DOI] [PubMed] [Google Scholar]
- Shin M-Y, Shin C, Choi JW, Lee J, Lee S, Kim S. 2019. Pharmacokinetic profile of propyl paraben in humans after oral administration. Environ Int 130:104917, PMID: 31234001, 10.1016/j.envint.2019.104917. [DOI] [PubMed] [Google Scholar]
- Shintani H. 2001. Determination of the endocrine disrupter bisphenol-A in the blood of uremia patients treated by dialysis. Chromatographia 53(5–6):331–333, 10.1007/BF02490435. [DOI] [Google Scholar]
- Søeborg T, Frederiksen H, Andersson AM. 2014. Considerations for estimating daily intake values of nonpersistent environmental endocrine disruptors based on urinary biomonitoring data. Reproduction 147(4):455–463, PMID: 24287425, 10.1530/REP-13-0458. [DOI] [PubMed] [Google Scholar]
- Soule HD, Vazguez J, Long A, Albert S, Brennan M. 1973. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst 51(5):1409–1416, PMID: 4357757, 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- Stroustrup A, Bragg JB, Andra SS, Curtin PC, Spear EA, Sison DB, et al. 2018a. Neonatal intensive care unit phthalate exposure and preterm infant neurobehavioral performance. PLoS One 13(3):e0193835, PMID: 29505594, 10.1371/journal.pone.0193835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroustrup A, Bragg JB, Busgang SA, Andra SS, Curtin P, Spear EA, et al. 2018b. Sources of clinically significant neonatal intensive care unit phthalate exposure. J Exp Sci Environ Epidemiol, PMID: 30242269, 10.1038/s41370-018-0069-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wada M, Kuroda N, Hirayama K, Nakazawa H, Nakashima K. 2001. Simultaneous determination of phenolic xenoestrogens by solid-phase extraction and high-performance liquid chromatography with fluorescence detection. Anal Sci 17(6):697–702, PMID: 11707938, 10.2116/analsci.17.697. [DOI] [PubMed] [Google Scholar]
- Taylor JA, Welshons WV, vom Saal FS. 2008. No effect of route of exposure (oral; subcutaneous injection) on plasma bisphenol A throughout 24 h after administration in neonatal female mice. Reprod Toxicol 25(2):169–176, PMID: 18295446, 10.1016/j.reprotox.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Térouanne B, Tahiri B, Georget V, Belon C, Poujol N, Avances C, et al. 2000. A stable prostatic bioluminescent cell line to investigate androgen and antiandrogen effects. Mol Cell Endocrinol 160(1–2):39–49, PMID: 10715537, 10.1016/s0303-7207(99)00251-8. [DOI] [PubMed] [Google Scholar]
- Turgut F, Sungur S, Okur R, Yaprak M, Ozsan M, Ustun I, et al. 2016. Higher serum bisphenol A levels in diabetic hemodialysis patients. Blood Purif 42(1):77–82, PMID: 27193155, 10.1159/000445203. [DOI] [PubMed] [Google Scholar]
- Vela-Soria F, Ballesteros O, Zafra-Gómez A, Ballesteros L, Navalón A. 2014. UHPLC-MS/MS method for the determination of bisphenol A and its chlorinated derivatives, bisphenol S, parabens, and benzophenones in human urine samples. Anal Bioanal Chem 406(15):3773–3785, PMID: 24710638, 10.1007/s00216-014-7785-9. [DOI] [PubMed] [Google Scholar]
- Wahl HG, Hong Q, Hildenbrand S, Risler T, Luft D, Liebich H. 2004. 4-Heptanone is a metabolite of the plasticizer di(2-ethylhexyl) phthalate (DEHP) in haemodialysis patients. Nephrol Dial Transplant 19(10):2576–2583, PMID: 15280519, 10.1093/ndt/gfh425. [DOI] [PubMed] [Google Scholar]
- Wang PG, Zhou W. 2013. Rapid determination of parabens in personal care products by stable isotope GC-MS/MS with dynamic selected reaction monitoring. J Sep Sci 36(11):1781–1787, PMID: 23494853, 10.1002/jssc.201201098. [DOI] [PubMed] [Google Scholar]
- Weuve J, Sánchez BN, Calafat AM, Schettler T, Green RA, Hu H, et al. 2006. Exposure to phthalates in neonatal intensive care unit infants: urinary concentrations of monoesters and oxidative metabolites. Environ Health Perspect 114(9):1424–1431, PMID: 16966100, 10.1289/ehp.8926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue J, Liu W, Kannan K. 2017. Bisphenols, benzophenones, and bisphenol A diglycidyl ethers in textiles and infant clothing. Environ Sci Technol 51(9):5279–5286, PMID: 28368574, 10.1021/acs.est.7b00701. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Lian M, Liu L, Cui H. 2005. High-performance liquid chromatographic assay of parabens in wash-off cosmetic products and foods using chemiluminescence detection. Anal Chim Acta 537(1–2):31–39, 10.1016/j.aca.2005.01.027. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.