Abstract
Background:
Although studies have provided estimates of premature mortality to either heat or cold in adult populations, and fetal exposure to ambient temperature may be associated with life expectancy, the effects of temperature on aging in early life have not yet been studied. Telomere length (TL) is a marker of biological aging, and a short TL at birth may predict lifespan and disease susceptibility later in life.
Objectives:
We studied to what extent prenatal ambient temperature exposure is associated with newborn TL.
Methods:
In the ENVIRONAGE (ENVIRonmental influence ON early AGEing) birth cohort in Flanders, Belgium, we measured cord blood and placental TL in 1,103 mother–newborn pairs (singletons with of gestation) using a quantitative real-time polymerase chain reaction (qPCR) method. We associated newborn TL with average weekly exposure to ambient temperature using distributed lag nonlinear models (DLNMs) while controlling for potential confounders. Double-threshold DLNMs were used to estimate cold and heat thresholds and the linear associations between temperature and TL below the cold threshold and above the heat threshold.
Results:
Prenatal temperature exposure above the heat threshold (19.5°C) was associated with shorter cord blood TL. The association with a 1°C increase in temperature was strongest at week 36 of gestation and resulted in a 3.29% [95% confidence interval (CI): , ] shorter cord blood TL. Consistently, prenatal temperature exposure below the cold threshold (5.0°C) was associated with longer cord blood TL. The association with a 1°C decrease in temperature was strongest at week 10 of gestation with 0.72% (95% CI: 0.46, 0.97) longer cord blood TL.
Discussion:
Our study supports potential effects of prenatal temperature exposure on longevity and disease susceptibility later in life. Future climate scenarios might jeopardize the potential molecular longevity of future generations from birth onward. https://doi.org/10.1289/EHP5153
Introduction
Awareness and concerns regarding global warming worldwide have focused recent research on the evaluation of climate change effects on human health (Patz et al. 2005). Climate change will affect human health in direct and indirect ways via increases in temperature, heatwaves, droughts, floods, changes in crop yield, and alterations in infectious diseases (Watts et al. 2018). It has been estimated that, globally, the average temperature will increase between 2.4 and 4.8°C by 2100 with, in addition, higher frequencies of hot days and longer heatwaves and less frequent cold days (IPCC 2013). Exposures to extreme temperatures, including both acute heat and cold exposures, have been associated with increased cerebrovascular, cardiovascular, and respiratory morbidity and mortality rates, especially in the elderly (Bunker et al. 2016; Mora et al. 2017; Ye et al. 2012). However, recent evidence suggests that also in utero, exposures to both cold and heat are associated with adverse birth outcomes, such as a low birth weight and preterm births (Cox et al. 2016; Strand et al. 2011). This indicates a potential role of ambient temperature in fetal programming in utero.
Animal-based studies suggest a link of increased temperature exposures and increased body temperature with decreases in lifespan (Conti 2008; Keil et al. 2015). Low human body temperature is associated with increased longevity in men from the Baltimore Longitudinal Study of Aging (Roth et al. 2002). Whether ambient temperature exposures may be involved in longevity in humans is unknown. However, Catalano et al. (2008) showed, using time-series methods in large birth cohort studies composed of Danish, Finnish, Norwegian, and Swedish participants, that low ambient temperature during gestation was associated with longer lifespan in males, attributed to an increased proportion of fitter males that survive gestation.
Telomeres are the nucleoprotein structures at the end of chromosomes consisting of up to several thousands of tandem-repeated TTAGGG sequences (Blackburn 1991). Due to the inability of DNA polymerase to fully replicate the lagging DNA strand during replication, telomeres shorten with each cellular division, and oxidative stress and inflammation caused by lifestyle, behavioral, and environmental factors may additionally increase telomere shortening. Most studies evaluated telomere length (TL) as a biological age marker in adults; however, recent evidence underscores the importance of telomeres at birth. TL is highly variable at birth (Factor-Litvak et al. 2016; Okuda et al. 2002), and both heritability and environmental factors operative in utero have been proposed to explain this variability (Entringer et al. 2018). As TL at birth may be predictive of later-life TL (Bijnens et al. 2017) and lifespan (Heidinger et al. 2012) and may indicate susceptibility for later-life diseases (Aviv and Shay 2018), important determinants of newborn TL have recently been explored. Factors associated with shorter newborn TL include prepregnancy body mass index (BMI) (Martens et al. 2016), maternal stress (Entringer et al. 2013; Send et al. 2017), prenatal air pollution exposure (Martens et al. 2017; Perera et al. 2018), and proximity to major roads (Bijnens et al. 2015).
However, identifying and evaluating other important factors related to newborn TL may gain further insights into the developmental origins of TL-associated health and disease conditions later in life. Because ambient temperature exposure may relate to longevity, we hypothesized that prenatal exposure to ambient temperature may be associated with the biological aging process in utero, as reflected by newborn TL. Up until now, no evidence exists on the association between ambient temperature and TL in humans. However, animal-based studies using different fish species indicate a negative association between temperature and TL (Debes et al. 2016; Noreikiene et al. 2017; Simide et al. 2016), and in an experimental study, TL of Saccharomyces cerevisiae was shorter after heat exposure (Romano et al. 2013). To test our hypothesis, we studied the association between newborn TL (measured in cord blood and placental tissue) and exposure to ambient temperature during pregnancy in the ENVIRONAGE (ENVIRonmental influence ON early AGEing) birth cohort.
Methods
Study Population and Data Collection
This study included 1,295 mother–newborn pairs (all singleton births with of gestation) from the ongoing population-based prospective ENVIRONAGE birth cohort study. Participants were recruited between February 2010 and December 2016 from Friday at 1200 hours to Monday at 0700 hours. Detailed study procedures are provided elsewhere (Janssen et al. 2017). The study protocol was approved by the ethical committees of Hasselt University and East-Limburg Hospital in Genk (Belgium) and has been carried out according to the Declaration of Helsinki. To obtain a population of individuals having full data on both cord blood and placental TL for statistical analysis (), we excluded 192 individuals due to lack of exposure data () and lack of TL in cord blood () or in placenta (), of which 3 individuals had no TL evaluated in both cord blood and placenta.
Maternal prepregnancy BMI () was determined at the first antenatal visit (weeks 7–9 of gestation). The date of conception was estimated using the first ultrasound exam. After delivery, mothers provided informed consent and completed the study questionnaires in the postdelivery ward. Detailed information on maternal age, paternal age, maternal education, smoking status, fruit and vegetables consumption, physical activity, parity, and newborn ethnicity was collected. Maternal education was coded “low” when mothers did not obtain any degree, “middle” when they obtained a high school degree, and “high” when they obtained a college or university degree. Mothers were categorized as “never smoker,” “former smoker” when they quit smoking before pregnancy, and “smoker” when smoking continued during pregnancy. Maternal consumption of fruit and vegetables was based on the number of portions as indicated by the mothers (estimated for the entire duration of pregnancy) and classified into the following categories: less than one portion per day, one portion per day, two portions per day, and three or more portions per day. Maternal physical activity was based on the amount of times a week (estimated for the entire duration of pregnancy) a mother indicated to be physically active for more than 20 min. This was coded “low” when mothers indicated to be active less than once a week, “middle” when indicated to be active once a week, and “high” when indicated to be active two or more times a week. Newborns were classified as “European-Caucasian” when two or more grandparents were European, and non-European when at least three grandparents were of non-European origin. Also, perinatal parameters such as birth date, newborn sex, and birth weight were obtained. Season of birth was defined based on date of birth and was categorized as “winter” (from 21 December to 20 March), “spring” (from 21 March to 20 June), “summer” (from 21 June to 20 September), and “autumn” (from 21 September to 20 December). Using the medical records obtained at the hospital, we identified pregnancy complications by the presence of one or more of the following conditions during pregnancy: gestational diabetes, hypertension, infectious disease, preeclampsia, vaginal bleeding, and hyper- or hypothyroidism. The ENVIRONAGE birth cohort is generalizable to the gestational segment of the population at large, as it was similar to all births (1999–2009) in Flanders, Belgium, with respect to maternal age, education, parity, newborn sex, ethnicity, and birth weight (Cox et al. 2013). Maternal plasma and cord blood plasma vitamin D levels were measured with an electro-chemiluminescence immunoassay using the Modular E170 automatic analyzer (Roche).
Exposure Assessment
Daily mean temperature (ºC) and relative humidity (%) were measured at a representative measuring station (Diepenbeek, Belgium), and these data were provided by the Belgian Royal Meteorological Institute. This measuring station is the nearest to the recruitment hospital (), and is representative for the participants who on the average (5th to 95th percentiles), lived (3.8 to 25.7 km) from the station. Starting from the date of conception, we calculated the mean weekly temperature for each week of pregnancy. As full exposure data are needed in distributed lag models, we restricted our analysis to 36 wk of gestation and excluded newborns with a gestational age less than 36 wk.
As exposure to ambient air pollution [particulate matter with aerodynamic diameter ()] is associated with newborn TL (Martens et al. 2017), and the daily variation in air pollution concentrations is related to meteorological conditions including ambient temperature, we considered ambient air pollution a confounder. We modeled daily mean concentrations (in ) using a high-resolution spatial-temporal interpolation method (kriging) (Janssen et al. 2008) in combination with a dispersion model (Lefebvre et al. 2011, 2013), as described previously (Janssen et al. 2017). More than 80% () of the temporal and spatial variability in concentrations in the Flemish region of Belgium was explained by this interpolation tool (Maiheu et al. 2012). For weeks 1 to 36 of pregnancy, from the date of conception onward, a weekly mean concentration was calculated using daily mean concentrations at the mother’s residence. Address changes of mothers (; 12.2%) during the period of pregnancy were taken into account when calculating the weekly exposures to .
Sample Collection and Average Relative Telomere Length Measurement
Standardized procedures for umbilical cord blood and placental tissue collection for TL assessment have been described in detail previously (Janssen et al. 2017; Martens et al. 2016). Umbilical cord blood was collected immediately after delivery in BD Vacutainer® plastic whole-blood tubes with spray-coated K2EDTA (BD). Samples were centrifuged at for 15 min, plasma was separated, and the remainder with the buffy coats were stored at . Placentas were collected and stored at within 10 min of delivery. Four different placental biopsies () were taken underneath the chorioamniotic membranes at the fetal side at from the umbilical cord and were stored at as described previously (Janssen et al. 2014). Care was taken by visual examination and dissection to avoid chorioamniotic membrane contamination. As the average variation of TL within one placenta was low, i.e., 11.7% (measured for 4 biopsies in 14 placentas), only one biopsy taken to the right of the main artery was used for placental TL measurement. DNA was extracted from cord blood buffy coat and placental tissue using the QIAamp DNA Mini Kit (Qiagen). Average relative TL was measured in triplicate using a real-time PCR method, as described previously (Martens et al. 2016). In brief, we amplified the telomeric region using telomere-specific primers (telg and telc), and one single-copy gene was amplified (36B4) on a 7900HT Fast Real-Time PCR System (Applied 0Biosystems). Cycle thresholds of the telomere amplifications were normalized relative to the cycle thresholds of the single-copy amplifications using qbase software (version qbase+, Biogazelle). TLs were measured in two separate batches. Average relative TLs were therefore expressed as the ratio of telomere copy number to single-copy gene number (T/S) relative to the average T/S ratio of the entire sample set within each batch. Reaction efficiency was assessed on each reaction plate, and interrun calibrators were used to account for interrun variability. In batch 1, we measured 785 cord blood and 648 placental telomeres; in batch 2, we measured 318 cord blood and 455 placental telomeres. For cord blood, the triplicates of the telomere runs, single-copy gene runs, and T/S ratios showed coefficients of variation (CV) of 0.69, 0.43, and 6.8%; while for placental telomeres, the CVs were 0.75, 0.45, and 7.1%, respectively.
Statistical Analysis
We used distributed lag nonlinear models (DLNMs) to model the association between TL and average weekly mean temperature during gestational weeks 1 to 36. A DLNM is defined through a cross-basis function, which allows the simultaneous estimation of a nonlinear exposure–response association and nonlinear effects across lags, the latter termed lag–response association (Gasparrini 2014). In the first stage, the temperature–TL association was modeled using a natural cubic spline with 5 degrees of freedom (df), placing spline knots at equally spaced values of the actual temperature range to allow enough flexibility in the two ends of the temperature distribution. The median weekly average temperature (11.1°C) was used as the reference value to calculate estimates. As the exposure–response curve obtained by the natural cubic spline DLNM was relatively flat at average temperatures, and associations between temperature and TL were only observed at both ends of the temperature distribution, we applied a double-threshold DLNM in the second stage. A double-threshold DLNM implies no association between temperature and the outcome for temperatures between the cold and the heat threshold, whereas the association between temperature and the outcome is assumed to be linear below the cold threshold and above the heat threshold. We tested multiple cold thresholds (in 0.5°C gaps) from (1st percentile) to 6.0°C (25th percentile) and heat thresholds from 15.0°C (75th percentile) to 22.0°C (99th percentile) and searched for the combination that minimized the residual deviance of the model. We then calculated the percent difference in TL for a 1°C decrease in temperature below the cold threshold and for a 1°C increase above the heat threshold. In all analyses, the lag structure was modeled using a natural cubic spline with 5 df, with knots at equally spaced values in the original lag scale (1 to 36 wk). We also included a cross basis for in the model (Martens et al. 2017). The function was assumed to be linear, and the lag structure was modeled using a natural cubic spline with 5 df (with knots at equally spaced values in the original lag scale). In addition, we accounted for a priori selected covariates that included known determinants of newborn or adult TL and variables with a potential link with ambient temperature and TL, such as date of delivery, season of birth, gestational age, maternal and paternal age, maternal prepregnancy BMI, newborn sex, ethnicity, parity, maternal smoking status, maternal education, pregnancy complications, and batch of TL measurement. The Akaike information criterion was used to compare the fit of models (Gasparrini et al. 2010).
Additionally, average effect estimates of the weekly-based estimates over the different weeks (weeks 1–36) of pregnancy were calculated and expressed as a percent difference in TL for a 1°C decrease below and above the cold and heat thresholds, respectively. In addition, we ran postnatal exposure models, using average weekly temperatures for 36 wk after delivery, to evaluate the presence or absence of postnatal exposure associations, using the same model specifications as described above. We calculated 36 wk averaged postnatal effect estimates, which were compared with the averaged prenatal effect estimates. In addition, the average prenatal effect estimates were compared in different sensitivity analyses. In the first sensitivity analysis, we used an unconstrained DLNM to define the lag structure, that is, a model in which the weekly mean exposures () are entered as separate variables (Schwartz 2000; Zanobetti et al. 2000). Also, we adjusted our model for month of delivery instead of season of birth, and we tested the exclusion of the cross basis for and the inclusion of a cross basis for humidity (linear exposure–response function and 5 df for the lag structure). We additionally adjusted our models for maternal fruit and vegetables consumption, physical activity, maternal plasma and cord blood plasma vitamin D levels, and our cord blood TL models for white blood cell counts and the percentage of neutrophils, lymphocytes, monocytes, and eosinophils. We also checked whether results were robust to the exclusion of newborns from non-European origin, mothers with low education, current or former smokers, mothers with complications during pregnancy, and those who had a caesarean section. Finally, we ran models by newborn sex. All analyses were performed with the statistical software R (Version 3.5.1, R Development Core Team) using the dlnm package (Gasparrini 2011).
Results
Characteristics of the Study Population
General characteristics of the study population () are provided in Table 1. The newborns, which included 529 (48.0%) girls, had a mean [standard deviation (SD)] gestational age of 39.4 (1.1) wk and mean (SD) birth weight of 3,455 (440) g. Most (86.9%) of the newborns were Europeans of Caucasian ethnicity. Mothers had a mean (SD) age of 29.3 (4.5) y and a prepregnancy BMI of . Most of the mothers (64.3%) did not smoke during pregnancy and obtained at least a secondary school degree (88.8%). A total of 575 (52.%) mothers were primiparous and 396 (36%) secundiparous. Fathers had a mean (SD) age of 31.7 (5.1) y. The average weekly mean (5th to 95th percentile) ambient temperature during pregnancy was 10.8°C (1.0, 19.6). In Table 2, we show temperature characteristics for the first week of conception presented by different seasons of conception to show temperature variability across individuals within different seasons. Average weekly mean (5th to 95th percentiles) residential exposure was (4.2–), and the annual mean (5th to 95th percentiles) concentrations of (9.4–) observed in the ENVIRONAGE birth cohort are currently above the World Health Organization guidelines for exposure ( for annual mean concentrations and 24-h mean exposure). Cord blood and placental TL ranged from 0.51 to 1.74 and from 0.40 to 2.0, respectively. Cord blood TL and placental TL were correlated (; ).
Table 1.
Mother–newborn characteristics () of a subset of live singleton births of gestation in Flanders, Belgium (February 2010 to December 2016) ENVIRONAGE (ENVIRonmental influence ON early AGEing) birth cohort.
| Characteristic | or (%) |
|---|---|
| Newborns | |
| Birth weight, g | |
| Sex | |
| Girls | 529 (48.0) |
| European-Caucasian | 958 (86.9) |
| Gestational age | |
| 37 wk | 57 (5.2) |
| 38 wk | 158 (14.3) |
| 39 wk | 316 (28.7) |
| 40 wk | 414 (37.5) |
| 41 wk | 158 (14.3) |
| Season of birth | |
| Winter | 253 (22.9) |
| Spring | 275 (24.9) |
| Summer | 271 (24.6) |
| Autumn | 304 (27.6) |
| Plasma vitamin D, ng/mLa | 24.5 (13.1) |
| Mothers | |
| Age, years | |
| BMI, | |
| Education | |
| Low | 124 (11.2) |
| Middle | 421 (38.2) |
| High | 558 (50.6) |
| Smoking status | |
| Never smoker | 709 (64.3) |
| Former smoker | 264 (23.9) |
| Current smoker | 130 (11.8) |
| Pregnancy complications | 139 (12.6) |
| Cesarean section | 43 (3.9) |
| Parity | |
| 1 | 575 (52.1) |
| 2 | 396 (35.9) |
| 132 (12.0) | |
| Physical activityb | |
| Low | 330 (31.5) |
| Middle | 223 (21.3) |
| High | 495 (47.2) |
| Fruit and vegetables consumptionc | |
| 120 (11.1) | |
| 1 portion/day | 331 (30.8) |
| 2 portions/day | 404 (37.6) |
| 221 (20.5) | |
| Weekly mean temperature, °Cd | 10.8 (1.0 to 19.6) |
| Weekly mean exposure, d | 12.6 (4.2 to 30.1) |
| Plasma vitamin D, ng/mLe | 17.5 (9.3) |
| Fathers | |
| Age, years | |
Note: Education was low when no degree was obtained, middle when a high school degree was obtained, and high when a college or university degree was obtained. Pregnancy complications include the presence of one or more conditions, including gestational diabetes, hypertension, infectious disease, preeclampsia, vaginal bleeding, and hyper- or hypothyroidism. BMI, body mass index; CI, confidence interval; European-Caucasian: three or more grandparents of European descent; , particulate matter with aerodynamic diameter ; SD, standard deviation.
Data missing on .
Data missing on and defined as low when active (at least 20 min) less than once a week, middle when active once a week, and high when active two or more times a week during pregnancy.
Data missing on .
Presented as weekly means (5th to 95th percentiles) and for 36 wk of gestation. Temperatures were derived from the Belgian Royal Meteorological Institute, and was modeled at the maternal residential address.
Data missing on .
Table 2.
Temperature characteristics of the first week of conception (in °C) for the entire population and presented by season of conception for participants of the ENVIRONAGE (ENVIRonmental influence ON early AGEing) birth cohort.
| Period | Mean | SD | 5th to 95th percentiles | |
|---|---|---|---|---|
| Entire population | 1,103 | 10.6 | 6.0 | 0.96 to 19.4 |
| Winter | 299 | 4.3 | 3.6 | to 9.2 |
| Spring | 252 | 12.9 | 3.6 | 6.5 to 18.4 |
| Summer | 276 | 17.0 | 2.6 | 13.5 to 22.2 |
| Autumn | 276 | 8.2 | 4.1 | 1.4 to 14.7 |
Association between Prenatal Temperature Exposure and Newborn Telomere Length
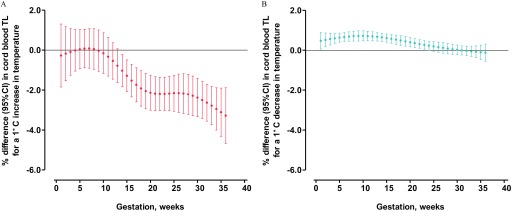
The nonlinear relationships of cord blood and placental TL with ambient temperature exposure for different gestational weeks (obtained by the natural cubic spline DLNM) are provided in Figures S1 and S2. The combination of temperatures minimizing the residual deviance in the double-threshold DLNM for cord blood TL were 5.0°C (cold threshold) and 19.5°C (heat threshold). These thresholds correspond to the 18th and 95th percentiles of the temperature distribution, respectively. Lag-specific (weekly) DLNM estimates of the difference in cord blood TL associated with a 1°C increase above the heat threshold and with a 1°C decrease in weekly ambient temperature below the cold threshold are presented in Figure 1. An increase in weekly ambient temperature above the heat threshold was associated with a shorter cord blood TL from gestational week 14 to 36 (Figure 1A). The association of a 1°C increase was strongest at week 36 (3.29% shorter TL; 95% CI: , ) and the weakest at week 14 of gestation (1.04% shorter TL; 95% CI: , ). Averaging the weekly effect estimates over the entire pregnancy showed that each 1°C increase in ambient temperature above the heat threshold was associated with 1.49% (95% CI: , ) shorter cord blood telomeres (Table 3).
Figure 1.
Heat and cold effect estimates on cord blood telomere length (TL). Week-specific estimates provided as percent (%) differences in average relative TL [with 95% confidence interval (CI)] for a 1°C increase in ambient temperature above the heat threshold (19.5°C) for associations with heat (A), and a 1°C decrease in ambient temperature below the cold threshold (5.0°C) for associations with cold (B). Models were adjusted for date of delivery, gestational age, maternal prepregnancy body mass index (BMI), maternal age, paternal age, newborn sex, newborn ethnicity, season of birth, parity, maternal smoking status, maternal education, pregnancy complications, maternal particulate matter with aerodynamic diameter () exposure, and batch.
Table 3.
Comparison between average prenatal effect estimates and average postnatal effect estimates for the association between ambient temperature and newborn telomere length (TL).
| Model | Percent difference (95% CI) | ||
|---|---|---|---|
| Heat effect | Cold effect | ||
| Cord blood | |||
| Prenatal model | 1,103 | (, ) | 0.37 (0.23, 0.51) |
| Postnatal model | 1,103 | 0.13 (, 0.81) | 0.04 (, 0.16) |
| Placenta | |||
| Prenatal model | 1,103 | (, 1.13) | 0.76 (0.47, 1.06) |
| Postnatal model | 1,103 | (, 1.99) | 0.45 (0.18, 0.73) |
Note: Estimates are averaged week-specific estimates over the entire prenatal period (36 wk after conception) and postnatal period (36 wk after delivery). Estimates provided as a percent difference [95% confidence interval (CI)] in TL for a 1°C increase in ambient temperature above the heat threshold (19.5°C for cord blood and 21.5°C for placenta) for associations with heat and a 1°C decrease in ambient temperature below the cold threshold (5.0°C for cord blood and 2.5°C for placenta) for associations with cold. Models were adjusted for date of delivery, gestational age, maternal prepregnancy body mass index (BMI), maternal age, paternal age, newborn sex, newborn ethnicity, season of birth, parity, maternal smoking status, maternal education, pregnancy complications, maternal particulate matter with aerodynamic diameter () exposure, and batch.
Among similar lines, a decrease in ambient temperature under the cold threshold was associated with longer telomeres from gestational weeks 1 to 24 (Figure 1B). The association of a 1°C decrease in weekly ambient temperature was strongest at week 10 (0.72% longer TL; 95% CI: 0.46, 0.97) and the weakest at week 24 (0.23% longer TL; 95% CI: 0.00, 0.45). Averaging the weekly effect estimates over the entire pregnancy showed that each 1°C decrease in ambient temperature below the cold threshold was associated with 0.37% (95% CI: 0.23 to 0.51) longer cord blood telomeres (Table 3).
Estimated cold (2.5°C) and heat thresholds (21.5°) for placental TL correspond to the 9th and 98th percentiles of the temperature distribution, respectively. Similar to cord blood TL, a decrease in ambient temperature below the cold threshold was associated with longer placental telomeres in weeks 3 to 34 of gestation (Figure S3B). Averaging the weekly effect estimates over the entire pregnancy showed that each 1°C decrease in ambient temperature below the cold threshold was associated with 0.76% (95% CI: 0.47, 1.06) longer placental TL (Table 3). However, depending on the gestational week, an increase in temperature above the heat threshold showed a positive or negative association with placental TL (Figure S3A), but no averaged effect was observed over the entire pregnancy (Table 3).
In models exploring the associations between newborn TL and postnatal exposures, no significant averaged effect estimates over the postnatal period (36 wk after delivery) were observed for the association of postnatal temperature exposure above and below the heat and cold thresholds with cord blood TL. For placenta, no significant heat effect estimates were observed, and a weaker association with postnatal temperatures below the cold threshold was observed compared with the prenatal cold effect estimates (Table 3).
Sensitivity Analyses
The robustness of our findings in cord blood TL was evaluated by comparing the average of the weekly estimates over the entire pregnancy in the main model with those from several sensitivity analyses (Table 4). Our results remained robust for model adjustments to relative humidity, month of delivery, maternal fruit and vegetables consumption, maternal physical activity, maternal and cord blood plasma vitamin D levels, and cord blood cell counts. Exclusion of non-European newborns, mothers with low education levels, current and former smokers, and mothers who experienced a pregnancy complication or underwent a cesarean section did not alter our results. Temperature was associated with newborn TL in both boys and girls. Sensitivity analyses for the placental models are provided in Table S1.
Table 4.
Sensitivity analyses for cord blood telomere length (TL).
| Model | Percent difference (95% CI) in cord blood TL | ||
|---|---|---|---|
| Heat effect | Cold effect | ||
| Main model | 1,103 | (, ) | 0.37 (0.23, 0.51) |
| Unconstrained lag structure | 1,103 | (, ) | 0.34 (0.20, 0.48) |
| Adjustment for month of delivery | 1,103 | (, ) | 0.44 (0.30, 0.59) |
| Adjustment for humidity | 1,103 | (, ) | 0.38 (0.23, 0.53) |
| No adjustment | 1,103 | (, ) | 0.20 (0.09, 0.31) |
| Adjustment for fruit and vegetables consumptiona | 1,076 | (, ) | 0.38 (0.24, 0.52) |
| Adjustment for physical activityb | 1,048 | (, ) | 0.35 (0.20, 0.49) |
| Adjustment for cord plasma vitamin Dc | 937 | (, ) | 0.32 (0.17, 0.46) |
| Adjustment for maternal plasma vitamin Dd | 764 | (, ) | 0.32 (0.16, 0.47) |
| Adjustment for cord blood cell countse | 847 | (, ) | 0.39 (0.23, 0.54) |
| Excluding non-European | 958 | (, ) | 0.43 (0.29, 0.58) |
| Excluding low educated | 979 | (, ) | 0.40 (0.25, 0.55) |
| Excluding current and former smokers | 709 | (, ) | 0.41 (0.23, 0.59) |
| Excluding pregnancy complications | 964 | (, ) | 0.40 (0.25, 0.54) |
| Excluding cesarean sections | 1,060 | (, ) | 0.37 (0.24, 0.51) |
| Excluding all of the above | 471 | (, ) | 0.54 (0.32, 0.75) |
| Newborn boys | 574 | (, ) | 0.40 (0.20, 0.59) |
| Newborn girls | 529 | (, ) | 0.32 (0.12, 0.52) |
Note: Estimates are averaged week-specific estimates over the entire pregnancy. Estimates provided as a percent difference [95% confidence interval (CI)] in TL for a 1°C increase in ambient temperature above the heat threshold (19.5°C) for associations with heat and a 1°C decrease in ambient temperature below the cold threshold (5.0°C) for associations with cold temperatures. Models were adjusted for date of delivery, gestational age, maternal prepregnancy body mass index (BMI), maternal age, paternal age, newborn sex, newborn ethnicity, season of birth, parity, maternal smoking status, maternal education, pregnancy complications, maternal particulate matter with aerodynamic diameter () exposure, and batch. The season of birth was removed from the model adjusting for month of delivery.
Missing data on .
Missing data on .
Missing data on .
Missing data on .
Adjusted for white blood cells ( count), neutrophils (%), lymphocytes (%), monocytes (%), and eosinophils (%); missing data on .
Discussion
TL at birth is a major contributor to adult TL (Bijnens et al. 2017). Therefore, unraveling the environmental determinants of TL at birth is important in the context of the developmental origins of health and disease. In our prospective birth cohort study, including more than 1,000 mother–newborn pairs, we found that in a temperate climate, gestational exposure to higher ambient temperature was associated with shorter cord blood TL at birth. In addition to the negative association between heat and newborn TL, we estimated a positive association between cold and TL. A decrease in temperature below a cold threshold was associated with both longer cord blood and placental TLs. Our results shed a new light on indirect prenatal risks of climate change, which may have further consequences in later life. Investigations show that climate change has the potential to result in a substantial increase in temperature-related mortality in most regions (Gasparrini et al. 2017). In these projections, the potential risk of the prenatal exposures on markers of longevity should be further considered.
The importance of our findings are underscored by the observation that early-life TL may relate to life expectancy as observed in an animal-based study on zebra finches (Heidinger et al. 2012), and secondly, by the observation that initial TL predicts later-life TL and therefore may be indicative of the susceptibility to later-life TL-associated diseases (Aviv and Shay 2018; Benetos et al. 2013; Bijnens et al. 2017). Indeed, recently, it has been shown that TL measured at birth determines a person’s TL measured approximately 20 y later in life (Bijnens et al. 2017).
Two lines of evidence support our current molecular epidemiological associations in newborns. First, studies in both poikilotherm and homeotherm animals suggest a link of increased temperature exposures and increased body temperature with decreases in lifespan (Conti 2008; Holloszy and Smith 1986; Keil et al. 2015; Zhang et al. 2015). In transgenic mice (overexpression of the uncoupling protein 2 in hypocretin neurons, Hcrt-UCP2) with a lower body temperature of 0.3°C , and independent of caloric intake, the median lifespans increased 12% in males and 20% in females, indicating a clear relationship between temperature and longevity (Conti et al. 2006). Second, epidemiological evidence suggests that season of birth predicts health and disease conditions later in life. For instance, based on population data for Austria, Denmark, and Australia, the month of birth and remaining life expectancy at age 50 were found to be related, with higher life expectancy for people born in winter (Doblhammer and Vaupel 2001). In addition, cold ambient temperatures during gestation are predicted to lead to longer lifespans of males (Catalano et al. 2008). In the Baltimore Longitudinal Study of Aging, a higher survival rate of men (age range: 19–95 y) in the lower half of the body temperature distribution was observed compared with men in the upper half (Roth et al. 2002). Evidence whether ambient temperature exposure may alter core body temperature is rather limited, although a study of women exposed for 48 h at 27°C showed a significant increase in skin and body temperature (van Marken Lichtenbelt et al. 2001). Clear metabolic alterations have been observed in relationship to low ambient temperature exposures. Independently of seasonal fluctuations, exposures to cold environments induce brown adipose tissue abundance and activity, impacting energy and substrate metabolism (Lee et al. 2014). Also, it has been shown that acute cold exposure may have immune-stimulating effects (Brenner et al. 1999; Jansky et al. 1996), and may both induce and reduce inflammatory responses (Gagnon et al. 2014; Halonen et al. 2010).
How can we explain our observations? During pregnancy, both alterations in maternal core body temperatures, as well as exposures to extreme ambient temperatures, are related to adverse neonatal outcomes. Ambient exposures to both low and high temperatures have been associated with preterm birth and birth weight, although inconclusive results have been reported (Strand et al. 2011). As reviewed by Edwards et al. (2003) maternal–fetal heat exchange mechanisms may lead to potential adverse effects of heat on birth outcomes, including embryonic death, abortion, growth retardation, and developmental defects. Different identified biological and molecular processes may underlie the adverse effects of heat on both mothers and newborns. Heat exposure may induce heat cytotoxicity, coagulopathies, and systemic inflammation responses, as it is associated with elevated production of proinflammatory cytokines (Leon and Helwig 2010a, 2010b). Upon heat exposure, increased muscle and skin blood flow has been observed in order to increase heat loss, which is compensated with decreases in blood flow in other parts of the human body, including visceral organs (Garcia-Trabanino et al. 2015). A reduced maternal blood flow may affect the maternal–fetal exchange of nutrients and heat, which may lead to the adverse effects observed in newborns upon heat exposure, including reduced fetal growth and a lower birth weight (Lawlor et al. 2005). This potential mechanism relies on animal-based studies, which suggest that chronic heat stress reduces placental weight and decreases uterine and umbilical blood flow, leading toward a reduction in both oxygen and glucose transportation to the fetus (Wells 2002).
The exact mechanisms underlying potential effects of heat exposure on the telomere biology system are still rather unclear. The aforementioned heat-induced biological mechanisms, however, support the inverse association between high ambient exposure and TL in our study population, as telomeres have been associated with inflammatory pathways (Jurk et al. 2014). A recent animal-based study of Siberian sturgeon showed that a 1-month heat exposure to 30°C was associated with 15% shorter telomeres (Simide et al. 2016). Heat stress has been shown to influence telomere integrity, and the shortening of telomeres in yeast cells after heat exposure has been shown (Romano et al. 2013). Heat shock induces the expression of heat shock factor 1, and this induces TElomeric Repeat containing RNA (TERRA) transcription, which protects against heat-induced telomeric integrity alterations, indicating clear heat effects on the telomere biology system (Koskas et al. 2017). In addition, human telomerase activity has been shown to be temperature dependent, with maximum activity at 37°C under experimental conditions (Sun et al. 1999). Our finding that longer telomeres are associated with decreasing temperatures below a cold threshold are indicative of a protective effect of cold exposure on TL. Along similar lines, caloric restriction may increase lifespan, and underlying effects include reduced body temperatures, lower metabolic rates, and alteration of the oxidative stress states (Sohal and Weindruch 1996).
Our results showed similar associations for cold with both cord blood and placental TL, with a slight difference in time windows during pregnancy. In contrast, we only observed an overall negative association (averaged over the entire pregnancy) between heat exposure and cord blood TL but not with placental TL. Regarding the latter, we need to acknowledge that our results of heat estimates in placenta should be interpreted with caution because we only have a low number of observations above the heat threshold (21.5°C). This is supported by the variable results for placental TL associations above the heat threshold in several sensitivity analyses and differences observed in the sex-specific analysis. Furthermore, we identified several differences in time windows for the cold and heat estimates in cord blood and placenta, and these might be different, first due to different cold and heat thresholds that were identified, which minimized the residual deviance in the double-threshold DLNM, and second, by differences in TL regulation in different tissues. We observed stronger associations with heat in cord blood late in pregnancy, which may be indicative of a decrease in heat stress–related compensating mechanisms making the telomere system more vulnerable to heat exposure late in gestation. We currently can only speculate about the biological meanings behind these observations, as this is the first report on ambient temperature and TL in humans. Although it has been shown that the telomere system is plastic in utero, and especially in early embryogenesis, strong changes in TL have been observed (Schaetzlein et al. 2004). Lengthening of telomeres were observed in early cleavage mice embryos (Liu et al. 2007), and in humans, a shortening in TL was observed around 6 wk of gestation with maintenance of length later throughout gestation (Cheng et al. 2013; Youngren et al. 1998). In conjunction with these observations, telomerase activity is high during embryonic and fetal development but decreases throughout gestation (Cheng et al. 2013; Ulaner and Giudice 1997). These dynamics in fetal telomere biology may therefore differentially interact with and respond to environmental exposures, which may, to some extent, account for the different vulnerability periods of telomeres during gestation. Furthermore, other TL-regulating factors, as stated previously, including telomerase activity and TERRA expression, may be temperature sensitive, and further investigation of these factors may help researchers gain insights in the understanding of vulnerable periods for telomeres to heat or cold exposures. Lastly, TL is regulated by epigenetic factors (Blasco 2007), and alterations in fetal epigenetic marks, especially subtelomeric methylation, may be important in setting TL (Ravlić et al. 2018). In this regard, ambient prenatal temperature during the first trimester was associated with the placental density of DNA methylation distribution (Abraham et al. 2018).
Other factors related to ambient temperature exposure that are associated with TL, including diet (Crous-Bou et al. 2014) and physical activity (Arsenis et al. 2017), might explain the observed associations. However, adjusting for maternal physical activity and fruit and vegetables consumption did not alter our results. Furthermore, ambient temperature is strongly associated with light or sun exposure, and several factors related to sun and light exposure, including vitamin D levels or psychological mood alterations and stress, may be important factors in our observations. This because maternal vitamin D status has been associated with newborn TL (Kim et al. 2018) and mood disorders (Muneer and Minhas 2019) have been linked with TL. These factors may warrant further attention, and in this regard, we were able to adjust our models, in a smaller subset, for maternal plasma and cord blood plasma vitamin D levels, and showed that our associations were independent of maternal or newborn vitamin D status.
This study has several strengths. First, we were able to assess the association between newborn TL and ambient temperature in a large-sample-size cohort of more than 1,000 newborns in two matrices. Second, we were able to adjust for important potential confounders including parental age, newborn sex, ethnicity, gestational age, maternal prepregnancy BMI, education, smoking status, parity, pregnancy complications, air pollution exposure, and season of birth. In addition, our estimates were found to be robust to changes in model specifications and exclusions of specific population groups. Third, by applying a double-threshold DLNM, we were able to identify cold and heat thresholds and critical periods during pregnancy for the association between newborn TL and ambient temperature exposures. Fourth, our study population is generalizable, as it is representative of the gestational segment of the population at large (Janssen et al. 2017). Fifth, our recruitment area is uniform for temperature due to very small differences in altitude (ranging from 10 to above sea level) and latitude (distance between the most southern and northern part, ) with a maximum spatial variation of 0 to 1.5°C observed based on monthly temperatures (Belgian Royal Meteorological Institute). This study needs to be interpreted within the following limitations. First, more accurate temperature measurements, such as personal exposures to ambient temperature and measures of body temperature, were not available. We acknowledge that pregnant women may spend a lot of their time indoors, where temperatures may be different from the ambient measured temperatures because of the use of air-conditioning and/or heating. This was not taken into account, as no information was available on time spent indoors or on the use of air-conditioning and heating. On warm days, however, outdoor temperature may be a better reflection of the actual exposure, since the proportion of houses equipped with air-conditioning in Belgium is relatively low. We also did not have information on the occurrence of maternal fever during pregnancy. Second, our results may only be generalizable for regions with a climate similar to our study population, especially regarding the identified heat and cold thresholds. Belgium is classified as a Cfb climate using the Köppen-Geiger climate classification (Peel et al. 2007). This climate is characterized as a temperate climate without a dry season and with a warm summer, and is referred to as an oceanic climate (with a monthly mean temperature below 22°C in the warmest month and above 0°C in the coldest month). This climate is representative for western European countries and other regions in southeastern Australia, Tasmania, New Zealand, and North and South America. Therefore, other heat and cold thresholds may be assumed for other populations, as acclimatization and different adaptation responses may apply for different population and climate conditions (Gasparrini et al. 2015). Third, we used the qPCR method, and our TL values are not directly comparable with other studies using qPCR; this is because normalization and the use of a reference (standard) sample are different across studies (Lin et al. 2019). Also, we expressed TL as a relative measure rather than an absolute measure that could be obtained with, for example, the terminal restriction fragment (TRF) method; therefore, we cannot translate our findings to actual telomere base pairs. In addition, a nonlinear association may exist between TLs measured with qPCR and TRF (Aviv et al. 2011), making it more difficult to compare our estimates in terms of cord blood telomere base pairs measured with TRF (Factor-Litvak et al. 2016). Nevertheless, we observed large effect estimates for cord blood TL in association with temperatures above the heat threshold (e.g., the largest estimate of a 3.29% reduction in TL with 1°C increase at week 36 of gestation). These estimates should be regarded with caution, as they represent the associations for increments in temperatures above a heat threshold defined as 19.5°C in weekly average temperatures, which represents the 95th percentile of the temperature distribution. These increments above the thresholds will not take place for an individual at each week of pregnancy. To put our estimates into context with other studies using newborn TL as measured with qPCR and expressed as percent difference, it has been shown that a doubling in maternal urinary cadmium and maternal urinary arsenic resulted in 6.83% shorter and 5.75% longer cord blood TL, respectively (Song et al. 2019; Zhang et al. 2019). Furthermore, an increment of folate is associated with 5.8% longer cord blood TL (Entringer et al. 2015). A study by Bijnens et al. showed that a twofold increase in distance to a major road was associated with 5.32% longer placental TL (Bijnens et al. 2015). In the ENVIRONAGE birth cohort, we previously showed that a increase in prepregnancy BMI was associated with 0.50 and 0.66% shorter cord blood and placental TL, respectively, and that a increment in over the entire pregnancy was associated with 8.8% and 13.2% shorter cord blood and placental TL, respectively (Martens et al. 2016, 2017). Fourth, our associations may gain strength in case no associations are observed when using postnatal exposures (a so-called negative control). However, due to the high temporal correlative nature of ambient temperatures, the presence of postnatal associations cannot be ruled out. Nevertheless, we evaluated the associations between postnatal temperatures (36 wk after delivery) and cord blood and placental TL (Table 3). We did not observe significant average effect estimates for postnatal temperatures (heat and cold models) and cord blood TL. In addition, we observed a less strong association with postnatal temperatures below the cold threshold in placenta and no associations with temperatures above the heat threshold. This strengthens our findings to some extent, especially on cord blood telomeres. Finally, as newborn TL may be highly heritable and determined by parental TL (Factor-Litvak et al. 2016), the potential mediating effect of parental TL on the observed association should warrant further evaluation. Unfortunately, this mediation could not be evaluated in the ENVIRONAGE study because data on parental TLs were not available.
How our results may be translated to later-life health and diseases can only be speculated at this moment; therefore, prospective follow-up studies are needed to fully understand later-life consequences in relationship to initial TL. However, it has been hypothesized that TL at birth may not fully represent the start point of a biological aging clock, as this may imply that the clock is set at zero at birth (Factor-Litvak et al. 2017). This latter is unlikely, as a high variation in TL at birth is observed (Factor-Litvak et al. 2016), and therefore, TL at birth may represent or underlie disease susceptibility, and an interplay between long and short telomeres may be predictive for the risk of developing cancers or cardiovascular diseases later in life (Factor-Litvak et al. 2017).
Conclusions
Exposure to ambient temperature during pregnancy may be an important environmental factor associated with the fetal programming of telomere biology. Prenatal exposure to low ambient temperatures was associated with longer TL, suggesting potential protection against future cardiovascular disease development, and this may relate to increased longevity. In line with this, exposure to high temperatures was associated with shorter cord blood TL, which adds to the known adverse effects of heat exposures during pregnancy. Our results add to the growing body of evidence that climate change and the subsequent temperature rise will negatively affect human health, even from birth onward. TL at birth may predict later-life health, and our study indicates that the origins of diseases may, to some extent, result from prenatal ambient temperature exposures. Reducing the rate of global warming, which will lead to decreased frequencies of the predicted extreme exposures, may enhance overall life expectancy and quality and may promote molecular longevity from birth.
Supplementary Material
Acknowledgments
The ENVIRONAGE birth cohort is supported by the EU program Ideas (ERC-2012-StG 310898) and by the Flemish Scientific Fund (FWO, G073315N). B.C. is a postdoctoral fellow of the FWO (12Q0517N). T.S.N. coordinates the ENVIRONAGE birth cohort and designed the current study together with D.S.M., B.C., and M.P. D.S.M. measured cord and placental TLs and performed quality control of the database. B.C. performed the statistical analysis. D.S.M. and T.S.N. wrote the first draft of the manuscript. All authors were involved in data interpretation and critical revision of the manuscript. Data is available from the corresponding author upon reasonable request.
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5153).
These authors contributed equally to this work.
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Abraham E, Rousseaux S, Agier L, Giorgis-Allemand L, Tost J, Galineau J, et al. 2018. Pregnancy exposure to atmospheric pollution and meteorological conditions and placental DNA methylation. Environ Int 118: 334–347, PMID: 29935799, 10.1016/j.envint.2018.05.007. [DOI] [PubMed] [Google Scholar]
- Arsenis NC, You T, Ogawa EF, Tinsley GM, Zuo L. 2017. Physical activity and telomere length: impact of aging and potential mechanisms of action. Oncotarget 8(27):45008–45019, PMID: 28410238, 10.18632/oncotarget.16726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. 2011. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by southern blots and qPCR. Nucleic Acids Res 39(20):e134, PMID: 21824912, 10.1093/nar/gkr634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Shay JW. 2018. Reflections on telomere dynamics and ageing-related diseases in humans. Philos Trans R Soc Lond B Biol Sci 373(1741):20160436, PMID: 29335375, 10.1098/rstb.2016.0436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benetos A, Kark JD, Susser E, Kimura M, Sinnreich R, Chen W, et al. 2013. Tracking and fixed ranking of leukocyte telomere length across the adult life course. Aging Cell 12(4):615–621, PMID: 23601089, 10.1111/acel.12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnens EM, Zeegers MP, Derom C, Martens DS, Gielen M, Hageman GJ, et al. 2017. Telomere tracking from birth to adulthood and residential traffic exposure. BMC Med 15(1):205, PMID: 29157235, 10.1186/s12916-017-0964-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bijnens E, Zeegers MP, Gielen M, Kicinski M, Hageman GJ, Pachen D, et al. 2015. Lower placental telomere length may be attributed to maternal residential traffic exposure; a twin study. Environ Int 79: 1–7, PMID: 25756235, 10.1016/j.envint.2015.02.008. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. 1991. Structure and function of telomeres. Nature 350(6319):569–573, PMID: 1708110, 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- Blasco MA. 2007. The epigenetic regulation of mammalian telomeres. Nat Rev Genet 8(4):299–309, PMID: 17363977, 10.1038/nrg2047. [DOI] [PubMed] [Google Scholar]
- Brenner IK, Castellani JW, Gabaree C, Young AJ, Zamecnik J, Shephard RJ, et al. 1999. Immune changes in humans during cold exposure: effects of prior heating and exercise. J Appl Physiol 87(2):699–710, PMID: 10444630, 10.1152/jappl.1999.87.2.699. [DOI] [PubMed] [Google Scholar]
- Bunker A, Wildenhain J, Vandenbergh A, Henschke N, Rocklöv J, Hajat S, et al. 2016. Effects of air temperature on climate-sensitive mortality and morbidity outcomes in the elderly; a systematic review and meta-analysis of epidemiological evidence. EBioMedicine 6:258–268, PMID: 27211569, 10.1016/j.ebiom.2016.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catalano R, Bruckner T, Smith KR. 2008. Ambient temperature predicts sex ratios and male longevity. Proc Natl Acad Sci USA 105(6):2244–2247, PMID: 18250336, 10.1073/pnas.0710711104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Kong F, Luan Y, Sun C, Wang J, Zhang L, et al. 2013. Differential shortening rate of telomere length in the development of human fetus. Biochem Biophys Res Commun 442(1–2):112–115, PMID: 24246679, 10.1016/j.bbrc.2013.11.022. [DOI] [PubMed] [Google Scholar]
- Conti B. 2008. Considerations on temperature, longevity and aging. Cell Mol Life Sci 65(11):1626–1630, PMID: 18425417, 10.1007/s00018-008-7536-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, et al. 2006. Transgenic mice with a reduced core body temperature have an increased life span. Science 314(5800):825–828, PMID: 17082459, 10.1126/science.1132191. [DOI] [PubMed] [Google Scholar]
- Cox B, Martens E, Nemery B, Vangronsveld J, Nawrot TS. 2013. Impact of a stepwise introduction of smoke-free legislation on the rate of preterm births: analysis of routinely collected birth data. BMI 346: f441, PMID: 23412829, 10.1136/bmj.f441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox B, Vicedo-Cabrera AM, Gasparrini A, Roels HA, Martens E, Vangronsveld J, et al. 2016. Ambient temperature as a trigger of preterm delivery in a temperate climate. J Epidemiol Community Health 70(12):1191–1999, PMID: 27261529, 10.1136/jech-2015-206384. [DOI] [PubMed] [Google Scholar]
- Crous-Bou M, Fung TT, Prescott J, Julin B, Du M, Sun Q, et al. 2014. Mediterranean diet and telomere length in Nurses' Health Study: population based cohort study. BMI 349:g6674, PMID: 25467028, 10.1136/bmj.g6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debes PV, Visse M, Panda B, Ilmonen P, Vasemagi A. 2016. Is telomere length a molecular marker of past thermal stress in wild fish? Mol Ecol 25(21):5412–5424, PMID: 27662607, 10.1111/mec.13856. [DOI] [PubMed] [Google Scholar]
- Doblhammer G, Vaupel JW. 2001. Lifespan depends on month of birth. Proc Natl Acad Sci U S A 98(5):2934–2939, PMID: 11226344, 10.1073/pnas.041431898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards MJ, Saunders RD, Shiota K. 2003. Effects of heat on embryos and foetuses. Int J Hyperthermia 19(3):295–324, PMID: 12745973, 10.1080/0265673021000039628. [DOI] [PubMed] [Google Scholar]
- Entringer S, de Punder K, Buss C, Wadhwa PD. 2018. The fetal programming of telomere biology hypothesis: an update. Philos Trans R Soc Lond B Biol Sci 373(1741):20170151, PMID: 29335381, 10.1098/rstb.2017.0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Blackburn EH, Buss C, Shahbaba B, et al. 2015. Maternal folate concentration in early pregnancy and newborn telomere length. Ann Nutr Metab 66(4):202–208, PMID: 26067849, 10.1159/000381925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, Blackburn EH, et al. 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am J Obstet Gynecol 208(2):134.e1–134.e7, PMID: 23200710, 10.1016/j.ajog.2012.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Susser E, Aviv A. 2017. Environmental exposures, telomere length at birth, and disease susceptibility in later life. JAMA Pediatr 171(12):1143–1144, PMID: 29049439, 10.1001/jamapediatrics.2017.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Factor-Litvak P, Susser E, Kezios K, McKeague I, Kark JD, Hoffman M, et al. 2016. Leukocyte telomere length in newborns: implications for the role of telomeres in human disease. Pediatrics 137(4):e20153927, PMID: 26969272, 10.1542/peds.2015-3927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagnon DD, Gagnon SS, Rintamäki H, Tormakangas T, Puukka K, Herzig KH, et al. 2014. The effects of cold exposure on leukocytes, hormones and cytokines during acute exercise in humans. PloS One 9(10):e110774, PMID: 25338085, 10.1371/journal.pone.0110774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Trabanino R, Jarquin E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, et al. 2015. Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador–a cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res 142:746–755, PMID: 26209462, 10.1016/j.envres.2015.07.007. [DOI] [PubMed] [Google Scholar]
- Gasparrini A. 2011. Distributed lag linear and non-linear models in R: the package dlnm. J Stat Softw 43(8):1–20, PMID: 22003319, 10.18637/jss.v043.i08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A. 2014. Modeling exposure-lag-response associations with distributed lag non-linear models. Stat Med 33(5):881–899, PMID: 24027094, 10.1002/sim.5963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Armstrong B, Kenward MG. 2010. Distributed lag non-linear models. Stat Med 29(21):2224–2234, PMID: 20812303, 10.1002/sim.3940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Hashizume M, Lavigne E, Zanobetti A, Schwartz J, et al. 2015. Mortality risk attributable to high and low ambient temperature: a multicountry observational study. Lancet 386(9991):369–375, PMID: 26003380, 10.1016/S0140-6736(14)62114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparrini A, Guo Y, Sera F, Vicedo-Cabrera AM, Huber V, Tong S, et al. 2017. Projections of temperature-related excess mortality under climate change scenarios. Lancet Planet Health 1(9):e360–e367, PMID: 29276803, 10.1016/S2542-5196(17)30156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halonen JI, Zanobetti A, Sparrow D, Vokonas PS, Schwartz J. 2010. Associations between outdoor temperature and markers of inflammation: a cohort study. Environ Health 9(1):42, PMID: 20653951, 10.1186/1476-069X-9-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidinger BJ, Blount JD, Boner W, Griffiths K, Metcalfe NB, Monaghan P. 2012. Telomere length in early life predicts lifespan. Proc Natl Acad Sci U S A 109(5):1743–1748, PMID: 22232671, 10.1073/pnas.1113306109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloszy JO, Smith EK. 1986. Longevity of cold-exposed rats: a reevaluation of the “rate-of-living theory. J Appl Physiol 61(5):1656–1660, PMID: 3781978, 10.1152/jappl.1986.61.5.1656. [DOI] [PubMed] [Google Scholar]
- IPCC (Intergovernmental Panel on Climate Change). 2013. Climate Change 2013: The Physical Science Basis: Working Group I Contribution to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Stocker TF, Qin D, Plattner GK, Tignor M, Allen SK, eds. Cambridge, UK and New York, NY, USA: Cambridge University Press. [Google Scholar]
- Jansky L, Pospisilová D, Honzová S, Ulicný B, Srámek P, Zeman V, et al. 1996. Immune system of cold-exposed and cold-adapted humans. Eur J Appl Physiol Occup Physiol 72(5–6):445–450, PMID: 8925815, 10.1007/BF00242274. [DOI] [PubMed] [Google Scholar]
- Janssen BG, Byun HM, Cox B, Gyselaers W, Izzi B, Baccarelli AA, et al. 2014. Variation of DNA methylation in candidate age-related targets on the mitochondrial-telomere axis in cord blood and placenta. Placenta 35(9):665–672, PMID: 25047690, 10.1016/j.placenta.2014.06.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen S, Dumont G, Fierens F, Mensink C. 2008. Spatial interpolation of air pollution measurements using CORINE land cover data. Atmos Environ 42(20):4884–4903, 10.1016/j.atmosenv.2008.02.043. [DOI] [Google Scholar]
- Janssen BG, Madhloum N, Gyselaers W, Bijnens E, Clemente DB, Cox B, et al. 2017. Cohort profile: the ENVIRonmental influence ON early AGEing (ENVIRONAGE): a birth cohort study. Int J Epidemiol 46(5):1386–1387, PMID: 28089960, 10.1093/ije/dyw269. [DOI] [PubMed] [Google Scholar]
- Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, et al. 2014. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat Commun 2:4172, PMID: 24960204, 10.1038/ncomms5172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keil G, Cummings E, de Magalhães JP. 2015. Being cool: how body temperature influences ageing and longevity. Biogerontology 16(4):383–397, PMID: 25832892, 10.1007/s10522-015-9571-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kim GJ, Lee D, Ko JH, Lim I, Bang H, et al. 2018. Higher maternal vitamin D concentrations are associated with longer leukocyte telomeres in newborns. Matern Child Nutr 14(1):e12475, PMID: 28598004, 10.1111/mcn.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koskas S, Decottignies A, Dufour S, Pezet M, Verdel A, Vourc'h C, et al. 2017. Heat shock factor 1 promotes TERRA transcription and telomere protection upon heat stress. Nucleic acids Res 45(11):6321–6333, PMID: 28369628, 10.1093/nar/gkx208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawlor DA, Leon DA, Davey Smith G. 2005. The association of ambient outdoor temperature throughout pregnancy and offspring birthweight: findings from the Aberdeen Children of the 1950s cohort. BJOG 112(5):647–657, PMID: 15842292, 10.1111/j.1471-0528.2004.00488.x. [DOI] [PubMed] [Google Scholar]
- Lee P, Smith S, Linderman J, Courville AB, Brychta RJ, Dieckmann W, et al. 2014. Temperature-acclimated brown adipose tissue modulates insulin sensitivity in humans. Diabetes 63(11):3686–3698, PMID: 24954193, 10.2337/db14-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre W, Vercauteren J, Schrooten L, Janssen S, Degraeuwe B, Maenhaut W, et al. 2011. Validation of the MIMOSA-AURORA-IFDM model chain for policy support: modeling concentrations of elemental carbon in Flanders. Atmos Environ 45(37):6705–6713, 10.1016/j.atmosenv.2011.08.033. [DOI] [Google Scholar]
- Lefebvre W, Degrawe B, Beckx C, Vanhulsel M, Kochan B, Bellemans T, et al. 2013. Presentation and evaluation of an integrated model chain to respond to traffic- and health-related policy questions. Environ Model Softw 40:160–170, 10.1016/j.envsoft.2012.09.003. [DOI] [Google Scholar]
- Leon LR, Helwig BG. 2010a. Heat stroke: role of the systemic inflammatory response. J Appl Physiol (1985) 109(6):1980–1988, PMID: 20522730, 10.1152/japplphysiol.00301.2010. [DOI] [PubMed] [Google Scholar]
- Leon LR, Helwig BG. 2010b. Role of endotoxin and cytokines in the systemic inflammatory response to heat injury. Front Biosci (Schol Ed) 2:916–938, PMID: 20515834, 10.2741/s111. [DOI] [PubMed] [Google Scholar]
- Lin J, Smith DL, Esteves K, Drury S. 2019. Telomere length measurement by qPCR - summary of critical factors and recommendations for assay design. Psychoneuroendocrinology 99:271–278, PMID: 30343983, 10.1016/j.psyneuen.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Bailey SM, Okuka M, Muñoz P, Li C, Zhou L, et al. 2007. Telomere lengthening early in development. Nat Cell Biol 9(12):1436–1441, PMID: 17982445, 10.1038/ncb1664. [DOI] [PubMed] [Google Scholar]
- Maiheu B, Veldeman N, Viaene P, De Ridder K, Lauwaet D, Smeets N, et al. 2012. Bepaling van de best beschikbare grootschalige concentratiekaarten uchtkwaliteit voor België. http://www.ademloos.be/sites/default/files/gezondheid_docs/Eindrapport_Concentratiekaarten_29_01_2013_TW.pdf [accessed 1 December 2012].
- Martens DS, Cox B, Janssen BG, Clemente DBP, Gasparrini A, Vanpoucke C, et al. 2017. Prenatal air pollution and newborns' predisposition to accelerated biological aging. JAMA Pediatr 171(12):1160–1167, PMID: 29049509, 10.1001/jamapediatrics.2017.3024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens DS, Plusquin M, Gyselaers W, De Vivo I, Nawrot TS. 2016. Maternal pre-pregnancy body mass index and newborn telomere length. BMC Med 14(1):148, PMID: 27751173, 10.1186/s12916-016-0689-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mora C, Dousset B, Caldwell IR, Powell FE, Geronimo RC, Bielecki CR, et al. 2017. Global risk of deadly heat. Nature Clim Change 7(7):501, 10.1038/nclimate3322. [DOI] [Google Scholar]
- Muneer A, Minhas FA. 2019. Telomere biology in mood disorders: an updated, comprehensive review of the literature. Clin Psychopharmacol Neurosci 17(3):343–363, PMID: 31352701, 10.9758/cpn.2019.17.3.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noreikiene K, Kuparinen A, Merila J. 2017. Age at maturation has sex- and temperature-specific effects on telomere length in a fish. Oecologia 184(4):767–777, PMID: 28730343, 10.1007/s00442-017-3913-5. [DOI] [PubMed] [Google Scholar]
- Okuda K, Bardeguez A, Gardner JP, Rodriguez P, Ganesh V, Kimura M, et al. 2002. Telomere length in the newborn. Pediatr Res 52(3):377–381, PMID: 12193671, 10.1203/00006450-200209000-00012. [DOI] [PubMed] [Google Scholar]
- Patz JA, Campbell-Lendrum D, Holloway T, Foley JA. 2005. Impact of regional climate change on human health. Nature 438(7066):310–317, PMID: 16292302, 10.1038/nature04188. [DOI] [PubMed] [Google Scholar]
- Peel MC, Finlayson BL, McMahon TA. 2007. Updated world map of the Köppen-Geiger climate classification. Hydrol Earth Syst Sci 11(5):1633–1644, 10.5194/hess-11-1633-2007. [DOI] [Google Scholar]
- Perera F, Lin CJ, Qu L, Tang D. 2018. Shorter telomere length in cord blood associated with prenatal air pollution exposure: benefits of intervention. Environ Int 113:335–340, PMID: 29395277, 10.1016/j.envint.2018.01.005. [DOI] [PubMed] [Google Scholar]
- Ravlić S, Škrobot Vidaček N, Nanić L, Laganović M, Slade N, Jelaković B, et al. 2018. Mechanisms of fetal epigenetics that determine telomere dynamics and health span in adulthood. Mech Ageing Dev 174: 55–62, PMID: 28847485, 10.1016/j.mad.2017.08.014. [DOI] [PubMed] [Google Scholar]
- Romano GH, Harari Y, Yehuda T, Podhorzer A, Rubinstein L, Shamir R, et al. 2013. Environmental stresses disrupt telomere length homeostasis. PLoS Genet 9(9):e1003721, PMID: 24039592, 10.1371/journal.pgen.1003721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, et al. 2002. Biomarkers of caloric restriction may predict longevity in humans. Science 297(5582):811, PMID: 12161648, 10.1126/science.1071851. [DOI] [PubMed] [Google Scholar]
- Schaetzlein S, Lucas-Hahn A, Lemme E, Kues WA, Dorsch M, Manns MP, et al. 2004. Telomere length is reset during early mammalian embryogenesis. Proc Natl Acad Sci U S A 101(21):8034–8038, PMID: 15148368, 10.1073/pnas.0402400101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz J. 2000. The distributed lag between air pollution and daily deaths. Epidemiology 11(3):320–326, PMID: 10784251, 10.1097/00001648-200005000-00016. [DOI] [PubMed] [Google Scholar]
- Send TS, Gilles M, Codd V, Wolf I, Bardtke S, Streit F, et al. 2017. Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology 42(12):2407–2413, PMID: 28397798, 10.1038/npp.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simide R, Angelier F, Gaillard S, Stier A. 2016. Age and heat stress as determinants of telomere length in a long-lived fish, the Siberian sturgeon. Physiol Biochem Zool 89(5):441–447, PMID: 27617363, 10.1086/687378. [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. 1996. Oxidative stress, caloric restriction, and aging. Science 273(5271):59–63, PMID: 8658196, 10.1126/science.273.5271.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song L, Liu B, Zhang L, Wu M, Wang L, Cao Z, et al. 2019. Association of prenatal exposure to arsenic with newborn telomere length: results from a birth cohort study. Environ Res 175:442–448, PMID: 31158562, 10.1016/j.envres.2019.05.042. [DOI] [PubMed] [Google Scholar]
- Strand LB, Barnett AG, Tong S. 2011. The influence of season and ambient temperature on birth outcomes: a review of the epidemiological literature. Environ Res 111(3):451–462, PMID: 21333980, 10.1016/j.envres.2011.01.023. [DOI] [PubMed] [Google Scholar]
- Sun D, Lopez-Guajardo CC, Quada J, Hurley LH, Von Hoff DD. 1999. Regulation of catalytic activity and processivity of human telomerase. Biochemistry 38(13):4037–4044, PMID: 10194316, 10.1021/bi982249n. [DOI] [PubMed] [Google Scholar]
- Ulaner GA, Giudice LC. 1997. Developmental regulation of telomerase activity in human fetal tissues during gestation. Mol Hum Reprod 3(9):769–773, PMID: 9358002, 10.1093/molehr/3.9.769. [DOI] [PubMed] [Google Scholar]
- van Marken Lichtenbelt WD, Westerterp-Plantenga MS, van Hoydonck P. 2001. Individual variation in the relation between body temperature and energy expenditure in response to elevated ambient temperature. Physiol Behav 73(1–2):235–242, PMID: 11399317, 10.1016/s0031-9384(01)00477-2. [DOI] [PubMed] [Google Scholar]
- Watts N, Amann M, Ayeb-Karlsson S, Belesova K, Bouley T, Boykoff M, et al. 2018. The Lancet Countdown on health and climate change: from 25 years of inaction to a global transformation for public health. Lancet 9(17):32464, PMID: 29096948, 10.1016/S0140-6736(17)32464-9. [DOI] [PubMed] [Google Scholar]
- Wells JC. 2002. Thermal environment and human birth weight. J Theor Biol 214(3):413–425, PMID: 11846599, 10.1006/jtbi.2001.2465. [DOI] [PubMed] [Google Scholar]
- Ye X, Wolff R, Yu W, Vaneckova P, Pan X, Tong S. 2012. Ambient temperature and morbidity: a review of epidemiological evidence. Environ Health Perspect 120(1):19–28, PMID: 21824855, 10.1289/ehp.1003198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngren K, Jeanclos E, Aviv H, Kimura M, Stock J, Hanna M, et al. 1998. Synchrony in telomere length of the human fetus. Hum Genet 102(6):640–643, PMID: 9703424, 10.1007/s004390050755. [DOI] [PubMed] [Google Scholar]
- Zanobetti A, Wand MP, Schwartz J, Ryan LM. 2000. Generalized additive distributed lag models: quantifying mortality displacement. Biostatistics 1(3):279–292, PMID: 12933509, 10.1093/biostatistics/1.3.279. [DOI] [PubMed] [Google Scholar]
- Zhang L, Song L, Liu B, Wu M, Wang L, Zhang B, et al. 2019. Prenatal cadmium exposure is associated with shorter leukocyte telomere length in Chinese newborns. BMC Med 17(1):27, PMID: 30722777, 10.1186/s12916-019-1262-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Xiao R, Ronan EA, He Y, Hsu AL, Liu J, et al. 2015. Environmental temperature differentially modulates C. elegans longevity through a thermosensitive TRP channel. Cell Rep 11(9):1414–1424, PMID: 26027928, 10.1016/j.celrep.2015.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.