Abstract
Objective
The 16-week, randomised, double-blind Sildenafil in Treatment-Naïve Children, Aged1–17 years, with Pulmonary Arterial Hypertension (STARTS-1) study assessed the effect of sildenafil on cardiopulmonary exercise testing (CPET) in treatment-naïve paediatric patients with pulmonary arterial hypertension (PAH) and included a long-term extension (STARTS-2). CPET has rarely been performed in paediatric patients and we assessed both aerobic capacity with peak oxygen consumption (PVO2) and ventilatory inefficiency with the slope of ventilation to carbon dioxide production (VE/VCO2 slope).
Methods
Patients (aged 1–17 year) were randomised to low (10 mg), medium (10–40 mg) and high (20–80 mg) sildenafil dose groups. Patients previously treated with placebo in STARTS-1 were randomised to one of three blinded sildenafil dose groups for STARTS-2. CPET was assessed by cycle ergometry at baseline, week 16, and year 1.
Results
Of the 234 children randomised, 115 could exercise. At week 16, the combined sildenafil dose group had a 7.7% increase in mean PVO2 percent change from baseline compared with placebo (95% CI −0.2% to 15.6%; p=0.056); at year 1, a significant increase in mean percent change in PVO2 from baseline was only observed in the low-dose group (mean of 12.4% and 95% CI 3% to 21.8%). For VE/VCO2 slope, at week 16, the combined dose group had a −9.7% mean change from baseline compared with placebo (95% CI −14.9% to −4.5%; p<0.001); at year 1, there were no significant changes for any dose group.
Conclusions
Sildenafil monotherapy (combined sildenafil dose group) appeared to improve short-term VE/VCO2 slope versus placebo but did not significantly improve PVO2 in treatment-naïve paediatric patients with PAH who were developmentally able to exercise.
Trial registration number
NCT00159913 for A1481131, NCT00159874 for A1481156.
Keywords: pulmonary arterial hypertension (PAH), paediatric cardiac function, exercise ECG
Key questions.
What is already known about this subject?
Sildenafil is well tolerated in paediatric patients with pulmonary hypertension with little change in functional capacity as measured by peak oxygen consumption.
What does this study add?
This is the largest, randomised placebo controlled study reporting the effects of sildenafil on metabolic stress test parameters in children with pulmonary hypertension who are able to exercise.
Sildenafil appeared to improve short-term VE/VCO2 slope versus placebo but did not significantly improve PVO2 in treatment-naïve paediatric patients with pulmonary arterial hypertensionwho were able to exercise.
Additionally, we report on the safety of metabolic stress testing in a young population of patients with pulmonary hypertension which may help inform future clinical trials in this patient population.
How might this impact clinical practice?
Metabolic stress test is safe and can be repeated multiple times in this population. Other endpoints such as VE/VCO2 slope should be considered when planning future clinical trials.
Introduction
Pulmonary arterial hypertension (PAH) is a rare and complex disease affecting both children and adults, resulting in an increase in pulmonary vascular resistance and subsequent right heart failure and death.1 In children, PAH has previously been shown to carry a poor prognosis with a median untreated survival of only 10 months.2 Although not wellstudied, the development of therapies for PAH and their subsequent use in children have been associated with a marked improvement in survival to over 60% at 10 years.3 4 The Sildenafil in Treatment-Naïve Children, Aged 1–17 years, with Pulmonary Arterial Hypertension(STARTS-1 and 2) studies were the first randomised, double-blind studies to evaluate the efficacy of a therapy in children with PAH. Previously published results showed a trend towards improvement in the primary endpoint of percent change in peak oxygen consumption (VO2) for the combined sildenafil dose groups against placebo.5 6
The 6 min walk (6 MW) test is used to evaluate the efficacy of therapies for PAH and has been shown to correlate with survival.7 8 One limitation of the 6 MW test is that it is effortdependent which can be variable in children.9 Cardiopulmonary exercise testing (CPET) is an objective assessment of both maximal and submaximal exercise tolerance and has been performed safely and repeatedly in children, including those with PAH, and correlates with survival.8 10 11 However, the peak VO2 is also dependent on exercise effort, and maximal effort can be difficult to attain in children. CPET however has the advantage of being able to determine whether a test is maximal, unlike the 6 MW test. Additionally, the slope of ventilation to carbon dioxide (VE/VCO2) production is unchanged throughout exercise, is effortindependent and predicts mortality in heart failure and patients with pulmonary hypertension.12–14 Changes in VE/VCO2 slope in response to treatment have not been evaluated in children being treated for PAH. In this study, exercise data from the STARTS-1 and 2 studies was examined. Specifically, we evaluate the impact of sildenafil on CPET measures including peak VO2 and VE/VCO2 slope and report the 16-week and 1-year results of exercise testing.
Methods
The study designs of the STARTS-1 and 2 trials have been previously published.5 6 In brief, STARTS-1 randomised patients with idiopathic, heritable or other (connective tissue disease or congenital heart disease) PAH between the ages of 1–17 to either placebo or low-dose, medium-dose or high-dose sildenafil, three times a day. Doses were dependent on body weight, with the goal to achieve inhibition of phosphodiesterase type 5 activity of 53%, 77% or 90%, respectively. The STARTS-2 trial was a continuation of STARTS-1. Patients who were initially randomised to receive sildenafil in STARTS-1 were maintained on their initial dose for STARTS-2; those initially receiving placebo in STARTS-1 were randomised to one of the three doses of sildenafil used in STARTS-1. During STARTS-2, changes in the sildenafil dose were allowed. In STARTS-1, the primary endpoint was percent change in peak VO2 normalised to body weight from baseline to 16 weeks. Secondary endpoints included change in PVO2 at 1 year, changes in VE/VCO2 slope at 16 weeks and 1 year, right heart haemodynamics, exercise duration and physical and psychosocial scales of the Child Health Questionnaire-Parent form 28.
To ensure high-quality data, CPET at each site was evaluated and qualified by a centralised exercise core laboratory. Sites were allowed to enrol patients only when approved by the core laboratory. Each site was required to acquire CPET data from two normal control (healthy) adults using a 20 W/min continuous ramp protocol. The gas exchange data were reviewed by the core laboratory and if values fell out of expected norms, the data were deemed unsuitable, and site qualification was rejected. Common reasons for a site to fail qualification included using the wrong ramp protocol, leaks in the gas exchange system or an improperlycalibrated ergometer. Sites that were rejected initially performed corrective changes and were retested until data were acceptable. Each site was required to retest one of their control subjects every 6 months to maintain quality.
Only children who were developmentally able to reliably perform CPET were evaluated for the primary endpoint. Developmental ability was determined by the investigator. Patients were able to be enrolled in the study if their peak VO2 was >10 and<28 mL/kg/min. Each test began with a rest phase of up to 3 min until the respiratory exchange ratio (RER) was less than 0.8 and stable. Patients then began a ‘warm-up’ phase of unloaded cycling for 1–3 min at 60 revolutions/min followed by a 5 W/min (<40 kg) or 10 W/min (>40 kg) continuous ramp protocol. All patients were encouraged to exercise to maximum while maintaining a cadence of 50–60 revolutions/min. Other reasons to stop exercise included chest pain, presyncope or exercise-induced hypotension. Patients were monitored for at least 10 min after completing exercise, including a 3 min warm-down phase of unloaded cycling. Vital signs, Borg rate of perceived exertion and ECG were monitored throughout exercise and recovery.
Once the exercise test was performed, test data were sent to the exercise core laboratory for initial review to ensure an adequate test was performed prior to randomisation into the trial (peak exercise RER >1.05 and/or an increase of 0.15 from baseline, linear increases in VO2, carbon dioxide production and ventilation). There was no predetermined goal for peak heart rate. CPET endpoints were determined by two independent, blinded physicians at the Core Lab. Disagreements were reconciled via discussion and mutual agreement.
Statistical analysis
The primary endpoint was evaluated using analysis of covariance (ANCOVA) in which the mean percent change from baseline in PVO2 at week 16 in the 3 sildenafil treatment groups (low, medium and high dose) was contrasted to that in the placebo group (with contrast coefficients 1/3, 1/3, 1/3 and −1). Comparisons of the individual dose groups to the placebo group were also made. The ANCOVA model included terms for treatment, baseline result, aetiology and weight group. Statistical hypothesis testing was performed at the two-sided 5% significance level. Similar analyses were conducted for treatment comparisons in percent change of VE/VCO2 slope from baseline at week 16. For STARTS-2, the percent change from baseline to 1 year for PVO2 and for VE/VCO2 slope was summarised with descriptive statistics for each STARTS-1treatment group. For other CPET parameters, including RER, end-tidal O2 and end-tidal CO2, percent change from baseline at week 16 and at year 1 was also summarised with descriptive statistics. VE/VCO2 slope was calculated as the slope of the best fit line for ventilation and carbon dioxide throughout the entire exercise duration.
Informed consent/assent was obtained from each subject (or subject’s legally authorised representative).
Results
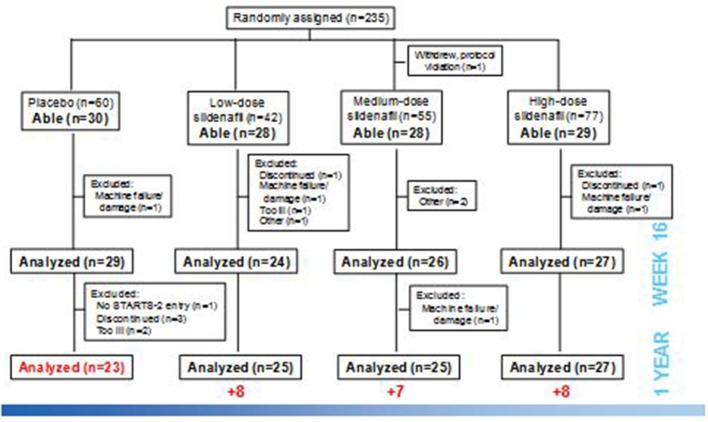
Of the 234 patients randomised and treated in the STARTS-1 trial, 115 patients (49%) were developmentally able to reliably perform CPET and thus had baseline readings. Reasons for not exercising included age <7 (63) and developmental issues (trisomy 21 (31), couldnot reach pedals and various reasons including intolerance of mouthpiece and low physical activity. One hundred and six of these patients (92%) had evaluable PVO2 data at week 16 (figure 1, machine failure/damage, n=3; discontinuation without final assessment, n=2; too ill, inadequate test data that were not repeated, lack of staff, not done in error, n=1 each). PVO2 data at year 1 were evaluable in 98 patients. One hundred and five patients had both evaluable PVO2 and VE/VCO2 slope data at week 16, and 100 patients had evaluable VE/VCO2 slope data at 1 year.
Figure 1.
Patients were assigned to one of three doses or placebo by weight and developmental ability to perform exercise testing. Not all patients able to perform exercise were tested due to a variety of factors.
Table 1 shows the baseline characteristics of the patients. The age range of patients who could exercise was 5–17, and half were between the ages of 5 and 12. Most patients were female and all had idiopathic or heritable PAH or PAH associated with congenital heart disease. The majority of patients were WHO functional class II at baseline. Mean PVO2 at baseline was 17.6 (43.9% predicted), 18.1 (45.2% predicted) and 17.3 mL/kg/min (45.5% predicted) for the low-dose, medium-dose and high-dose sildenafil groups, respectively, versus 20.0 mL/kg/min (51.2% predicted) for the placebo group; similarly, the mean baseline VE/VCO2 slope was 50.1, 47.6 and 50.2 for the low-dose, medium-dose and high-dose sildenafil groups, respectively, versus 46.3 for the placebo group.
Table 1.
Demographic characteristics of patients able to exercise reliably
| Characteristic, mean (SD) | Placebo* (n=30) |
Sildenafil dose | ||
| Low (n=28) |
Medium (n=28) |
High (n=29) |
||
| Female sex, n (%) | 19 (63) | 17 (61) | 15 (54) | 21 (72) |
| Age, y, n (%) | ||||
| 5–12 | 18 (60) | 15 (54) | 13 (46) | 11 (38) |
| 13–17 | 12 (40) | 13 (46) | 15 (54) | 18 (62) |
| Race—n (%) | ||||
| White | 8 (27) | 10 (36) | 11 (39) | 6 (21) |
| Black | 2 (7) | 0 | 1 (4) | 0 |
| Asian | 4 (13) | 6 (21) | 7 (25) | 7 (24) |
| Other | 16 (53) | 12 (43) | 9 (32) | 16 (55) |
| Weight, kg, mean (range) | 37 (20–60) | 42 (21–105) |
42 (23–106) |
37 (15–61) |
| BMI, kg/m2 | 18 (3) | 19 (6) | 18 (4) | 17 (3) |
| WHO functional class, n (%) | ||||
| I | 10 (33) | 5 (18) | 8 (29) | 6 (21) |
| II | 17 (57) | 15 (54) | 13 (46) | 18 (62) |
| III | 3 (10) | 8 (29) | 7 (25) | 5 (17) |
| Aetiology, n (%) | ||||
| IPAH/HPAH | 10 (33) | 9 (32) | 10 (36) | 12 (41) |
| APAH | 20 (67) | 19 (68) | 18 (64) | 17 (59) |
| Surgical repair† | 7 (23) | 7 (25) | 7 (25) | 8 (28) |
| Congenital systemic-to-pulmonary shunt withSaO2 ≥88% at rest | 12 (40) | 11 (39) | 10 (36) | 9 (31) |
| Postrepair D-transpositionof great arteries | 1 (3) | 1 (4) | 1 (4) | 0 |
| PVO2, mL/kg/min | 20.0 (3.8) | 17.6 (4.4) | 18.1 (4.8) | 17.3 (3.6) |
| VE/VCO2 slope | 46.3 (15.2) | 50.1 (14.8) | 47.6 (13.6) | 50.2 (14.5) |
| RER | 1.10 (0.13) | 1.09 (0.08) | 1.10 (0.12) | 1.09 (0.09) |
| Time to PVO2, s | 460 (141) | 433 (127) | 453 (140) | 436 (106) |
| Total ventilation, L/min | 36.3 (13.0) | 38.0 (13.4) | 37.2 (16.2) | 34.2 (14.1) |
| End-tidal O2, mmHg | 106.7 (20.2) | 117.5 (15.7) | 113.8 (14.7) | 105.9 (21.3) |
| End-tidal CO2, mmHg | 26.3 (5.9) | 23.5 (6.1) | 27.9 (4.5) | 23.4 (5.4) |
| Anaerobic threshold, mL/kg/min | 13.1 (2.8) | 13.1 (3.8) | 12.2 (4.4) | 11.8 (2.9) |
| Percent predicted PVO2 | 51.2 (11.9) | 43.9 (10.0) | 45.2 (12.0) | 45.5 (10.7) |
*Twenty-nine subjects randomised to sildenafil low, medium and high dose in Sildenafil in Treatment-Naïve Children, Aged 1–17 years, with Pulmonary Arterial Hypertension.
†Surgical repairs included atrial septal defect, ventricular septal defect, patent ductus arteriosus, aortopulmonary window and others.
APAH, associated pulmonary arterial hypertension; BMI, body mass index; CPET, cardiopulmonary exercise test; HPAH, heritable PAH; IPAH, idiopathic pulmonary arterial hypertension; PAH, pulmonary arterial hypertension; PVO2, peak VO2; RER, respiratory exchange ratio; SaO2, systemic arterial oxygen saturation; VE/VCO2, ratio of ventilation to CO2 output; VO2, oxygen consumption.
Week 16 outcomes
The primary endpoint, mean change in PVO2 in the combined sildenafil dose groups at week 16 compared with placebo, increased by 7.7%±4.0% (SE); p=0.056, 95% CI −0.2% to 15.6%). Compared with placebo, there were significant increases in mean PVO2 for the medium-dose group, but not for the low-dose or high-dose group.(table 2) There was no change in PVO2 compared with baseline for the placebo group (figure 2a). Similarly, compared with placebo, the VE/VCO2 slope improved, with a mean reduction in the combined sildenafil dose groups (±SE) of −9.7±2.6% (p<0.001, 95% CI of the mean difference: −14.9% to −4.5%, figure 3a).
Table 2.
Percent change from baseline in cardiopulmonary exercise parameters at week 16 and year 1 of Sildenafil in Treatment-Naïve Children, Aged 1–17 years, with Pulmonary Arterial Hypertension (STARTS-1/-2)
| CPET parameter | Week 16 | Year 1 | ||||||
| Placebo (n=30) |
Low (n=28) |
Medium (n=28) |
High (n=29) |
Placebo* (n=29) |
Low (n=28) |
Medium (n=28) |
High (n=29) |
|
| Peak VO2 | ||||||||
| N | 29 | 24 | 26 | 27 | 23 | 25 | 25 | 27 |
| Mean | 0.5 | 6.4 | 13.4 | 10.6 | −0.2 | 12.4 | 7.3 | 5.3 |
| SD | 15.9 | 20.2 | 19.5 | 15.5 | 19.9 | 22.8 | 33.8 | 23.5 |
| VE/VCO2 slope | ||||||||
| N | 29 | 23 | 26 | 27 | 23 | 25 | 24 | 26 |
| Mean | 2.7 | −8 | −8.2 | −9.6 | 2.3 | −5.2 | −5.5 | −0.3 |
| SD | 12.3 | 13.4 | 11.1 | 14.5 | 14.5 | 17.9 | 17.1 | 29.1 |
| Respiratory exchange ratio | ||||||||
| N | 29 | 24 | 26 | 27 | 23 | 25 | 25 | 27 |
| Mean | −2.7 | 0 | −4.0 | −2.0 | 2.5 | 2.2 | 5.6 | 0.7 |
| SD | 10.4 | 11.7 | 10.7 | 10.3 | 13.8 | 8.7 | 13.4 | 11.5 |
| Time to peak VO2 | ||||||||
| N | 29 | 24 | 26 | 27 | 23 | 25 | 25 | 27 |
| Mean | 4.5 | 15.2 | 16.0 | 11.2 | 15.2 | 25.5 | 13.1 | 7.7 |
| SD | 34.9 | 26.3 | 22.9 | 28.6 | 65.7 | 35.7 | 33.4 | 33.0 |
| Total ventilation | ||||||||
| N | 29 | 23 | 26 | 27 | 23 | 25 | 25 | 26 |
| Mean | 4.0 | −2.8 | 4.5 | 6.1 | 14.2 | 15.3 | 14.7 | 11.3 |
| SD | 20.9 | 24.7 | 19.1 | 21.4 | 23.7 | 22.6 | 35.1 | 19.4 |
| End-tidal O2 | ||||||||
| N | 19 | 12 | 14 | 15 | 12 | 14 | 10 | 11 |
| Mean | –1.5 | –2.2 | –1.2 | –2.3 | –1.5 | 0 | 0.5 | –0.3 |
| SD | 3.9 | 3.8 | 3.2 | 5.8 | 4.3 | 3.1 | 3.5 | 4.0 |
| End-tidal CO2 | ||||||||
| N | 19 | 12 | 12 | 13 | 12 | 14 | 9 | 8 |
| Mean | − 1.6 | 13.0 | − 0.3 | 4.3 | 6.9 | 9.1 | 4.1 | 10.3 |
| SD | 9.3 | 18.2 | 6.6 | 13.9 | 14.2 | 18.9 | 17.8 | 33.4 |
| Anaerobic threshold | ||||||||
| N | 26 | 20 | 22 | 25 | 20 | 22 | 21 | 22 |
| Mean | 5.7 | 8.3 | 9.7 | 6.6 | 4.6 | −1.2 | 2.0 | 3.3 |
| SD | 25.2 | 26.6 | 28.5 | 24.0 | 25.0 | 23.1 | 29.5 | 29.4 |
| Percent predicted peak VO2 | ||||||||
| N | 29 | 24 | 26 | 27 | 23 | 25 | 25 | 27 |
| Mean | 0.7 | 6.6 | 12.9 | 10.9 | 1.0 | 12.8 | 7.7 | 5.8 |
| SD | 16.0 | 19.7 | 19.4 | 16.4 | 20.2 | 22.7 | 34.5 | 23.6 |
*Parenthesis show values for placebo-treated STARTS-1 patient subsequently randomised in STARTS-2 to sildenafil low, medium or high dose.
CO2, carbon dioxide production; CPET, cardiopulmonary exercise test; VE, ventilation; VO2, oxygen consumption.
Figure 2.
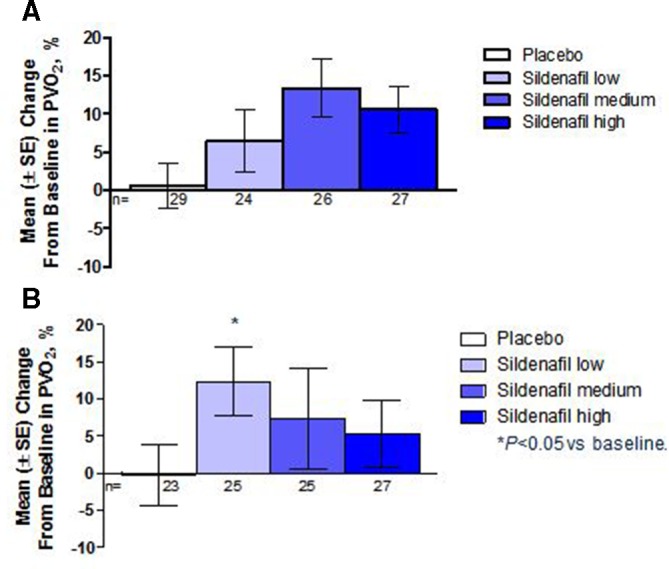
Mean change in peak oxygen consumption from baseline at (a) week 16 and (b) 1 year. At week 16, significant improvement was achieved in patients treated with medium-dose and high-dose sildenafil and there was no change in placebo-treated patients. At year 1, the placebo-treated patients had received sildenafil at low, medium or high dose since week 16. Only the low-dose patients had a significant increase in change in peak VO2 at 1 year.
Figure 3.
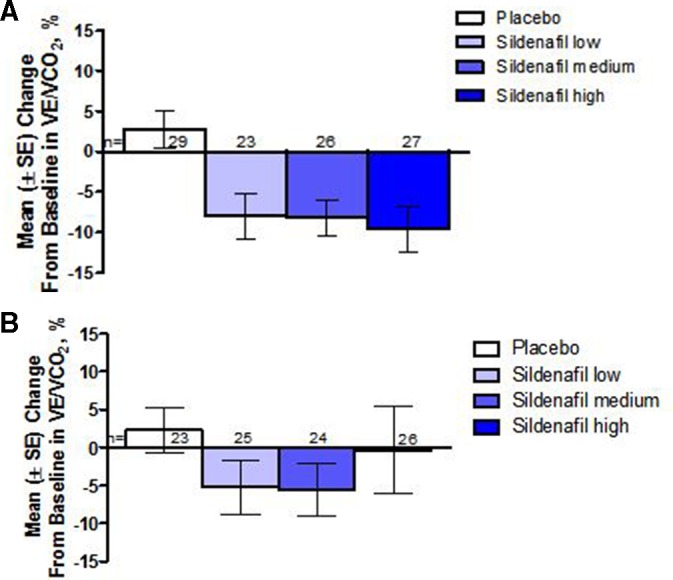
Mean change in the VE/VCO2slope at (a) week 16 and (b) 1 year in patients. Mean VE/VCO2 slope decreased (improved) compared with baseline in all sildenafil-treated patients at week 16. The change from baseline in the sildenafil groups combined versus placebo was statistically significant overall, and within each treatment group. At year 1, the placebo-treated patients had received sildenafil at low, medium or high dose since week 16. There was a sustained mean reduction in VE/VCO2 slope in sildenafil low and medium group but was not statistically significant.
There were 76 patients who received sildenafil and were developmentally able to exercise, and among them 43 (56.6%) had both an increase in PVO2 and a decrease in VE/VCO2 slope compared with their baseline. Twenty-nine developmentally able patients received placebo and among them 7 (24.1%) had both an increase in PVO2 and a decrease in VE/VCO2 slope compared with baseline.
1-year outcomes
Two hundred and twenty (94%) out of 234 patients who randomised and treated in the STARTS-1 trial continued into STARTS-2. As shown in figure 2b, there was a sustained increase in mean PVO2 at 1 year in all of the individual sildenafil dose groups compared with baseline, but only the low-dose group had a statistically significant mean increase of 12.4% (95% CI 3.0% to 21.8%,) (Table 2). Both the low-dose and medium-dose groups had a sustained numerical reduction in mean percent change in VE/VCO2 slope compared with baseline, but neither of these changes was statistically significant (figure 3b); and there was no appreciable numerical change in the high-dose group in VE/VCO2 slope at year 1.
At 1 year, only 36 patients (36.7% out of 98 patients who had both PVO2 and VE/VCO2 slope data) had both an increase in PVO2 and a decrease in VE/VCO2 slope compared with baseline, suggesting progression of disease in the majority of the subjects. Consistency in changes from week 16 to 52 was observed for PVO2 with 73% of patients showing improvements or worsening at both week 16 and year 1. Patients who showed improvement at year 1 only included the placebo and low-dose sildenafil groups in STARTS-1. Only 63% of the patients had a consistent improvement or worsening for VE/VCO2 slope at weeks 16 and 52.
Safety
The two primary reasons that patients were deemed ‘unable to exercise’ were age <7 years or Down syndrome. There were no reports of difficulty performing the test or adverse events (ischaemia, arrhythmia) that prevented completion of the exercise test.
Discussion
In the first placebo-controlled, randomised trial of sildenafil versus placebo in children with PAH developmentally able to exercise, the combined sildenafil treatment groups did not show statistically significant improvement over placebo for PVO2 at week 16. However, the improvement in VE/VCO2 slope was statistically significantly better in the combined sildenafil group than placebo at week 16.
There have been few trials assessing exercise capacity in children with pulmonary hypertension. In adults, 6 MW distance has been used as the primary endpoint in almost all clinical trials of PAH drugs as it is believed to be an important predictor of outcome.15 The major limitations to using the 6 MW test include variable effort, a learning effect from performing multiple times, day-to-day variation and the impact of comorbidities, all of which may be more important in children. CPET has been shown to be reproducible, predictive of outcomes and allows one to determine if a maximal test was performed.16 Because of this, CPET might be a more objective test of exercise capacity in children. Additionally, the VE/VCO2 slope, an effort-independent value, might be especially useful for children who may not give a maximal effort. For adults, several measures obtained with CPET predict survival in patients with PAH, and CPET provides an objective assessment of true functional capacity as well as ventilatory inefficiency.8 15 17 Miyamoto et al performed both tests in 27 patients and found a strong correlation between distance walked and PVO2, VE/VCO2 slope and oxygen pulse.8 Lammers et al performed both tests in 41 children with PAH at an average age of 13 years.17 They found a similar correlation between the exercise endpoints and distance walked. In our study, we found that PVO2 and VE/VCO2 slope both changed (improved) in the appropriate directions compared with placebo. Patients as young as 7 years old were able to perform maximal exercise tests as shown by achieving a high RER and the changes that occurred at week 16 were sustained over time. Clearly, CPET can be safely and adequately performed in children and thus measures made with CPET might be a better endpoint than a 6 MW test in this population.
The finding of an improvement in exercise capacity in children with PAH receiving sildenafil is similar to what has been demonstrated in prior studies. Humpl et al performed an open-label study of 14 children with PAH and found an increase in walk distance from 278+114 to 443+107 m at 6 months.18 An improvement in 6 MW distance was also found in adults with sildenafil alone or as an addition to epoprostenol.19 20 Oudiz et al evaluated the effects of 3 months of sildenafil on VE/VCO2 in 14 adults compared with 14 matched controls, showing a significant decrease in VE/VCO2 of 9±2 (mean±SE) in the sildenafil group compared with a non-significant increase in the controls.20
The effect of other PAH drugs on changes in exercise capacity in children has not been wellstudied. Intravenous epoprostenol has been studied in children and has been shown to be associated with an improvement in functional class and 6 MW distance.21–23 Similarly, iloprost and treprostinil have been studied in children, although in very small studies. Three studies showed that children with PAH who had symptomatic improvement from these drugs all had an improvement in their 6 MW distance.24–26 Finally, bosentan and ambrisentan have also been shown to produce improvements in functional class for those who also increased 6 MW distance.27–29 Symptomatic improvement appears to track improvement in functional capacity for patients with PAH regardless of the therapy used.
This study is limited by the lack of a placebo control group beyond 16 weeks, and the inability of half of the patients to perform exercise. Furthermore, even though some children received therapy beyond 1 year, we do not have exercise data to assess if functional capacity improvements persisted beyond this point.
In conclusion, we have demonstrated that some children with PAH as young as 7 years of age are able to exercise and perform interpretable CPET. At 16 weeks compared with placebo, sildenafil tended to increase exercise capacity as measured by peak oxygen consumption and also showed a statistically significant improvement in ventilatory inefficiency, although these changes were not sustained by year 1. Larger and longerterm studies of sildenafil and other PAH drugs in the paediatric PAH population to assess long-term efficacy, optimal dosing and predictors of prognosis would be valuable. Ventilatory inefficiency may be a more reliable measure of change in exercise gas exchange in children with PAH. Finally, metabolic stress testing is safe and may be repeated multiple times in children with PAH.
Footnotes
Contributors: All authors have made substantial contributions to the manuscript. All authors were involved in the conception and design of the study, the interpretation of the data, the manuscript outline and final revision of the manuscript. All authors confirm that the study objectives and procedures are honestly disclosed. All authors have agreed to be accountable for all aspects of the work including any questions related to the accuracy or integrity of the manuscript. SR drafted the manuscript and all authors have approved the final version to be published. MZ and CB personally reviewed the safety and efficacy data and they have also reviewed the study execution data and confirm that procedures were followed to an extent that convinces all authors that the results are valid and generalisable to a population similar to that enrolled in this study. MZ drafted the statistical analysis section and understands the statistical methods employed and confirms that she understands this analysis, that the methods are clearly described and that they are a fair way to report the results. Furthermore, she and SR have personally reviewed the serious adverse events and confirm that these are disclosed and analysed even in the presence of uncertainty with respect to relationship to treatment. DI contributed to design analysis and all subsequent drafts of the manuscript.
Funding: This study was sponsored by Pfizer. The sponsor designed and managed the STARTS-1 and STARTS-2 studies. The sponsor or its designated representatives performed clinical monitoring, data management, statistical analysis and reporting.
Competing interests: SR was the head of the exercise core lab for this trial and received research support. MB is a consultant for Actelion Pharmaceuticals, Bayer Healthcare, GlaxoSmithKline, Eli Lilly and Pfizer; and has received grant support and personal fees from Actelion Pharmaceuticals, GlaxoSmithKline, Eli Lilly, Pfizer and Bayer Healthcare. RO is a speaker/consultant for Actelion Pharmaceuticals, Arena, Bayer Healthcare, Gilead Pharmaceuticals, Medtronic, Reata Pharmaceuticals, SteadyMed and United Therapeutics, and has received grant support for research from AAdi, Actelion Pharmaceuticals, Arena, Gilead Pharmaceuticals, GSK, Liquidia, Reata Pharmaceuticals and United Therapeutics. CB and MZ are employees of Pfizer and own Pfizer stock. DI was previously a consultant for Pfizer. The University of Colorado receives fees for DI to be a consultant for Actelion, Bayer, Lilly and United Therapeutics.
Patient consent for publication: Not required.
Ethics approval: The study was approved by local Institutional Review Boards.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data are available upon reasonable request. Data may be obtained from a third party and are not publicly available.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension a report of the American College of cardiology Foundation Task force on expert consensus documents and the American heart association developed in collaboration with the American College of chest physicians; American thoracic Society, Inc.; and the pulmonary hypertension association. J Am Coll Cardiol 2009;53:1573–619. 10.1016/j.jacc.2009.01.004 [DOI] [PubMed] [Google Scholar]
- 2.D'Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. results from a national prospective registry. Ann Intern Med 1991;115:343–9. 10.7326/0003-4819-115-5-343 [DOI] [PubMed] [Google Scholar]
- 3.Zijlstra WMH, Douwes JM, Rosenzweig EB, et al. Survival differences in pediatric pulmonary arterial hypertension: clues to a better understanding of outcome and optimal treatment strategies. J Am Coll Cardiol 2014;63:2159–69. 10.1016/j.jacc.2014.02.575 [DOI] [PubMed] [Google Scholar]
- 4.Humbert M, Sitbon O, Simonneau G. Treatment of pulmonary arterial hypertension. N Engl J Med 2004;351:1425–36. 10.1056/NEJMra040291 [DOI] [PubMed] [Google Scholar]
- 5.Barst RJ, Ivy DD, Gaitan G, et al. A randomized, double-blind, placebo-controlled, dose-ranging study of oral sildenafil citrate in treatment-naive children with pulmonary arterial hypertension. Circulation 2012;125:324–34. 10.1161/CIRCULATIONAHA.110.016667 [DOI] [PubMed] [Google Scholar]
- 6.Barst RJ, Beghetti M, Pulido T, et al. STARTS-2: long-term survival with oral sildenafil monotherapy in treatment-naive pediatric pulmonary arterial hypertension. Circulation 2014;129:1914–23. 10.1161/CIRCULATIONAHA.113.005698 [DOI] [PubMed] [Google Scholar]
- 7.Guyatt GH, Sullivan MJ, Thompson PJ, et al. The 6-minute walk: a new measure of exercise capacity in patients with chronic heart failure. Can Med Assoc J 1985;132:919–23. [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000;161:487–92. 10.1164/ajrccm.161.2.9906015 [DOI] [PubMed] [Google Scholar]
- 9.Garofano RP, Barst RJ. Exercise testing in children with primary pulmonary hypertension. Pediatr Cardiol 1999;20:61–4. 10.1007/s002469900399 [DOI] [PubMed] [Google Scholar]
- 10.Yetman AT, Taylor AL, Doran A, et al. Utility of cardiopulmonary stress testing in assessing disease severity in children with pulmonary arterial hypertension. Am J Cardiol 2005;95:697–9. 10.1016/j.amjcard.2004.10.056 [DOI] [PubMed] [Google Scholar]
- 11.Abumehdi MR, Wardle AJ, Nazzal R, et al. Feasibility and safety of cardiopulmonary exercise testing in children with pulmonary hypertension. Cardiol Young 2016;26:1144–50. 10.1017/S1047951115001961 [DOI] [PubMed] [Google Scholar]
- 12.Francis DP, Shamim W, Davies LC, et al. Cardiopulmonary exercise testing for prognosis in chronic heart failure: continuous and independent prognostic value from VE/VCO(2)slope and peak VO(2). Eur Heart J 2000;21:154–61. 10.1053/euhj.1999.1863 [DOI] [PubMed] [Google Scholar]
- 13.Keteyian SJ, Patel M, Kraus WE, et al. Variables measured during cardiopulmonary exercise testing as predictors of mortality in chronic systolic heart failure. J Am Coll Cardiol 2016;67:780–9. 10.1016/j.jacc.2015.11.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oudiz RJ, Midde R, Hovanesyan A, et al. Usefulness of right-to-left shunting and poor exercise gas exchange for predicting prognosis in patients with pulmonary arterial hypertension. Am J Cardiol 2010;105:1186–91. 10.1016/j.amjcard.2009.12.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McLaughlin VV, Gaine SP, Howard LS, et al. Treatment goals of pulmonary hypertension. J Am Coll Cardiol 2013;62:D73–81. 10.1016/j.jacc.2013.10.034 [DOI] [PubMed] [Google Scholar]
- 16.Bensimhon DR, Leifer ES, Ellis SJ, et al. Reproducibility of peak oxygen uptake and other cardiopulmonary exercise testing parameters in patients with heart failure (from the heart failure and a controlled trial investigating outcomes of exercise traiNing). Am J Cardiol 2008;102:712–7. 10.1016/j.amjcard.2008.04.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lammers AE, Diller G-P, Odendaal D, et al. Comparison of 6-min walk test distance and cardiopulmonary exercise test performance in children with pulmonary hypertension. Arch Dis Child 2011;96:141–7. 10.1136/adc.2009.169904 [DOI] [PubMed] [Google Scholar]
- 18.Humpl T, Reyes JT, Holtby H, et al. Beneficial effect of oral sildenafil therapy on childhood pulmonary arterial hypertension: twelve-month clinical trial of a single-drug, open-label, pilot study. Circulation 2005;111:3274–80. 10.1161/CIRCULATIONAHA.104.473371 [DOI] [PubMed] [Google Scholar]
- 19.Galiè N, Ghofrani HA, Torbicki A, et al. Sildenafil citrate therapy for pulmonary arterial hypertension. N Engl J Med 2005;353:2148–57. 10.1056/NEJMoa050010 [DOI] [PubMed] [Google Scholar]
- 20.Oudiz RJ, Roveran G, Hansen JE, et al. Effect of sildenafil on ventilatory efficiency and exercise tolerance in pulmonary hypertension. Eur J Heart Fail 2007;9:917–21. 10.1016/j.ejheart.2007.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barst RJ, Maislin G, Fishman AP. Vasodilator therapy for primary pulmonary hypertension in children. Circulation 1999;99:1197–208. 10.1161/01.CIR.99.9.1197 [DOI] [PubMed] [Google Scholar]
- 22.Lammers AE, Hislop AA, Flynn Y, et al. Epoprostenol treatment in children with severe pulmonary hypertension. Heart 2007;93:739–43. 10.1136/hrt.2006.096412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nakayama T, Shimada H, Takatsuki S, et al. Efficacy and limitations of continuous intravenous epoprostenol therapy for idiopathic pulmonary arterial hypertension in Japanese children. Circ J 2007;71:1785–90. 10.1253/circj.71.1785 [DOI] [PubMed] [Google Scholar]
- 24.Alehan D, Yıldırım I, Sahin M, et al. Long-Term inhaled iloprost use in children with pulmonary arterial hypertension. Cardiol Young 2012;22:396–403. 10.1017/S1047951111001843 [DOI] [PubMed] [Google Scholar]
- 25.Ivy DD, Doran AK, Smith KJ, et al. Short- and long-term effects of inhaled iloprost therapy in children with pulmonary arterial hypertension. J Am Coll Cardiol 2008;51:161–9. 10.1016/j.jacc.2007.09.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levy M, Celermajer DS, Bourges-Petit E, et al. Add-On therapy with subcutaneous treprostinil for refractory pediatric pulmonary hypertension. J Pediatr 2011;158:584–8. 10.1016/j.jpeds.2010.09.025 [DOI] [PubMed] [Google Scholar]
- 27.Maiya S, Hislop AA, Flynn Y, et al. Response to bosentan in children with pulmonary hypertension. Heart 2006;92:664–70. 10.1136/hrt.2005.072314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hislop AA, Moledina S, Foster H, et al. Long-Term efficacy of bosentan in treatment of pulmonary arterial hypertension in children. Eur Respir J 2011;38:70–7. 10.1183/09031936.00053510 [DOI] [PubMed] [Google Scholar]
- 29.Takatsuki S, Rosenzweig EB, Zuckerman W, et al. Clinical safety, pharmacokinetics, and efficacy of ambrisentan therapy in children with pulmonary arterial hypertension. Pediatr Pulmonol 2013;48:27–34. 10.1002/ppul.22555 [DOI] [PMC free article] [PubMed] [Google Scholar]