Abstract
Background:
High out-of-pocket (OOP) cost is a barrier to health care access and treatment compliance. Our study examined high OOP health care cost and burden trends in adults with kidney disease.
Methods:
Using Medical Expenditure Survey 2002–2011 data, we examined the proportion of people greater than 17 years old with kidney disease whose OOP burden were high. Trends by insurance status i.e. private, public or none and trends by income level i.e. poor, low, middle or high income were also examined in this study.
Results:
Approximately 16% of people with kidney disease faced high OOP burden in 2011. The proportion of adults with high OOP burden between 2002 and 2011 fell by 9.7% points. The proportion of privately insured adults facing high OOP burden decreased by 4.7, those who were publicly insured 22.4, and those who were uninsured, 3.1 percentage points. The proportion of those facing high OOP burden who were poor/near poor fell by 26.5, those who had low income 13.4, and those who had middle income, 9 percentage points.
Conclusions:
Though high OOP burden declined between 2002 and 2011 in the US population with kidney disease, most of the decline was among the publicly insured, so that the uninsured populations with kidney disease remain vulnerable. Providers and policy makers should be aware of the vulnerability of uninsured individuals with kidney disease to high OOP burden.
Keywords: kidney disease, Out of Pocket Burden, MEPS
Introduction
Kidney disease is one of the leading causes of morbidity and mortality in the United States (US) and globally.1,2 More than 20 million adults in the US have kidney disease (KD), and 15.1 deaths per 100,000 population result from KD annually.3,4 The financial burden of KD on families and the country is substantial, with an estimated cost of $25 to $46 billion/year.5,6
Healthcare costs in the US have risen tremendously in the past 6 decades, with national health expenditures growing to a record $3 trillion, accounting for 17.5% of the gross domestic product in 2014.7 Furthermore, 11% of total national health expenditures, approximately $330 billion, are related to out-of-pocket (OOP) spending. Consequently, families spend 5 – 14% of their annual income on health care, while 21 – 46% of families experience some financial burden of medical care.8 The impact of healthcare costs on families, irrespective of insurance coverage status, cannot be overstated.
High OOP costs affect medication adherence,9,10,11 access, and utilization of health care.10,12,13 In addition, people with chronic diseases are particularly vulnerable to high OOP cost.13,14,15 For example, 23% of people with diabetes16 and 28% of Medicare beneficiaries with cancer10 faced high OOP burden. Similarly, people with other chronic diseases such as hypertension, osteoarthritis, dementia and inflammatory bowel disease experience one to three times higher OOP expenditures compared to those without these diseases.13,15,17,18
In the last decade policies such as the Medicare part D, Medicare Advantage, Medicare Special Needs Plans (SNPS) and H.R. 4814 The Chronic Kidney Disease Improvement in Research and Treatment Act have all been geared towards lowering the financial burden of health care, the OOP cost for individuals with End Stage Renal Disease (ESRD) and giving more Medicare options for dialysis patients.19 However, individuals with Medicare due to ESRD, continue to experience substantial health related OOP cost and burden, even with Medicare Part D enrollment.8,20
Even so, OOP cost in Medicare recipients with chronic kidney disease (CKD) and ESRD has been described,5,8 but no study has examined trends in OOP burden in non-Medicare recipients with KD in a nationally representative sample. The aim of our study is to examine high OOP health care cost and burden trends in adults with kidney disease by income and insurance coverage status.
METHODS
Data source and Sample
The analysis is based on the dataset from the Medical Expenditure Panel Survey Household Component (MEPS-HC) for 2002–2011. This survey administered by the Agency for Healthcare Research and Quality is a nationally representative of the U.S. civilian non-institutionalized population.21 The household survey contains information on health status, healthcare expenditures and use of services, payment sources, insurance coverage, and other socio demographic details for individuals and families.22 The HC component is based on self-report, therefore in order to validate information received from MEPS-HC respondents, the Medical Provider Component (MPC) requests data on medical and financial characteristics from physicians, pharmacies, home health care providers, and hospitals.23 As part of the MPC, International Classification of Disease, Ninth Revision, Clinical Modification (ICD-9-CM) coded diagnosis are also collected. Interviewers recorded reported medical conditions and procedures related to KD was recorded as verbatim text which were converted to ICD-9-CM codes by professional coders. Upon verification the error rate for coders did not exceed 2.5%. Fully specified ICD-9-CM codes were condensed to three digits in order protect respondent’s confidentiality.23
We used clinical classification categories (CCCs), generated using Clinical Classification Software24, at the person level to identify individuals with KD in the MEPS-HC medical condition. “The CCCs aggregates the ICD-9-CM conditions and V-codes per individual into 260 mutually exclusive clinically homogeneous categories”24. Data from the HC survey of the medical condition and full-year consolidated files for each year were merged using the unique person identifier (DUPERSID) on a one-to-one match.24 We merged 10 years of data in order to ensure a large sample size for a robust estimation. This resulted in an unweighted sample of 2,966 (weighted sample of 2,747,806) adults aged 18–64 with KD. We excluded people aged ≥65 years due to Medicare eligibility, since elderly people have different healthcare needs than younger people.16,25
We combined 10 years of MEPS data because they have a common variance structure relevant for compatibility and comparatively of our study variables.26 The analytic sampling weight variable was adjusted by dividing it by the number of years merged. In order to report the “average annual” basis rather than the entire combined period, we used the sum total of adjusted weights which corresponds to the average annual population size for the combined period and estimates of totals.27 To this end, this study accounted for the clustering, sampling weights and stratification design for nationally representative estimates on the proportion of demographics, mean annual OOP burden and additional OOP burden on health care for the US population.23 Some of the advantages of MEPS data includes the ability to capture all the medical events including emergency room, outpatient, inpatient etc., for each medical care sought, medical events are linked to the primary conditions accordingly, costs incurred by society were derivable from all payers covered in the MEPS and finally, its complex sampling design which enables extrapolation of estimates to the broader U.S civilian non-institutionalized population. The consumer price index was used to adjust the family’s direct 2002–2011 medical costs to a common 2014-dollar value.28
Measures
Variables of interest
The outcome variable in this study is the OOP burden. In MEPS, OOP expenditures refers to self-reported coinsurance and deductible payments plus cash outlays for supplies, services and items not covered by the health insurance.29 Health insurance premiums were not included in our analysis as our interest is to evaluate the financial burden related to medical care utilization.29 “OOP burden was calculated by dividing total family OOP spending on healthcare for all members in a given year by the family’s self-reported pre-tax income for that year”.16 Income and financial burden for healthcare were both measured at the family level. Likewise, we calculated the OOP burden at the family level prior designating to an individual level.16 We considered individuals with KD as having a high OOP burden if their family total OOP healthcare spending exceeded 10% of the family income.16 Those individuals with OOP burden >1 (0.56%) were excluded to control for outliers. Negative spending was discarded as implausible.16 “Medical expenditures according to MEPS include office-based medical provider, hospital outpatient, emergency room, inpatient hospital (including zero night stays), pharmacy and other medical expenses (vision aids, medical supplies and equipment)”.23 Individuals with KD were identified from the MEPS-HC medical condition files with “CCCs 156 (nephritis, nephrosis, renal sclerosis), 157 (acute and unspecified renal failure), 158 (Chronic renal failure), 160 (calculus or urinary tract) and 161 (other diseases of kidney and ureters)”.22
Controlled covariates
All controlled covariates used for analysis were based on self-report:
Co-morbidities: Co-morbidities were dichotomized as yes or no based-on response to the following questions “Have you ever been diagnosed with hypertension, stroke, emphysema, joint pain, arthritis and asthma?” Presence of Cardiovascular Disease (CVD) represents a positive response to the question “Have you ever been diagnosed with coronary heart disease or angina or myocardial infarction or other heart diseases?” Race/ethnic was dichotomized into three groups: Non-Hispanic White (NHW), Non-Hispanic Black (NHB), Hispanic and Others. Education was dichotomized as: less than high school (≤ grade 11), high school (grade 12) and college or more (grade ≥ 13). Marital status was categorized as: married, non-married and never married. Gender was categorized into 2 groups – male and female while age was dichotomized into two groups: 18–44 and 45–64. Census region was categorized as: Northeast, Midwest, South and West. Metropolitan Statistical Area (MSA) was coded as yes =1 if individual resided in a MSA at December 31st of that year. Health insurance was categorized into private only, public only and uninsured during the entire year. Income level was defined based on percentage poverty level and categorized into four groups: poor/negative (<125%), low-income (125% to less than 200%), middle-income (200% to less than 400%) and high-income (≥ 400%). Calendar year was categorized into 2002/2003, 2004/05, 2006/07, 2008/09, and 2010/11 consecutively for the pooled data.
Definition of Insurance
In the US, under 65, to be eligible for Medicare, an individual has to have a permanent disability as determined by the Social Security Administration or be on dialysis.30 The eligibility for Medicaid differs state by state by income as well as other medical criteria.30 We classified insurance status into three groups: privately insured, publicly insured (Medicare and Medicaid) and uninsured. We classified individuals without insurance coverage during the entire as uninsured and everyone else was assigned to one of two mutually exclusive insurance categories based on the type and length of coverage held during the entire (measured monthly).16,25 To ensure sufficient sample size, we also incorporated the remaining persons covered with other types of insurance (other public and Tricare) as part of the overall analysis.
Analyses
We estimated over the proportion of adults with KD with high OOP burden over time and subsequently stratified by insurance status and family income level. The demographic characteristics of individuals with kidney disease are presented by OOP burden status (OOP Ratio >10% vs OOP Ratio <10%), as percentages for categorical variables, with differences tested using chi square (χ2) tests taking into account the complex survey design. To explore the drivers behind the changes in high OOP burden, we estimated the annual mean family OOP spending on different types of health care services (prescription, inpatient care, office care, emergency visit care and other health services), and family income with stratification by insurance.16 Mean changes from the benchmark year (2002/03) compared to other time periods were examined using t-tests. In addition, we calculated OOP spending for all healthcare services for adults with KD.
We used a two-part model that accounted for the complex survey design to estimate the proportion of people with KD facing high OOP burden. The two-part model entails first, a probit model which estimates the probability of having a zero versus positive OOP burden and second, a generalized linear model (GLM) gamma distribution and log link estimate which is contingent on having a positive OOP burden.31,32 The two-part model offers users the advantage to calculate marginal effects and their standard errors given the availability of margins in STATA.32 We performed kernel density plot to verify the skewness of the OOP burden. Thus, we used GLM gamma distribution and log link in the second part of the model in order to transform the OOP burden into log scale thereby to account the skewness of the OOP burden. The advantage of using GLM gamma family and log link over log OLS includes ability to avoid bias associated with retransformation to raw scale, homoscedasticity assumptions and its ability to relax normality.32 Finally, we controlled for confounders such as socio-demographic factors (including age, marital status, sex, education, race), health insurance status, MSA, region, income level, and comorbidities in the models. In addition, we estimated a model with interaction terms between time and insurance coverage.
We performed all analysis using STATA 14 (StataCorp LP College Station, TX) and statistically significance was set at p<0.05 for this study.
Results
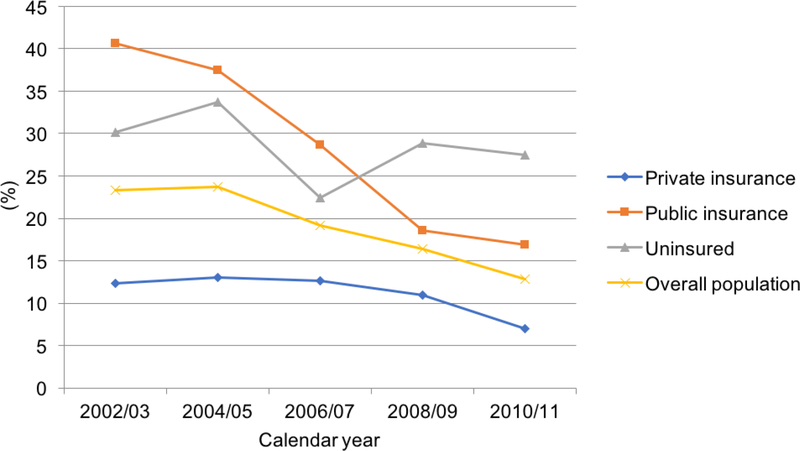
The proportion of adults <65 years with KD and high OOP burden between 2002 and 2011 fell by 9.7% points (Fig. 1). The proportion adults with KD facing a high OOP burden fell continuously from 22.6% during the reference year, 2002/2003 to 12.9% in 2011. The estimates and changes in proportion of adults with KD who had a high OOP burden varied by insurance (Fig. 1) and income (Fig. 2) following stratification. Figure 1 shows trends in OOP burden in individuals with KD stratified by insurance status. For privately insured individuals, high OOP burden initially increased to 13.1% in 2004/2005 and then declined continuously by 4.7 percentage points from 11.9% in 2002/2003 to 7.19% in 2010/2011. For publicly insured individuals, high OOP burden declined continuously by 22.4 percentage points from 39.6% in 2002/2003 to 17.2% in 2010/2011. For uninsured individuals, high OOP burden initially increased to 32.9% in 2004/2005 and then declined continuously by 3.1 percentage points from 30.1% in 2002/2003 to 27.0% in 2010/2011.
Figure 1:
Adults aged 18–64 with kidney disease facing high OOP burden from 2002–2011 by insurance status.
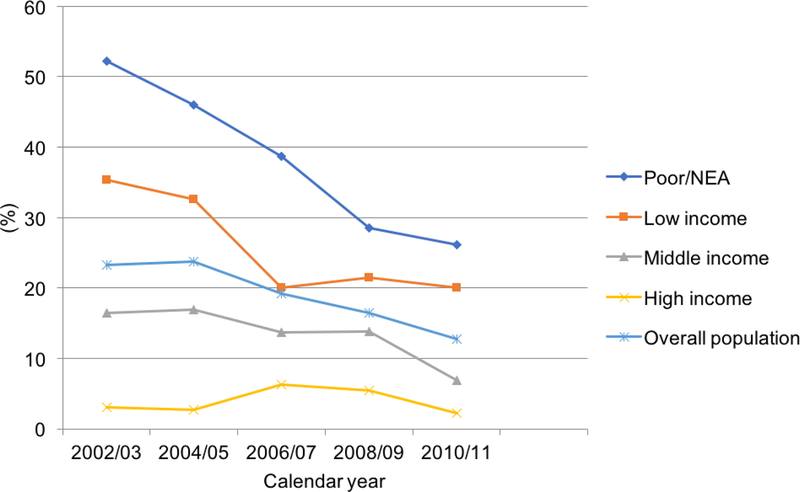
Figure 2:
Adults aged 18–64 with kidney disease facing high OOP burden from 2002–2011 by income status.
Figure 2 illustrates trends in OOP burden for adults with KD stratified by income level. High OOP burden fell continuously by 26.5 percentage points from 51.9% in 2002/2003 to 25.4% in 2010/2011 for the poor and near poor group. For the low-income group, high OOP burden decreased continuously by 13.4 percentage points from 33.9% in 2002/2003 to 20.5% in 2010/2011. For the middle-income group, the OOP burden initially increased to 17.0% in 2004/2005 and then declined continuously by 9.0 percentage points from 16.1% in 2002/2003 to 7.1% in 2010/2011. For the high-income group, high OOP burden decreased to 2.65% in 2003/2004 and then increased to 6.23% in 2005/2006, then declining to 2.22% by 0.43 percentage points in 2010/2011 from a baseline of 2.65%.
Table 1 shows the differences in demographics by OOP burden status in the KD population. Significant differences in high OOP status were found by specific demographic, clinical, and time characteristics. High OOP burden was more likely in age 45–64, females, non-married and never married, less than high school and high school education, publicly insured and uninsured, Southern and Western regions, and poor/near poor and low-income individuals with KD. The comorbidities diabetes, hypertension, CVD, emphysema, joint pain, arthritis, and asthma all were more likely to have high OOP burden for individuals with KD. High OOP burden was more likely in 2002/2003, and 2004/2005 in individuals with KD (see table 1).
Table 1:
Sample demographics by family out-of-pocket (OOP) burden aged 18–64 among people with kidney disease.
| Variables | All (%) | OOP Ratio >10% (%) | OOP Ratio <10% (%) | P-value |
|---|---|---|---|---|
| N(n) | 2,747,806 (2,966) | 491,526 (565) | 2,256280 (2,401) | |
| Age category | 0.010 | |||
| Age 18–44 | 44.1 | 37.1 | 45.7 | |
| Age 45–64 | 55.9 | 62.9 | 54.3 | |
| Gender | 0.002 | |||
| Male | 50.9 | 43.3 | 52.5 | |
| Female | 49.1 | 56.7 | 47.5 | |
| Race/ethnicity | 0.071 | |||
| Non-Hispanic White | 71.3 | 71.3 | 71.3 | |
| Non-Hispanic Black | 11.2 | 14.1 | 10.6 | |
| Hispanic | 13.1 | 10.4 | 13.7 | |
| Others | 4.4 | 4.2 | 4.4 | |
| Marital status | <0.001 | |||
| Married | 59.7 | 45.6 | 62.8 | |
| Non-married+ | 20.1 | 31.9 | 17.5 | |
| Never married | 20.2 | 22.5 | 19.7 | |
| Education category | 0.001 | |||
| <High school | 18.6 | 22.7 | 17.6 | |
| High school | 34.1 | 38.7 | 33.2 | |
| College or more | 47.3 | 38.6 | 49.2 | |
| Insurance | <0.001 | |||
| Private | 69.3 | 48.5 | 73.8 | |
| Public | 19.5 | 33.0 | 16.6 | |
| Uninsured | 11.2 | 18.5 | 9.6 | |
| Metropolitan statistical status | 0.121 | |||
| MSA | 17.9 | 21.2 | 17.2 | |
| Non-MSA | 82.1 | 78.8 | 82.8 | |
| Census region | 0.013 | |||
| Northeast | 19.6 | 17.2 | 20.1 | |
| Midwest | 21.4 | 15.4 | 22.7 | |
| South | 40.4 | 46.8 | 39.0 | |
| West | 18.6 | 20.6 | 18.2 | |
| Poverty category | <0.001 | |||
| Poor/NEA | 19.2 | 47.9 | 13.0 | |
| Low Income | 13.4 | 22.3 | 11.5 | |
| Middle Income | 29.8 | 22.1 | 31.5 | |
| High Income | 37.6 | 7.7 | 44.0 | |
| Chronic conditions | ||||
| Diabetes | 23.0 | 33.9 | 20.6 | <0.001 |
| Hypertension | 45.5 | 56.6 | 43.1 | <0.001 |
| CVD | 20.5 | 34.3 | 17.6 | <0.001 |
| Emphysema | 2.4 | 3.9 | 2.1 | 0.072 |
| Joint pain | 46.2 | 65.6 | 42.0 | <0.001 |
| Arthritis | 32.1 | 48.1 | 28.6 | <0.001 |
| Asthma | 11.9 | 19.1 | 10.4 | <0.001 |
| Year category | 0.002 | |||
| Year 2002/03 | 19.2 | 24.4 | 18.1 | |
| Year 2004/05 | 17.9 | 22.0 | 16.9 | |
| Year 2006/07 | 20.2 | 19.3 | 20.3 | |
| Year 2008/09 | 22.4 | 18.4 | 20.9 | |
| Year 2010/11 | 22.3 | 15.9 | 23.8 |
N - weighted sample size; n - unweighted sample size; %, weighted percentage.
Non-married stands for widowed/divorced and separated. OOP burden ratio is computed total family’s OOP spending on healthcare for all members divided by family’s self-reported pre-tax income.
The components of OOP burden in the KD population, stratified by insurance type and time is displayed in Table 2. The total mean unadjusted OOP expenditures for individuals with KD declined significantly from $1,707 in 2002/2003 to $1,218 (P<0.05) in 2010/2011. By insurance status, among the privately insured individuals with KD, the total mean unadjusted OOP expenditures increased significantly by 63.1% from $1,224 in 2002/2003 to $1,996 (P<0.05) in 2006/2007. Among the publicly insured individuals with KD, the total mean unadjusted OOP expenditures significantly decreased by 60.8% from $3,113 in 2002/2003 to $1,219 (P<0.01) in 2010/2011. Among the uninsured individuals with KD, it is notable that mean unadjusted OOP inpatient expenditures increased from $6 in 2002/2003 to $484 (P<0.05) in 2010/2011. There was an opposite significant declining trend in prescription drugs OOP expenditures by 39.8% for the privately insured from $1,096 in 2002/2003 to $660 (P<0.001) in 2010/2011.
Table 2:
Mean annual family income and OOP burden for kidney disease services by insurance coverage 2002–2011.
| Mean annual total family OOP spending ($) | Mean OOP (Person level) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Sample size | Mean annual family income | All healthcare services | Prescription drugs | Inpatient care | Outpatient care | Office care | Emergency visit care | Other care | People with kidney disease | |
| Overall | ||||||||||
| 2002–03 | 647 | 53,133 | 2,867 (5) | 1,460 (3) | 136 (0.3) | 113 (0.2) | 619 (1) | 78 (0.1) | 460 (1) | 1,707 |
| 2004–05 | 544 | 56,809 | 3,080 (5) | 1,475 (3) | 227 (0.4) | 151 (0.3) | 542 (1) | 134 (0.2) | 551 (1) | 2,068 |
| 2006–07 | 584 | 61,650* | 2,651 (4) | 1,043 (2) | 117 (0.2) | 116 (0.2) | 605 (1) | 109 (0.2) | 662 (1) | 1,617 |
| 2008–09 | 575 | 64,980** | 2,421 (4) | 928** (1) | 183 (0.3) | 97 (0.1) | 561 (1) | 170** (0.3) | 481 (1) | 1,437 |
| 2010–11 | 616 | 69,542*** | 2,082* (3) | 730*** (1) | 134 (0.2) | 77 (0.1) | 454 (1) | 131 (0.2) | 556* (1) | 1,218* |
| Privately insured | ||||||||||
| 2002–03 | 366 | 64,620 | 2,487 (4) | 1,096 (2) | 145 (0.2) | 108 (0.2) | 540 (1) | 95 (0.1) | 503 (1) | 1,224 |
| 2004–05 | 291 | 72,462** | 2,991 (4) | 1,333 (2) | 176 (0.2) | 197 (0.3) | 546 (1) | 120 (0.2) | 619 (1) | 1,996 |
| 2006–07 | 293 | 80,589*** | 2,932 (4) | 926 (1) | 73 (0.1) | 171 (0.2) | 760 (1) | 124 (0.2) | 878* (1) | 1,656* |
| 2008–09 | 303 | 79,928** | 2,574 (3) | 871 (1) | 199 (0.2) | 134 (0.2) | 666 (1) | 131 (0.2) | 571 (1) | 1,270 |
| 2010–11 | 316 | 88,171*** | 2,284 (3) | 660*** (1) | 101 (0.1) | 112 (0.1) | 554 (1) | 175 (0.2) | 682 (1) | 1,188 |
| Publicly insured | ||||||||||
| 2002–03 | 197 | 26,978 | 4,049 (15) | 2,480 (9) | 163 (0.6) | 137 (0.5) | 861 (3) | 11 (0.04) | 397 (1) | 3113 |
| 2004–05 | 166 | 22,016 | 3,421 (16) | 2,206 (10) | 141 (0.6) | 55 (0.2) | 655 (3) | 22 (0.1) | 342 (2) | 2,293 |
| 2006–07 | 195 | 26,568 | 1,865 (7) | 1,203 (5) | 71 (0.3) | 27 (0.1) | 240 (1) | 32 (0.1) | 292 (1) | 1,284** |
| 2008–09 | 172 | 27,667 | 1,718 (6) | 1,144 (4) | 77 (0.3) | 27 (0.1) | 249 (1) | 54 (0.2) | 167* (1) | 1,463 |
| 2010–11 | 201 | 36,852 | 1,708* (5) | 921 (2) | 69 (0.2) | 31 (0.1) | 249 (1) | 34 (0.1) | 404 (1) | 1,219** |
| Uninsured | ||||||||||
| 2002–03 | 73 | 44,426 | 2,297 (5) | 1,126 (3) | 6 (0.01) | 92 (0.2) | 574 (1) | 157 (0.4) | 348 (1) | 1,428 |
| 2004–05 | 83 | 39,215 | 2,736 (7) | 855 (2) | 603 (2) | 63 (0.2) | 280 (1) | 383 (1) | 564 (1) | 1,903 |
| 2006–07 | 85 | 40,136 | 3,049 (8) | 1,312 (3) | 446 (1) | 38 (0.1) | 655 (2) | 154 (0.4) | 443 (1) | 2,180 |
| 2008–09 | 90 | 47,938 | 2,861 (6) | 879 (2) | 298 (0.6) | 29 (0.1) | 538 (1) | 592 (1) | 525 (1) | 2,329 |
| 2010–11 | 80 | 42,182 | 2,249 (5) | 771 (2) | 484* (1) | 27 (0.1) | 488 (1) | 166 (0.4) | 313 (1) | 1,571 |
Note: Data are mean (%), the percentages represent the relative portion of the family’s OOP expenditures paid from total family’s income.
Significantly different from estimates in 2001–2002 at p<0.05,
Significantly different from estimates in 2001–2002 at p<0.01,
Significantly different from estimates in 2001–2002 at p<0.001. Cost is adjusted in 2014 dollar.
The estimated incremental effects are presented in table 3. After adjusting for socio-demographic and comorbidity covariates as well as time in the two-part model, individuals with KD between the ages of 45 and 64 had a significant 0.009 (95% CI −0.001 – 0.015) higher OOP burden relative to individuals between the ages of 18 and 44 (see Table 3). For example, it would imply an increase from 20% to 21% burden for individuals who had a baseline of 20% OOP burden. Non-Hispanic Black (−0.012; 95% CI −0.020–0.004) and Hispanic (−0.016; 95% CI −0.025 - −0.006) individuals with KD had lower OOP burdens relative to non-Hispanic White. Uninsured individuals with KD had a 0.017 (95% CI 0.005–0.028) higher OOP burden than those with private insurance. Relative to the poorest individuals with KD, low (−0.05; 95% CI −0.071 - −0.029), middle (−0.08; 95% CI −0.101 - −0.059) and high (−0.098; 95% CI −0.118 - −0.076) income individuals had lower OOP burdens. Among comorbidities, individuals with KD and comorbid diabetes (0.017; 95% CI 0.008 – 0.027), hypertension (0.009; 0.002–0.015), CVD (0.019; 95% CI 0.011–0.027), and joint pain (0.009; 95% CI 0.003–0.016) had higher OOP burdens. Over time, 2008/2009 (−0.015; 95% CI −0.023 - −0.007) and 2010/2011 (−0.022; 95% CI −0.031 - −0.014) had lower OOP burdens relative to 2002/2003. We found significant negative interaction effects between public insurance and 2008/09 (−0.030; 95% CI −0.055- −0.004) and public insurance and 2010/11 (−0.023; 95% CI −0.045- −0.001).
Table 3:
Two-part regression model: Incremental effects of OOP burden ratio for people with kidney disease!
| Variables | Incremental Effect | 95% Cl | P-value |
|---|---|---|---|
| Covariates | |||
| Age 18–44 | -- | -- | -- |
| Age 45–64 | 0.009* | −0.001–0.015 | 0.022 |
| Male | -- | -- | -- |
| Female | 0.005 | −0.001 – 0.011 | 0.076 |
| Non-Hispanic White | -- | -- | -- |
| Non-Hispanic Black | −0.012** | −0.020 – 0.004 | 0.002 |
| Hispanic | −0.016** | −0.025 – −0.006 | 0.002 |
| Others | −0.011 | −0.024 – 0.0004 | 0.059 |
| Married | -- | -- | -- |
| Non-married+ | 0.001 | −0.007 – 0.008 | 0.830 |
| Never married | 0.008 | −0.002 – 0.018 | 0.118 |
| <High school | -- | -- | -- |
| High school | 0.003 | −0.004 – 0.010 | 0.410 |
| College or more | 0.008 | −0.0001 – 0.016 | 0.052 |
| Private | -- | -- | -- |
| Public insured | −0.006 | −0.014 – 0.003 | 0.177 |
| Uninsured | 0.017** | 0.005 – 0.028 | 0.005 |
| Non-MSA | -- | -- | -- |
| MSA | 0.003 | −0.004 – 0.010 | 0.399 |
| Northeast | -- | -- | -- |
| Midwest | −0.008 | −0.019 – 0.002 | 0.113 |
| South | 0.002 | −0.008 – 0.012 | 0.668 |
| West | 0.001 | −0.011 – 0.012 | 0.910 |
| Poor/NEA | -- | -- | -- |
| Low Income | −0.050*** | −0.071 – −0.029 | <0.001 |
| Middle Income | −0.080*** | −0.101 – −0.059 | <0.001 |
| High Income | −0.098*** | −0.118 – −0.076 | <0.001 |
| No Diabetes | -- | -- | -- |
| Diabetes | 0.017*** | 0.008 – 0.027 | <0.001 |
| No hypertension | -- | -- | -- |
| Hypertension | 0.009** | 0.002 – 0.015 | 0.007 |
| No CVD | -- | -- | -- |
| CVD | 0.019*** | 0.011 – 0.027 | <0.001 |
| No emphysema | -- | -- | -- |
| Emphysema | −0.009 | −0.028 – 0.010 | 0.362 |
| No joint pain | -- | -- | -- |
| Joint pain | 0.009** | 0.003 – 0.016 | 0.003 |
| No arthritis | -- | -- | -- |
| Arthritis | 0.004 | −0.003 – 0.011 | 0.213 |
| No asthma | -- | -- | -- |
| Asthma | 0.008 | −0.001 – 0.018 | 0.086 |
| Year 2002/03 | -- | -- | -- |
| Year 2004/05 | −0.0008 | −0.009 – 0.007 | 0.840 |
| Year 2006/07 | −0.009 | −0.018 – 0.0007 | 0.070 |
| Year 2008/09 | −0.015*** | −0.023 – −0.007 | <0.001 |
| Year 2010/11 | −0.022*** | −0.031 – −0.014 | <0.001 |
Level of significance p< 0.05;
level of significance p<0.01,
level of significance p < 0.001
Non-married stands for widowed/divorced and separated.
Cost is adjusted in 2014 dollar
Discussion
This study on trends of OOP burden from a nationally representative sample of US adults with KD shows that unadjusted mean OOP expenditures have declined between 2002 and 2011, most of the decline was among the publicly insured, so that the uninsured populations with kidney disease remain vulnerable. Approximately 15.9% of adults with KD faced a high OOP burden in the US in 2010/2011. The uninsured, age 45 – 64 and certain comorbidities (diabetes, hypertension, cardiovascular disease and joint pain) had higher OOP burden, while non-Hispanic blacks, Hispanics and the poor had lower OOP burden independent of relevant covariates.
Overall, high OOP burden was stable from 2002 to 2005 and then consistently decreased from 2004 through 2011. Varying trends were also observed by insurance and income category. While the trends in OOP burden observed among privately insured individuals mirrored the overall population, publicly insured and poor/near-poor income individuals experienced a robust decline in high OOP burden from 2002 – 2011. Uninsured individuals had a decline in high OOP burden from 2004/2005 – 2006/2007, then experienced an increase during most part of the study period. It is possible that the Obamacare plan has since offset this trend for the uninsured, but this was not examined in this analysis. We also noticed the gap in high OOP burden observed between the publicly insured/poor income and privately insured/high income categories at beginning of the study period narrowed substantially by the end of the study period.
The trend observed among publicly insured could be due to the significant decline in out of pocket expenditures for all healthcare services from 2002 to 2011. For example, there was a significant decline in prescription drug out of pocket expenditures from 2002 to 2010, resulting in a significant decline in OOP expenditures for all healthcare services from 2002 to 2011. The decline in prescription drugs OOP expenditures could be due to the impact of Medicare part D on all drug prices which has benefited all individuals beyond Medicare beneficiaries. It could also be due to the significant increase in mean annual family income from 2002 to 2011 (P<0.001). In addition, the results of the interaction effects between public insurance and time reinforces the decline in OOP burden observed between 2008 and 2011. On the other hand, the trend observed among the privately insured could be due to the significant increase in “other care” OOP expenditures from 2002 to 2007, even though they also had a significant increase in mean annual family income from 2002 to 2011.
Our findings are comparable to Li et al who examined OOP burden in patients with diabetes.16 Like our study, they reported a decline in high OOP burden between 2001 and 2002 – 2011, even though we observed a higher percentage point decline. After stratification by income and insurance status, while our study revealed uninsured individuals had the highest OOP burden followed by the publicly and privately insured, they found that privately and uninsured individuals had the highest OOP burden followed by publicly insured individuals.16 In addition, we observed that poor/near-poor individuals had the highest OOP burden followed by low, middle and high-income individuals, while they showed the poor/near-poor and low-income individuals had the highest OOP burden, followed by middle and high-income individuals. These subtle differences could be related to study design. For example, while both studies pooled adjacent years to examine trends in OOP burden, the baseline year and final year differ between studies. Also, we did not include insurance premiums in our analyses.
Another study examined OOP burden in non-elderly adults with hypertension, and in contrast to our study, revealed that individuals with private non-group insurance had the highest OOP burden, followed by individuals that are uninsured, publicly insured and those with private group insurance.12 Unlike our study, they included insurance premiums in computing total OOP burden, and they also defined OOP burden differently.
Our study findings have enormous clinical implications since high OOP burden leads to medication non-adherence,9 decreases access to care,10,13 delays recommended care13 and ultimately leads to poor health outcomes.10 There should be more patient-provider discussions with regards to cost of care33 - especially cost of prescription medications, to enable early detection of barriers to be recommended care. More importantly, multinational research has shown that while differences exist in OOP spending, these differences arise from nation-specific policies, rather than patient-specific factors such clinical, demographics and economic characteristics.9 Hence, initiation of policies by policy makers to reduce OOP burden in adults with KD, most especially for individuals with early CKD is of the essence. More so, providers need to be aware of the vulnerability of uninsured individuals with kidney disease to high OOP burden, which negatively impacts medication adherence and clinical outcomes.
The unique strengths of this study include being the first study to examine trends in OOP burden in adults with KD, using nationally representative data and the best cost data available to examine expenditure by service type. However, some limitations cannot be ignored. First, even though we pooled 10-years of data this study cannot be interpreted as longitudinal data. Second, we did not include health insurance premiums and individuals aged 65 and over given their eligibility for Medicare, hence a potential for under estimation of OOP burden in this population and limited generalizability respectively. Third, comorbidities were based on self-report and are thus prone to bias. Fourth, it is difficult to attribute findings of high OOP burden to KD solely, since care and treatment of KD is intertwined with management of other comorbid conditions, such as diabetes and hypertension. Fifth, the small sample size of adults with KD limits the power of group comparison. Sixth, the kidney disease group was heterogeneous and included individuals with varying degrees of disease acuity and potentially different economic impact. Nevertheless, MEPS is the only valid national survey that captures expenditures in adults with KD, so we believe we have provided an important contribution to the literature.
In conclusion, we found that trends in the OOP burden for individuals with kidney disease decreased by approximately 10 percentage points between 2002 and 2011. Even with this observed decline, a significant proportion of adults with KD, continue to face a high OOP burden.
Acknowledgement:
Funding Source: This study was supported by Grant K24DK093699 from The National Institute of Diabetes and Digestive and Kidney Disease (PI: Leonard Egede) and the Advancing Healthier Wisconsin/Clinical and Translational Science Award program at the Medical College of Wisconsin (UL1TR001436 and KL2TR001438, KL2 award to Ozieh).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: The authors report no competing interest relevant to this article.
Ethics Approval and consent to participate: This study was based on a publicly available dataset, MEPS (as described in the methods). The authors did not have direct contact with survey participants.
Publisher's Disclaimer: Disclaimer: This manuscript represents the views of the authors and not those of NIH, VHA or HSR&D
References
- 1.Eknoyan G, Lameire N, Barsoum R et al. The burden of kidney disease: improving global outcomes. Kidney Int. 2004;66(4):1310–4. [DOI] [PubMed] [Google Scholar]
- 2.Lozano R, Naghavi M, Foreman K. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention (CDC). National Chronic Kidney Disease Fact Sheet: General Information and National Estimates on Chronic Kidney Disease in the United States, 2014. Atlanta, GA: US Department of Health and Human Services, Centers for Disease Control and Prevention, 2014 [Google Scholar]
- 4.Xu JQ, Murphy SL, Kochanek KD et al. Deaths: Final data for 2013. National vital statistics reports; vol 64 no 2. Hyattsville, MD: National Center for Health Statistics; 2016. [PubMed] [Google Scholar]
- 5.U.S. Renal Data System(USRDS): USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 6.Soni A and Wright J Average Annual Health Care Use and Expenditures for Kidney Disease among Adults 18 and Older, U.S. Civilian Noninstitutionalized Population, 2003–2007; Statistical Brief #306. December 2010. Agency for Healthcare Research and Quality. Rockville, MD, 2010 [Google Scholar]
- 7. [November 18th, 2016]; https://www.cms.gov/Research-Statistics-Data-and-Systems/Statistics-Trends-and-Reports/NationalHealthExpendData/NHE-Fact-Sheet.html accessed.
- 8.Cohen RA, Kirzinger WK. Financial burden of medical care: A family perspective NCHS data brief, no 142 Hyattsville, MD: National Center for Health Statistics. 2014. [PubMed] [Google Scholar]
- 9.Hirth RA, Greer SL, Albert JM et al. Out-of-pocket spending and medication adherence among dialysis patients in twelve countries. Health Aff (Millwood). 2008;27(1):89–102. [DOI] [PubMed] [Google Scholar]
- 10.Kaisaeng N, Harpe SE, Carroll NV. Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J Manag Care Spec Pharm. 2014;20(7):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Piette JD, Wagner TH, Potter MB et al. Health insurance status, cost-related medication underuse, and outcomes among diabetes patients in three systems of care. Med Care. 2004;42(2):102–9. [DOI] [PubMed] [Google Scholar]
- 12.Bernard DM, Johansson P, Fang Z. Out-of-pocket healthcare expenditure burdens among nonelderly adults with hypertension. Am J Manag Care. 2014. May;20(5):406–13. [PubMed] [Google Scholar]
- 13.Merlis M Family Out-of-Pocket Spending for Health Services: A Continuing Source of Financial Insecurity, The Commonwealth Fund, June 2002. [Google Scholar]
- 14.Harman JS, Kelleher KJ, Reynolds CF et al. Out-of-pocket healthcare expenditures of older Americans with depression. J Am Geriatr Soc. 2004;52(5):809–13. [DOI] [PubMed] [Google Scholar]
- 15.Kotlarz H, Gunnarsson CL, Fang H et al. Insurer and out-of-pocket costs of osteoarthritis in the US: evidence from national survey data. Arthritis Rheum. 2009;60(12):3546–53. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Barker LE, Shrestha S et al. Changes over time in high out-of-pocket health care burden in U.S. adults with diabetes, 2001–2011. Diabetes Care. 2014;37(6):1629–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Delavande A, Hurd MD, Martorell P et al. Dementia and out-of-pocket spending on health care services. Alzheimers Dement. 2013;9(1):19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gunnarsson C, Chen J, Rizzo JA et al. Direct health care insurer and out-of-pocket expenditures of inflammatory bowel disease: evidence from a US national survey. Dig Dis Sci. 2012;57(12):3080–91. [DOI] [PubMed] [Google Scholar]
- 19.https://www.kidney.org/blog/advocacy-action/medicare-beneficiaries-kidney-failure-have-highest-out-pocket-spending accessed November 18th, 2016.
- 20.Cubanski J, Swoope C, Damico A et al. Health Care on a Budget: The Financial Burden of Health Spending by Medicare Households. The Henry J. Kaiser Family Foundation. January 2014 [Google Scholar]
- 21.Agency for Healthcare Research and Quality (AHRQa). Methodology Report # 27, Sample design of the 2011 Medical Expenditure Panel Survey Insurance Component 2013a, Available from http://meps.ahrq.gov/data_files/publications/mr27/mr27.pdf accessed 22 August 2014. [Google Scholar]
- 22.Agency for Healthcare Research and Quality (AHRQb). Medical Expenditure Panel Survey, 2011 Medical conditions 2013b, Available from http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/h146/h146doc.pdf Accessed 20 August 2014. [Google Scholar]
- 23.Agency for Healthcare Research and Quality (AHRQc). Medical Expenditure Panel Survey. 2011 Full year consolidated data file 2013c, Available from http://meps.ahrq.gov/mepsweb/data_stats/download_data_files.jsp. Accessed 18 August 2014.
- 24.Agency for Healthcare Research and Quality (AHRQ). Household Component-Insurance Component Linked Data 1999, Research file 2003, Available from http://meps.ahrq.gov/mepsweb/data_stats/download_data/pufs/link_99hcic/hc_ic99link_doc.pdf. Accessed 22 August 2014. [Google Scholar]
- 25.Banthin JS, Bernard DM. Changes in Financial Burdens for Health Care: National Estimates for the Population Younger Than 65 Years, 1996 to 2003. JAMA 2006; 296:2712–2719. [DOI] [PubMed] [Google Scholar]
- 26.DeVoe JE, Tillotson CJ, Wallace LS. Children’s receipt of health care services and family health insurance patterns. Ann Fam Med. 2009; 7: 406–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Agency for Healthcare Research and Quality (AHRQd). MEPS HC-036: 1996–2011 Pooled Linkage Variance Estimation File 2013d. MEPS file, Available from http://meps.ahrq.gov/data_stats/download_data/pufs/h36/h36u11doc.shtml. Accessed 18 August 2014. [Google Scholar]
- 28.Bureau of Labor Statistics, CPI Inflation Calculator”. Available from http://data.bls.gov/cgi-bin/cpicalc.pl [Google Scholar]
- 29.Hwang W, Weller W, Ireys H et al. 2001. Out-of-Pocket Medical Spending For health Care of Chronic Conditions. Health Affairs 2001; 20(6): 267–278. [DOI] [PubMed] [Google Scholar]
- 30. https://www.cms.gov/About-CMS/Agency-Information/Aboutwebsite/Privacy-Policy.html.
- 31.Manning WG, Mullahy J. Estimating log models: to transform or not to transform? J Health Econ. 2001; 20: 461–494. [DOI] [PubMed] [Google Scholar]
- 32.Belotti F, Deb P, Manning WG et al. Tpm: estimating two-part models. The Stat Journal. 2012; 5(2):1–13. [Google Scholar]
- 33.Alexander GC, Casalino LP, Meltzer DO. Patient-physician communication about out-of-pocket costs. JAMA. 2003;290(7):953–8. [DOI] [PubMed] [Google Scholar]