Abstract
Gliomas are a highly heterogeneous tumor, refractory to treatment and the most frequently diagnosed primary brain tumor. Although the current WHO grading system (2016) demonstrates promise towards identifying novel treatment modalities and better prediction of prognosis over time, to date, existing targeted and mono therapy approaches have failed to elicit a robust impact on disease progression and patient survival. It is possible that tumor heterogeneity as well as specifically targeted agents fail because redundant molecular pathways in the tumor make it refractory to such approaches. Additionally, the underlying metabolic pathology, which is significantly altered during neoplastic transformation and tumor progression, is unaccounted for. With several molecular and metabolic pathways implicated in the carcinogenesis of CNS tumors, including glioma, we postulate that a systemic, broad spectrum approach to produce robust targeting of relevant and multiple molecular and metabolic regulation of growth and survival pathways, critical to the modulation of hallmarks of carcinogenesis, without clinically limiting toxicity, may provide a more sustained impact on clinical outcomes compared to the modalities of treatment evaluated to date. The objective of this review is to examine the emerging hallmark of reprogramming energy metabolism of the tumor cells and the tumor microenvironment during carcinogenesis, and to provide a rationale for exploiting this hallmark and its biological capabilities as a target for secondary chemoprevention and treatment of glioma. This review will primarily focus on interventions to induce ketosis to target the glycolytic phenotype of many cancers, with specific application to secondary chemoprevention of low grade glioma-to halt the progression of lower grade tumors to more aggressive subtypes, as evidenced by reduction in validated intermediate endpoints of disease progression including clinical symptoms.
Keywords: Gliomas, Energy metabolism, Ketosis, Ketogenic diet, Kecondary chemoprevention, Molecular pathways, Metabolic pathways, Aerobic glycolysis, Glucose, Insulin
1. Introduction
Cancers are one of the leading causes of morbidity and mortality worldwide, accounting for 8.8 million deaths in 2015 [1,2]. In the US, it is estimated that 1,688,780 new cancers will be diagnosed in 2017 [3] and 600,920 people will die from this disease in 2017. Gliomas are a highly heterogeneous tumor, refractory to treatment and the most frequently diagnosed primary brain tumor. In 2017, an estimated 23,800 new cases of brain tumors will be diagnosed, and 16,700 will die from a brain tumor, most attributed to glioma [3]. Gliomas are neuroepithelial tumors that originate from the supporting glial cells of the central nervous system (CNS). Glial tumors mostly consist of astrocytomas and oligodendrogliomas. The 2016 WHO classification of CNS tumors uses molecular genetic parameters in addition to histology to define many tumor entities [4]. The routine assessment of isocitrate dehydrogenase (IDH) mutation status, which are frequent in grade II and III infiltrating gliomas and a small subset of glioblastomas (GBM), improves histological diagnostic accuracy and has been observed to have a favorable prognostic implication for all glial tumors [5–7] and to be predictive for chemotherapeutic responses in anaplastic oligodendrogliomas with codeletion of 1p/19q chromosomes. Glial tumors that contain chromosomal codeletion of 1p/19q, also defined as tumors of oligodendroglial lineage, have favorable prognosis. GBM typically lack IDH mutations and are instead characterized by EGFR, PTEN, TP53, PDGFRA, NF1, and CDKN2A/B alternations and TERT promoter mutations [5]. The revised classification thus provides a model that reflects malignant characteristics based on histopathlogical and molecular features of the tumors, offering additional opportunities for improved diagnosis, treatment, and estimating prognosis in the molecular era. Lower grade diffuse gliomas (LGGI and II) (WHO Grade I–II) have fewer malignant characteristics than high-grade gliomas (WHO Grade III-IV), and a relatively better clinical prognosis. However, the majority of LGGs eventually progress to high grade gliomas (HGG, WHO Grade III or IV), with death an inevitable outcome [8]. The life expectancy following diagnosis with Grade IV GBM is 2–4 months without treatment. Survival at 5 years for patients with GBM who receive treatment with concurrent chemoradiotherapy followed by maintenance temozolomide is around 8–14% [9]. Progression free survival for low grade glioma with standard treatment (LGG, WHO Grade I or II) is 8–35 months depending on patient age, tumor size, functional scores, and symptoms [8]. Treatment for LGG includes surgical resection followed by either radiation and chemotherapy or chemotherapy alone, but average survival is still approximately seven (7) years from diagnosis [8]. Although LGG have a less aggressive course than do HGG, both tumor, its treatment, and the ultimate poor prognosis contribute to increased patient burden with disabling morbidity including decline in neurocognitive functions, seizures and compromised quality of life [8]. Significant gaps exist in how best to manage LGG during active surveillance, a period when patients report significant anxiety, eagerness to reduce disease progression, treatment-related symptoms, and demonstrate significant interest in interventions that can extend their years and quality of survival. Patients with LGG may thus represent an ideal cohort for the evaluation of interventions for secondary chemoprevention and symptom management.
Although the current WHO grading system (2016) [4] demonstrates promise towards identifying novel treatment modalities and better prediction of prognosis over time, to date, existing targeted and mono therapy approaches have failed to elicit a robust impact on disease progression and patient survival. It is possible that tumor heterogeneity as well as specifically targeted agents fail because redundant molecular pathways in the tumor make it refractory to such approaches. Additionally, the underlying metabolic pathology, which is significantly altered during neoplastic transformation and tumor progression, is unaccounted for. Although LGG have a less aggressive course than do high-grade gliomas, both tumor and its treatment contribute to increased patient burden with disabling morbidity including decline in neurocognitive functions, seizures and ultimately to progression to HGG. There is thus an urgent need and opportunity for the development of novel, adjunct, secondary chemopreventative strategies targeting patients with LGG to slow or halt progression of LGG to HGG.
The recognition and broad applicability of the concepts described by Hanahan and Weinberg [10] – identifying the hallmarks of cancer – has transformed the landscape of cancer prevention and treatment. The hallmarks of cancer constitute an organizing principle for rationalizing the complexities and multi-step development of neoplastic disease. They include sustaining proliferative signaling, evading growth suppressors, resisting cell death, enabling replicative immortality, inducing angiogenesis, and activating invasion and metastasis. Additional hallmarks include genome instability, which underlies these hallmarks and generates the genetic diversity that is permissive for their acquisition, and inflammation, which fosters multiple hallmark functions. Based on the conceptual progress made in the last decade, two emerging hallmarks of potential generality were added to this list: reprogramming energy metabolism and evading immune destruction [11]. Abnormal energy metabolism is a consistent feature of most tumor cells across all tissue types [12]. The characteristic metabolic phenotype of tumor cells as compared to non-transformed cells has been well documented. In the 1930s, Otto Warburg observed that tumors exhibit a unique metabolic phenotype characterized by high rates of aerobic glycolysis, or fermentation in the presence of oxygen [13]. Following glycolysis, pyruvate is primarily fermented to lactate despite availability of oxygen. This feature, known as the Warburg effect, is characterized by tumorhypoxia, genetic mutations, and mitochondrial abnormalities within proliferating cancer cells [14]. The rapid and unbridled proliferation characteristic of tumor growth is an energy and resource-consuming process, and thus predictably, metabolism is significantly altered during neoplastic transformation and tumor progression [15]. The Warburg effect confers multiple growth promoting effects onto the tumor, including provision of ATP in the face of hypoxia, acidification of the tumor microenvironment, regeneration of endogenous antioxidants, and provision of carbon sources for biomass production, among others [15]. These alterations cause most cancers to induce unregulated glucose fermentation pathways for energy and to fuel growth, a factor that underlies the use of FDG-PET scans as an important diagnostic tool for oncologists.
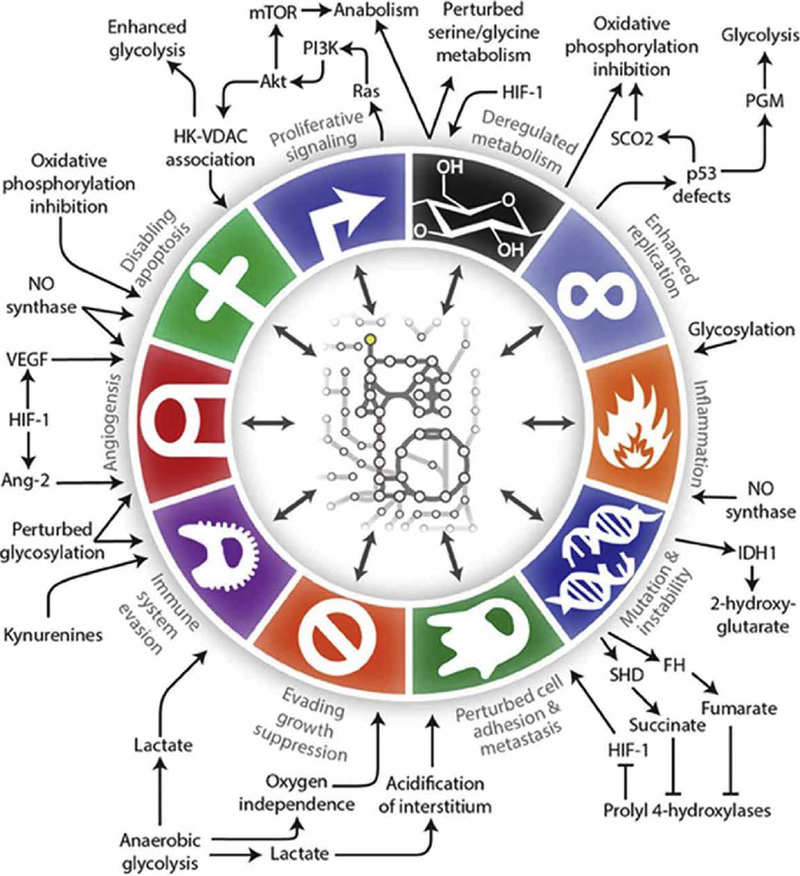
In the years since the discovery of the Warburg effect, researchers have elucidated other common metabolic alterations co-opted by tumors to support optimal growth conditions, including major changes in the regulation and activity of lipid and amino acid metabolism [16]. Glutamine, the most abundant amino acid in the blood, serves as a critical fermentable energy substrate for tumors, as well as an anaplerotic source for the TCA cycle which feeds de novo lipid and protein synthesis pathways [16–19]. Serine and one-carbon metabolism is heightened to support purine synthesis for nucleotide production, and is also important for methylation and subsequent epigenetic control of gene expression [20–22]. Acetyl co-A precursors such as glucose, glutamine, and acetate supply a readily available pool of metabolites for fatty acid synthesis, a critical pathway for many cancer types [23–25]. Acetyl co-A is also an important acetyl group donor for epigenetic acetylation which regulates oncogene and tumor suppressor gene expression. These are but a few of the known common metabolic alterations of tumors, which have been expertly reviewed in recent years [16,26,27]. Importantly [28,29], many classic oncogene mutations confer a metabolic phenotype which supports survival and proliferation [15], and thus it is impossible to always separate genetic expression and metabolic signaling in tumors; indeed, these pathways are most often one in the same. In fact, each of Hanahan and Weinberg’s hallmarks of cancer can be linked to specific metabolic alterations (Fig. 1). Acknowledging the importance of metabolic regulation of growth and survival pathways has led to multiple new lines of inquiry for the discovery of novel anti-neoplastic agents [27,30–33]. For these reasons, cancer metabolism is a rapidly growing area of research.
Fig. 1.
Metabolic regulation of the hallmarks of cancer. (http://journal.frontiersin.org/article/10.3389/fnmol.2016.00122/full#B62) [69].
With several molecular and metabolic pathways implicated in the carcinogenesis of CNS tumors, including glioma [34–36], we postulate that a systemic, broad spectrum approach [37] to produce robust targeting of relevant and multiple molecular and metabolic regulation of growth and survival pathways, critical to the modulation of hallmarks of carcinogenesis, without clinically limiting toxicity, may provide a more sustained impact on clinical outcomes compared to the modalities of treatment evaluated to date.
The objective of this review is to examine the emerging hallmark of reprogramming energy metabolism of the tumor cells and the tumor microenvironment during carcinogenesis, and to provide a rationale for exploiting this hallmark and its biological capabilities as a target for secondary chemoprevention and treatment of glioma. This review will primarily focus on interventions to induce ketosis to target the glycolytic phenotype of many cancers, with specific application to secondary chemoprevention of LGG - to halt the progression of lower grade tumors to more aggressive subtypes, as evidenced by reduction in validated intermediate endpoints of disease progression including clinical symptoms.
2. Ketogenic diets and/or exogenous supplementation to induce ketosis
Ketones are energy metabolites that are naturally produced in the body under certain physiological conditions, including starvation, fasting, prolonged exercise, or during the consumption of a ketogenic diet (KD). The KD is a high fat, moderate protein, very low carbohydrate diet, classically consisting of a 4:1 macronutrient ratio of fats: protein and carbohydrate by weight. It is most well-characterized clinically for its use in treating pediatric refractory epilepsy [38], but may also have therapeutic efficacy for disorders such as obesity, diabetes, hypertension, Amyotrophic Lateral Sclerosis (ALS), Alzheimer’s, and Parkinson’s disease [39–50]. Like cancer, many of these diseases exhibit underlying metabolic pathology [51,52] and chronic inflammation which has been linked to a state of hyperglycemia [53–55]. The KD mimics a metabolic state of starvation, inducing a physiologic shift towards fat metabolism. The carbohydrate restriction of the KD keeps glucose and insulin levels low and stable, activating gluconeogenesis and forcing acetyl CoA generated by fatty acid β-oxidation to shunt towards ketogenesis in the liver. Acetoacetate (AcAc) and β-hydroxybutyrate (βHB) are the two primary ketones found in blood. The third ketone, acetone (ACE), is produced by the spontaneous decarboxylation of AcAc and is rapidly exhaled. As a result of these conditions, ketones replace glucose as a primary energy substrate for extra-hepatic tissues, including the brain. The term ketones used in the context of physiology and metabolism refers to these three molecules. This is despite the fact that the structure of βHB is not classified as a ketone in the chemistry nomenclature sense of the word, as it does not contain a carbonyl group attached to two carbon atoms. They are also referred to as ketone bodies, although this phrase is not as commonly used as it was in the past.
It is possible to elevate blood ketones artificially with exogenous ketones or ketogenic agents (KA). KAs are either enzymatically cleaved or metabolized to produce ketones. They can therefore raise blood ketone levels without the need for severe dietary restrictions or modifications which may not be feasible in certain patient populations. For example, R, S-1, 3-butanediol acetoacetate diester (BD-AcAc2) is a non-ionized, water-soluble precursor to ketones. Gastric esterase rapidly catalyzes the release of AcAc, liberating R, S-1, 3-butanediol which is metabolized by the liver to produce βHB. Thus, this KA causes a rapid elevation in blood AcAc followed by a more sustained production of βHB [56,57]. In rats, single dose administration of BD-AcAc2 via intragastric gavage raised ketone levels to therapeutic levels (> 3 mM βHB and > 3mM AcAc), and mimicked the anti-convulsant effects of the KD by increasing latency to oxygen toxicity-induced seizures [57]. It is possible that an exogenous ketone-supplemented KD would be easier to maintain and offer a more efficacious therapy than a standard KD or ketone supplementation alone. Indeed, exogenous BD-AcAc2 supplementation delivered in addition to standard high carbohydrate rodent chow was shown to be as effective as the KD in a glioma-derived model of metastatic cancer [58]. These effects were enhanced by using the BD-AcAc2 in addition to the KD [59].
3. Role of ketosis in carcinogenesis and tumor progression
Due to its propensity to suppress glucose and insulin signaling, ketosis is being explored as a potential anti-cancer therapy [60–62]. However, it is becoming increasingly clear that ketosis, unlike most cancer therapies that target a single vulnerability in the cancer cell, induces widespread metabolic, genetic, and immune changes [62–64]. Although glucose deprivation was the original intent of KD as a potential cancer treatment [65], further investigation has suggested multiple other mechanisms by which ketosis may elicit anti-cancer effects [58,66–70]. Many of these are now thought to be mechanistically attributable to the ketone bodies themselves, which in addition to serving as energy substrate, also have important signaling properties [71]. Indeed, ketones appear to demonstrate intrinsic anti-cancer properties in some cancer subtypes as they have been shown to inhibit cancer in vitro and in vivo [58,67,72,73]. Perhaps the earliest report of direct anti-cancer effects of ketones, by Magee et al. in 1979, demonstrated that βHB exerted a dose-dependent inhibition of proliferation in lymphoma, cervical cancer, and melanoma cells and a significant reduction of metastatic spread in a murine melanoma model [67]. Similarly, AcAc was shown to produce a dose-dependent inhibition in ATP production and proliferation in four colon and three breast cancer cell lines, while eliciting no similar effect on three control fibroblast cell lines [66]. Another report demonstrated that both AcAc and βHB inhibited viability and induced apoptosis in neuroblastoma cells, but had no effect on control fibroblasts [73]. Most recently, we demonstrated that physiologic concentrations of βHB reduced viability and proliferation in glioma cells despite the presence of high glucose media [58]. The extent to which these effects in vitro contribute to postulated anti-cancer effects of ketosis in vivo is unknown, although a beneficial effect is suggested in most pre-clinical models tested [74]. It is becoming increasingly clear that benefits and possible risks may be cancer type-specific, as recently a small number of studies have reported that ketones supplied without suppression of dietary carbohydrate, may serve as a substrate to fuel tumor growth, or in the presence of the BRAFV600E mutation, may enhance signaling cascades that promote tumor progression [75,76]. Thus, it will be critical to evaluate this potential therapy in multiple cancer types.
We discuss below evidence for a role of ketosis in preventing carcinogenesis or slowing tumor progression in sensitive tumors through the following mechanisms: 1) reduction of glucose and insulin; 2) modulation of oxidative stress; 3) reduction of inflammation; 4) enhancement of anti-tumor immunity; 5) alteration of gene expression; and 6) sensitization of tumors to standard of care and adjuvant therapies.
3.1. Reduction of glucose and insulin
Due to the Warburg effect, glucose from dietary carbohydrates acts as a primary metabolic fuel for many tumors. This observation prompted initial research into the KD as a cancer therapy, with carbohydrate restriction-induced glucose deprivation thought to be the major mechanism by which the KD might slow tumor progression. It is well established that hyperglycemia increases tumor growth rate in animals and in humans [77–79]. Indeed, Seyfried and colleagues showed that blood glucose is directly correlated to glioma growth in vivo [78]. Furthermore, hyperglycemic episodes following resection of malignant astrocytoma is independently associated with decreased survival [80–82]. Diabetes has been linked to decreased risk of glioma onset [83] whereas type 2 diabetes mellitus [84] and obesity [84,85] are independent risk factors for poor outcome in patients with HGG. Glucose uptake is a rate-limiting step of glucose metabolism and is mediated by the glucose transporter (GLUT) proteins. GLUT expression is consistently elevated in cancer cells of varying tissue origins [6]. Multiple major oncogenes are known to directly upregulate GLUT transporters and glycolytic proteins, including c-Myc, K-Ras, and BRAF [87]. Of the 14 known GLUT isoforms, GLUT 1 and GLUT 3 overexpression is most often associated with malignant transformation and progression, including in glioma, and both have been correlated to poor prognosis clinically [86,88]. The low Km of these proteins allow tumors to import glucose from the blood even at relatively low plasma concentrations [89]. Excess glucose availability provides the glycolytic substrates to fuel the Warburg effect and biosynthetic processes [15]. It also provides an energy substrate to support cellular functions despite tumor hypoxia or reduced mitochondrial function which is often associated with cancers [35,90,91]. Enhanced glycolytic flux also increases activity of the pentose phosphate pathway (PPP), a divergent metabolic pathway from glycolysis which provides precursors to support the potent antioxidant defense system that allows tumors to survive and thrive in a state of heightened oxidative stress [92]. Therefore, the KD is thought to work in large part by decreasing glucose availability to the tumor. As described, the KD has been shown to decrease glucose uptake by tumors in humans [65].
Hyperglycemia stimulates insulin secretion from the pancreas. High levels of circulating insulin, or hyperinsulinemia, is also associated with an increased risk of colorectal, pancreatic, and breast cancer, among others [93]. High serum concentrations of IGF-I have been associated with onset of LGG [94]. Insulin binds to the insulin receptor (IR) to mediate the cellular effects of glucose metabolism, but it also exerts a potent growth factor response. IR expression and signaling is commonly over-activated in cancers and is considered to play an important role in promoting tumor growth and progression [95]. IR activation stimulates Ras and the MAPK cascade which mediate the mitogenic effects of insulin, promoting cell proliferation. IR also works through the PI3 K pathway to promote cell survival through Akt and mTOR. Furthermore, Akt promotes expression of NFκB which activates pro-inflammatory and anti-apoptotic programs. Growth promotion by insulin signaling crosstalks with the β-catenin/Wnt pathway, and therefore may play a role in dedifferentiation and malignant progression [96]. Insulin also induces vascular endothelial growth factor (VEGF) expression, a potent activator of angiogenesis [97,98]. Angiogenesis is required for tumor growth and plays a critical role in cancer progression and metastasis. Emerging evidence suggests that attempts to target a single pathway within the insulin signaling network, such as with the use of PI3K inhibitors, are ineffective due to reactive hyperglycemia. It has been proposed that simultaneous lowering of systemic glucose levels, such as with the KD and pharmacologic inhibition of insulin signaling may offer a better therapeutic response.
The KD decreases pancreatic insulin production by restricting carbohydrate intake. It has also been shown to enhance insulin sensitivity in healthy tissues, which leads to decreased circulating insulin in the blood [99]. In fact, in clinical studies, a switch from a standard diet to a low carbohydrate diet reduced total insulin levels by 50% in healthy subjects [100] and 41% in type-1 diabetics [101], while improving insulin sensitivity up to 75% in type-2 diabetics [102]. The KD has been reported to reduce vascularization in a xenograft model of gastric cancer, possibly through its inhibition of insulin signaling [103]. In a mouse model of prostate cancer, a reduction in tumor growth rate and prolonged survival was associated with a decrease in serum insulin in mice fed the KD [104]. In a small human trial, patients with end-stage cancer receiving a KD for 28 days exhibited a significant drop in blood insulin [105]. This decrease in insulin was inversely correlated to the degree of relative ketosis, which was positively correlated to therapeutic response [105]. Therefore, the KD may work in part by inhibiting insulin signaling in tumors.
Interestingly, exogenous ketone supplements may also suppress glucose and insulin systemically, via mechanisms currently unknown. Rats fed a standard diet supplemented with a ketone monoester for 14 days exhibited a reduction in both glucose and insulin compared to control animals [106]. In this study, glucose was reduced from 5 mM to 2.8 mM, and insulin was reduced from 0.54 ng/mL to 0.26 ng/mL. In a separate study, the same research group also reported that a ketone ester-supplemented standard diet increased the Quantitative Insulin Sensitivity Check Index (QUICKI), a marker of insulin sensitivity, by 73% – even when calories were controlled [106]. In this study, fasting insulin levels were reduced by 85% in ketone ester-treated animals. Our group has also observed a similar reduction in blood glucose following oral administration of various exogenous ketogenic agents [107]. In a mouse model of metastatic cancer, animals fed standard rodent chow supplemented with 20% BD-AcAc2 by weight exhibited a 30% reduction in blood glucose [58]. In healthy rats, we also observed a reduction in blood glucose following an oral bolus of exogenous ketone supplementation. This hypoglycemic effect mirrored a rise in blood ketones and lasted between 30 min to 12 h, depending on the KA and dose used [107]. Thus, it appears that exogenous administration of ketones can suppress glucose and insulin, perhaps working through an enhancement of systemic insulin sensitivity.
3.2. Modulation of oxidative stress
It is well established that basal elevation of reactive oxygen species (ROS) production and oxidative stress is a consistent phenotype in cancers across tissue types [108]. This is likely attributable in part to mitochondrial damage which incites increased free radical production [109]. Furthermore, chronic inflammation from sustained hyperglycemia, infection, or exposure to chemicals like tobacco, is also a major source of ROS production in tumors [108]. Elevated ROS confer a growth advantage to the tumor while playing a significant role in tumorigenesis and progression [110]. However, this elevation in oxidative stress is kept at sub-lethal levels by an up-regulation of endogenous antioxidant systems [111,112], allowing cancers to survive and thrive in a redox state which would be toxic to healthy cells. There still exists, however, a threshold point above which ROS will irreversibly damage and induce apoptosis in cancer cells [113]. Cancer-promoting effects of ROS have led to the proposal and testing of anti-oxidant therapies for cancer. At the same time, recognition that oxidative stress fuels cancer susceptibility has simultaneously spurred development of pro-oxidant therapies with the intention of pushing cells past their oxidative breaking point to induce cell death [114]. All current non-surgical standard therapies, including radiation and chemotherapy, work in part by enhancing ROS production [114]. This paradoxical phenomenon has led to the common understanding that ROS is truly a double-edged sword for cancer, which can be exploited to either promote or inhibit tumor progression [115].
Ketosis elicits an intriguing effect on redox balance in tumors. Evidence suggests the KD lowers basal oxidative stress levels within tumors, but enhances oxidative stress-induced damaged in response to chemotherapy and radiation [116,117]. It has been clearly established that in healthy tissues, ketosis protects against oxidative stress by simultaneously decreasing ROS production and enhancing endogenous antioxidant capacity [118,119]. Veech and colleagues studied the effects of ketone metabolism by investigating energy efficiency and mitochondrial respiration after administering a glucose-containing perfusate supplemented with 5 mM βHB in a working rat heart [120]. Their studies showed that ketone metabolism increases the oxidation of co-enzyme Q in the electron transport chain, thus reducing production of the superoxide anion (O2-%), an important precursor for the generation of many ROS [120]. Ketone metabolism also caused a reduction of the mitochondrial NAD and cytoplasmic NADP couples which are necessary for regenerating reduced glutathione (GSH). Verdin et al. also reported that βHB functions as an endogenous histone deacetylase inhibitor (HDACI) to increase transcriptional activation of oxidative stress resistance factors which upregulate expression of antioxidants such as mitochondrial superoxide dismutase (MnSOD) and catalase (CAT) [68]. In this report, pre-treatment with exogenous βHB prior to an oxidative challenge reduced protein carbonylation by 54% and completely suppressed lipid peroxidation, supporting a potent anti-oxidant potential of ketosis. Similarly, the KD has been shown to reduce ROS production and enhance endogenous antioxidant expression in glioma in vivo [117]. Thus, it is possible that the KD works in part by reducing basal levels of oxidative stress in tumors.
A striking differential effect occurs when pro-oxidant therapies are applied to tumors in the presence of ketosis. Fath et al. examined the effects of the KD, radiation, and carboplatin chemotherapy on oxidative stress in two lung cancer xenograft models [116]. They showed that the animals fed a KD in combination with radiation therapy exhibited increased lipid peroxidation and oxidative damage. The explanation for these results is unclear, although they may reflect reduced metabolic substrates necessary for a significant antioxidant response. As mentioned, a major consequence of the glycolytic phenotype of cancer is increased flux through the PPP, which generates NADPH that is used for regeneration of reduced glutathione. This is a major source of antioxidant protection for tumors in response to ROS-inducing radiation and chemotherapy. With reduced flux through glycolysis and subsequently PPP, it is possible the tumor is more vulnerable to rapid onset of oxidative stress. Indeed, preliminary studies have demonstrated that the KD enhances OxS-mediated radiation-induced DNA damage in glioma, but reduces it in surrounding healthy tissue [58]. However, Scheck and colleagues recently presented findings that βHB itself potentiates radiation therapy and increases radiosensitivity of glioma cells in vitro, regardless of glucose concentration [121]. A reduction in c-Myc expression, an oncogene known to play a vital role in DNA repair, was also observed thus suggesting ketone-enhancement of radiotherapy may be mediated via a signaling mechanism [116].
Clearly the role of OxS in cancer in response to ketosis is complex and not well-understood, but preliminary work suggests this novel therapy induces modulation in these pathways that confer anti-cancer effects.
3.3. Reduction of inflammation
Systemic inflammation is a negative prognostic factor for cancer patients independent of tumor type and stage [122–124]. It can be initiated through the acute-phase response by the liver in response to tumor, while the tumor can also secrete inflammatory cytokines such as TNF-α, IL-6, and IL-1β, among others. Increased inflammation reduces patient appetite, initiates catabolism and inhibits synthesis signaling in the skeletal muscle, and contributes to cancer-induced wasting (cachexia) directly and indirectly, contributing to reduced quality of life [122–124]. Ketosis may inhibit progression and induce cell death in cancers in part by inhibiting inflammation. Studies have shown that the KD reduces circulating inflammatory markers in humans [125], and preliminary studies with the ketone ester suggest similar findings [126].
The glioma microenvironment contains a large number of infiltrating immune cells that secrete pro-inflammatory cytokines, creating an inflamed state which promotes tumor progression and invasive capacity [127]. A major player in the inflammatory process of brain tumors is the NLRP3 inflammasome – a component of the innate immune system responsible for the activation of inflammatory processes in response to pathogens or injury. NLRP3 inflammasome activation contributes to tumor growth and radiotherapy resistance in mouse models of glioma, while NLRP3 inhibition slows tumor growth and prolongs survival in these models [128]. We have demonstrated that chronic feeding of BD-AcAc2 reduces circulating inflammatory markers in healthy rats [126]. In collaboration with Youm and colleagues, we reported that the ketone β-hydroxybutyrate directly inhibits assembly of the NLRP3 inflammasome, reducing NLRP3-mediated inflammatory cytokine production and blocking NLRP3-mediated inflammatory disease [70]. Furthermore, it has been demonstrated that radiation induces NLRP3 inflammasome activation which contributes to radio-resistance in glioma, and that inhibition of NLRP3 slows tumor growth and restores radio-sensitivity [128]. It is likely that ketone inhibition of NLRP3-mediated inflammation in glioma contributes to its efficacy and the reported synergy between KD and radiation [60]. Inflammation also contributes to glioma pathology by inducing peritumoral edema which is a significant contributor to morbidity and mortality in patients. The KD was demonstrated to reduce peritumoral edema in the GL-261 mouse glioma model by approximately 50%, even when comparing tumors of similar size [62]. This effect was accompanied by an alteration in expression of multiple genes associated with inflammation and vascular permeability. Specifically, activation of NFκB, a transcription factor activated in response to inflammation which upregulates tumor-promoting cytokines and survival genes, was reduced by KD therapy. Similarly, COX-2, a potent promotor of inflammation, was reduced. Aquaporins and MMPs related to leaky vasculature and brain tumor edema were also decreased, suggesting a preservation of the blood brain barrier with KD treatment.
3.4. Enhancement of anti-tumor immunity
It has recently been demonstrated that ketosis induced by the KD enhances anti-tumor immunity [63]. The KD increased tumor-reactive innate and adaptive immune responses in the GL-261 model of glioma. Both an enhancement in cytotoxic T cell anti-tumor activity and a reduction in immune suppression were observed. The KD also increased the ability of CD8+ T cells to produce cytokines upon tumor stimulation, as well as the cytotoxic activity of those immune cells. Tumor infiltrating lymphocytes (TILs) from KD-fed mice had significantly reduced levels of the inhibitory receptors PD-1 and CTLA-4, and tumors from these mice expressed lower levels of inhibitory ligands CD86 and PD-L1. Furthermore, tumors from KD-fed mice had increased tumor-reactive CD4+ T cell infiltration while immunosuppressive T regulatory (Treg) presence did not change. Depletion of CD8+ T cells attenuated much of the therapeutic effects of the KD. Collectively, these findings strongly suggest that ketosis exerts its effects, at least in part, by enhancing anti-tumor immunity.
3.5. Alteration of gene expression
Studies have demonstrated that the gene expression profile of tumors grown in animals treated with the KD differs markedly from tumors grown in animals fed a standard diet [62,64]. In a study in the GL-261 glioma model [129], the KD was shown to reduce the expression of genes involved in the hypoxic response, including carbonic ahdydrase IX and HIF-1α. Phosphorylated NF-kB was also decreased, suggesting a reduction of NF-kB activation. Genes involved in angiogenesis and vascular remodeling were also reduced, including VEGFR2, VEGFB, PLAU, TIMP-1, TEK, and ITGB1. Several genes related to invasive potential were also altered, including reductions in MMP-2 and vimention. Since peritumoral endema was reduced by the KD, expression of genes related to this phenomenon were also measured. Indeed, the tight-junction protein ZO-1 was increased, and the water channel AQPN-4 was reduced. Studies have further suggested that the KD normalizes gene expression such that it more closely resembles that of healthy tissue [4,130]. Gene expression profiling was performed on tumor and contralateral healthy brain from mice with GL-261 glioma treated with and without the KD [64]. Approximately half of the > 1000 gene changes were diet-induced. A two-way ANOVA for interaction revealed that the KD induced tumor gene expression to be more normal, or closer to that of the healthy brain tissue. Gene changes of interest included those involved in oxidative stress and signal transduction pathways.
Cancers exhibit widespread differences in epigenetic patterns compared to their normal tissue counterparts, allowing them to increase expression of oncogenes and inhibit the expression of tumor suppressor genes [131]. Histone deacetylases (HDACs) catalyze the removal of acetyl groups from DNA histones and are known to play critical roles in mediating gene expression changes in tumors. As such, histone deacetylase inhibitors (HDACI) are being investigated for their use as antineoplastic agents. Preclinical evidence suggests that HDACIs may be efficacious in a number of different malignancies, and there are currently four FDA-approved HDACIs for cancer [132–135]. HDACIs elicit a plethora of anti-cancer effects in vitro including inhibition of anti-apoptotic proteins, activation of pro-apoptotic proteins, induction of ROS generation and DNA damage, and inhibition of DNA repair [136]. As mentioned, Verdin and colleagues demonstrated that the βHB acts as an endogenous HDACI both in vitro and in vivo at milimolar levels easily achievable with the KD or KA [68]. Thus, it is possible that the effects of ketosis on gene expression in tumors, and its potential anti-cancer efficacy, is mediated at least in part by the HDACI actions of βHB [68].
3.6. Sensitization of tumors to standard of care and adjuvant therapies
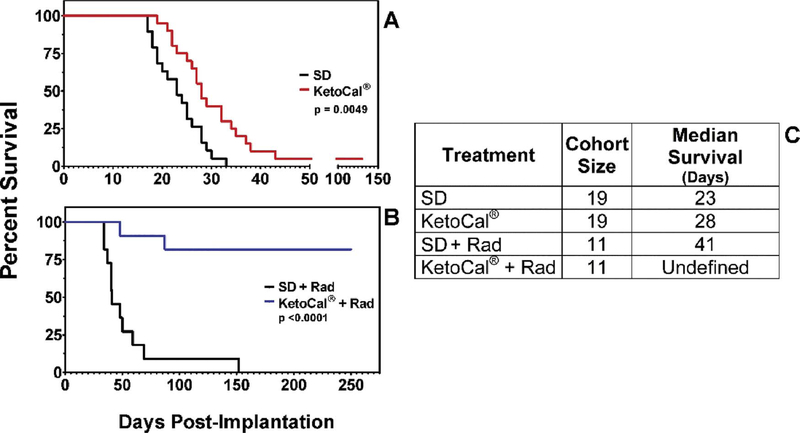
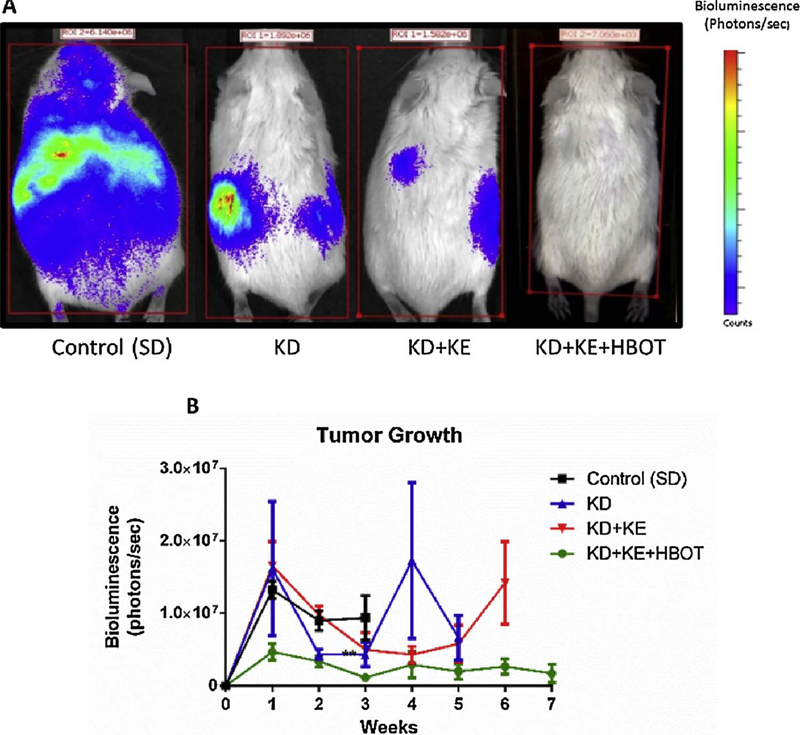
Importantly, ketosis appears to be particularly effective when used as an adjuvant to standard of care (SOC) or other metabolically-targeted treatments, increasing tumor sensitivity to such therapies. Scheck and colleagues showed a significant synergistic response to the combination of KD and radiation therapy in the GL-261 mouse model of malignant glioma (Fig. 3) [60]. The KD and radiotherapy both independently increased survival time; however, when administered concomitantly, 82% of mice exhibited complete and permanent tumor remission, even after returning to a standard diet. These investigators have also presented data showing a synergistic effect of combining KD with temozolomide chemotherapy in this model [137]. Also, as previously described, Fath et al. reported a synergistic effect between the KD and radiochemotherapy in a lung cancer model [116]. Similarly, fasting, a condition which induces physiologic ketosis, increases therapeutic response to chemotherapy in melanoma, glioma, and breast cancer cells, as well as in a mouse model of neuroblastoma [138]. Furthermore, fasting and the KD have been demonstrated to reduce SOC toxicity and improve quality of life indices during treatment [138,139]. This potential synergy with SOC is critically important as ketogenic therapies would only be investigated clinically as an adjuvant to SOC. Other adjuvant therapies under investigation may also synergize with ketosis, including hyperbaric oxygen therapy (Fig. 4) [12,59] and metabolically-targeted drugs [140,141].
Fig. 3.
Ketogenic diet enhances efficacy of radiotherapy in mouse glioma model. Radiation therapy and ketogenic diet prolonged survival as monotherapies, but complete and permanent remission was observed in 82% of mice when the two therapies were delivered concomitantly. This study suggests that ketosis may provide an effective adjuvant to standard of care therapy in glioma. Reprinted with permission from Abdelwahab, et al. PLOS One, 2012 [60].
Fig. 4.
Ketosis is synergistic with hyperbaric oxygen therapy in a mouse model of metastatic cancer. In a 2015 report by Poff et al. the ketogenic diet (KE), BD-AcAC2 supplementation (KE), and hyperbaric oxygen (HBOT) elicited a potent synergistic effect on suppressing tumor growth and metastatic spread in a glioblastoma-derived model of metastatic cancer, supporting the idea that ketosis may serve as a useful adjuvant to other non-toxic, metabolic-targeted adjuvant therapies under investigation. Fig. 1 from Poff et al., 2015; http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0127407. Reprinted with permission from Poff, et al. PLOS One, 2015 [59].
In summary, in addition to multiple genetic, epigenetic, and growth signaling alterations, cancer cells exhibit reprogramming of several metabolic pathways. Importantly, many classic oncogene mutations confer a metabolic phenotype which supports survival and proliferation. Indeed, each of Hanahan and Weinberg’s hallmarks of cancer can be linked to specific metabolic alterations. Thus, it may be logical to utilize a systemic, broad spectrum approach [37] to robusty target miltiple relevant molecular and metabolically regulation of growth and survival pathways that modulate the hallmarks of carcinogenesis, without clinically limiting toxicity. However, to date, there continues to be a paucity of research that exploits these mechanisms comprehensively to improve prognosis and outcomes relevant to tumor progression and reduction of related symptom burden.
4. Rationale of ketogenic diets targeting metabolic dysregulation observed in glioma
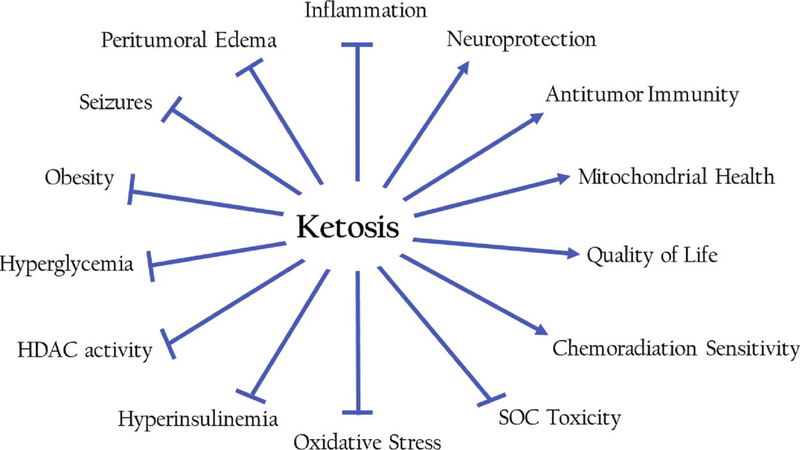
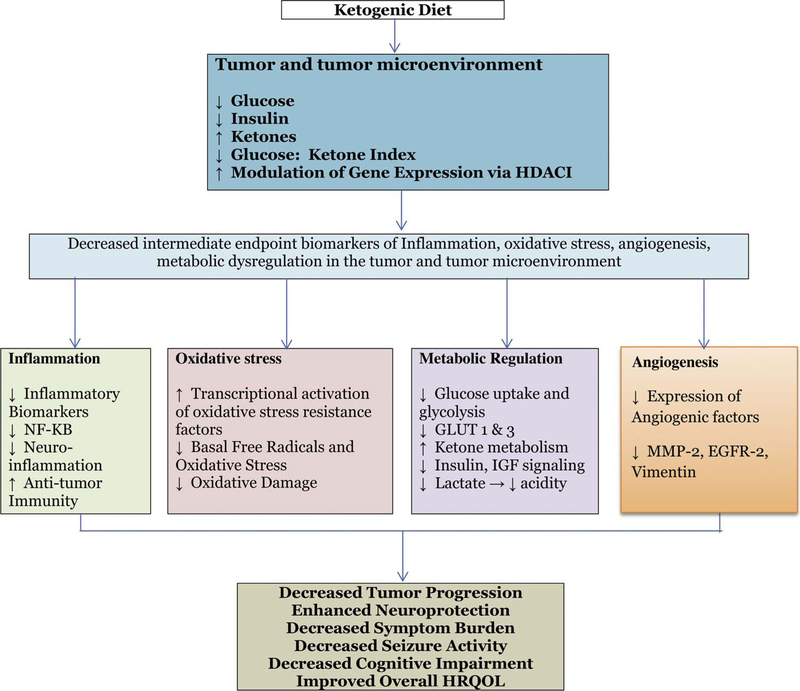
Tumors including CNS tumors often express a strong glycolytic or Warburg phenotype, both prior to and in response to chemoradiation intervention [105]. Prolonged exposure of ketone bodies (KBs) to the blood-brain barrier (BBB) endothelial cells upregulates expression of monocarboxylate transporters that enhance uptake of ketones by the brain. However, brain tumor cells appear to have a high obligatory need for glucose but may be metabolically inflexible, and thus have limited capacity to oxidize KBs as an energy source. Thus ketosis, a metabolic state characterized by decreased blood glucose and elevated blood ketones, may potentially deprive the tumor of key metabolic substrates and have the potential to control further growth and associated symptoms [142,143]. Indeed, several cancers have been found to lack expression of one or more ketone utilization enzymes [27,28,106,107], a feature also observed in malignant glioma [106]. Interestingly, it has been demonstrated that some glioma cells that do express ketolytic enzymes are unable to metabolize βHB to compensate for glucose restriction like healthy neurons [142,143]. In the section below, we review the evidence from in vitro, preclinical and early phase trials by our team and others have shown that ketogenic diets KD target metabolic dysregulation to elicit a number of distinct yet simultaneous anti-cancer effects (Fig. 2) targeting the Warburg effect, significantly reducing glucose and insulin, increasing ketone bodies, reducing oxidative stress, reducing inflammation, attenuating the acidic tumor microenvironment, and downregulating proteins associated with angiogenesis, ultimately resulting in reduced tumor progression and potentially symptom burden.
Fig. 2.
Summary of relevant effects of ketosis.
4.1. In vitro studies in gliomas
In a study evaluating ketolytic and glycolytic enzyme expression in 22 malignant glioma biopsies, the rate limiting mitochondrial ketolytic enzymes were low or very low in 14 of 17 GBMs and in 1 of 5 anaplastic gliomas, while at least one glycolytic enzyme was overexpressed in 13 of 17 GBMs and all 5 anaplastic gliomas [144]. Another study involving 5 different glioma cell lines concluded that glioma cells are unable to metabolize βHB to compensate for glucose restriction like healthy brain cells, even when ketolytic enzymes were expressed [142,143]. Normal ketone metabolism has been reported in some glioma cell lines in vitro [66,145,146], but interestingly, the effects of the KD in vivo revealed no effect on tumor growth [147]. Furthermore and of importance, mitochondrial dysfunction is a hallmark of gliomas [35,148–154]. Ketones bypass glycolysis and are oxidized completely within the mitochondria, an observation further supporting the hypothesis that ketones may beless efficient energy substrates within glioma compared to the healthy brain. Thus, even in tumors where ketolytic enzymes are expressed or ketone oxidation may be occurring, if there is dysfunction at the level of the mitochondria such that oxidative phosphorylation is inefficient, fuels that are metabolized solely through this pathway will likely provide an inefficient energy source. This may explain why the growth of some tumors that demonstrate carbon-labeled utilization of ketones are still not promoted by the KD [66]. It is also quite plausible that in tumors where ketones are oxidized efficiently for energy, the multiple signaling effects we have outlined in this report are still able to produce an overall neutral or anti-tumor effect. Still, we posit that tumors which consistently express abnormalities in mitochondrial structure and function would not be as efficient utilizers of ketones compared to tissues with normal mitochondria. This may also explain studies such as the previously described Maurer et al., wherein ketolytic enzyme expression was normal, but the tumor cells were still unable to utilize ketones to compensate during glucose deprivation [143]. The in vitro evidence has thus demonstrated that KD creates a metabolic state that limits glucose and insulin, and provides KBs as an alternative energy source, which is a less useful fuel for the tumor, including glioma, that may lead to suppression of cancer progression.
4.2. Preclinical evidence demonstrating the effectiveness of KD in treating gliomas
A number of preclinical studies have demonstrated therapeutic effects of the KD for glioma, many of which have been previously described in this review. Seyfried and colleagues have published several reports on the calorie-restricted ketogenic diet (R-KD) as potential therapy for glioma. The R-KD decreased tumor weight when administered as a stand-alone therapy in the CT-2A malignant mouse astrocytoma model; however, it elicited potent synergistic anti-cancer effects when administered in conjunction with the glycolytic inhibitor 2-deoxy-D-glucose (2-DG) [140]. In the same model, Seyfried et al. showed that blood glucose levels had a direct and positive correlation with tumor growth and that the R-KD significantly decreased plasma glucose [78]. The R-KD also decreased plasma insulin-like growth factor-1 (IGF-1) levels, a known biomarker for cancer progression and angiogenesis.
Scheck and colleagues reported in a series of studies that the KD fed ad libitum increases median survival time by approximately 20–30% in the GL-261 malignant glioma model [63,117,121]. Poff et al. reported that two KDs tested prolonged survival time by approximately 45% and 55% in a glioma-derived mouse model of metastatic cancer [12,126]. Similarly, Reynolds and colleagues demonstrated that the KD reduced tumor growth and prolonged progression free survival in patient-derived GBM in both sub-cutaneous and orthotropic implantation models [155]. As previously mentioned, the KD prolonged survival time in the GL-261 mouse model of malignant glioma, but synergized with radiation therapy to promote complete and permanent remission in most animals when used concomitantly [60]. These investigators also reported a synergistic effect between KD and temozolomide therapy in the GL-261 model [137]. In summary, KD diets alone and as an adjuvant therapy have shown promise in reducing tumor progression in preclinical models of glioma via targeting several metabolic and molecular intermediate endpoints of carcinogenesis.
4.3. Clinical/Case studies evaluating effectiveness of KD in brain tumors
The earliest report of the KD as a cancer therapy in humans was published by Nebeling et al. in 1995 [65]. This study was designed to determine if the KD could decrease glucose uptake by tumors in two pediatric patients with late stage malignant astrocytomas. FDG-PET imaging showed decreased glucose uptake by an average of 21.8% in the two patients’ tumors. One patient responded remarkably well to dietary therapy, exhibiting clinical improvement in quality of life and motor function. At the time of the report, she had continued the KD therapy for an additional year with no disease progression. The other patient responded well initially, but was lost to follow-up. In 2011, Zuccoli et al. published a case report on a 65 year old woman with multicentric glioblastoma multiforme (GBM) treated with R-KD given concomitantly with standard care [118]. The patient responded well to dietary therapy, and after two months of treatment, her tumors were no longer visible by FDG-PET or MRI imaging. Ten weeks after discontinuing the dietary therapy, however, her tumors returned and she eventually succumbed to the disease. Currently, there are a small number of clinical trials evaluating the effects of the KD on GBM. A small pilot trial in recurrent GBM patients demonstrated that KD was safe and feasible, but may not elicit significant benefit as a monotherapy in these patients [156]. The trial was also complicated by difficulties with compliance and poor tolerability to the diet. Patients who were able to achieve stable ketosis exhibited a trend of improved progression free survival, but the effect was not statistically significant in this small group. It is possible that ketogenic therapy imparts a more significant impact in patients with earlier stage disease; however, to the authors’ knowledge, no trials have yet assessed this therapy in patients with LGG.
Thus, evidence from early observational, pilot and case reports provide a strong mechanistic rationale for KD in reducing tumor progression of CNS tumors. Based on laboratory studies, it appears that ketosis is a promising therapy that substantially alters metabolic physiology to elicit a number of distinct yet simultaneous anti-cancer efects, including a reduction of glucose and insulin, modulation of oxidative stress, reduction of inflammation, enhancement of anti-tumor immunity, and alteration of gene expression, among others. However, much of this early evidence in clinical trials comes from non-randomized trials and short-term interventions in advanced cancer patients and thus only provide data on feasibility and short-term safety. Given the absence of long-term safety and clear evidence of efficacy, it is clear that randomized clinical studies are warranted to further evaluate the potential therapeutic role, the effectiveness, safety and the feasibility of KDs targeting cancer.
4.4. Other related effects of ketosis
Aside from these direct anti-cancer effects of ketosis, preclinical studies suggest that KD also sensitizes tumors to chemoradiation therapy, eliciting a potent synergistic effect in various animal models, including glioma [60]. Furthermore, ketosis is unique amongst most proposed or known cancer therapies in its ability to protect healthy tissue from damage. The neuroprotective effects of ketosis are well-documented [118,157], and have been reported in varied etiologies of neurological damage, including acute insults such as stroke and traumatic brain injury (TBI), conditions of neurodegeneration such as Alzheimer’s disease and Parkinson’s, and pre-clinical models of chemical-induced injury, among others [118,157]. Indeed, in multiple published reports, ketosis has been shown to prevent neuronal death and enhance cognitive function following insult. While not fully-understood, it is thought that ketosis elicits neuroprotective effects in part by enhancing brain energy reserves and reducing oxidative stress and neuroinflammation [157,158]. In glioma, where standard of care therapies produce substantial and enduring cognitive and quality of life deficits, the potential implications of this are significant. Thus, ketosis induced by the KD and/or KA may provide a powerful, broad spectrum approach to simultaneously target multiple aspects of cancer, while enhancing the effects of current standard of care chemoradiation, and protecting healthy tissue from the adverse side effects of treatment.
4.5. Potential contraindications of ketogenic diets in cancer
Much of the evidence of potential contraindications of KD related to cancer comes from laboratory studies and a few early phase clinical trials. In a preclinical model of the rare genetic disorder Tuberous Sclerosis Complex (TSC), Eker rats (Tsc2+/−) subjected to ad libitum prolonged feeding of a KD (4, 6 and 8 months) demonstrated promotion of growth of renal tumors by recruiting ERK1/2 and mTOR which are associated with the accumulation of oleic acid and the overproduction of growth hormone. Simultaneously, Nrf2, p53 and 8-oxoguanine glycosylase α-dependent antitumor mechanisms were launched by the KD, suggesting that this therapy may be contraindicated for TSC patients [159]. As previously noted, tumors containing the BRAFV600E mutation may also be contraindicated for ketogenic diet therapy [66]. The ketogenic diet promoted the growth of human melanoma cells expressing this mutation in a xenograft mouse model by selectively enhancing BRAF V600E mutant-dependent MEK1 activation. Finally, the Lisanti group has published a set of papers proposing that ketones can promote tumor growth via a reverse Warburg effect and two-compartment metabolism between stromal and tumor cells in pre-clinical breast cancer models [75,160–163]. The research team generated hTERT-immortalized fibroblasts overexpressing rate-limiting ketogenesis enzymes, and co-cultured them with MDA-MB-231 breast cancer cells which were genetically altered to over-express rate-limiting ketolytic enzymes [162]. In this model system, ketone administration promoted tumor growth and metastasis. This proof of concept experiment is interesting but deserves further investigation, especially as Fine et al. demonstrated that ketone administration suppressed ATP production and growth in MDA-MB-231 cells that were not genetically engineered for enhanced ketone metabolism [66]. The Lisanti group has also reported that ketones can induce a gene signature associated with increased “stemness” in MCF7 breast cancer cells [163]. Although a small number of studies suggest the KD may have beneficial effects in breast carcinogenesis [66], the data in this cancer type is sparse and deserves critical attention in light of the Lisanti lab’s reports. To date, there have been no studies demonstrating a tumor-promoting effect of the ketogenic diet on glioma.
Evidence from early clinical trials on potential short-term side effects of KDs include gastrointestinal distress, acidosis, hypoglycemia, dehydration, lethargy, [164] hypomagnesemia, and elevated circulating cholesterol and free fatty acid concentrations [165,166]. Potential long-term side effects include hyperlipidemia, hypercholesterolemia, nephrolithiasis, cardiomyopathy, and bone mineral loss. There are also known contraindications to the KD, which include genetic disorders of fatty acid oxidation, carnitine deficiency disorders, and porphyria. KDs can also be deficient in certain vitamins and minerals, such as calcium, selenium, copper, zinc, and vitamin D. Although these adverse effects have been reported to be minor in short-term interventions and have been reported in patients who are on KD for more than 1 year, it has also been shown that several of these adverse effects can be prevented or corrected with appropriate patient selection, supplementations, well-fomulated KD and adjustments in food choices. For example, studies have demonstrated that a well-formulated KD taking into consideration composition of saturated versus monosaturated fats and protein elicits multiple positive effects on blood lipids and cardiovascular biomarkers, including a reduction in triglycerides, an elevation in HDL:LDL cholesterol, a reduction in body fat mass, and a suppression of circulating inflammatory markers [2,142,143,167–169]. Still, it is important that adverse events and related biomarkers be closely monitored in early phase randomized clinical trials, to inform future evaluation of the efficacy of KD in specific cancers including patient selection, KD formulation and the biomarkers to monitor for safety.
While several studies are evaluating therapeutic use of the KD for varied disease states, a consistent obstacle is dietary compliance. Additionally, it is likely that cancer patients may face unique obstacles to dietary therapy due to treatment modalities, including anorexia, mucositis, cachexia, etc. Patients can have difficulty restricting carbohydrate and increasing fat intake to the degree necessary to induce therapeutic levels of ketosis. In such cases, pharmacological modulators or KA supplementation to KD provides a useful tool for initiating and supplementation to sustain ketosis. Utilizing KAs as an adjuvant to KD reduces the severity of carbohydrate restriction necessary and will almost certainly enhance patient compliance. Furthermore, KAs such as the BD-AcAc2 ketone ester [5[58] are unique amongst known KA in their ability to elevate both βHB and AcAc [57]. Although not evaluated for safety in randomized clinical trials, other ketones have been shown to possess anti-cancer properties in different model systems [58,66,67].
In summary, although several laboratory studies have provided evidence of safety and potential contrainidcations data from randomized clinical trials of safety and efficacy of KD targeting cancer patient populations continue to remain relatively sparse. Well-powered, rigorously designed clinical trials to evaluate safety and effectiveness of KD in interfering in carcinogenesis, while maintaining safety, in carefully selected cancer patient populations are urgently needed.
5. Conclusions
A promising approach to cancer control is to develop interventions to slow or halt progression of early stages of carcinogenesis to invasive disease as indicated by biomarkers of disease progression as well as related symptom burden. Our goal is to utilize a broad-spectrum approach targeting multiple signaling pathways that result in modulation of apoptosis, proliferation, inflammation and related pathologies, relevant to progression in early stage disease to advanced cancers. The metabolic pathways which support rapid growth of tumors represent a promising therapeutic target for cancer. Ketogenic therapies in the form of a KD and/or pharmacological modulators using exogenous KAs represent one potential tool to exploit the metabolic vulnerabilities of tumors. A number of distinct yet interrelated effects of ketosis may be implicated in the potential anti-tumor effects of ketosis (Fig. 2). Ketogenic therapy for CNS tumors, including glioma, extends far beyond the originally proposed mechanism of reducing glucose availability, and may work through multiple and yet distinct mechanisms such as reducing inflammation, altering in oxidative stress, enhancing anti-tumor immunity, altering gene expression, sensitizing tumors to standard of care and adjuvant therapies, among others (Fig. 5). Some of these mechanisms may depend directly on ketone signaling, supporting the notion that an elevation in blood ketones is a significant component of ketogenic therapy for CNS tumors. To date, case reports and pilot projects have reported moderate success evaluating KD in patients with advanced stage disease (HGG). However, it is important to acknowledge that advanced stage disease, including GBM, display significant increases in genomic complexity and a variety of cell-signaling redundancies. Thus, clinicians face multiple patient-related (due to illness, compliance and other co-morbidities) and metastatic disease-related challenges. It is possible that the KD would be more effective in earlier stage disease (LGG) and provide an opportunity to potentially increase the likelihood of pinpointing targets for transformational therapies. However, to date, there continues to be a paucity of research that exploits these mechanisms and systematically examines interventions such as the KD, relevant to tumor progression in glioma. If successful, these novel treatments could provide a non-toxic, cost-effective adjuvant therapy to standard of care in a disease currently with a grim prognosis.
Fig. 5.
Hypothesized Mechanism by Which a Ketogenic Diet may Reduce Disease Progression and Symptom Burden in Low Grade Glioma.
Acknowledgments
Grant support
NIH/NCIP30 CA076292Moffitt CCSG Grant P30 CA076292.
Abbreviations:
- 2-DG
glycolytic inhibitor 2-deoxy-D-glucose
- ACAC
acetoacetate
- ACE
acetone
- Akt
strain AK, thymoma
- ALS
amyotrophic lateral sclerosis
- ATP
adenosine triphosphate
- βHB
β-hydroxybutyrate
- β-catenin/Wnt
β-catenin/wingless-related integration site
- CD4+
cluster of differentiation 4
- CD8+
cluster of differentiation 8
- CDKN2A/B
cyclin-dependent kinase Inhibitor 2A
- CoA
coenzyme A
- CT-2A
CT-2A glioma model
- EGFR
epidermal growth factor receptor
- FDG-PET
fluorodeoxyglucose positron emission tomography
- GBM
glioblastoma multiforme
- GL-261
GL-261 glioma model
- GLUT
glucose transporter proteins
- GLUT 1
glucose transporter 1
- GLUT 3
glucose transporter 3
- GSH
glutathione
- HGG
high grade glioma
- IGF-1
insulin-like growth factor-1
- IDH
isocitrate dehydrogenase
- IR
insulin receptor
- KA
ketogenic agent
- KB
ketone bodies
- KD
ketogenic diet
- KE
3-butanediol acetoacetate diester
- Km
Michaelis constant
- LGG
low grade glioma
- MAPK
mitogen-activated protein kinases
- mTOR
mammalian target of rapamycin
- NAD
nicotinamide adenine dinucleotide
- NADP
nicotinamide adenine dinucleotide phosphate (oxidized form)
- NADPH
nicotinamide adenine dinucleotide phosphate (reduced form)
- NF1
neurofibromin
- NFκB
nuclear factor kappa-light-chain-enhancer of activated B cells
- NLRP3
NLR family pyrin domain containing 3
- OxS
oxidative stress
- PDGFRA
platelet derived growth factor receptor alpha
- PPP
pentose phosphate pathway
- PTEN
phosphatase and tensin homolog
- Ras
protein family of GTP kinases discovered in rat sarcoma
- R-KD
calorie-restricted ketogenic diet
- ROS
reactive oxygen species
- SOC
standard of care
- TERT
telomerase reverse transcriptase
- TILs
tumor infiltrating lymphocytes
- TP53
tumor promoter protein 53
- TSC
tuberous sclerosis complex
- Treg
T regulatory
- VEGF
vascular endothelial growth factor
- WHO
World Health Organization
Footnotes
Disclosure
Angela Poff is a scientific advisor to Pruvit Ventures, LLC, and is an inventor on the following patent: Dominic P. D’Agostino; Angela M. Poff; Patrick Arnold; “Targeting Cancer with Metabolic Therapy and Hyperbaric Oxygen” (Patent Number: 9801903).
Dominic P. D’Agostino is an inventor on the following patents:
Dominic P. D’Agostino; Angela M. Poff; Patrick Arnold; “Targeting Cancer with Metabolic Therapy and Hyperbaric Oxygen” (Patent Number: 9801903).
Dominic P. D’Agostino; Patrick Arnold; Shannon L. Kesl; “Compositions and Methods for Producing Elevated and Sustained Ketosis” (Patent Number: 20170266148).
Conflict of interest
There is no conflict of interest.
References
- [1].Cancer: Key Facts. Geneva, Switzerland: : World Health Organization; 2015. [Google Scholar]
- [2].Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, et al. , CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009–2013, Neuro Oncol. 18 (2016) v1–v75, 10.1093/neuonc/now207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cancer Facts & Figures. Atlanta, GA : American Cancer Society; 2017. [Google Scholar]
- [4].Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. , The 2016 World Health Organization classification of tumors of the central nervous system: a summary, Acta Neuropathol. 2016 (131) (2016) 803–820, 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- [5].Appin CL, Brat DJ, Molecular pathways in gliomagenesis and their relevance to neuropathologic diagnosis, Adv. Anat. Pathol. 22 (2015) 50–58, 10.1097/PAP.0000000000000048. [DOI] [PubMed] [Google Scholar]
- [6].Duffau H, Taillandier L, New concepts in the management of diffuse low-grade glioma: proposal of a multistage and individualized therapeutic approach, Neuro Oncol. 17 (2015) 332–342, 10.1093/neuonc/nou153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Powell RT, Olar A, Narang S, Rao G, Sulman E, Fuller GN, et al. , Identification of histological correlates of overall survival in lower grade gliomas using a bag-of-words paradigm: a preliminary analysis based on hematoxylin & eosin stained slides from the lower grade glioma cohort of the cancer genome atlas, J. Pathol. Inform. 8 (2017) 9, 10.4103/jpi.jpi_43_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Claus EB, Walsh KM, Wiencke JK, Molinaro AM, Wiemels JL, Schildkraut JM, et al. , Survival and low-grade glioma: the emergence of genetic information, Neurosurg. Focus 38 (2015) E6, 10.3171/2014.10.FOCUS12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. , Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial, Lancet Oncol. 10 (2009) 459–466. [DOI] [PubMed] [Google Scholar]
- [10].Hanahan D, Weinberg RA, The hallmarks of cancer, Cell 100 (2000) 57–70, 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- [11].Hanahan D, Weinberg RA, Hallmarks of cancer: the next generation, Cell 144 (2011) 646–674, 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- [12].Poff AM, Ari C, Seyfried TN, D’Agostino DP, The ketogenic diet and hyperbaric oxygen therapy prolong survival in mice with systemic metastatic cancer, PLoS One 8 (2013) e65522, 10.1371/journal.pone.0065522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Warburg O, On the origin of cancer cells, Science 123 (1956) 309–314, 10.1126/science.123.3191.309. [DOI] [PubMed] [Google Scholar]
- [14].Seyfried TN, Shelton LM, Cancer as a metabolic disease, Nutr. Metab. (Lond.) 7 (2010) 7, 10.1186/743-7075-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Gillies RJ, Robey I, Gatenby RA, Causes and consequences of increased glucose metabolism of cancers, J. Nucl. Med. 49 (Suppl. 2) (2008) 24S–42S, 10.2967/jnumed.107.047258. [DOI] [PubMed] [Google Scholar]
- [16].Strickland M, Stoll EA, Metabolic reprogramming in glioma, Front. Cell Dev Biol. 5 (2017) 43, 10.3389/fcell.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen L, Cui H, Targeting glutamine induces apoptosis: a cancer therapy approach, Int. J. Mol. Sci. 16 (2015) 22830–22855, 10.3390/ijms160922830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Shelton LM, Huysentruyt LC, Seyfried TN, Glutamine targeting inhibits systemic metastasis in the VM-M3 murine tumor model, Int. J. Cancer 127 (2010) 2478–2485, 10.1002/ijc.25431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Venneti S, Dunphy MP, Zhang H, Pitter KL, Zanzonico P, Campos C, et al. , Glutamine-based PET imaging facilitates enhanced metabolic evaluation of gliomas in vivo, Sci. Transl. Med. 7 (2015) 274ra17, 10.1126/scitranslmed.aaa009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Labuschagne CF, van den Broek NJ, Mackay GM, Vousden KH, Maddocks OD, Serine, but not glycine, supports one-carbon metabolism and proliferation of cancer cells, Cell Rep. 7 (2014) 1248–1258, 10.1016/j.celrep.2014.04.045. [DOI] [PubMed] [Google Scholar]
- [21].Obst R, Jannakopulos E, Urbaschek B, Huth K, Muller-Berghaus G, Blood coagulation and serum lipids in pregnant rats following endotoxin in-jection–studies of the Sanarelli-Shwartzmann phenomenon, Thromb. Diath. Haemorrh. 26 (1971) 474–487. [PubMed] [Google Scholar]
- [22].Potter GD, Sclerosis of the base of the skull as a manifestation of nasopharyngeal carcinoma, Radiology 94 (1970) 35–38, 10.1148/10./94.1.35. [DOI] [PubMed] [Google Scholar]
- [23].Grube S, Dunisch P, Freitag D, Klausnitzer M, Sakr Y, Walter J, et al. , Overexpression of fatty acid synthase in human gliomas correlates with the WHO tumor grade and inhibition with Orlistat reduces cell viability and triggers apoptosis, J. Neurooncol. 118 (2014) 277–287, 10.1007/s11060-014-1452-z. [DOI] [PubMed] [Google Scholar]
- [24].Hopperton KE, Duncan RE, Bazinet RP, Archer MC, Fatty acid synthase plays a role in cancer metabolism beyond providing fatty acids for phospholipid synthesis or sustaining elevations in glycolytic activity, Exp. Cell Res. 320 (2014) 302–310, 10.1016/j.yexcr.2013.10.016. [DOI] [PubMed] [Google Scholar]
- [25].Yoshii Y, Furukawa T, Saga T, Fujibayashi Y, Acetate/acetyl-CoA metabolism associated with cancer fatty acid synthesis: overview and application, Cancer Lett. 356 (2015) 211–216, 10.1016/j.canlet.2014.02.019. [DOI] [PubMed] [Google Scholar]
- [26].Pavlova NN, Thompson CB, The emerging hallmarks of cancer metabolism, Cell Metab. 23 (2016) 27–47, 10.1016/j.cmet.2015.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Zhao Y, Butler EB, Tan M, Targeting cellular metabolism to improve cancer therapeutics, Cell. Death. Dis. 4 (2013) e532, 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Lee N, Kim D, Cancer metabolism fueling more than just growth, Mol. Cells 39 (2016) 847–854, 10.14348/molcells.2016.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Vazquez A, Kamphorst JJ, Markert EK, Schug ZT, Tardito S, Gottlieb E, Cancer metabolism at a glance, J. Cell Sci. 129 (2016) 3367–3373, 10.1242/jcs.181016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Ciavardelli D, Rossi C, Barcaroli D, Volpe S, Consalvo A, Zucchelli M, et al. , Breast cancer stem cells rely on fermentative glycolysis and are sensitive to 2-deoxyglucose treatment, Cell Death Dis. 5 (2014) e1336, 10.1038/cddis.2014.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].DiDonato KL, Liu Y, Lindsey CC, Hartwig DM, Stoner SC, Community pharmacy patient perceptions of a pharmacy-initiated mobile technology app to improve adherence, Int. J. Pharm. Pract. 23 (2015) 309–319, 10.1111/ijpp.12168. [DOI] [PubMed] [Google Scholar]
- [32].Nakano I, Therapeutic potential of targeting glucose metabolism in glioma stem cells, Expert Opin. Ther. Targets 18 (2014) 1233–1236, 10.1517/14728222.2014.944899. [DOI] [PubMed] [Google Scholar]
- [33].Yu Z, Xie G, Zhou G, Cheng Y, Zhang G, Yao G, et al. , NVP-BEZ235, a novel dual PI3K-mTOR inhibitor displays anti-glioma activity and reduces chemoresistance to temozolomide in human glioma cells, Cancer Lett. 367 (2015) 58–68, 10.1016/j.canlet.2015.07.007. [DOI] [PubMed] [Google Scholar]
- [34].Galarraga J, Loreck DJ, Graham JF, DeLaPaz RL, Smith BH, Hallgren D, et al. , Glucose metabolism in human gliomas: correspondence of in situ and in vitro metabolic rates and altered energy metabolism, Metab. Brain Dis. 1 (1986) 279–291. [DOI] [PubMed] [Google Scholar]
- [35].Oudard S, Boitier E, Miccoli L, Rousset S, Dutrillaux B, Poupon MF, Gliomas are driven by glycolysis: putative roles of hexokinase, oxidative phosphorylation and mitochondrial ultrastructure, Anticancer Res. 17 (1997) 1903–1911. [PubMed] [Google Scholar]
- [36].Roslin M, Henriksson R, Bergstrom P, Ungerstedt U, Bergenheim AT, Baseline levels of glucose metabolites, glutamate and glycerol in malignant glioma assessed by stereotactic microdialysis, J. Neurooncol. 61 (2003) 151–160. [DOI] [PubMed] [Google Scholar]
- [37].Block KI, Gyllenhaal C, Lowe L, Amedei A, Amin AR, Amin A, et al. , Designing a broad-spectrum integrative approach for cancer prevention and treatment, Semin. Cancer Biol. 35 (Suppl) (2015) S276–S304, 10.1016/j.semcancer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Groesbeck DK, Bluml RM, Kossoff EH, Long-term use of the ketogenic diet in the treatment of epilepsy, Dev. Med. Child Neurol. 48 (2006) 978–981, 10.1017/S0012162206002143. [DOI] [PubMed] [Google Scholar]
- [39].Brownlow ML, Benner L, D’Agostino D, Gordon MN, Morgan D, Ketogenic diet improves motor performance but not cognition in two mouse models of Alzheimer’s pathology, PLoS One 8 (2013) e75713, 10.1371/journal.pone.0075713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Cheng B, Yang X, An L, Gao B, Liu X, Liu S, Ketogenic diet protects dopaminergic neurons against 6-OHDA neurotoxicity via up-regulating glutathione in a rat model of Parkinson’s disease, Brain Res. 1286 (2009) 25–31, 10.1016/j.brainres.2009.06.060. [DOI] [PubMed] [Google Scholar]
- [41].Cicero AF, Benelli M, Brancaleoni M, Dainelli G, Merlini D, Negri R, Middle and long-term impact of a very low-carbohydrate ketogenic diet on cardiometabolic factors: a multi-center, cross-sectional, clinical study, High Blood Press Cardiovasc Prev. 22 (2015) 389–394, 10.1007/s40292-015-0096-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Henderson ST, Vogel JL, Barr LJ, Garvin F, Jones JJ, Costantini LC, Study of the ketogenic agent AC-1202 in mild to moderate Alzheimer’s disease: a randomized, double-blind, placebo-controlled, multicenter trial, Nutr. Metab. (Lond.) 6 (2009) 31, 10.1186/743-7075-6-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hite AH, Berkowitz VG, Berkowitz K, Low-carbohydrate diet review: shifting the paradigm, Nutr. Clin. Pract. 26 (2011) 300–308, 10.1177/0884533611405791. [DOI] [PubMed] [Google Scholar]
- [44].Kashiwaya Y, Bergman C, Lee JH, Wan R, King MT, Mughal MR, et al. , A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease, Neurobiol. Aging 34 (2013) 1530–1539, 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Paoli A, Rubini A, Volek JS, Grimaldi KA, Beyond weight loss: a review of the therapeutic uses of very-low-carbohydrate (ketogenic) diets, Eur. J. Clin. Nutr. 67 (2013) 789–796, 10.1038/ejcn.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shaafi S, Najmi S, Aliasgharpour H, Mahmoudi J, Sadigh-Etemad S, Farhoudi M, et al. , The efficacy of the ketogenic diet on motor functions in Parkinson’s disease: a rat model, Iran. J. Neurol. 15 (2016) 63–69. [PMC free article] [PubMed] [Google Scholar]
- [47].Volek J, Sharman M, Gomez A, Judelson D, Rubin M, Watson G, et al. , Comparison of energy-restricted very low-carbohydrate and low-fat diets on weight loss and body composition in overweight men and women, Nutr. Metab. (Lond.) 1 (2004) 13, 10.1186/743-7075-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Wood RJ, Volek JS, Davis SR, Dell’Ova C, Fernandez ML, Effects of a carbohydrate-restricted diet on emerging plasma markers for cardiovascular disease, Nutr. Metab. (Lond.) 3 (2006) 19, 10.1186/743-7075-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wood RJ, Volek JS, Liu Y, Shachter NS, Contois JH, Fernandez ML, Carbohydrate restriction alters lipoprotein metabolism by modifying VLDL, LDL, and HDL subfraction distribution and size in overweight men, J. Nutr. 136 (2006) 384–389. [DOI] [PubMed] [Google Scholar]
- [50].Zhao Z, Lange DJ, Voustianiouk A, MacGrogan D, Ho L, Suh J, et al. , A ketogenic diet as a potential novel therapeutic intervention in amyotrophic lateral sclerosis, BMC Neurosci. 7 (2006) 29, 10.1186/471-2202-7-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lin MT, Beal MF, Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases, Nature 443 (2006) 787–795, 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- [52].Picard M, Turnbull DM, Linking the metabolic state and mitochondrial DNA in chronic disease, health, and aging, Diabetes 62 (2013) 672–678, 10.2337/db12-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Esposito K, Marfella R, Giugliano D, Stress hyperglycemia, inflammation, and cardiovascular events, Diabetes Care 26 (2003) 1650–1651, 10.2337/diacare.26.5.1650-a. [DOI] [PubMed] [Google Scholar]
- [54].Gyurko R, Siqueira CC, Caldon N, Gao L, Kantarci A, Van Dyke TE, Chronic hyperglycemia predisposes to exaggerated inflammatory response and leukocyte dysfunction in Akita mice, J. Immunol. 177 (2006) 7250–7256, 10.4049/jimmunol.177.10.7250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lin Y, Berg AH, Iyengar P, Lam TK, Giacca A, Combs TP, et al. , The hyperglycemia-induced inflammatory response in adipocytes: the role of reactive oxygen species, J. Biol. Chem. 280 (2005) 4617–4626, 10.1074/jbc.M411863200. [DOI] [PubMed] [Google Scholar]
- [56].D’Agostino D, Pilla R, Held H, Landon C, Ari C, Arnold P, et al. , Development, testing and therapeutic applications of ketone esters (KE) for CNS oxygen toxicity (CNS-OT); i.e., hyperbaric oxygen (HBO2)-induced seizures, FASEB J. 26 (2012) 711. [Google Scholar]
- [57].D’Agostino DP, Pilla R, Held HE, Landon CS, Puchowicz M, Brunengraber H, et al. , Therapeutic ketosis with ketone ester delays central nervous system oxygen toxicity seizures in rats, Am. J. Physiol. Regul. Integr. Comp. Physiol. 304 (2013) R829–R836, 10.1152/ajpregu.00506.2012. [DOI] [PubMed] [Google Scholar]
- [58].Poff AM, Ari C, Arnold P, Seyfried TN, D’Agostino DP, Ketone supplementation decreases tumor cell viability and prolongs survival of mice with metastatic cancer, Int. J. Cancer 135 (2014) 1711–1720, 10.1002/ijc.28809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Poff AM, Ward N, Seyfried TN, Arnold P, D’Agostino DP, Non-toxic metabolic management of metastatic cancer in VM mice: novel combination of ketogenic diet, ketone supplementation, and hyperbaric oxygen therapy, PLoS One 10 (2015) e0127407, 10.1371/journal.pone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Abdelwahab MG, Fenton KE, Preul MC, Rho JM, Lynch A, Stafford P, et al. , The ketogenic diet is an effective adjuvant to radiation therapy for the treatment of malignant glioma, PLoS One 7 (2012) e36197, 10.1371/journal.pone.0036197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Champ CE, Palmer JD, Volek JS, Werner-Wasik M, Andrews DW, Evans JJ, et al. , Targeting metabolism with a ketogenic diet during the treatment of glioblastoma multiforme, J. Neurooncol. 117 (2014) 125–131, 10.1007/s11060-014-1362-0. [DOI] [PubMed] [Google Scholar]
- [62].Woolf EC, Curley KL, Liu Q, Turner GH, Charlton JA, Preul MC, et al. , The ketogenic diet alters the hypoxic response and affects expression of proteins associated with angiogenesis, invasive potential and vascular permeability in a mouse glioma model, PLoS One 10 (2015) e0130357, 10.1371/journal.pone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Lussier DM, Woolf EC, Johnson JL, Brooks KS, Blattman JN, Scheck AC, Enhanced immunity in a mouse model of malignant glioma is mediated by a therapeutic ketogenic diet, BMC Cancer 16 (2016) 310, 10.1186/s12885-016-2337-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Scheck AC, Abdelwahab MG, Fenton KE, Stafford P, The ketogenic diet for the treatment of glioma: insights from genetic profiling, Epilepsy Res. 100 (2012) 327–337, 10.1016/j.eplepsyres.2011.09.022. [DOI] [PubMed] [Google Scholar]
- [65].Nebeling LC, Miraldi F, Shurin SB, Lerner E, Effects of a ketogenic diet on tumor metabolism and nutritional status in pediatric oncology patients: two case reports, J. Am. Coll. Nutr. 14 (1995) 202–208, 10.1080/07315724.1995.10718495. [DOI] [PubMed] [Google Scholar]
- [66].Fine EJ, Miller A, Quadros EV, Sequeira JM, Feinman RD, Acetoacetate reduces growth and ATP concentration in cancer cell lines which over-express uncoupling protein 2, Cancer Cell Int. 9 (2009) 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Magee BA, Potezny N, Rofe AM, Conyers RA, The inhibition of malignant cell growth by ketone bodies, Aust. J. Exp. Biol. Med. Sci. 57 (1979) 529–539, 10.1038/icb.979.54. [DOI] [PubMed] [Google Scholar]
- [68].Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. , Suppression of oxidative stress by beta-hydroxybutyrate, an endogenous histone deacetylase inhibitor, Science 339 (2013) 211–214, 10.1126/science.1227166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Woolf EC, Syed N, Scheck AC, Tumor metabolism, the ketogenic diet and beta-hydroxybutyrate: novel approaches to adjuvant brain tumor therapy, Front. Mol. Neurosci. 9 (2016) 122, 10.3389/fnmol.2016.00122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Youm YH, Nguyen KY, Grant RW, Goldberg EL, Bodogai M, Kim D, et al. , The ketone metabolite beta-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease, Nat. Med. 21 (2015) 263–269, 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Newman JC, Verdin E, Ketone bodies as signaling metabolites, Trends Endocrinol. Metab. 25 (2014) 42–52, 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sawai M, Yashiro M, Nishiguchi Y, Ohira M, Hirakawa K, Growth-inhibitory effects of the ketone body, monoacetoacetin, on human gastric cancer cells with succinyl-CoA: 3-oxoacid CoA-transferase (SCOT) deficiency, Anticancer Res. 24 (2004) 2213–2217. [PubMed] [Google Scholar]
- [73].Skinner R, Trujillo A, Ma X, Beierle EA, Ketone bodies inhibit the viability of human neuroblastoma cells, J. Pediatr. Surg. 44 (2009) 212–216, 10.1016/j.jpedsurg.2008.10.042discussion6. [DOI] [PubMed] [Google Scholar]
- [74].Klement RJ, Champ CE, Otto C, Kammerer U, Anti-tumor effects of ketogenic diets in mice: a meta-analysis, PLoS One 11 (2016) e0155050, 10.1371/journal.pone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Bonuccelli G, Tsirigos A, Whitaker-Menezes D, Pavlides S, Pestell RG, Chiavarina B, et al. , Ketones and lactate fuel tumor growth and metastasis: evidence that epithelial cancer cells use oxidative mitochondrial metabolism, ABBV Cell Cycle 9 (2010) 3506–3514, 10.4161/cc.9.17.12731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Xia S, Lin R, Jin L, Zhao L, Kang HB, Pan Y, et al. , Prevention of dietary-fat-fueled ketogenesis attenuates BRAF V600E tumor growth, Cell Metab. 25 (2017) 358–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Iguchi T, Takasugi N, Nishimura N, Kusunoki S, Correlation between mammary tumor and blood glucose, serum insulin, and free fatty acids in mice, Cancer Res. 49 (1989) 821–825. [PubMed] [Google Scholar]
- [78].Seyfried TN, Sanderson TM, El-Abbadi MM, McGowan R, Mukherjee P, Role of glucose and ketone bodies in the metabolic control of experimental brain cancer, Br. J. Cancer 89 (2003) 1375–1382, 10.1038/sj.bjc.6601269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Stattin P, Bjor O, Ferrari P, Lukanova A, Lenner P, Lindahl B, et al. , Prospective study of hyperglycemia and cancer risk, Diabetes Care 30 (2007) 561–567, 10.2337/dc06-0922. [DOI] [PubMed] [Google Scholar]
- [80].Adeberg S, Bernhardt D, Foerster R, Bostel T, Koerber SA, Mohr A, et al. , The influence of hyperglycemia during radiotherapy on survival in patients with primary glioblastoma, Acta Oncol. 55 (2016) 201–207, 10.3109/0284186X.s015.1043397. [DOI] [PubMed] [Google Scholar]
- [81].Chaichana KL, McGirt MJ, Woodworth GF, Datoo G, Tamargo RJ, Weingart J, et al. , Persistent outpatient hyperglycemia is independently associated with survival, recurrence and malignant degeneration following surgery for hemispheric low grade gliomas, Neurol. Res. 32 (2010) 442–448, 10.1179/174313209X431101. [DOI] [PubMed] [Google Scholar]
- [82].McGirt MJ, Chaichana KL, Gathinji M, Attenello F, Than K, Ruiz AJ, et al. , Persistent outpatient hyperglycemia is independently associated with decreased survival after primary resection of malignant brain astrocytomas, Neurosurgery 63 (2008) 286–291, 10.1227/01.NEU.0000315282.61035.48discussion91. [DOI] [PubMed] [Google Scholar]
- [83].Seliger C, Ricci C, Meier CR, Bodmer M, Jick SS, Bogdahn U, et al. , Diabetes, use of antidiabetic drugs, and the risk of glioma, Neuro Oncol. 18 (2016) 340–349, 10.1093/neuonc/nov100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Chambless LB, Parker SL, Hassam-Malani L, McGirt MJ, Thompson RC, Type 2 diabetes mellitus and obesity are independent risk factors for poor outcome in patients with high-grade glioma, J. Neurooncol. 106 (2012) 383–389, 10.1007/s11060-011-0676-4. [DOI] [PubMed] [Google Scholar]
- [85].Siegel EM, Nabors LB, Thompson RC, Olson JJ, Browning JE, Madden MH, et al. , Prediagnostic body weight and survival in high grade glioma, J. Neurooncol. 114 (2013) 79–84, 10.1007/s11060-013-1150-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Szablewski L, Expression of glucose transporters in cancers, Biochim. Biophys. Acta 1835 (2013) 164–169, 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [87].Calvo MB, Figueroa A, Pulido EG, Campelo RG, Aparicio LA, Potential role of sugar transporters in cancer and their relationship with anticancer therapy, Int. J. Endocrinol. (2010), 10.1155/2010/205357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Flavahan WA, Wu Q, Hitomi M, Rahim N, Kim Y, Sloan AE, et al. , Brain tumor initiating cells adapt to restricted nutrition through preferential glucose uptake, Nat. Neurosci. 16 (2013) 1373–1382, 10.1038/nn.3510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Frayn KN, Metabolic Regulation: A Human Perspective, John Wiley & Sons, New York, NY, 2009. [Google Scholar]
- [90].Modica-Napolitano JS, Singh KK, Mitochondrial dysfunction in cancer, Mitochondrion 4 (2004) 755–762, 10.1016/j.mito.2004.07.027. [DOI] [PubMed] [Google Scholar]
- [91].Yang C, Jiang L, Zhang H, Shimoda LA, DeBerardinis RJ, Semenza GL, Analysis of hypoxia-induced metabolic reprogramming, Methods Enzymol. 542 (2014) 425–455, 10.1016/B978-0-12-416618-9.00022-4. [DOI] [PubMed] [Google Scholar]
- [92].Patra KC, Hay N, The pentose phosphate pathway and cancer, Trends Biochem. Sci. 39 (2014) 347–354, 10.1016/j.tibs.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Belfiore A, Malaguarnera R, Insulin receptor and cancer, Endocr. Relat. Cancer 18 (2011) R125–R147, 10.1530/ERC-11-0074. [DOI] [PubMed] [Google Scholar]
- [94].Rohrmann S, Linseisen J, Becker S, Allen N, Schlehofer B, Overvad K, et al. , Concentrations of IGF-I and IGFBP-3 and brain tumor risk in the European prospective investigation into cancer and nutrition, Cancer Epidemiol. Biomarkers Prev. 20 (2011) 2174–2182, 10.1158/055-9965.EPI-11-0179. [DOI] [PubMed] [Google Scholar]
- [95].Boyd DB, Insulin and cancer, Integr. Cancer Ther. 2 (2003) 315–329, 10.1177/1534735403259125. [DOI] [PubMed] [Google Scholar]
- [96].Liang J, Slingerland JM, Multiple roles of the PI3 K/PKB (Akt) pathway in cell cycle progression, ABBV Cell Cycle 2 (2003) 339–345. [PubMed] [Google Scholar]
- [97].Lu M, Amano S, Miyamoto K, Garland R, Keough K, Qin W, et al. , Insulin-induced vascular endothelial growth factor expression in retina, Invest. Ophthalmol. Vis. Sci. 40 (1999) 3281–3286. [PubMed] [Google Scholar]
- [98].Miuma S, Ichikawa T, Arima K, Takeshita S, Muraoka T, Matsuzaki T, et al. , Branched-chain amino acid deficiency stabilizes insulin-induced vascular endothelial growth factor mRNA in hepatocellular carcinoma cells, J. Cell. Biochem. 113 (2012) 3113–3121, 10.1002/jcb.24188. [DOI] [PubMed] [Google Scholar]
- [99].Volek JS, Sharman MJ, Cardiovascular and hormonal aspects of very-low-carbohydrate ketogenic diets, Obes. Res. 12 (Suppl. 2) (2004) 115S–123S, 10.1038/oby.2004.276. [DOI] [PubMed] [Google Scholar]
- [100].Harber MP, Schenk S, Barkan AL, Horowitz JF, Effects of dietary carbohydrate restriction with high protein intake on protein metabolism and the somatotropic axis, J. Clin. Endocrinol. Metab. 90 (2005) 5175–5181, 10.1210/jc.2005-0559. [DOI] [PubMed] [Google Scholar]
- [101].Nielsen JV, Jonsson E, Ivarsson A, A low carbohydrate diet in type 1 diabetes: clinical experience—a brief report, Ups. J. Med. Sci. 110 (2005) 267–273, 10.3109/2000-1967-074. [DOI] [PubMed] [Google Scholar]
- [102].Boden G, Sargrad K, Homko C, Mozzoli M, Stein TP, Effect of a low-carbohydrate diet on appetite, blood glucose levels, and insulin resistance in obese patients with type 2 diabetes, Ann. Intern. Med. 142 (2005) 403–411, 10.7326/0003-4819-142-6-200503150-00006. [DOI] [PubMed] [Google Scholar]
- [103].Otto C, Kaemmerer U, Illert B, Muehling B, Pfetzer N, Wittig R, et al. , Growth of human gastric cancer cells in nude mice is delayed by a ketogenic diet supplemented with omega-3 fatty acids and medium-chain triglycerides, BMC Cancer 8 (2008) 122, 10.1186/471-2407-8-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Freedland SJ, Mavropoulos J, Wang A, Darshan M, Demark-Wahnefried W, Aronson WJ, et al. , Carbohydrate restriction, prostate cancer growth, and the insulin-like growth factor axis, Prostate 68 (2008) 11–19, 10.1002/pros.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Fine EJ, Segal-Isaacson CJ, Feinman RD, Herszkopf S, Romano MC, Tomuta N, et al. , Targeting insulin inhibition as a metabolic therapy in advanced cancer: a pilot safety and feasibility dietary trial in 10 patients, Nutrition 28 (2012) 1028–1035, 10.1016/j.nut.2012.05.001. [DOI] [PubMed] [Google Scholar]
- [106].Srivastava S, Kashiwaya Y, King MT, Baxa U, Tam J, Niu G, et al. , Mitochondrial biogenesis and increased uncoupling protein 1 in brown adipose tissue of mice fed a ketone ester diet, FASEB J. 26 (2012) 2351–2362, 10.1096/fj.11-200410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kesl SL, Poff AM, Ward NP, Fiorelli TN, Ari C, Van Putten AJ, et al. , Effects of exogenous ketone supplementation on blood ketone, glucose, triglyceride, and lipoprotein levels in Sprague-Dawley rats, Nutr. Metab. (Lond.) 13 (2016) 9, 10.1186/s12986-016-0069-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Fruehauf JP, Meyskens FL Jr., Reactive oxygen species: a breath of life or death? Clin. Cancer Res. 13 (2007) 789–794, 10.1158/078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- [109].Hamanaka RB, Chandel NS, Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes, Trends Biochem. Sci. 35 (2010) 505–513, 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Wiseman H, Halliwell B, Damage to DNA by reactive oxygen and nitrogen species: role in inflammatory disease and progression to cancer, Biochem. J 313 (Pt 1) (1996) 17–29, 10.1042/bj3130017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Hileman EO, Liu J, Albitar M, Keating MJ, Huang P, Intrinsic oxidative stress in cancer cells: a biochemical basis for therapeutic selectivity, Cancer Chemother. Pharmacol. 53 (2004) 209–219, 10.1007/s00280-003-0726-5. [DOI] [PubMed] [Google Scholar]
- [112].Portakal O, Ozkaya O, Erden Inal M, Bozan B, Kosan M, Sayek I, Coenzyme Q10 concentrations and antioxidant status in tissues of breast cancer patients, Clin. Biochem. 33 (2000) 279–284, 10.1016/S0009-9120(00)00067-9. [DOI] [PubMed] [Google Scholar]
- [113].Kong Q, Beel JA, Lillehei KO, A threshold concept for cancer therapy, Med. Hypotheses 55 (2000) 29–35, 10.1054/mehy.999.0982. [DOI] [PubMed] [Google Scholar]
- [114].Wang J, Yi J, Cancer cell killing via ROS: to increase or decrease, that is the question, Cancer Biol. Ther. 7 (2008) 1875–1884, 10.4161/cbt.7.12.7067. [DOI] [PubMed] [Google Scholar]
- [115].Schumacker PT, Reactive oxygen species in cancer cells: live by the sword, die by the sword, Cancer Cell 10 (2006) 175–176, 10.1016/j.ccr.2006.08.015. [DOI] [PubMed] [Google Scholar]
- [116].Allen BG, Bhatia SK, Buatti JM, Brandt KE, Lindholm KE, Button AM, et al. , Ketogenic diets enhance oxidative stress and radio-chemo-therapy responses in lung cancer xenografts, Clin. Cancer Res. 19 (2013) 3905–3913, 10.1158/078-0432.CCR-12-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Stafford P, Abdelwahab MG, Kim DY, Preul MC, Rho JM, Scheck AC, The ketogenic diet reverses gene expression patterns and reduces reactive oxygen species levels when used as an adjuvant therapy for glioma, Nutr. Metab. (Lond.) 7 (2010) 74, 10.1186/743-7075-7-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Maalouf M, Rho JM, Mattson MP, The neuroprotective properties of calorie restriction, the ketogenic diet, and ketone bodies, Brain Res. Rev. 59 (2009) 293–315, 10.1016/j.brainresrev.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Veech RL, The therapeutic implications of ketone bodies: the effects of ketone bodies in pathological conditions: ketosis, ketogenic diet, redox states, insulin resistance, and mitochondrial metabolism, Prostaglandins Leukot. Essent. Fatty Acids 70 (2004) 309–319, 10.1016/j.bbcan.2012.12.004. [DOI] [PubMed] [Google Scholar]
- [120].Kashiwaya Y, Sato K, Tsuchiya N, Thomas S, Fell DA, Veech RL, et al. , Control of glucose utilization in working perfused rat heart, J. Biol. Chem. 269 (1994) 25502–25514. [PubMed] [Google Scholar]
- [121].Rossi AP, Woolf EC, Brooks KS, Fairres MJ, Scheck AC, The ketone body b-hydroxybutyrate increases radiosensitivity in glioma cell lines in vitro, Cancer Res. 75 (2015) 3346, 10.1158/538-7445.AM2015-3346. [DOI] [Google Scholar]
- [122].Blum D, Omlin A, Baracos VE, Solheim TS, Tan BH, Stone P, et al. , Cancer cachexia: a systematic literature review of items and domains associated with involuntary weight loss in cancer, Crit. Rev. Oncol. Hematol. 80 (2011) 114–144, 10.1016/j.critrevonc.2010.10.004. [DOI] [PubMed] [Google Scholar]
- [123].McMillan DC, An inflammation-based prognostic score and its role in the nutrition-based management of patients with cancer, Proc. Nutr. Soc. 67 (2008) 257–262, 10.1017/S0029665108007131. [DOI] [PubMed] [Google Scholar]
- [124].Wallengren O, Lundholm K, Bosaeus I, Diagnostic criteria of cancer cachexia: relation to quality of life, exercise capacity and survival in unselected palliative care patients, Support. Care Cancer 21 (2013) 1569–1577, 10.1007/s00520-012-1697-z. [DOI] [PubMed] [Google Scholar]
- [125].Forsythe CE, Phinney SD, Fernandez ML, Quann EE, Wood RJ, Bibus DM, et al. , Comparison of low fat and low carbohydrate diets on circulating fatty acid composition and markers of inflammation, Lipids 43 (2008) 65–77, 10.1007/s11745-007-3132-7. [DOI] [PubMed] [Google Scholar]
- [126].Poff A, Ward N, D’Agostino D, Metabolic effects of exogenous ketone supplementation − an alternative or adjuvant to the ketogenic diet as a cancer therapy? Keystone Symposia − New Frontiers in tumor Metabolism. Banff, Alberta, Canada, 2016. [Google Scholar]
- [127].Sowers JL, Johnson KM, Conrad C, Patterson JT, Sowers LC, The role of inflammation in brain cancer, Adv. Exp. Med. Biol. 816 (2014) 75–105, 10.1007/978-3-0348-837-8_4. [DOI] [PubMed] [Google Scholar]
- [128].Li L, Liu Y, Aging-related gene signature regulated by Nlrp3 predicts glioma progression, Am. J. Cancer. Res. 5 (2015) 442–449. [PMC free article] [PubMed] [Google Scholar]
- [129].Dawes JD, The postero-superior quadrant, J. Laryngol. Otol. 88 (1974) 955–967. [DOI] [PubMed] [Google Scholar]
- [130].Brown KM, Rosenstreich DL, Mechanism of action of a human interleukin 1 inhibitor, Cell. Immunol. 105 (1987) 45–53. [DOI] [PubMed] [Google Scholar]
- [131].Hassler MR, Egger G, Epigenomics of cancer—emerging new concepts, Biochimie 94 (2012) 2219–2230, 10.1016/j.biochi.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Ceccacci E, Minucci S, Inhibition of histone deacetylases in cancer therapy: lessons from leukaemia, Br. J. Cancer 114 (2016) 605–611, 10.1038/bjc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Richardson PG, Mitsiades CS, Laubach JP, Hajek R, Spicka I, Dimopoulos MA, et al. , Preclinical data and early clinical experience supporting the use of histone deacetylase inhibitors in multiple myeloma, Leuk. Res. 37 (2013) 829–837, 10.1016/j.leukres.2013.03.006. [DOI] [PubMed] [Google Scholar]
- [134].Richardson PG, Schlossman RL, Alsina M, Weber DM, Coutre SE, Gasparetto C, et al. , PANORAMA 2: panobinostat in combination with bortezomib and dexamethasone in patients with relapsed and bortezomib-refractory myeloma, Blood 122 (2013) 2331–2337, 10.1182/blood-2013-01-481325. [DOI] [PubMed] [Google Scholar]
- [135].Watanabe T, Investigational histone deacetylase inhibitors for non-Hodgkin lymphomas, Expert Opin. Investig. Drugs 19 (2010) 1113–1127, 10.1517/13543784.2010.504710. [DOI] [PubMed] [Google Scholar]
- [136].Bose P, Dai Y, Grant S, Histone deacetylase inhibitor (HDACI) mechanisms of action: emerging insights, Pharmacol. Ther. 143 (2014) 323–336, 10.1016/j.pharmthera.2014.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Scheck AC, Abdelwahab M, Stafford P, Kim D-Y, Iwai S, Preul MC, et al. , Mechanistic studies of the ketogenic diet as an adjuvant therapy for malignant gliomas, American Association for Cancer Research 101 st Annual Meeting, Washington, D.C.: AACR, 2010. p. 638. [Google Scholar]
- [138].Lee C, Raffaghello L, Brandhorst S, Safdie FM, Bianchi G, Martin-Montalvo A, et al. , Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy, Sci. Transl. Med. 4 (2012) 124–127, 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Schmidt M, Pfetzer N, Schwab M, Strauss I, Kammerer U, Effects of a ketogenic diet on the quality of life in 16 patients with advanced cancer: a pilot trial, Nutr. Metab. (Lond.) 8 (2011) 54, 10.1186/743-7075-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Marsh J, Mukherjee P, Seyfried TN, Drug/diet synergy for managing malignant astrocytoma in mice: 2-deoxy-D-glucose and the restricted ketogenic diet, Nutr. Metab. (Lond.) 5 (2008) 33, 10.1186/743-7075-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Zhuang Y, Chan DK, Haugrud AB, Miskimins WK, Mechanisms by which low glucose enhances the cytotoxicity of metformin to cancer cells both in vitro and in vivo, PLoS One 9 (2014) e108444, 10.1371/journal.pone.0108444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Fredericks M, Ramsey RB, 3-Oxo acid coenzyme A transferase activity in brain and tumors of the nervous system, J. Neurochem. 31 (1978) 1529–1531, 10.1111/j.1471-4159.1978.tb06581.x. [DOI] [PubMed] [Google Scholar]
- [143].Maurer GD, Brucker DP, Bahr O, Harter PN, Hattingen E, Walenta S, et al. , Differential utilization of ketone bodies by neurons and glioma cell lines: a rationale for ketogenic diet as experimental glioma therapy, BMC Cancer 11 (2011) 315, 10.1186/471-2407-11-315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Jackson SH, Shah K, Debbas NM, Johnston A, Peverel-Cooper CA, Turner P, The interaction between i: v. theophylline and chronic oral dosing with slow release nifedipine in volunteers, Br. J. Clin. Pharmacol. 21 (1986) 389–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Eloqayli H, Melo TM, Haukvik A, Sonnewald U, [2,4-(13)C]beta-hydroxybutyrate metabolism in astrocytes and C6 glioblastoma cells, Neurochem. Res. 36 (2011) 1566–1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Roeder LM, Poduslo SE, Tildon JT, Utilization of ketone bodies and glucose by established neural cell lines, J. Neurosci. Res. 8 (1982) 671–682. [DOI] [PubMed] [Google Scholar]
- [147].De Feyter HM, Behar KL, Rao JU, Madden-Hennessey K, Ip KL, Hyder F, et al. , A ketogenic diet increases transport and oxidation of ketone bodies in RG2 and 9L gliomas without affecting tumor growth, Neuro Oncol. 18 (2016) 1079–1087, 10.1093/neuonc/now088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Arismendi-Morillo G, Castellano-Ramirez A, Seyfried TN, Ultrastructural characterization of the mitochondria-associated membranes abnormalities in human astrocytomas: functional and therapeutics implications, Ultrastruct. Pathol. 41 (2017) 234–244, 10.1080/01913123.2017.1300618. [DOI] [PubMed] [Google Scholar]
- [149].Arismendi-Morillo GJ, Castellano-Ramirez AV, Ultrastructural mitochondrial pathology in human astrocytic tumors: potentials implications pro-therapeutics strategies, J. Electron. Microsc. (Tokyo) 57 (2008) 33–39, 10.1093/jmicro/dfm038. [DOI] [PubMed] [Google Scholar]
- [150].Dmitrenko V, Shostak K, Boyko O, Khomenko O, Rozumenko V, Malisheva T, et al. , Reduction of the transcription level of the mitochondrial genome in human glioblastoma, Cancer Lett. 218 (2005) 99–107, 10.1016/j.canlet.2004.07.001. [DOI] [PubMed] [Google Scholar]
- [151].Katsetos CD, Anni H, Draber P, Mitochondrial dysfunction in gliomas, Semin. Pediatr. Neurol. 20 (2013) 216–227, 10.1016/j.spen.2013.09.003. [DOI] [PubMed] [Google Scholar]
- [152].Liang BC, Grootveld M, The importance of mitochondria in the tumourigenic phenotype: gliomas as the paradigm (review), Int. J. Mol. Med. 27 (2011) 159–171, 10.3892/ijmm.2010.579. [DOI] [PubMed] [Google Scholar]
- [153].Liang BC, Hays L, Mitochondrial DNA copy number changes in human gliomas, Cancer Lett. 105 (1996) 167–173. [DOI] [PubMed] [Google Scholar]
- [154].Vidone M, Clima R, Santorsola M, Calabrese C, Girolimetti G, Kurelac I, et al. , A comprehensive characterization of mitochondrial DNA mutations in glioblastoma multiforme, Int. J. Biochem. Cell Biol. 63 (2015) 46–54, 10.1016/j.biocel.2015.01.027. [DOI] [PubMed] [Google Scholar]
- [155].Martuscello RT, Vedam-Mai V, McCarthy DJ, Schmoll ME, Jundi MA, Louviere CD, et al. , A supplemented high-fat low-carbohydrate diet for the treatment of glioblastoma, Clin. Cancer Res. 22 (2016) 2482–2495, 10.1158/078-0432.CCR-15-916. [DOI] [PubMed] [Google Scholar]
- [156].Rieger J, Bahr O, Maurer GD, Hattingen E, Franz K, Brucker D, et al. , ERGO: a pilot study of ketogenic diet in recurrent glioblastoma, Int. J. Oncol. 44 (2014) 1843–1852, 10.3892/ijo.2014.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Gasior M, Rogawski MA, Hartman AL, Neuroprotective and disease-modifying effects of the ketogenic diet, Behav. Pharmacol. 17 (2006) 431–439, 10.1097/00008877-200609000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [158].Stafstrom CE, Rho JM, The ketogenic diet as a treatment paradigm for diverse neurological disorders, Front. Pharmacol. 3 (2012) 59, 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [159].Liskiewicz AD, Kasprowska D, Wojakowska A, Polanski K, Lewin-Kowalik J, Kotulska K, et al. , Long-term high fat ketogenic diet promotes renal tumor growth in a rat model of tuberous sclerosis, Sci. Rep. 6 (2016) 21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [160].Capparelli C, Guido C, Whitaker-Menezes D, Bonuccelli G, Balliet R, Pestell TG, et al. , Autophagy and senescence in cancer-associated fibroblasts metabolically supports tumor growth and metastasis via glycolysis and ketone production, ABBV Cell Cycle 11 (2012) 2285–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Lisanti MP, Sotgia F, Ketone bodies and two-compartment tumor metabolism: stromal ketone production fuels mitochondrial biogenesis in epithelial cancer cells, ABBV Cell Cycle 11 (2012) 3956–3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Martinez-Outschoorn UE, Lin Z, Whitaker-Menezes D, Howell A, Sotgia F, Lisanti MP, Ketone body utilization drives tumor growth and metastasis, ABBV Cell Cycle 11 (2012) 3964–3971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [163].Martinez-Outschoorn UE, Prisco M, Ertel A, Tsirigos A, Lin Z, Pavlides S, et al. , Ketones and lactate increase cancer cell stemness, driving recurrence, metastasis and poor clinical outcome in breast cancer: achieving personalized medicine via metabolo-genomics, ABBV Cell Cycle 10 (2011) 1271–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Vergati M, Krasniqi E, Monte GD, Riondino S, Vallone D, Guadagni F, et al. , Ketogenic diet and other dietary intervention strategies in the treatment of cancer, Curr. Med. Chem. 24 (2017) 1170–1185. [DOI] [PubMed] [Google Scholar]
- [165].Kossoff EH, Hartman AL, Ketogenic diets: new advances for metabolism-based therapies, Curr. Opin. Neurol. 25 (2012) 173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Kwiterovich PO Jr., E.P. Vining, P. Pyzik, R. Skolasky Jr., J.M. Freeman, Effect of a high-fat ketogenic diet on plasma levels of lipids, lipoproteins, and apolipoproteins in children, JAMA 290 (2003) 912–920. [DOI] [PubMed] [Google Scholar]
- [167].Volek JS, Gomez AL, Love DM, Weyers AM, Hesslink R Jr., J.A. Wise, et al. , Effects of an 8-week weight-loss program on cardiovascular disease risk factors and regional body composition, Eur. J. Clin. Nutr. 56 (2002) 585–592, 10.1038/sj.ejcn.1601362. [DOI] [PubMed] [Google Scholar]
- [168].Volek JS, Phinney SD, Forsythe CE, Quann EE, Wood RJ, Puglisi MJ, et al. , Carbohydrate restriction has a more favorable impact on the metabolic syndrome than a low fat diet, Lipids 44 (2009) 297–309, 10.1007/s11745-008-3274-2. [DOI] [PubMed] [Google Scholar]
- [169].Volek JS, Sharman MJ, Gomez AL, DiPasquale C, Roti M, Pumerantz A, et al. , Comparison of a very low-carbohydrate and low-fat diet on fasting lipids, LDL subclasses, insulin resistance, and postprandial lipemic responses in overweight women, J. Am. Coll. Nutr. 23 (2004) 177–184, 10.1080/07315724.2004.10719359. [DOI] [PubMed] [Google Scholar]