Abstract
Objective
Previous animal studies have shown that the oxytocin system might affect glucose homeostasis through the hypothalamus–pituitary–adrenal (HPA) axis and peripheral organs. Moreover, whether the effect is stratified by the polymorphism of oxytocin receptor gene (OXTR) remains unclear.
Methods
In this study, we recruited 89 non-diabetic participants. Their plasma oxytocin and serum insulin profiles were obtained, and the polymorphism of OXTR rs53576 was genotyped.
Results
There were significant correlations between the oxytocin level and fasting glucose level (r = –0.29, P <0.01), insulin level (r = –0.26, P = 0.01), and homeostasis model assessment-estimated insulin resistance (HOMA-IR) (r = –0.25, P = 0.01), when adjusted for age, gender, and body mass index (BMI). When further considering the stratification effects of OXTR variation, we found that the oxytocin level was significantly correlated with the fasting glucose level (r = –0.25, P = 0.04), insulin level (r = –0.35, P = 0.03), and HOMA-IR (r = –0.35, P < 0.01) in subjects with the OXTR A allele (n = 75) after adjustment for age, gender, and BMI. In addition, the oxytocin level in those with the GG genotype of OXTR was significantly negatively correlated with the leptin level (n = 14, r = –0.66, P = 0.02).
Conclusion
The results demonstrated that the polymorphism of OXTR plays an important role in individual differences in the correlation of oxytocin and glucose homeostasis in non-diabetic subjects.
Keywords: glucose, insulin, oxytocin, OXTR, polymorphism
Introduction
Oxytocin, secreted by the paraventricular nucleus and supraoptic nucleus of the hypothalamus, is well-known for its roles in parturition and lactation. Recent reports have suggested that oxytocin is also important for energy homeostasis and regulation of feeding behavior.1,2 The oxytocin receptor (OXTR) is expressed in the brain, heart, kidneys, pancreas, and adipose tissue.3 Animal studies have shown that direct injection of oxytocin centrally suppressed the desire for food consumption and influenced glucose metabolism.4–6 Knock-out OXT- or OXTR-gene mice developed hyperphagia and obesity phenotypes.7,8 In addition, a feedback of oxytocin secretion can be stimulated by changes in glucose metabolism.9 Not only have animal studies shown that obesity is associated with impaired oxytocin release in the brain,10 but also human study has shown decreased circulating levels of oxytocin in obese and newly-diagnosed type 2 diabetes mellitus patients.11 Subsequent studies demonstrated that oxytocin administration intranasally might influence the food reward system, inversely regulate the insulin level and glucose metabolism, and affect food preference.12–14
Moreover, individual oxytocin biological activity can be affected by age, body mass index (BMI), and genetic variations in either the OXT or OXTR gene. OXTR is located on chromosome 3p25 and contains three introns and four exons, and spans 17 kb of DNA. OXTR contains several single nucleotide polymorphisms (SNPs) that have been genotyped in association studies of various traits and behaviors in humans, such as physiological reactivity to stress, amygdala and hypothalamus functioning, and benefit from social support.15,16 Among the OXTR variations, a common OXTR polymorphism, rs53576 (G/A), in intron 3, has been demonstrated to modulate stress-coping and rewarding behaviors.17–19 In addition, our previous study demonstrated that this polymorphism is associated with striatal dopaminergic activity, which is critical for metabolic regulation.20 Although a recent study indicated that genotype frequencies of the OXTR rs53576 polymorphism were associated with susceptibility to type 2 diabetes,21 the contribution of OXTR rs53576 polymorphism to glucose homeostasis in humans is unclear. In the current study, therefore, we aimed to investigate not only the correlation of oxytocin and glucose homeostasis, but also the stratification effect of OXTR polymorphism on the homeostasis in non-diabetic participants.
Methods
Ethic Statement
The research protocol was approved by the Ethical Committee for Human Research at the National Cheng Kung University Hospital, and written informed consent was obtained from each subject before any procedures were performed. This study was conducted in accordance with the Declaration of Helsinki.
Subjects
The non-diabetic participants were enrolled from the community through advertisement and had been recruited.20 Individuals were excluded if they were found to have an endocrine illness, mental illness, or neurological disorder after a diagnostic interview.22 Participants (i) with any acute or unstable medical condition, such as acute coronary syndrome, a recent history of brain hemorrhage, or any admission to hospital in the most recent 3 months; (ii) with a history of head trauma or neurological disease; (iii) using medication with an effect on the central dopamine or serotonin system; (iv) with a history of alcohol abuse or other substance abuse; and (iv) who had previously been diagnosed with type 2 diabetes mellitus or had HbA1c >6% were excluded from our study. Body weight and body high of each subject were measured, and BMI (kg/m2) were calculated accordingly.
Oxytocin Level and OXTR Genotyping
Fasting blood samples were collected from the antecubital vein between 08:00 and 10:00 am in heparinized plain tubes then centrifuged at 2400 ×g for 15 min at 4°C. Plasma was then aspirated and stored at −80°C. The oxytocin immunoreactivity level was quantified in duplicate using a commercial oxytocin ELISA kit (Elisa Kit for oxytocin, USCN Life Science, Houston, TX). The detectable range for this assay was 12.35–1000 pg/mL. The intra-assay coefficient of variation (CV) was 10%, and the inter-assay CV was 12%. The minimum detectable dose of oxytocin was typically less than 4.87 pg/mL. There was no significant cross-reactivity or interference between oxytocin and the analogues observed. We validated the assay by taking a pool of 10 plasma samples from our subjects and spiking it with a series of oxytocin levels in the physiological range (dilutions from 2–50 pg/mL). The assay reported the increments in the spiked plasma samples accurately (R2 = 0.998).
Genomic DNA was extracted from each blood sample using a QIAamp DNA blood kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. The quality of the extracted genomic DNA was checked by agarose gel electrophoresis analysis. The DNA was stored at −80°C until use. The SNP of OXTR rs53576 was analyzed using commercially-available TaqMan SNP Genotyping Assays (Applied Biosystems, Foster City, CA) according to the manufacturer’s instructions, and amplification and dissociation were carried out using an ABI 7900HT Fast Real-Time PCR System (Applied Biosystems). The PCR system automatically calculated the negative derivative of the change in fluorescence. The SNP genotype of each tested sample was determined using the STEPONE software program and confirmed manually. In cases of disagreement, the analysis was repeated.
Fasting Glucose Profile Measurements
The participants were instructed to fast for at least nine hours prior to each examination. Blood samples were collected between 08:00 and 10:00 am. Fasting plasma glucose values were determined using the glucose oxidase method (Synchron CX3, Beckman, Brea, CA). The HbA1c value was measured using the automated boronate affinity high performance liquid chromatography method (CLC385; Primus corp., Kansas City, MO). The fasting serum insulin concentration was measured using a solid-phase radioimmunoassay method (Diagnostic Products Corporation, Los Angeles, CA). The insulin resistance index, which indicated the homeostasis model assessment-estimated insulin resistance (HOMA-IR), was calculated as fasting serum insulin value (μIU/mL) × fasting plasma glucose value (mg/dL)/405.23 The fasting plasma leptin level was measured using an ELISA method (Linco Research, St Louis, MO).
Statistical Analysis
The data were analyzed using SPSS software v17 (SPSS Inc., Chicago, IL). Means and standard deviations (SDs) were calculated for descriptive analysis of the participants’ demographic data and metabolic profiles by the t-test. The literature concerning the SNP that is most studied (rs53576) has usually compared the GG individuals with AG and AA combined. Because A allele carriers have similar social phenotypic presentations of stress,24 and one recent study showed that the base pairing of the AA genotype has a protective role against type 2 diabetes mellitus.21 Thus, carriers with one or two copies of the A allele were combined into a dominant A allele model group to examine the dominant effect of the A allele of the OXTR in the current study. Metabolic profiles were tested for significant differences between genotypes using the Student’s t-test. Pearson’s correlations were used to examine the correlations between oxytocin level and metabolic profile. Partial correlations of adjusting age, sex, and BMI were performed, because oxytocin is an age, gender, and BMI-specific circulating hormone.25,26 The threshold for statistical significance was 0.05.
Results
We recruited 89 participants, the demographic data of whom are shown in Table 1. Participants were on average 32.7 ± 11.9 years of age, and 43.8% were male. The frequencies of the OXTR polymorphisms fitted the Hardy-Weinberg equilibrium (P = 0.98). In addition, the genotypic distribution of the participants (AA: 37.1%; AG: 47.2%; GG: 15.7%) was consistent with that presented in the Ensembl project (http://asia.ensembl.org) for participants of Asian ethnicity (AA: 47.2%, AG: 42.3% and GG: 10.5%). In addition, the demographic characteristics, including the glucose level, insulin indices, leptin level and oxytocin level, were not significantly different between subjects subgrouped by the polymorphisms of OXT (Table 1).
Table 1.
Demographic Data and Fasting Glucose Profile in All of the Subjects
| All of the Subjects | P valuea | P valueb | P valuec | |||
|---|---|---|---|---|---|---|
| ALL (n = 89) | AA+AG (n = 75) | GG (n = 14) | ||||
| Sex (male, %) | 39 (43.8%) | 31 (41.3%) | 8 (57.1%) | 0.27 | – | – |
| Age (years) | 32.7 ± 11.9 | 32.1 ± 11.5 | 36.1 ± 13.7 | 0.25 | – | – |
| BMI (kg/m2) | 22.8 ± 3.7 | 22.6 ± 3.5 | 23.9 ± 4.3 | 0.21 | 0.41 | |
| Fasting glucose (mg/dL) | 88.1 ± 8.1 | 88.2 ± 8.0 | 87.6 ± 8.7 | 0.80 | 0.31 | 0.12 |
| Insulin (µIn/mL) | 6.8 ± 5.5 | 7.0 ± 6.4 | 5.8 ± 4.3 | 0.51 | 0.34 | 0.09 |
| HbA1c (%) | 5.4 ± 0.3 | 5.4 ± 0.3 | 5.5 ± 0.3 | 0.37 | 0.63 | 0.84 |
| HOMA-IR | 1.5 ± 1.4 | 1.5 ± 1.7 | 1.3 ± 0.8 | 0.60 | 0.42 | 0.15 |
| Leptin (ng/mL) | 12.0 ±16.2 | 11.3 ± 16.0 | 15.8 ± 17.3 | 0.34 | 0.62 | 0.76 |
| Oxytocin (pg/mL) | 25.9 ±5.8 | 25.7 ± 5.9 | 27.2 ± 5.7 | 0.39 | 0.61 | 0.51 |
Notes: aUnadjusted model. bAdjusted for age and gender. cAdjusted for age, gender, and BMI.
Abbreviations: BMI, body mass index; HOMA-IR, homeostasis model assessment-estimated insulin resistance.
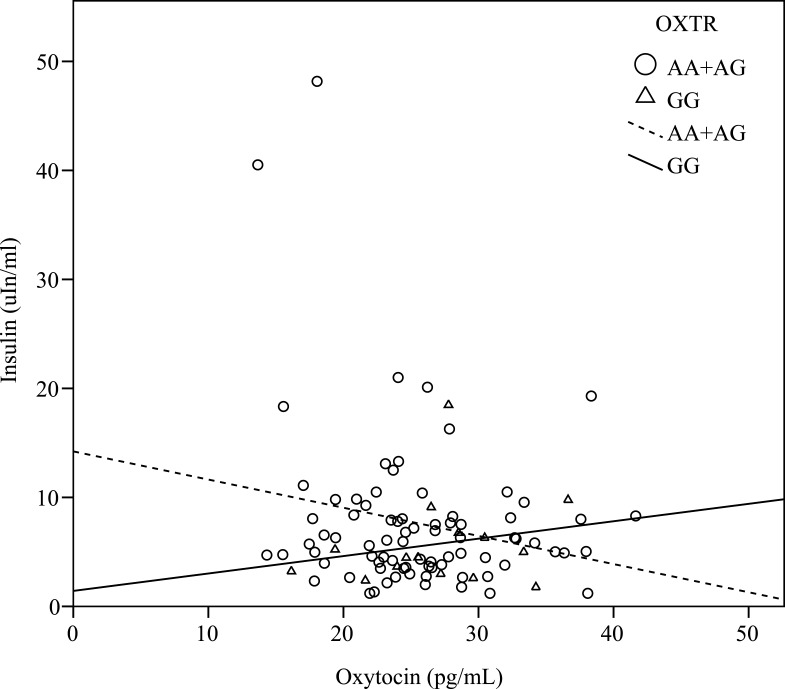
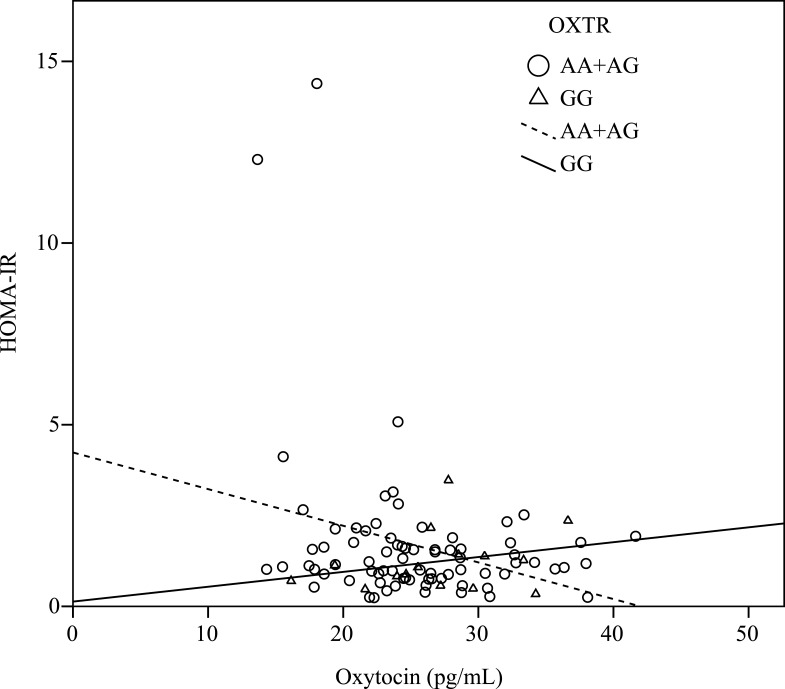
There were significant correlations between the oxytocin level and fasting glucose level (r = –0.29, P < 0.01), insulin level (r = –0.26, P = 0.01), and HOMA-IR (r = –0.25, P = 0.01), when adjusted for age, gender and BMI (Table 2). Furthermore, when considering the stratification effects of the OXTR variation, we found that the oxytocin level was significantly correlated with the fasting glucose level (r = –0.25, P = 0.04), insulin level (r = –0.35, P= 0.03) (Figure 1), and HOMA-IR (r = –0.35, P < 0.01) (Figure 2) in participants with the A allele (n = 75) of OXTR after adjustment for age, gender and BMI. Furthermore, the oxytocin level in those with the GG genotype was significantly negatively correlated with the leptin level (n = 14, r = –0.66, P = 0.02) (Table 2).
Table 2.
Correlation Between Initial Plasma Oxytocin Level and Fasting Glucose Profile Subgrouped by OXTR Variation
| ALL (n = 89) | AA+AG (n = 75) | GG (n = 14) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| r (P)a | r (P)b | r (P)c | r (P)a | r (P)b | r (P)c | r (P)a | r (P)b | r (P)c | |
| Fasting glucose (mg/dL) | –0.10 (0.39) | –0.28 (0.56) | –0.29 (<0.01*) | –0.23 (0.07) | –0.26 (0.07) | –0.25 (0.04*) | –0.16 (0.59) | –0.02 (0.98) | –0.04 (0.89) |
| Insulin (µIn/mL) | –0.13 (0.28) | –0.24 (0.04) | –0.26 (0.01*) | –0.27 (0.03*) | –0.33 (0.03*) | –0.35 (0.03*) | 0.20 (0.49) | 0.15 (0.86) | 0.17 (0.60) |
| HbA1c (%) | –1.5 (0.20) | –0.12 (0.09) | –0.11 (0.30) | –0.18 (0.14) | –0.08 (0.14) | –0.09 (0.71) | –0.01 (0.98) | –0.07 (0.86) | –0.08 (0.83) |
| HOMA-IR | –0.20 (0.09) | –0.20 (0.04) | –0.25 (0.01*) | –0.26 (0.03*) | –0.34 (0.03*) | –0.35 (<0.01*) | 0.26 (0.38) | 0.15 (0.73) | 0.21 (0.52) |
| Leptin (ng/mL) | 0.12 (0.32) | –0.10 (0.97) | –0.09 (0.89) | 0.08 (0.52) | 0.07 (0.52) | 0.08 (0.59) | –0.53 (0.05) | –0.64 (0.02*) | –0.66 (0.02*) |
Notes: *P < 0.05. aUnadjusted model. bAdjusted for age and gender. cAdjusted for age, gender, and BMI.
Abbreviations: HOMA-IR, homeostasis model assessment-estimated insulin resistance; BMI, body mass index.
Figure 1.
Correlation between the fasting level of oxytocin and insulin. The oxytocin level was significantly negatively correlated with insulin level (r = –0.27, P = 0.03) in carriers with the A allele of the OXTR, and the result was consistent even after adjustment for age, gender, and BMI (r = –0.35, P = 0.03). However, there was positively correlated in carriers with the GG genotype.
Figure 2.
Correlation between the level of oxytocin and the homeostasis model assessment-estimated insulin resistance (HOMA-IR). The oxytocin level was significantly negatively correlated with HOMA-IR (r = –0.26, P = 0.03) in carriers with the A allele of the OXTR, and the result was consistent even after adjustment for age, gender, and BMI (r = –0.35, P < 0.01). However, there was positively correlated in carriers with the GG genotype.
Discussion
The results of this study were the first to show a significant stratification effect of OXTR variation on the correlations of oxytocin and glucose homeostasis in a human study, while there were significant correlations between the oxytocin level and fasting glucose profiles in our study and recent reports.9,10 Furthermore, the directionality of this association in A allele carriers differed from that in GG carriers. In recent years, oxytocin has been suggested to be a potential therapeutic target against obesity; however, divergent effects on adiposity and diabetes have been observed. The results of the current study suggested that identification of the OXTR genotype would be beneficial in terms of oxytocin treatment and gaining an understanding of the human pathology of obesity.
The direct causative role of OXTR rs53576 polymorphism, involving an adenine (A)/guanine (G) transition in intron 3, on oxytocin receptors have not been identified and currently difficult to examine in vivo in humans.27 Although oxytocin levels were not different between OXTR rs53576 variant groups, however, an increasing body of research suggests that variation of OXTR rs53576 for key players involved in oxytocin signaling substantially contribute to individual differences in empathy, reflection of sensitivity to familial environment, and mental health issues.17–19,28 In addition, studies have demonstrated that the OXTR rs53576 variations are associated with the amygdala volumes and with the amount of oxytocin receptors in the amygdala, consequently affecting amygdala function and anxiety-related trait.27,29 Moreover, recent studies indicated that G carriers are more sensitive to the surroundings, both in favorable or negative contexts, and this elevated susceptibility to the environment may affect neural plasticity in mechanisms involving the oxytocin system.24,27,28 These individuals with GG genotype exhibited altered blood pressure and cortisol levels following rejection, effects not apparent among A carriers.24 Thus, evidence from previous reports and the current study suggest that the polymorphism of OXTR rs53576 could influence glucose homeostasis and be associated with susceptibility to type 2 diabetes in humans through the regulation stress and HPA axis.21,30 Subjects with the GG genotype of the OXTR rs53576 polymorphism may be not beneficial to glucose homeostasis compared with A allele carriers.
The stratification effect on energy homeostasis might arise from both central and peripheral oxytocin action. Centrally, oxytocin could affect the emotional response to stress that determines the blood glucose level in nondiabetic subjects.31–33 Moreover, previous reports also showed that A allele carriers of polymorphism of OXTR rs53576 are more resilient and present lower physiological reactions under stress.24 Peripherally, oxytocin administration improved glucose intolerance in a mouse model,34 and oxytocin administration has an acute glucose regulatory effect in healthy subjects.35 Although the central–peripheral oxytocinergic effects on energy homeostasis are still unclear, the polymorphism of OXTR could stratify glucose homeostasis. The results of the current study suggested that the peripheral oxytocin–glucose association in terms of fasting glucose level, insulin level and HOMA-IR, was stratified by the polymorphism of OXTR rs53576 in non-diabetic subjects. Interestingly, a recent study showed that subjects with the A allele of the OXTR rs53576 polymorphism may be protected against diabetes mellitus formation.21,30 Thus, additional research is needed to elucidate the function of OXTR in the physiological reaction to stress and to clarify the role of OXTR in glucose homeostasis.
Oxytocin might play an important role in terms of interacting with leptin and insulin for metabolic control.36,37 The adipokine leptin is another energy-regulated neuropeptide that impacts hypothalamic insulin signaling.38 Interestingly, evidence suggests that oxytocin interacts with leptin centrally to inhibit food intake.39 Animal studies have demonstrated that oxytocin deficiency may lead to hyperleptinemia and metabolic syndrome,40 while direct central or peripheral oxytocin administration could decrease fat mass, demonstrating an effect of oxytocin on leptin deficiency or resistance.41 An obese rodent study also showed that direct central infusion of leptin affected OXT gene expression and mediated body weight.36 Moreover, our study here demonstrated the different directionalities of the correlations of the oxytocin and leptin level in individuals with the A allele and those with the GG genotype of OXTR, although the causal relationship and the mechanism should be confirmed in further study.
Limitation
There were several limitations to our study. First, small sample sizes weakened the reliability of the results. Second, further challenge tests to validate the associations and test whether the associations could be reversed were lacking. Third, we did not collect factors, such as diet and exercise, that may influence glucose metabolism.17,42 We analyzed the correlation between oxytocin level and fasting glucose profile adjusted for age, gender, and BMI. The value of BMI is not only associated with fat deposition and pro-inflammatory cytokine production, but also as an important role represented for evaluation of diet intake and physical activity.43 In addition, we have excluded those who previously been diagnosed with type 2 diabetes mellitus or had HbA1c >6% to exclude other possible confounding factors. Finally, we did not test the functional assessment of the OXTR polymorphism on glucose homeostasis. Therefore, the findings may be considered exploratory, further larger sample sizes should be employed to confirm our results.
Conclusion
In conclusion, our study demonstrated significant correlations between oxytocin and glucose metabolism in non-diabetic participants. We also identified a significant stratification effect of OXTR polymorphism on glucose homeostasis. The results suggested that the polymorphism of OXTR might play an important role in individual differences in the biological oxytocin effect on glucose homeostasis.
Acknowledgments
This study was financially supported by the National Science Council of Taiwan (MOST 103-2320-B-006 -013, MOST 104-2320-B-006-024, MOST 105-2320-B-006-014, MOST 105-2321-B-006-020, MOST 106-2320-B-006-040, MOST 107-2320-B-006-071, MOST 108-2320-B-006-004, and MOST 108-2320-B-006-047-MY3). This research also received funding (NCKUH-10301003, NCKUH-10509004, and NCKUH-10703057) from the National Cheng Kung University Hospital. The authors thank Mr. Chien Ting Lin for his administrative support.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Arora S. Role of neuropeptides in appetite regulation and obesity–a review. Neuropeptides. 2006;40:375–401. doi: 10.1016/j.npep.2006.07.001 [DOI] [PubMed] [Google Scholar]
- 2.Olszewski PK, Klockars A, Olszewska AM, Fredriksson R, Schioth HB, Levine AS. Molecular, immunohistochemical, and pharmacological evidence of oxytocin’s role as inhibitor of carbohydrate but not fat intake. Endocrinology. 2010;151:4736–4744. doi: 10.1210/en.2010-0151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chaves VE, Tilelli CQ, Brito NA, Brito MN. Role of oxytocin in energy metabolism. Peptides. 2013;45:9–14. doi: 10.1016/j.peptides.2013.04.010 [DOI] [PubMed] [Google Scholar]
- 4.Arletti R, Benelli A, Bertolini A. Influence of oxytocin on feeding behavior in the rat. Peptides. 1989;10:89–93. doi: 10.1016/0196-9781(89)90082-X [DOI] [PubMed] [Google Scholar]
- 5.Agius M, Rogers J, Bongards E, O’Connor S, Verdolini N, Elisei S. Assessing and staging bipolar disorder. Br J Psychiatry. 2014;204:493–494. doi: 10.1192/bjp.204.6.493a [DOI] [PubMed] [Google Scholar]
- 6.Roh SG, Koiwa K, Sato K, Ohtani Y, Takahashi T, Katoh K. Actions of intravenous injections of AVP and oxytocin on plasma ACTH, GH, insulin and glucagon concentrations in goats. Anim Sci J. 2014;85:286–292. doi: 10.1111/asj.12142 [DOI] [PubMed] [Google Scholar]
- 7.Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9 [DOI] [PubMed] [Google Scholar]
- 8.Kasahara Y, Sato K, Takayanagi Y, et al. Oxytocin receptor in the hypothalamus is sufficient to rescue normal thermoregulatory function in male oxytocin receptor knockout mice. Endocrinology. 2013;154:4305–4315. doi: 10.1210/en.2012-2206 [DOI] [PubMed] [Google Scholar]
- 9.Altszuler N, Fuchs AR. Oxytocin secretion is stimulated by changes in glucose metabolism. Proc Soc Exp Biol Med. 1994;207:38–42. doi: 10.3181/00379727-207-43788 [DOI] [PubMed] [Google Scholar]
- 10.Zhang G, Bai H, Zhang H, et al. Neuropeptide exocytosis involving synaptotagmin-4 and oxytocin in hypothalamic programming of body weight and energy balance. Neuron. 2011;69:523–535. doi: 10.1016/j.neuron.2010.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qian W, Zhu T, Tang B, et al. Decreased circulating levels of oxytocin in obesity and newly diagnosed type 2 diabetic patients. J Clin Endocrinol Metab. 2014;99:4683–4689. doi: 10.1210/jc.2014-2206 [DOI] [PubMed] [Google Scholar]
- 12.Leng G, Sabatier N. Oxytocin - the sweet hormone? Trends Endocrinol Metab. 2017;28:365–376. doi: 10.1016/j.tem.2017.02.007 [DOI] [PubMed] [Google Scholar]
- 13.Ott V, Finlayson G, Lehnert H, et al. Oxytocin reduces reward-driven food intake in humans. Diabetes. 2013;62:3418–3425. doi: 10.2337/db13-0663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker JA, Bloom SR. Hypothalamic neuropeptides and the regulation of appetite. Neuropharmacology. 2012;63:18–30. doi: 10.1016/j.neuropharm.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 15.Myers AJ, Williams L, Gatt JM, et al. Variation in the oxytocin receptor gene is associated with increased risk for anxiety, stress and depression in individuals with a history of exposure to early life stress. J Psychiatr Res. 2014;59:93–100. doi: 10.1016/j.jpsychires.2014.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.LoParo D, Waldman ID. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: a meta-analysis. Mol Psychiatry. 2015;20:640–646. doi: 10.1038/mp.2014.77 [DOI] [PubMed] [Google Scholar]
- 17.Olff M, Frijling JL, Kubzansky LD, et al. The role of oxytocin in social bonding, stress regulation and mental health: an update on the moderating effects of context and interindividual differences. Psychoneuroendocrinology. 2013;38:1883–1894. doi: 10.1016/j.psyneuen.2013.06.019 [DOI] [PubMed] [Google Scholar]
- 18.Saphire-Bernstein S, Way BM, Kim HS, Sherman DK, Taylor SE. Oxytocin receptor gene (OXTR) is related to psychological resources. Proc Natl Acad Sci U S A. 2011;108:15118–15122. doi: 10.1073/pnas.1113137108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thompson SM, Hammen C, Starr LR, Najman JM. Oxytocin receptor gene polymorphism (rs53576) moderates the intergenerational transmission of depression. Psychoneuroendocrinology. 2014;43:11–19. doi: 10.1016/j.psyneuen.2014.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang WH, Lee IH, Chen KC, et al. Oxytocin receptor gene rs53576 polymorphism modulates oxytocin-dopamine interaction and neuroticism traits–a SPECT study. Psychoneuroendocrinology. 2014;47:212–220. doi: 10.1016/j.psyneuen.2014.05.020 [DOI] [PubMed] [Google Scholar]
- 21.Saravani R, Esmaeeli E, Kordi Tamendani M, Nejad MN. Oxytocin receptor gene polymorphisms in patients with diabetes. Gene Cell Tissue. 2015;2:e60171. doi: 10.17795/gct-27904 [DOI] [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The mini-international neuropsychiatric interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(Suppl 20):22–33. [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883 [DOI] [PubMed] [Google Scholar]
- 24.McQuaid RJ, McInnis OA, Matheson K, Anisman H. Distress of ostracism: oxytocin receptor gene polymorphism confers sensitivity to social exclusion. Soc Cogn Affect Neurosci. 2015;10:1153–1159. doi: 10.1093/scan/nsu166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elabd C, Cousin W, Upadhyayula P, et al. Oxytocin is an age-specific circulating hormone that is necessary for muscle maintenance and regeneration. Nat Commun. 2014;5:4082. doi: 10.1038/ncomms5082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rilling JK, Demarco AC, Hackett PD, et al. Sex differences in the neural and behavioral response to intranasal oxytocin and vasopressin during human social interaction. Psychoneuroendocrinology. 2014;39:237–248. doi: 10.1016/j.psyneuen.2013.09.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishina K, Takagishi H, Fermin ASR, et al. Association of the oxytocin receptor gene with attitudinal trust: role of amygdala volume. Soc Cogn Affect Neurosci. 2018;13:1091–1097. doi: 10.1093/scan/nsy075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Flasbeck V, Moser D, Kumsta R, Brüne M. The OXTR single-nucleotide polymorphism rs53576 moderates the impact of childhood maltreatment on empathy for social pain in female participants: evidence for differential susceptibility. Front Psychiatry. 2018;9:359. doi: 10.3389/fpsyt.2018.00359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J, Qin W, Liu B, et al. Neural mechanisms of oxytocin receptor gene mediating anxiety-related temperament. Brain Struct Funct. 2014;219:1543–1554. doi: 10.1007/s00429-013-0584-9 [DOI] [PubMed] [Google Scholar]
- 30.Aschbacher K, Kornfeld S, Picard M, et al. Chronic stress increases vulnerability to diet-related abdominal fat, oxidative stress, and metabolic risk. Psychoneuroendocrinology. 2014;46:14–22. doi: 10.1016/j.psyneuen.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Faulenbach M, Uthoff H, Schwegler K, Spinas GA, Schmid C, Wiesli P. Effect of psychological stress on glucose control in patients with type 2 diabetes. Diabet Med. 2012;29:128–131. doi: 10.1111/j.1464-5491.2011.03431.x [DOI] [PubMed] [Google Scholar]
- 32.Chiodera P, Volpi R, Capretti L, Cataldo S, Speroni G, Coiro V. Effect of systemic oxytocin administration on dexamethasone-induced leptin secretion in normal and obese men. J Clin Endocrinol Metab. 2000;85:3683–3686. doi: 10.1210/jcem.85.10.6890 [DOI] [PubMed] [Google Scholar]
- 33.Wing RR, Epstein LH, Blair E, Nowalk MP. Psychologic stress and blood glucose levels in nondiabetic subjects. Psychosom Med. 1985;47:558–564. doi: 10.1097/00006842-198511000-00005 [DOI] [PubMed] [Google Scholar]
- 34.Maejima Y, Iwasaki Y, Yamahara Y, Kodaira M, Sedbazar U, Yada T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging (Albany NY). 2011;3:1169–1177. doi: 10.18632/aging.100408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klement J, Ott V, Rapp K, et al. Oxytocin improves beta-cell responsivity and glucose tolerance in healthy men. Diabetes. 2017;66:264–271. doi: 10.2337/db16-0569 [DOI] [PubMed] [Google Scholar]
- 36.Perello M, Raingo J, Castro MG. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. PLoS One. 2013;8:e59625. doi: 10.1371/journal.pone.0059625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gao ZY, Drews G, Henquin JC. Mechanisms of the stimulation of insulin release by oxytocin in normal mouse islets. Biochem J. 1991;276(Pt 1):169–174. doi: 10.1042/bj2760169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kleinridders A, Lauritzen HP, Ussar S, et al. Leptin regulation of Hsp60 impacts hypothalamic insulin signaling. J Clin Invest. 2013;123:4667–4680. doi: 10.1172/JCI67615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Blevins JE, Schwartz MW, Baskin DG. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am J Physiol Regul Integr Comp Physiol. 2004;287:R87–R96. doi: 10.1152/ajpregu.00604.2003 [DOI] [PubMed] [Google Scholar]
- 40.Camerino C. Low sympathetic tone and obese phenotype in oxytocin-deficient mice. Obesity (Silver Spring). 2009;17:980–984. doi: 10.1038/oby.2009.12 [DOI] [PubMed] [Google Scholar]
- 41.Altirriba J, Poher AL, Rohner-Jeanrenaud F. Chronic oxytocin administration as a treatment against impaired leptin signaling or leptin resistance in obesity. Front Endocrinol (Lausanne). 2015;6:119. doi: 10.3389/fendo.2015.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rich-Edwards JW, Spiegelman D, Lividoti Hibert EN, et al. Abuse in childhood and adolescence as a predictor of type 2 diabetes in adult women. Am J Prev Med. 2010;39:529–536. doi: 10.1016/j.amepre.2010.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tchernof A, Després J-P. Pathophysiology of human visceral obesity: an update. Physiol Rev. 2013;93:359–404. doi: 10.1152/physrev.00033.2011 [DOI] [PubMed] [Google Scholar]