Abstract
Background and Aim
The relationship between subclinical hypothyroidism (SHYPO) and sleep quality is still unclear. Our objective was to compare the sleep quality between SHYPO patients and a control group with normal thyroid function.
Methods
A total of 2224 patients with SHYPO and 12,622 euthyroid (EUTH) control group patients were included in the present study. The Pittsburgh Sleep Quality Index (PSQI) was used to assess sleep quality. The sleep outcomes were compared to explore the association between SHYPO and sleep quality. Furthermore, we tried to identify the risk factors of poor sleep in SHYPO patients.
Results
Compared to the EUTH control patients, SHYPO patients had a higher PSQI score (6.83 ± 2.67 vs 6.64 ± 2.63, p =0.004) and a higher proportion of poor sleepers (67.09% vs 64.75%, p =0.033). Moreover, subjects with SHYPO were associated with poorer sleep (Odd Ratio (OR) 1.120, 95% Confidence Intervals (CI) 1.016 to 1.235, p =0.023), longer sleep latency (OR 1.162, 95% CI 1.053 to 1.282, p =0.003), and shorter sleep duration (OR 1.148, 95% CI 1.019 to 1.293, p =0.023) after adjusting for potential confounders. Furthermore, we found that lower age, lower body mass index, and women were risk factors for poor sleep quality in SHYPO patients.
Conclusion
Our findings suggest a relationship between SHYPO and poor sleep quality in a large Chinese population.
Keywords: subclinical hypothyroidism, sleep quality, PSQI
Introduction
Subclinical hypothyroidism (SHYPO) is defined as elevated levels of thyroid-stimulating hormone (TSH) with normal levels of free triiodothyronine (fT3) and free thyroxine (fT4).1 It is a common disorder with a high prevalence rate of up to 18% in the general population, especially among elderly people and women.2,3 SHYPO has been associated with an increased risk of adverse cardiovascular events.4,5 In addition, SHYPO may include nonspecific symptoms, including fatigue, depression, weakness, and cognitive impairment.6,7
Sleep disturbances have become serious public health problems that are frequently found in the general population.8,9 It has a negative impact on quality of life and health behaviors.8 Thyroid disorders are often related to sleep problems.10–12 Several endocrine functions are altered during the process from awake to sleep. One of the most conspicuous endocrine alterations is the activity of the hypothalamic–pituitary–thyroid axis.13 For example, when an individual is experiencing sleep curtailment, an increase in TSH levels released by the hypothalamus corresponds to an increase in thyroid hormone levels has been detected.14 However, the association between SHYPO and sleep quality is still not clear, with limited evidence. Haruko Akatsu et al explored the relationship between thyroid function and sleep quality in older men, and no significant association was found.15 However, all the individuals included in this study were men over the age of 65 years. Another study by Benedetta Demartini et al also found no significant difference in sleep disturbances between SHYPO patients and healthy control group patients.16 However, only 246 participants were included in that study, and no specific sleep scales were used.
Therefore, a study based on the general population is essential. In the present study, we investigated the relationship between SHYPO and sleep quality with a total of 14,846 individuals.
Methods
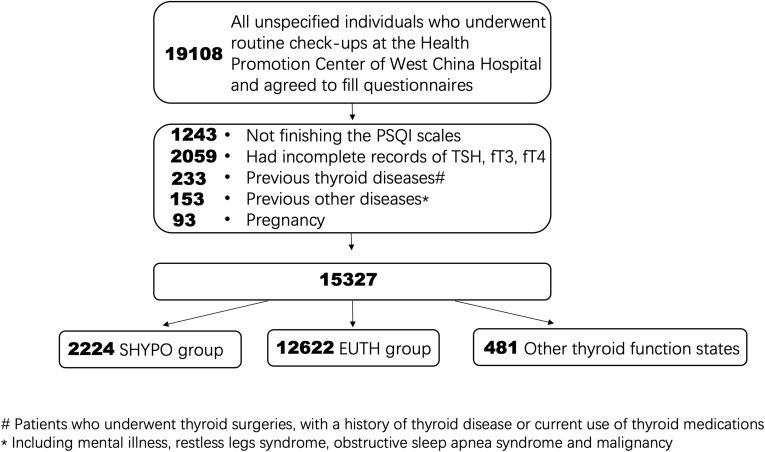
The Health Promotion Center of West China Hospital is an institute providing physical examination for any social individuals or groups. From October 2014 to October 2018, all unspecified individuals who underwent routine check-ups at the Health Promotion Center of West China Hospital were indiscriminately invited to complete the Pittsburgh sleep quality index (PSQI) questionnaire. There were 19,108 individuals willing to fill in the questionnaire (Figure 1). After written informed consent were read and signed up, participants were leaded to a separate comfortable place and completed questionnaires. Sleep quality of participants were obtained by the scores of PSQI questionnaire (one week version). PSQI were developed in 1988 by Buyse et al17 and was confirmed to have good reliability and validity in clinical and non-clinical settings.18 The PSQI contains 19 items that can be grouped into 7 components: a. subjective sleep quality; b. sleep latency; c. sleep duration; d. sleep efficiency; e. sleep disturbance; f. sleep medication use; and g. daytime dysfunction and sleepiness. Each component is weighted equally ranging from 0 (no difficulty) to 3 (severe difficulty); thus, the total score is 0 to 21. A cut-off point of a total score greater than 5 was used to define poor sleepers.17,18
Figure 1.
Study design. There were 19,108 individuals who underwent routine check-ups at our center willing to fill the PSQI questionnaires. After exclusion and grouping, subjects with euthyrid function (n=12,622) and subclinical hypothyroidism (n=2224) were included in this study.
The laboratory normal reference ranges used in our hospital were as follows: thyroid stimulating hormone (TSH), 0.27–4.2 mU/L; free thyroxin (fT4), 12–22 pmol/L; and free triiodothyronine (fT3), 3.6–7.5 pmol/L. The study protocol was approved by the Institutional Review Board of West China Hospital of Sichuan University. All participants provided written informed consent. Individuals with the values of TSH, fT3 and fT4 within the normal reference range would be gathered in EUTH group. People with TSH over 4.2 mU/L but normal fT3 and fT4 values were collected into SHYPO group.1
The exclusion criteria were listed as follow: Participants who did not finish the PSQI scales; had incomplete records of TSH, fT4 and fT3; underwent thyroid surgeries, with a history of thyroid disease or current use of thyroid medications (for the thyroid hormone values are under human intervention); previous diagnoses of mental illness, restless legs syndrome, obstructive sleep apnea syndrome and malignancy (for the clear relationship existed with lower sleep quality19–21); and pregnancy for the different hormone levels. Eventually, 14,846 individuals aging from 16 to 77 years old were included in the study. Two groups were included in the present study: subjects with euthyrid function (EUTH) group SHYPO group (Figure 1).
This study was approved by the Clinical Research Ethics Committee of Sichuan University West China Hospital. The study complied with the standards set by the Declaration of Helsinki.
Statistical Analysis
Continuous variables were compared using the t-tests for normally distributed data and the Mann–Whitney U-test for skewed data. Categorical variables were compared using chi-square analyses. Logistic regressions were used to assess the relationship between sleep outcome and SHYPO. The sleep measures were classified as follows: PSQI score (>5 vs ≤5), sleep latency (>30 min vs ≤30 min), sleep duration (≥5 h vs <5 h), sleep efficiency (≥85% vs <85%), and sleep medication (use vs nonuse). We further generated two adjusted models for the correlation analyses. Baseline variables that were found clinically relevant or that showed univariate relationships with SHYPO were included in adjusted models.13,22 Model 1 was adjusted for gender, age, and BMI. Model 2 was adjusted for gender, age, BMI, hypertension, diabetes, alcohol consumption, and smoking. Then, in the patients with SHYPO, logistic regressions were conducted to explore the association between sleep quality (PSQI score >5 vs ≤5) and potential risk factors. Odds ratios (ORs) with 95% confidence intervals (95%13 CIs) were calculated to estimate the relative risks. A P <0.05 was considered statistically significant. SPSS software (SPSS 22; SPSS Inc., Chicago, IL, USA) was used for all statistical analyses.
Result
Eventually, 2224 SHYPO patients and 12,622 EUTH control group patients were included in the present study. The demographic and clinical characteristics of the participants are shown in Table 1. Overall, of the 14,846 anticipants, 9665 (65.10%) had poor sleep quality. Importantly, the proportion of poor sleepers was higher in the SHYPO group (67.09%) than in the EUTH control group (64.75%). Furthermore, the average PSQI score was also higher in the SHYPO patients than in the control group patients (6.83 ± 2.67 vs 6.64 ± 2.63, p =0.004). When we compared the components of the PSQI score, the SHYPO patients had a lower subjective sleep quality (p =0.023), longer sleep latency (p <0.001), and increased sleep disturbance (p =0.001). In addition, the scores of sleep duration, sleep efficiency, and sleep medication use were also higher in the SHYPO group, but without significance. Surprisingly, the SHYPO patients had less daytime dysfunction and sleepiness than the control group patients (p =0.020). In addition, both age and sex differed significantly between the two groups. The SHYPO group had a higher average age and female population than the control group (both p <0.001). Correspondingly, the percentage of smokers and drinkers in the SHYPO group was also smaller than the control group (both p <0.001). The SHYPO group had higher TSH, and thus lower FT3 and FT4 (all p <0.001).
Table 1.
Comparison of Characteristics Between Subclinical Hypothyroidism and Euthyroid Individuals
| EUTH (n=12,622) | SHYPO (n=2224) | P | |
|---|---|---|---|
| Age(years) | 42.95 ± 8.85 | 43.77 ± 9.48 | |
| Gender | |||
| Male | 7573 | 994 | <0.001* |
| Female | 5049 | 1230 | <0.001* |
| BMI(kg/m2) | 23.83 ± 3.31 | 23.69 ± 3.37 | 0.074 |
| Hypertension | |||
| Yes | 891 | 169 | 0.362 |
| No | 11,731 | 2055 | |
| Diabetes | |||
| Yes | 380 | 49 | 0.036 |
| No | 12,242 | 2175 | |
| Alcohol consumption | |||
| Yes | 6409 | 879 | <0.001* |
| No | 6213 | 1345 | |
| Smoking | |||
| Yes | 3645 | 366 | <0.001* |
| No | 8977 | 1858 | |
| TSH (mU/L) | 2.31 ± 0.86 | 6.02 ± 2.68 | <0.001* |
| fT3 (pmol/L) | 5.02 ± 0.60 | 4.92 ± 0.60 | <0.001* |
| fT4 (pmol/L) | 16.71 ± 2.02 | 15.92 ± 2.01 | <0.001* |
| Poor sleeper (PSQI score>5) | |||
| Yes | 8173 | 1492 | 0.033* |
| No | 4449 | 732 | |
| PSQI score | 6.64 ± 2.63 | 6.83 ± 2.67 | 0.004* |
| A | 1.08 ± 0.80 | 1.12 ± 0.80 | 0.023* |
| B | 1.28 ± 0.94 | 1.36 ± 0.94 | <0.001* |
| C | 1.63 ±0.89 | 1.65 ± 0.90 | 0.341 |
| D | 0.35 ± 0.71 | 0.37 ± 0.73 | 0.093 |
| E | 0.88 ± 0.55 | 0.93 ± 0.56 | 0.001* |
| F | 0.10 ± 0.47 | 0.12 ± 0.52 | 0.064 |
| G | 1.35 ± 0.94 | 1.30 ± 0.93 | 0.020* |
Note: *Represents P<0.05.
Abbreviations: BMI, Body Mass Index; A, subjective sleep quality; B, sleep latency; C, sleep duration; D, sleep efficiency; E, sleep disturbance; F, sleep medication use; G, daytime dysfunction and sleepiness.
The regression analyses were then performed to determine the associations between SHYPO and poor sleep, as shown in Table 2. Compared with the EUTH group, the SHYPO group was associated with poorer sleep (OR 1.110, 95% CI 1.008 to 1.221, p =0.033) and longer sleep latency (OR 1.143, 95% CI 1.037 to 1.259, p =0.007) in the unadjusted analyses. Moreover, after adjusting for potential confounders in different models, SHYPO was still significantly associated with a higher PSQI score and longer sleep latency. Subjects with SHYPO were more likely to have a sleep duration of <5 h when using adjusted regression models (both OR 1.148, 95% CI 1.019 to 1.293, p =0.023). In addition, SHYPO was significantly associated with the use of sleep medication in the unadjusted analyses (OR 1.224, 95% CI 1.018 to 1.473, p =0.032); however, no significance remained after adjusting for covariates. In terms of sleep efficiency, no interaction with SHYPO was found in either the unadjusted or adjusted analyses.
Table 2.
Associations of Subclinical Hypothyroidism with Poor Sleep Indicators
| Sleep Measures | EUTH (n=12,622) | SHYPO (n=2224) | P |
|---|---|---|---|
| Poor sleeper (PSQI score >5) | |||
| Unadjusted | Ref. | 1.110 (1.008, 1.221) | 0.033* |
| Model 1 | Ref. | 1.107 (1.004, 1.220) | 0.041* |
| Model 2 | Ref. | 1.120 (1.016, 1.235) | 0.023* |
| Sleep latency >30 min | |||
| Unadjusted | Ref. | 1.143 (1.037, 1.259) | 0.007* |
| Model 1 | Ref. | 1.129 (1.024, 1.245) | 0.015* |
| Model 2 | Ref. | 1.162 (1.053, 1.282) | 0.003* |
| Sleep duration <5h | |||
| Unadjusted | Ref. | 1.116 (0.993, 1.255) | 0.066 |
| Model 1 | Ref. | 1.148 (1.019, 1.293) | 0.023* |
| Model 2 | Ref. | 1.148 (1.019, 1.293) | 0.023* |
| Sleep efficiency <85% | |||
| Unadjusted | Ref. | 1.105 (0.996, 1.226) | 0.060 |
| Model 1 | Ref. | 1.053 (0.947, 1.170) | 0.341 |
| Model 2 | Ref. | 1.051 (0.946, 1.168) | 0.356 |
| Use of sleep medication | |||
| Unadjusted | Ref. | 1.224 (1.018, 1.473) | 0.032* |
| Model 1 | Ref. | 1.052 (0.871, 1.271) | 0.596 |
| Model 2 | Ref. | 1.055 (0.873, 1.276) | 0.577 |
Notes: Model 1 was adjusted for gender, age, and BMI. Model 2 was adjusted for gender, age, BMI, hypertension, diabetes, alcohol consumption, and smoking. *Represents P<0.05.
Then, we tried to explore the possible risk factors of poor sleep quality in patients with SHYPO. As shown in Table 3, the risk of poor sleep quality decreased as age (OR 0.984, 95% CI 0.975 to 0.993, p =0.001) and BMI (OR 0.958, 95% CI 0.933 to 0.983, p =0.001) increased. In addition, in the SHYPO group, the female patients (OR 1.278, 95% CI 1.070 to 1.526, p =0.007) were significantly associated with a higher risk of poor sleep. Surprisingly, no association was found between TSH (OR 0.985, 95% CI 0.954 to 1.017, p =0. 359) and poor sleep quality in the SHYPO subjects.
Table 3.
Associations of Thyroid Functions and Other Clinical Characteristics of Poor Sleep in Subclinical Hypothyroidism Patients
| Sleep Measure | Regression Coefficient (B) | OR (95% Confidence Interval) | P |
|---|---|---|---|
| TSH | −0.015 | 0.985 (0.954, 1.017) | 0.359 |
| Age | –0.016 | 0.984 (0.975, 0.993) | 0.001* |
| BMI | −0.043 | 0.958 (0.933, 0.983) | 0.001* |
| Gender Male Female |
0.245 |
Ref. 1.278 (1.070, 1.526) |
0.007* |
| Hypertension No Yes |
−0.125 |
Ref. 0.883 (0.636, 1.226) |
0.456 |
| Diabetes No Yes |
−0.261 |
Ref. 0.770 (0.430, 1.377) |
0.379 |
| Alcohol consumption No Yes |
−0.031 |
Ref. 0.969 (0.809, 1.161) |
0.733 |
| Smoking No Yes |
0.127 |
Ref. 1.136 (0.891, 1.447) |
0.303 |
Note: *Represents P<0.05.
Discussion
To the best of our knowledge, this is the first study to investigate the relationship between SHYPO and sleep quality based on a large general population. We found that, when compared to EUTH, SHYPO was significantly associated with an increased risk of poor sleep quality in the Chinese population. Specifically, SHYPO was related to longer sleep latency, shorter sleep duration, and increased sleep disturbance. We also found a significant association between lower age, lower BMI, and women with poor sleep quality in SHYPO patients.
In the present study, not only the SHYPO patients had significantly higher PSQI scores and a higher proportion of poor sleepers, even the EUTH group, individuals with PSQI scores greater than 5 had higher TSH levels than those with good sleep. A previous study by Haruko Akatsu et al also found a similar trend when comparing EUTH (n =629) and SHYPO (n =38). The SHYPO group also had higher PSQI scores (6.0 ± 3.6 vs 5.6 ± 3.2), and a higher proportion of poor sleepers (50.0% vs 46.1%) than the EUTH group.15 However, due to the relatively small number of participants, there was no significant difference. Another investigation by Benedetta Demartini et al found no significant difference in sleep disturbances between the EUTH and SHYPO subjects.16 However, 117 of the 123 (95.1%) SHYPO patients included had levothyroxine treatment, which may have some effect on their sleep. In addition, the scales they used in the study were not specialized for sleep surveys. The relationship between SHYPO and sleep quality may be explained by the inhibitory effect of sleep on TSH secretion.13 Sleep loss in humans can affect the function of the human hypothalamo-pituitary-thyroid axis and is associated with increased TSH.23,24 The direct relationship between TSH and sleep is still under researching. However, there are two hypothesizes to explain the phenomenon of increasing TSH value with low sleep qualities. On the one hand, when a subject is about to sleep, increasing activity of the hypothalamic–pituitary–thyroid axis is the most conspicuous endocrine changes that occur under the physiological conditions.13 When the hypothalamus increases TSH release, the sympathetic nervous system is activated meanwhile and directly stimulates the thyroid gland,25 which may cause the sleep disorders under some pathological condition. On the other hand, it is reported that there are two different TSH forms, free TSH and macro TSH, existed in the human body. Different with the free TSH, macro TSH derived from the pars tuberalis, and is under the control of the suprachiasmatic nucleus, which is known to regulate sleep behavior. An increase in serum macro TSH is associated with low sleep quality and regulated in a manner distinct from free TSH, potentially due to an altered glycosylation structure.26
Compared to EUTH controls, the SHYPO individuals were older and had a higher proportion of women. These results are consistent with previous findings.27 Since there was a higher proportion of women, it was not surprising that there were fewer smokers and drinkers and a lower BMI in the SHYPO group. These confounders, along with hypertension and diabetes, were adjusted in the regression models to minimize the interference in assessing the relationship between SHYPO and sleep quality. Furthermore, for the first time, we found that youth, thinness, and women were risk factors for poor sleep quality in patients with SHYPO.
There are still some limitations in our study. First, due to its cross-sectional design, the present study could not show a causal relationship between SHYPO and sleep quality even after adjustment. In addition, the PSQI used in our study is a self-report scale, although it has been proven to have good reliability and validity. More objective measures, such as wrist actigraphy and polysomnography, can be used for further investigation.
In conclusion, we found a significant correlation between SHYPO and poor sleep quality in a large Chinese population. Furthermore, youth, thinness, and women with SHYPO are more likely to suffer from poor sleep.
Acknowledgment
This study was supported by grants from the National Key R&D Program of China (2017YF0907504).
Disclosure
The authors report no conflicts on interest in this work.
References
- 1.Peeters RP, Solomon CG. Subclinical hypothyroidism. N Engl J Med. 2017;376(26):2556–2565. doi: 10.1056/NEJMcp1611144 [DOI] [PubMed] [Google Scholar]
- 2.Biondi B, Cappola AR, Cooper DS. Subclinical hypothyroidism: a review. JAMA. 2019;322(2):153–160. doi: 10.1001/jama.2019.9052 [DOI] [PubMed] [Google Scholar]
- 3.Canaris GJ, Manowitz NR, Mayor G, et al. The colorado thyroid disease prevalence study. Arch Intern Med. 2000;160(4):526–534. doi: 10.1001/archinte.160.4.526 [DOI] [PubMed] [Google Scholar]
- 4.Nicolas R, Den Elzen, WP, Bauer DC, et al. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA. 2010;304(12):1365–1374. doi: 10.1001/jama.2010.1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss IA, Bloomgarden N, Frishman WH. Subclinical hypothyroidism and cardiovascular risk: recommendations for treatment. Cardiol Rev. 2011;19(6):291–299. doi: 10.1097/CRD.0b013e318227df87 [DOI] [PubMed] [Google Scholar]
- 6.Salman R, Lorna I, Gill K, Crispian O, Carolyn MM, Weaver JU. The beneficial effect of L-thyroxine on cardiovascular risk factors, endothelial function, and quality of life in subclinical hypothyroidism: randomized, crossover trial. J Clin Endocrinol Metab. 2007;92(5):1715–1723. doi: 10.1210/jc.2006-1869 [DOI] [PubMed] [Google Scholar]
- 7.Giuseppe P, Gennaro P, Giuseppe R, Nicola F, Fabio M. Subclinical hypothyroidism and cognitive impairment: systematic review and meta-analysis. J Clin Endocrinol Metab. 2015;100(11):4240–4248. doi: 10.1210/jc.2015-2046 [DOI] [PubMed] [Google Scholar]
- 8.Irwin MR. Why sleep is important for health: a psychoneuroimmunology perspective. Annu Rev Psychol. 2015;66(1):143. doi: 10.1146/annurev-psych-010213-115205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shi L, Chen SJ, Ma MY, et al. Sleep disturbances increase the risk of dementia: a systematic review and meta-analysis. Sleep Med Rev. 2017;40:S1087079217300114. [DOI] [PubMed] [Google Scholar]
- 10.Bahammam SA, Sharif MM, Jammah AA, Bahammam AS. Prevalence of thyroid disease in patients with obstructive sleep apnea. Respir Med. 2011;105(11):1755–1760. doi: 10.1016/j.rmed.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 11.Lin CC, Tsan KW, Chen PJ. The relationship between sleep apnea syndrome and hypothyroidism. Chest. 1992;102(6):1663–1667. doi: 10.1378/chest.102.6.1663 [DOI] [PubMed] [Google Scholar]
- 12.José Carlos P, Andersen, ML. The role of thyroid hormone in sleep deprivation. Med Hypotheses. 2014;82(3):350–355. doi: 10.1016/j.mehy.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 13.Parker DC, Rossman LG, Pekary AE, Hershman JM. Effect of 64-hour sleep deprivation on the circadian waveform of thyrotropin (TSH): further evidence of sleep-related inhibition of TSH release. J Clin Endocrinol Metab. 1987;64(1):157–161. doi: 10.1210/jcem-64-1-157 [DOI] [PubMed] [Google Scholar]
- 14.AC G. Textbook of Medical Physiology. 7th ed. Philadelphia, PA: WBSaunders Company; 1981. [Google Scholar]
- 15.Akatsu H, Ewing SK, Stefanick ML, et al. Association between thyroid function and objective and subjective sleep quality in older men: the osteoporotic fractures in men (MrOS) study. Endocr Pract. 2014;20(6):576–586. doi: 10.4158/EP13282.OR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Demartini B, Ranieri R, Masu A, Selle V, Scarone S, Gambini O. Depressive symptoms and major depressive disorder in patients affected by subclinical hypothyroidism: a cross-sectional study. J Nerv Ment Dis. 2014;202(8):603–607. doi: 10.1097/NMD.0000000000000168 [DOI] [PubMed] [Google Scholar]
- 17.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4 [DOI] [PubMed] [Google Scholar]
- 18.Liu XCT, Hu MQ, Wang L, et al. Reliability and validity of the Pittsburgh sleep quality index. Chin J Psychiatry. 1996;29(2):5. [Google Scholar]
- 19.Bielicki P, Przybylowski T, Kumor M, Barnas M, Wiercioch M, Chazan R. Thyroid hormone levels and TSH activity in patients with obstructive sleep apnea syndrome. Adv Exp Med Biol. 2016;878:67–71. [DOI] [PubMed] [Google Scholar]
- 20.Burman D. Sleep disorders: restless legs syndrome. FP Essent. 2017;460:29–32. [PubMed] [Google Scholar]
- 21.Palagini L, Bastien CH, Marazziti D, Ellis JG, Riemann D. The key role of insomnia and sleep loss in the dysregulation of multiple systems involved in mood disorders: a proposed model. J Sleep Res. 2019;28(6):e12841. doi: 10.1111/jsr.v28.6 [DOI] [PubMed] [Google Scholar]
- 22.Stone GW, Maehara A, Lansky AJ, et al. A prospective natural-history study of coronary atherosclerosis. N Engl J Med. 2011;364(3):226–235. doi: 10.1056/NEJMoa1002358 [DOI] [PubMed] [Google Scholar]
- 23.Brabant G, Prank K, Ranft U, et al. Physiological regulation of circadian and pulsatile thyrotropin secretion in normal man and woman. J Clin Endocrinol Metab. 1990;70(2):403–409. doi: 10.1210/jcem-70-2-403 [DOI] [PubMed] [Google Scholar]
- 24.Kuhs H, Farber D, Tolle R. Serum prolactin, growth hormone, total corticoids, thyroid hormones and thyrotropine during serial therapeutic sleep deprivation. Biol Psychiatry. 1996;39(10):857. doi: 10.1016/0006-3223(95)00240-5 [DOI] [PubMed] [Google Scholar]
- 25.Pereira JC Jr., Andersen ML. The role of thyroid hormone in sleep deprivation. Med Hypotheses. 2014;82(3):350–355. doi: 10.1016/j.mehy.2014.01.003 [DOI] [PubMed] [Google Scholar]
- 26.Kadoya M, Koyama S, Morimoto A, et al. Serum macro TSH level is associated with sleep quality in patients with cardiovascular risks - HSCAA study. Sci Rep. 2017;7:44387. doi: 10.1038/srep44387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vanderpump MP, Tunbridge WM. Epidemiology and prevention of clinical and subclinical hypothyroidism. Thyroid. 2002;12(10):839–847. doi: 10.1089/105072502761016458 [DOI] [PubMed] [Google Scholar]