Abstract
Ferroptosis is a new form of programmed cell death with characteristic accumulation of reactive oxygen species (ROS) resulting from iron accumulation and lipid peroxidation. Ferroptosis is involved in many diseases, including cancer, and induction of ferroptosis has shown attractive antitumour activities. In this review, we summarize recent findings on the regulatory mechanisms of key regulators of ferroptosis, including the catalytic subunit solute carrier family 7 member 11 (SLC7A11), the glutathione peroxidase 4 (GPX4), p53 and non-coding RNAs, the correlations between ferroptosis and iron homeostasis or autophagy, ferroptosis-inducing agents and nanomaterials and the diagnostic and prognostic value of ferroptosis-associated genes in TCGA data.
Keywords: ferroptosis, cancer, iron, autophagy, p53
Introduction
Ferroptosis is a new form of programmed cell death that is different from apoptosis, necroptosis and autophagy at both the morphological and biochemical levels and has characteristic accumulation of reactive oxygen species (ROS) resulting from iron accumulation and lipid peroxidation.1,2 Ferroptosis is involved in many diseases, such as ischaemia/reperfusion-induced organ injury, stroke and cancer. Inhibition of ferroptosis has been shown to be effective in treating ischaemia/reperfusion-induced organ injury and stroke in several experimental models,3–5 and inducing ferroptosis is considered a promising method for cancer therapy.6–8 As hotspots of cancer research, cancer immunotherapy and tumour cell interactions are also associated with ferroptosis. Immunotherapy-activated CD8+ T cells augment lipid peroxidation and ferroptosis and ultimately promote immunotherapy efficacy.9 Intercellular interactions between cancer cells were shown to suppress ferroptosis by activating NF2 and regulating the Hippo signalling pathway, and blocking NF2 sensitized cancer cells to ferroptosis.10 In this article, we reviewed the recent findings on ferroptosis in cancer, especially the correlation between ferroptosis and iron homeostasis or autophagy, the molecular regulatory mechanisms of ferroptosis, and ferroptosis-inducing agents/nanomaterials.
Dysregulation of Iron Homeostasis Regulates Ferroptosis in Cancer Cells
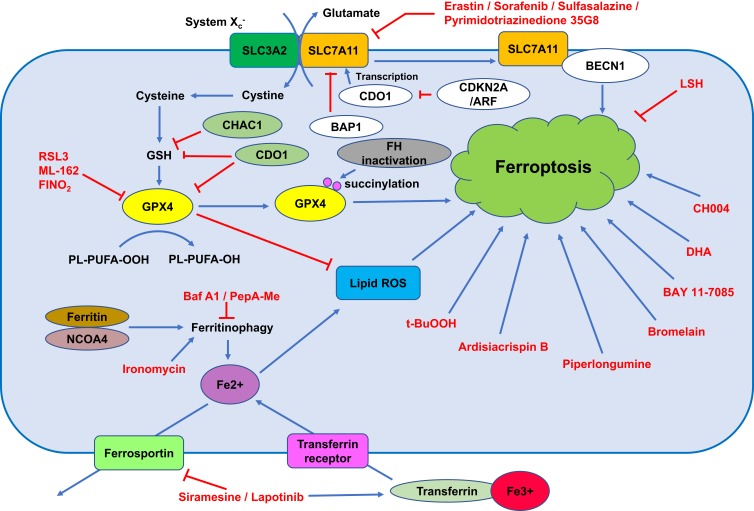
Iron dependency is a characteristic of cancer cells, and its dysregulation leads to ferroptosis.11 Cellular iron homeostasis is associated with the uptake and efflux of iron and the release of the cellular iron storage protein ferritin.12 Transferrin (TF) is an abundant and high-affinity iron-binding protein that can carry iron in the plasma and transfer it to cells that have transferrin receptors (TFRCs) by receptor-mediated endocytosis.12 In breast cancer cells, TFRCs participate in sulfasalazine-induced ferroptosis.13 Ferroportin is an iron efflux pump and exports iron out of the cell.14 Ferroportin upregulation inhibited ferroptosis, and silencing of ferroportin sensitized cancer cells to ferroptosis.15,16 Ferritin is the critical intracellular iron storage protein complex and is composed of two proteins, FTL (ferritin light polypeptide 1) and FTH1 (ferritin heavy polypeptide 1). Ferritin has a cage-like structure with a central cavity for iron storage.
Transferrin and its receptor are required for ferroptosis (Figure 1).17,18 Treatment of breast cancer cells with siramesine (a lysosome-disrupting agent) and lapatinib (a tyrosine kinase inhibitor) increased transferrin expression, decreased ferroportin-1 expression, upregulated FeCl3 levels, and ultimately induced ferroptosis in breast cancer cells. In addition, knockdown of transferrin or overexpression of ferroportin-1 downregulated ROS and inhibited ferroptosis.19 In both high-grade serous ovarian cancer tissues and ovarian cancer tumour-initiating cells (TICs), ferroportin is downregulated, and the transferrin receptor (TFR1) is overexpressed, reflecting that fact that ovarian cancer growth is highly dependent on iron; in addition, forced decreases in intracellular iron concentrations inhibited tumour growth and intraperitoneal dissemination of tumour cells in vivo.20 Knockdown of ferroportin also promoted erastin-induced ferroptosis in neuroblastoma cells.16 Inhibitors of lysosome activity, including Baf A1 and PepA-Me, decrease the intracellular iron level and lysosomal ROS and prevent erastin- or RSL3-induced ferroptosis in human fibrosarcoma HT1080 cells partially by attenuating intracellular transport of transferrin or autophagic degradation of ferritin.21
Figure 1.
The mechanism of ferroptosis.
Note: This figure shows the process of ferroptosis and summarizes the molecules and pathways in the regulation of ferroptosis.
Abbreviation: PUFA, polyunsaturated fatty acids.
Ferritinophagy refers to the process of autophagic degradation of ferritin, and it is required for ferroptosis.22,23 Ironomycin is a selective agent against breast cancer stem cells, and it can accumulate and sequester iron in lysosomes, trigger ferritinophagy and finally induce ferroptosis.24,25 NCOA4, as a selective cargo receptor for ferritin, is involved in ferritinophagy.26 Silencing NCOA4 upregulates ferritin expression, decreases Fe2+ levels and limits erastin-induced ferroptosis.27,28 In summary, dysregulation of iron homeostasis participates in the occurrence of ferroptosis, and inducing ferroptosis by upregulating the iron level might become a new anticancer strategy.
The Correlation Between Autophagy and Ferroptosis in Cancer Cells
Autophagy is an important catabolic process that transfers cytoplasmic material to lysosomes for degradation. The role of autophagy in cell death is inconsistent. In some cases, autophagy promotes cell survival in response to environmental stresses, including energy depletion and nutritional starvation; in other cases, abnormal autophagy, such as excessive or impaired autophagy, leads to cell death. The correlation between ferroptosis and autophagy is still largely unclear. On the one hand, suppression of autophagy by quinacrine induced ferroptosis and further enhanced the susceptibility of glioblastoma stem cells to temozolomide;29 on the other hand, activation of ferroptosis has been linked to enhanced autophagy.28 When autophagy is activated, the cellular iron stock protein ferritin is degraded, and the cellular iron level is rapidly upregulated via ferritinophagy. The high levels of cellular labile iron promote the accumulation of cellular ROS, which induce the onset of ferroptosis. Interestingly, a recent study reported that selective autophagic degradation of ARNTL/BMAL1, which is a circadian clock regulator, enhanced the ferroptosis of cancer cells both in vitro and in vivo.30,31
Atg5 and Atg7 are key genes in the formation of the autophagosome, and knockout or knockdown of Atg5 and Atg7 reduces Fe2+ and lipid peroxidation levels by inhibiting the degradation of the iron storage protein ferritin and further inhibits erastin-induced ferroptosis.28 However, autophagy inhibitors such as bafilomycin A1 and chloroquine (CQ) cannot inhibit erastin-induced ferroptosis in some cancer cell lines (e.g., HT-1080, BJeLR and PANC1).1,28 Some possible reasons are that both CQ and bafilomycin A1 are non-specific autophagy inhibitors, and they also inhibit lysosomal processes and endocytic pathways.32
BECN1 (beclin 1) is a key component of the class III phosphatidylinositol 3-kinase (Ptdlns3K) complex and is involved in autophagosome formation. BECN1 interacts with several proteins and regulates many processes, including autophagy, endocytosis and phagocytosis. Song et al reported that BECN1 promoted ferroptosis by interacting with SLC7A11 and upregulating lipid peroxidation in an autophagy-independent manner, and the formation of the BECN1-SLC7A11 complex was regulated by AMPK-mediated phosphorylation of BECN1 at Ser90/93/96.33
In addition to autophagy, ferroptosis crosstalks with apoptosis. Inducers of ferroptotic erastin or artesunate (ART) could promote tumour necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis, induce endoplasmic reticulum (ER) stress and upregulate the expression of p53-upregulated modulator of apoptosis (PUMA) induced by CHOP. The synergistic effects of erastin/ART and TRAIL occur via the p53-independent CHOP/PUMA pathway.34 CA9, a membrane-associated α-CA (carbonic anhydrase), is upregulated in the formation of malignant mesothelioma. Inhibition of CA9 induced the ferroptosis and apoptosis of cancer cells by increasing catalytic Fe2+ in lysosomes and mitochondria and lipid peroxidation.35 However, the regulatory hub and network connecting ferroptosis and apoptosis are still unknown.
Key Regulators in the Regulation of Ferroptosis in Cancer Cells
SLC7A11, GPX4 and p53 were identified as the key regulators in cancer cell ferroptosis. In addition, an increasing number of studies have focused on the roles of non-coding RNAs and other mechanisms in ferroptosis regulation.
SLC7A11
System Xc− is a heterodimeric cystine/glutamate antiporter and consists of two key members, including the catalytic subunit solute carrier family 7 member 11 (SLC7A11) and solute carrier family 3 member 2 (SLC3A2). System Xc− mainly transports extracellular cystine to cells (Figure 1). Intracellular cystine is rapidly converted to cysteine and then functions as the precursor for glutathione biosynthesis. System Xc− is the upstream molecule in the process of ferroptosis. Drugs that target SLC7A11 and block cystine uptake could induce ferroptosis (Figure 1). Erastin, sulfasalazine and sorafenib could induce ferroptosis via targeting and inhibiting system Xc−.7 In particular, erastin, which is different from other inhibitors of system Xc−, caused strong and persistent inhibition of system Xc− when exposed to cells for a very short duration at low erastin concentrations. These inhibitory effects of erastin on system Xc− are not caused by modification of the transporter.36
There are at least two ways to control system Xc- activity in ferroptosis. First, SLC7A11 could interact with BECN1 and further induce ferroptosis. Second, SLC7A11 could be regulated at the transcriptional level, and a decrease in SLC7A11 could consequently induce ferroptosis.37 In vitro and in vivo studies revealed that cyclin-dependent kinase inhibitor 2A (CDKN2A/ARF) sensitized cancer cells to ferroptosis by inhibiting the ability of NRF2 and its transcriptional target SLC7A11.37 Sulfasalazine induced ferroptosis via inhibiting SLC7A11. CISD2 overexpression enabled resistance to sulfasalazine-induced ferroptosis, and silencing CISD2 or pioglitazone treatment sensitized resistant head and neck cancer cells to sulfasalazine-induced ferroptosis.38 Pyrimidotriazinedione 35G8, a protein disulfide isomerase inhibitor, induced ferroptosis and autophagy by upregulating the transcription and protein expression of SLC7A11.39 In addition, SLC7A11 can also be regulated at the post-translational level. The tumour suppressor BRCA1-associated protein 1 (BAP1) is a nuclear deubiquitinating enzyme and can reduce histone 2A ubiquitination (H2Aub) on chromatin. In human cancers, BAP1 reduces H2Aub occupancy on the SLC7A11 promoter and suppresses SLC7A11 expression in a deubiquitination-dependent manner and then inhibits cystine uptake, promotes lipid peroxidation, and finally induces ferroptosis. Importantly, mutants (C91A, S10T, E31A, L49V, C91G, H169Q, F170C and G185R) in the UCH domain of BAP1 lose the function of repressing SLC7A11 expression and the ability to inhibit anchorage-independent growth.40
GPX4 in the Regulation of Ferroptosis in Cancer Cells
GPX4 is an antioxidant enzyme (Figure 1). As the key regulator of ferroptosis, GPX4 catalyses and reduces lipid peroxides.41 GPX4 is expressed in 35.5% (n=93) of diffuse large B cell lymphoma, and its positive expression is positively associated with poor overall survival (OS) and progression-free survival (PFS) and negatively correlated with 8-hydroxydeoxyguanosine (8-OHdG), which is an oxidative stress marker. Overexpression of GPX4 confers resistance to ROS-induced cell death to tumour cells, and silencing GPX4 sensitizes tumour cells to ROS-induced cell death.42 The hypoxia-inducible factor (HIF) pathway confers with sensitivity to ferroptosis to clear cell carcinoma cells via regulation of GPX4 expression.43 GPX4 is also reported to have interactions and be regulated via protein degradation by HSPA5 in cancer cells.44
The GPX4 inhibitors RSL3 and ML-162 induced ferroptosis of head and neck cancer cells and increased the expression of p62 and NRF2 in cisplatin-resistant cancer cells and cancer cells with acquired RSL3 resistance. Overexpression of NRF2 renders chemosensitive NH3 cells resistant to RSL3, and silencing NRF2 has opposite effects.45 RSL3 promotes ROS accumulation and induces cell death in colorectal cancer (CRC) cells, increases transferrin expression and decreases GPX4 expression. Overexpression of GPX4 rescued RSL3-induced ferroptosis in CRC cells.46 Importantly, GPX4 can also be regulated at the post-translational level. Hereditary leiomyomatosis and renal cell cancer (HLRCC) is a hereditary cancer with characteristic inactivation of the Krebs cycle enzyme fumarate hydratase (FH). A recent study reported that a ferroptosis inducer could target and selectively kill HLRCC cells, and FH inactivation sensitized HLRCC cells to ferroptosis by promoting intracellular fumarate accumulation and further modifying the C93 succinylation of GPX4 and inhibiting its activity.47 FINO2 is an endoperoxide-containing 1,2-dioxolane that can effectively induce ferroptosis by indirectly inhibiting GPX4 enzymatic function and directly oxidizing iron, ultimately inducing widespread lipid peroxidation and ferroptosis with no effects on GPX4 expression.48 In summary, the expression, activity and post-translational modification of GPX4 are regulated in cancer formation and development.
p53 in the Regulation of Ferroptosis in Cancer Cells
Traditionally, p53 is a well-known tumour suppressor and elicits cell cycle arrest, apoptosis and senescence in response to cellular stress. A recent study reported that p53 inhibited cystine uptake and induced ferroptosis; mechanistically, p53 bound to the promoter of the SLC7A11 gene, and transcriptionally, p53 repressed SLC7A11 expression. The p533KR mutant retained the ability to regulate SLC7A11 expression and induce ferroptosis but failed to induce cell cycle arrest, senescence and apoptosis (Figure 2). Notably, SLC7A11 overexpression abrogated p533KR-mediated tumour growth suppression in a xenograft model.49 Meanwhile, N-terminal mutants of p53 (L25Q, F53Q, W26S and F54S) had no effect on SLC7A11 expression and ROS levels and failed to facilitate ferroptosis.50 The P47S variant of p53 conferred human cells with resistance to RSL3-induced ferroptosis.51
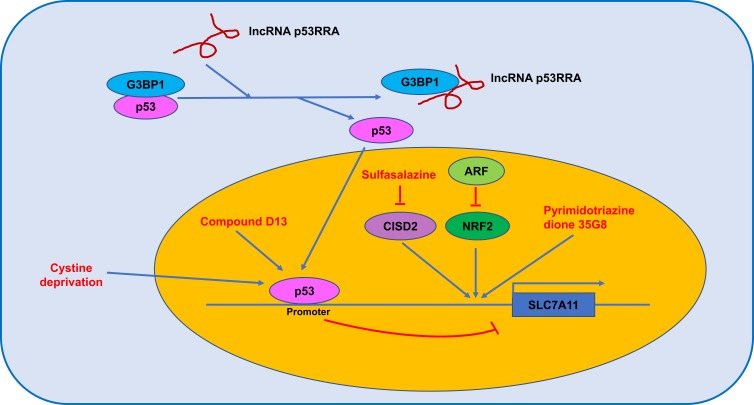
Figure 2.
The regulated mechanism of SLC7A11.
Spermidine/spermine N1-acetyltransferase 1 (SAT1) is a transcription target of p53 and is a rate-limiting enzyme in polyamine catabolism, and its activation induces lipid peroxidation, sensitizes cells to ferroptosis in response to ROS-induced stress, and suppresses tumour growth in a xenograft tumour model. Importantly, knockout of SAT1 partially abrogates p53-mediated ferroptosis.52 Compound D13, a derivative of albiziabioside A, possesses strong inhibitory activity against cancer cells, is especially efficacious against multidrug-resistant (MDR) cancer cells in vitro, and suppresses tumorigenesis without causing toxicity in normal organs in vivo. Mechanistically, compound D13 activates p53 and induces ferroptosis and apoptosis via the mitochondrial pathway.53 Erastin induces ferrroptotic and apoptotic cell death in A549 cells and upregulates p53 in a ROS-dependent manner. Meanwhile, p53 activation suppressed SLC7A11 and further promoted ROS accumulation via positive feedback.54 The lncRNA P53RRA, which is downregulated in cancers, interacts with Ras GTPase-activating protein-binding protein 1 (G3BP1) and displaces p53 from the G3BP1 complex, causes p53 accumulation in the nucleus and ultimately resulting in ferroptosis and apoptosis (Figure 2).55 Cystine deprivation induces ferroptosis of certain cancer cells, and stabilization of wild-type p53 attenuates ferroptosis in response to cystine deprivation via upregulation of the p53 transcriptional target p21, slowing the depletion of intracellular glutathione and reducing the accumulation of lipid ROS.56 Loss of p53 prevents nuclear accumulation of dipeptidyl peptidase-4 (DPP4) and promotes plasma membrane-associated DPP4-dependent lipid peroxidation, ultimately leading to ferroptosis.57
Non-Coding RNAs in the Regulation of Ferroptosis in Cancer Cells
In addition to the lncRNA P53RRA, which was mentioned above, many other non-coding RNAs also play important roles in ferroptosis. miR-7-5p conferred cancer cell radioresistance by decreasing mitoferrin and Fe2+ and ultimately inhibiting ferroptosis.58 miR-137 blocked the erastin- and RSL3-induced ferroptosis of human melanoma cells that harboured the BRAFV600E mutation by targeting SLC1A5, and knockdown of miR-137 enhanced the antitumour activity of erastin both in vitro and in vivo.59 miR-9 reduced erastin- and RSL3-induced ferroptosis by targeting the glutamic-oxaloacetic transaminase GOT1 in melanoma.60 miR-27a regulated cisplatin resistance by directly targeting SLC7A11 in bladder cancer, and miR-27a overexpression or silencing of SLC7A11 resensitized tumour cells to cisplatin (Table 1).61 Using an RNA sequencing method, Yu et al revealed that differentially expressed lncRNAs were enriched in the ferroptotic pathway in non-small-cell lung cancer (NSCLC).62 LINC00336 was reported to inhibit ferroptosis in lung cancer cells by sponging miR-6852 and positively regulating its target, CBS (cystathionine β-synthase).63
Table 1.
The Roles of miRNAs in Cancer Cell Ferroptosis
| miRNA Name | Target | Function | Cancer Types | References |
|---|---|---|---|---|
| miR-7-5p | NA | Inhibiting ferroptosis and inducing resistance to irradiation | Cervical cancer, tongue cancer and liver cancer | 58 |
| miR-9 | GOT1 | Inhibiting erastin- and RSL3-induced ferroptosis | Melanoma | 60 |
| miR-27a | SLC7A11 | Regulating cisplatin resistance | Bladder cancer | 61 |
| miR-137 | SLC1A5 | Inhibiting erastin- and RSL3-induced ferroptosis | Melanoma | 59 |
| miR-6852 | CBS | Inhibiting ferroptosis and being sponged by LINC00336 | Lung cancer | 63 |
Other Mechanisms Underlying Ferroptosis Regulation
Many genes, such as NFS1, ΔNp63α and LSH, are involved in the regulation of ferroptosis. NFS1 activity is fundamental for maintaining iron-sulfur cluster biogenesis in high-oxygen environments such as the lung. NFS1 depletion suppresses the growth of metastatic and primary lung tumours by inducing ferroptosis, with no effects on the growth of mammary or subcutaneous tumours. In a subset of lung adenocarcinomas, NFS1 is amplified, and its loss inhibits lung cancer growth.64 ΔNp63α protected the p53−/- MEFs from erastin-induced ferroptosis, and these findings indicated that ΔNp63α negatively regulated ferroptosis in a p53-independent manner.65 CHAC1 degradation of GSH enhanced cystine starvation-induced ferroptosis by activating the GCN2-eIF2a-ATF4 pathway in triple-negative breast cancer cells.66 c-Jun O-GlcNAcylation-regulated GSH synthesis participates in the ferroptosis process in liver cancer cells.67 Silencing CDO1 inhibited erastin-induced ferroptosis in gastric cancer cells both in vitro and in vivo by increasing GSH levels, preventing ROS production, reducing lipid peroxidation and upregulating GPX4 expression.68 Lymphoid-specific helicase (LSH) is a DNA methylation modifier that is directly regulated by EGLN1 and c-Myc. LSH inhibits ferroptosis by interacting with WDR76, regulating iron and lipid ROS.69 A recent study also identified ferroptosis upregulated factors (FUFs), which were transcriptionally regulated by HIC1, and ferroptosis downregulated factors (FDFs), which were transcriptionally regulated by HUF4A, in liver cancer.70 In artesunate-induced ferroptosis, GRP78 was upregulated, and silencing GRP78 sensitized pancreatic cancer cells to artesunate.71 ATM was found to be an essential factor in ferroptosis, and inactivation or silencing of ATM suppressed cystine deprivation- or erastin-induced ferroptosis.15
Ferroptosis-Inducing Agents and Nanomaterials
Inducing ferroptosis is a promising method for cancer therapy; therefore, many studies have focused on the exploration of ferroptosis-inducing agents. Recent studies reported that many agents induced ferroptosis of cancer cells by regulating different downstream genes. Tertiary-butyl hydroperoxide (t-BuOOH), which is a widely used inducer of oxidative stress, increased lipid peroxidation and cytosolic ROS and ultimately induced ferroptosis.72 Dihydroartemisinin (DHA) effectively induced the ferroptosis of cancer cells by regulating the AMPK/mTOR/p70S6k signalling pathway, promoting the degradation of ferritin and further increasing the iron pool.73,74 Ardisiacrispin B, a naturally occurring oleanane-type triterpene saponin that is extracted from the fruit of Ardisia kivuensis Taton (of the family Myrsinaceae), also effectively induced the ferroptosis of cancer cells.75 Bromelain treatment inhibited the cell growth and proliferation of Kras-mutant colorectal cancer cells by upregulating the expression of the ferroptosis responder ACSL-4.76 CH004, a bioactive inhibitor of human cystathionine β-synthase (CBS), which is the key enzyme in the transsulfuration pathway for sulfur amino acids, was reported to induce the ferroptosis of HepG2 cells and inhibit tumour growth in a liver tumour xenograft mouse model.77 BAY 11–7085 induced ferroptosis by activating the Nrf2-SLC7A11-HO-1 signalling pathway, especially promoting HO-1 translocation from the cytosol to the nucleus and mitochondria.78 In addition, Betula etnensis Raf. (of the family Betulaceae) extract promoted ferroptosis by inducing HO-1 expression.79 Piperlongumine (PL) is a natural product, and Yamaguchi et al reported that PL and CN-A (a plant growth regulator) synergistically induced the ferroptosis of pancreatic cancer cells. Additionally, sulfasalazine (SSZ, a ferroptosis inducer) further improved the cancer cell-killing activities of PL and CN-A treatment.80 Molecularly, sulfasalazine upregulated ROS and downregulated GPX4 and xCT in breast cancer cells.13 Sorafenib treatment induced ferroptosis by increasing the mRNA and protein expression of MT-1G via NRF2.81 In addition, epunctanone and artemisinin derivatives have also been reported to induce ferroptosis in tumour cells.82,83 Taken together, these results suggest that future studies should focus on the antitumour activity of ferroptosis-inducing agents in animal models, including a patient-derived xenograft model (PDX).
Exploiting ferroptosis-inducing nanomaterials is an attractive area of study. Wang et al produced arginine-rich manganese silicate nanobubbles (AMSNs) via a one-pot reaction with arginine (Arg) as the surface ligand for tumour homing. The AMSNs had a highly efficient glutathione (GSH)-depleting ability and thereby effectively induced ferroptosis by inactivating GPX4. Moreover, AMSN degradation during GSH depletion led to T1-weighted magnetic resonance imaging (MRI) enhancement.84 SRF@FeIIITA nanoparticles are formed with the Fe3+ ion and naturally derived tannic acid (TA) as the outer surface and sorafenib (SRF) as the core. In lysosomes, the outer surface of SRF@FeIIITA nanoparticles was dissociated, and SRF was released to inhibit GPX4 activity and induce ferroptosis. The TA was arranged to reduce the liberated and ferroptosis-generated Fe3+ to Fe2+, and the sustained Fe2+ levels led to the death of H2O2-overloaded cancer cells and strongly inhibited tumour proliferation but had minimal effects on normal cells. Typically, photosensitizer-adsorbed SRF@FeIIITA nanoparticles allow for rapid tumour imaging owing to the acid-responsive fluorescence recovery, and these nanoparticles are very useful for future applications.85 Shen et al reported cisplatin (CDDP)-loaded Fe3O4/Gd2O3 hybrid nanoparticles conjugated to lactoferrin (LF) and an RGD dimer (RGD2) (FeGd-HN@Pt@LF/RGD2). These nanoparticles crossed the blood-brain barrier, were internalized by cancer cells via integrin αvβ3-mediated endocytosis, released Fe2+, Fe3+ and CDDP, and then directly or indirectly participated in the Fenton reaction and induced cancer cell death.86 Yue et al designed a novel ferroptosis agent, FePt-PTTA-Eu3+-FA (FPEF), which consists of the luminescent lanthanide complex PTTA-Eu3+ and folic acid (FA) in FePt nanoparticles. In vitro and in vivo studies confirmed that these nanoparticles showed satisfactory anticancer activity.87
Dysregulation and Prognostic Value of Ferroptosis-Associated Genes in Cancer
To further explore the roles of ferroptosis-associated genes in carcinoma, we analysed the dysregulation and prognostic value of these genes using TCGA data by analysing the starBase v2.0 database88 (Table 2). As SLC7A11 is the key regulator of ferroptosis, silencing SLC7A11 or inhibiting its activity can induce ferroptosis. SLC7A11 is overexpressed in many types of cancers (Table 2), including cholangiocarcinoma (CHOL), head and neck squamous cell carcinoma (HNSC), liver hepatocellular carcinoma (LIHC), lung squamous cell carcinoma (LUSC) and kidney renal papillary cell carcinoma (KIRP). Zhu et al confirmed SLC7A11 upregulation in oral tongue squamous cell carcinoma (OTSCC), and downregulation of its expression via administration of Ce6-erastin nanoparticles showed satisfactory antitumour effects.89 Importantly, high expression of SLC7A11 is significantly associated with poor prognosis in LIHC and KIRP patients (Table 2, Figure 3A and B). These results suggest that the resistance to ferroptosis caused by SLC7A11 overexpression is involved in the tumorigenesis of many types of cancer. TCGA data showed that GPX4 was upregulated in uterine corpus endometrial carcinoma (UCEC) and correlated with unfavourable survival outcome in colon adenocarcinoma (COAD) and acute myeloid leukaemia (LAML). GPX4 was confirmed to be overexpressed and correlated with poor prognosis in NSCLC.90 However, its diagnostic and prognostic value in other types of cancer still need to be evaluated. Transferrin is underexpressed in breast invasive carcinoma (BRCA), and its underexpression is related to an unfavourable survival outcome (Table 2 and Figure 4A). Lapatinib alone or in combination with siramesine increased transferrin expression and resulted in ferroptosis and autophagy-related cell death.91 However, transferrin is overexpressed in kidney renal clear cell carcinoma (KIRC), and its overexpression is significantly linked with poor prognosis in KIRC patients (Table 2 and Figure 4B). These inconsistent findings suggest that transferrin plays different roles in different types of cancers. LINC00472 (lncRNA P53RRA) is downregulated in several types of cancers, such as bladder urothelial carcinoma (BLCA), lung adenocarcinoma (LUAD), HNSC and KIRC. Interestingly, its low expression is associated with poor prognosis in KIRC patients (Figure 5). In lung cancer cells, the interaction between LINC00472 and G3BP1 promoted p53 translocation from the cytoplasm to the nucleus, enhanced the suppressive effects of p53 on SLC7A11 expression, and ultimately induced ferroptosis.55 In other tumours, whether low expression of LINC00472 affects p53-based SLC7A11 suppression still needs to be explored. Future studies should focus on the detailed regulatory mechanisms of these genes in ferroptosis.
Table 2.
The Dysregulation and Prognostic Value of Ferroptosis Associated Genes in TCGA Data
| Gene | Name | Dysregulation in Cancer | Correlation with Survival in Cancer |
|---|---|---|---|
| BAP1 | BRCA1 associated protein 1 | Up: LIHC | Unfavourable: PRAD, LIHC |
| Down: NO | Favourable: UVM, THYM, LUAD | ||
| BECN1 | Beclin 1 | Up: CHOL | Unfavourable: NO |
| Down: NO | Favourable: UCEC | ||
| CDO1 | Cysteine dioxygenase type 1 | Up: NO | Unfavourable: STAD |
| Down: CHOL, KICH, BRCA, LUSC, UCEC, LUAD, PRAD, BLCA, KIRC, COAD, HNSC, STAD | Favourable: UVM, LIHC, LUAD | ||
| CHAC1 | ChaC glutathione specific gamma-glutamylcyclotransferase 1 | Up: BLCA, LUSC, COAD, CHOL, BRCA, LUAD | Unfavourable: UVM, KIRC, KIRP, THYM, LGG |
| Down: KIRP, KIRC | Favourable: MESO | ||
| CISD2 | CDGSH iron sulfur domain 2 | Up: NO | Unfavourable: LUAD, LGG, KICH, UVM |
| Down: NO | Favourable: KIRC | ||
| DPP4 | Dipeptidyl peptidase 4 | Up: THCA | Unfavourable: LGG, BLCA, PRAD, LUSC |
| Down: KICH, BRCA, LUSC | Favourable: KIRC, THYM, MESO, KIRP | ||
| FTH1 | Ferritin heavy chain 1 | Up: CHOL, LIHC | Unfavourable: HNSC, UVM, LGG, LIHC, THYM, KIRP |
| Down: NO | Favourable: NO | ||
| FTL | Ferritin light chain | Up: HNSC, LIHC | Unfavourable: LGG, UCEC, MESO, KIRC |
| Down: NO | Favourable: NO | ||
| GPX4 | Glutathione peroxidase 4 | Up: UCEC | Unfavourable: COAD, LAML |
| Down: NO | Favourable: UCEC, THCA, LUAD | ||
| LINC00472 (LncRNA P53RRA) | Long intergenic non-protein coding RNA 472 | Up: CHOL | Unfavourable: PAAD |
| Down: LUSC, KIRC, KIRP, BLCA, KICH, LUAD, HNSC, THCA, ESCA | Favourable: UVM, BRCA, KIRC, LGG | ||
| miR-137 | Up: LUAD, COAD, LIHC, LUSC, BRCA, KICH | Unfavourable: HNSC | |
| Down: NO | Favourable: ACC | ||
| NCOA4 | Nuclear receptor coactivator 4 | Up: NO | Unfavourable: TGCT |
| Down: NO | Favourable: KIRC, LGG, LUAD, SARC, MESO, | ||
| NFE2L2 (NRF2) | Nuclear factor, erythroid 2 like 2 | Up: LUSC | Unfavourable: LGG |
| Down: NO | Favourable: KIRC, MESO, SARC | ||
| NFS1 | NFS1 cysteine desulfurase | UP: LUSC | Unfavourable: LAML |
| Down: NO | Favourable: LGG | ||
| SAT1 | Spermidine/spermine N1-acetyltransferase 1 | Up: UCEC | Unfavourable: LGG, KIRC |
| Down: NO | Favourable: UCEC, MESO, ACC | ||
| SLC1A5 | Solute carrier family 1 member 5 | Up: CHOL, LIHC, THCA, LUSC, STAD, COAD, ESCA, UCEC, PAAD | Unfavourable: LGG, MESO, LIHC, KIRC, UVM |
| Down: NO | Favourable: STAD | ||
| SLC3A2 | Solute carrier family 3 member 2 | Up: CHOL, ESCA, KICH, LUSC, HNSC, COAD, BLCA | Unfavourable: SKCM, LIHC, HNSC, LGG, ACC, LUAD, |
| Down: NO | Favourable: UCS | ||
| SLC7A11 | Solute carrier family 7 member 11 | Up: CHOL, LIHC, LUSC, KIRP, KICH, LUAD, COAD, PAAD, BRCA, HNSC, KIRC, ESCA, PRAD, STAD | Unfavourable: ACC, UVM, LIHC, KIRP, MESO, THCA, SARC |
| Down: NO | Favourable: OV | ||
| SLC11A1 (LSH) | Solute carrier family 11 member 1 | Up: COAD, KIRC, CHOL, KIRP, STAD, HNSC, UCEC, BRCA | Unfavourable: LGG, KIRC, LIHC, THYM, |
| Down: PAAD, LUAD, LUSC | Favourable: PCPG | ||
| TF | Transferrin | Up: LUAD, KIRC, LUSC, STAD, UCEC | Unfavourable: KIRC, STAD |
| Down: CHOL, HNSC, BRCA, KICH | Favourable: BRCA, LAML, LGG | ||
| TFRC | Transferrin receptor | Up: CHOL, ESCA, HNSC, UCEC, LUSC, LIHC, STAD, BLCA | Unfavourable: LGG, LIHC, THCA, KICH, ACC, TGCT, |
| Down: NO | Favourable: STAD, LUSC |
Abbreviations: ACC, adrenocortical carcinoma; BLCA, bladder urothelial carcinoma; BRCA, breast invasive carcinoma; CHOL, cholangiocarcinoma; COAD, colon adenocarcinoma; ESCA, esophageal carcinoma; HNSC, head and neck squamous cell carcinoma; KICH, kidney chromophobe; KIRC, kidney renal clear cell carcinoma; KIRP, kidney renal papillary cell carcinoma; LGG, brain lower grade glioma; LIHC, liver hepatocellular carcinoma; LUAD, lung adenocarcinoma; LUSC, lung squamous cell carcinoma; MESO, mesothelioma; OV, ovarian serous cystadenocarcinoma; PAAD, pancreatic adenocarcinoma; PRAD, prostate adenocarcinoma; SARC, sarcoma; SKCM, skin cutaneous melanoma; STAD, stomach adenocarcinoma; THCA, thyroid carcinoma; THYM, thymoma; UCEC, uterine corpus endometrial carcinoma; UVM, uveal melanoma.
Figure 3.

The dysregulation and prognostic value of SLC7A11 in cancers.
Notes: (A) The expression of SLC7A11 was higher in LIHC tissues than that in normal tissues (Fold change = 22.44, p < 0.001) and its high expression was positively associated with poor prognosis of LIHC patients (p < 0.001). (B) The expression of SLC7A11 was higher in KIRP tissues than that in normal tissues (Fold change = 15.31, p < 0.001) and its high expression was positively associated with poor prognosis of KIRP patients (p = 0.0016).
Figure 4.
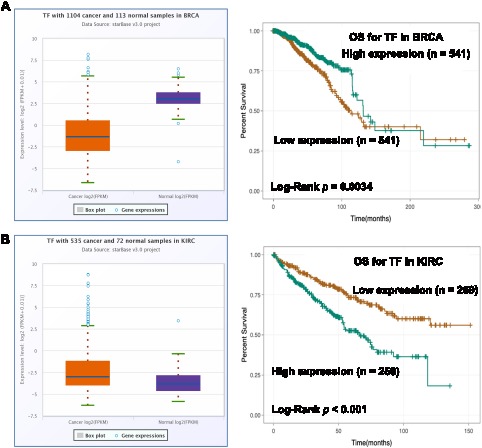
The dysregulation and prognostic value of TF in cancers.
Notes: (A) The expression of TF was lower in BRCA tissues than that in normal tissues (Fold change = 0.24, p < 0.001) and its low expression was positively associated with poor prognosis of BRCA patients (p = 0.0034). (B) The expression of TF was higher in KIRC tissues than that in normal tissues (Fold change = 16.96, p < 0.001) and its high expression was positively associated with poor prognosis of KIRC patients (p < 0.001).
Figure 5.
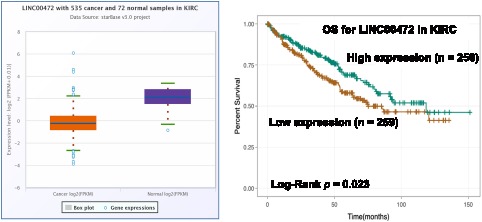
The dysregulation and prognostic value of LINC00472 in KIRC.
Notes: The expression of LINC00472 was lower in KIRC tissues than that in normal tissues (Fold change = 0.27, p < 0.001) and its low expression was positively associated with poor prognosis of KIRC patients (p = 0.023).
Concluding Remarks
Although there have recently been many proceedings and new findings on ferroptosis, the detailed mechanisms are largely unknown. 1) The roles of circRNAs in the regulation of ferroptosis are still unknown. 2) Except for limited ferroptosis-associated genes, such as SLC7A11, GPX4 and p53, the regulatory networks underlying ferroptosis are still unclear. 3) The interactome and post-translational modifications of SLC7A11 and GPX4 still need to be identified. 4) Future studies should focus on the diagnostic and prognostic value of ferroptosis-associated genes. 5) Cancer therapy targeting ferroptosis should be further studied and validated, and inducing ferroptosis might become a promising cancer treatment method.
Funding Statement
This study was funded by the National Natural Science Foundation of China (No. 81760526 and No. 81802794) and Yunnan Provincial Research Foundation for Basic Research, China (No. 2018FB135).
Data sharing statement
The data that support the findings of this study are openly available in starBase database at http://starbase.sysu.edu.cn/index.php, DOI: 10.1093/nar/gkt1248, reference number [PMID: 24297251].
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Dixon SJ, Lemberg KM, Lamprecht MR, et al. Ferroptosis: an iron-dependent form of nonapoptotic cell death. Cell. 2012;149(5):1060–1072. doi: 10.1016/j.cell.2012.03.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xie Y, Hou W, Song X, et al. Ferroptosis: process and function. Cell Death Differ. 2016;23(3):369–379. doi: 10.1038/cdd.2015.158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedmann Angeli JP, Schneider M, Proneth B, et al. Inactivation of the ferroptosis regulator Gpx4 triggers acute renal failure in mice. Nat Cell Biol. 2014;16(12):1180–1191. doi: 10.1038/ncb3064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Linkermann A, Skouta R, Himmerkus N, et al. Synchronized renal tubular cell death involves ferroptosis. Proc Natl Acad Sci U S A. 2014;111(47):16836–16841. doi: 10.1073/pnas.1415518111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alim I, Caulfield JT, Chen Y, et al. Selenium drives a transcriptional adaptive program to block ferroptosis and treat stroke. Cell. 2019;177(5):1262–1279 e1225. doi: 10.1016/j.cell.2019.03.032 [DOI] [PubMed] [Google Scholar]
- 6.Shen Z, Song J, Yung BC, Zhou Z, Wu A, Chen X. Emerging strategies of cancer therapy based on ferroptosis. Adv Mater. 2018;30(12):e1704007. doi: 10.1002/adma.v30.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stockwell BR, Friedmann Angeli JP, Bayir H, et al. Ferroptosis: a regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171(2):273–285. doi: 10.1016/j.cell.2017.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nagpal A, Redvers RP, Ling X, et al. Neoadjuvant neratinib promotes ferroptosis and inhibits brain metastasis in a novel syngeneic model of spontaneous HER2(+ve) breast cancer metastasis. Breast Cancer Res. 2019;21(1):94. doi: 10.1186/s13058-019-1177-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang W, Green M, Choi JE, et al. CD8(+) T cells regulate tumour ferroptosis during cancer immunotherapy. Nature. 2019;569(7755):270–274. doi: 10.1038/s41586-019-1170-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wu J, Minikes AM, Gao M, et al. Intercellular interaction dictates cancer cell ferroptosis via NF2-YAP signalling. Nature. 2019;572(7769):402–406. doi: 10.1038/s41586-019-1426-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hassannia B, Vandenabeele P, Vanden Berghe T. Targeting ferroptosis to iron out cancer. Cancer Cell. 2019;35(6):830–849. doi: 10.1016/j.ccell.2019.04.002 [DOI] [PubMed] [Google Scholar]
- 12.Andrews NC, Schmidt PJ. Iron homeostasis. Annu Rev Physiol. 2007;69:69–85. doi: 10.1146/annurev.physiol.69.031905.164337 [DOI] [PubMed] [Google Scholar]
- 13.Yu H, Yang C, Jian L, et al. Sulfasalazineinduced ferroptosis in breast cancer cells is reduced by the inhibitory effect of estrogen receptor on the transferrin receptor. Oncol Rep. 2019;42(2):826–838. doi: 10.3892/or.2019.7189 [DOI] [PubMed] [Google Scholar]
- 14.Ward DM, Kaplan J. Ferroportin-mediated iron transport: expression and regulation. Biochim Biophys Acta. 2012;1823(9):1426–1433. doi: 10.1016/j.bbamcr.2012.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen PH, Wu J, Ding CC, et al. Kinome screen of ferroptosis reveals a novel role of ATM in regulating iron metabolism. Cell Death Differ. 2019. doi: 10.1038/s41418-019-0393-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geng N, Shi BJ, Li SL, et al. Knockdown of ferroportin accelerates erastin-induced ferroptosis in neuroblastoma cells. Eur Rev Med Pharmacol Sci. 2018;22(12):3826–3836. doi: 10.26355/eurrev_201806_15267 [DOI] [PubMed] [Google Scholar]
- 17.Gao M, Monian P, Quadri N, Ramasamy R, Jiang X. Glutaminolysis and transferrin regulate ferroptosis. Mol Cell. 2015;59(2):298–308. doi: 10.1016/j.molcel.2015.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao M, Monian P, Jiang X. Metabolism and iron signaling in ferroptotic cell death. Oncotarget. 2015;6(34):35145–35146. doi: 10.18632/oncotarget.v6i34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ma S, Henson ES, Chen Y, Gibson SB. Ferroptosis is induced following siramesine and lapatinib treatment of breast cancer cells. Cell Death Dis. 2016;7:e2307. doi: 10.1038/cddis.2016.208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basuli D, Tesfay L, Deng Z, et al. Iron addiction: a novel therapeutic target in ovarian cancer. Oncogene. 2017;36(29):4089–4099. doi: 10.1038/onc.2017.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Torii S, Shintoku R, Kubota C, et al. An essential role for functional lysosomes in ferroptosis of cancer cells. Biochem J. 2016;473(6):769–777. doi: 10.1042/BJ20150658 [DOI] [PubMed] [Google Scholar]
- 22.Mancias JD, Wang X, Gygi SP, Harper JW, Kimmelman AC. Quantitative proteomics identifies NCOA4 as the cargo receptor mediating ferritinophagy. Nature. 2014;509(7498):105–109. doi: 10.1038/nature13148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sui S, Zhang J, Xu S, Wang Q, Wang P, Pang D. Ferritinophagy is required for the induction of ferroptosis by the bromodomain protein BRD4 inhibitor (+)-JQ1 in cancer cells. Cell Death Dis. 2019;10(5):331. doi: 10.1038/s41419-019-1564-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mai TT, Hamai A, Hienzsch A, et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat Chem. 2017;9(10):1025–1033. doi: 10.1038/nchem.2778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hamai A, Caneque T, Muller S, et al. An iron hand over cancer stem cells. Autophagy. 2017;13(8):1465–1466. doi: 10.1080/15548627.2017.1327104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Santana-Codina N, Mancias JD. The role of NCOA4-mediated ferritinophagy in health and disease. Pharmaceuticals (Basel). 2018;11(4):114. doi: 10.3390/ph11040114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao M, Monian P, Pan Q, Zhang W, Xiang J, Jiang X. Ferroptosis is an autophagic cell death process. Cell Res. 2016;26(9):1021–1032. doi: 10.1038/cr.2016.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hou W, Xie Y, Song X, et al. Autophagy promotes ferroptosis by degradation of ferritin. Autophagy. 2016;12(8):1425–1428. doi: 10.1080/15548627.2016.1187366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buccarelli M, Marconi M, Pacioni S, et al. Inhibition of autophagy increases susceptibility of glioblastoma stem cells to temozolomide by igniting ferroptosis. Cell Death Dis. 2018;9(8):841. doi: 10.1038/s41419-018-0864-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Yang M, Kang R, Klionsky DJ, Tang D. Autophagic degradation of the circadian clock regulator promotes ferroptosis. Autophagy. 2019;15(11)1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang M, Chen P, Liu J, et al. Clockophagy is a novel selective autophagy process favoring ferroptosis. Sci Adv. 2019;5(7):eaaw2238. doi: 10.1126/sciadv.aaw2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klionsky DJ, Abdelmohsen K, Abe A, et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy. 2016;12(1):1–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Song X, Zhu S, Chen P, et al. AMPK-mediated BECN1 phosphorylation promotes ferroptosis by directly blocking system Xc(-) activity. Curr Biol. 2018;28(15):2388–2399 e2385. doi: 10.1016/j.cub.2018.05.094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hong SH, Lee DH, Lee YS, et al. Molecular crosstalk between ferroptosis and apoptosis: emerging role of ER stress-induced p53-independent PUMA expression. Oncotarget. 2017;8(70):115164–115178. doi: 10.18632/oncotarget.23046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li Z, Jiang L, Chew SH, Hirayama T, Sekido Y, Toyokuni S. Carbonic anhydrase 9 confers resistance to ferroptosis/apoptosis in malignant mesothelioma under hypoxia. Redox Biol. 2019;26:101297. doi: 10.1016/j.redox.2019.101297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sato M, Kusumi R, Hamashima S, et al. The ferroptosis inducer erastin irreversibly inhibits system xc- and synergizes with cisplatin to increase cisplatin’s cytotoxicity in cancer cells. Sci Rep. 2018;8(1):968. doi: 10.1038/s41598-018-19213-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen D, Tavana O, Chu B, et al. NRF2 is a major target of ARF in p53-independent tumor suppression. Mol Cell. 2017;68(1):224–232 e224. doi: 10.1016/j.molcel.2017.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim EH, Shin D, Lee J, Jung AR, Roh JL. CISD2 inhibition overcomes resistance to sulfasalazine-induced ferroptotic cell death in head and neck cancer. Cancer Lett. 2018;432:180–190. doi: 10.1016/j.canlet.2018.06.018 [DOI] [PubMed] [Google Scholar]
- 39.Kyani A, Tamura S, Yang S, et al. Discovery and mechanistic elucidation of a class of PDI inhibitors for the treatment of glioblastoma. ChemMedChem. 2017;13(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang Y, Shi J, Liu X, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181–1192. doi: 10.1038/s41556-018-0178-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yang WS, SriRamaratnam R, Welsch ME, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156(1–2):317–331. doi: 10.1016/j.cell.2013.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kinowaki Y, Kurata M, Ishibashi S, et al. Glutathione peroxidase 4 overexpression inhibits ROS-induced cell death in diffuse large B-cell lymphoma. Lab Invest. 2018;98(5):609–619. doi: 10.1038/s41374-017-0008-1 [DOI] [PubMed] [Google Scholar]
- 43.Zou Y, Palte MJ, Deik AA, et al. A GPX4-dependent cancer cell state underlies the clear-cell morphology and confers sensitivity to ferroptosis. Nat Commun. 2019;10(1):1617. doi: 10.1038/s41467-019-09277-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhu S, Zhang Q, Sun X, et al. HSPA5 regulates ferroptotic cell death in cancer cells. Cancer Res. 2017;77(8):2064–2077. doi: 10.1158/0008-5472.CAN-16-1979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shin D, Kim EH, Lee J, Roh JL. Nrf2 inhibition reverses resistance to GPX4 inhibitor-induced ferroptosis in head and neck cancer. Free Radic Biol Med. 2018;129:454–462. doi: 10.1016/j.freeradbiomed.2018.10.426 [DOI] [PubMed] [Google Scholar]
- 46.Sui X, Zhang R, Liu S, et al. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9:1371. doi: 10.3389/fphar.2018.01371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kerins MJ, Milligan J, Wohlschlegel JA, Ooi A. Fumarate hydratase inactivation in hereditary leiomyomatosis and renal cell cancer is synthetic lethal with ferroptosis induction. Cancer Sci. 2018;109(9):2757–2766. doi: 10.1111/cas.13701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaschler MM, Andia AA, Liu H, et al. FINO2 initiates ferroptosis through GPX4 inactivation and iron oxidation. Nat Chem Biol. 2018;14(5):507–515. doi: 10.1038/s41589-018-0031-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jiang L, Kon N, Li T, et al. Ferroptosis as a p53-mediated activity during tumour suppression. Nature. 2015;520(7545):57–62. doi: 10.1038/nature14344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jiang L, Hickman JH, Wang SJ, Gu W. Dynamic roles of p53-mediated metabolic activities in ROS-induced stress responses. Cell Cycle. 2015;14(18):2881–2885. doi: 10.1080/15384101.2015.1068479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jennis M, Kung CP, Basu S, et al. An African-specific polymorphism in the TP53 gene impairs p53 tumor suppressor function in a mouse model. Genes Dev. 2016;30(8):918–930. doi: 10.1101/gad.275891.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ou Y, Wang SJ, Li D, Chu B, Gu W. Activation of SAT1 engages polyamine metabolism with p53-mediated ferroptotic responses. Proc Natl Acad Sci U S A. 2016;113(44):E6806–E6812. doi: 10.1073/pnas.1607152113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei G, Sun J, Hou Z, et al. Novel antitumor compound optimized from natural saponin Albiziabioside A induced caspase-dependent apoptosis and ferroptosis as a p53 activator through the mitochondrial pathway. Eur J Med Chem. 2018;157:759–772. doi: 10.1016/j.ejmech.2018.08.036 [DOI] [PubMed] [Google Scholar]
- 54.Huang C, Yang M, Deng J, Li P, Su W, Jiang R. Upregulation and activation of p53 by erastininduced reactive oxygen species contribute to cytotoxic and cytostatic effects in A549 lung cancer cells. Oncol Rep. 2018;40(4):2363–2370. doi: 10.3892/or.2018.6585 [DOI] [PubMed] [Google Scholar]
- 55.Mao C, Wang X, Liu Y, et al. A G3BP1-interacting lncRNA promotes ferroptosis and apoptosis in cancer via nuclear sequestration of p53. Cancer Res. 2018;78(13):3484–3496. doi: 10.1158/0008-5472.CAN-17-3454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tarangelo A, Magtanong L, Bieging-Rolett KT, et al. p53 suppresses metabolic stress-induced ferroptosis in cancer cells. Cell Rep. 2018;22(3):569–575. doi: 10.1016/j.celrep.2017.12.077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xie Y, Zhu S, Song X, et al. The tumor suppressor p53 limits ferroptosis by blocking DPP4 activity. Cell Rep. 2017;20(7):1692–1704. doi: 10.1016/j.celrep.2017.07.055 [DOI] [PubMed] [Google Scholar]
- 58.Tomita K, Fukumoto M, Itoh K, et al. MiR-7-5p is a key factor that controls radioresistance via intracellular Fe(2+) content in clinically relevant radioresistant cells. Biochem Biophys Res Commun. 2019;518:712–718. doi: 10.1016/j.bbrc.2019.08.117 [DOI] [PubMed] [Google Scholar]
- 59.Luo M, Wu L, Zhang K, et al. miR-137 regulates ferroptosis by targeting glutamine transporter SLC1A5 in melanoma. Cell Death Differ. 2018;25(8):1457–1472. doi: 10.1038/s41418-017-0053-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhang K, Wu L, Zhang P, et al. miR-9 regulates ferroptosis by targeting glutamic-oxaloacetic transaminase GOT1 in melanoma. Mol Carcinog. 2018;57(11):1566–1576. doi: 10.1002/mc.v57.11 [DOI] [PubMed] [Google Scholar]
- 61.Drayton RM, Dudziec E, Peter S, et al. Reduced expression of miRNA-27a modulates cisplatin resistance in bladder cancer by targeting the cystine/glutamate exchanger SLC7A11. Clin Cancer Res. 2014;20(7):1990–2000. doi: 10.1158/1078-0432.CCR-13-2805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yu H, Han Z, Xu Z, An C, Xu L, Xin H. RNA sequencing uncovers the key long non-coding RNAs and potential molecular mechanism contributing to XAV939-mediated inhibition of non-small cell lung cancer. Oncol Lett. 2019;17(6):4994–5004. doi: 10.3892/ol.2019.10191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang M, Mao C, Ouyang L, et al. Long noncoding RNA LINC00336 inhibits ferroptosis in lung cancer by functioning as a competing endogenous RNA. Cell Death Differ. 2019;26:2329–2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Alvarez SW, Sviderskiy VO, Terzi EM, et al. NFS1 undergoes positive selection in lung tumours and protects cells from ferroptosis. Nature. 2017;551(7682):639–643. doi: 10.1038/nature24637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wang GX, Tu HC, Dong Y, et al. DeltaNp63 inhibits oxidative stress-induced cell death, including ferroptosis, and cooperates with the BCL-2 family to promote clonogenic survival. Cell Rep. 2017;21(10):2926–2939. doi: 10.1016/j.celrep.2017.11.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen MS, Wang SF, Hsu CY, et al. CHAC1 degradation of glutathione enhances cystine-starvation-induced necroptosis and ferroptosis in human triple negative breast cancer cells via the GCN2-eIF2alpha-ATF4 pathway. Oncotarget. 2017;8(70):114588–114602. doi: 10.18632/oncotarget.23055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chen Y, Zhu G, Liu Y, et al. O-GlcNAcylated c-Jun antagonizes ferroptosis via inhibiting GSH synthesis in liver cancer. Cell Signal. 2019;63:109384. doi: 10.1016/j.cellsig.2019.109384 [DOI] [PubMed] [Google Scholar]
- 68.Hao S, Yu J, He W, et al. Cysteine dioxygenase 1 mediates erastin-induced ferroptosis in human gastric cancer cells. Neoplasia. 2017;19(12):1022–1032. doi: 10.1016/j.neo.2017.10.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Jiang Y, Mao C, Yang R, et al. EGLN1/c-Myc induced lymphoid-specific helicase inhibits ferroptosis through lipid metabolic gene expression changes. Theranostics. 2017;7(13):3293–3305. doi: 10.7150/thno.19988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Du L, Qiao Y, et al. Ferroptosis is governed by differential regulation of transcription in liver cancer. Redox Biol. 2019;24:101211. doi: 10.1016/j.redox.2019.101211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang K, Zhang Z, Wang M, et al. Role of GRP78 inhibiting artesunate-induced ferroptosis in KRAS mutant pancreatic cancer cells. Drug Des Devel Ther. 2019;13:2135–2144. doi: 10.2147/DDDT.S199459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wenz C, Faust D, Linz B, et al. t-BuOOH induces ferroptosis in human and murine cell lines. Arch Toxicol. 2018;92(2):759–775. doi: 10.1007/s00204-017-2066-y [DOI] [PubMed] [Google Scholar]
- 73.Du J, Wang T, Li Y, et al. DHA inhibits proliferation and induces ferroptosis of leukemia cells through autophagy dependent degradation of ferritin. Free Radic Biol Med. 2019;131:356–369. doi: 10.1016/j.freeradbiomed.2018.12.011 [DOI] [PubMed] [Google Scholar]
- 74.Chen GQ, Benthani FA, Wu J, Liang D, Bian ZX, Jiang X. Artemisinin compounds sensitize cancer cells to ferroptosis by regulating iron homeostasis. Cell Death Differ. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mbaveng AT, Ndontsa BL, Kuete V, et al. A naturally occuring triterpene saponin ardisiacrispin B displayed cytotoxic effects in multi-factorial drug resistant cancer cells via ferroptotic and apoptotic cell death. Phytomedicine. 2018;43:78–85. doi: 10.1016/j.phymed.2018.03.035 [DOI] [PubMed] [Google Scholar]
- 76.Park S, Oh J, Kim M, Jin EJ. Bromelain effectively suppresses Kras-mutant colorectal cancer by stimulating ferroptosis. Anim Cells Syst (Seoul). 2018;22(5):334–340. doi: 10.1080/19768354.2018.1512521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang L, Cai H, Hu Y, et al. A pharmacological probe identifies cystathionine beta-synthase as a new negative regulator for ferroptosis. Cell Death Dis. 2018;9(10):1005. doi: 10.1038/s41419-018-1063-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chang LC, Chiang SK, Chen SE, Yu YL, Chou RH, Chang WC. Heme oxygenase-1 mediates BAY 11-7085 induced ferroptosis. Cancer Lett. 2017;416:124–137. doi: 10.1016/j.canlet.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 79.Malfa GA, Tomasello B, Acquaviva R, et al. Betula etnensis Raf. (Betulaceae) extract induced HO-1 expression and ferroptosis cell death in human colon cancer cells. Int J Mol Sci. 2019;20:11. doi: 10.3390/ijms20112723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yamaguchi Y, Kasukabe T, Kumakura S. Piperlongumine rapidly induces the death of human pancreatic cancer cells mainly through the induction of ferroptosis. Int J Oncol. 2018;52(3):1011–1022. doi: 10.3892/ijo.2018.4259 [DOI] [PubMed] [Google Scholar]
- 81.Sun X, Niu X, Chen R, et al. Metallothionein-1G facilitates sorafenib resistance through inhibition of ferroptosis. Hepatology. 2016;64(2):488–500. doi: 10.1002/hep.28574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mbaveng AT, Fotso GW, Ngnintedo D, et al. Cytotoxicity of epunctanone and four other phytochemicals isolated from the medicinal plants Garcinia epunctata and Ptycholobium contortum towards multi-factorial drug resistant cancer cells. Phytomedicine. 2018;48:112–119. doi: 10.1016/j.phymed.2017.12.016 [DOI] [PubMed] [Google Scholar]
- 83.Ooko E, Saeed ME, Kadioglu O, et al. Artemisinin derivatives induce iron-dependent cell death (ferroptosis) in tumor cells. Phytomedicine. 2015;22(11):1045–1054. doi: 10.1016/j.phymed.2015.08.002 [DOI] [PubMed] [Google Scholar]
- 84.Wang S, Li F, Qiao R, et al. Arginine-rich manganese silicate nanobubbles as a ferroptosis-inducing agent for tumor-targeted theranostics. ACS Nano. 2018;12:12380–12392. doi: 10.1021/acsnano.8b06399 [DOI] [PubMed] [Google Scholar]
- 85.Liu T, Liu W, Zhang M, et al. Ferrous-supply-regeneration nanoengineering for cancer-cell-specific ferroptosis in combination with imaging-guided photodynamic therapy. ACS Nano. 2018;12:12181–12192. doi: 10.1021/acsnano.8b05860 [DOI] [PubMed] [Google Scholar]
- 86.Shen Z, Liu T, Li Y, et al. Fenton-reaction-acceleratable magnetic nanoparticles for ferroptosis therapy of orthotopic brain tumors. ACS Nano. 2018;12(11):11355–11365. doi: 10.1021/acsnano.8b06201 [DOI] [PubMed] [Google Scholar]
- 87.Yue L, Dai Z, Chen X, et al. Development of a novel FePt-based multifunctional ferroptosis agent for high-efficiency anticancer therapy. Nanoscale. 2018;10(37):17858–17864. doi: 10.1039/C8NR05150J [DOI] [PubMed] [Google Scholar]
- 88.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–D97. doi: 10.1093/nar/gkt1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhu T, Shi L, Yu C, et al. Ferroptosis promotes photodynamic therapy: supramolecular photosensitizer-inducer nanodrug for enhanced cancer treatment. Theranostics. 2019;9(11):3293–3307. doi: 10.7150/thno.32867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lai Y, Zhang Z, Li J, et al. STYK1/NOK correlates with ferroptosis in non-small cell lung carcinoma. Biochem Biophys Res Commun. 2019;519(4):659–666. doi: 10.1016/j.bbrc.2019.09.032 [DOI] [PubMed] [Google Scholar]
- 91.Ma S, Dielschneider RF, Henson ES, et al. Ferroptosis and autophagy induced cell death occur independently after siramesine and lapatinib treatment in breast cancer cells. PLoS One. 2017;12(8):e0182921. doi: 10.1371/journal.pone.0182921 [DOI] [PMC free article] [PubMed] [Google Scholar]