ABSTRACT
A Cry1Ac-expressing sugarcane cultivar, CTC91087-6, has been developed by Centro de Tecnologia Canavieira (CTC) to be resistant to the sugarcane borer (Diatraea saccharalis). This genetically modified event was developed using Agrobacterium-mediated transformation and the help of the selectable marker phosphinothricin N-acetyltransferase (PAT) expressed from bar gene. We describe here a detailed characterization of CTC91087-6 event with respect to protein expression, nutritional composition, and assessment of its derived DNA and proteins in raw sugar. Expression of the Cry1Ac and PAT (bar) proteins produced by CTC91087-6 was evaluated in different tissues and at different times during the growing season. The new proteins are preferentially expressed in leaves, are produced at low levels in stalks, and are near the limits of detection in root tissues. The levels of Cry1Ac were much higher than PAT in all evaluated tissues. Furthermore, Cry1Ac levels in CTC91087-6 leaves are stable at various times during sugarcane cultivation cycle, assuring borer control throughout the complete crop cycle. Assessment of CTC91087-6 tissues for key food and feed nutrients as recommended by OECD to assess the safety of new varieties of sugarcane showed compositional equivalence to the conventional counterpart CTC9001 and to other commercial sugarcane varieties used as references. Raw sugar samples produced from CTC91087-6 did not contain DNA corresponding to cry1Ac and bar genes nor DNA specifically derived from CTC91087-6. In a similar way, there is no detection of Cry1Ac and PAT proteins in raw sugar produced from CTC91087-6. Taken together these results show that CTC91087-6 stably expresses Cry1Ac and PAT proteins and is substantially equivalent to the conventional counterpart CTC9001.
KEYWORDS: cry1Ac, genetically modified, insect resistance, nutritional composition, sugar, sugarcane
INTRODUCTION
Sugarcane is cultivated in more than 100 countries mostly located in tropical and sub-tropical regions of the world. According to FAO, Brazil accounted for 40% of world´s sugarcane production,1 making the country the largest sugarcane producer and an important player in the international sugar market. The sugarcane industry plays a key role in the Brazilian economy due to its importance in generating income, jobs, and foreign earnings. This sector is responsible for approximately two percent of the country’s entire Gross Domestic Product (GDP) and annually grosses US$ 43.6 billion dollars through the sale of sugar, ethanol fuel, and bioelectricity.2
Sugarcane production is harmed by diseases and pests commonly found in tropical regions. The sugarcane borer, Diatraea saccharalis (Fabricius, 1794) (Lepidoptera – Crambidae), is considered the major pest occurring in the Brazilian sugarcane fields. After mating, each borer female lays 200 to 400 eggs on both sides of sugarcane leaves. The neonate larvae feed on the leaves’ parenchyma, migrating to the sheath region looking for shelter and, after one ecdysis, borer larvae drill the stalk bark and starts feeding on sugarcane stalks. In this phase of development, the insect causes substantial economic damages to the crop.3,4 Borer infestation may cause yield losses of over 10% and reduce sugar quality due to the presence of undesirable secondary metabolites and poor color characteristics.5,6
Genetic manipulation through modern biotechnology makes feasible the insertion of insect resistance genes into the sugarcane genome, thereby providing more favorable characteristics and helping the Brazilian sugarcane industry. In 1998, Brazil approved a herbicide-tolerant soybean, the first biotech-derived crop approved by the Brazilian biosafety authority.7 Since then, many other genetically modified (GM) crops including soybeans, maize, cotton, common beans, and eucalyptus have been approved and currently, Brazil’s area planted with GM crops adds up to 50.2 million hectares, the second largest genetically modified crop area in the world.8
The development of GM sugarcane was delayed due to the complexity of sugarcane genome that delayed the molecular characterization of selected events and, most importantly, still today prevents the use of conventional breeding techniques to introgress the desirable GM trait into the several sugarcane genetic backgrounds needed by sugarcane mills. In 2017, CTNBio approved the cultivation of CTC175-A, the first GM sugarcane developed to control the sugarcane borer in Brazilian cultivation fields.7 CTC175-A expresses Cry1Ab protein and it was developed by the Centro de Tecnologia Canavieira (CTC) to be planted in rich soils found in the Brazilian Center-South, the main Brazilian producer region. To support the Brazilian sugarcane industry with an additional GM sugarcane variety suitable to more diverse cultivation conditions, CTC has developed CTC91087-6 event, a Cry1Ac-expressing sugarcane that is also resistant to the borer. This event was approved for cultivation in Brazil in 2018.7
Before the commercial release of any genetically modified crop, several tests are performed and submitted for evaluation by regulatory authorities. These tests include the experiments to develop and verify the efficacy of the cultivar and assays to ensure environmental, food and feed safety of the new variety. Here we report some of the tests performed on CTC91087-6 variety, including the evaluation of the presence of the new proteins, Cry1Ac and PAT (bar) in sugarcane tissues throughout the crop cycle. This assessment is critical to evaluate the potential of Diatraea saccharalis control as well as human and animal exposure from food and feed consumptions. We also report the evaluation of the composition of CTC91087-6 stalks and forage to assess substantial equivalence to the conventional counterpart CTC9001 and commercial sugarcane references as substantial equivalence is a key step in the food and feed safety assessment of new GM cultivars. Additionally, as sugarcane is mostly consumed by humans through sugar ingestion, we also investigate the presence of new proteins and foreign DNA in the final product, raw sugar.
Results present here indicate that CTC91087-6 expresses Cry1Ac preferentially in leaves at levels required to control borer at its initial life-cycle developmental stage throughout sugarcane cultivation cycle and that this new GM cultivar is substantially equivalent to its conventional counterpart. Sugar produced from CTC91087-6 is indistinguishable from sugar produced from conventional sugarcane. Therefore, there is no evidence that the consumption of CTC91087-6 derived food and feed should pose any additional risk to human and animal health.
MATERIALS AND METHODS
Material
CTC91087-6 was developed by Agrobacterium tumefaciens mediated transformation of CTC9001 sugarcane variety. CTC9001 is a modern sugarcane hybrid that holds several desirable agronomic characteristics such as adaptability to mechanical harvest system adopted by the majority of the Brazilian sugarcane growers. CTC9001 has also the genetic potential for high ratoon cane sprouting vigor, high yield, excellent ratooning, low fiber, and high sugar content. Although CTC9001 cultivar is resistant to leaf scald, smut, brown and orange rust diseases, it is susceptible to D. saccharalis attack as are all modern sugarcane hybrids.
The selection of CTC91087-6 as an elite event as well as the molecular characterization of this event was performed by CTC researchers (Almeida, manuscript in press). Briefly, CTC91087-6 presents a single T-DNA insertion in its genome containing the intact cassettes for Cry1Ac and PAT (bar) expression (Figure 1).
FIGURE 1.
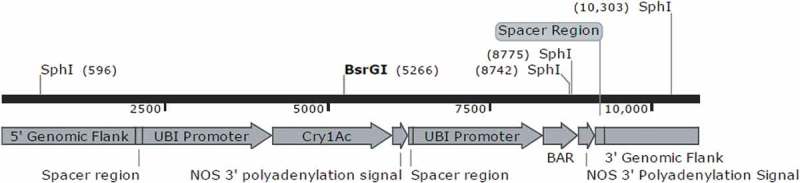
Map (10,710 bp) of the full T-DNA insert flanked by genomic regions of CTC91087-6 event.
The cry1Ac gene produces a delta-endotoxin protein originated from Bacillus thuringiensis, that is toxic to Lepidoptera insects, including the sugarcane borer.9 The cry1Ac gene encodes a 615 amino acid toxin with an estimated molecular weight of 68 KDa and insecticidal tryptic core of 52 KDa. The gene was synthetically synthetized using sugarcane-preferred codons.9 The mode of action of this protein requires the specific interaction with insect gut receptors disrupting gut function and integrity and resulting in insect toxicity and death.10 The expression of Cry1Ac in CTC91087-6 is regulated by the ubi-1 promoter of polyubiquitin protein from maize11 and the nos terminator from Agrobacterium tumefaciens.12
CTC91087-6 also expresses the phosphinothricin-N-acetyl transferase (PAT) from bar gene, isolated from Streptomyces hygroscopicus. PAT protein catalyzes the acetylation and detoxication of glufosinate used as in vitro selectable marker.13 The PAT (bar) expression in CTC91087-6 is regulated by the same genetic regulatory elements, ubi-1 and nos, used in the Cry1Ac cassette.
Field Trials
After transformation, insect-resistant events were planted in the field during one crop season when CTC91087-6 was selected as the lead event based on its molecular profile (single insertion with well-defined flanking sequences and the presence of intact protein expression cassettes) and superior efficacy in borer control. CTC91087-6 plants from this first field trial were harvested and used to produce seedlings for next season regulatory trials. Therefore, regulatory trials were conducted using T1 (one generation after transformation) plants.
Regulatory field trials were established, during the 2017/18 crop season, in five Brazilian representative regions of CTC9001 cultivations areas: Barrinha, Piracicaba, Valparaíso (São Paulo State) and Quirinópolis (Goiás State) in the Brazilian South Center regions, and Camamu (Bahia State) in the Brazilian Northeast. The trials were carried out using a randomized complete block design (RCBD) with four replicates. At each experiment, plots of genetically modified CTC91087-6, parental control CTC9001, as well as four sugarcane commercial varieties were planted. All plots were comprised of four rows, spaced 1.5 m apart. The row length ranged from 4 to 12 m, depending on the location.
The efficacy of CTC91087-6 in controlling D. saccharalis was measured as infestation intensity (%I.I.). All tillers from 5 selected clumps per plot were artificially infested with approximately 30 borer eggs, 5 times at 30-day intervals. After 10 months all tillers in the selected clumps were split longitudinally to quantify damage. The infestation rate was reported as damaged internodes/total internodes per tiller.
Sampling for Composition and ELISA Analysis
All regulatory experiments were sampled for composition and expression analysis.
Stalks and whole plants were sampled for compositional analysis according to OECD14 recommendation. For whole-plant sampling, 10 plants were randomly harvested from the plots. After discarding old leaves and crushing, samples were frozen. Stalk samples were also harvested from 10 randomly chosen plants, crushed and frozen. All frozen samples were sent to the laboratory and stored at −80°C until processing.
Expression analyses were performed in CTC91087-6 leaf, stalk, and root tissues. Leaf Cry1Ac and PAT protein expressions were monitored at several time points to evaluate expression stability required for borer control throughout the sugarcane cultivation cycle. In the plant cane experiment, leaf samples were collected at 100, 200, and 300 Days After Planting (DAP). In the ratoon cane experiment, leaves were collected at various times before (60 and 120 DAP) and after cutting (60 and 120 DAC – Days After Cutting). Stalk and root Cry1Ac and PAT expression were assessed at the end of the sugarcane cultivation cycle to evaluate environmental and dietary exposure to the newly expressed proteins.
Leaves were sampled by harvesting 30 cm of tissue from leaf top, from five to ten leaves. After discarding the midrib, leaves were cut into pieces and frozen. Stalk samples were produced the same way as described previously for stalk composition samples. Roots were sampled by digging up a complete ratoon, washing root system to remove adhered soil, cutting exposed roots into small pieces and then frozen. All frozen samples were sent to the laboratory and stored at −80°C until processing.
Compositional Analysis
Whole plant samples and stalk samples of CT91087-6, CTC9001 and commercial reference cultivars (CTC4, CTC20, RB855156, RB867515) were collected at 330 ± 5 days after planting in all five replicated field trials. The composition of samples was analyzed based on the recommendations of OECD Guidance Document for nutritional assessment of new varieties of sugarcane.14 OECD stands that there are no toxins, anti-nutrients or known allergens in sugarcane and that the main contribution of sugarcane to the human diet is sugar (sucrose). Therefore, OECD recommends that only major constituents be measured in new sugarcane varieties, and that these constitutes should be measured in whole cane (comprising stalks and leaves). The exception to this is sucrose content, which is traditionally measured in the stalk only.
Recommended parameters and analytical methods used for compositional analysis were: moisture (AOAC 935.29), dry weight (AOAC 935.29), crude protein (AOAC 2001.11), total fat/ether extract (AOAC 2003.06), crude fiber (Ankom, Method 1), neutral detergent fiber – NDF (Ankom Method 13), acid detergent fiber – ADF (Ankom Method 12), ashes (AOAC 942.05), and sucrose (ICUMSA, Method GS7/8/4-24, 2011). In addition to OECD recommendation, the concentrations of fructose (ICUMSA, Method GS7/8/4-24, 2011) and glucose (ICUMSA, Method GS7/8/4-24, 2011) were also measured.
Protein Expression Analysis
Detailed expression analysis of Cry1Ac and PAT (bar) proteins was carried out in leaf, stalk and root tissues of CTC91087-6 event, collected in all five replicated field trials, during the 2017/18 crop season. Total protein extraction for ELISA used 30 mg of leaf, 60 mg of stalk and 200 mg of root tissues. After extraction, total protein samples in triplicate were quantified by Bradford or BCA methodologies and equalized to a concentration of 150 µg/mL to normalize the amount of protein per sample.
For subsequent analysis of Cry1Ac and PAT (bar) proteins, samples were normalized to the concentrations of 1 and 30 µg/mL of total protein, respectively, to ensure protein concentrations within the range of reliable quantification. Finally, Cry1Ac and PAT (bar) expression levels were determined by ELISA assays using the commercially available kits “Envirologix AP003 CRBS, (Portland, Maine, USA)” and “Envirologix AP013 BAR (Portland, Maine, USA)”, according to manufacturer’s instructions. Since these kits do not provide direct quantification, the analysis was based on the comparison of absorbance values of test samples with values predicted from a standard curve of absorbance measurements of commercial synthetic proteins of known concentration.
ELISA assays were validated according to Armbruster and Pry.15 The theoretical LOD (Limit Of Detection) for ELISA assays was calculated using the average OD values obtained for 12 non-GM samples (CTC9001) plus three standard deviations. The Limit Of Quantification (LOQ) was estimated using CTC9001 sample extracts spiked with known concentrations of reference proteins and measuring the standard deviation (SD), coefficient of variation (CV), and relative error (RE) for each concentration. The lowest reliable values are considered the LOQ of the assay.
Preferentially, protein expression data were reported as µg protein/g dry weight (DW) tissue to simplify treatment comparisons. The exceptions are the measurement of protein expression in roots that were expressed as µg protein/g fresh weight (FW) tissue. The levels of stalk proteins, in FW basis, were also reported when used to infer food and feed exposure.
DNA and Protein Detection in Raw Sugar
Fully mature stalks of CTC91087-6 and CTC9001 were collected from plots of Piracicaba trial for sugar production. Eighty to 100 stalks per plot were collected and processed into raw sugar using a laboratory-scale method that mimics industrial sugar production. Briefly, harvested stalks were shredded and pressed to obtain 40 L of extracted juice that was clarified, concentrated until approximately 65° Brix, and crystallized.16–18 These sugar samples were used for further analysis of cry1Ac and bar target DNA using conventional PCR analysis, detection of Cry1Ac and PAT (bar) proteins using ELISA and for specific detection of the DNA from CTC91087-6 event using a qPCR analysis. Four independent samples from both treatments, CTC91087-6 and CTC9001, were used for each analysis.
Detection of DNA sequences from cry1Ac and bar genes was performed by EUROFINS do Brasil (https://www.eurofins.com.br/) using conventional PCR. Briefly, DNA was extracted using the CTAB methodology, quantified by spectrophotometry and evaluated in a 1.5% agarose gel. Amplifications were performed using EUROFINS proprietary detection kit (GBA74 kit). The detection limit of the method is 0.01% determined with DNA from pure and unprocessed flour. The PCR method was performed in 30 cycles of amplification.
An event-specific assay was also employed to search for specific nucleotide sequences from CTC91087-6 event in raw sugar. This was performed using TaqMan® technology and a set of primers and probes that specifically detect sequences at the junction of T-DNA 3ʹ-end and CTC91087-6 genome flanking sequence.
Briefly, DNA extraction from raw sugar was performed using the NucleoSpin® Plant II commercial kit following the manufacturer’s recommendations (MACHEREY-NAGEL GmbH & Co. KG, Germany). Amplifications used the forward primer (5ʹ-CGTTTCCCGCCTTCAGTTTA-3ʹ), the reverse primer (5ʹ-GCCGTTATGTTGGAAGTAGG -3ʹ) and the specific probe (FAM -5ʹ- CGTGTTCCTATCGCCAGC-3ʹ-MGB). TaqMan® real-time PCR reactions were performed using a 7500 real-time PCR System (Applied Biosystems, USA). CTC91087-6 specific DNA sequences were amplified using one cycle of 2 min at 50°C for uracil-N-glycosylase activation; one cycle of 20 s at 95°C for DNA polymerase activation; and 40 cycles of 95°C for 3 s (denaturation) and 60°C for 30 s (annealing and extension). The sugarcane polyubiquitin gene was used as endogenous control, in a multiplex reaction, to confirm the presence and quality of the DNA used (forward primer, 5ʹ TCGCCCGCCGTAATAAATAG 3ʹ; reverse primer, 5ʹ ATCTGGTTGTGTGTGTGTGCG 3ʹ; probe, VIC -5ʹ CTCCACACCCTCTTT 3ʹ-MGB).
The event-specific assay was validated according to Armbruster and Pry.15 Briefly, the LOB (Limit Of Blank) of the assay was estimated using 95 percentile of detectable Cts values from CTC9001 sugar samples without target DNA spiking. LOD and LOQ estimation were assisted by a dilution curve with raw samples spiked with 5, 1, 0.1 and 0.01 ng of CTC91087-6 DNA, in triplicate. The dilution of lower value, compliant with the following criteria, was determined as the LOD of the assay: 1) to be greater than the limit of blank (LOB); 2) to present standard deviation lower than 1 Ct and, 3) to present detectable values greater than 95% of the total replicates. LOQ was estimated from the lowest concentration that could be accurately quantified, namely, , where is the standard deviation of from the assay.
ELISA detection of Cry1Ac and PAT (bar) proteins in raw sugar were performed in a similar way as described previously for stalk, root and leaf analysis using the same commercially available kits “Envirologix AP003 CRBS” for Cry1Ac and “Envirologix AP013 BAR” for PAT (bar) according to manufacturer’s instructions.
Statistical Analysis
Prior to statistical analysis of composition and protein expression data, a studentized PRESS residual and graphical analysis was applied to each data set to identify influential data. After that, data were analyzed using a linear mixed-model approach. For each data set, data across all sites were combined for statistical analysis. Combined site analysis was done using the following statistical model:
wherein is the measurement of replicate j on site i for treatment k; is the overall mean; is the effect of site i (i = 1 to 5); is the effect of replicate j (j = 1 to 4); is the effect of replicate j on site i (j = 1 to 4); is the effect of the treatment k (k = 1 to 8); is the interaction between site i and treatment k; is the experimental residual error. The statistical model used to evaluate sugar composition data used the same statistical model to data from Piracicaba field trial (i = 1).
The main effects for cultivar were treated as fixed, whereas site and replicates across sites were treated as random. All calculations were performed using R software version 3.4.2.19 The significance of the differences between the genotypic mean values was assessed using statistical tests at a significance level of p ≤ 0.05.
RESULTS AND DISCUSSION
Nutritional Composition of Sugarcane Event CTC91087-6
The main goal of the compositional analysis is to assess whether the production of the biotechnology-improved cultivars leads to the arising of unintended differences in compositional parameters that might render the new variety less nutritious or safe. Compositional comparative characterization of genetically modified and control (non-GM) plants considers the principle of substantial equivalence, i.e., except for the purpose of the genetic modification (the presence of Cry1Ac and PAT (bar) proteins in this particular case) other important parameters are not significantly altered beyond known and acceptable variation. If no significant differences are detected between plants derived from biotechnology and conventional counterpart plants, it is concluded that the new cultivar is compositionally similar to, or substantially equivalent to, the appropriate genetic, non-transformed comparator. If unintended differences are observed, additional testing may be required to confirm safety.
Here we analyzed CTC91087-6 compositional parameters related to nutrition and the use of sugarcane in the diet, as defined by the OECD Guidance Document.14 This reference document concluded, from the literature and experience with sugarcane cultivation and consumption, that sugarcane does not contain any known antinutrients, toxins or allergens; consequently, the compositional analysis described here focused on key parameters related to nutrition and use of sugarcane as food and feed.
Based on the results of combined data analysis, there were no statistically significant differences (p ≤ 0.05) in any comparison of nutritional components between CTC91087-6 and the conventional counterpart CTC9001. Also, all observed CTC91087-6 compositional parameter mean values were within the range of values observed for the commercial reference cultivars grown at the same sites and agronomic conditions, with only one exception: the CTC91087-6 dry matter was slightly above the range of the references; however, the mean value was not statistically different from the mean observed for CTC9001 (Table 1). This result is explained by the higher biomass yield conferred by the CTC9001 genetic background compared to the commercial cultivar references tested.
TABLE 1.
Mean values of compositional parameters measured in genetically modified CTC91087-6 and conventional counterpart CTC9001. Mean values represent five experiments (4 repetitions each) planted at Brazilian sugarcane growing regions (Barrinha, Piracicaba, Valparaíso (São Paulo State); Quirinópolis (Goiás State); and Camamu (Bahia State)).
| Mean ± SEM |
Range of Commercial reference cultivarsc |
|||
|---|---|---|---|---|
| Analyte | CTC91087-6 | CTC9001 | Min | Max |
| Dry matter | 23.48 ± 1.06 | 22.73 ± 1.06 | 20.20 | 22.77 |
| Moisture | 76.29 ± 0.81 | 76.33 ± 0.81 | 76.05 | 78.96 |
| Crude protein1 | 3.38 ± 0.23 | 3.56 ± 0.23 | 2.79 | 4.65 |
| Crude fat1 | 1.19 ± 0.11 | 1.10 ± 0.11 | 0.63 | 1.27 |
| Ash1 | 3.05 ± 0.41 | 3.26 ± 0.41 | 3.02 | 4.34 |
| Crude fiber1 | 25.82 ± 0.79 | 27.33 ± 0.79 | 23.91 | 31.32 |
| NDF1 | 47.79 ± 1.27 | 50.91 ± 1.27 | 46.07 | 56.89 |
| ADFa | 31.10 ± 0.77 | 32.95 ± 0.77 | 29.30 | 37.29 |
| Sucroseb | 11.63 ± 0.84 | 12.17 ± 0.84 | 9.20 | 12.28 |
| Glucoseb | 0.91 ± 0.12 | 0.91 ± 0.12 | 0.62 | 1.11 |
| Fructoseb | 0.75 ± 0.09 | 0.76 ± 0.09 | 0.55 | 0.84 |
a Results are expressed on dry weight basis.
b Values expressed sugarcane stalk basis.
c Minimum and maximum mean values of four commercial reference cultivars; SEM: Standard Error of the Mean. No significant difference between CTC91087-6 event and conventional counterpart CTC9001 according to t-test at p ≤ 0.05.
The key components analyzed are used as indicators of whether the unintended effects of the genetic modification influencing plant metabolism have occurred or not. In conclusion, the results of combined data analysis of compositional of CTC91087-6 grown in five representative locations in the Brazilian sugarcane growing regions establish that this GM sugarcane cultivar is substantially equivalent to the non-GM conventional comparator CTC9001.
Cry1Ac and PAT (bar) Protein Expression in Tissues of CTC91087-6 Sugarcane Event
Leaf concentrations of Cry1Ac remained relatively constant or increased slightly over the plant cane experiment (100, 200 and 300 DAP) with levels of 102.8, 93.7 and 107.6 µg/g DW. In contrast, the expression levels of PAT (bar) were much lower reaching their highest concentration at 100 DAP at 0.64 µg/g DW and statistically decreased at the 200 and 300 DAP time points with mean values of 0.37 µg/g DW leaf (Table 2).
TABLE 2.
Comparisons of mean leaf Cry1Ac and PAT (bar) expression dry weight of genetically modified CTC91087-6 in plant cane cycle (100, 200 and 300 DAP). Values are from combined statistical analysis. Mean values represent five experiments (4 repetitions each) planted at Brazilian sugarcane growing regions (Barrinha, Piracicaba, Valparaíso (São Paulo State); Quirinópolis (Goiás State); and Camamu (Bahia State)).
| Time | Mean ± SEM (µg/g DW) |
|
|---|---|---|
| Cry1Ac | PAT (bar) | |
| 100 DAP | 102.8 ± 5.91 a | 0.64 ± 0.045 a |
| 200 DAP | 93.7 ± 5.91 a | 0.37 ± 0.045 b |
| 300 DAP | 107.6 ± 5.91 a | 0.37 ± 0.045 b |
Mean values ± SEM (Standard Error of the Mean). Values followed by the same letter do not differ from each other according to Tukey’s test at p ≤ 0.05.
In the ratoon cycle experiment, the effect of cutting on leaf expression levels showed that the combined-site leaf Cry1Ac concentrations at the time points were 147.0 and 139.5 µg/g DW at 60 and 120 DAP and 77.5 and 96.1 µg/g DW at 60 and 120 DAC. The PAT expression levels were 0.46, 0.59, 0.54 and 0.64 µg/g DW for the 60 and 120 DAP and 60 and 120 DAC timepoints, respectively. Comparing the times 60 and 120 DAP with 60 and 120 DAC, Cry1Ac levels were statistically lower at 60 and 120 DAC, while PAT levels, even showing statistical differences, were relatively stable (Table 3). However, expression results from the post-cutting time intervals (60 and 120 DAC) for both Cry1Ac and PAT (Table 3) showed levels consistent with the results of plant cane experiment (Table 2), suggesting that the expression levels after cutting were similar to levels observed before cutting.
TABLE 3.
Comparison of means of leaf Cry1Ac and PAT (bar) expression of genetically modified CTC91087-6 before (60 and 120 DAP) and after cutting (60 and 120 DAC). Values are from combined statistical analysis. Mean values represent five experiments (4 repetition each) planted at Brazilian sugarcane growing regions (Barrinha, Piracicaba, Valparaíso (São Paulo State); Quirinópolis (Goiás State); and Camamu (Bahia State)).
| Time | Mean ± SEM (µg/g DW) |
|
|---|---|---|
| Cry1Ac | PAT (bar) | |
| 60 DAP | 147.0 ± 5.17 a | 0.46 ± 0.031 a |
| 120 DAP | 139.5 ± 5.17 a | 0.59 ± 0.031 bc |
| 60 DAC | 77.5 ± 5.17 b | 0.54 ± 0.031 ab |
| 120 DAC | 96.1 ± 5.17 b | 0.64 ± 0.031 c |
Mean values ± SEM (Standard Error of the Mean). Values followed by the same letter do not differ according to Tukey’s test at p ≤ 0.05.
Protein expression values in several crops are known to vary at different time points because of experimental variability. The overall conclusion from the plant cane experiment is that the combined-site Cry1Ac leaf expression ranges observed (93.7–107.6 µg/g DW) are generally replicated in the cutting experiment at 60 and 120 DAC. The values in ratoon cane experiment at 60 and 120 DAP, which were higher than the plant cane experiment values at 100, 200 and 300 DAP, are probably due to the natural variability of measured levels and do not represent a meaningful reduction in Cry1Ac expression as a result of cutting and regrowth. Collectively, the results from the post-cutting time intervals for both Cry1Ac and PAT (bar) showed levels consistent with the results of the plant cane experiment and suggest that the expression levels after cutting were similar to levels observed before cutting.
The Cry1Ac levels found in leaves of CTC91087-6 event confers resistance to D. saccharalis to plants evaluated close to maturity (10 months) (Table 4). In field, the sugarcane borer lays its eggs in sugarcane leaves and the neonates must feed on leaf parenchyma, pass through one ecdysis and, only then, pierce the sugarcane stalks. Due to the insect life cycle, the leaf Cry1Ac levels are more relevant to event efficacy to prevent or lessen the number of pests that enter the stalks, where the relevant economic damage occurs.
TABLE 4.
Mean values of infestation intensity (I.I.%) of four independent field trials (Piracicaba, Barrinha, Valparaíso (São Paulo State) and Quirinópolis (Goiás State)).
| Genotype | Infestation Intensity (I.I.%) Average ± SD |
Tukey 5% |
|---|---|---|
| CTC9001 TC | 29.62 ± 8.17 | a |
| CTC9001 | 30.11 ± 7.83 | a |
| CTC91087-6 | 0.13 ± 0.12 | b |
The combined-site expression data for stalk, which was harvested 330 DAP, showed that mean Cry1Ac and PAT (bar) expression levels were 15.4 µg/g DW and 0.06 µg/g DW, respectively. These results clearly demonstrate that the expression of both proteins at CTC91087-6 stalks was much lower than their expression in CTC91087-6 leaf tissues. The expression values for root tissue harvested at 330 DAP showed that Cry1Ac and PAT (bar) levels were generally below the limit of detection of the methodology (Table 5).
TABLE 5.
Mean values of root and stalk Cry1Ac and PAT (bar) expression at 330DAP in CTC91087-6 event. Mean values represent five experiments (4 repetition each) planted at Brazilian sugarcane growing regions (Barrinha, Piracicaba, Valparaíso (São Paulo State); Quirinópolis (Goiás State); and Camamu (Bahia State)).
| Roota µg/g FW |
Stalkb µg/g DW |
|||
|---|---|---|---|---|
| Site | Cry1Ac | PAT (bar) | Cry1Ac | PAT (bar) |
| Barrinha | <LOQ | <LOD | 16.2 | 0.058 |
| Piracicaba | <LOQ | <LOD | 10.0 | 0.087 |
| Valparaíso | <LOQ | <LOD | 17.6 | 0.062 |
| Quirinópolis | 0.053c | <LOD | 16.0 | 0.035 |
| Camamu | <LOQ | <LOD | 17.1 | 0.061 |
aCry1Ac: LOD ≤ 0.0006 μg/g; LOQ ≤ 0.0012 μg/g; Bar: LOD ≤ 0.00032 μg/g; LOQ ≤ 0.0004 μg/g.
b Cry1Ac: LOD ≤ 0.002 μg/g; LOQ ≤ 0.004 μg/g; Bar: LOD ≤ 0.0011 μg/g; LOQ ≤ 0.0012 μg/g.
c One replicate was higher than the LOQ.
Cry and PAT proteins are among the most studied and used proteins in modern biotechnology products. Both classes of proteins have been used in several genetically modified crop events worldwide for over 20 years, and have a documented history of safe use. Specifically, the toxicologic properties of the Cry1Ac and PAT (bar) protein amino acid sequences or homologs have been comprehensively studied and widely accepted by scientists and regulators worldwide. Toxicology studies of orally administered purified Cry1Ac and PAT (pat) proteins have established very high No Observed Adverse Effect Levels (NOAEL) of approximately 5,000 mg/kg body weight, for both proteins.20,21
The intakes of these expressed proteins resulting from the ingestion of event CTC91087-6 stalks are very low compared with the NOAELs resulting in very high safety margins. As in natura sugarcane is mostly consumed at the informal market in Brazil, there are no official figures for the typical intakes of unprocessed sugarcane or juice. However, given the known expression level of the Cry1Ac and PAT (bar) proteins in stalk of 3.65 and 0.01 µg/g FW, and the NOAELs for both proteins reported in the literature, it is possible to calculate the amount of sugarcane consumption locally in Brazil required to meet the NOAEL value for each protein. Specifically, a 60 kg Brazilian consumer would need to consume approximately 82 and 30,000 metric tons of CTC91087-6 stalks to meet the NOAELs for the Cry1Ac and PAT proteins, respectively. In conclusion, the direct intakes of these proteins from locally prepared and consumed products derived from CTC91087-6 are trivial compared with the amounts shown to be safe in animals.
In Brazil, sugarcane is also harvested to produce forage for cattle during the dry season. Like the human intake example for unprocessed sugarcane, the amount of sugarcane forage needed to exceed the NOAEL values is extremely high. For example, a 200 kg cow would need to consume 1,000 g of each protein to reach the NOAEL value for the Cry1Ac or PAT proteins. Using the stalk protein expression value of 3.65 µg Cry1Ac/g FW stalk, a 200 kg cow would need to consume 273 metric tons of event CTC91087-6 sugarcane stalk forage to exceed the NOAEL.
The most relevant tissue for plant protection is the leaf tissue and times studied throughout the plant cane and ratoon cane experiments showed high and stable Cry1Ac expression over time, consistent with the intended sugarcane borer protection over the growing season and crop harvests. As expected, Cry1Ac and PAT (bar) expression levels were much lower in stalks than in leaves and were virtually not detected in roots.
DNA and Protein Detection in CTC91087-6 Raw Sugar Fractions
Assessment of four lot samples of sugar produced from CTC91087-6 and the conventional counterpart CTC9001 were negative for the presence of DNA sequences with homology to cry1Ac and bar gene. Additionally, the search for CTC91087-6 event specific sequences in raw sugar demonstrated the lack of detection of such sequences in raw sugar (Table 6).
TABLE 6.
Results for the detection of Cry1Ac and PAT (bar) proteins in sugar samples from sugarcane stalks from the GM CTC91087-6 and non-GM CTC9001. Data for protein detection correspond to three technical ELISA assay replicates (n = 3). Samples harvest from Piracicaba (São Paulo State), 4 repetitions.
| Target | Assay | LOD | Lot Number** | Results |
|---|---|---|---|---|
| DNA | Probe Cry1Ac - Eurofins | 0.01% | 1 | NEGATIVE |
| DNA | Probe Cry1Ac - Eurofins | 0.01% | 2 | NEGATIVE |
| DNA | Probe Cry1Ac - Eurofins | 0.01% | 3 | NEGATIVE |
| DNA | Probe Cry1Ac - Eurofins | 0.01% | 4 | NEGATIVE |
| DNA | Probe PAT - Eurofins | 0.01% | 1 | NEGATIVE |
| DNA | Probe PAT - Eurofins | 0.01% | 2 | NEGATIVE |
| DNA | Probe PAT - Eurofins | 0.01% | 3 | NEGATIVE |
| DNA | Probe PAT - Eurofins | 0.01% | 4 | NEGATIVE |
| DNA | Event Specific Assay | 0.008 ng/mg sugar | 1 | < LOD |
| DNA | Event Specific Assay | 0.008 ng/mg sugar | 2 | < LOD |
| DNA | Event Specific Assay | 0.008 ng/mg sugar | 3 | < LOD |
| DNA | Event Specific Assay | 0.008 ng/mg sugar | 4 | < LOD |
| Protein | ELISA Cry1Ac | 0.0085µg/g sugar | 1 | < LOD |
| Protein | ELISA Cry1Ac | 0.0085µg/g sugar | 2 | < LOD |
| Protein | ELISA Cry1Ac | 0.0085µg/g sugar | 3 | < LOD |
| Protein | ELISA Cry1Ac | 0.0085µg/g sugar | 4 | < LOD |
| Protein | ELISA PAT | 0.006µg/g sugar | 1 | < LOD |
| Protein | ELISA PAT | 0.006µg/g sugar | 2 | < LOD |
| Protein | ELISA PAT | 0.006µg/g sugar | 3 | < LOD |
| Protein | ELISA PAT | 0.006µg/g sugar | 4 | < LOD |
Cry1Ac and PAT (bar) proteins were analyzed by ELISA; the results were negative for the presence of these proteins with values below the LOD of the method (LOD Cry1Ac 0.0085 µg/g; LOD PAT (bar) 0.006 µg/g) (Table 6).
These results shown here agree to various studies that have established that sugarcane stalk DNA and proteins are removed from the final product because of the processing at high temperatures, pH modification and flocculation required in sugar production. For example, Cullis et al.22 examined the loss of total DNA and protein in Brazilian sugarcane processing plants and found that neither DNA nor protein was quantifiable in refined sugar. The limits of detection of the methods used were low and were estimated to be 1 ng/g and 1 µg/g of refined sugar for DNA and protein, respectively. Similarly, Cheavegatti-Gianotto et al.23 who examined the loss of DNA and protein of the highly prevalent protein Rubisco, showed that sugarcane processing removed Rubisco DNA and protein to levels that were below the limit of detection of highly sensitive methods. In other words, the processing and refining of sugarcane produces a highly purified food ingredient. Similar findings have been reported for the removal of newly-expressed proteins in GM sugarcane and sugar beets.24,25
The primary human food consumed produced from sugarcane is sugar for both domestic and international consumption. Consequently, the projected “worst-case” theoretical intakes of any sugarcane plant protein, including Cry1Ac and PAT (bar) proteins (at the limit of detection for total stalk protein of 1 µg/g refined sugar), for a 60 kg Brazilian consumer eating 47.6 g of sugar/person/day (Euromonitor Inc, 2016), is 0.79 µg of protein/kg body weight (bw)/day. It is possible to calculate safety margins for both proteins using the NOAEL value divided by the projected “worst-case” daily intakes. Because both proteins have oral NOAEL values of 5,000 mg/kg bw/day, the safety margins for both proteins are 6.3 × 106. Kennedy et al.26 have conducted a similar case study of possible intakes in eight representative countries worldwide, including Brazil, and have shown similar safety margins for these proteins. However, the “worst-case” assumption for the presence of these proteins in the sugar of 1 µg/g sugar is undoubtedly a gross overestimation. The results of analysis of raw sugar produced from event CTC91087-6 sugarcane show that neither protein was detectable in any of the four lots using limits of detection from 0.0085 to 0.006 µg/g sugar compared with the assumed value of 1 µg/g. Therefore, in the case of sugar produced from CTC91087-6 sugarcane, the safety margins for both proteins would approach 1 × 109. Such safety margins reflect the facts that these proteins are non-toxic and are virtually absent from sugar. Therefore, we conclude that processed food ingredients produced from event CTC91087-6 sugarcane are as safe as products processed from conventional sugarcane.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
References
- 1.FAOSTAT Statistic database. Rome (Italy) [accessed 2019 February] http://www.fao.org/faostat/en/#data. [Google Scholar]
- 2.Vasconcelos SP, Carpio GTL.. Bagasse, straws, tips, and vinasse: from sugarcane waste to a clean and renewable bioenergy source. Int J Adv Social Sci Humanities. 2017;5:27–37. [Google Scholar]
- 3.Garcia JF, Botelho PSM.. Diatraea saccharalis (Fabricius, 1794) (Lepidoptera – crambidae) In: Simões Neto DE, Garcia Garcia JF, editors. Cana-de-açúcar: pragas e doenças: desafios fitossanitários e manejo sustentável. Jaboticabal, Brazil: Gráfica Multipress Ltda; 2016. p. 160. [Google Scholar]
- 4.de Pinto AS, Botelho PSM, de Oliveira HN.. Guia ilustrado de pragas e insetos benéficos da cana-de-açúcar. Piracicaba: CP2; 2009. p. 157. [Google Scholar]
- 5.Botelho PSM, Macedo N. Cotesia flavipes para o controle de Diatraea saccharalis In: Parra JRP, Botelho PSM, Correaferreira BS, Bento JMS, editors. Controle biológico no Brasil: parasitoides e predadores. São Paulo, Brazil: Manole; 2002. p. 409–25. [Google Scholar]
- 6.Precetti AACM, Téran FO, Sanchez AG. Alterações nas características tecnológicas de algumas variedades de cana-de-açúcar, devidas ao dano da broca Diatraea saccharalis. Boletim Técnico Copersucar. 1988;40:3–8. [Google Scholar]
- 7.CTNBio (2019) Comissão Técnica Nacional de Biossegurança. [accessed 2019 February] http://ctnbio.mcti.gov.br/en/liberacao-comercial#/liberacao-comercial/consultar-processo.
- 8.ISAAA (2017) Global status of commercialized biotech/GM crops in 2017: biotech crop adoption surges as economic benefits accumulate in 22 years. [accessed 2019 February] http://www.isaaa.org/resources/publications/briefs/53/download/isaaa-brief-53-2017.pdf.
- 9.Weng LX, Deng HH, Xu JL, Li Q, Zhang YQ, Jiang ZD, Zhang LH. Transgenic sugarcane plants expressing high levels of modified cry1Ac provide effective control against stem borers in field trials. Transgenic Res. 2011;20(4):759–72. doi: 10.1007/s11248-010-9456-8. [DOI] [PubMed] [Google Scholar]
- 10.OECD (2007) Consensus document on safety information on transgenic plants expressing Bacillus thuringiensis-derived insect control proteins. ENV/JM/MONO(2007)14. [accessed 2019 February] http://www.oecd.org/env/ehs/biotrack/46815888.pdf.
- 11.Christensen AH, Sharrock RA, Quail PH. Maize polyubiquitin genes: structure, thermal perturbation of expression and transcript splicing, and promoter activity following transfer to protoplasts by electroporation. Plant Mol Biol. 1992;18:675–89. [DOI] [PubMed] [Google Scholar]
- 12.Bevan M, Sarnes WM, Chilton MD. Structure and transcription of the nopaline synthase gene region of T-DNA. Nucl Acid Res. 1983;11(2):369–85. doi: 10.1093/nar/11.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D’Halluin KL, de Block M, Denecke J, Janssens J, Leemans J, Reynaerts A, Botterman J. The bar gene as selectable and screenable marker in plant engineering. Methods Enzymol. 1992;216:415–26. doi: 10.1016/0076-6879(92)16038-l. [DOI] [PubMed] [Google Scholar]
- 14.OECD Consensus document on compositional considerations for new varieties of sugarcane (Saccharum ssp. hybrids): key food and feed nutrients, anti-nutrients and toxicants Series on the safety of novel foods and feeds no. 23. Paris, France: OECD Environment Dictorate; 2011. [accessed 2018 March] https://www.oecd.org/env/ehs/biotrack/48962816.pdf. [Google Scholar]
- 15.Armbruster DA, Pry T. Limit of blank, limit of detection and limit of quantitation. Clin Biochem Rev. 2008;29:S49. [PMC free article] [PubMed] [Google Scholar]
- 16.Merheb GA, Oliveira N, Giulietti M, Bernardo A. Combined effect of starch and dextran in sucrose crystallization. Sugar Ind. 2016;141:697–704. [Google Scholar]
- 17.Novello AP. (2015). Seleção de leveduras para fermentação com alta pressão osmótica usando processo de fermentação extrativa. São Paulo, Brazil: Esalq; PhD Thesis, Esalq, Piracicaba. doi: 10.11606/T.11.2015.tde-29042015-102100. [DOI] [Google Scholar]
- 18.Merheb GA. (2014). Influência da contaminação combinada de dextrana e amido na cristalização do açúcar. PhD Thesis, UFSCar, São Carlos, Brazil: [accessed 2016 August11] https://repositorio.ufscar.br/handle/ufscar/3961. [Google Scholar]
- 19.R Core Team R: A language and environment for statistical computing. Vienna (Austria): R Foundation for Statistical Computing; 2017. https://www.R-project.org/. [Google Scholar]
- 20.EFSA Scientific opinion on an application by Dow AgroSciences (EFSA-GMO-NL-2013-116) for placing on the market of genetically modified insect-resistant soybean DAS-81419-2 for food and feed uses, import and processing under regulation (EC) No 1829/2003. Efsa J. 2016;14:1–23. [Google Scholar]
- 21.Spencer TM, Orozco EM, Doyle RM. Petition for determination of non-regulated status: insect protected corn (Zea mays L.) with the cry1Ac gene from bacillus thuringiensis subsp kurstaki. Dekalb Genetics Corporation; 1996. October 14, 1986. [Google Scholar]
- 22.Cullis C, Contento AL, Schell MA. DNA and protein analysis throughout the industrial process of refining sugar cane. Int J Agric Food Res. 2014;3:1–15. [Google Scholar]
- 23.Cheavegatti-Gianotto A, Gentile A, Oldemburgo DA, Merheb GA, Sereno ML, Lirette RP, Ferreira THS, Oliveira W. Lack of detection of bt sugarcane Cry1Ab and NptII DNA and proteins in sugarcane processing products including raw sugar. Front Bioeng Biotechnol. 2018;6:24. doi: 10.3389/fbioe.2018.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oguchi T, Onishi M, Chikagawa Y, Kodama T, Suzuki E, Kasahara M, AKIYAMA H, TESHIMA R, FUTO S, HINO A, et al. Investigation of DNA in residual sugar from sugar beet (Beta vulgaris l.). Shokuhin Eiseigaku Zasshi. 2009;50:41–46. doi: 10.3358/shokueishi.50.41. [DOI] [PubMed] [Google Scholar]
- 25.Joyce PA, Dinh S-Q, Burns EM, O’Shea MG. Sugar from genetically modified sugarcane: tracking transgenes, transgene products and compositional analysis. Proc Int Soc Sugar Cane Technol. 2013;28:1–9. [Google Scholar]
- 26.Kennedy DR, Gianotto A, de Oliveira SW, Lirette PR, Hjelle JJ. A general safety assessment for purified food ingredients derived from biotechnology crops: case study of Brazilian sugar and beverages produced from insect-protected sugarcane. Frontiers; 2018;6:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- CTNBio (2019) Comissão Técnica Nacional de Biossegurança. [accessed 2019 February] http://ctnbio.mcti.gov.br/en/liberacao-comercial#/liberacao-comercial/consultar-processo.
- ISAAA (2017) Global status of commercialized biotech/GM crops in 2017: biotech crop adoption surges as economic benefits accumulate in 22 years. [accessed 2019 February] http://www.isaaa.org/resources/publications/briefs/53/download/isaaa-brief-53-2017.pdf.
- OECD (2007) Consensus document on safety information on transgenic plants expressing Bacillus thuringiensis-derived insect control proteins. ENV/JM/MONO(2007)14. [accessed 2019 February] http://www.oecd.org/env/ehs/biotrack/46815888.pdf.


