ABSTRACT
Background: Preeclampsia is a pregnancy-related complication and the major cause to maternal and fetal mortality. Despite extensive studies, the pathogenesis of this disease still remains unknown. Here we explored the roles of HIF-1α and Notch1/ETBR in preeclampsia.
Methods: Immunohistochemistry, RT-qPCR and western blot were used to measure levels of Notch1 and ETBR in placentas of preeclampsia patients. Transwell invasion assay and in vitro Matrigel assay were used to test the functions of Notch1, HIF-1α and ETBR in invasion and angiogenesis of trophoblast cells. In addition, we used reduced uterine perfusion pressure (RUPP) rat model to study preeclampsia in vivo.
Results: We found that Notch1 and ETBR were down-regulated in the placenta of patients with preeclampsia. Hypoxia promoted invasion and angiogenesis of trophoblast cells, and up-regulated expressions of HIF-1α, Notch1/ETBR. Overexpression of Notch1 facilitated invasion and angiogenesis of trophoblast cells while HIF-1α inhibitor suppressed. Furthermore, Notch1 or ETBR could promote angiogenesis of trophoblast cells in RUPP rats.
Conclusions: Our study reveals that HIF-1α and Notch1/ETBR play important roles in preeclampsia. Hypoxia-induced HIF-1αregulated Notch1/ETBR signaling, thereby modulating invasion and angiogenesis of trophoblast cells. These results shed light on molecular mechanisms of preeclampsia and provide potential targets for preeclampsia therapy.
KEYWORDS: Preeclampsia, HIF-1α, Notch1
Introduction
Preeclampsia (PE) is a multisystem disorder characterized by hypertension (HTN) and proteinuria and usually develops after 20 weeks of gestation [1,2]. It affects 3–5% of pregnancies worldwide and severe PE is a major cause of maternal and fetal morbidity and mortality. Shallow implantation and defective spiral artery remodeling are the pathological features of PE, leading to placental hypoxia and dysfunction [3]. Many studies have shown that trophoblastic dysfunction, such as inadequate migration and invasion, is closely related to shallow implantation and defective spiral artery remodeling, and thus, is believed as the major cause to PE development [2]. The pathogenesis of PE is poorly understood despite extensive studies and there is clear need to understand the underlying mechanisms to provide better therapy.
Embryogenesis takes place in a hypoxia environment during the first 10 weeks of pregnancy, since plugs of cytotrophoblasts block maternal spiral arteries that supply the placental site [4]. Events that happen later and help convert the maternal–fetal interface from a hypoxic environment to one with adequate oxygen are critical for normal cytotrophoblast cell development [4]. Disruptions in those events could lead to trophoblastic dysfunction and failures in placentation. Placental ischemia causes the release of various factors in the maternal circulation including cytokines (TNFα, IL-6), anti-angiogenic factors (soluble Fms-like tyrosine kinase-1, sFlt-1, soluble endoglin, sENG), reactive oxygen species (ROS), and decreases the pro-angiogenic factors, such as vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β), resulting in systemic endothelial dysfunction [5,6]. Previous studies have implicated Hypoxia-inducible factor 1 (HIF-1) as a key mediator of the hypoxia-induced regulation of those factors [7].
HIF-1 is a master regulator of the cellular response to low oxygen environment and plays crucial role in regulating trophoblast differentiation and invasion, as well as spiral artery remodeling [7]. HIF-1 is heterodimeric consisting of two subunits, α and β [8]. HIF-1α expression is increased in placenta tissues of PE patients and many studies indicate that HIF-1α is an important molecular link between placental hypoxia and downstream mediators of PE [9].
Endothelin-1 (ET-1) is a major vasoconstrictor and has been shown as a key downstream effector in the pathophysiology of PE [10]. ET-1 activates endothelin receptor type A (ETAR) in vascular smooth muscle (VSM) to induce vasoconstriction but activates endothelin receptor type B (ETBR) largely in the endothelium to induce the release of vasodilators [11]. Although the role of ETAR in vascular contraction is well studied, the role of ETBR, particularly during normal pregnancy and hypertension pregnancy, is poorly understood. Here, we hypothesized that ETBR might be a downstream effector of HIF-1α. Previous research suggests that Notch1-STAT3-ETBR signaling is involved in regulating vasodilatation while HIF-1α regulates Delta-like ligand 4 (DLL4)/Notch1 signaling [12,13]. In addition, some studies have reported that ETBR expression is up-regulated in normal pregnant rats and that the ability of blood vessels to relax mediated by ET-1/ETBR is enhanced [14]. Further, in PE rat model, ETBR expression is down-regulated and the ability of blood vessels to relax is reduced as well compared to normal pregnant mice [15]. Administration of ETBR agonist could alleviate the HTN [15]. Therefore, ETBR might be involved in PE although its role remains elusive.
In this study, we fully investigated the functions of HIF-1α and ETBR in PE. We showed that both ETBR and Notch1 were down-regulated in placentas from PE patients. Hypoxia treatment promoted invasion and angiogenesis of trophoblast cells and increased expressions of HIF-1α, Notch1-STAT3-ETBR, and pro-angiogenic factors, but decreased anti-angiogenic factors. Further Notch1 and HIF-1α ovexpression facilitated invasion and angiogenesis of trophoblast cells while HIF-1α over expression could obviouslyreversethe effectcausedbyknockdownofNotch1. Last but not the least, we demonstrated that Notch1 and ETBR promoted angiogenesis of placenta in PE rat model. Together, our study elucidates that HIF-1α regulates angiogenesis via Notch1-STAT3-ETBR pathway in trophoblast cells.
Materials and methods
Placental tissues and human primary trophoblast cell culture
Human placental tissues were collected from 10 patients diagnosed as preeclampsia and 10 age-matched normal pregnant women from Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology from May 2016 to May 2018. There is no statistical difference between two groups in woman age, gestational age and body mass index (BMI). Written informed consent was obtained from each patient and the study was reviewed and approved by the Ethics Committee of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology.
All specimens were collected within 5 min after cesarean birth and from normal areas in the center of placenta maternal surface with exclusion of calcification and bleeding sites. Two specimens were collected from one patient and each specimen was 1 cm × 1 cm × 1 cm. All samples were snap-frozen in liquid nitrogen after wash cleaning and stored in −80°C for further experiments.
For human primary trophoblast cell culture, the placenta tissues were collected under sterile conditions and washed with cold PBS for 3 times. The minced tissues were digested with trypsin and DNAse (200U/ml) for 3 times and supernatant cells were collected, followed by wash with PBS twice. The villous cytotrophoblast cells were isolated in density centrifugation media (Percoll, 300 r/min, 20 min). The isolated trophoblast cells were cultured in RPMI-1640 culture media for 1 h until macrophages were attached to the plate. Seed the supernatant cells onto 24-well plates and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA, USA) and 1% pen-strep in a humidified atmosphere containing 5% CO2.
Plasmids and transfection
Short hairpin (sh) RNA and overexpression vectors were designed and synthesized by Shanghai GenePharma (Shanghai, China), and they were cloned into the lentivirus vectors. For Notch1 or HIF-1α overexpression, Notch1 or HIF-1αfull-length sequence was used and the empty lentivirus vector was used as control. For ETBR shRNA, it was purchased from Santa Cruz USA.
The transfection was performed using lipofectamine 2000 (Invitrogen, USA) according to the manufacturer’s protocol.
Immunohistochemistry
Placenta tissues were embedded in paraffin, cut into 5 μm thick sections and then mounted on glass. The sections were then dried overnight at 37°C and deparaffinized in xylene and rehydrated through a graded concentrations of alcohol. The tissue sections were then quenched in 3% hydrogen peroxide and blocked with 5% bovine serum albumin (BSA) for 1 h, followed by primary antibodies staining overnight at 4°C. After several washed in PBS, the sections were incubated with secondary antibodies for 1 h at room temperature. All slices were then incubated with substrates for Envision system-HRP using the standard kit (Dako REAL Ebision kit; DAKO, Glostrup, Denmark) according to manufacture’s protocol. Images were taken using a light microscope. The following primary antibodies were used: Rabbit polyclonal anti-Notch-1 antibody (1: 200; Santa Cruz, USA), Rabbit polyclonal anti-ETBR antibody (1: 200; Santa Cruz, USA), rabbit polyclonal anti-Von Willebrand factor (vWF; 1: 200; DAKO, Glostrup, Denmark).
Transwell invasion assay
Transfected cells or cells treated with hypoxia were plated on the upper part of a transwell chamber (pre-coated with Matrigel) in serum-free RPMI-1640 and RPMI-1640 with 10% fetal bovine serum was added in the lower part of the chamber as chemoattractant. After 6 h or 12 h of incubation at 37°C, cells inside the upper part were removed. Migrated cells on the lower membrane surface were analyzed by crystal violet staining.
In vitro matrigel assay
Twenty-four-well plates were coated at 4°C with Matrigel solution (BD, Biosciences) and incubated at 37°C for 1 h to allow for polymerization of the Matrigel. Trophoblast cells treated with hypoxia (exposure to 2% oxygen atmosphere) were seeded in the Matrigel-coated wells. After incubation at 37°C for indicated time, images were taken under a phase-contrast inverted microscope at 4× magnification. Tube formation was quantified by ImageJ software.
RNA extraction and Rt-qPCR
Total RNA was isolated from the cells or placenta tissue using Trizol reagent (Invitrogen, Missouri, USA) according to the manufacturer’s instructions. DNaseI was used to avoid DNA contamination. One micrograms total RNA from each sample was subjected to reverse transcription using the SuperScript® IV First-Strand Synthesis System (Invitrogen, Massachusetts, USA) before PCR amplification using 1× Power SYBR® Green PCR Master Mix (Invitrogen, Massachusetts, USA). Relative expression levels of mRNAs were normalized to GAPDH mRNA as internal controls. The following primers were used for analysis:
Notch1 forward: 5Ê1-CTGGTCAGGGAAATCGTG-3Ê1
Notch1 reverse: 5Ê1-TGGGCAGTGGCAGATGTAG-3Ê1;
ETBR forward: 5Ê1-AACTTCCGCTCCAGCAAT-3Ê1;
ETBR reverse: 5Ê1-TCCCGAGGCTTCATTCAT-3Ê1;
GAPDH forward: 5Ê1-AGGTCGGTGTGAACGGATTTG-3Ê1;
GAPDH reverse: 5Ê1-GGGGTCGTTGATGGCAACA-3Ê1;
Western blot
Polyacrylamide gel electrophoresis and immunodetection were performed as standard techniques. Briefly total proteins were extracted from cultured cells by RIPA buffer (Beyotime Institute of Biotechnology, Nantong, China) with protease inhibitor cocktail. Protein concentration was determined by Pierce BCA protein Assay (Thermo Fisher Scientific, Massachusetts, USA). Equal amounts of protein samples were separated by SDS-PAGE and then transferred to PVDF membranes (Millipore, Massachusetts, USA). Selected proteins were detected with specific antibodies. The following antibodies were used: rabbit polyclonal anti-Notch-1 antibody (1: 500; Santa Cruz, USA), rabbit polyclonal anti-ETBR antibody (1: 500; Santa Cruz, USA), mouse monoclonal anti-VEGF antibody (1: 500, ThermoFisher, USA), rabbit polyclonal anti-Phospho-STAT3 (Tyr705) antibody (1: 200, Cell Signaling, USA), rabbit polyclonal anti-STAT3 antibody (1: 500, Abcam, USA), rabbit polyclonal anti-HIF-1α antibody (1: 500, Abcam, USA), mouse monoclonal anti-β-actin antibody (1: 1000, Santa Cruz, USA).
Enzyme-linked immunosorbent assay (ELISA)
Culture medium was collected for the measurement of cytokine concentration by ET-1 ELISA kit (R&D Systems, USA), sFlt-1 ELISA kit (R&D Systems, USA), IL-6 ELISA kit (Abcam, USA), TNF-α ELISA kit (Abcam, USA) according to manufacturer’s instructions.
Reduced uterine perfusion pressure (RUPP) rat model
All animal studies have been approved by the Ethics Committee of Tongji Hospital of Tongji Medical College, Huazhong University of Science and Technology, and performed under the guidance of the Ethics Committee. On gestational day 14, pregnant rats were anesthetized and maintained with 3% isoflurane (Webster, MA, USA). A midline abdominal incision was made and the lower abdominal aorta was isolated. A silver surgical clip (0.203 mm) was placed on the lower abdominal aorta above the iliac bifurcation and two other sliver surgical clips (0.1 mm) were placed on both the left and right ovarian arteries that supply the uterus separately.
RUPP rats were divided randomly into five groups (Vehicle, shNC, shETBR, NC, Notch1) as indicated in figures and received intraperitoneal injection of shETBR (2 × 1010 pfu), shRNA-NC (2 × 1010 pfu), NC (2 × 1010 pfu) or Notch1 (2 × 1010 pfu) every 2 days for 4 days.
Statistical analysis
All animal and cell experiments were performed at least three times and analyzed using Graphpad Prism 7. The human experiment results (n = 10) were also analyzed in Graphpad Prism 7. Statistical significance was determined by unpaired two-tailed Student t test for two groups or one-way ANOVA followed by Tukey’s post hoc test for multiple groups. *P< 0.05, **P< 0.01, ***P< 0.001. Data were presented as Mean ± SD (standard deviation).
Results
HI1-1α, ETBR and Notch1 are down-regulated in placenta of patients with PE
To investigate the functions of HIF-1α,ETBR and Notch1 in PE, we examined expressions of HIF-1α, ETBR and Notch1 in patients with PE. First, we used placenta specimens from PE patients and normal pregnant women to do immunohistochemistry experiment. Compared with normal pregnant women, ETBR and Notch1 levels were significantly lower in PE patients (Figure 1(a)). Next, we did primary trophoblast cell culture with placenta tissues and measured the mRNA and protein levels of HIF-1α, ETBR and Notch1 in PE patients. Consistently, we found that both mRNA and protein expressions of HIF-1α, ETBR and Notch1 were significantly reduced in PE patients compared to normal pregnant women (Figure 1(b,c)). These results indicate that HIF-1α, ETBR and Notch1 might be involved in PE development.
Figure 1.
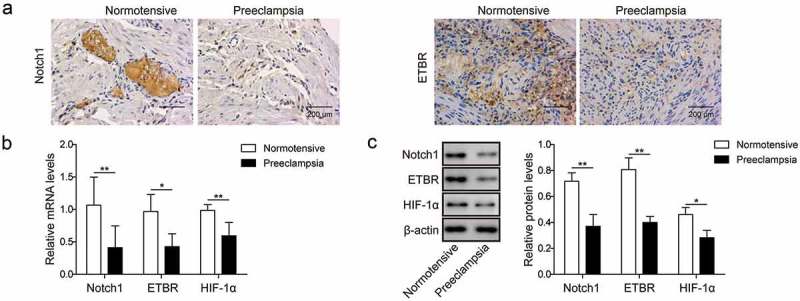
Notch1 and ETBR are down-regulated in placentas of PE patients. (a) Representative images of immunohistochemistry analysis of Notch1 and ETBR expression in placentas from normal pregnant women and PE women. (b) RT-qPCR analysis of Notch1 and ETBR in placentas from normal pregnant women and PE women. (c) Western blot analysis of Notch1 and ETBR in placentas from normal pregnant women and PE women. * P< 0.05; ** P< 0.01.
Hypoxia promotes invasion and angiogenesis of trophoblast cells
Hypoxia is an important signal that guides placental development and trophoblast cell differentiation and placentation [5]. To further examine the roles of ETBR and Notch1 in trophoblast cell placentation, we used human trophoblast cell lines, HTR-8/Svneo and JEG-3,and cultured them under hypoxia. Using transwell assay, we observed that the invasive capability of trophoblast cells was greatly enhanced 6 h after hypoxia and were further increased at 12 h(Figure 2(a)). Moreover, using Matrigel assay, we found that the angiogenesis capability of trophoblast cells was also increased at 6 h after hypoxia and showed larger increase at 12 h (Figure 2(b)). Molecularly, we found that HIF-1α, ETBR, Notch1, p-STAT3 and pro-angiogenic factor VEGF were all up-regulated with time after hypoxia while proteins including ET-1, TNF-α, anti-angiogenic factor sFlt-1, and IL-6 were all down-regulated with time following hypoxia treatment (Figure 2(c,d)). These data suggest that HIF-1α and Notach1/STAT3/ETBR signaling might play some roles in regulating invasion and angiogenesis of trophoblast cells during placental development.
Figure 2.
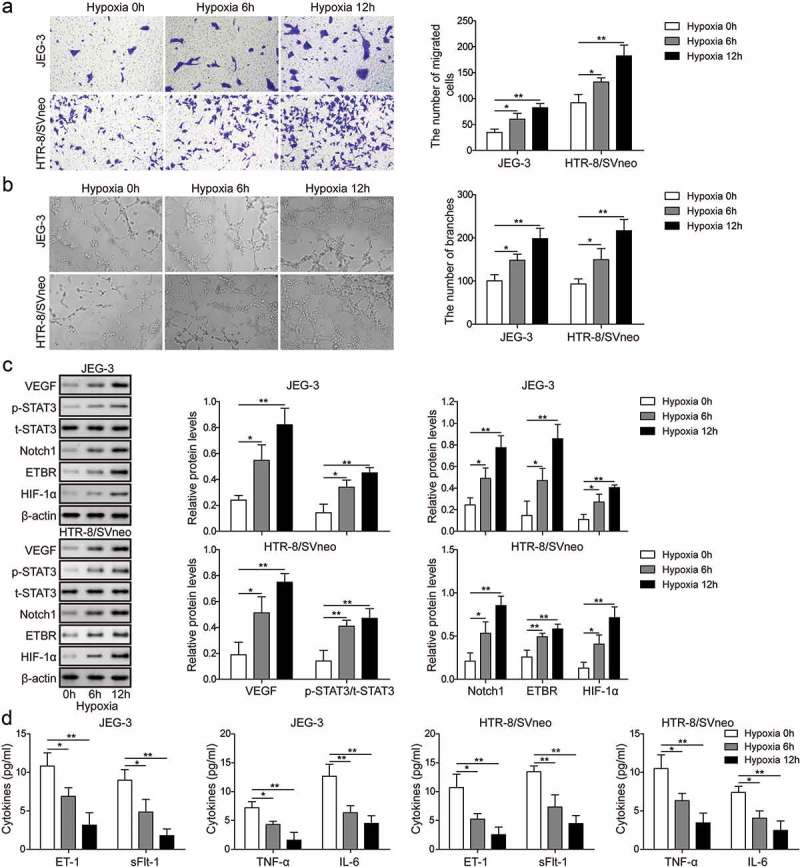
Hypoxia promotes invasion and angiogenesis of trophoblast cells. (a) Transwell invasion assay was used to determine cell invasion of trophoblast cells at 0 h, 6 h and 12 h after hypoxia. (b) Matrigel assay was used to measure angiogenesis of trophoblast cells at 0 h, 6 h and 12 h time after hypoxia. (c) Western blot analysis of related proteins indicated in figures after hypoxia. (d) ELISA analysis of ET-1, TNF-α, sFlt-1 and IL-6 levels at 0 h, 6 h and 12 h after hypoxia. * P< 0.05; ** P< 0.01.
Overexpression of Notch1 increases invasion and angiogenesis of trophoblast cells
To directly examine whether Notch1 regulates invasion and angiogenesis capabilities of trophoblast cells, we overexpressed Notch1 through transfection and measured its effects on invasion and angiogenesis. With transwell assay and Matrigel assay, we found that both invasion and angiogenesis capabilities of trophoblast cells were greatly enhanced in cells transfected with Notch1 compared to control cells or cells transfected with NC (Figure 3(a,b)). Similarly, protein levels of ETBR, p-STAT3 and VEGF were higher in Notch1 overexpression cells while ET-1, TNF-α, and IL-6 expressions were lower than in control cells (Figure 3(c,d)). Together, these demonstrate that Notch1 promotes invasion and angiogenesis of trophoblast cells.
Figure 3.
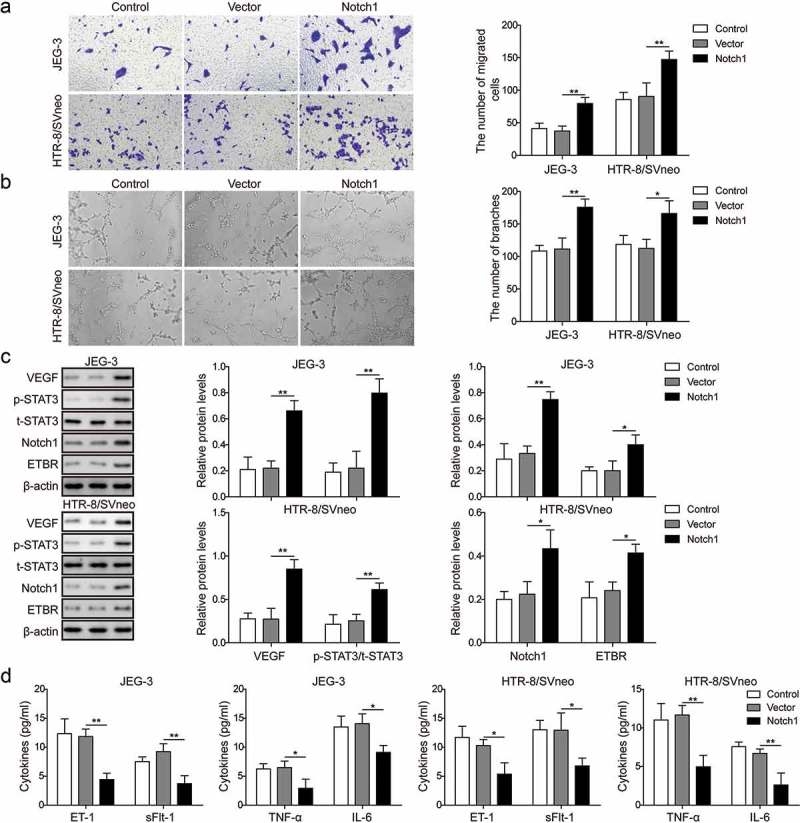
Notch1 promotes invasion and angiogenesis of trophoblast cells. (a) Transwell invasion assay was used to determine cell invasion of control trophoblast cells or cells transfected with NC or Notch1. (b) Matrigel assay was used to measure angiogenesis of control trophoblast cells or cells transfected with NC or Notch1. (c) Western blot analysis of related proteins in control cells or transfected cells. (d) ELISA analysis of ET-1, TNF-α, sFlt-1 and IL-6 levels in control cells or transfected cells. * P< 0.05; ** P< 0.01.
HIF-1α promotes invasion and angiogenesis of trophoblast cellsviaNotch1/STAT3/ETBR pathway
We next investigated whether HIF-1α is also involved in the trophoblast cell invasion and angiogenesis. Firstly, we treated trophoblast cells with shNotch1and/orHIF-1α-overexpressed plasmid and observed that knockdown of Notch1 suppressed invasion of trophoblast cells, while HIF-1α overexpression facilitated invision, and could reverse the suppression by knockdown of Notch1 (Figure 4(a)). Similarly, angiogenesis of trophoblast cells wassuppressedbyknockdown of Notch1, and promoted by HIF-1α overexpression. The suppressionbyknockdowntpdel of Notch1couldrelievedbyHIF-1α overexpression (Figure 4(b)). We then measured the expressions of related proteins and found that shNotch1 significantly decreased ETBR, p-STAT3, Notch1 and VEGF but increased levels of ET-1, TNF-α, sFlt-1 and IL-6, whileHIF-1α overexpression effectively promoted HIF-1α,ETBR, p-STAT3, Notch1 and VEGF but reduced levels of ET-1, TNF-α, sFlt-1 and IL-6, which partly reversed the effect by shNotch1 (Figure 4(c,d)). Therefore, we conclude that HIF-1α promotes invasion and angiogenesis of trophoblast cells, which is similar to hypoxia treatment or Notch1 overexpression. And the results demonstratedHIF-1αmightexerttheaboveeffect via Notch1/STAT3/ETBR pathway.
Figure 4.
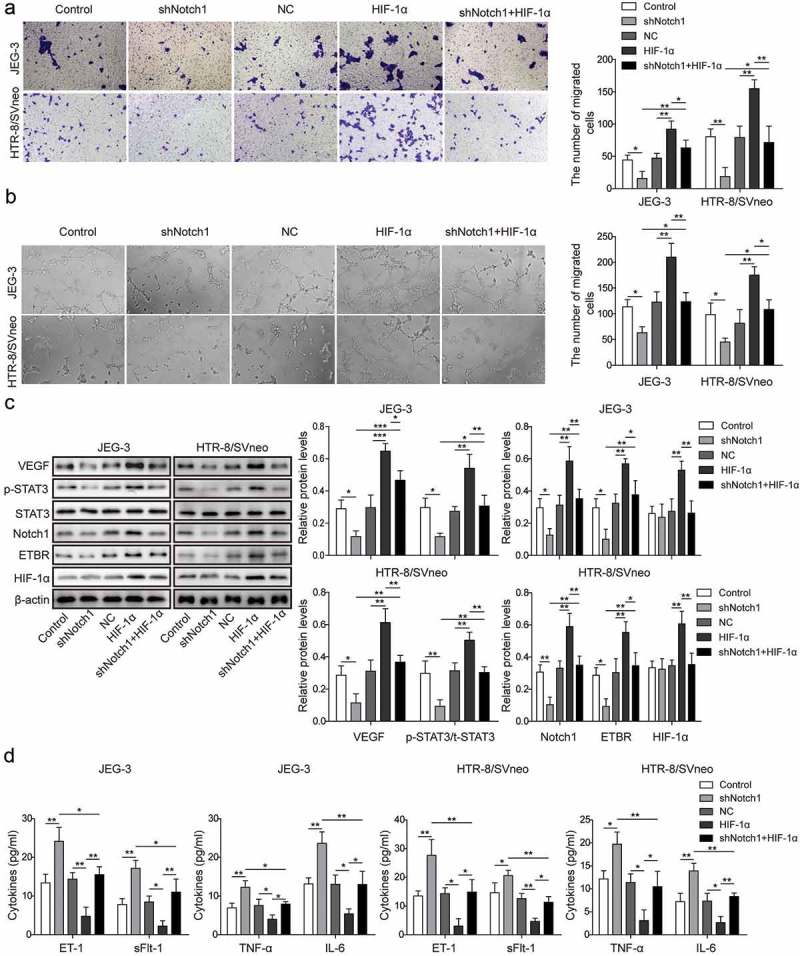
HIF-1α promotes invasion and angiogenesis of trophoblast cells via Notch1/STAT3/ETBR pathway. (a) Transwell invasion assay was used to determine cell invasion of control trophoblast cells or cells treated with shNotch1and/or HIF-1α-overexpressed plasmid. (b) Matrigel assay was used to measure angiogenesis of control trophoblast cells or cells treated withshNotch1and/or HIF-1α-overexpressed plasmid. (c) Western blot analysis of related proteins in control cells or cells treated with shNotch1and/or HIF-1α-overexpressed plasmid. (d) ELISA analysis of ET-1, TNF-α, sFlt-1 and IL-6 levels in control cells or cells treated with shNotch1and/or HIF-1α-overexpressed plasmid. * P< 0.05; ** P< 0.01; ***P< 0.001.
ETBR1 and Notch1 promote angiogenesis in placenta vivo
In the end, we examined the role of ETBR and Notch1 in PE in vivo. We used PE rat model by performing reduced uterine perfusion pressure (RUPP) surgery [16]. We found that knockdown ETBR with shETBR significantly reduced microvessel density in RUPP rats compared to RUPP rats injected with shNC or control (Figure 5(a)). However, overexpression of Notch1 significantly increased microvessel density in RUPP rats compared with RUPP rats injected with NC or control mice (Figure 5(c)). Further, we measured the levels of angiogenesis-related proteins and showed that knockdown ETBR up-regulated Notch1 and VEGF expressions while overexpression of Notch1 down-regulated their levels (Figure 5(b,d)). Altogether, these results prove that ETBR and Notch1 promote angiogenesis in placenta in vivo.
Figure 5.
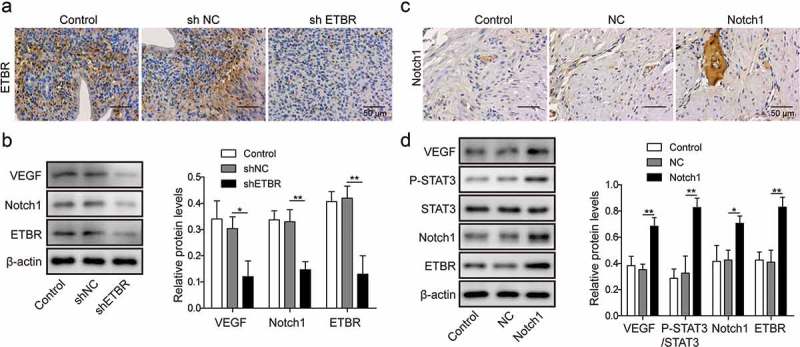
Notch1 and ETBR facilitate angiogenesis of trophoblast cells in RUPP rats. (a) Immunohistochemical staining analysis of ETBR expression in RUPP rats injected with shNC or shETBR. (b) Western blot analysis of related proteins in RUPP rats injected with shNC or shETBR. (c) Immunohistochemical staining analysis of Notch1 expression in RUPP rats injected with NC or Notch1. (d) Western blot analysis of related proteins in RUPP rats injected with NC or Notch1. *P < 0.05; **P < 0.01.
Discussion
Despite numerous studies and great advances in research and therapy, the pathogenesis and molecular mechanisms of PE is still largely unknown. Therefore, it is very important to understand underlying mechanisms that drive development and progression of PE. Emerging evidence implicates the existence of hypoxia in PE [7]. In this study, we evaluated how hypoxia-regulated trophoblast cell function and the underlying molecular mechanisms. We show that hypoxia promotes invasion and angiogenesis of trophoblast cells. HIF-1α, a key protein that protects cytophoblast cells from hypoxia during the early development, also facilitates invasion and angiogenesis of trophoblast cells via Notch1/STAT3/ETBR signaling.
It is well known that shallow placentation and lack of spiral artery remodeling are the two major causes of placental hypoxia in PE, which subsequently leads to up-regulations of anti-angiogenic factors and maternal syndrome of PE such as proteinuria and hypertension [17]. HIF-1α is a key mediator of this regulation. During normal pregnancy, HIF-1α is transiently expressed in trophoblast cells during the early gestation due to the hypoxia environment but falls at around 9 week of gestation [18]. However, HIF-1α is highly expressed in placentas of PE women and a growing body of evidence show that HIF-1α, as a transcription factor, is the molecular bridge between placental hypoxia and the downstream mediators of PE [19]. HIF-1α has been shown to induce the anti-angiogenic factors like sFlt-1, sENG, and TGF-β [18,20,21]. Here, we showed that during the early stage of PE, HIF-1α was still low and the downstream targtes Notch1/ETBR were also down-regulated. Due to the hypoactivity of HIF-1α/Notch1/ETBR pathway, the angiogenesis of trophoblast cells gets inhibited and subsequently contributes to the development of PE. HIF-1α might get increased at the later stage of PE, which is a compensatory response to prolonged hypoxia, and future studies examining the time course of HIF-1α change during PE might be interesting. Moreover, previous study implicates the association between HIF-1α and Dll4/Notch1 in missed abortion [13], and in PE we showed that hypoxia and HIF-1α modulated Notch1/ETBR expression as well. As aberrant angiogenesis plays essential roles in PE and missed abortion [22], there may be many shared mechanisms by the two diseases, such as hypoxia-induced signaling pathways [23,24].
Hypertension is one major manifestation of PE and ET-1 plays a key role in mediating hypertension in the pathophysiology of PE [25–27]. ET-1 is up-regulated in PE patients and correlated with sFlt-1 and sENG levels [28]. Moreover, ET-1 antagonist attenuates hypertension in a pregnant rat model of uteroplacental ischemia. ET-1 is known as a potent vasoconstrictor; however, there are two types of receptors for ET-1, ETAR and ETBR [29]. Activation of ETAR in VSM causes vasoconstriction while ETBR mediates vasodilation and is predominantly expressed in the endothelium. In the present study, we observed reduced expression of ETBR in placentas from PE patients and found that hypoxia and HIF-1α regulated ETBR levels in trophoblast cells. Further, we provided strong evidence demonstrating that ETBR promotes invasion and angiogenesis of trophoblast cells both in vitro and in vivo. Our data, together with other findings, support a crucial role of ETBR down-regulation in PE [25,30].
In conclusion, we show that ETBR and Notch1 are down-regulated in PE patients. HIF-1α expression induced by hypoxia promotes invasion and angiogenesis of trophoblast cells via targeting Notch1/STAT3/ETBR pathway. Our work reveals an essential role of HIF1-α and Notch1/STAT3/ETBR in PE development and suggests that they could serve as a target for potential therapy development.
Funding Statement
This work is supported by National Natural Science Foundation of China (NSFC 81701476 and 81701530) and Hubei Provincial Natural Science Foundation of China (2017CFB626 and 2016CFB321).
Article highlights
ETBR and Notch1 are down-regulated in PE patients;
Hypoxia increases invasion and angiogenesis of trophoblast cells;
HIF-1 α and Notch1 promote invasion and angiogenesis of trophoblast cells;
Notch1 and ETBR promote angiogenesis in RUPP rat model.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Mol BWJ, Roberts CT, Thangaratinam S, et al. Pre-eclampsia. Lancet. 2016;387:999–1011. [DOI] [PubMed] [Google Scholar]
- [2].Sircar M, Thadhani R, Karumanchi SA.. Pathogenesis of preeclampsia. Curr Opin Nephrol Hypertens. 2015;24:131–138. [DOI] [PubMed] [Google Scholar]
- [3].Esteve-Valverde E, Ferrer-Oliveras R, Gil-Aliberas N, et al. Pravastatin for preventing and treating preeclampsia: a systematic review. Obstet Gynecol Surv. 2018;73:40–55. [DOI] [PubMed] [Google Scholar]
- [4].Hutter D, Kingdom J, Jaeggi E. Causes and mechanisms of intrauterine hypoxia and its impact on the fetal cardiovascular system: a review. Int J Pediatr. 2010;2010:401323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Tal R. The role of hypoxia and hypoxia-inducible factor-1alpha in preeclampsia pathogenesis. Biol Reprod. 2012;87:134. [DOI] [PubMed] [Google Scholar]
- [6].Soares MJ, Iqbal K, Kozai K. Hypoxia and placental development. Birth Defects Res. 2017;109:1309–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Macklin PS, McAuliffe J, Pugh CW, et al. Hypoxia and HIF pathway in cancer and the placenta. Placenta. 2017;56:8–13. [DOI] [PubMed] [Google Scholar]
- [8].Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1). Mol Pharmacol. 2006;70:1469–1480. [DOI] [PubMed] [Google Scholar]
- [9].Rajakumar A. Evidence for the functional activity of hypoxia-inducible transcription factors overexpressed in preeclamptic placentae. Placenta. 2004;25:763–769. [DOI] [PubMed] [Google Scholar]
- [10].Saleh L, Verdonk K, Visser W, et al. The emerging role of endothelin-1 in the pathogenesis of pre-eclampsia. Ther Adv Cardiovasc Dis. 2016;10:282–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Saleh L, Danser JA, van den Meiracker AH. Role of endothelin in preeclampsia and hypertension following antiangiogenesis treatment. Curr Opin Nephrol Hypertens. 2016;25:94–99. [DOI] [PubMed] [Google Scholar]
- [12].LeComte MD, Shimada IS, Sherwin C, et al. Notch1-STAT3-ETBR signaling axis controls reactive astrocyte proliferation after brain injury. Proc Natl Acad Sci U S A. 2015;112:8726–8731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fang Y, Yu S, Ma Y, et al. Association of Dll4/notch and HIF-1a -VEGF signaling in the angiogenesis of missed abortion. PLoS One. 2013;8:e70667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Mazzuca MQ, Dang Y, Khalil RA. Enhanced endothelin receptor type B-mediated vasodilation and underlying [Ca(2)(+)]i in mesenteric microvessels of pregnant rats. Br J Pharmacol. 2013;169:1335–1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mazzuca MQ, Li W, Reslan OM, et al. Downregulation of microvascular endothelial type B endothelin receptor is a central vascular mechanism in hypertensive pregnancy. Hypertension. 2014;64:632–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li J, LaMarca B, Reckelhoff JF. A model of preeclampsia in rats: the reduced uterine perfusion pressure (RUPP) model. Am J Physiol Heart Circ Physiol. 2012;303:H1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Armaly Z, Jadaon JE, Jabbour A, et al. Preeclampsia: novel mechanisms and potential therapeutic approaches. Front Physiol. 2018;9:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Caniggia I, Mostachfi H, Winter J, et al. Hypoxia-inducible factor-1 mediates the biological effects of oxygen on human trophoblast differentiation through TGFbeta(3). J Clin Invest. 2000;105:577–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Korkes HA, De Oliveira L, Sass N, et al. Relationship between hypoxia and downstream pathogenic pathways in preeclampsia. Hypertens Pregnancy. 2017;36:145–150. [DOI] [PubMed] [Google Scholar]
- [20].Lee SB, Wong AP, Kanasaki K, et al. Preeclampsia: 2-methoxyestradiol induces cytotrophoblast invasion and vascular development specifically under hypoxic conditions. Am J Pathol. 2010;176:710–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Nevo O, Soleymanlou N, Wu Y, et al. Increased expression of sFlt-1 in in vivo and in vitro models of human placental hypoxia is mediated by HIF-1. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Chen DB, Zheng J. Regulation of placental angiogenesis. Microcirculation. 2014;21:15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu F, Tian FJ, Lin Y, et al. Oxidative stress: placenta function and dysfunction. Am J Reprod Immunol. 2016;76:258–271. [DOI] [PubMed] [Google Scholar]
- [24].Wu F, Tian FJ, Lin Y. Oxidative stress in placenta: health and diseases. Biomed Res Int. 2015;2015:293271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Granger JP, Spradley FT, Bakrania BA. The endothelin system: a critical player in the pathophysiology of preeclampsia. Curr Hypertens Rep. 2018;20:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Garovic VD, August P. Preeclampsia and the future risk of hypertension: the pregnant evidence. Curr Hypertens Rep. 2013;15:114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Parikh NI, Gonzalez J. Preeclampsia and hypertension: courting a long while: time to make it official. JAMA Intern Med. 2017;177:917–918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Aggarwal PK, Chandel N, Jain V, et al. The relationship between circulating endothelin-1, soluble fms-like tyrosine kinase-1 and soluble endoglin in preeclampsia. J Hum Hypertens. 2012;26:236–241. [DOI] [PubMed] [Google Scholar]
- [29].Houde M, Desbiens L, D’Orleans-Juste P. Endothelin-1: biosynthesis, signaling and vasoreactivity. Adv Pharmacol. 2016;77:143–175. [DOI] [PubMed] [Google Scholar]
- [30].Jain A, Olovsson M, Burton GJ, et al. Endothelin-1 induces endoplasmic reticulum stress by activating the PLC-IP(3) pathway: implications for placental pathophysiology in preeclampsia. Am J Pathol. 2012;180:2309–2320. [DOI] [PubMed] [Google Scholar]


