ABSTRACT
Although several effective treatments exist against Varicella zoster virus (VZV), resistant strains have emerged and the treatment is usually not definite and may have various undesired side effects. Thus, alternative treatment options are necessary. Here we studied the inhibitory effects of natural polysaccharides (PSs) obtained from renewable sources, varied by their structure and charge, on VZV infection in vitro, using a plaque assay. In terms of selectivity indices, almost all the tested PSs were very active; in the order of λ > ἰ > G > κ > P against VZV compared to Acyclovir as a reference drug and exhibited dose-dependent behavior. Our results, which showed a strong inhibition of VZV infection when the cells were treated with ἰ only at the time of infection or only post infection may indicate a multistep inhibitory effect. It seems that ἰ may block different stages of the virus replication cycle including early steps such as absorption and penetration to the host cells and also late steps after the penetration into the host cells. These results are part of an on-going research that highlights the PSs as potential novel nontoxic candidates that can be used against VZV, and contributes to the elucidation of their mode of action.
KEYWORDS: Anti-viral, carrageenan, renewable polysaccharides, sulfated polysaccharides, Varicella zoster virus
1. Introduction
Viruses are considered as one the main causes of human and others infectious diseases, which may lead to death despite the significant progress made in antiviral drug development [1–3]. Herpes viruses are double stranded DNA viruses belonging to the family Herpesviridae which are responsible for a variety of mild to severe human diseases, sometimes life threatening, particularly in immune-comprised patients and neonates [4]. After the acute infection, herpes viruses establish latency and persist in different cells of the body, according to the infecting virus. The latent virus is reactivated spontaneously, causing recurrent infections in infected patients [5]. Herpes viruses are very common among mammals and about 80% of the adult population has antibodies to most, if not all of them [6]. Varicella zoster virus (VZV), belongs to the α-subgroup of herpes viruses and causes two different diseases. The first, varicella, which is a result of primary infection with VZV (usually occurs during childhood) and the second is zoster, which results due to reactivation of the latent virus and provokes severe pain in the area of latently infected ganglia [7,8].
VZV-associated diseases can be effectively treated with nucleoside analogs such as acyclovir (ACV) and famciclovir, which inhibit viral DNA synthesis [7,9]. However, the effectiveness of these drugs to treat VZV infections in immunocompromised and in immunodeficient patients is limited due to the development of resistant VZV strains [7,10]. In addition, ACV and other available nucleoside analogs have different undesired side effects (like nausea, vomiting, headache and others) [11], and are not highly effective against reactivated herpes viruses [12,13]. Furthermore, a VZV vaccine is available, however, significant concerns still exist regarding its efficiency in individuals carrying latent VZV and in immunocompromised individuals [14,15]. Therefore, there is a need for novel effective drugs to treat VZV-associated diseases with different modes of action to that of nucleoside derivatives.
A variety of natural products possess antiviral activity and some of them have already been used for the treatment of human viral infections with RNA and DNA viruses [13,16–19]. Some studies on various strains and groups of viruses have shown that renewable polysaccharides (PSs), that are present in a wide variety of organisms, especially the sulfated ones (SPSs), possess the ability to inhibit replication of various viruses [20–24]. It was suggested that they mainly interfere with the attachment of the viruses to the host cell membrane, and as a result, they prevent their entrance into the host cells [25,26]. In this study, for the first time, the antiviral activity of several renewable PSs against VZV was tested and compared in accordance with their structures and contents. The studied PSs are produced by different organisms that are mainly known for their beneficial physicochemical properties, in light of which they are used in basic industries as thickeners, emulsifiers and stabilizers. The PSs can be divided into two groups: 1) SPSs that are derived from red algae: iota, kappa and lambda carrageenan (ἰ, κ and λ, respectively) and SPSs which dissolve during growth of the red alga Porphyridium cruentum (P); 2) Non-sulfated PSs, including: Guar Gum (G), Locust bean gum (LB)- which belong to the glucomannans family derived from plants, and Xanthan Gum (X)- derived from the bacterium Xanthomonas campestris. Each one of the PSs has its characteristic composition and structure, as demonstrated in Table 1.
Table 1.
Representative description of the investigated PSs.
| PS | Functional Groups | Branched/ Linear |
Repeating unit | Side chains |
|---|---|---|---|---|
| ἰ | -OH, -OSO3- |
Linear | D-Gal-4-sulfate,3,6-anhydro-D-Gal-2-sulfate | - |
| κ | -OH, -OSO3- |
Linear | D-Gal-4-sulfate,3,6-anhydro-D-Gal | - |
| λ | -OH, -OSO3- |
Linear | D-Gal-2-sulfate, D-Gal-2,6 disulfate | - |
| P | -OH, -OSO3- -COO- |
Branched | Not finally defined. A disaccharide aldobiouronic acid 3-O-(-d-glucopyranosyluronic acid)-l-galactopyranose and a larger linear composed of the same disaccharide were determined | Not available |
| G | -OH | Branched | β-(1→4)-linked D-mannose | single α-D-galactose at the C6 position of D-mannose at every second mannose |
| LB | -OH | Branched | β-(1→4)-linked mannose | single α-D-galactose at the C6 position of D-mannose and highly unevenly substituted |
| X | -OH -CH2OCOCH3 -COO− |
Branched | β-(1→4)-D-glucopyranose | a trisaccharide composed of d‐mannose (β‐1,4), d‐glucuronic acid (β‐1,2) and d‐mannose, which are attached to alternate glucose residues in the backbone by α‐1,3 linkages. |
It was already demonstrated that the red algae SPSs hold promising potential as antiviral agents in various systems [21–23]. For instance, different types of carrageenan act as inhibitors of several viruses such as papillomavirus [27], rhinovirus [28] and herpes simplex virus (HSV-1, HSV-2) [29–32]. It was also reported that P has antiviral activity against HSV-1, HSV-2 and VZV in vitro [26,33,34], and also against HSV-1 in vivo [33,34]. Since these SPSs are anionic, it was suggested they interact with the positive charges present on the surface of the virus and inhibit virus adsorption to the host cells [20,35]. Moreover, the type of the ionic group also plays a crucial role in the antiviral potency [21]. However, the mechanism of action of these SPSs action is believed not to be related solely to the interaction with the virus due to its negatively charged characteristics; e.g it was suggested that P is involved in inhibiting a late step during the herpes virus replication cycle, having a pleiotropic mode of action [33]. Although neutral PSs were less studied than their charged counterparts, their lower rate or lack of antiviral activity compared to SPSs was reported [36].
The main goal of this study was to test the inhibitory effects of different natural PSs, which are obtained from various organisms (differing by their structure and charge) on VZV infection; Moreover, a primary investigation concerning their mode of action as antiviral agents was also employed.
2. Materials and methods
2.1. PS preparations
PSs were purchased from Aldrich, unless otherwise stated. The PS derived from Porphyridium cruentum (P) was separated from the growth media of Porphyridium cruentum (UTEX 161). Briefly, the microalgae were grown in artificial sea water with f/2 fertilizer [37], with the addition of 10 mM NaNO3. The cultures were kept in 500 mL flasks and placed on an orbital shaker in an incubator with 1.5% CO2 and constant lighting of 70 µmol photons/m2 *s−1 (16/8 light regime) using 6500K LEDs at 20°C. After acquiring ~2 gDC/L, the cultures were transferred to a polyethylene bag (2 L total volume) and kept under high light conditions (~500 µmol photons/m2 *s−1) with artificial water without f/2 fertilizer for 2 weeks. Then, 500 mL of Porphyridium cruentum culture was centrifuged at 10,000 g and the cell-algae pellet was discarded. Ethanol (Sigma Aldrich) was added to the supernatant at a volume ratio of 3:1, respectively. During the addition of the solvent the medium was firmly stirred using a magnetic stirrer for 15 min. The mixture was centrifuged at 24,000 X g and 4°C for 15 min., and the PS pellet was re-dissolved and dialyzed (MW cutoff 8,000) against DDW at 4°C until the conductivity of the water reached 300 µs*cm−1. The dialyzed fraction was then freeze-dried and the PS powders were re-dissolved in DDW to reach 0.5% w/v, comprising P stock which was further autoclaved.
2.2. Cells and viruses
African green monkey kidney (Vero) cells were purchased from the American Type Culture Collection, Rockville, MD, USA. The cells were grown in RPMI medium with 10% fetal calf serum, 1% glutamine, 50 µg/mL antibiotics mixture (penicillin and streptomycin) and incubated at 37 ° C in a humidified air containing 5% CO2. VZV was obtained from ATCC (VR-1433). A stock of the virus was prepared by propagating it to > 104 plaque forming units (PFU) per mL in Vero cells and stored as VZV cell- associated stock at −80°C.
2.3. Cytotoxicity assay
Vero cells were treated with different concentrations of the PSs (1–5000 µg/mL) and their cytotoxicities were evaluated by the following methods: 1. Counting the cells by Neubauer hemocytometer after 3 days of treatment. 2. Daily morphological observations by optical inverted microscope. 3. MTT assay was performed as previously described [38]. Briefly, 50 µg/mL MTT solution was added to every well and the plate was incubated at 37°C for 5 h. Then the MTT solution was removed and replaced with solubilization buffer (SDS 10% in 0.01 N HCl). After overnight incubation at 37°C, absorbance was measured at 570 nm, indicating the metabolic activity of the cells. The cytotoxic effect was calculated as a percentage of the surviving cells under treatment in relation to the number of the living cells in the control untreated cells.
2.4. Antiviral activity assay
The antiviral activity of the tested PSs was evaluated by plaque assay [26]. Briefly, in a typical procedure Vero cells were seeded at 2 × 105 cells/well in 24-well culture plates, as detailed in the Material and Methods section. After overnight incubation, the medium was removed and each well was infected with 0.01 multiplicity of infection (m.o.i) of VZV (from VZV cell associated stock) for 2 h at 37°C. Then the medium was removed and cells were covered with a layer of medium containing carboxymethylcellulose (CMC). Two days post-infection the CMC overlay was removed, cell monolayers were fixed with 10% formaldehyde in saline, stained with crystal violet and plaques were counted. The PSs were added at different stages in accordance to the different experimental settings that are designated as: 1) infected cells: cells that were infected and not treated with PSs 2) pre-incubation: infected cells that were pre-cultured with ἰ for 2 h and then washed twice with 0.9% NaCl solution 3) incubation during infection: the cells were infected and treated with ἰ during the 2 h infection time and then washed twice with 0.9% NaCl solution 4) prolonged-incubation: infected cells that were treated with the PS during the infection and 2-days post infection 5) post- incubation: infected cells that were incubated with ἰ during the 2-days post-infection 6) Effect of i on intracellular VZV replication (Endogenous virus): In order to examine a possible effect of ἰ on intracellular VZV replication, Vero cells were infected with 0.01 m.o.i. of VZV without treatment with ἰ for 2 h. Then, the medium was removed and replaced with fresh medium with or without the appropriate concentrations of ἰ. Subsequently, the infected cells were removed from the wells by treatment with trypsin at 20 h post infection. The obtained cells were pelleted by centrifugation at 1500 rpm for 5 min., and washed three times with saline solution. Each pellet was re-suspended with 100 µL of physiological saline solution and the cells were broken by freezing and thawing. Cell debris were removed by centrifugation at 1500 rpm for 5 min., the mixture containing the endogenous virus was then used for infecting cell monolayers and the antiviral effect was evaluated by the standard plaque assay. 7) Direct incubation of ἰ with the virus (without Vero Cells): 100 µL of VZV cell-associated stock was incubated with 100 µL of 100 µg/mL ἰ or with 100 µL of medium (as a control) for 1 h at 37°C. Then the VZV- ἰ mixture was diluted 1:103 and 1:104 prior to infection, which highly reduces the PS concentration to anti-viral non effective concentrations. Subsequently, Vero cells were infected by the diluted virus mixtures for 2 h and the antiviral effect was evaluated by the standard plaque assay.
The fold inhibition percentage of the antiviral effect was calculated according to the following formula: Fold inhibition effect = (A0-AT)/A0 × 100, where A0 is the plaque numbers counted in the infected untreated cells, and AT is the plaque numbers counted in each treatment experiment.
2.5. Statistical analysis
All experiments were repeated five times. The data are expressed as the mean ± SEM. The significance of the differences between the means of various subgroups was assessed by unpaired two-tailed Student’s t test. The statistical analysis was performed with Graph-Pad Prism 7.02 Software (San Diego, CA). P < 0.05 was considered statistically significant.
3. Results
In this study different types of commercial natural polysaccharides were tested for their antiviral activity against VZV infection: red macroalgae carrageenans – ἰ, κ, λ; red microalga polysaccharide – P; plant polysaccharide – G, LB; and bacteria polysaccharide – X.
3.1. Cytotoxicity of the PSs
Vero cells were treated with different concentrations of the tested PSs and their viability was examined for 3 days after the treatment. Similar cytotoxicity results were obtained by both used methods counting the number of living cells and MTT assay. It is evident that up to a concentration of 10 µg/mL all the PSs had no significant cytotoxic effect on the Vero cells (Figure 1). The ἰ, λ, G, LB and X polysaccharides did not demonstrate any cytotoxic effect on the cells even at concentrations up to 5000 µg/mL. However, treatments of cells with concentration of P and κ above 100 µg/mL resulted in a lower cell viability rate in comparison to control untreated cells (Figure 1). At a concentration of 1000 µg/mL, κ and the P dramatically reduced the cell viability rates (~50% reduction in viable cells).
Figure 1.
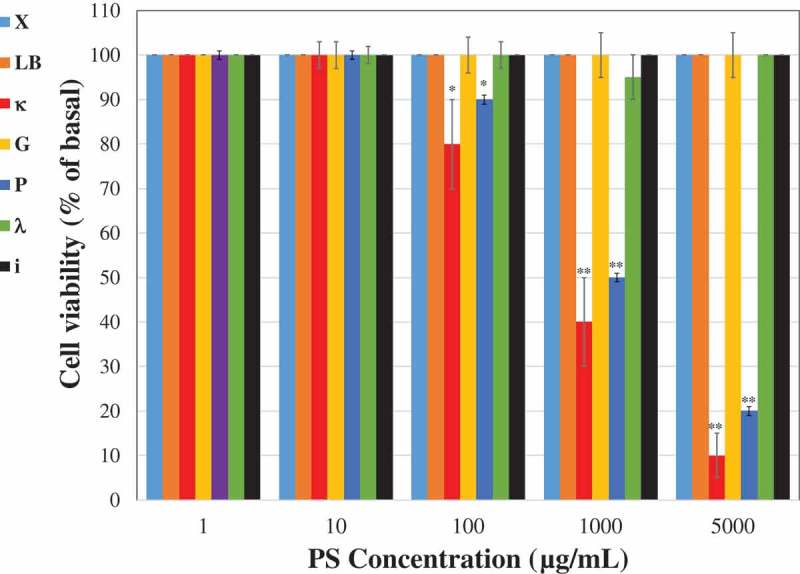
Cytotoxic effect of PSs. Vero cells (2x105) were plated in 24 well plates and treated with different concentrations of the tested PSs. The cell viability was measured 3 days after the treatment. The results are presented as a percentage of the control untreated cells. Data are expressed as the mean ± SEM percentage (n = 5); *p < 0.05; **p < 0.001 vs. control untreated cells.
Representative descriptive morphological observations of Vero cells that were treated with 1000 µg/mL κ, in comparison to the control untreated cells are shown in Figure 2, as demonstrated by optical inverted microscopy. The treated cells showed a typical morphological texture of damaged cells, i.e the cells were shriveled and by round-shaped (Figure 2(b)) in comparison to the control untreated cells that were spindle-shaped (Figure 2(a)).
Figure 2.
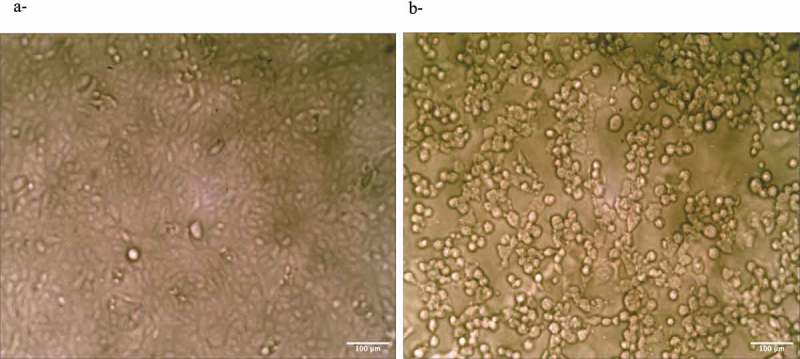
(a) Control untreated Vero cells. (b) Cells after 3 days of treatment with 1000 µg/mL κ.
3.2. Antiviral activity of different PSs on VZV infection
Vero cells were infected with VZV in the presence of various concentrations of the PSs and the treatments were continued up to the end of experiment (2-days post infection). Although all the various tested PSs demonstrated a significant antiviral effect against VZV (Figure 3), it seems that LB and X had the weakest inhibition impact; at a concentration of 100 µg/mL they rendered only 35 and 45% inhibition of the viral infection, respectively, while the other tested PSs almost completely inhibited the viral infection at this concentration. In addition, a dose-dependent effect was observed for all PSs. Noteworthy, focusing on the low dose range treatments (< 10 µg/mL), is that SPSs of all the carrageenan forms yielded the strongest inhibitory effect. Among the carrageenans, at the lowest concentration (1 µg/mL), λ had the strongest inhibitory effect on VZV infection, i.e it rendered ~ 90% inhibition. κ and the ἰ also demonstrated a significant inhibitory effect (at 1 µg/mL they rendered ~ 75% and 70% inhibition respectively, Figure 3). The other PSs that also exhibited antiviral activity, but weaker than those of the carrageenan group treatments were P and G (at 1 µg/mL they rendered ~ 55% inhibition effect.
Figure 3.

Vero cells were infected with 0.01 m.o.i of VZV in the absence or presence of various doses of the tested PSs. The infected cells were treated with the PS during the infection and 2-days post infection. The effect is presented as inhibition percentage compared to the infected untreated cells. Data are expressed as the mean ± SEM percentage (n = 5); **p < 0.001 vs. the infected untreated cells.
The selectivity indices (SI) of these tested polysaccharides, calculated as the ratio of the dose that reduced the number of viable Vero cells to 50% (CC50) to the effective dose that inhibited 50% of viral activity (IC50) are shown in Table 2.
Table 2.
CC50, IC50 and SI values of different polysaccharides against VZV.
| Parameter Evaluation |
|||
|---|---|---|---|
| Tested Molecule | CC50a | IC50b | SIc |
| X | >5000 | 800 | >6.25 |
| LB | >5000 | 800 | >6.25 |
| G | >5000 | 1 | >5,000 |
| κ | 1000 | 0.5 | 2,000 |
| λ | >5000 | 0.1 | >50,000 |
| ἰ | >5000 | 0.8 | >6,250 |
| P | 1000 | 1 | 1000 |
| ACV | 70 | 0.1 | 700 |
aFifty percent cytotoxic concentration (µg/mL).
bFifty percent inhibitory concentration (µg/mL).
cSelective index (CC50/IC50).
In terms of SI values almost all the PSs are very active, in the order of λ > ἰ > G > κ > P against VZV relative to ACV as a reference drug.
3.3. Primary investigation of ἰ antiviral mode of action
To expand our knowledge regarding the mode of action of ἰ, cells were treated with ἰ at various stages of the infection cycle, including: pre-infection, during infection, and post infection. As demonstrated, when the ἰ was only pre-incubated with Vero cells prior to VZV infection, there was no significant inhibitory effect on plaque formation compared with the control untreated cells (Figure 4). However, when ἰ was added only at the time of the infection period or during and post infection, a significant virus inhibition was demonstrated (Figure 4).
Figure 4.
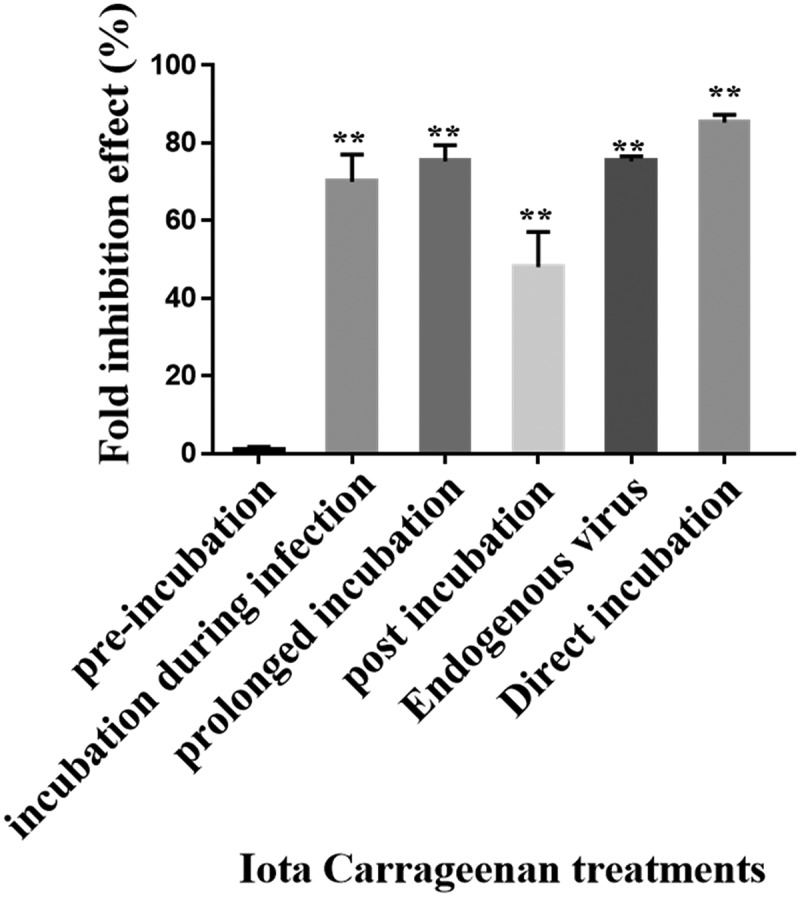
Antiviral activity of i on VZV infection at different stages of the infection. Vero cells infected with 0.01 m.o.i of VZV and treated with 1 µg/mL of ἰ at different periods of time. Furthermore, direct effect of ἰ on the virus infectivity (direct incubation) and its effect on the endogenous virus production were examined (n = 5); **p < 0.001 vs. the infected untreated cells.
A significant inhibitory effect was also observed when i was added to the cells only post infection, although to a lesser extent (~48% inhibition) compared to that observed in PS prolong-incubated infected cells (~75% inhibition). In order to examine whether ἰ has an effect on VZV replication after its penetration into the host cells, the effect of i on intracellular VZV replication (endogenous virus) was tested as detailed in “Materials and Methods” section. Our results (Figure 4) showed that treatment with i caused ~75% inhibition of VZV infection development. Furthermore, when the viral particles were pre-incubated with ἰ only before infection, there was about 85% inhibition of the viral infection (Figure 4).
4. Discussion
In the last decade, renewable polysaccharides obtained from natural sources have gained a lot of interest for their invaluable application across a variety of fields including textile, food, cosmetics, and pharmaceutical industries [39]. Many studies that were focused on elucidation of their beneficial bioactivities, have proven that some of the polysaccharides, especially the sulfated forms, have the capacity to act as antiviral molecules against various viruses. Herein we studied the inhibitory effects of natural polysaccharides obtained from renewable sources, varied by their structure and charge, on VZV infection in vitro.
All the tested PSs showed a significant antiviral effect against VZV, depending on their dose-concentration treatment. All the SPSs as well as G exhibited significantly higher antiviral bioactivity rates in comparison to X and LB. This finding is in agreement with previous studies which suggested that the antiviral bioactivity of polysaccharides strongly depends on their level of sulfation [21,22,24,40,41]. Moreover, members of the carrageenan family were already proven to act as antiviral agents against herpes viruses, including HSV-1 and HSV-2 [26,29,30,36,42]. Among the carrageenans, λ had the strongest inhibitory effect on VZV infection. The primary differences between the carrageenan types, which tailor their specific properties, including their antiviral bioactivities, are the number and the position of ester sulfate groups, their molecular weight, as well as the content of 3,6-anhydro-galactose. The λ form contains the highest sulfate content- three sulfate ester groups, resulting in calculated sulfate content of approximately 41% w/w [43,44]. Whereas, κ and ἰ contain one and two sulfate ester groups respectively, resulting in a calculated sulfate content of approximately 20 and 33% w/w, respectively [43,44]. In addition, λ does not contain the 3,6-anhydro-galactose that is present in the κ and ἰ forms. The sulfate content may be one of the reasons for the enhanced inhibitory effect, as was already suggested in several reports [21,22,24,40,41]. However, different composition and structure may also affect the SPS’s bioactivities. Although ἰ has a higher sulfate content than κ, no significant differences were observed in their antiviral effect. Therefore, it can be suggested that the linkage and the position of the ester sulfate may also have a crucial role in activating the PS as an antiviral agent; e.g the ester sulfate residues in the building blocks of ἰ are attached to the 2nd position of the 3,6-anhydro-galactose (Table 1). P has already been reported as an antiviral agent in vivo and in vitro against various viruses, including herpes simplex and VZV [26,34]. The weaker antiviral impact of the P on the VZV infection in comparison to the carrageenan group may be attributed to its relatively low sulfate content (~9%w/w) [45]. In addition, P’s structure is different than that of the carrageenans; It is a branched heteropolymer, composed of different monosugars, among them xylose, glucose and galactose, and the negatively charged glucuronic acid residue. It is noteworthy that G which is a neutral branched PS, significantly inhibited VZV infection. These results demonstrate that the sulfate is not the only factor that can affect the antiviral activity. Indeed, it was reported that the neutral polysaccharide scleroglucan acts as an effective inhibitor against HSV-1 and HSV-2 [36]. Interestingly, even though the LB structure is very similar to that of G (Table 1), its antiviral activity was less effective. It can be suggested that the highly uneven distribution of the α-D-galactose at the C6 position of D-mannose reduced the PS antiviral activity. It was previously suggested that the antiviral capacity of some polysaccharides can be attributed to their negatively charge nature. However, X which is known by its negatively charged nature due to glucuronic acid residues did not possess antiviral bioactivity as observed for G or the SPSs studied. Therefore it can be suggested that the type of the ionic group can play a crucial role in the antiviral potency [21], or probably the negative charge is not critically required for the antiviral activity of these PSs.
In order to evaluate the safety of using these PSs as antiviral drugs, their cytotoxicities were evaluated. In the dose-range that was tested the ἰ, λ, G, LB and X polysaccharides did not show any cytotoxic effect on the cells, and therefore were considered as safe additives. However, in agreement with former publications, P and κ can reduce Vero cell viability depending on their concentration [26]. Notably, although the basic repeating units of ἰ and κ are similar, both being composed of D-Gal-4-sulfate,3,6-anhydro-D-Gal, κ was still cytotoxic to Vero cells in comparison to ἰ.
Theoretically evaluation of the use of these PSs as an alternative treatment in terms of effectiveness and safety in comparison to ACV against VZV was performed by calculating the SI values. According to their SI values, it was found that the effectivity and safety of PSs are in the order of λ > ἰ > G > κ > P against VZV. Notably, all these PSs yielded very high SI values in comparison to ACV and therefore they can be considered as potential candidates for antiviral therapy.
The next step of the study was to gain comprehensive knowledge concerning the mechanism of antiviral action of ἰ. The ἰ was chosen for further investigation based on its effectiveness and its beneficial overall safety profile, as follows: 1) It demonstrated high SI values 2) It is characterized by unique rheological properties (gel-forming and viscosifying properties). These properties make the ἰ more attractive as an applicative topical gel (easy to use on the skin). 3) ἰ is considered as nontoxic and has been used for decades as an additive in many food products and in different pharmaceutical and cosmetics agents. Even though the λ yielded higher SI values compared to ἰ, we did not focus in our investigation on its mode of action due to its reported pro-inflammatory potential [46] in contrast to i [47].
The significant inhibitory effect of ἰ when it was added either only at the time of the infection period or only post infection may indicate that ἰ affects different stages during the viral infection cycle. It can be suggested that ἰ may inhibit early step/s in the virus infection such as virus attachment or penetration to host cells and late step/s after the penetration of the virus into the host cells. Indeed, previous reports showed that ἰ can inhibit replication of Human papillomavirus [27], influenza A H1N1 virus [48], dengue virus [49], and Rhinovirus [28] by blocking the binding of the virus to the host cells. This finding proves that ἰ has a direct impact on the virus, probably by strongly interacting with the viral particles and thus preventing their binding to the host cells. This is in agreement with different previous studies which utilized in vitro human papillomavirus (HPV) infection experiments [27]. However, ἰ did not show any significant inhibition of the viral infection when it was only pre-incubated with cells. This result may indicate a non-or a weak and reversible attachment of this PS with the host cell membrane.
In addition, our findings prove the involvement of ἰ in a late stage of the viral replication cycle after its penetration into the cells. It strongly reduced the number of infective viruses inside the host cells when it was added only post infection. Indeed, several previous reports demonstrated that ἰ inhibits various virus types in late stage/s of the viral replication cycle; For example Gonzalez et al. [50] showed that ἰ inhibited a step in HSV-1 replication subsequent to viral internalization but prior to the onset of late viral protein synthesis. In addition, a potent inhibitory effect of ἰ on the replication of hepatitis A virus in the human hepatoma cell line PLC/PRF/5 was also observed [51]. Further studies are required for deep understanding of the exact mechanism of action of the i polysaccharide and probably the other studied PSs.
These findings are part of an on-going research, whose ultimate goal is to elucidate the exact PS inhibitory mechanism of action. Herein, renewable PSs were proven to act as a beneficial natural agent that can inhibit VZV infection.
Funding Statement
This work was supported by the Sami Shamoon College of Engineering [internal grant # 06/Y15/T1/D1].
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- [1].Elion GB, Furman PA, Fyfe JA, et al. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl)guanine. Proc Natl Acad Sci USA. 1977;74:5716–5720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Meyers JD, Wade JC, Mitchell CD, et al. Multicenter collaborative trial of intravenous acyclovir for treatment of mucocutaneous herpes simplex virus infection in the immunocompromised host. Am J Med. 1982;73:229–235. [DOI] [PubMed] [Google Scholar]
- [3].Müller V, Chávez JH, Reginatto FH, et al. Evaluation of antiviral activity of South American plant extracts against herpes simplex virus type 1 and rabies virus. Phytother Res. 2007;21:970–974. [DOI] [PubMed] [Google Scholar]
- [4].Khan MTH, Ather A, Thompson KD, et al. Extracts and molecules from medicinal plants against herpes simplex viruses. Antiviral Res. 2005;67:107–119. [DOI] [PubMed] [Google Scholar]
- [5].Whitley RJ, Roizman B.. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. [DOI] [PubMed] [Google Scholar]
- [6].Freer G, Pistello M. Varicella-zoster virus infection: natural history, clinical manifestations, immunity and current and future vaccination strategies. New Microbiol. 2018;41:95–105. [PubMed] [Google Scholar]
- [7].Sasivimolphan P, Lipipun V, Likhitwitayawuid K, et al. Inhibitory activity of oxyresveratrol on wild-type and drug-resistant varicella-zoster virus replication in vitro. Antiviral Res. 2009;84:95–97. [DOI] [PubMed] [Google Scholar]
- [8].Galetta KM, Gilden D. Zeroing in on zoster: A tale of many disorders produced by one virus. J Neurol Sci. 2015;358:38–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Docherty JJ, Sweet TJ, Bailey E, et al. Resveratrol inhibition of varicella-zoster virus replication in vitro. Antiviral Res. 2006;72:171–177. [DOI] [PubMed] [Google Scholar]
- [10].Bao B, Meng Z, Li N, et al. Design, synthesis and antiviral activity studies of schizonepetin derivatives. Int J Mol Sci. 2013;14:17193–17203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Field AK, Biron KK. “The end of innocence” revisited: resistance of herpesviruses to antiviral drugs. Clin Microbiol Rev. 1994;7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Devrim I, Tezer H, Haliloğlu G, et al. Relapsing Herpes simplex virus encephalitis despite high-dose acyclovir therapy: a case report. Turk J Pediatr. 2008;50:380–382. [PubMed] [Google Scholar]
- [13].Picton SF, Flatt PR, McClenaghan NH. Differential acute and long term actions of succinic acid monomethyl ester exposure on insulin-secreting BRIN-BD11 cells. Int J Exp Diabetes Res. 2001;2:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Choi EJ, Lee CH, Kim YC, et al. Wogonin inhibits Varicella‑Zoster (shingles) virus replication via modulation of type I interferon signaling and adenosine monophosphate‑ activated protein kinase activity. J Funct Foods. 2015;17:399–409. [Google Scholar]
- [15].Gilden D, Mahalingam R, Nagel MA, et al. Review: the neurobiology of varicella zoster virus infection. Neuropathol Appl Neurobiol. 2011;37:441–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Yarmolinsky L, Zaccai M, Ben-Shabat S, et al. Antiviral activity of ethanol extracts of Ficus binjamina and Lilium candidum in vitro. New Biotechnol. 2009;26:307–313. [DOI] [PubMed] [Google Scholar]
- [17].Yarmolinsky L, Huleihel M, Zaccai M, et al. Potent antiviral flavone glycosides from Ficus benjamina leaves. Fitoterapia. 2012;83:362–367. [DOI] [PubMed] [Google Scholar]
- [18].Clark A. Natural products as a resource for new drugs. Pharm Res. 1996;3:1133–1141. [DOI] [PubMed] [Google Scholar]
- [19].Bailly C, Perrine D, Lacelot JC, et al. Sequence-selective binding to DNA of bis(amidinophenoxy)alkanes related to propamidine and pentamidine. Biochem J. 1997;323:23–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Baba M, Snoeck R, Pauwels R, et al. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob Agents Chemother. 1988;32:1742–1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Ghosh T, Chattopadhyay K, Marschall M, et al. Focus on antivirally active sulfated polysaccharides: from structure–activity analysis to clinical evaluation. Glycobiology. 2008;19:2–15. [DOI] [PubMed] [Google Scholar]
- [22].Ahmadi A, Zorofchian Moghadamtousi S, Abubakar S, et al. Antiviral potential of algae polysaccharides isolated from marine sources: a review. BioMed Res Int. 2015;825203. DOI: 10.1155/2015/825203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Jiao G, Yu G, Zhang J, et al. Chemical structures and bioactivities of sulfated polysaccharides from marine algae. Mar Drugs. 2011;9:196–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Wang W, Wang SX, Guan HS. The antiviral activities and mechanisms of marine polysaccharides: an overview. Mar Drugs. 2012;10:2795–2816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Harden EA, Falshaw R, Carnachan SM, et al. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antiviral Res. 2009;83:282–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Huleihel M, Ishanu V, Tal J, et al. Antiviral effect of red microalgal polysaccharides on Herpes simplex and Varicella zoster viruses. J Appl Phycol. 2001;13:127–134. [Google Scholar]
- [27].Buck CB, Thompson CD, Roberts JN, et al. Carrageenan is a potent inhibitor of papillomavirus infection. PLoS Pathog. 2006;2:e69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Grassauer A, Weinmuellner R, Meier C, et al. Iota-Carrageenan is a potent inhibitor of rhinovirus infection. Virol J. 2008;5:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Zacharopoulos VR, Phillips DM. Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diagn LabImmunol. 1997;4:465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Carlucci MJ, Scolaro LA, Noseda MD, et al. Protective effect of a natural carrageenan on genital herpes simplex virus infection in mice. Antiviral Res. 2004;64:137–141. [DOI] [PubMed] [Google Scholar]
- [31].de Sf-tischer PC, Talarico LB, Noseda MD, et al. Chemical structure and antiviral activity of carrageenans from Meristiella gelidium against herpes simplex and dengue virus. Carb Polym. 2006;63:459–465. [Google Scholar]
- [32].Carlucci MJ, Pujol CA, Ciancia M, et al. Antiherpetic and anticoagulant properties of carrageenans from the red seaweed Gigartina skottsbergii and their cyclized derivatives: correlation between structure and biological activity. Int J Biol Macromol. 1997;20:97–105. [DOI] [PubMed] [Google Scholar]
- [33].Arad (Malis) S, Ginzberg A, Huleihel M. Antiviral activity of sulfated polysaccharides of marine red algae, . In: Fingerman M, editor. Recent advances in marine biotechnology: biomaterials from aquatic and terrestrial organisms. Enfield, UK,: Science Publishers Inc; 2006. p. 37–62. [Google Scholar]
- [34].Huleihel M, Ishanu V, Tal J, et al. Activity of porphyridium sp polysaccharide against herpes simplex viruses in vitro and in vivo. J Biochem Biophys Meth. 2002;50:189–200. [DOI] [PubMed] [Google Scholar]
- [35].Ehresmann D, Deig EF, Hatch MT. Antiviral properties of algal polysaccharides and related compounds In: Hoppe H, Levring T, Tanaka Y, editors. Marine algae in pharmaceutical science. New York, NY: Walter de Gruyter; 1979. p. 293–302. [Google Scholar]
- [36].Marchetti M, Pisani S, Pietropaolo V, et al. Inhibition of herpes simplex virus infection by negatively charged and neutral carbohydrate polymers. J.Chemotherapy. 1995;7:90–96. [DOI] [PubMed] [Google Scholar]
- [37].Guillard RRL. Culture of marine invertebrate animals In: Smith W.L., Chanley M.H., editors. Culture of phytoplankton for feeding marine invertebrates. New York, NY: Plenum Book Publ. Corp; 1975. p. 29–60. [Google Scholar]
- [38].Shi Y, Kornovski BS, Savani R, et al. A rapid, multiwell colorimetric assay for chemotaxis. J Immunol Methods. 1993;164:149–154. [DOI] [PubMed] [Google Scholar]
- [39].Xu SY, Huang X, Cheong KL. Recent advances in marine algae polysaccharides: isolation, structure, and activities. Mar Drugs. 2017;15:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Arad (Malis) S, Levy-Ontman O. Red microalgal cell-wall polysaccharides: biotechnological aspects. Curr Opin Biotech. 2010;21:358–364. [DOI] [PubMed] [Google Scholar]
- [41].Bandyopadhyay SS, Navid MH, Ghosh T, et al. Structural features and in vitro antiviral activities of sulfated polysaccharides from Sphacelaria indica. Phytochemistry. 2011;72:276–283. [DOI] [PubMed] [Google Scholar]
- [42].Gomaa HHA, Elshoubaky GA. Antiviral activity of sulfated polysaccharides carrageenan from some marine seaweeds. Int J Curr Pharm Res. 2016;7:34–42. [Google Scholar]
- [43].Nanaki S, Karavas E, Kalantzi L, et al. Miscibility study of carrageenan blends and evaluation of their effectiveness as sustained releasecarriers. Carb Polym. 2010;79:1157–1167. [Google Scholar]
- [44].De Ruiter GA, Rudolph B. Carrageenan biotechnology. Trends Food Sci Technol. 1997;8:389–395. [Google Scholar]
- [45].Arad (Malis) S, Levy-Ontman O. Sulfated polysaccharides in the cell wall of red microalgae In: Sabu T, Dominique D, Christophe C, et al, editors. Handbook of biopolymer-based materials: from blends and composites to gels and complex networks. Wiley-VCH, Verlag: John Wiley & Sons Ltd; 2013. p. 351–370. [Google Scholar]
- [46].Morris CJ. Carrageenan-induced paw edema in the rat and mouse. Methods Mol Biol. 2003;225:115–121. [DOI] [PubMed] [Google Scholar]
- [47].Hebar A, Koller C, Seifert JM, et al. Non-clinical safety evaluation of intranasal iota-carrageenan. PLoS One. 2015;10:e0122911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Leibbrandt A, Meier C, König-Schuster M, et al. Iota carrageenan is a potent inhibitor of influenza A virus infection. PLoS ONE. 2010;5:e14320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Talarico LB, Damonte EB. Interference in dengue virus adsorption and uncoating by carrageenans. Virology. 2007;363:473–485. [DOI] [PubMed] [Google Scholar]
- [50].Gonzalez ME, Alarcon B, Carrasco L. Polysaccharides as antiviral agents: antiviral activity of carrageenan. Antimicrob Agents Chemother. 1987;31:1388–1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Girond S, Crance JM, Van Cuyck-Gandre H, et al. Antiviral activity of carrageenan on hepatitis A virus replication in cell culture. Res Virol. 1991;142:261–270. [DOI] [PubMed] [Google Scholar]


