ABSTRACT
Cardamonin (CAD) is a member of the aromatic ketones family that is closely related to anti-bacterial, anti-inflammatory and anti-cancer effects. Nevertheless, the physiological function of cardamonin in chronic colitis and colon cancer has not been well verified. We found that cardamonin treatment alleviates intestinal disease, including recurring colitis and colitis-associated tumorigenesis, along with the reduced secretion of IL-1β and TNF-α. Further, cardamonin inhibits cell viability and inflammation factors of colorectal cancer cells in vitro. In tumor cells, the inhibitory effect of cardamonin on cell proliferation is closely related to decreased phosphorylation of signal transducers and activators of transcription (STAT) signals. This study reveals the crucial role of cardamonin in sustaining gastrointestinal homeostasis and offers a new strategy for colon cancer therapy.
KEYWORDS: Cardamonin, colon, inflammation, tumorigenesis, STAT
Introduction
Inflammation is deemed as a biological reaction to eliminate infection and injury to host. However, uncontrolled inflammation is a high-risk factor for colon cancer [1]. Previous studies found patients with chronic colitis are more susceptible to colorectal cancer than the general population [2,3]. Colon cancer is the third common malignant tumors both in estimated new cases and estimated deaths worldwide [4]. As the surgical techniques have developed rapidly, the cure rate and five-year survival rate after surgery still needs to be further improved [5]. Although various chemical-based therapies are carried out in clinical application, novel agents targeting proliferation-related pathway are urgently needed for enhancing effective treatment for colon cancer.
Cardamonin (CAD) is a natural chalcone extracted from the plant named Alpinia rafflesiana [6]. It has displayed anti-inflammatory, anti-tumor, anti-microbial effects as well as other biological activities. CAD interacts with various cellular signaling including NRF2, ERK, β-Catenin, mTOR and other targets during tumor progression [7–12]. Also, CAD modulates cell apoptosis via influencing death receptors and cell survival proteins [13,14]. Recent studies have revealed that CAD decreased activation of STAT3 and NF-κB [15–18]; these studies indicate that it may present a new kind of transcription factor inhibitor. However, the function and potential mechanism of CAD in chronic colon inflammation process and colitis-associated carcinoma remain to be well elucidated.
In our current study, we concentrated on evaluating the physiological role of CAD on inflammation and colorectal tumorigenesis. The results indicate that CAD relieves recurring colitis and inhibits carcinogenesis via inhibiting STATs pathway in colon cancer cells.
Materials and methods
Cell lines and chemicals
The human colon cancer cell line HT-29 and SW-460 were cultured in DMEM (Invitrogen, USA) containing 10% fetal bovine serum (Invitrogen, USA) and penicillin/streptomycin (Invitrogen, USA). All cells were cultured in 5% CO2 at 37°C. Cardamonin (Sigma, USA) was dissolved in several concentrations and stored at −20°C. Cells were treated with IL-6 (Sigma, USA) for 30 min.
Induction of colitis and inflammation-associated tumor
C57BL/6 mice were obtained from Vital River (Beijing, China). All animal studies were approved by China Association for accreditation of laboratory animal care. The C57BL/6 mice were introduced to 3% dextran sulfate sodium (DSS) for five days to induce acute colitis. Recurring colitis was established by three cycles of 2.5% DSS treatment [19]. To induce colitis-associated colon cancer (CACC), mice were injected one i.p. of Azoxymethane (AOM) for 10mg/kg and received three cycles of DSS exposure (2.5%). Each CAD group was given CAD (40mg/kg) every other day throughout the process. The mice were sacrificed and harvested at the end of each course.
Average clinical score was assessed by weight loss value, stool consistency and rectal bleeding. Each value was calculated and get a comprehensive score [20].
Tumor histopathology and immunofluorescence
The colon of each mouse was removed to analysis polyp formation as reported in the previous study [21]. Colon sample was fixed in paraformaldehyde (4%) and then was entrapped in paraffin for H&E staining and anti-Ki67 (BD Pharmingen, USA) immunohistochemical staining. Frozen samples were incubated with anti-p-STAT3 (BD Pharmingen, USA) for immunofluorescence staining.
Proinflammatory mediator evaluation
Colon was isolated and the wet weight was measured. Inflammatory factors from tumor and supernatants of HT-29 and SW-460 cells were assayed using enzyme-linked immunosorbent assay (ELISA).
Quantitative polymerase chain reaction (qPCR)
Total RNA was isolated from samples using Trizol (Invitrogen, USA) according to instructions from the manufacturer. qPCR was performed using SYBR Premix Ex Taq TM Kit (Takara, Japan), analyzed by 2−ΔΔCt method and normalized to Gapdh.
Analysis of cell viability
For assay of cell viability, cells (1.0 × 104/well) were seeded to a 24-well plate. The following day, cells were exposed to various concentrations of chemicals. One day after incubation, cells were measured by using Cell Counting Kit-8 (CCK8, Dojindo Laboratories, Japan).
Western blotting
Cultured cells and dissected transplanted tumors were collected; protein was extracted using RIPA lysis buffer (Beyotime, China) supplemented with cocktail protease inhibitor (biotool, USA). According to protein concentration tested by BCA kit (Bio-rad, China). Equivalent amounts of samples (30μg) were blended with 5× SDS loading buffer (Beyotime, China) and ddH2O. Samples were heated at 99°C for 5 min and then ran on an SDS-PAGE (10%) gel at 115 V for 1.2 h. Proteins were transferred to a PVDF membrane. PVDF Membrane was blocked using 5% BSA in TBST at room temperature for 1 h and incubated in antibodies p-JAK2 (Y1007), JAK2, p-STAT1 (Y701), STAT1, p-STAT3 (Tyr705), STAT3, p-STAT5(Y694), STAT5 and β-actin (Cell Signaling Technology, USA) for 16h at 4°C. The membrane was washed in 1X TBST and then treated with appropriate secondary antibodies for 1h at RT. Membrane was washed in TBST. After incubation with the ECL Plus system (Amersham Biosciences, Sweden), signals were determined using Image Quant LAS 4000 mini system (GE Healthcare Bio-sciences, England).
Statistical analysis
The significant differences between average values were confirmed by three independent experiments. Unless otherwise stated, each value represents mean ±SEM. The results were assessed by GraphPad Prism 6 (GraphPad Software) and unpaired t-test, One-way ANOVA tests with Bonferroni method was used for multiple comparisons between values. Data were considered to be statistically significant when p value < 0.05.
Results
CAD attenuates experimental colitis in mice
To figure out whether CAD functions as an anti-inflammatory agent, we attempted to observe the effect of CAD on acute colitis in mice. Mice were exposed to 3% DSS for five days to establish an acute colitis model. During the process, mice without CAD administration showed a reduction in colonic length (Figure 1(a,b)) as well as weight loss (Figure 1(c)). The clinical parameters that reflect disease course (weight loss, hemoccult positivity and stool consistency) were monitored to gain clinical scores. A decrease in clinical severity was detected in the CAD-treated mice (Figure 1(d)).
Figure 1.
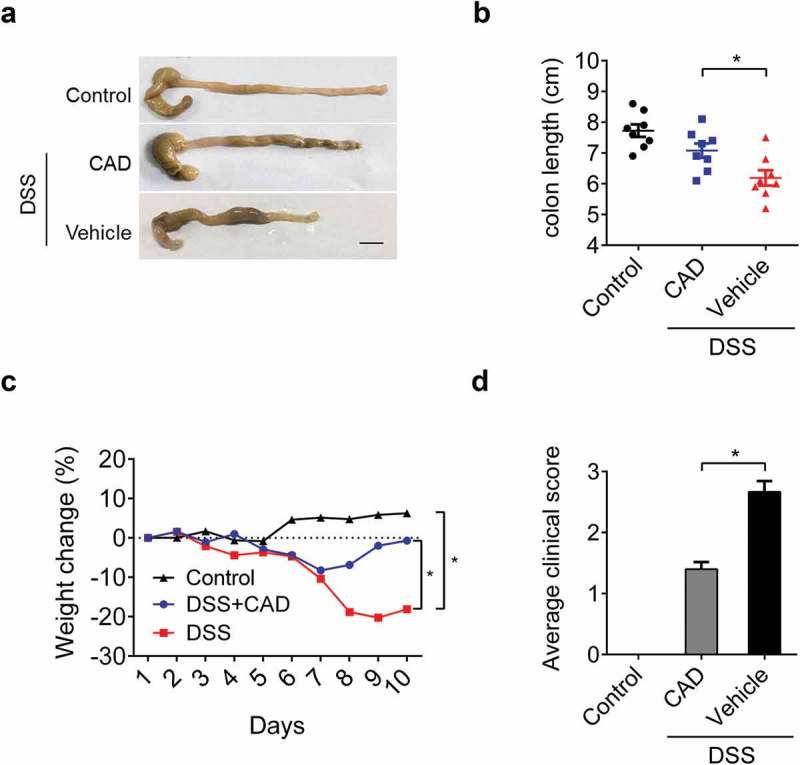
Effects of cardamonin (CAD) in acute colitis. Wild-type mice were challenged with 3% DSS for five days; CAD (40mg/kg) or vehicle was administrated every other day starting from day 0. Disease progression was assessed daily. (a) Representative morphological changes of the colon at day 10. (b) Colon length of mice was measured after sacrifice. *p < 0.05 by ANOVA. (c) Weight loss was monitored every day. *p < 0.05 by Two-way ANOVA. D Average clinical score including weight loss value, stool consistency and rectal bleeding. *p < 0.05 by ANOVA. Black bar, 1cm; Data represent means±SEM.
We next probed the anti-inflammatory role of CAD on recurring colitis model. Over the course mice were exposed to 2.5% DSS for three rounds to induce recurring colitis, after each round of DSS mice were allowed to recover for two weeks. During the whole process, animals treated with CAD exhibited less weight loss compared to mice received vehicles only (Figure 2(a)), which is in consistent with the results observed in the acute model. In colitis model, colon shortening is closely related to the progression of the disease. The colon length from CAD treated group was increased than those in the DSS group (Figure 2(b)). Similar to this, histopathology analysis revealed that CAD protected colons from DSS-induced severe mucosa damage (Figure 2(c)). CAD treatment during DSS challenge demonstrated significantly reduced the release of inflammatory factors compared to vehicle controls (Figure 2(d–e)). These results proved that CAD acts as a negative regulator of inflammation in chronic colitis.
Figure 2.
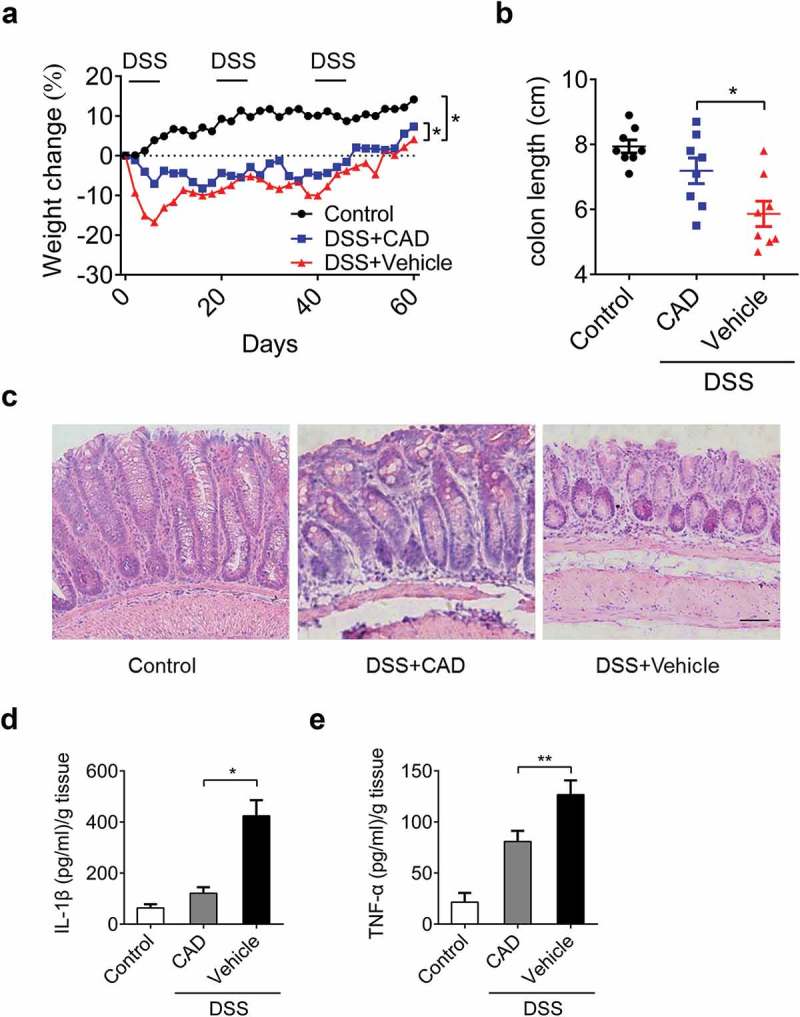
Cardamonin attenuates the development of recurring colitis. Mice were administrated with three rounds of 2.5% DSS for five days; each round was followed by two weeks of normal water to induce recurring colitis. (a) Weight change was monitored in the recurring colitis model. *p < 0.05 by Two-way ANOVA. (b) The length of colon was measured on day 60. *p < 0.05 by ANOVA. (c) Representative histopathology of colon tissues. Original magnification, ×200. (d-e) Colon was homogenized and amounts of IL-1β and TNF-α in supernatants were assayed by ELISA. *p < 0.05, **p < 0.01 by ANOVA. Data represent means±SEM.
CAD alleviates disease progression in colitis-associated colon cancer(Cacc)
To observe the function of CAD in the origin and development of colitis-driven colon carcinoma, mice were injected with Azoxymethane (AOM) followed by three rounds of DSS (2.5%) to establish CACC model (Figure 3(a)). Mice administrated with AOM/DSS showed weight loss compared to the control group, while CAD intervention group exhibited less weight loss than vehicle treated group (Figure 3(b)). Consistent with this, the length of colons obtained from CAD treated mice were longer than vehicle treated mice (Figure 3(c)). During CACC, levels of multiple inflammatory cytokines were increased. CAD caused decreased levels of IL-1β and TNF-α in mice suffering from CACC (Figure 3(d–e)). These are consistent with previous results obtained from experimental colitis in mice.
Figure 3.
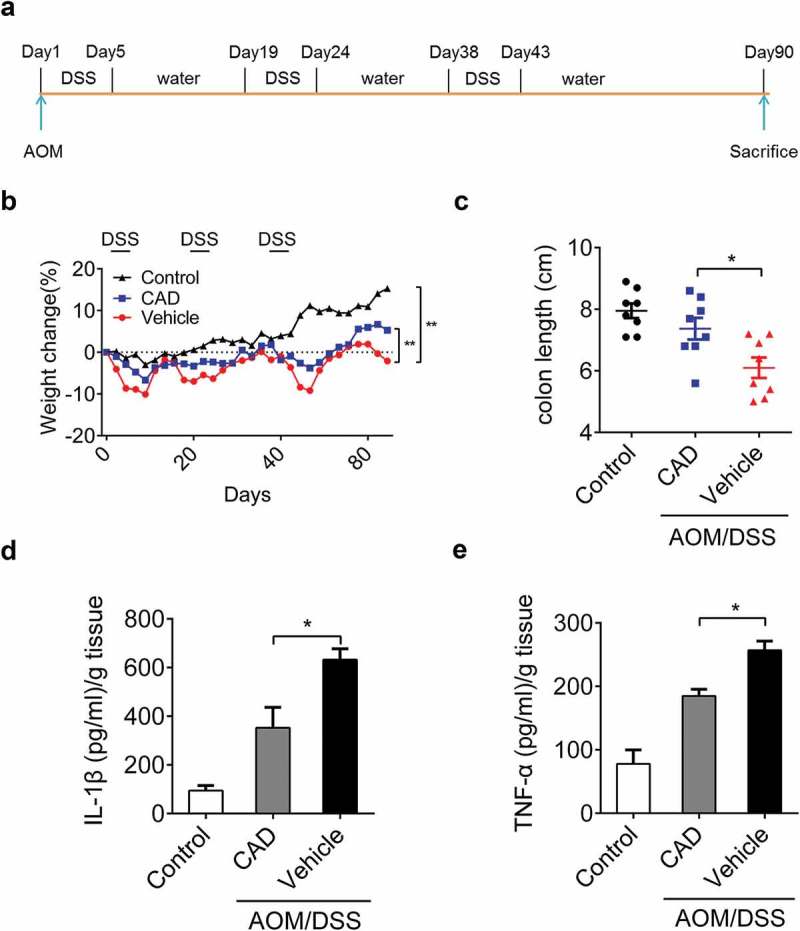
CAD can protect mice in colitis-associated colon carcinoma. (a) Experimental protocol for colitis-associated colon carcinoma model. (b) Weight changes were measured during the whole course. **p < 0.01 by Two-way ANOVA. (c) Length of the colon on day 90. *p < 0.05 by ANOVA. (d-e) Colon was homogenized and amounts of IL-1β and TNF-α in supernatants were assayed by ELISA. *p 0.05 by ANOVA. Data represent means ± SEM.
Effects of CAD on tumorigenesis in CACC
CAD treatment decreased colon inflammation in experimental colitis, and there has been evidence that colon inflammation is often associated with colonic tumorigenesis. The number and average size of macroscopic polyps focus in the colon of CAD treated mice were decreased compared to vehicle controls (Figure 4(a,b)). CAD administration reduced polyps and ulceration structure in the colon (Figure 4(c,d)). Ki67 staining showed the percentage of proliferating cells in the colon was decreased by CAD treatment compared with vehicle group (Figure 4(e,f)); these all suggested the protective role of CAD in CACC.
Figure 4.
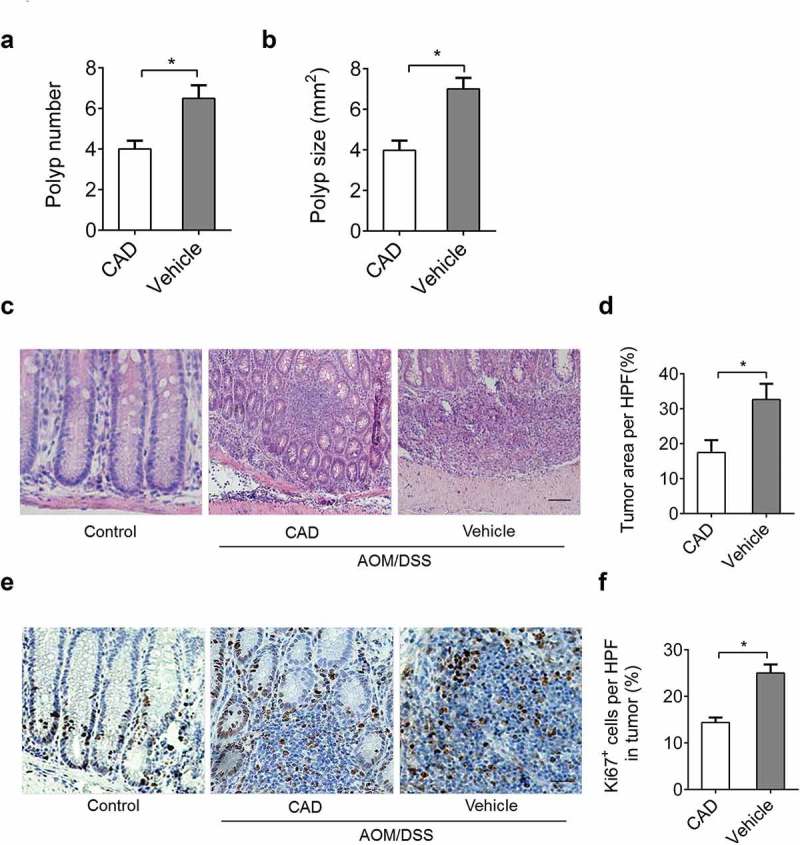
CAD attenuates tumorigenesis during CACC. The number (a) and average size (b) of polyps in colons harvested from mice in the CACC model. (c) Representative histopathology of paraffin-embedded colon sections. (d) Percent of tumor area in representative histopathology sections by Image pro plus (IPP). (e) Ki67 expression was assessed by IHC from colon tissues. (f) Percent of Ki67 positive cells in each HPF was quantified by IPP. Scale bar, 50 μm; Data represent means±SEM, *p < 0.05 by two-tailed Student’s t test.
In vitro research suggests CAD reduced cell viability in both HT-29 (Figure 5(a)) and SW-460 cells (Figure 5(b)) while qPCR assay of Ki67 also supported this result (Figure 5(c,d)). Similar to in vivo study, CAD treatment also decreased levels of IL-1β and TNF-α in supernatants of HT-29 cells (Figure 5(e)) as well as SW-460 cells (Figure 5(f)).
Figure 5.
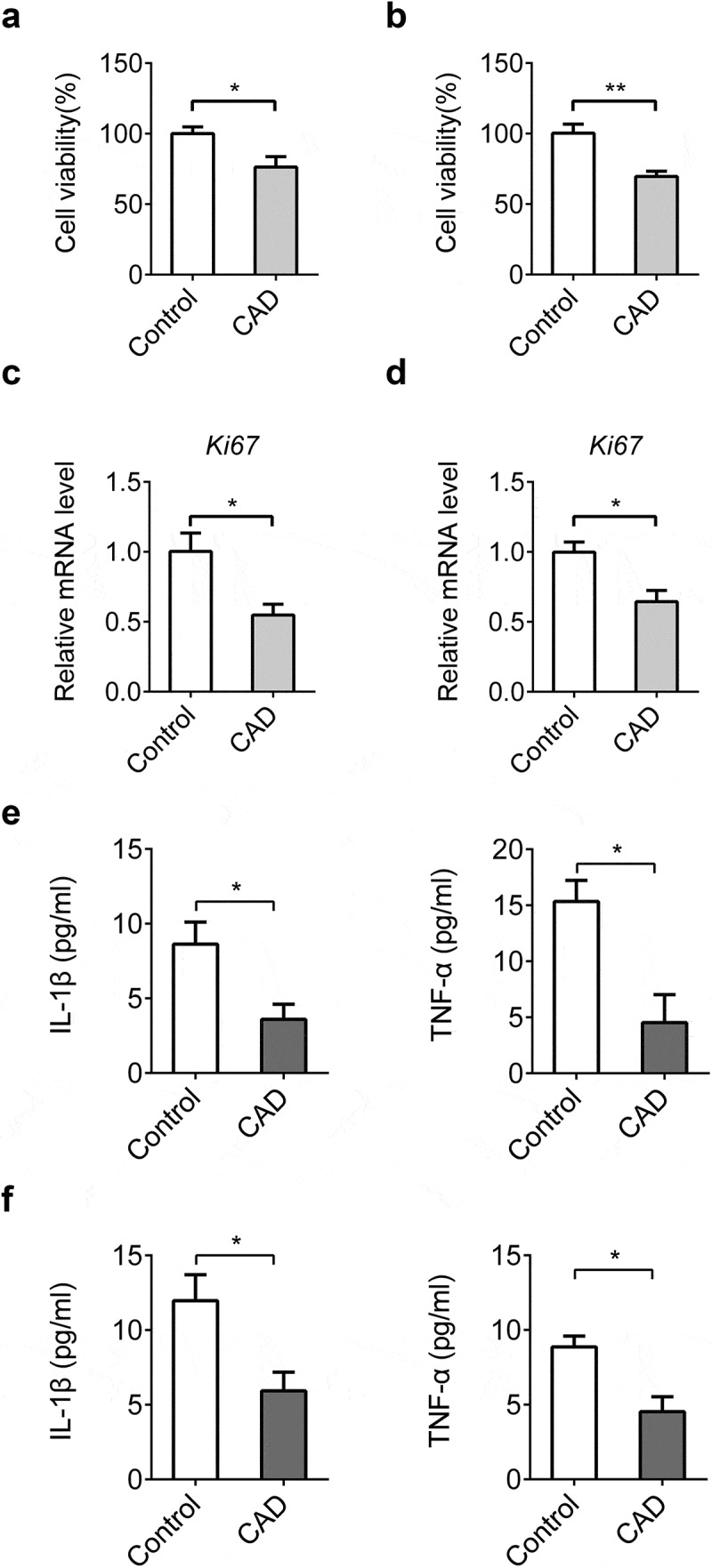
CAD inhibits cell proliferation and chemokine secretion in vitro. CAD (30 μM) was given to colon cancer cells. Cell viability of HT-29 (a) and SW-460 cells (b) was assessed by CCK-8 assay. mRNA levels of Ki67 in HT-29 (c) and SW-460 cells (d) were detected by qPCR. Levels of IL-1β and TNF-α in supernatants of HT-29 (e) and SW-460 cells (f) were assayed by ELISA. Data represent means±SEM, *p < 0.05, **p < 0.01 by two-tailed Student’s t test.
CAD negatively regulates STATs signaling in colitis-associated tumorigenesis
Previous observations have explored the negative roles of CAD on signal transducers and activators of transcription (STAT) proteins among which STAT3 signaling activation is often associated with various carcinomas including CACC [22]. Initial immunofluorescence test showed reduced localization of STAT3 in CAD treated group (Figure 6(a)). To further explore the impact of CAD on STATs activity, levels of upstream p-JAK2 and p-STATs were analyzed in colon tissue from mice over the course of the CACC model. In general, p-JAK2, p-STAT1, p-STAT3 and p-STAT5 were all decreased in CAD treated mice compared with their vehicle controls (Figure 6(b,c)). Together, these data showed that CAD inhibited STAT1, STAT3 and STAT5 activation mediated hindering colon tumorigenesis during CACC.
Figure 6.
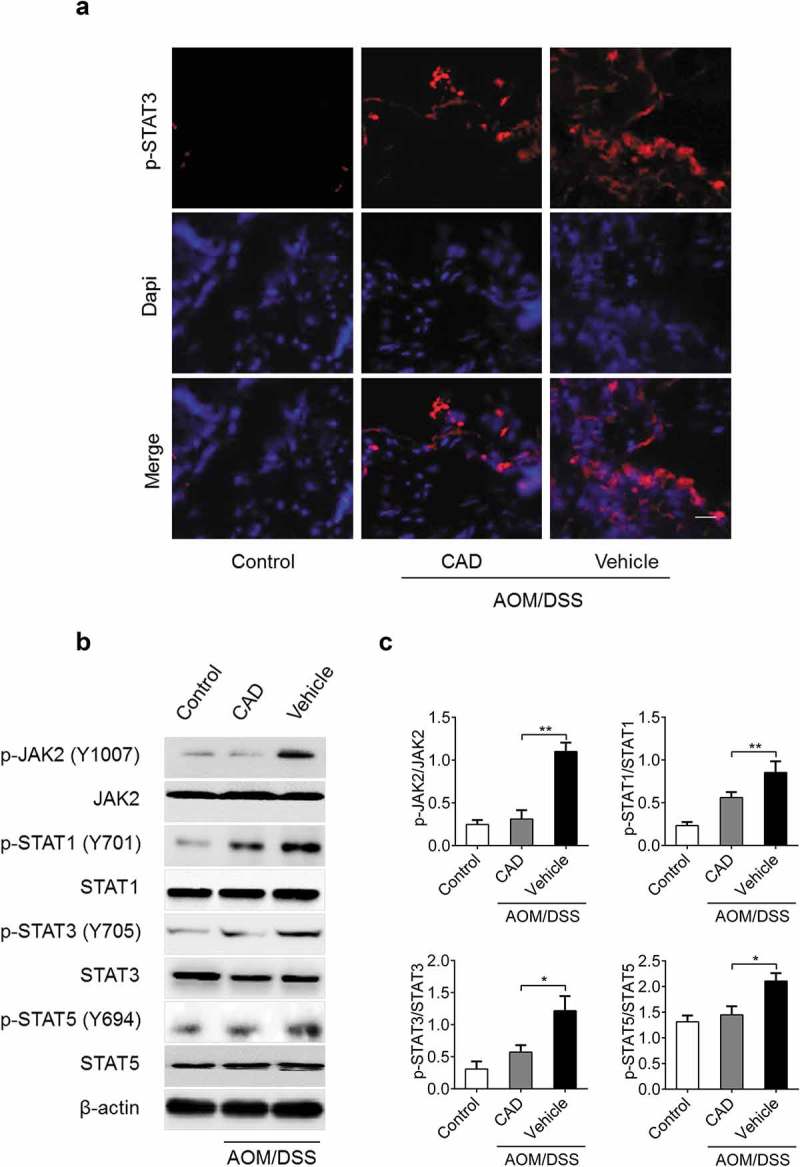
STAT3 activation was reduced in the colon tumors from CAD group. (a) Immunofluorescence assay showing localization of activated STAT3 in colon tissue. (b) Levels of p-JAK2, p-STAT1, p-STAT3 and p-STAT5 in colon tissue were analyzed by western blotting. (c) The western blot data were quantified by using Image J software. Scale bar, 25 μm; Data represent means±SEM, *p < 0.05, **p < 0.01 by ANOVA.
STAT3 signaling is essential for the protective role of CAD in colon cancer cells
To further study the role of CAD on STATs during CACC, we concentrated on the function of CAD in IL-6 stimulated activation of STAT1, STAT3 and STAT5 in HT-29 and SW-460 cells. Consistent with in vivo results, we found that STATs activation caused by IL-6 stimulation was also suppressed by CAD (Figure 7(a,b)). Restraining of STATs signals by CAD caused a decreased secretion of tumor-associated inflammatory factors in both HT29 cell line (Figure 7(c)) and SW-460 cell line (Figure7(d)). A further assay indicated that the reduced cytokines were associated with decreased cell viability by CAD in colorectal cancer cells (Figure 7(e,h)). These data suggested the important role of STAT3 activation during CAD inhibited colon neoplastic cell proliferation and chemokine secretion.
Figure 7.
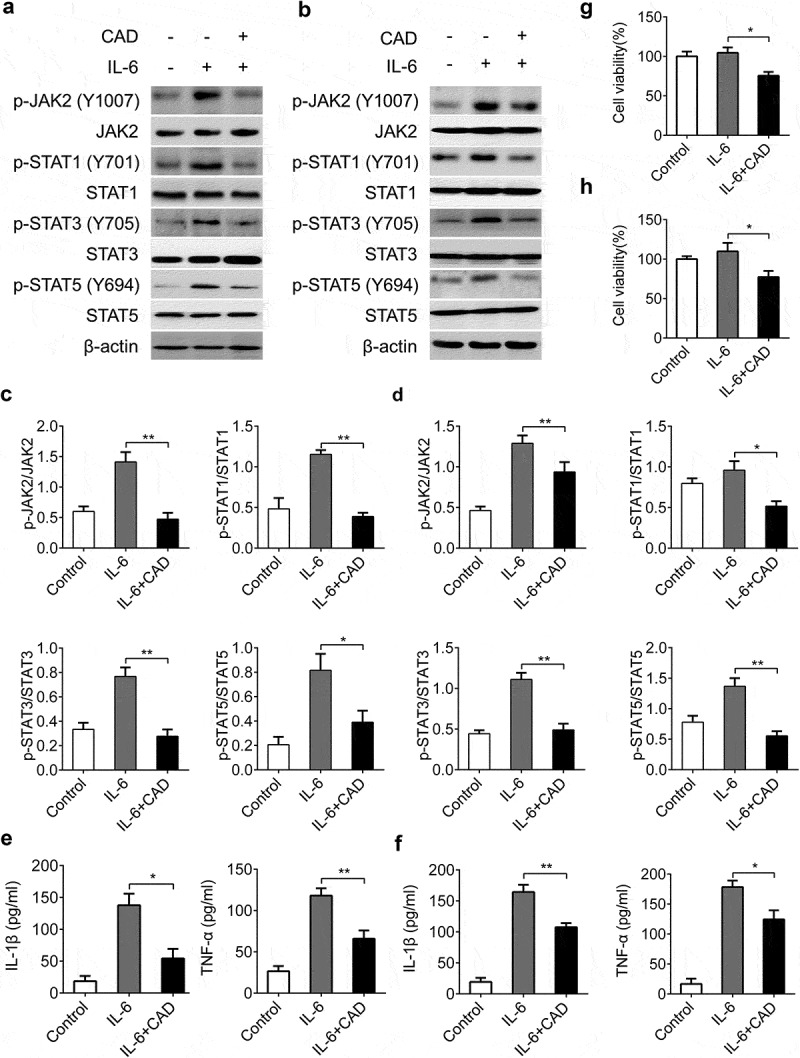
Reduction of cell viability and inflammation factors in colon tumor cells was associated with inhibited STAT3 pathway. CAD was added to HT-29 and SW-460 cell cultures after stimulated by IL-6 (50 ng/ml). Expressions of p-JAK2, p-STAT1, p-STAT3 and p-STAT5 in HT-29 (a) and SW-460 cells (b) were detected by western blotting. The western blot data of HT-29 (c) and SW-460 cells (d) were quantified by using Image J software. (e) Levels of IL-1β and TNF-α in supernatants of HT-29 cells were assayed by ELISA. (f) Levels of IL-1β and TNF-α in SW-460 cells. Cell viabilities of HT-29 (g) and SW-460 cells (h) were analyzed by CCK-8 assay. Data represent means±SEM, *p < 0.05, **p < 0.01 by ANOVA.
Discussion
A recent study has verified the effect of CAD on inflammation in acute colitis [23]. In accordance with this, we also confirmed this effect in short term colitis model. Further, our data suggest that CAD suppresses recurring colitis and CACC and negatively regulates STATs signaling.
Inflammation refers to a biological process during which several molecular arresters are needed to protect body tissues from excessive responses that lead to tissue destruction [24–26]. A series of chalcones including CAD was found to suppress inflammatory activities that contribute to carcinogenesis [27–30]. In the present study, our data proved that chronic colitis was attenuated by CAD therapy. Besides, we assessed the inhibitory effect of CAD on colonic tumorigenesis in the CACC model, which further certified that CAD suppresses the proliferation of colonic tumor cells from an in vivo angle. CAD decreased both inflammatory factors including TNF-α in mice-derived macrophages and transcription levels of IL-1β, IL-6 and TNF-α in neuroinflammatory cells in proinflammatory state [23,31]. Similarly, reduced secretions of IL-1β and TNF-α in recurring colitis by CAD in our study indicated the anti-inflammation role of CAD. In further in vitro research, decreased IL-1β and TNF-α level were closely related to decreased cell viability by CAD. According to previous research and our data, CAD is also likely to inhibit inflammation through affecting cell viability in addition to directly restraining inflammation. However, the detailed mechanism underlying the direct and indirect inhibitory role of CAD on modulating cytokines in inflammation remains to be further elucidated.
Constitutive STAT family activation is highly related with inflammation, cell proliferation and apoptosis [32–40]. Since there is a close link between the development of chronic inflammation and cancer, various researches have concentrated upon the deregulations of STAT3 signaling in various carcinogenesis processes (including CACC) [41–44]. Given that CAD was previously reported to modulating activation of STATs [22], we detected whether phosphorylated STAT1, STAT3 and STAT5 were influenced by CAD both in vitro and in vivo. Our findings indicated that either cell viability or activation of STAT1, STAT3 and STAT5 was restrained after CAD administration, the results support previous studies in which CAD promotes apoptosis through depressing STAT signaling [45,46]. Besides, decreased amounts of inflammatory cytokines in CAD treated colon carcinoma cells indicated inhibited STATs activities is necessary for reducing the secretion of tumor-related cytokines in CACC.
In summary, this study provides evidence for the anti-tumor role of CAD through negatively modulating STAT family. Given the well-known relationship between anti-inflammatory/anti-tumor signals and homeostasis, our work provides a comprehending of the role of CAD to identify new agents in dealing with gastrointestinal disorder problems.
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. The manuscript does not contain clinical studies or patient data.
Informed consent
For this type of study, formal consent is not required.
References
- [1].Mantovani A, Allavena P, Sica A, et al. Cancer-related inflammation. Nature. 2008;454:436–444. [DOI] [PubMed] [Google Scholar]
- [2].Saleh M, Trinchieri G.. Innate immune mechanisms of colitis and colitis-associated colorectal cancer. Nat Rev Immunol. 2011;11:9–20. [DOI] [PubMed] [Google Scholar]
- [3].Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345:235–241. [DOI] [PubMed] [Google Scholar]
- [4].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. [DOI] [PubMed] [Google Scholar]
- [5].Francescone R, Hou V, Grivennikov SI. Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis. 2015;21:409–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Yadav VR, Prasad S, Sung B, et al. The role of chalcones in suppression of NF-kappaB-mediated inflammation and cancer. Int Immunopharmacol. 2011;11:295–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].De Spirt S, Eckers A, Wehrend C, et al. Interplay between the chalcone cardamonin and selenium in the biosynthesis of Nrf2-regulated antioxidant enzymes in intestinal Caco-2 cells. Free Radic Biol Med. 2016;91:164–171. [DOI] [PubMed] [Google Scholar]
- [8].Hatziieremia S, Gray AI, Ferro VA, et al. The effects of cardamonin on lipopolysaccharide-induced inflammatory protein production and MAP kinase and NFkappaB signalling pathways in monocytes/macrophages. Br J Pharmacol. 2006;149:188–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Zhang T, Yamamoto N, Yamashita Y, et al. The chalcones cardamonin and flavokawain B inhibit the differentiation of preadipocytes to adipocytes by activating ERK. Arch Biochem Biophys. 2014;554:44–54. [DOI] [PubMed] [Google Scholar]
- [10].Shrivastava S, Jeengar MK, Thummuri D, et al. Cardamonin, a chalcone, inhibits human triple negative breast cancer cell invasiveness by downregulation of Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Biofactors. 2016;43(2):152–169. [DOI] [PubMed] [Google Scholar]
- [11].Liao Q, Shi DH, Zheng W, et al. Antiproliferation of cardamonin is involved in mTOR on aortic smooth muscle cells in high fructose-induced insulin resistance rats. Eur J Pharmacol. 2010;641:179–186. [DOI] [PubMed] [Google Scholar]
- [12].Jiang FS, Tian SS, Lu JJ, et al. Cardamonin regulates miR-21 expression and suppresses angiogenesis induced by vascular endothelial growth factor. Biomed Res Int. 2015;2015:501581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Ohtsuki T, Kikuchi H, Koyano T, et al. Death receptor 5 promoter-enhancing compounds isolated from Catimbium speciosum and their enhancement effect on TRAIL-induced apoptosis. Bioorg Med Chem. 2009;17:6748–6754. [DOI] [PubMed] [Google Scholar]
- [14].Yadav VR, Prasad S, Aggarwal BB. Cardamonin sensitizes tumour cells to TRAIL through ROS- and CHOP-mediated up-regulation of death receptors and down-regulation of survival proteins. Br J Pharmacol. 2012;165:741–753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Zhang J, Sikka S, Siveen KS, et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis. 2016;22:158–168. [DOI] [PubMed] [Google Scholar]
- [16].Jia D, Tan Y, Liu H, et al. Cardamonin reduces chemotherapy-enriched breast cancer stem-like cells in vitro and in vivo. Oncotarget. 2016;7:771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Qin Y, Sun CY, Lu FR, et al. Cardamonin exerts potent activity against multiple myeloma through blockade of NF-kappaB pathway in vitro. Leuk Res. 2012;36:514–520. [DOI] [PubMed] [Google Scholar]
- [18].Lee MY, Seo CS, Lee JA, et al. Alpinia katsumadai H(AYATA) seed extract inhibit LPS-induced inflammation by induction of heme oxygenase-1 in RAW264.7 cells. Inflammation. 2012;35:746–757. [DOI] [PubMed] [Google Scholar]
- [19].Neufert C, Becker C, Neurath MF. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat Protoc. 2007;2:1998–2004. [DOI] [PubMed] [Google Scholar]
- [20].Siegmund B, Lehr HA, Fantuzzi G, et al. IL-1 beta -converting enzyme (caspase-1) in intestinal inflammation. Proc Natl Acad Sci U S A. 2001;98:13249–13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Allen IC, Wilson JE, Schneider M, et al. NLRP12 suppresses colon inflammation and tumorigenesis through the negative regulation of noncanonical NF-kappaB signaling. Immunity. 2012;36:742–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Takahashi A, Yamamoto N, Murakami A. Cardamonin suppresses nitric oxide production via blocking the IFN-gamma/STAT pathway in endotoxin-challenged peritoneal macrophages of ICR mice. Life Sci. 2011;89:337–342. [DOI] [PubMed] [Google Scholar]
- [23].Ren G, Sun A, Deng C, et al. The anti-inflammatory effect and potential mechanism of cardamonin in DSS-induced colitis. Am J Physiol Gastrointest Liver Physiol. 2015;309:G517–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Nasef NA, Mehta S, Ferguson LR. Susceptibility to chronic inflammation: an update. Arch Toxicol. 2017;91:1131–1141. [DOI] [PubMed] [Google Scholar]
- [25].Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15:551–567. [DOI] [PubMed] [Google Scholar]
- [26].Raposo TP, Beirao BC, Pang LY, et al. Inflammation and cancer: till death tears them apart. Vet J. 2015;205:161–174. [DOI] [PubMed] [Google Scholar]
- [27].Lopez SN, Castelli MV, Zacchino SA, et al. In vitro antifungal evaluation and structure-activity relationships of a new series of chalcone derivatives and synthetic analogues, with inhibitory properties against polymers of the fungal cell wall. Bioorg Med Chem. 2001;9:1999–2013. [DOI] [PubMed] [Google Scholar]
- [28].Vogel S, Barbic M, Jurgenliemk G, et al. Synthesis, cytotoxicity, anti-oxidative and anti-inflammatory activity of chalcones and influence of A-ring modifications on the pharmacological effect. Eur J Med Chem. 2010;45:2206–2213. [DOI] [PubMed] [Google Scholar]
- [29].Makita H, Tanaka T, Fujitsuka H, et al. Chemoprevention of 4-nitroquinoline 1-oxide-induced rat oral carcinogenesis by the dietary flavonoids chalcone, 2-hydroxychalcone, and quercetin. Cancer Res. 1996;56:4904–4909. [PubMed] [Google Scholar]
- [30].Herencia F, Ferrandiz ML, Ubeda A, et al. Novel anti-inflammatory chalcone derivatives inhibit the induction of nitric oxide synthase and cyclooxygenase-2 in mouse peritoneal macrophages. FEBS Lett. 1999;453:129–134. [DOI] [PubMed] [Google Scholar]
- [31].Kim YJ, Ko H, Park JS, et al. Dimethyl cardamonin inhibits lipopolysaccharide-induced inflammatory factors through blocking NF-kappaB p65 activation. Int Immunopharmacol. 2010;10:1127–1134. [DOI] [PubMed] [Google Scholar]
- [32].Han J, Theiss AL. Stat3: friend or foe in colitis and colitis-associated cancer? Inflamm Bowel Dis. 2014;20:2405–2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Wake MS, Watson CJ. STAT3 the oncogene - still eluding therapy? Febs J. 2015;282:2600–2611. [DOI] [PubMed] [Google Scholar]
- [34].Furtek SL, Backos DS, Matheson CJ, et al. Strategies and approaches of targeting STAT3 for cancer treatment. ACS Chem Biol. 2016;11:308–318. [DOI] [PubMed] [Google Scholar]
- [35].Yu H, Lee H, Herrmann A, et al. Revisiting STAT3 signalling in cancer: new and unexpected biological functions. Nat Rev Cancer. 2014;14:736–746. [DOI] [PubMed] [Google Scholar]
- [36].Bivona TG, Hieronymus H, Parker J, et al. FAS and NF-kappaB signalling modulate dependence of lung cancers on mutant EGFR. Nature. 2011;471:523–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhang X, Blaskovich MA, Forinash KD, et al. Withacnistin inhibits recruitment of STAT3 and STAT5 to growth factor and cytokine receptors and induces regression of breast tumours. Br J Cancer. 2014;111:894–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Netchiporouk E, Litvinov IV, Moreau L, et al. Deregulation in STAT signaling is important for cutaneous T-cell lymphoma (CTCL) pathogenesis and cancer progression. Cell Cycle. 2014;13:3331–3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Frank DA. StAT signaling in cancer: insights into pathogenesis and treatment strategies. Cancer Treat Res. 2003;115:267–291. [DOI] [PubMed] [Google Scholar]
- [40].Jin BR, Chung KS, Cheon SY, et al. Rosmarinic acid suppresses colonic inflammation in dextran sulphate sodium (DSS)-induced mice via dual inhibition of NF-kappaB and STAT3 activation. Sci Rep. 2017;7:46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].De Simone V, Franze E, Ronchetti G, et al. Th17-type cytokines, IL-6 and TNF-alpha synergistically activate STAT3 and NF-kB to promote colorectal cancer cell growth. Oncogene. 2015;34:3493–3503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Lin C, Wang L, Wang H, et al. Tanshinone IIA inhibits breast cancer stem cells growth in vitro and in vivo through attenuation of IL-6/STAT3/NF-kB signaling pathways. J Cell Biochem. 2013;114:2061–2070. [DOI] [PubMed] [Google Scholar]
- [43].Zhang L, Shao L, Creighton CJ, et al. Function of phosphorylation of NF-kB p65 ser536 in prostate cancer oncogenesis. Oncotarget. 2015;6:6281–6294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xu ZS, Zhang HX, Li WW, et al. FAM64A positively regulates STAT3 activity to promote Th17 differentiation and colitis-associated carcinogenesis. Proc Natl Acad Sci U S A. 2019;116:10447–10452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Wu N, Liu J, Zhao X, et al. Cardamonin induces apoptosis by suppressing STAT3 signaling pathway in glioblastoma stem cells. Tumour Biol. 2015;36:9667–9676. [DOI] [PubMed] [Google Scholar]
- [46].Zhang J, Sikka S, Siveen KS, et al. Cardamonin represses proliferation, invasion, and causes apoptosis through the modulation of signal transducer and activator of transcription 3 pathway in prostate cancer. Apoptosis. 2017;22:158–168. [DOI] [PubMed] [Google Scholar]


