ABSTRACT
Liver cancer stem cells contribute to tumorigenesis, progression, recurrence and drug resistance of hepatocellular carcinoma (HCC). However, the underlying mechanism for the propagation of liverCSCs is not fully understood yet. Here we show that miR-219 is upregulated in liver CSCs. Knockdown of miR-219 attenuates the self-renewal and tumorigenicity of liver CSCs. Conversely, miR-219 overexpressing enhances the self-renewal and tumorigenicity of liver CSCs.Mechanistically,miR-219 downregulates E-cadherin via itsmRNA 3ʹUTR in liver CSCs. The correlation between miR-219 and E-cadherin is validated in human HCC tissues. Furthermore, the miR-219 expression determines the responses of hepatoma cells to sorafenib treatment. Our findings indicate that miR-219 plays a critical role in liver CSCs expansion and sorafenib response, rendering miR-219 as an optimal target for the prevention and intervention of HCC.
Abbreviations: HCC: Hepatocellular carcinoma; CSCs: cancer stem cells; DMEM: Dulbecco’s modified Eagle’s medium; FBS: fetal bovine serum; OS: overall survival
KEYWORDS: Hepatocellular carcinoma, liver cancer stem cell, miR-219, E-cadherin, sorafenib
Introduction
Hepatocellular carcinoma (HCC) related mortality is one of the highest among all cancers [1]. Most HCC patients were diagnosed at a late stage and lost their surgical opportunity. What’s worse is that traditional treatment, targeted therapy, and immunological therapy are unsatisfactory [2]. Sorafenib is first FDA-approved HCC targeted drugs for advanced HCC patients, which provides very limited therapeutic effect [3]. Increasing evidence shows that there are a small part of cells called liver cancer stem cells (CSCs) [4,5]. Liver CSCs have self-renewal ability, tumorigenicity capacity, resistance to chemotherapy and recurrence characteristics [6]. Numerous researchers showed that chemo-resistance and recurrence of HCC were closely associated with the existence of liver CSCs [7]. Therefore, identification of the underlying mechanisms governing liver CSCs propagation may lead to the discovery of promising therapeutic strategies for HCC patients.
MicroRNAs (miRNAs) have participated in regulation of pathogenesis of human tumors and could be potential diagnostic markers and therapeutic target [8]. miRNAs can regulate RNA silencing and post-transcriptional of gene expression in general by binding to the 3ʹUTR of target mRNAs [9]. Recent studies have demonstrated that miRNAs were also involved in regulating liver CSCs self-renewal, tumorigenicity capacity and resistance to chemotherapy [10,11]. For instance, miR-429 was upregulated in liver CSCs and promoted liver CSCs expansion via targeting Rb binding protein 4 [12]. miR-194 was downregulated in liver CSCs and inhibits liver CSCs self-renewal and tumorigenicity by targeting RAC1 [13]. Thus, liver CSCs-specific miRNAs might be promising targets for cancer therapy. Previous studies found that miR-219 was upregulated in HCC tissues and promotes tumor growth and metastasis of HCC by regulating Cadherin 1 [14]. However, the role of miR-219 in liver CSCs is unclear.
In this study, we first find that miR-219 is markedly upregulated in liver CSCs. Next, by using loss-of-function analysis and gain-of-function analysis in liver CSCs, we demonstrate that miR-219 promotes the self-renewal capacity, tumorigenicity, and chemo-resistance of liver CSCs. Further mechanism study reveals that miR-219 downregulates E-cadherin via its mRNA 3ʹUTR. More importantly, we find that miR-219 plays a role in the sensitivity of HCC cells to sorafenib. Taken together, our findings revealed the crucial role of the miR-219 in liver CSCs expansion and sorafenib response, rendering miR-219 as an optimal target for the prevention and intervention of liver CSCs.
Materials and methods
Clinical tissues and cell culture
Forty-eight HCC patients’ samples were collected at the Department of Surgical Oncology, Jinling Hospital(Nanjing, China). Patient informed consent was also obtained and the procedure of human sample collection was approved by the Ethical Committee of Jinling Hospital. Four HCC patients’ tissues were used for isolated primary HCC cells. Forty HCC patients’ tissues were used for analysis the relationship between miR-219 and E-cadherin. Four HCC patients’ tissues were used for PDXs analysis. Detailed clinicopathological features of these patients is described in online supplementary table1.
HCC cell lines HCCLM3 and hepG2 were obtained from the Chinese Academy of Sciences (Shanghai, China). HCCLM3 and hepG2were cultured with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal bovine serum (FBS) and 2 mM L-glutamine and 25 µg/ml gentamicin, and maintained at 37°C in a 5% CO2 incubator. miR-219 mimic or miR-219 sponge lentivirus and their control lentivirus were purchased from Shanghai GenePharma(Shanghai, China). Special siE-cadherin and its negative control siRNA were purchased from Ribobio(Shanghai, China).
Spheroid formation assay
miR-219 sponge or miR-219 mimic and control hepatoma cells (300 cells per well) were cultured in a six-well ultra-low attachment culture plate in DMEM/F12 (Gibco) supplemented with 1% FBS, 20 ng/mL bFGF and 20 ng/mL EGF for 1 week, and the total number of spheres was counted under the microscope. The results were repeated for three times.
In vitro limiting dilution assay
Various numbers of hepatoma cells (2, 4, 8, 16, 32, 64 cells per well) were seeded into 96-well ultra-low attachment culture plate in DMEM/F12 (Gibco) supplemented with 1% FBS, 20 ng/mL bFGF and 20 ng/mL EGF for 1 week. The proportion of CSCs was analyzed using Poisson distribution statistics and the L-Calc Version 1.1 software program (Stem Cell Technologies, Inc., Vancouver, Canada) as described [15].
In vivo limiting dilution assay
HCCLM3 miR-219 sponge or HCCLM3 miR-219 mimic and their control hepatoma cells were diluted serially to the desired doses, and were mixed with matrigel gel (1:1). Then, the mixed cells were injected subcutaneously into 10 NOD-SCID mice each group. After 2 months, the number of tumors was counted.
Flow-cytometric analysis
For CD133+ and OV6+ cells sorting, primary HCC patients’ cells and hepatoma cells were incubated with the primary anti–CD133 (Cat. no. 18,470-1-AP; Proteintech) or anti-OV6 for 30 min at room temperature. The cells were then subjected to flow cytometry using a MoFlo XDP cell sorter from Beckman Coulter (Indianapolis, IN, USA) according to the manufacturer’s instructions. The sorted cells from three independent experiments were subjected to Real-time PCR assay.
HCCLM3 miR-219 mimic or miR-219 sponge and control cells were incubated with the primary anti-EpCAMor CD133 for 30 min at room temperature. Flow-cytometric analysis was performed using a MoFlo XDP from Beckman Coulter according to the manufacturer’s instructions.
Cell proliferation assays
The cell proliferation assay was conducted with Counting Kit-8 (CCK-8) (Tongren,Shanghai, China). HCCLM3 or hepG2 miR-219 sponge and their control cells were seeded into 96-well plates (3 × 103 cells per well). The hepatoma cells were treated with 2 μM sorafenib or not for 72 h.Then 10% CCK-8 solution was added. The absorbance of each sample was assessed by a microplate reader set at 450nM. Each sample was performed three times.
Apoptosis assay
HCCLM3 or hepG2 miR-219 sponge and their control cells were treated with sorafenib (10 μM) for 48 h, followed by staining with Annexin V and 7-AAD for 15 min at room temperature in the dark. Apoptotic cells were determined by an Annexin VFITC Apoptosis Detection Kit I (BD Pharmingen, San Diego, CA) and flow cytometer according to the manufacturer’s instructions.
Luciferase reporter assay
The wild-type sequence containing the predicted target sites of miR-219 in the3ʹUTR of E-cadherin mRNA was synthesized by Shanghai Genechem Co.Ltd. We mutated the target sites from CUCCACto GACCGA. After plasmid transfection, luciferase activities were measured using a Synergy 2 Multidetection Microplate Reader (BioTek Instruments, Inc.). All samples were independently repeated three times.
Real-time PCR
RNA of samples was obtained by TRIzol reagent (Invitrogen, USA). Total RNA was subjected to reverse transcription using a TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems). qRT-PCR analysis of miR-219 expression was carried out using TaqMan MicroRNA assay kits (Applied Biosystems). Results were normalized to U6 snRNA using the comparative threshold cycle (Ct) method. The miR-219 primer sequences were forward: 5ʹ CGGTGATTGTCCAAACGCAATTC 3ʹ; E-cadherin primer sequences were forward: 5ʹ ATTCTGATTCTGCTGCTCTTG 3ʹ, reverse: 5ʹ AGTCCTGGTCCTCTTCTCC 3ʹ; NANOG primer sequences were forward: 5ʹ AATACCTCAGCCTCCAGCAGATG 3ʹ, reverse: 5ʹ TGCGTCACACCATTGCTATTCTTC 3ʹ; SOX-2 primer sequences were forward: 5ʹ TGGAGAAGGAATGGTCCACTTC 3ʹ, reverse: 5ʹ GGATAAGTACACGCTGCCCG 3ʹ; Oct4 primer sequences were forward: 5ʹ ATGTGCGCGTAACTGTCCAT 3ʹ, reverse: 5ʹ CTGCAGTGTGGGTTTCGGGCA 3ʹ; c-Myc primer sequences were forward: 5ʹ CCCTCCACTCGGAAGGACTA 3ʹ, reverse: 5ʹ GCTGGTGCATTTTCGGTTGT 3ʹ; β-catenin primer sequences were forward: 5ʹ CGCTGGATTTTCAAAACAGT 3ʹ, reverse: 5ʹ CTGAGGAGCAGCTTCAGTCC 3ʹ; Bmi-1 primer sequences were forward: 5ʹ TGGAGAAGGAATGGTCCACTTC 3ʹ, reverse: 5ʹ GTGAGGAAACTGTGGATGAGGA 3ʹ. The β-actin was used as a reference for relative expression calculation and its primer sequences were forward: 5ʹ GGCCCAGAATGCAGTTCGCCTT 3ʹ, reverse: 5ʹ AATGGCACCCTGCTCACGCA 3ʹ.
Western blotting assay
Samples were obtained with cell lysis buffer and disposed as we described before [16]. After quantification with bicinchoninic acid (BCA) assay (Weiao, Shanghai,China), we separated each protein through 10% SDS-page and then moved them onto PVDF membranes (Millipore,USA). Then, samples were blocked with 5% nonfat milk. After incubation with primary antibodies against PARP, E-cadherin and GAPDH (Cell Signaling Technology, Danvers, MA, USA) and secondary antibodies, protein levels were detected withImageQuant LAS 4000 (GEHealthcare Life Sciences). Each sample was analyzed three times.
PDX model
For the patient-derived xenograft (PDX) model, primary HCC tumor samples were obtained for xenograft establishment as described previously [17]. The mice with xenografts were given sorafenib (60 mg/kg) or vehicle daily orally for 30 days (n = 5 for each group). Tumor volumes were measured at the indicated time points. All procedures and protocols were approved by the Ethical Committee of Jinling Hospital.
Statistical analysis
GraphPad Prism (GraphPad Software, Inc. La Jolla, USA) was used for all statistical analyses. Statistical analysis was carried out using t-test or Bonferroni Multiple Comparisons Test: *p < 0.05. A p-value of less than 0.05 was considered statistically significant.
Results
Mir-219 expression is upregulated in liver CSCs
To examine the expression of miR-219 in liver CSCs, we enriched liver CSCs by flow cytometry sorting or sphere formation. As shown in (Figure 1(a,b)), the expression of miR-219 was dramatically increasing in sorted CD133+ or OV6+ primary HCC cells. Consistently, miR-219 level was also upregulated in sorted CD133+ or OV6+hepatoma cell lines (Figure 1(c,d)). Moreover, miR-219 expression was notably increasing in HCC spheres derived from human primary HCC cells (Figure 1(e)). We also observed the similar result in hepatoma cell lines (Figure 1(f)). Intriguingly, the miR-219 level could be partially restored during reattachment in parallel with the differentiation (Figure 1(g)). Taken together, these results demonstrated that miR-219 expression was upregulated in liver CSCs.
Figure 1.
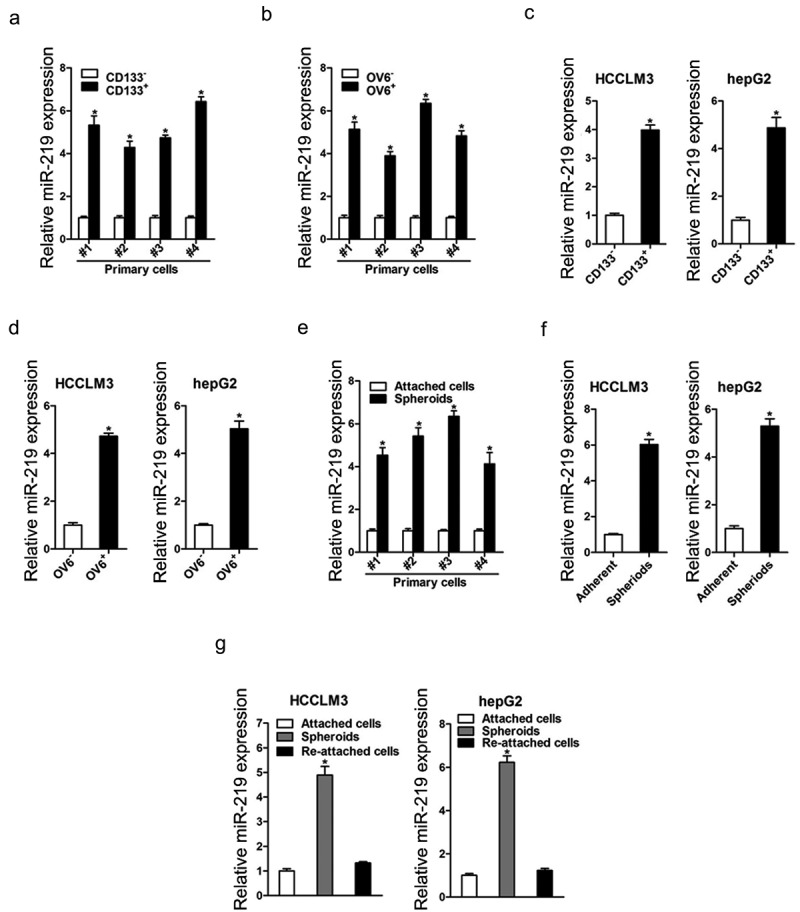
miR-219 is upregulated in liver CSCs.
a. C133 positive primary HCC cells were sorted and subjected to real-time PCR assay (n = 3). b. OV6 positive primary HCC cells were sorted and subjected to real-time PCR assay (n = 3). c. Real-time PCR analysis of miR-219 in CD133 positive HCC cells and control negative HCC cells (n = 3). d. Real-time PCR analysis of miR-219 in OV6 positive HCC cells and control negative HCC cells (n = 3). e. Real-time PCR analysis of miR-219 in primary HCC adherent and spheroids cells (n = 3). f. Real-time PCR was performed to examine the expression of miR-219 in hepatoma spheroids (n = 3). g. HCCLM3 and hepG2 cell–derived spheroids were trypsinized and cultured in attachment conditions. miR-219 expression in spheroids versus reattached cells was compared by real-time PCR (n = 3).(Data are represented as mean±s.d.; *P < 0.05; two-tailed Student’s t-test.)
Mir-219 is required for the maintenance of liver CSCs
To investigate the potential function of miR-219 in liver CSCs, we treated hepatoma cell lines with miR-219 sponge virus or negative control virus (Figure 2(a)). As expected, we found that miR-219 interference downregulated the expression of stemness-associated genes in hepatoma cells (Figure 2(b)). Moreover, flow-cytometric analysis showed that the proportion of liver CSCs was decreased in miR-219 knockdown hepatoma cells (Figure 2(c,d)). Additionally, miR-219 interference hepatoma cells formed fewer and smaller spheres (Figure 2(e)). Furthermore, in vitro and in vivo limiting dilution assay indicated that miR-219 knockdown significantly decreased the tumor incidence and CSCs frequency in hepatoma cells (Figure 2(f,g)).
Figure 2.
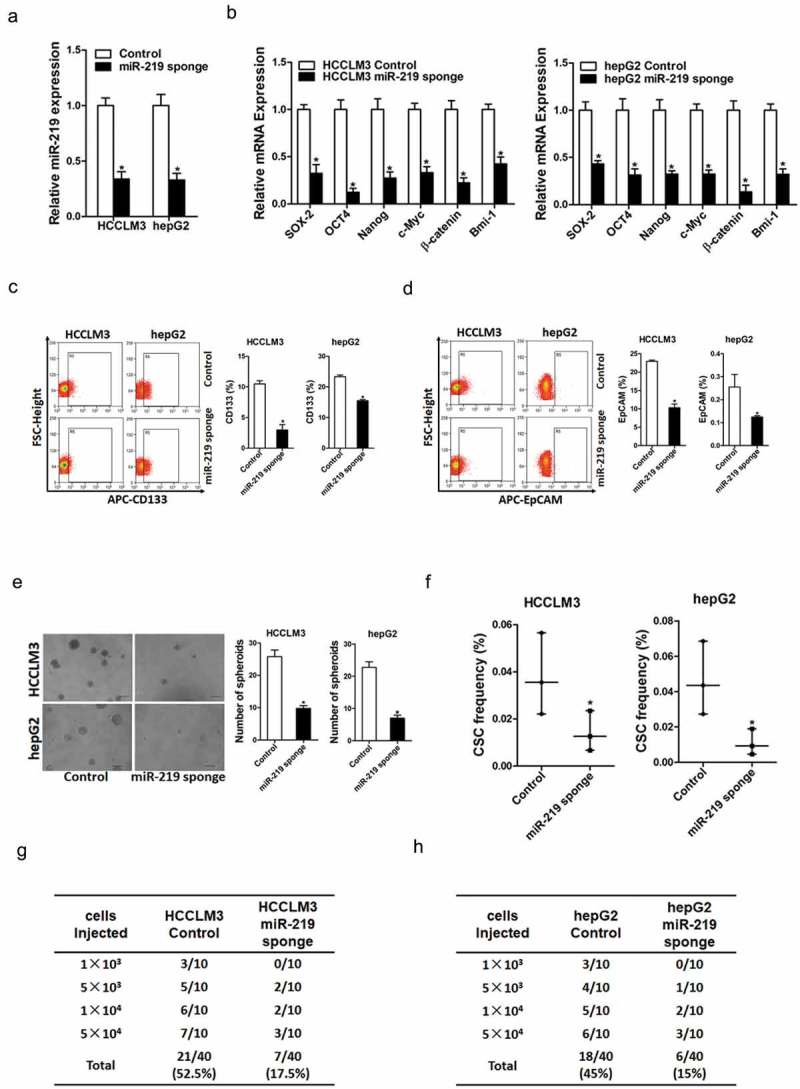
miR-219 is required for the maintenance of liver CSCs.
a. HCCLM3 and hepG2 cells were infected with miR-219 sponge virus and control virus. The stable infectants were checked by real-time PCR (n = 3). b. The expression of liver CSCs associated genes were examined in miR-219 sponge and control HCC cells by real-time PCR (n = 3). c. Flow-cytometric analysis of the proportion of CD133+cells inmiR-219 sponge and control HCC cells (n = 3). d. Flow cytometric analysis of the proportion of EpCAM+ cells inmiR-219 sponge and control HCC cells (n = 3). e. Spheres formation assay of miR-219 sponge and control HCC cells (n = 3). f. The frequency of liver CSCs in miR-219 sponge and control HCC cells was compared by in vitro limiting dilution assay (n = 8). g. The frequency of liver CSCs in HCCLM3 miR-219 sponge and its control cells was compared by in vivo limiting dilution assay. Tumors were observed over 2 months; n = 10 for each group. h. The frequency of liver CSCs in hepG2miR-219 sponge and its control cells were compared by in vivo limiting dilution assay. Tumors were observed over 2 months; n = 10 for each group.(Data are represented as mean±s.d.; *P < 0.05; two-tailed Student’s t-test.)
Mir-219 promotes liver CSC expansion
To further validate promoting roles of miR-219 in liver CSCs expansion, miR-219 stable overexpressing infectants of hepatoma cells were used (Figure 3(a)). As expected, we found that miR-219 overexpression increased the expression of stemness-associated genes in hepatoma cells (Figure 3(b)). Moreover, the flow-cytometric analysis showed that the proportion of liver CSCs was upregulated in miR-219 overexpressing hepatoma cells (Figure 3(c,d)). Additionally, miR-219 overexpressing hepatoma cells formed much more and bigger spheres (Figure 3(e)). Furthermore, in vitro and in vivo limiting dilution assay indicated that miR-219 overexpressing dramatically increased the tumor incidence and CSCs frequency in hepatoma cells (Figure 3(f,g)).
Figure 3.
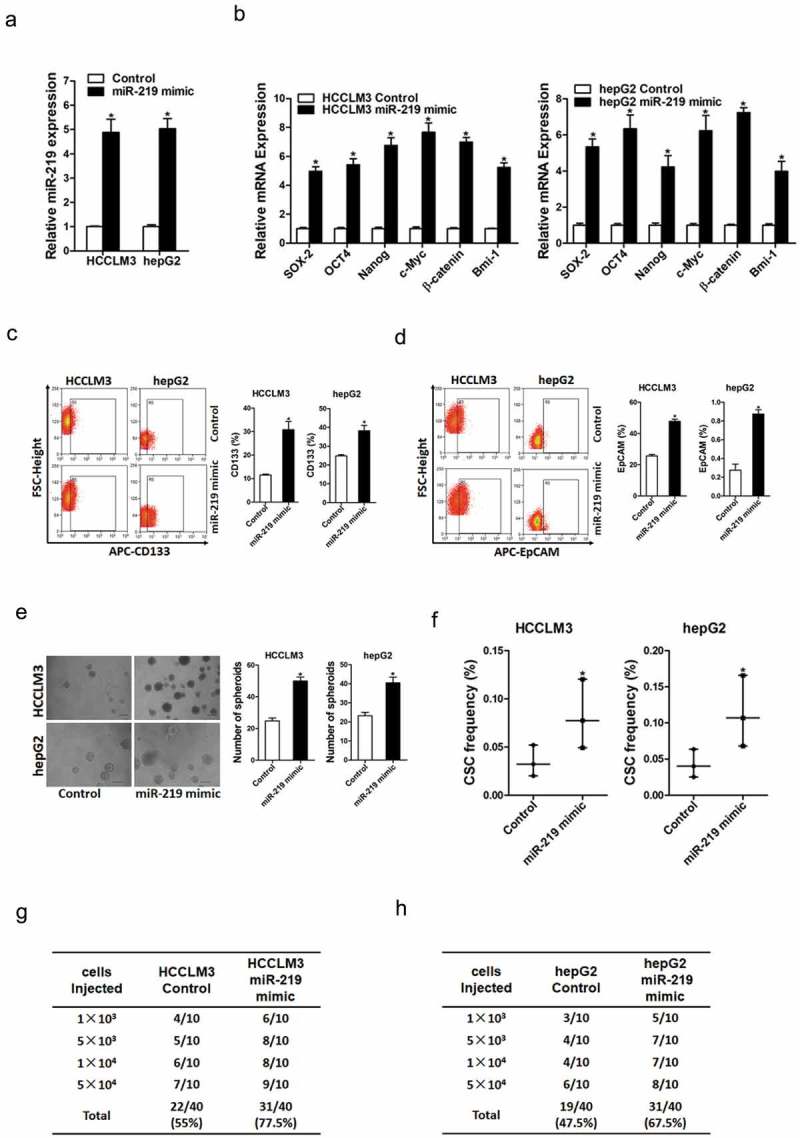
miR-219 promotes the expansion of liver CSCs.
a. HCCLM3 and hepG2 cells were infected with miR-219 mimic virus and control virus. The stable infectants were checked by real-time PCR (n = 3). b. The expression of liver CSCs associated genes were examined in miR-219 mimic and control HCC cells by real-time PCR (n = 3). c. Flow cytometric analysis of the proportion of CD133+ cells in miR-219 mimic and control HCC cells (n = 3). d. Flow cytometric analysis of the proportion of EpCAM+ cells in miR-219 mimic and control HCC cells (n = 3). e. Spheres formation assay of miR-219 mimic and control HCC cells (n = 3). f. The frequency of liver CSCs in miR-219 mimic and control HCC cells was compared by in vitro limiting dilution assay (n = 8). g. The frequency of liver CSCs in HCCLM3 miR-219 mimic and its control cells was compared by in vivo limiting dilution assay. Tumors were observed over 2 months; n = 10 for each group. h. The frequency of liver CSCs in hepG2 miR-219 mimic and its control cells was compared by in vivo limiting dilution assay. Tumors were observed over 2 months; n = 10 for each group.(Data are represented as mean±s.d.; *P < 0.05; two-tailed Student’s t-test.)
E-cadherinisrequired for mir-219 in liver CSCs
It was reported that miR-219 targeted the 3ʹ-UTRs of GPC3 and E-cadherin in hepatoma cells [14,18]. So we doubted whether GPC3 and E-cadherin was involved in miR-219 mediated liver CSCs expansion. Our data found that GPC3 expression was unchanged in miR-219 knockdown or overexpressing liver CSCs. However, E-cadherin mRNA expression was increased in miR-219 knockdown liver CSCs and decreased in miR-219 overexpressing liver CSCs (Figure 4(a,b)). Consistently, the E-cadherin protein level was also downregulated in miR-219 overexpressingliver CSCs (Figure 4(c)). Additionally, the wild-type or mutant E-cadherin 3ʹ-UTR reporter plasmids were transfected into miR-219 overexpressing liver CSCs. The mutation of miR-219 binding site in the E-cadherin 3ʹ-UTR abrogated the distinct activation of E-cadherin 3ʹ-UTR between miR-219 overexpressing and control hepatoma cells (Figure 4(d)). Moreover, there was a significant negative correlation between miR-219 and E-cadherin mRNA expression in human HCC tissues (Figure 4(e)).To investigate the role of E-cadherin in miR-219-mediated liver CSCs expansion, special E-cadherin siRNA was used (Figure 4(f)). Special E-cadherin siRNA abolished the difference in the proportion of liver CSCs, self-renewalability, and tumorigenesis capacity between miR-219 overexpression and control hepatoma cells (Figure 4(g,I)). Collectively, these results indicated that E-cadherin was required for miR-219 in liver CSCs.
Figure 4.
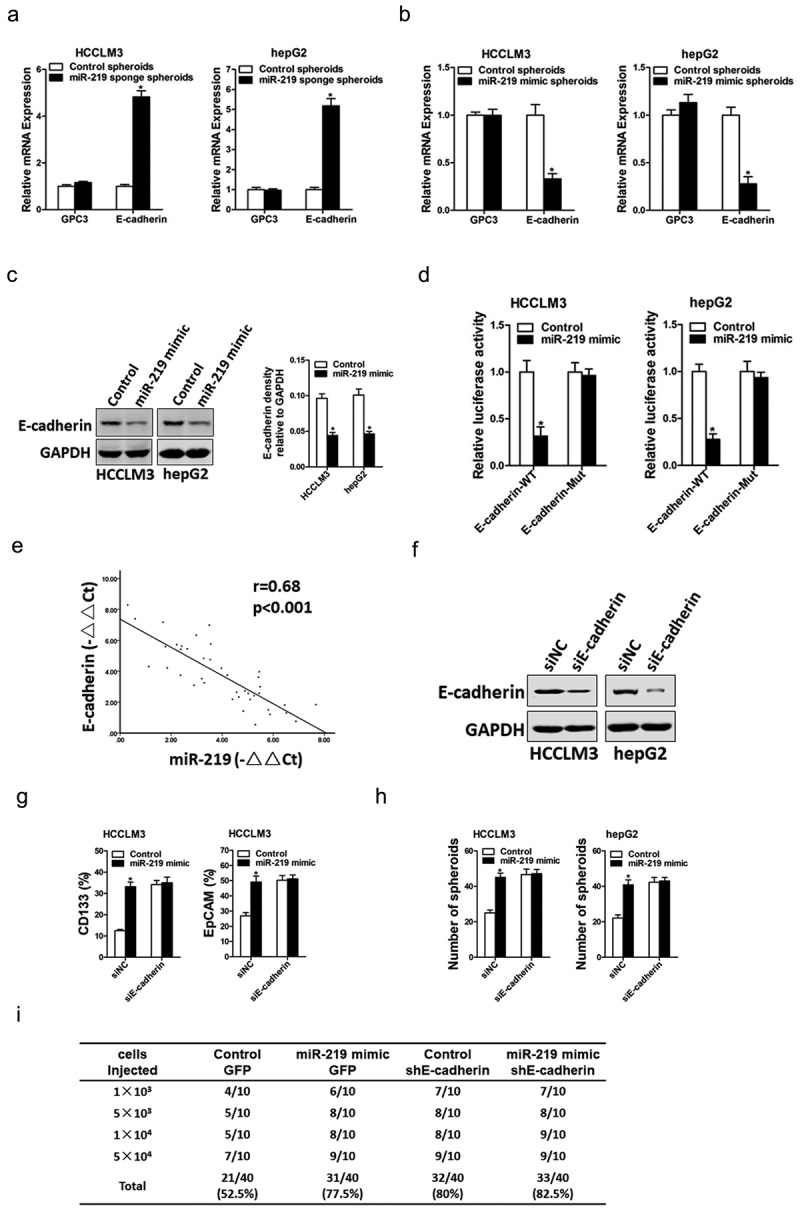
E-cadherinis required for miR-219 in liver CSCs.
a. The mRNA expression of GPC3 and E-cadherin was checked in miR-219 sponge and control liver CSCs by real-time PCR (n = 3). b. The mRNA expression of GPC3 and E-cadherin was checked in miR-219 mimic and control liver CSCs by real-time PCR (n = 3). c. The protein level of E-cadherin was checked in miR-219 mimic and control liver CSCs by western blot assay. d. Luciferase reporter assays performed in miR-219 mimic and control hepatoma cells transfected with wild-type or mutant E-cadherin 3ʹ-UTR plasmids (n = 3). e. Significant correlation was observed between miR-219 andE-cadherin expression in HCC tissues (n = 40). f. Hepatoma cells were transfected with E-cadherin siRNA or negative control, and then subjected to western blot assay. g. HCCLM3miR-219 mimic and its control cells were transfected with E-cadherin siRNA or negative control, and the CD133+ orEpCAM+ HCC cells were checked by flow-cytometric assay (n = 3). h. miR-219 mimic and control hepatoma cells were transfected with E-cadherin siRNA or negative control, and then subjected to spheroid formation (n = 3). i. HCCLM3 miR-219 mimic and its control cells were transfected with E-cadherin siRNA or negative control, and then subjected to in vivo limiting dilution assay. Tumors were observed over 2 months; n = 10 for each group. (Data are represented as mean±s.d.; *P < 0.05; two-tailed Student’s t-test.)
Mir-219 plays a role in the sensitivity of HCC cells to sorafenib
Increasing researches shows that liver CSCs were involved in HCC chemo-resistant and recurrence [19,20]. So we next explore the potential role of miR-219 in the sensitivity of HCC cells to sorafenib. As shown in (Figure 5(a)), miR-219 expression was markedly upregulated in sorafenib-resistant HCC xenografts. Consistently, we also found that miR-219 expression was increasing in sorafenib-resistant hepatoma cells (Figure 5(b)). miR-219interference sensitized hepatoma cells to sorafenib-induced growth inhibition and cell apoptosis (Figure 5(c,d)).In addition, western blot analysis also showed that the protein expression of PARP in miR-219 knockdown hepatoma cells was significantly increased when they were exposed to the same doses of sorafenib when compared with normal HCC cells (Figure 5(e)).Furthermore, we found that the PDXs derived from HCC tumors with high miR-219 levels were resistant to sorafenib treatment. In contrast, the PDXs derived from HCC tumors with low miR-219 levels were sensitive to sorafenib treatment (Figure 5(f,h)). More importantly,the interference of E-cadherin abolished sorafenib sensitive in miR-219knockdown hepatoma cells (Figure 5(i)). Taken together, the above data demonstrated that the miR-219plays a role in the sensitivity of HCC cells to sorafenib.
Figure 5.
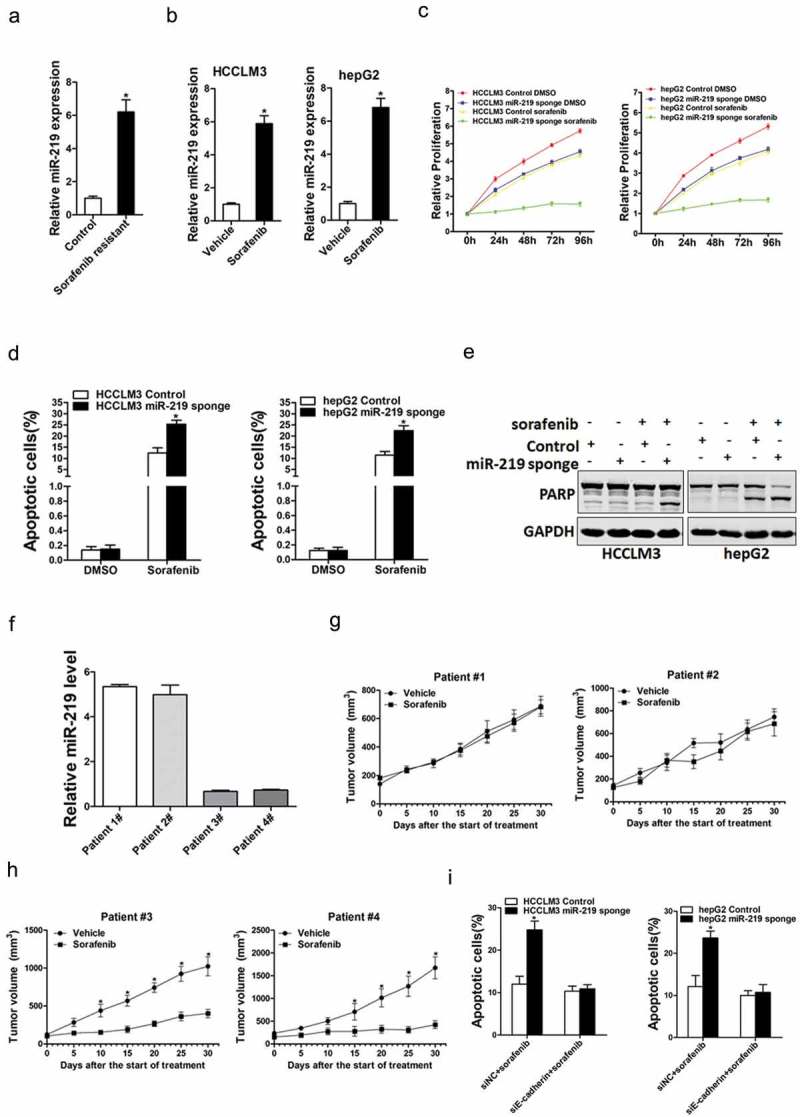
The effect of miR-219 on drug resistance of HCC to sorafenib.
a. Real-time PCR was performed to examine the expression of miR-219 in the sorafenib-resistant HCC xenografts (n = 3). b. The expression of miR-219 in sorafenib resistant and control hepatoma cells (n = 3). c. miR-219 mimic and control hepatoma cells cultured in 96-well plates were treated with 2 μM sorafenib, and cell viability was measured at indicated times using Cell Counting Kit-8 (n = 6). d. miR-219 mimic and control hepatoma cells were treated with 10 μM sorafenib as indicated for 48 h. Percentage of apoptotic cells was determined by fluorescence-activated cell sorting (n = 3). e. miR-219 mimic and control hepatoma cells were treated with 10 μM sorafenib as indicated for 48 h. The protein of cleaved-PARP was determined by western blot. f. The expression of miR-219 in PDXs primary tumors was determined by TaqMan MicroRNA assay. g. PDXs with high miR-219 levels in their primary tumors were treated with sorafenib (60 mg/kg body weight) or vehicle for 30 days (n = 5 for each group). The xenograft growth was recorded. h. PDXs with low miR-219 levels in their primary tumors were treated with sorafenib (60 mg/kg body weight) or vehicle for 30 days (n = 5 for each group). The xenograft growth was recorded. i. miR-219 sponge and control HCC cells were transfected with siE-cadherin or siNC. The cells were then treated with sorafenib (10 μM) for 48 h and their apoptosis was examined by flow cytometry (n = 3).(Data are represented as mean±s.d.; *P < 0.05; two-tailed Student’s t-test.)
Discussion
Hepatocellular carcinoma is one of the most death tumors in the world. Postoperative metastasis and recurrence remains the major reasons for the poor prognosis of HCC [21]. Transcatheter arterial chemoembolization (TACE) and sorafenib are always used for advanced HCC patients, while the effect is not satisfactory [22]. Many studies have suggested that liverCSCs are closely related to the recurrence, metastasis and drug resistance of HCC [23]. However, the mechanism for the propagation of liver CSCs remains unclear. Thus, characterizing the liver CSCs-related molecules and signaling pathways may provide more clues to the understanding of HCC. In this study, we elaborated on the critical role of miR-219 in liver CSCs and the underlying mechanism. We also demonstrated the value of miR-219 plays a role in the sensitivity of HCC cells to sorafenib. To our knowledge, this is the first report for miR219 in the regulation of liver CSCs.
Accumulating evidence shows that miRNA expression was closely associated with the biological functions of many human tumors [24,25]. In previous study, miR-219-5p was found to promote tumor growth and metastasis of HCC by regulating Cadherin 1 [14]. However, some studies also demonstrated inconsistent results in liver cancers. For example,miR-219-5p was reported to inhibit HCC cell proliferation by targetingglypican-3 [18].The real reason is not clear. These results stimulate us to investigate the role of miR-219 in liver CSCs. It was found in the present study that miR-219 was upregulated in liver CSCs. We also found that miR-219 knockdown inhibited self-renewal and tumorigenicity of liver CSCs. Consistently, miR-219 overexpression could promote the self-renewal and tumorigenicity of liver CSCs. These results remains us that miR-219 was an oncogene and played an important role in liver CSCs.
E-cadherin is one of the most well-studied members of the cadherin family [26]. Loss of E-cadherin function or expression has been implicated in numerous cancer progression and metastasis [27,28], but the exact mechanism beneath E-cadherin inactivation in HCC remains vague. Previous studies have elucidated that E-cadherin expression changes in epithelial–mesenchymal transition (EMT) and mesenchymal–epithelial transition (MET) [29,30]. In addition, E-cadherin was reported to be involved in the modulation of CSCs [31]. In the present study, we found that overexpressing miR-219 in liver CSCs downregulated E-cadherin expression through binding to its 3ʹUTR.Furthermore, special E-cadherin siRNA could diminish the discrepancy of the self-renewal ability and tumorigenicity capacity between miR-219 overexpression liver CSCs and control cells. The correlation between miR-219 and E-cadherin is further validated in human HCC tissues.
Sorafenib, a receptor tyrosine kinase inhibitor, is the first FDA-approved targeted drug which used for advanced HCC patients [32]. However, only a small proportion of patients benefited from sorafenib treatment. So it’s urgent to find a drug marker for sorafenib treatment in HCC. In this study, our finding revealed that miR-219 interference liver cells are more sensitive to sorafenib treatment. Sorafenib PDX studies further indicated that low miR-219 level in HCC patients can serve as a reliable predictor for sorafenib response.
In summary, we demonstrated that miR-219 is increased in liver CSCs, which in turn promotes the self-renewal and tumorigenicity of liver CSCs. In addition, miR-219 promotes liver CSC expansion via directly regulating E-cadherin. In conclusion, our findings provide insight into the miR-219/E-cadherin axis as a potential therapeutic target against liver CSCs and a potential predictor for sorafenib treatment of HCC patients.
Funding Statement
This work was supported by the grand from the State Key Project on Infectious Diseases of China (2018ZX10732202-003).
Authors’ contributions
Anfeng Si, Longqi Wang, and Kun Miao conducted all experiments and analyzed the data. Anfeng Si, Rongrong Zhang, andHuiyu Jicollected clinical samples. Zhengqing Lei provided pathology evaluation and analyzed clinical data. Zhangjun Cheng provided support with experimental techniques. Anfeng Si wrote the manuscript and Baobing Haocontributed to the revision. Baobing Hao andXiangchun Fang conceived the project and supervised all experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Supplementary material
Supplemental data for this article can be accessed here.
References
- [1].Forner A, Reig M, Bruix J.. Hepatocellular carcinoma. Lancet. 2018;391:1301–1314. [DOI] [PubMed] [Google Scholar]
- [2].Llovet JM, Zucman-Rossi J, Pikarsky E, et al. Hepatocellular carcinoma. Nat Rev Dis Primers. 2016;2:16018. [DOI] [PubMed] [Google Scholar]
- [3].Han T, Xiang DM, Sun W, et al. PTPN11/Shp2 overexpression enhances liver cancer progression and predicts poor prognosis of patients. J Hepatol. 2015;63:651–660. [DOI] [PubMed] [Google Scholar]
- [4].Li XF, Chen C, Xiang DM, et al. Chronic inflammation-elicited liver progenitor cell conversion to liver cancer stem cell with clinical significance. Hepatology. 2017;66:1934–1951. [DOI] [PubMed] [Google Scholar]
- [5].Singh AK, Arya RK, Maheshwari S, et al. Tumor heterogeneity and cancer stem cell paradigm: updates in concept, controversies and clinical relevance. Int J Cancer. 2015;136:1991–2000. [DOI] [PubMed] [Google Scholar]
- [6].Magee JA, Piskounova E, Morrison SJ. Cancer stem cells: impact, heterogeneity, and uncertainty. Cancer Cell. 2012;21:283–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mo DC, Jia RR, Zhong JH. Hepatic Resection Compared to Chemoembolization in Intermediate to Advanced-Stage HCC: a comment for moving forward. Hepatology. 2018;68(3):977–993. [DOI] [PubMed] [Google Scholar]
- [8].Bayoumi AS, Sayed A, Broskova Z, et al. Crosstalk between Long Noncoding RNAs and MicroRNAs in Health and Disease. Int J Mol Sci. 2016;17:356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].DeSano JT, Xu L. MicroRNA regulation of cancer stem cells and therapeutic implications. Aaps J. 2009;11:682–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jiang C, Yu M, Xie X, et al. miR-217 targeting DKK1 promotes cancer stem cell properties via activation of the Wnt signaling pathway in hepatocellular carcinoma. Oncol Rep. 2017;38:2351–2359. [DOI] [PubMed] [Google Scholar]
- [11].Zhao J, Fu Y, Wu J, et al. The Diverse Mechanisms of miRNAs and lncRNAs in the Maintenance of Liver Cancer Stem Cells. Biomed Res Int. 2018;2018:8686027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Li L, Tang J, Zhang B, et al. Epigenetic modification of MiR-429 promotes liver tumour-initiating cell properties by targeting Rb binding protein 4. Gut. 2015;64:156–167. [DOI] [PubMed] [Google Scholar]
- [13].Ran RZ, Chen J, Cui LJ, et al. miR-194 inhibits liver cancer stem cell expansion by regulating RAC1 pathway. Exp Cell Res. 2019;378:66–75. [DOI] [PubMed] [Google Scholar]
- [14].Yang J, Sheng YY, Wei JW, et al. MicroRNA-219-5p Promotes Tumor Growth and Metastasis of Hepatocellular Carcinoma by Regulating Cadherin 1. Biomed Res Int. 2018;2018:4793971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Xiang D, Cheng Z, Liu H, et al. Shp2 promotes liver cancer stem cell expansion by augmenting beta-catenin signaling and predicts chemotherapeutic response of patients. Hepatology. 2017;65:1566–1580. [DOI] [PubMed] [Google Scholar]
- [16].Xiang DM, Sun W, Ning BF, et al. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. Gut. 2018;67(9):1704–1715. [DOI] [PubMed] [Google Scholar]
- [17].Xiang DM, Sun W, Zhou T, et al. Oncofetal HLF transactivates c-Jun to promote hepatocellular carcinoma development and sorafenib resistance. Gut. 2019;68:1858–1871. [DOI] [PubMed] [Google Scholar]
- [18].Huang N, Lin J, Ruan J, et al. MiR-219-5p inhibits hepatocellular carcinoma cell proliferation by targeting glypican-3. FEBS Lett. 2012;586:884–891. [DOI] [PubMed] [Google Scholar]
- [19].Fan Z, Duan J, Wang L, et al. PTK2 promotes cancer stem cell traits in hepatocellular carcinoma by activating Wnt/beta-catenin signaling. Cancer Lett. 2019;450:132–143. [DOI] [PubMed] [Google Scholar]
- [20].Sheng X, Sun X, Sun K, et al. Inhibitory effect of bufalin combined with Hedgehog signaling pathway inhibitors on proliferation and invasion and metastasis of liver cancer cells. Int J Oncol. 2016;49:1513–1524. [DOI] [PubMed] [Google Scholar]
- [21].Chen JC, Chen MS. [Multidisciplinary therapeutic strategies for recurrence and metastasis of liver cancer]. Zhonghua Gan Zang Bing Za Zhi. 2016;24:327–329. [DOI] [PubMed] [Google Scholar]
- [22].Lencioni R, Llovet JM, Han G, et al. Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: the SPACE trial. J Hepatol. 2016;64:1090–1098. [DOI] [PubMed] [Google Scholar]
- [23].Wang D, Fu L, Sun H, et al. Prostaglandin E2 Promotes Colorectal Cancer Stem Cell Expansion and Metastasis in Mice. Gastroenterology. 2015;149:1884–1895 e1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kai K, Dittmar RL, Sen S. Secretory microRNAs as biomarkers of cancer. Semin Cell Dev Biol. 2018;78:22–36. [DOI] [PubMed] [Google Scholar]
- [25].Afonso MB, Rodrigues PM, Simao AL, et al. Circulating microRNAs as Potential Biomarkers in Non-Alcoholic Fatty Liver Disease and Hepatocellular Carcinoma. J Clin Med. 2016;5(3). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gul IS, Hulpiau P, Saeys Y, et al. Evolution and diversity of cadherins and catenins. Exp Cell Res. 2017;358:3–9. [DOI] [PubMed] [Google Scholar]
- [27].Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer. 2000;36:1607–1620. [DOI] [PubMed] [Google Scholar]
- [28].Canel M, Serrels A, Frame MC, et al. E-cadherin-integrin crosstalk in cancer invasion and metastasis. J Cell Sci. 2013;126:393–401. [DOI] [PubMed] [Google Scholar]
- [29].Azizi R, Salemi Z, Fallahian F, et al. Inhibition of didscoidin domain receptor 1 reduces epithelial-mesenchymal transition and induce cell-cycle arrest and apoptosis in prostate cancer cell lines. J Cell Physiol. 2019;234:19539–19552. [DOI] [PubMed] [Google Scholar]
- [30].Luo T, Wang L, Wu P, et al. Downregulated vimentin and upregulated E-cadherin in T1 stage non-small-cell lung cancer: does it suggest a mesenchymal-epithelial transition?. Neoplasma. 2017;64:693–699. [DOI] [PubMed] [Google Scholar]
- [31].Tamura S, Isobe T, Ariyama H, et al. Ecadherin regulates proliferation of colorectal cancer stem cells through NANOG. Oncol Rep. 2018;40:693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Gauthier A, Ho M. Role of sorafenib in the treatment of advanced hepatocellular carcinoma: an update. Hepatol Res. 2013;43:147–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


