ABSTRACT
Long noncoding RNA (lncRNA) regulate many biological processes ranging from tumorigenesis to cancer metastasis. MicroRNA-223 (miR-223) acts as a novel tumor suppressor in bladder cancer (BC), however its target genes involved in BC, the molecular mechanisms governing its expression remain largely unknown. Both gain-of-function and loss of function experiments were performed to investigate the role of miR-223 in BC cells. The effects of miR-223 on BC progression were assessed using in vivo subcutaneous xenografts. The luciferase reporter assays were utilized to confirm the putative miR-223-binding site in the 3′-UTR of oncogene HSP90B1. The luciferase reporter assays and RNA immunoprecipitation assays were used to analyze the association between miR-223 and lncRNA DXL6-AS1 in BC cells. The expression of miR-223 was remarkably decreased in BC samples and BC cells. High miR-223 expression was correlated with favorable patient survival. BC cell growth in vivo was delayed by miR-223 overexpression. HSP90B1 was a direct target of miR-223 in BC cells, and the suppression of BC cell growth and invasion induced by miR-223 could be rescued by overexpression of HSP90B1. Moreover, lncRNA DXL6-AS1 was upregulated in BC tissues and functioned as a sponge for miR-223 and reduced its expression in BC cells, thereby enhancing cell proliferation and invasion. Forced expression of miR-223 could reverse the oncogenic effects of DXL6-AS1 on BC cell proliferation and invasion. Our study suggested that DLX6-AS1-mediated silencing of miR-223 promotes BC progression through the upregulation of HSP90B1.
KEYWORDS: Mir-223, DXL6-AS1, HSP90B1
Instruction
Bladder cancer (BC) is one of the most common urological malignancies in the world, and its incidence rates have been gradually increasing in China [1,2]. Despite many efforts have been made to develop multiple treatments, the 5-year survival rate of BC patients remains dissatisfied [3]. Both genetic and epigenetic changes are involved in the development and progression of BC [4], although the detailed mechanisms are still unknown.
The human transcriptome contains both protein-coding mRNAs and noncoding RNAs [5]. Long noncoding RNAs (lncRNAs), ranging from 200 nucleotides to 100 kilobases, participate in regulating a wide range of biological processes, through their interactions with miRNA, DNA and proteins [6,7]. Many lncRNAs are dysregulated in human cancers, and have tumor suppressive or oncogenic functions, thereby serving as potential biomarkers or therapeutic targets in cancer diagnosis and treatment.
MicroRNAs (miRNAs) are endogenous small non-coding regulatory RNAs with sizes of 19–24 nucleotides [8]. At the post-transcriptional level, miRNAs negatively regulate the expression of their target genes by binding directly to the 3′-untranslated region (3′-UTR) of target messenger RNAs (mRNAs), inducing mRNA degradation or protein translation repression [8]. Increasing evidence has shown that miRNAs play critical roles in pathological processes of multiple types of cancers, including BC [9,10]. Among the miRNAs detected in BC, miR-223 was able to inhibit the growth and invasiveness of BC cells in vitro, at least via targeting fibroblast growth factor receptor 2 [11–13]. However, the effects of miR-223 on BC cell growth in vivo, the downstream target genes of miR-223, and the mechanisms that control the expression of miR-223 in BC still remain largely unclear.
Here, we found that miR-223 was significantly downregulated in BC samples and that decreased miR-223 expression was associated with worse survival in patients with BC. Overexpression of miR-223 suppressed BC cell proliferation and invasion by targeting HSP90B1. Furthermore, ectopic expression of miR-223 dramatically suppressed the ability of BC cells to develop tumors in nude mice. Importantly, lncRNA DLX6-AS1 could bind to miR-223 and reduce its expression in BC cells. These findings show that the DLX6-AS1/miR-223/HSP90B1 signaling pathway plays a crucial role in BC development. DLX6-AS1, miR-223 and HSP90B1 could be promising prognostic biomarkers, and targeting this pathway may provide a promising therapeutic strategy for BC treatment.
Materials and methods
Patients and tissues
Eighty BC tissues and adjacent tissues were collected from patients, who underwent radical resection at the Ruijin Hospital Affiliated to Medical College of Shanghai Jiao Tong University, Shanghai, China. All BC tissues and adjacent tissues were snap-frozen in liquid nitrogen immediately after extraction and stored at −80°C until total RNA was extracted. The study was approved by the Ethics Committee of the Ruijin Hospital Affiliated to Medical College of Shanghai Jiao Tong University. Written informed consent was obtained from all patients.
Cell culture, chemicals, plasmids, and siRNAs
Human BC cell lines (T24 and SW780) and human normal human urothelial cell line SV-HUC-1 were purchased from the Shanghai Institute of Cell Biology (Shanghai, China). Cells were cultured in RPMI1640 media (catalog number: 61870044; Gilbco, USA) supplemented with 10% fetal bovine serum (FBS; catalog number: A3382001). All cell lines were maintained at 37 °C with 5% CO2.
DLX6-AS1 (pEX-3-DLX6-AS1) and HSP90B1 (pEX-3-HSP90B1) expression vectors were purchased from Genepharma (Shanghai, China). DLX6-AS1 siRNAs, HSP90B1 siRNAs, the nonspecific control siRNA, miR-223 mimic, control mimic, miR-223 inhibitor and control inhibitor were obtained from IGEbio (Guangzhou, China). The transfection was performed using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA; catalog number: 11668019) according to the manufacturer’s protocol.
Qrt-pcr assay
Total RNA was isolated using TRIzol reagent (Invitrogen, Carlsbad, CA, USA) and assessed using NanoDrop equipment (NanoDrop Technologies, Wilmington, DE, USA). Then, total RNA was converted to cDNA using an M-MLV Reverse Transcriptase Kit (Invitrogen, Carlsbad, CA, USA; catalog number: 28025013). Real-time PCR was performed on an ABI 7500 system with the SYBR-Green-quantitative real-time PCR Master Mix kit (Toyobo, Osaka, Japan; catalog number: QPK-212). The mirVanaTM qRT-PCR microRNA Detection Kit (Ambion, Austin, TX, USA; catalog number: AM1558) was used for miR-223 detection according to the manufacturer’s instructions. The results were normalized to the level of GAPDH or U6.
Western blot analysis
Total protein from cells was extracted using RIPA buffer (Beyotime, Jiangsu, China). Equal amount protein was separated by a 10% SDS-PAGE gel and transferred to polyvinylidene difluoride membrane (Millipore, Bedford, MA, USA). The membrane was blocked with 5% nonfat dry milk at room temperature for 1 h, and incubated with corresponding primary antibodies, HSP90B1 (1:1000, Santa Cruz, CA, USA; catalog number: sc-1794) and GAPDH (1:5000, Santa Cruz, CA, USA; sc-32233) overnight. The membranes were incubated with HRP-conjugated secondary antibodies (1:1000, Santa Cruz, CA, USA: catalog number: sc-2005) for 2 h at room temperature. Detection was performed by the ECL western blotting kit (Amersham Biosciences, Buckinghamshire, UK; catalog number: RPN2106). GAPDH was used as the loading control.
CCK-8 assay
CCK-8 assay (Beyotime Institute of Biotechnology, Jiangsu, China; catalog number: C0038) was used for BC cell proliferation analysis. BC cells were seeded in 96-well plate at a density of 1 × 103 cells per well. After 24 h, 48 h and 72 h of incubation, CCK-8 solution (10 μl) was added to the wells, and the absorbance was measured at 450 nm by a microplate reader (Bio-Rad, Hercules, CA, USA).
In vitro invasion assay
The invasive ability of BC cells was measured using the Matrigel transwell chambers (Corning, New York, USA; catalog number: 354,480) as described previously [14]. BC cells (2 × 104) that were suspended in serum-free medium were transferred to the upper chamber. The medium containing 10% FBS was added as chemokine to the lower chamber. After 24 h, the invaded cells on the lower surface of the membrane were fixed with 75% methanol (Sigma, St. Louis, MO, USA), and stained with crystal violet (Sigma, St. Louis, MO, USA). The evaluation of invasive ability was conducted by counting invaded cells under a microscope, and ten random fields of view were analyzed for each chamber. All experiments were performed in triplicate.
In vivo proliferation assay
BALB/c nude mice (4 weeks old) were purchased from Beijing HFK Bioscience (Beijing, China) and maintained in a specific pathogen-free facility. The protocol was approved by the Institutional Animal Care and Use Committee of Ruijin Hospital Affiliated to Medical College of Shanghai Jiao Tong University. BC cells (2 × 106) were inoculated subcutaneously in the right flank of the nude mice. Tumors were measured every 3 days and tumor volume was calculated using the following formula: volume = length (mm) x width2 (mm2)/2. Three weeks after BC cells inoculation, the mice were killed and the tumors were collected. The xenograft tumor tissues were fixed in 10% formaldehyde, embedded in paraffin and then sectioned. Anti-Ki-67 antibodies (Abcam, Cambridge, UK, dilution: 1:1000; catalog number: ab15580) were used for immunohistochemical analysis.
Dual-luciferase reporter assay
The full length of wild-type (WT) DLX6-AS1 or mutant (MUT) DLX6-AS1 with a mutated miR-223 binding site, the wild-type (WT) HSP90B1 3′-UTR fragment or mutant (MUT) HSP90B1 3′-UTR fragment with a mutated miR-223 binding site, were obtained from Genepharma (Shanghai, China). Mutations of DLX6-AS1 or HSP90B1 3′-UTR in the luciferase reporter vectors were generated by PCR mutagenesis using a QuickChange site-directed mutagenesis kit (Stratagene, La Jolla, CA, USA; catalog number: 200518) according to the manufacturer’s directions. BC cells (5 × 104) were seeded in 24-well plate overnight. Then, cells were co-transfected with the luciferase reporter vectors containing DLX6-AS1 (WT or MUT) or HSP90B1 3′-UTR (WT or MUT), together with miR-223 mimic, miR-223 inhibitor or the corresponding negative controls using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA; catalog number: 11668019). After 24 h, cells were harvested for luciferase detection using the dual-luciferase reporter assay system (Promega, Madison, WI, USA).
RNA immunoprecipitation (RIP) assay
RIP assays were performed using the Magna RIP RNA-Binding Protein Immunoprecipitation Kit (Millipore, Billerica, MA, USA; catalog number: 17–700). BC cells were lysed in RIP lysis buffer, and 100 μl whole-cell extracts were incubated with magnetic beads conjugated with antibodies that recognized Argonaute2 (Ago2, Millipore, Billerica, MA, USA; catalog number: 11A9) or control IgG (Millipore, Billerica, MA, USA; catalog number: 12–370) for 6 h at 4 °C. Then, the beads were incubated with proteinase K for 30 min at 55°C to remove proteins. Finally, the immunoprecipitated RNAs were used for the qRT-PCR analysis.
Statistical analysis
All statistical analysis was performed using the SPSS 22.0 statistical software package (SPSS, Chicago, USA). The data are presented as mean ± SEM from multiple individual experiments each performed in triplicate. Student’s t-test (two-tailed), one-way ANOVA test and Wilcoxon signed-rank test were applied to compare the significant differences. The value of P < 0.05 was considered significant.
Results
Mir-223 expression is downregulated in BC tissues and BC cells
First, we analyzed the expression profiles of miR-223 in multiple tumor types using a publicly available database (dbDEMC 2.0, http://www.picb.ac.cn/dbDEMC/) and found that miR-223 expression was reduced in various tumors, including BC (Figure 1(a)). The expression levels of miR-223 in two BC cell lines (T24 and SW780) and a normal human urothelial cell line SV-HUC-1 were examined by qRT-PCR analysis. Our results suggested that miR-223 expression was significantly reduced in BC cells compared to normal cells (Figure 1(b)). Then, we tested the levels of miR-223 in BC tissues and normal tissues using qRT-PCR assay. We found that miR-223 was significantly decreased in BC tissues (Figure 1(c)). We further accessed the prognostic value of miR-223 expression in 80 patients with BC, which were split into low- and high-expression groups by the median of miR-223 expression values. Kaplan-Meier analysis revealed that lower levels of miR-223 were associated with shorter overall survival (Figure 1(d)). These results indicated that loss of miR-223 expression was correlated with worse prognosis in BC patients.
Figure 1.
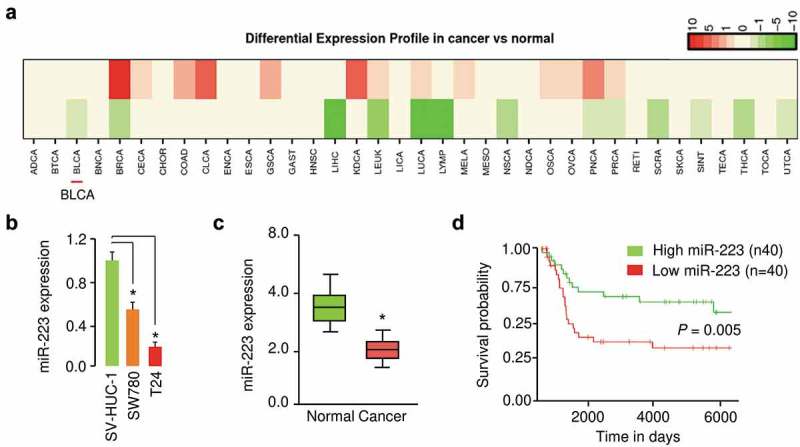
MiR-223 expression was downregulated in BC samples. (a) Differential expression patterns of miR-223 across different tumor types (dbDEMC 2.0 database). Green or red indicates downregulated or upregulated miR-223 expression in tumor tissues compared to normal tissues. Bladder cancer: BLCA. (b) qRT-PCR analysis for miR-223 in human BC cell lines (T24 and SW780) and human normal human urothelial cell line SV-HUC-1. (c) qRT-PCR assays for miR-223 in BC tissues and normal tissues. (d) The probability of overall survival in BC patients expressing high or low miR-223 levels. *P < 0.05.
The tumor suppressive effects of mir-223 on BC cell proliferation and invasion
The significant reduction of miR-223 expression in BC samples allowed us to explore the possible biological significance of miR-223 in the modulation of BC cell growth and invasion. T24 cells that have relatively lower miR-223 expression were transfected with miR-223 mimic, and SW780 cells that express relatively higher levels of miR-223 were transfected with miR-223 inhibitor (Figure 2(a)). We found that miR-223 mimic-transfected T24 cells displayed much slower growth rates and weaker invasive abilities compared with the control cells transfected with control mimic (Figure 2(b,c)). The downregulation of miR-223 in SW780 cells could increase the growth and invasion of BC cells (Figure 2(b,c)). We also tested whether silencing of miR-223 in SV-HUC-1 cells could induce cell proliferation and invasion. The inhibition of miR-223 significantly increased the growth and invasion of SV-HUC-1 cells (Figure 2(a,b,e)). Furthermore, overexpression of miR-223 reduced the proliferation and invasion of SW780 cells (Figure 2(f,g,h)). These data confirmed the growth and invasion-inhibitory roles of miR-223 in BC cells.
Figure 2.
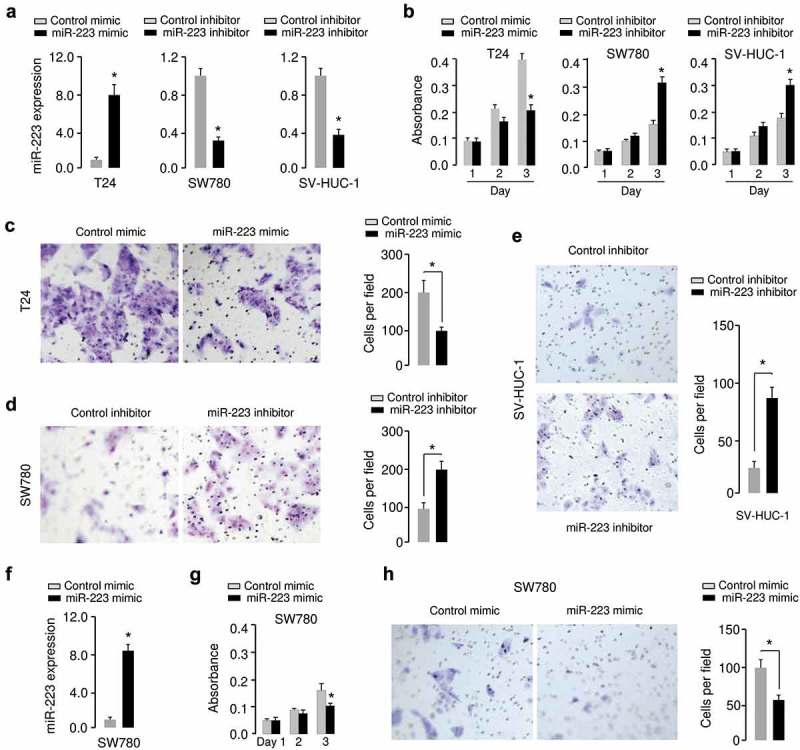
MiR-223 represses proliferation and invasion of BC cells. (a) Verification of miR-223 expression in T24, SW780 and SV-HUC-1 cells using qRT-PCR assay. (b) Proliferation assay in T24, SW780 and SV-HUC-1 cells transfected with miR-223 mimic or miR-223 inhibitor as indicated. (c, d, e) Cell invasion assay in T24 (c), SW780 (d) and SV-HUC-1(e) cells transfected as indicated. (f) Verification of miR-223 overexpression in SW780 cells using qRT-PCR assay. (g, h) Proliferation (g) and invasion (h) assay in SW780 cells transfected with miR-223 mimic or control mimic. *P < 0.05.
In order to verify whether the tumor suppressive effects of miR-223 observed in BC cells could influence BC growth in vivo, T24 cells that were transfected with miR-223 mimic or control mimic, and SW780 cells transfected with miR-223 inhibitor or control inhibitor, were subcutaneously inoculated into nude mice, respectively. As shown in Figure 3(a,b,c), the overexpression of miR-223 significantly delayed tumor growth, and the suppression of miR-223 significantly accelerated tumor growth in vivo. Furthermore, we examined the expression of cell proliferation marker Ki-67 in xenograft tumor sections using immunohistochemical staining. We found lower levels of Ki-67 in sections from tumors overexpressing miR-223, and observed higher levels of Ki-67 in sections from tumors with miR-223 knockdown (Figure 3(d)). Moreover, we found that overexpression of miR-223 in SW780 cells reduced the proliferation of SW780 cells in vivo (Figure 3(c,e)). Taken together, our data indicated that miR-223 represses the malignant phenotypes of BC cells in vivo and in vitro.
Figure 3.
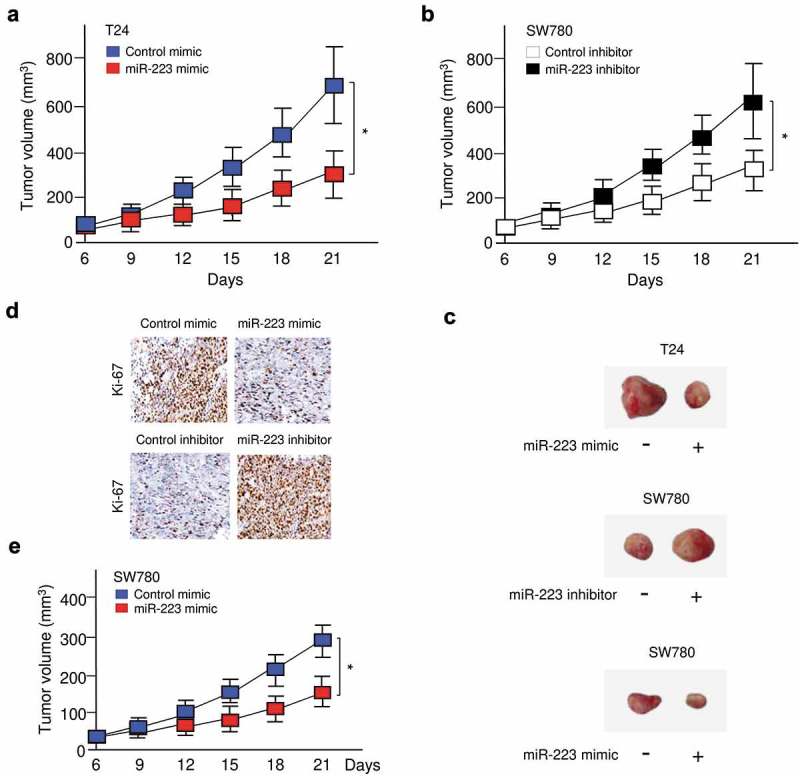
Restoration of miR-223 expression suppressed BC growth in vivo. (a, b) T24 (a) or SW780 (b) cells were transfected with miR-223 mimic or miR-223 inhibitor, respectively. Then, these cells were injected into nude mice. Tumor growth curves were measured after injection. (c) Representative pictures of tumors formed. (d) Immunohistochemical staining demonstrated that the overexpression of miR-223 in T24 cells (upper panel) inhibits, while the suppression of miR-223 in SW780 cells (bottom panel) induces tumor growth in vivo, as indicated by the expression of Ki-67. (e) SW780 cells were transfected with miR-223 mimic or control mimic. Then, these cells were injected into nude mice. Tumor growth curves were measured after injection. *P < 0.05.
MiR-223 directly targets HSP90B1 in BC cells
In order to elucidate the target genes of miR-223 in BC cells, we performed informatics analysis using online database TargetScan. We have detected a binding site for miR-223 in the 3′-UTR of HSP90B1 (Figure 4(a)). Interestingly, our meta-analysis using BioXpress database [15] revealed that the expression of HSP90B1 was elevated in many tumor types such as BC (Figure 4(b)). In a survival analysis of the TCGA BC data for 404 patients, we found that patients with higher HSP90B1 expression had poorer overall survival than those patients with lower HSP90B1 expression (Figure 4(c)). We then measured HSP90B1 levels in BC cell lines cells and a normal cell line SV-HUC-1 using qRT-PCR analysis. As shown in Figure 4(d), HSP90B1 expression was significantly higher in two BC cell lines than in normal cells. These data support a negative correlation between miR-223 and HSP90B1 expression in BC cells.
Figure 4.
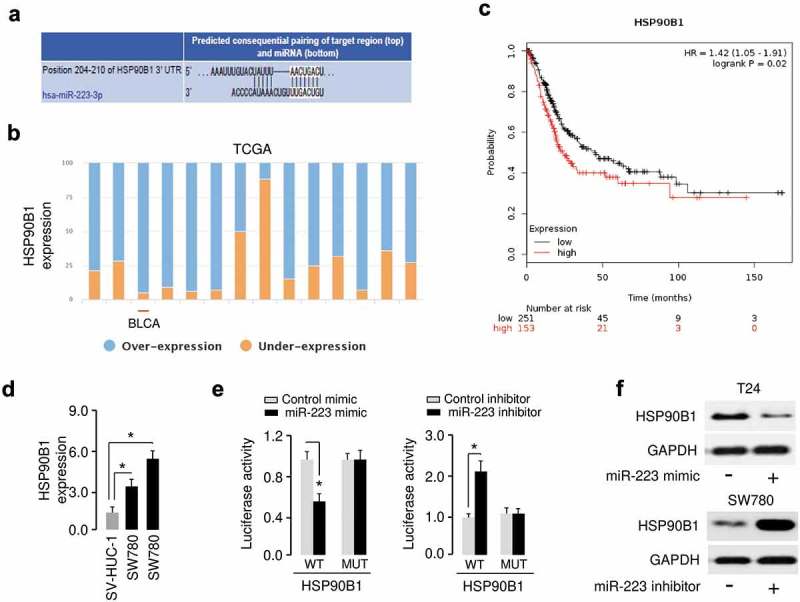
MiR-223 directly targets HSP90B1 in BC cells. (a) Bioinformatics analysis showed that miR-223 could directly target the HSP90B1 3′-UTR. (b) Exploring miR-223 expression in several human cancers using the BioXpress database. Bladder cancer: BLCA. (c) Prognostic value of HSP90B1 mRNA expression in BC patients was analyzed using the KM lotter database. (d) Expression of HSP90B1 mRNA in human BC cell lines (T24 and SW780) and human normal human urothelial cell line SV-HUC-1. (e) The luciferase-reporter plasmid containing WT or mutant HSP90B1 3′-UTR was co-transfected into BC cells, along with miR-223 mimic or miR-223 inhibitor as indicated. Then, the relative luciferase activity was performed by dual-luciferase reporter assays. (f) The protein expression of HSP90B1 was examined in BC cells transfected with miR-223 mimic or miR-223 inhibitor as indicated. *P < 0.05.
To test whether miR-223 could interact with the HSP90B1 3′-UTR, we performed the luciferase assays and found that the luciferase activity of WT HSP90B1 3′-UTR was significantly decreased in T24 cells transfected with miR-223 mimic compared with those transfected with control mimic, and was significantly increased in SW780 cells transfected with miR-223 inhibitor compared with those transfected with control inhibitor (Figure 4(e)). However, miR-223 had no significant impact on the luciferase activity MUT HSP90B1 3′-UTR, suggesting that the predicted fragment at the 3′-UTR of the HSP90B1 mRNA was the complementary site for the miR-223 seed region. Moreover, western blot analysis showed that miR-223 overexpression inhibited, and miR-223 knockdown increased the protein expression of HSP90B1. All these data suggested that HSP90B1 was a direct target of miR-223 in BC cells.
HSP90B1 modulates the tumor suppressive roles of miR-223 in BC cells
Based on the above findings, we speculated that HSP90B1 could function downstream of miR-223 and mediate the functions of miR-223 in BC cells. To validate this hypothesis, we conducted the rescue experiments by transfecting miR-223 mimic with HSP90B1 expression vector into T24 cells, or by transfecting miR-223 inhibitor with siRNAs targeting HSP90B1 (Figure 5(a)). As indicated in Figure 5(b), miR-223-suppressed cell proliferation and invasion was rescued by HSP90B1 overexpression (Figure 5(b)). The knockdown of miR-223 significantly increased cell proliferation and invasion, while the introduction of HSP90B1 siRNAs obviously abrogated these effects (Figure 5(c,d)), suggesting that miR-223 inhibited cell proliferation and invasion by downregulating HSP90B1 expression.
Figure 5.
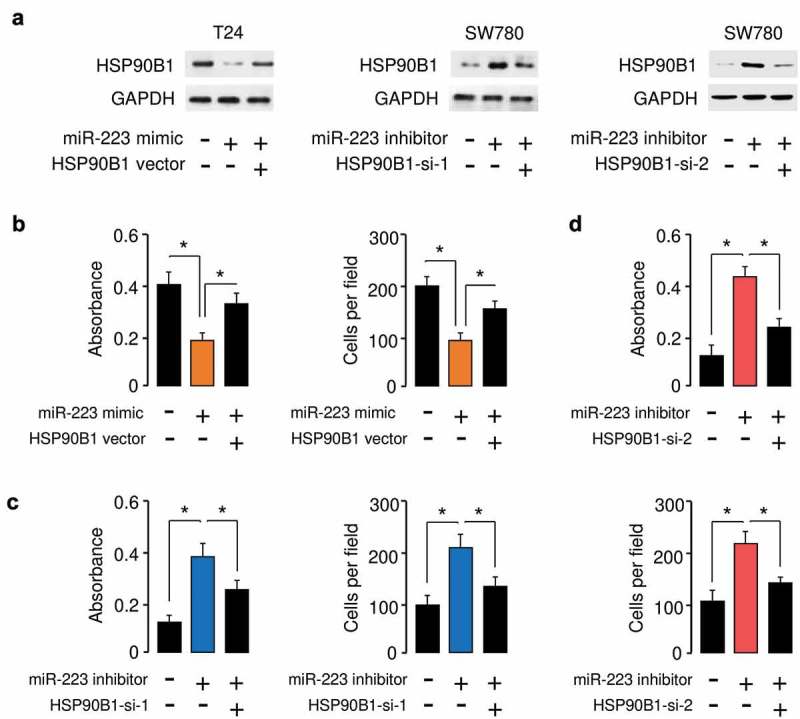
HSP90B1 modulates the tumor suppressive roles of miR-223 in BC cells. (a) The protein expression of HSP90B1 in T24 cells transfected with miR-223 mimic together with the HSP90B1 expression vector, and in SW780 cells transfected with miR-223 inhibitor together with HSP90B1 siRNAs. (b) Cell proliferation and invasion assays in T24 cells transfected with miR-223 mimic or control mimic, together with or without HSP90B1 expression vector. Cell proliferation and invasion assays in SW780 cells transfected with miR-223 inhibitor or control inhibitor, together with or without HSP90B1 siRNA 1 (c) or siRNA 2 (d). *P < 0.05.
DLX6-AS1 functions as a sponge for miR-223 and downregulates its expression
To determine whether lncRNAs might act as a sponge for miR-223 in BC cells, we used the starBase v2.0 database [16] to find the possible interactions between miR-223 and lncRNAs. Subsequent analysis using KM lotter database (http://kmplot.com/analysis/) was performed to screen out key lncRNA candidates associated with prognosis in BC patients. Based on the above screening, the most significant survival-related lncRNAs in BC were identified. Among them, LncRNA DLX6-AS1 was predicted to contain a sequence complementary to miR-223 (Figure 6(a)). The expression levels of DLX6-AS1 were examined in BC cell lines and normal cells using qRT-PCR assays. As compared to normal cells, the expression of DLX6-AS1 was significantly elevated in BC cells (Figure 6(b)). To confirm whether there was a direct interaction between miR-223 and DLX6-AS1, we used luciferase vectors containing either WT DLX6-AS1 or MUT DLX6-AS1, and transfected these reporter vectors into BC cells along with miR-223 mimic or miR-223 inhibitor, respectively. The ectopic expression of miR-223 led to a reduction in luciferase activity of WT DLX6-AS1, and the downregulation of miR-223 significantly increased the luciferase activity of WT DLX6-AS1 (Figure 6(c)). We noticed that miR-223 had no evident effects on MUT DLX6-AS1 (Figure 6(c)), suggesting that miR-223 interacted with DLX6-AS1 in BC cells.
Figure 6.
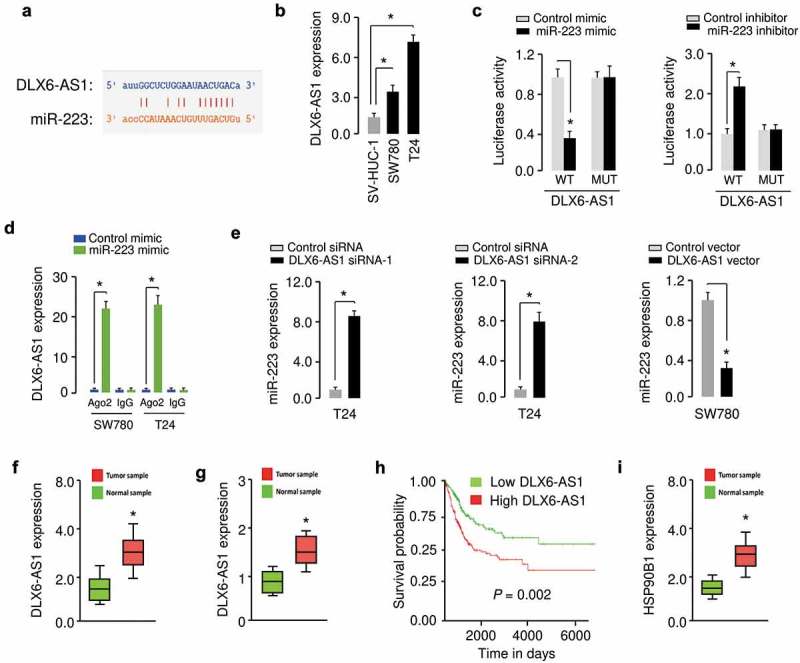
DLX6-AS1 acts as a sponge for miR-223 in BC cells. (a) Illustration of the predicted interaction between miR-223 and lncRNA DLX6-AS1. (b) Expression of DLX6-AS1 in human BC cell lines and human normal human urothelial cells, as measured by qRT-PCR assay. (c) Luciferase assays in T24 cells transfected with the reporter plasmid containing WT or mutant DLX6-AS1 together with or without miR-223 mimic, and in SW780 cells transfected with the reporter plasmid containing WT or mutant DLX6-AS1 together with or without miR-223 inhibitor. (d) RIP assay was performed in T24 and SW780 cells transfected with miR-223 mimic or control mimic. DLX6-AS1 expression was detected using qRT-PCR assay. (e) Expression of miR-223 in BC cells after knockdown or overexpression of DLX6-AS1. (f) qRT-PCR analysis of DLX6-AS1 levels in BC tissues and normal tissues. (g) DLX6-AS1 expression was analyzed in BC tissues (red) and normal tissues (green) using the data obtained from the MethHC database. (h) Kaplan-Meier curves for overall survival of BC patients with high or low levels of DLX6-AS1. (i) qRT-PCR analysis of HSP90B1 expression in BC tissues and normal tissues. *P < 0.05.
To test whether DLX6-AS1 and miR-223 were present in the Ago2-containing RNA-induced silencing complex, we performed the RIP assays in BC cells transfected with miR-223 mimic or control mimic. Endogenous DLX6-AS1 pull-down by Ago-2 was specifically enriched in cells overexpressing miR-223 (Figure 6(d)). Our qRT-PCR assay showed that the expression of miR-223 was upregulated when DLX6-AS1 was knocked down by DLX6-AS1 siRNAs, and the levels of miR-223 were decreased in BC cells transfected with the DLX6-AS1 expression vector (Figure 6(e)). The clinical significance of DLX6-AS1 in human BC was investigated using qRT-PCR analysis. The expression of DLX6-AS1 in BC tissues was significantly higher than that in normal tissues (Figure 6(f)). We examined the expression of DLX6-AS1 in BC tissues and normal tissues using the TCGA dataset from the MethHC database [17]. We found higher levels of DLX6-AS1 in BC samples (Figure 6(g)). 80 patients with BC were categorized into DLX6-AS1 low and DLX6-AS1 high groups based on the median DLX6-AS1 expression value. Overall survival was worse in the DLX6-AS1 high group than in the DLX6-AS1 low group (Figure 6(h)). Importantly, we also verified the upregulation of HSP90B1 in BC tissues compared with normal tissues using qRT-PCR assays (Figure 6(i)). These results showed that high DLX6-AS1 expression predicts poor patient survival, and that DLX6-AS1 might promote BC progression through upregulation of HSP90B1 via targeting miR-223.
DLX6-AS1 facilitates BC cell proliferation and invasion in a miR-223-mediated manner
To elucidate whether DLX6-AS1 functioned in BC cells in a miR-223-mediated mechanism, we utilized rescue experiments by transfecting DLX6-AS1 siRNAs together with miR-223 inhibitor into T24 cells, also by transfecting DLX6-AS1 expression vector together with miR-223 mimic into SW780 cells. Our results suggested that downregulation of DLX6-AS1 significantly decreased the proliferation and invasion of T24 cells, and the knockdown of miR-223 recovered the proliferation and invasion potential of T24 cells (Figure 7(a,b)). Consistently, the promoting effects of DLX6-AS1 on the proliferation and migration of SW780 cells were partially attenuated by the overexpression of miR-223 (Figure 7(c)). Thus, we concluded that DLX6-AS1 acted as an oncogenic lncRNA to facilitate BC cell proliferation and invasion in a miR-223-mediated manner.
Figure 7.
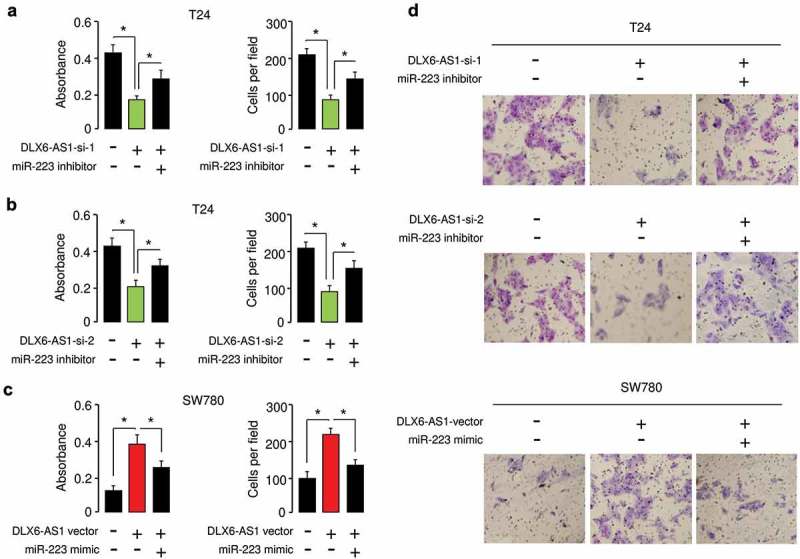
DLX6-AS1 facilitates BC cell proliferation and invasion in a miR-223-mediated manner.Cell proliferation and invasion assays in T24 cells transfected with (or without) DLX6-AS1 siRNA 1 (a) or DLX6-AS1 siRNA 2 (b), together with (or without) miR-223 inhibitor. (c) Cell proliferation and invasion assays in SW780 ells transfected with or without DLX6-AS1 expression vector, together with (or without) miR-223 mimic. (d) Representative images from the invasion assays. *P < 0.05.
Discussion
Understanding the genetic and epigenetic alterations that serve as key determinants for cancer initiation and progression may provide a critical opportunity for diagnosis and the therapeutic intervention of BC. An increasing number of studies have shown that miRNAs play important roles in most human cancers by functioning as either tumor suppressors or oncogenes [9,10]. Numerous miRNAs were dysregulated in BC, and their functional significance has been reported [18]. However, the cellular roles and underlying mechanisms of miR-223 in BC have not been fully investigated [11–13]. In this study, we revealed that miR-223 significantly suppressed BC cell invasion in vitro and inhibited BC cell proliferation in vitro and in vivo, suggesting that miR-223 could be a therapeutic target of BC.
Previously, increased expression of HSP90B1 has been considered as an indicator of worse prognosis in lung cancer, chronic lymphocytic leukemia and hepatocellular carcinoma [19–21]. In osteosarcoma cells, the downregulation of HSP90B1 inhibited tumor growth in vitro and in vivo, possibly through the regulation of the PI3K/AKT/mTOR pathway [22]. Using bioinformatics methods and reporter assays, we identified a novel oncogene HSP90B1, as a direct target of miR-223 in BC cells. We found that miR-223-induced cancer suppression was reversed by the ectopic overexpression of HSP90B1, indicating that HSP90B1 was a functional target of miR-223 and mediated the tumor suppressor functions of miR-223 in BC cells. Of note, PU-H71, an HSP90B1 inhibitor, has demonstrated considerable efficacy in triple-negative breast cancer, diffuse large B-cell lymphoma and myeloma with limited hematopoietic toxicity [23–25]. However, the efficacy of PU-H71 has not been investigated in BC.
LncRNAs were shown to associate with miRNAs, forming thee lncRNA-miRNA-mRNA regulatory network, where lncRNAs can act as competing endogenous RNAs to negatively regulate the expression of tumor suppressor miRNAs, enhancing malignant features of tumor cells [6,7]. DLX6-AS1 has been reported to be overexpressed in osteosarcoma [26], glioma [27], pancreatic cancer [28], lung cancer [29,30], ovarian cancer [31,32], gastric cancer [33] and liver cancer [34]. In gastric cancer, increased DLX6-AS1 expression was associated with T3/T4 invasion, distant metastasis and poor clinical prognosis [33]. Also, elevated expression of DLX6-AS1 was found to be associated with tumor size and advanced clinical stage in patients with lung cancer [30]. Higher DLX6-AS1 expression was negatively correlated with the survival of pancreatic cancer patients [28]. High expression of DLX6-AS1 was significantly correlated with advanced TNM stage, high tumor grade, and distant metastasis of patients with osteosarcoma [26]. DLX6-AS1 have been reported to play critical roles in regulating diverse cellular processes, such as cell growth, apoptosis, migration, EMT, invasion and cancer stemness through several mechanisms [26–34]. For instance, DLX6-AS1 acted as a competing endogenous RNA to upregulate some oncogenes through binding to tumor suppressor miRNAs, including miR-641 [26], miR-197-5p [27], miR-497-5p [28], miR-144 [29], miR-27b [30], miR-613 [32] and miR-204-5p [33]. In addition, DLX6-AS1 was shown to promote the stem cell properties of liver cancer stem cells through downregulation of CADM1 by increasing the methylation of the CADM1 promoter and activation of the STAT3 signaling pathway [34], suggesting that DLX6-AS1 has a tumor-promoting role in human tumors. However, its biological function and underlying mechanisms in BC have not been investigated. Here, we reported that DLX6-AS1 could enhance migration and invasion of BC cells. Furthermore, using the luciferase reporter assays combined with RIP experiments, we demonstrated a new link between miR-223 and lncRNA DLX6-AS1 in BC cells. The overexpression of DLX6-AS1 increased BC cell proliferation and invasion, and these effects were largely reversed by the restoration of miR-223. Thus, our data suggested that DLX6-AS1 regulated the growth and invasion via a miR-223-dependent mechanism, contributing to a better understanding of the mechanisms responsible for BC progression. Therefore, DLX6-AS1 sponged multiple tumor suppressor miRNAs to upregulate their target genes, leading to the promotion of tumor pathogenesis.
In summary, this study has uncovered a DLX6-AS1/miR-223/HSP90B1 regulatory pathway that participated in the regulation of BC progression and suggested that targeting this pathway may provide a promising prognostic and therapeutic strategy for BC treatment.
Disclosure statement
No potential conflict of interest was reported by the authors.
Ethical approval
All procedures performed in studies involving human tissues and mice were in accordance with the ethical standards of Ruijin Hospital Affiliated to Medical College of Shanghai Jiao Tong University.
References
- [1].Wong MCS, Fung FDH, Leung C, et al. The global epidemiology of bladder cancer: a joinpoint regression analysis of its incidence and mortality trends and projection. Sci Rep. 2018;8:1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Pang C, Guan Y, Li H, et al. Urologic cancer in China. Jpn J Clin Oncol. 2016;46:497–501. [DOI] [PubMed] [Google Scholar]
- [3].Abdollah F, Gandaglia G, Thuret R, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: a trend analysis. Cancer Epidemiol. 2013;37:219–225. [DOI] [PubMed] [Google Scholar]
- [4].Jung I, Messing E.. Molecular mechanisms and pathways in bladder cancer development and progression. Cancer Control. 2000;7:325–334. [DOI] [PubMed] [Google Scholar]
- [5].Diamantopoulos MA, Tsiakanikas P, Scorilas A.. Non-coding RNAs: the riddle of the transcriptome and their perspectives in cancer. Ann Transl Med. 2018;6:241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Balas MM, Johnson AM. Exploring the mechanisms behind long noncoding RNAs and cancer. Noncoding RNA Res. 2018;3:108–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Dong P, Xiong Y, Yue J, et al. Exploring lncRNA-mediated regulatory networks in endometrial cancer cells and the tumor microenvironment: advances and challenges. Cancers (Basel). 2019;11(2):E234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zabolotneva AA, Zhavoronkov A, Garazha AV, et al. Characteristic patterns of microRNA expression in human bladder cancer. Front Genet. 2013;3:310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dong F, Xu T, Shen Y, et al. Dysregulation of miRNAs in bladder cancer: altered expression with aberrant biogenesis procedure. Oncotarget. 2017;8:27547–27568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Dong P, Xiong Y, Yue J, et al. miR-34a, miR-424 and miR-513 inhibit MMSET expression to repress endometrial cancer cell invasion and sphere formation. Oncotarget. 2018;9:23253–23263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Guo J, Cao R, Yu X, et al. MicroRNA-223-3p inhibits human bladder cancer cell migration and invasion. Tumour Biol. 2017;39:1010428317691678. [DOI] [PubMed] [Google Scholar]
- [12].Sugawara S, Yamada Y, Arai T, et al. Dual strands of the miR-223 duplex (miR-223-5p and miR-223-3p) inhibit cancer cell aggressiveness: targeted genes are involved in bladder cancer pathogenesis. J Hum Genet. 2018;63:657–668. [DOI] [PubMed] [Google Scholar]
- [13].Yang C, Wu S, Wu X, et al. Overexpression of FGFR2 reversed miR-223-induced UM-UC-3 cell growth and migration inhibition in vitro Silencing circular RNA UVRAG inhibits bladder cancer growth and metastasis by targeting the microRNA-223/fibroblast growth factor receptor 2 axis. Cancer Sci. 2019;110:99–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Dong P, Xiong Y, Yu J, et al. Control of PD-L1 expression by miR-140/142/340/383 and oncogenic activation of the OCT4-miR-18a pathway in cervical cancer. Oncogene. 2018;37:5257–5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Wan Q, Dingerdissen H, Fan Y, et al. BioXpress: an integrated RNA-seq-derived gene expression database for pan-cancer analysis. Database (Oxford). 2015;2015:bav019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li JH, Liu S, Zhou H, et al. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42(Database issue):D92–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Huang WY, Hsu SD, Huang HY, et al. MethHC: a database of DNA methylation and gene expression in human cancer. Nucleic Acids Res. 2015;43(Databaseissue): D856–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Enokida H, Yoshino H, Matsushita R, et al. The role of microRNAs in bladder cancer. Investig Clin Urol. 2016;57(Suppl 1):S60–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Xu Y, Chen Z, Zhang G, et al. HSP90B1 overexpression predicts poor prognosis in NSCLC patients. Tumour Biol. 2016;37:14321–14328. [DOI] [PubMed] [Google Scholar]
- [20].Rodríguez-Vicente AE, Quwaider D, Benito R, et al. MicroRNA-223 is a novel negative regulator of HSP90B1 in CLL. BMC Cancer. 2015;15:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Yang Z, Zhuang L, Szatmary P, et al. Upregulation of heat shock proteins (HSPA12A, HSP90B1, HSPA4, HSPA5 and HSPA6) in tumour tissues is associated with poor outcomes from HBV-related early-stage hepatocellular carcinoma. Int J Med Sci. 2015;12:256–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li G, Cai M, Fu D, et al. Heat shock protein 90B1 plays an oncogenic role and is a target of microRNA-223 in human osteosarcoma. Cell Physiol Biochem. 2012;30:1481–1490. [DOI] [PubMed] [Google Scholar]
- [23].Caldas-Lopes E, Cerchietti L, Ahn JH, et al. Hsp90 inhibitor PU-H71, a multimodal inhibitor of malignancy, induces complete responses in triple-negative breast cancer models. Proc Natl Acad Sci U S A. 2009;106:8368–8373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Cerchietti LC, Lopes EC, Yang SN, et al. A purine scaffold Hsp90 inhibitor destabilizes BCL-6 and has specific antitumor activity in BCL-6-dependent B cell lymphomas. Nat Med. 2009;15:1369–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Usmani SZ, Bona RD, Chiosis G, et al. The anti-myeloma activity of a novel purine scaffold HSP90 inhibitor PU-H71 is via inhibition of both HSP90A and HSP90B1. J Hematol Oncol. 2010;3:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Zhang N, Meng X, Mei L, et al. LncRNA DLX6-AS1 promotes tumor proliferation and metastasis in osteosarcoma through modulating miR-641/HOXA9 signaling pathway. J Cell Biochem. 2019. March 6 [Epub ahead of print] DOI: 10.1002/jcb.28426 [DOI] [PubMed] [Google Scholar]
- [27].Li X, Zhang H, Wu X. Long noncoding RNA DLX6-AS1 accelerates the glioma carcinogenesis by competing endogenous sponging miR-197-5p to relieve E2F1. Gene. 2019;686:1–7. [DOI] [PubMed] [Google Scholar]
- [28].Yang J, Ye Z, Mei D, et al. Long noncoding RNA DLX6-AS1 promotes tumorigenesis by modulating miR-497-5p/FZD4/FZD6/Wnt/β-catenin pathway in pancreatic cancer. Cancer Manag Res. 2019;11:4209–4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Huang Y, Ni R, Wang J, et al. Knockdown of lncRNA DLX6-AS1 inhibits cell proliferation, migration and invasion while promotes apoptosis by downregulating PRR11 expression and upregulating miR-144 in non-small cell lung cancer. Biomed Pharmacother. 2019;109:1851–1859. [DOI] [PubMed] [Google Scholar]
- [30].Sun W, Zhang L, Yan R, et al. LncRNA DLX6-AS1 promotes the proliferation, invasion, and migration of non-small cell lung cancer cells by targeting the miR-27b-3p/GSPT1 axis. Onco Targets Ther. 2019;12:3945–3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhao J, Liu HR. Down-regulation of long noncoding RNA DLX6-AS1 defines good prognosis and inhibits proliferation and metastasis in human epithelial ovarian cancer cells via Notch signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23(8):3243–3252. [DOI] [PubMed] [Google Scholar]
- [32].You Q, Shi HY, Gong CF, et al. Long non-coding RNA DLX6-AS1 acts as an oncogene by targeting miR-613 in ovarian cancer. Eur Rev Med Pharmacol Sci. 2019. August;23(15):6429–6435. . [DOI] [PubMed] [Google Scholar]
- [33].Liang Y, Zhang CD, Zhang C, et al. DLX6-AS1/miR-204-5p/OCT1 positive feedback loop promotes tumor progression and epithelial-mesenchymal transition in gastric cancer. Gastric Cancer. 2019. August 28 Epub ahead of print DOI: 10.1007/s10120-019-01002-1 [DOI] [Google Scholar]
- [34].Wu DM, Zheng ZH, Zhang YB, et al. Down-regulated lncRNA DLX6-AS1 inhibits tumorigenesis through STAT3 signaling pathway by suppressing CADM1 promoter methylation in liver cancer stem cells. J Exp Clin Cancer Res. 2019;38(1):237. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


