ABSTRACT
Therapeutic monoclonal antibodies (mAbs) are commonly administered to patients through intravenous (IV) infusion, which involves diluting the medication into an infusion solution (e.g., saline and 5% dextrose). Using the wrong diluent can cause product aggregation, which may compromise patient safety. We and others have shown that Herceptin® (trastuzumab) and Avastin® (bevacizumab) undergo rapid aggregation upon mixing with dextrose and human plasma in vitro. In this study, we evaluated the compatibility of a panel of 11 therapeutic mAbs with dextrose or saline and human serum. These mAbs were randomly selected for their distinct formulations and IgG isotypes (IgG1, IgG2, IgG4, and Fc-fusion protein). All the mAbs appeared to be compatible with saline and human serum. However, mAbs that were formulated at acidic pH (≤ 6.5) exclusively formed insoluble aggregates upon mixing with dextrose and serum. Such aggregation was not detected for the mAbs that are at neutral pH (7.2–7.5) or in buffers containing sodium chloride. Mass spectrometric analysis revealed that the insoluble aggregates were composed of mAb molecules and several serum proteins (e.g., complement proteins, apolipoprotein, fibronectin) that are characterized by an isoelectric point of pH 5.4–6.7. At proximate pH to the isoelectric point values, those abundant serum proteins appeared to undergo isoelectric precipitation with mAb molecules. Our observations highlight a potential risk of protein aggregation at the blood-IV interface if a diluent is incompatible with a specific mAb formulation. This information has implications in guiding the design of product formulations and the selection of the right diluent for intravenous infusion of therapeutic mAbs.
Abbreviations: ADC: antibody-drug conjugate; D5W: 5% dextrose in water; IM: intramuscular; IV: intravenous; LC-MS/MS: liquid chromatography-tandem mass spectrometry; mAb: monoclonal antibody; SC: subcutaneous; pI: isoelectric point
KEYWORDS: Therapeutic monoclonal antibody, intravenous infusion, diluent, dextrose, saline, compatibility, plasma proteins, protein aggregation, isoelectric precipitation, formulation, pH, ionic strength
Introduction
Therapeutic antibodies have become a vital component of therapy for various diseases and conditions including, but not limited to, cancer, autoimmune and infectious diseases, and inflammatory conditions.1–4 As of December 2018, 97 therapeutic antibodies have been approved by the U.S. Food and Drug Administration (FDA), comprising 79% monoclonal antibodies (mAbs), 6% antibody-drug conjugates (ADCs and radiolabelled mAbs), 9% Fc fusion proteins, and 6% antibody antigen-binding fragment (Fab)-related proteins (Fab and Fab fusion proteins). All these products are administered to patients through parenteral routes: 63% (n = 61) by intravenous (IV) infusion, 34% (n = 33) by subcutaneous injection (SC), and 3% (n = 3) by intramuscular (IM) and intravitreal injection (Figure 1a).
Figure 1.
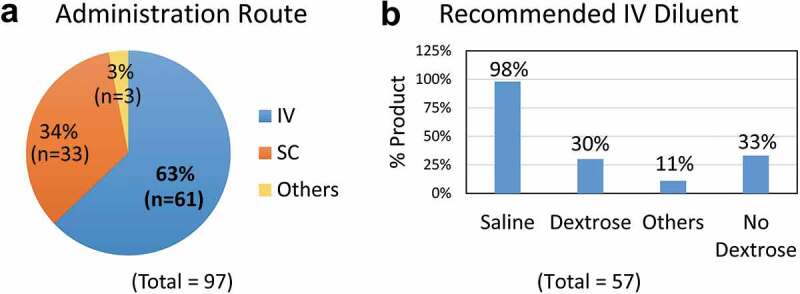
Routes of administration for therapeutic antibodies.
(a) Routes of administration for 97 therapeutic antibodies that were approved by the U.S. Food and Drug Administration (as of December 2018): intravenous infusion (63%, n = 61), subcutaneous (SC) injection (34%, n = 33), intramuscular (IM) and intravitreal injection (3%, n = 3). (b) Among the 57 mAbs that require further dilution for IV infusion, saline solution is acceptable for use with 98% (n = 56) products, while 5% dextrose solution and other diluents (0.45% sodium chloride or lactated ringer’s solution) are only recommended for 30% (n = 17) and 11% (n = 6) therapeutic antibodies, respectively. Remarkably, dextrose solution is prohibited for use with 33% (n = 19) antibody products. Note that four of the 61 antibodies that are administered through IV infusion require no dilution. Data were obtained from the Drugs@FDA website (https://www.accessdata.fda.gov/scripts/cder/daf/).
The IV infusion procedure involves diluting a medication into a diluent solution in a fluid bag or bottle. The use of diluent(s) is product-specific and is usually stipulated on the approved product labels (Table 1). While normal saline (0.9% sodium chloride) solution appears to be acceptable for use with 98% of therapeutic antibodies, the use of only 5% dextrose solution or other specific diluents (half normal saline solution and lactated Ringer’s solution) is recommended for some products (Figure 1b). Notably, dextrose solution is prohibited for use with 33% of the antibody products (Figure 1b). For instance, the prescribing information for Herceptin® and Avastin® specifies diluting with 0.9% sodium chloride injection and “Do not use dextrose (5%) solution”.5,6 Remicade was originally approved for infusion with 0.9% sodium chloride injection,7 and its label was recently updated to stress “Do not use with any other diluent”.8 It is clear that a diluent must be compatible with a specific mAb to ensure product stability and patient safety.
Table 1.
Diluents approved for intravenous infusion of therapeutic antibodies.
| Number of products with specific diluentsc |
|||||||
|---|---|---|---|---|---|---|---|
| Formulation pHa | Number of products | Formulation NaCl concentration | Number of products | Saline | Dextrose | Othersd | No dextrosee |
| 5.0–6.5 | 38 | 0 | 25 | 24 | 3 | 4 | 12 |
| 40 − 154 mM | 13 | 13 | 5 | 0 | 5 | ||
| 6.6–8.0 | 14 | 0 | 6 | 6 | 2 | 1 | 2 |
| 10 − 154 mM | 8 | 8 | 4 | 1 | 0 | ||
| Total | 52b | 52 | 51 | 14 | 6 | 19 | |
aTherapeutic antibodies (n = 52) are divided into two groups based on formulation pH in the range of pH 5.0–6.5 (n = 38) and pH 6.6–8.0 (n = 14) which are further grouped for salt concentrations in the formulations. Information were obtained from FDA approved product labels that are publicly available.
bFifty-seven mAbs require a diluent for intravenous infusion, five are excluded due to the lack of public information on their formulation pH values.
cSome products are compatible with more than one diluent.
d0.45% sodium chloride or Lactated Ringer’s Solution
eDextrose is prohibited for use with those products.
During IV infusion, the diluted mAb proteins are continuously introduced into a patient’s blood stream where they directly interact with blood components. Arvinte et al9 showed that Avastin® (bevacizumab) or Herceptin® (trastuzumab) underwent rapid aggregation after mixing with 5% dextrose and human plasma under in vitro conditions that simulate the interface of IV infusion. No aggregation was detected after mixing the mAbs with saline and human plasma. We previously identified abundant plasma proteins in the insoluble protein aggregates,10 namely complement proteins C3, C4, factor H, fibronectin, and apolipoprotein, which are characterized by an isoelectric point (pI) of 5.5–6.7.
To gain further insight into this phenomenon, we tested additional therapeutic mAbs from different IgG isotypes (IgG1, IgG2, IgG4, and a Fc-fusion protein) with a range of formulation pH (pH 5.2–7.5) and salt concentrations (0–154 mM NaCl) (Table 2). As noted, all commercial human plasma products used in our previous studies had an approximate pH of 8.1. To mimic the physiological pH of human blood, we used a commercial human serum product (pH 7.4) in this study. Our results show that the mAb protein stability is contingent to the pH and ionic strength in the product formulation. Mass spectrometric analysis identified complement proteins and several other abundant serum proteins in the insoluble protein aggregates. Our data suggest a higher risk of protein aggregation for therapeutic mAbs that are present in acidic formulations (pH ≤ 6.5). Although our conclusions are based on observations of in vitro studies that mimic the blood-IV interface, it is plausible that the findings might have implications for guiding the selection of the right diluent for IV infusion of therapeutic mAbs.
Table 2.
Therapeutic antibodies tested for compatibility with dextrose and human serum in vitro.
| Formulation pH* | Number of products | Formulation NaCl concentration* | Number of products | Number of products tested in this study | Number of products aggregated when mixed with dextrose and serum |
|---|---|---|---|---|---|
| 5.0–6.5 | 38 | 0 | 25 | 5 | 5 |
| 40 − 154 mM | 13 | 4 | 1 | ||
| 6.6–8.0 | 14 | 0 | 6 | 1 | 0 |
| 10 − 154 mM | 8 | 1 | 0 | ||
| Total | 52** | 52 | 11 | 6 |
*Therapeutic antibodies that require a diluent for IV infusion are divided into two groups based on formulation pH in the range of pH 5.0–6.5 and pH 6.6–8.0, which are further grouped for salt concentrations in the formulations. Information were obtained from FDA approved product labels that are publicly available.
**Only included the mAbs (n = 52) with formulation information (pH and salt concentrations) that are publicly available, out of 57 mAbs that require a diluent for IV infusion (Figure 1b).
Results
Aggregation of therapeutic mAbs upon mixing with dextrose and human serum in vitro
We tested a panel of 11 therapeutic mAbs for their compatibility with dextrose and human serum under in vitro conditions that simulate the interface of IV infusion with blood (Table 2). These mAbs were representatives of the different IgG isotypes (IgG1, IgG2, IgG4, and Fc fusion protein) with varying formulation pH values (5.2–7.5) and salt concentrations (0–154 mM sodium chloride) (Figure 2a). Equal concentrations of individual mAbs, which were present in their original formulations, were diluted into 5% dextrose, saline, or lactated Ringer’s solution, and then mixed with human serum followed by incubation at 25°C for 30 minutes (see details in Materials and Methods). The resultant protein aggregates, if any, were collected by centrifugation and resolved onto SDS-PAGE (Figure 2). Duplicate samples were processed without centrifugation to determine the level of nonspecific protein binding to the inner surface of the microtube. Results showed that there was little non specific protein adsorption to the microtube inner surface (Supplement I). Of the mAbs tested, all five mAbs (mAb-1, −3, −5, −7, −8) that were formulated in acidic buffers (pH 5.2–6.5) and lacking sodium chloride uniformly produced insoluble aggregates upon mixing with dextrose and human serum. A lower amount of aggregates was also detected for mAb-2, which was formulated in pH 5.5 buffer containing 50 mM sodium chloride. In contrast, little or no aggregates were detected for mAb-10 and mAb-11 that were formulated in neutral pH (7.2–7.5) and other three mAbs (mAb-4, mAb-6, and mAb-9) which were present in acidic formulations (pH 5.8–6.5) containing sodium chloride (50–154 mM). Notably, none of the 11 mAbs formed insoluble aggregates when mixed with serum and saline (Figure 2b) or lactated Ringer’s solution (Figure 2c). These data are in agreement with our previous observation that therapeutic mAbs presented in acidic and nonionic formulations (pH ≤ 6.5 and < 50 mM NaCl), are susceptible to insoluble aggregation upon mixing with dextrose and human serum or plasma10 in vitro.
Figure 2.
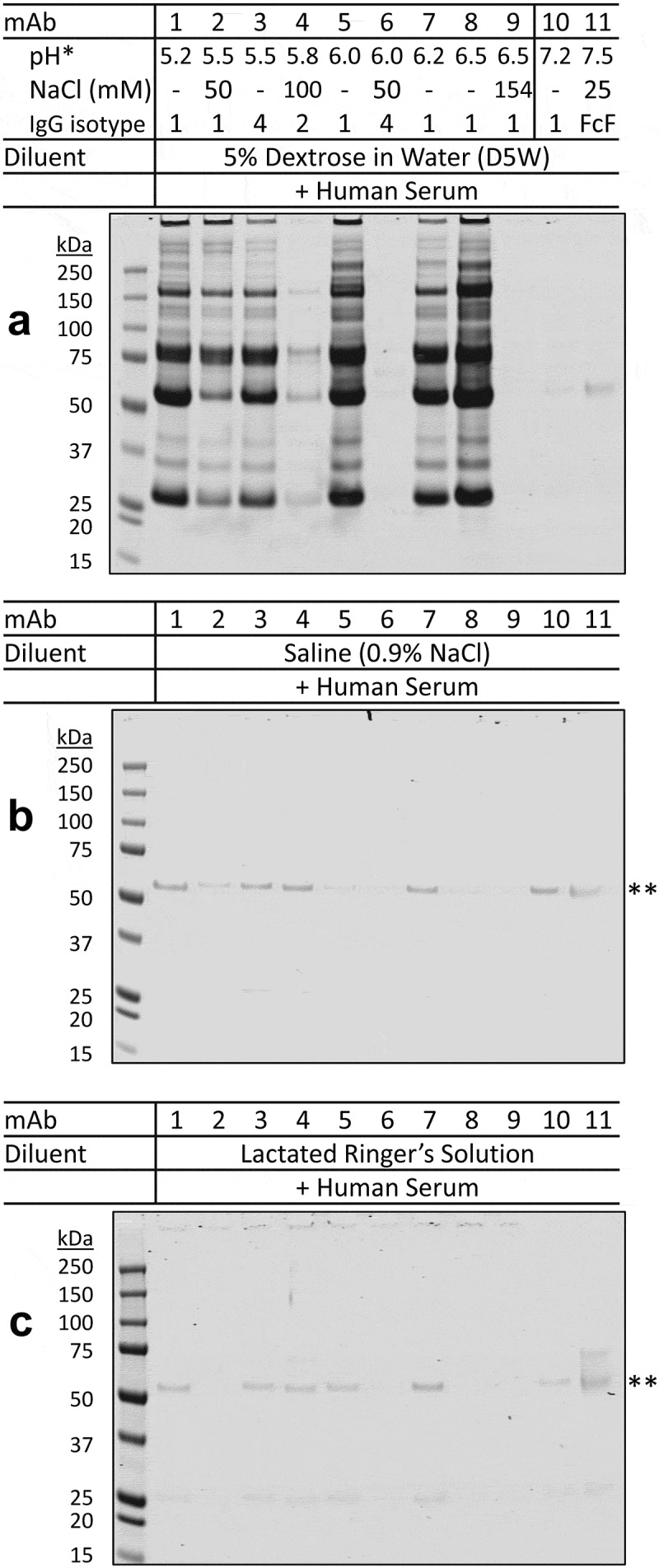
Aggregation of therapeutic mAbs after mixing with dextrose and human serum in vitro.
A panel of 11 therapeutic mAbs, named mAb-1 to mAb-11, were tested for their compatibility with diluents and human serum under in vitro conditions (see Materials and Methods). Aliquots of therapeutic mAbs, which were prepared from their original formulations, were diluted into (a) 5% dextrose in water (D5W), (b) saline (0.9% sodium chloride), and (c) lactated Ringer’s solution (containing 0.6% sodium chloride), followed by mixing with human serum. After incubation at 25°C for 30 minutes, the resultant insoluble pellets, if any, were collected by centrifugation and analyzed by SDS-PAGE electrophoresis. When diluted with 5% dextrose (a), insoluble protein aggregates were detected for therapeutic mAbs (mAb-1, mAb-3, mAb-5, mAb-7, and mAb-8) which were present in acidic pH (5.2 to 6.5) and nonionic formulation buffers. A lower amount of aggregates was also detected for mAb-2, which was formulated in pH 5.5 buffer containing 50 mM sodium chloride. In contrast, little or no aggregates were detected for mAb-10 and mAb-11 that were both formulated in pH 7.2 to 7.5 or other mAbs (mAb-4, mAb-6, and mAb-9) whose formulations were acidic (pH 5.8–6.5) and contained sodium chloride. When diluted with ionic diluent (saline or lactated Ringer’s solution) (b and c), no protein aggregates were detected across the 11 mAbs when mixed with human serum. * Shown are the pH values and NaCl concentrations of individual mAb formulation buffers and the corresponding IgG isotypes. ** The faint bands indicate basal protein adsorption to the microtube inner surface (Supplement I). Shown are representative images of duplicate experiments.
Comparative effects of pH and ionic strength on stability of therapeutic mAbs in the mixtures of dextrose and serum
We determined the stability of therapeutic mAbs when mixed with dextrose and serum as a function of pH and solution ionic strength. Samples were first tested in buffers with a range of pH 5.0–7.6. When diluted in dextrose solution at acidic pH 5.3–5.7, all 11 mAbs formed insoluble protein aggregates upon mixing with serum (Figure 3a). The levels of aggregation decreased when solution pH was raised and were almost undetectable at pH 7.3–7.7 (Figure 3b–d). A more rapid decline in aggregate formation was observed for samples prepared from therapeutic mAbs in formulations containing sodium chloride (mAb-2, −4, −6, −9, and −11). Next, we tested if protein aggregates formed in acidic conditions could be reversed by raising solution pH. As shown in Figure 3e (lanes 1–6), the indicated mAbs (1, 2, 3, 5, 7, 8) in acidic formulations formed large aggregates upon incubation with dextrose and serum at 25°C for 15 minutes. Duplicate samples were incubated for an additional 15 minutes after adjusting to pH 7.3–7.6. In these post-treatment samples, the pre-formed aggregates were no longer detectable in the resultant samples (Figure 3e, lanes 7–12).
Figure 3.
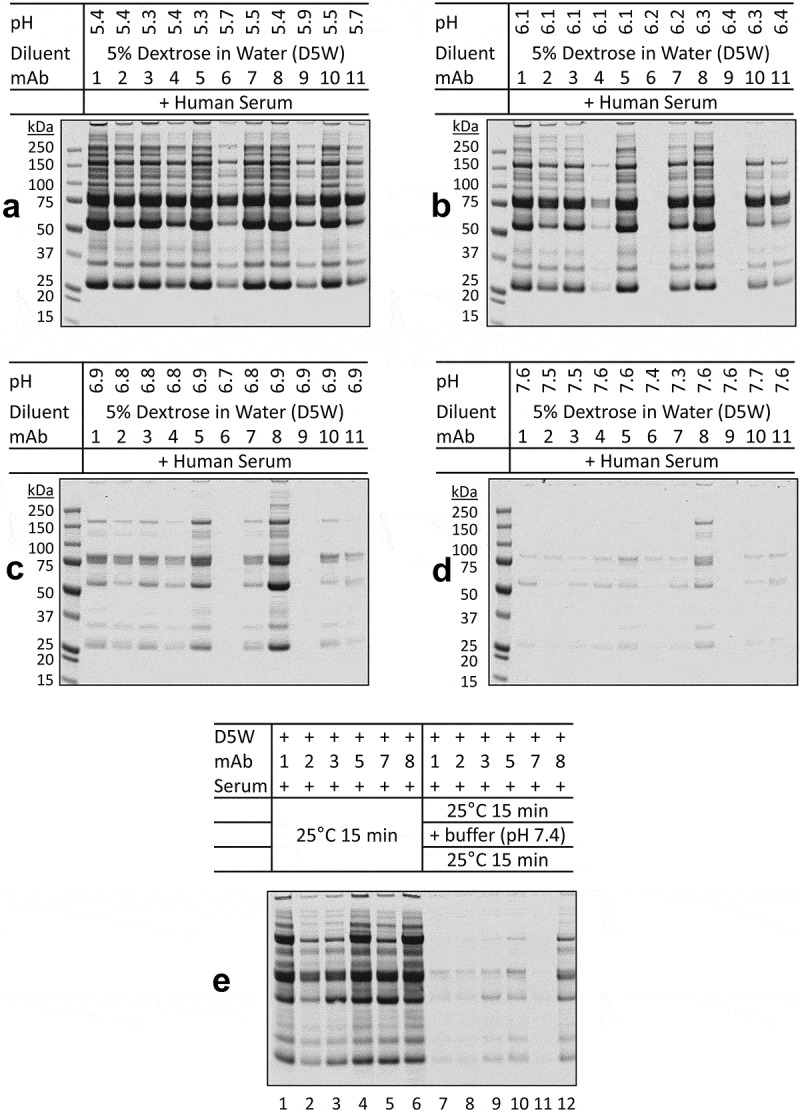
pH-dependence of protein aggregation of therapeutic mAbs in mixtures with dextrose and human serum.
The indicated D5W-mAb mixtures were adjusted to (a) pH 5.3–5.9, (b) pH 6.1–6.4, (c) pH 6.7–6.9, and (d) pH 7.3–7.7 with citrate-phosphate buffers (pH 5.0, 5.8, 6.6, and 7.6). The resultant samples were incubated with human serum and analyzed for potential protein aggregates. (a) At pH 5.3–5.9, all 11 mAbs formed insoluble aggregates, displaying similar band patterns on SDS-PAGE, with lower levels of aggregates being seen for mAbs in ionic formulations (mAbs 2, 4, 6, 9, and 11). (b-d) The levels of protein aggregates decreased by raising pH in the D5W-buffer-mAb mixtures. (e) Lanes 1–6, the indicated mAbs in acidic formulations formed massive aggregates after incubation with dextrose and serum at 25°C for 15 minutes. Lanes 7–12, duplicate samples were incubated for an additional 15 minutes after adjusting to pH 7.3–7.6. The preformed aggregates were no longer detectable in the resultant samples.
The impact of ionic strength was tested by including sodium chloride in the mixed solutions. Addition of 0.45% sodium chloride decreased dextrose-mediated mAb aggregation in serum (Figure 4a) in a dose-dependent manner (Figure 4b). We further showed that pH-dependent protein aggregation was reversible upon raising the ionic strength in the mixed solutions. All six mAbs (1, 2, 3, 5, 7, 8) tested formed large aggregates under the assay conditions (Figure 4c, lanes 1–6), which was effectively disrupted by adding 0.9% sodium chloride to individual samples (Figure 4c, lanes 7–12).
Figure 4.
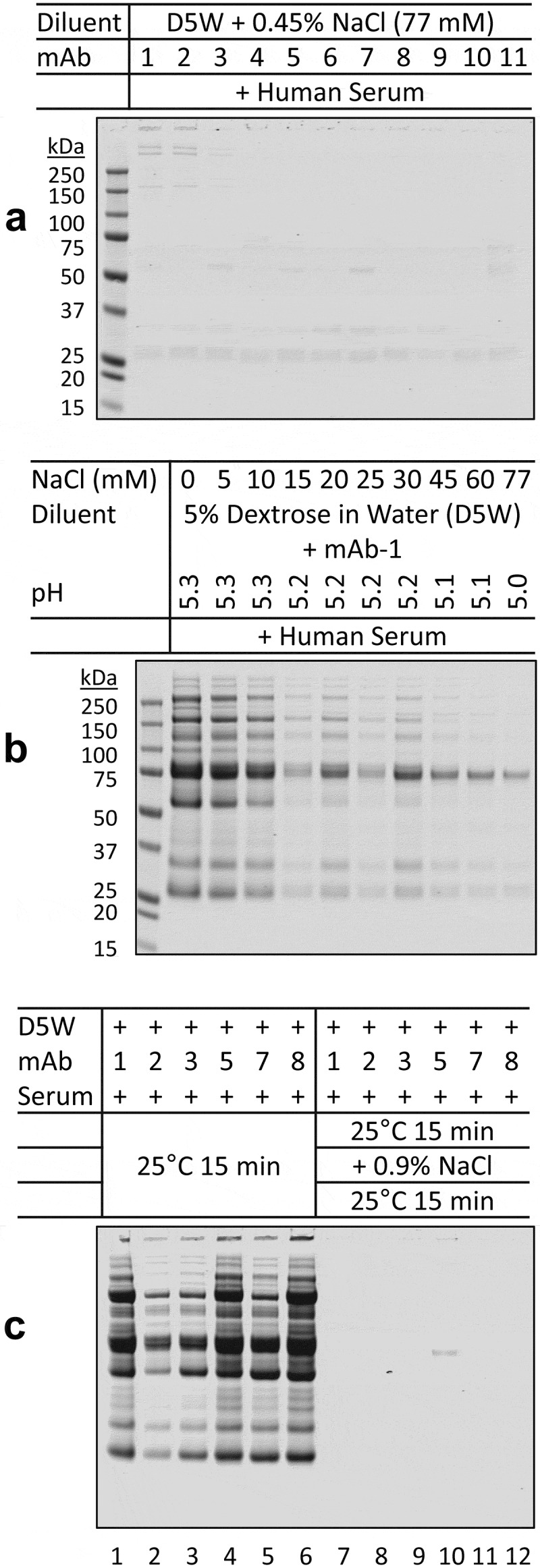
Ionic strength-dependence of aggregation of therapeutic mAbs in mixtures with dextrose and human serum.
(a) The indicated mAbs were mixed with a NaCl-containing dextrose solution (D5W + 0.45% NaCl (77 mM)) and human serum. At such ionic strength, little or no aggregates were detected across all the samples. (b) Aliquots of mAb-1 were diluted into D5W solutions with varying amounts of NaCl. The mixed solutions were adjusted to pH 5.0–5.3 with citrate-phosphate buffer (pH 5.0). When incubated with serum, the presence of NaCl suppressed aggregate formation in a dose-dependent manner. (c) Aliquots of 10% NaCl were added to the six D5W-mAb-serum solutions that contained the most aggregates (lanes 1–6) to mimic physiological NaCl concentration (final concentration 0.9% or 154 mM). After an additional 15 minutes of incubation, little or no aggregates were detected across the samples (lanes 7–12).
Taken together, the data demonstrate that stability of therapeutic mAbs is contingent to the pH and ionic strength in the resulting solutions of dextrose and serum. Although the assay conditions used in our study do not exactly replicate the in vivo environment, it is plausible that the findings might have implications for assessing product stability at the interface of blood and the IV infusion where both the mAb and diluent molecules come in direct contact with blood components.
Protein composition of dextrose-mediated insoluble aggregates of therapeutic mAbs with serum or plasma
The protein aggregates derived from Avastin and Herceptin in dextrose and plasma were shown to contain several plasma proteins.10 While plasma is similar in composition to serum, the measured pH values of commercial plasma products (pH~ 8.1) were higher than the physiological pH of serum products (pH 7.4). We compared aggregation patterns for therapeutic mAbs after mixing with serum versus plasma in dextrose solutions. Samples of insoluble protein aggregates were prepared from mAb-1, mAb-5 and mAb-7, which were in acidic buffers (pH < 6.5), and mAb-10, which was adjusted to approximately pH 5.5 from its original formulation pH 7.2, followed by incubation with dextrose and serum or plasma. Under both conditions, the resultant aggregates displayed comparable band patterns on SDS-PAGE (Figure 5a). Duplicate samples were subjected to in-solution trypsin digestion and liquid chromatography–tandem mass spectrometry (LC-MS/MS) analysis. Fourteen proteins were identified as the most abundant proteins in the insoluble aggregates across all samples (Table 3). In separate experiments, samples of insoluble aggregates were prepared from mAb-7 and mAb-10 and resolved onto SDS-PAGE (Figure 5b). Individual protein bands (total 12 bands as noted) were excised and analyzed by LC-MS/MS after in-gel trypsin digestion. The results confirmed the presence and identification of the 14 most abundant proteins in the corresponding bands, including the mAb molecules (as evidenced by IgG1 heavy and light chains), complement factor H, complement proteins C3, C4, and C5, apolipoprotein B-100, fibronectin, plasminogen, prothrombin, C4b-binding protein, serum albumin, and IgM (Figure 5c). As expected, fibrinogen was only identified in the aggregates formed in plasma (Figure 5b, band #12), but was absent in the aggregates derived from serum (Table 3). Notably, almost all of the serum/plasma proteins identified by LC-MS/MS are characterized by an acidic pI of 5.4 to 6.7 (Figure 5c). It is probable that, when exposed to approximate pH of the pI values (5.4 − 6.7), which was the case for mAbs in acidic formulations (pH 5.2 to 6.5), abundant serum or plasma proteins undergo isoelectric precipitation with mAb molecules to form insoluble protein aggregates.
Figure 5.
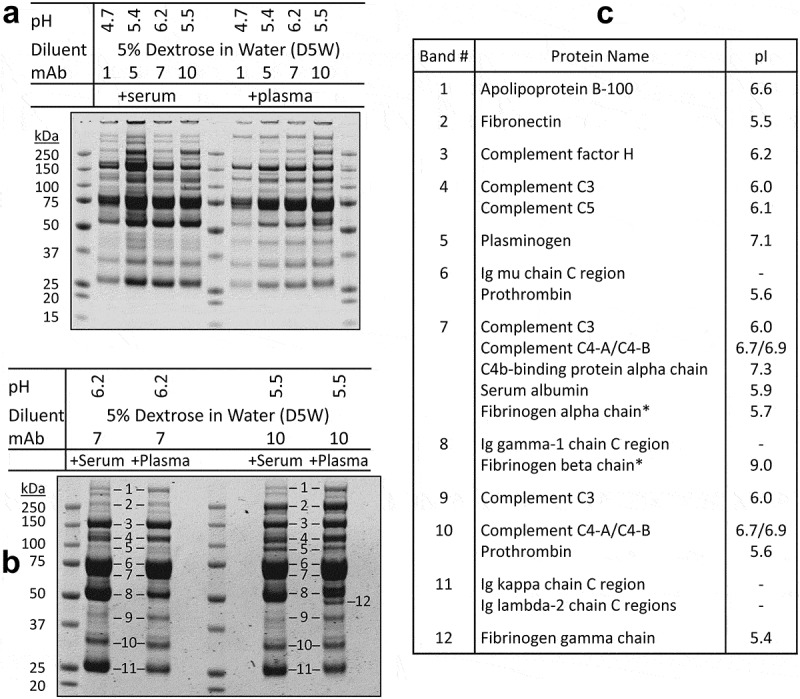
Comparison of protein aggregates in the mAb-dextrose mixtures with serum and plasma.
The indicated mAbs were diluted into dextrose at pH 4.7–6.2 and incubated with human plasma and serum, respectively, as in Figures 2a and 3a. (a) Serum mediated protein aggregates (left panel) showed similar band patterns as those of plasma (right panel) on SDS-PAGE. Duplicate aggregate samples were subjected to in-solution trypsin digestion followed by LC-MS/MS analysis revealing 14 of the most abundant proteins (Table 3). (b) Insoluble protein aggregates were prepared using mAb-7 and mAb-10 after mixing with dextrose at indicated pH and incubation with human serum and plasma, respectively. After resolving by SDS-PAGE, a set of 12 major protein bands (labeled 1 to 12) were retrieved for in-gel trypsin digestion followed by protein identification. (c) Shown are the most abundant protein(s) identified in the corresponding bands. * Proteins that were found in the aggregates from using plasma, but were absent in those of serum.
Table 3.
Comparison of protein aggregates in the mAb-dextrose mixtures with human serum and plasma.
| Total Protein Spectral Intensity (×107)*** |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Aggregates Formed in Serum |
Aggregates Formed in Plasma |
|||||||||||
| # | Protein Name* | pI** | MW (kDa) | Uniprot # | mAb1 | mAb5 | mAb7 | mAb10 | mAb1 | mAb5 | mAb7 | mAb10 |
| Common Proteins Identified in All Eight Insoluble Aggregates | ||||||||||||
| 1 | Apolipoprotein B-100 | 6.6 | 517 | P04114 | 16 | 19 | 7 | 7 | 17 | 13 | 10 | 22 |
| 2 | Fibronectin | 5.5 | 266 | P02751 | 3 | 19 | 7 | 29 | 1 | 5 | 5 | 55 |
| 3 | Complement C4-A/C4-B | 6.7/6.9 | 194 | P0C0L4/P0C0L5 | 56 | 44 | 61 | 79 | 50 | 48 | 56 | 62 |
| 4 | Complement C5 | 6.1 | 190 | P01031 | 6 | 5 | 8 | 12 | 5 | 7 | 8 | 12 |
| 5 | Complement C3 | 6.0 | 189 | P01024 | 29 | 31 | 31 | 30 | 93 | 111 | 118 | 122 |
| 6 | Complement factor H | 6.2 | 144 | P08603 | 72 | 52 | 63 | 55 | 90 | 88 | 84 | 91 |
| 7 | Plasminogen | 7.1 | 93 | P00747 | 2 | 2 | 3 | 26 | 4 | 3 | 3 | 13 |
| 8 | Prothrombin | 5.6 | 72 | P00734 | 2 | 4 | 2 | 4 | 8 | 24 | 15 | 15 |
| 9 | Serum albumin | 5.9 | 71 | P02768 | 12 | 6 | 6 | 2 | 2 | 4 | 6 | 4 |
| 10 | C4b-binding protein alpha chain | 7.3 | 69 | P04003 | 55 | 22 | 45 | 52 | 50 | 45 | 55 | 51 |
| 11 | Ig mu chain C region | – | – | P01871 | 119 | 77 | 67 | 64 | 102 | 65 | 90 | 90 |
| 12 | Ig gamma-1 chain C region | – | – | P01857 | 134 | 46 | 77 | 95 | 55 | 142 | 59 | 38 |
| 13 | Ig kappa chain C region | – | – | P01834 | 122 | 55 | 157 | 67 | 38 | 68 | 48 | 27 |
| 14 | Ig lambda-2 chain C regions | – | – | P0CG05 | 24 | 11 | 19 | 20 | 12 | 13 | 7 | 19 |
| Unique Proteins Identified only in Aggregates Formed in Plasma**** | ||||||||||||
| 1 | Fibrinogen alpha chain | 5.7 | 96 | P02671 | none | none | none | none | 3 | 3 | 5 | 46 |
| 2 | Fibrinogen beta chain | 9.0 | 57 | P02675 | none | none | none | none | 4 | 4 | 8 | 50 |
| 3 | Fibrinogen gamma chain | 5.4 | 52 | P02679 | none | none | none | none | 2 | 2 | 6 | 38 |
*Proteins were identified by the presence of at least three distinct peptides in the replicated samples from two independent experiments.
**Theoretical protein isoelectric point (pI), molecular weight (MW), and database accession number were obtained from UniProtKB/Swiss-Prot database using the Agilent Spectrum Mill mass spectrometry data analysis software. The proteins were sorted by their MWs.
***Total protein spectral intensity is the sum of all peptides that were assigned to the specific protein. Peptide intensity was determined on Spectrum Mill using extracted ion chromatograms. Listed proteins are those with an intensity value greater than 10 × 107 in at least one sample.
****Proteins that were found in the aggregates from using plasma but were absent in those of serum.
Discussion
Many therapeutic mAbs are administered to patients through IV infusion after dilution into a diluent solution such as saline or 5% dextrose. The diluent(s) must be compatible with specific product formulations to ensure product stability and patient safety. While much emphasis has been placed on product stability in the diluent solution, little published evidence is associated with assessing the compatibility of the mAb with diluent and blood components that simulate the interface of blood with the IV infusion diluent. Here we show that the stability of therapeutic mAbs is contingent to the pH and ionic strength in the mixed solutions with a diluent and human plasma or serum in vitro. Therapeutic mAbs that are formulated in acidic buffers (pH ≤ 6.5) with low ionic strength are found to be more susceptible to forming insoluble protein aggregates under the in vitro conditions. While the order of events remains to be defined, the insoluble aggregates isolated from the dextrose-mAb-serum (or plasma) mixtures are composed of abundant serum or plasma proteins that are characterized by a pI of ~5.4–6.7. When exposed to pH ~6.0, those serum or plasma proteins may undergo isoelectric precipitation, which recruit mAb molecules into insoluble protein aggregates (Figure 6). It is noted that a similar in-vitro dilution approach has also been used by other researchers for predicting stability of clinical candidate mAbs in the subcutaneous environment.11 Our data thus have implications for guiding the selection of the right diluent for IV infusion of therapeutic mAbs. The experimental procedures described can be adopted by pharmaceutical companies to assess compatibility of therapeutic mAbs with diluent solutions and human plasma or serum.
Figure 6.
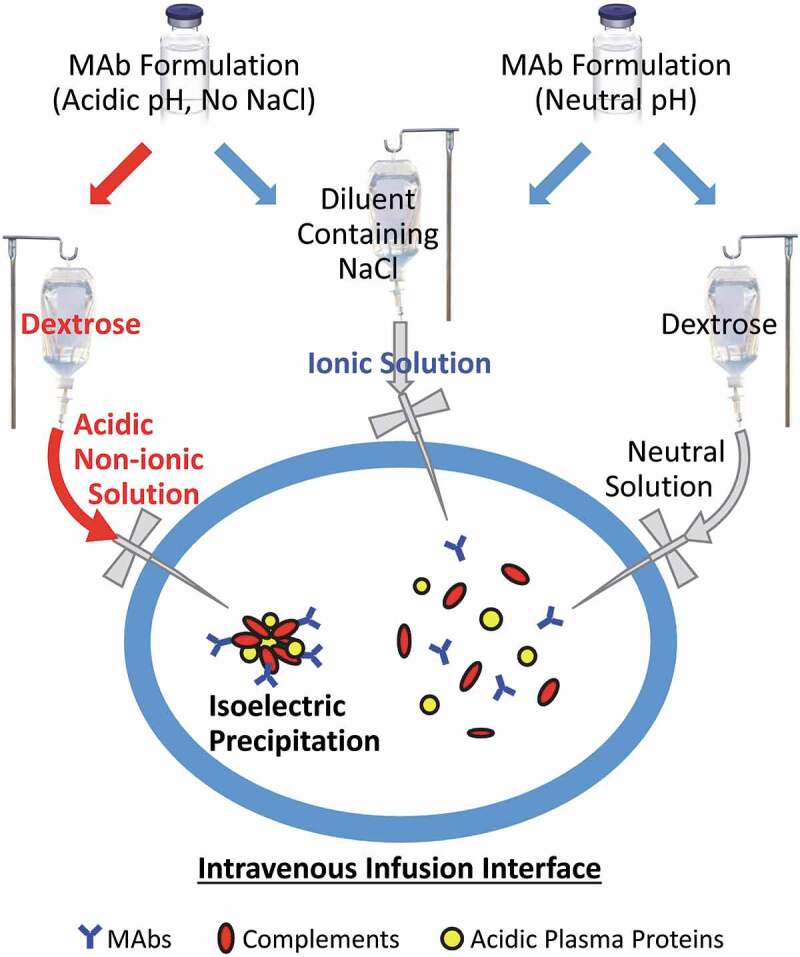
Potential biochemical pathways driving protein aggregation at the IV infusion interface.
Therapeutic mAbs, especially those formulated in acidic buffers (pH ≤ 6.5) with low ionic strength, could form insoluble protein aggregates at the interface of IV infusion with blood components when diluted into a nonionic dextrose for intravenous infusion (Figure 2). The underlying biochemical pathways may involve isoelectric precipitation of abundant plasma proteins (e.g., complement proteins) whose pI values are proximate to the formulation pH of the mAb (Table 3). This hypothesis is supported by the evidence that protein aggregation was effectively prevented by increasing ionic strength and/or pH in the mAb-diluent solution (Figures 3 and 4). By contrast, no protein aggregation was detected for mAbs that are formulated at physiological pH (mAb-10 and mAb-11) even when mixed with nonionic dextrose and human serum (Figure 2).
A panel of 11 therapeutic mAbs were tested, which are representatives of different IgG isotypes (IgG-1, IgG-2, IgG-4, and Fc-fusion protein) and formulations with varying pH (5.2–7.5) and sodium chloride concentrations (0–154 mM). All five mAbs that were present in acidic buffers (pH ≤ 6.5) and low salt concentrations, regardless of the IgG isotypes, were shown to rapidly form insoluble protein aggregates upon mixing with dextrose and human serum in vitro (Figure 2). By contrast, little or no aggregates were detected for mAbs (mAb-10, mAb-11) that were formulated at neutral pH (7.2–7.5) or in buffers containing sodium chloride (mAb-4, mAb-6 and mAb-9) (Figure 2). Consistently, protein aggregation was effectively abrogated by raising the pH or salt concentrations in the mixed solution (Figures 3 and 4). When dextrose, which lacks buffering capacity, is used as a diluent, the resulting mixture essentially preserves the pH of the reconstituted mAb solution (Supplement II).
Our data show that the pH and salt concentration of mAb formulations are critical determinants of product stability at the interface between the diluent and human serum or plasma. Notably, all the insoluble protein aggregates from the dextrose-mAb-serum (or plasma) mixtures at acidic pH (pH ≤ 6.5) were composed of abundant plasma proteins that are characterized by a pI of 5.4–6.7 (Table 3). This includes complement proteins C3, C4, and C5, complement factor H, apolipoprotein B-100, fibronectin, plasminogen, prothrombin, C4b-binding protein, serum albumin, and IgM (Figure 5c). These abundant plasma proteins may have aggregated and/or precipitated at the pI and low ionic strength as a result of reduced electrostatic repulsive force between molecules.12,13 Apolipoproteins (e.g., apolipoprotein B-100) may also contribute to the aggregation through interaction with the small “seeding” mAb aggregates that are potentially presented after dilution into dextrose solution.14–16
Precautions should be taken when interpreting the data presented herein because the experimental conditions used in this study are not necessarily reflective of the true microenvironment at the blood-IV interface. In clinical settings, a fluid (5% dextrose) containing mAb proteins is continuously delivered into a vein and eventually mixed with blood. A small amount of protein aggregates, if transiently formed at the interface, is expected to be reversible under physiological pH 7.4 and ionic strength (Figures 3E and 4C). However, the insoluble protein aggregates may be sustainable in the bloodstream; if this occurs, it may trigger adverse infusion reactions.17 In support of this notion, there is evidence that IV solutions do not mix rapidly with blood due to an intravascular streaming phenomenon, which was linked to focal toxicity in vivo.18,19 Our in vitro data showed an increase of cytokine release from healthy human peripheral blood mononuclear cells when incubated with protein aggregates derived from therapeutic mAbs (mAb-1, mAb-3, mAb-7 and mAb-8) (Supplement III). Additional studies are warranted to develop more clinically relevant models resembling the dynamic nature of the bloodstream, which can help assess product stability at the blood-IV interface and functional outcomes if protein aggregation occurs in the clinical settings. Our work highlights the importance of assessing compatibility of therapeutic mAbs with IV diluents and human plasma during product development.
Materials and methods
Therapeutic mAbs and reagents
Therapeutic mAbs and the United States Pharmacopoeia (USP) grade infusion solutions (0.9% sodium chloride, 5% dextrose, 5% dextrose and 0.45% sodium chloride, and lactated Ringer’s solution that contains 0.6% sodium chloride) were purchased from the National Institutes of Health (NIH) Pharmacy. Pooled normal human sera (Cat# S1-100ML) were from Millipore and pooled normal human plasma containing Na-EDTA as anticoagulant (Cat# IPLA-N) from Innovative Research. Aliquots were stored at −80°C. Prior to use, frozen vials were thawed at 37°C for 5 minutes and centrifuged at 4°C at 21,000 g for 5 minutes to remove particulates, if any, and low-density lipids. The resultant supernatants were immediately used in the following experiments. All chemicals were from Sigma unless specified otherwise.
In vitro assays for assessing compatibility of mAbs with diluent and human serum or plasma
The assays were performed as described previously10 in which aliquots of therapeutic mAbs were diluted into a diluent solution followed by addition of human serum or plasma at ratios that simulate the interface of blood and the IV infusion solution. The pH and salt concentrations were adjusted as indicated. Briefly, aliquots of mAb solutions (10 µL of mAbs formulated at 20–25 mg/mL or 20 µL of mAbs formulated at 10 mg/mL) were diluted into 300 µL of diluent in 1.5 mL Eppendorf tubes, and then mixed with 10 µL human serum or plasma by gently vortexing. The mixed solutions were incubated at 25°C for 30 minutes in an Eppendorf ThermoMixer at 300 rpm mixing frequency. The resultant insoluble protein pellets, if any, were collected by centrifugation at 25°C and 21,000 g for 3 minutes and washed three times with 500 µL of the same diluent solution. The pellets were dissolved in 20 µL NuPAGE LDS sample buffer (ThermoFisher, cat. no. NP0007) containing 50 mM dithiothreitol (DTT) (ThermoFisher, cat. no. A39255), incubated at 70°C for 10 minutes, and resolved using NuPAGE 4-12% Bis-Tris gels (ThermoFisher, cat. no. NP0322BOX). The Odyssey One-Color Protein Molecular Weight Marker (LI-CORE, Cat# 928–40000) was used as a protein ladder. Gels were stained using the Pierce Mini Gel Power Staining Kit (ThermoFisher, cat. no. 22840) and scanned by an Odyssey infrared imager (LI-COR).
Liquid chromatography–tandem mass spectrometry
To identify protein composition, insoluble protein aggregates were subjected to in-solution or in-gel digestion followed by LC-MS/MS analysis. For in-solution digestion, protein pellets were dissolved in 100 µL of 6 M guanidine hydrochloride in 100 mM Tris, 1 mM EDTA, pH 8.5. Reduction and alkylation of cysteine residues were achieved by adding 1 µL 0.5 M DTT and incubation at 37°C for 30 minutes followed by adding 6 µL 375 mM iodoacetamide (ThermoFisher, cat. no. A39271) and incubating for an additional 30 minutes in dark. After adding 2 µL 0.5 M DTT and incubation at room temperature for 5 minutes to terminate the reaction, 110 µL ice cold 20% trichloroacetic acid was mixed with the solution on ice for 5 minutes to allow precipitation of both proteins and guanidine. After centrifugation at 4°C and 21,000 g for 3 minutes, guanidine in the resultant pellets was removed by washing three times with an ice-cold 0.5 mL 1:1 (v/v) mixture of ethanol and ethyl acetate. The protein pellets were air dried and dissolved in 100 µL 25 mM ammonium bicarbonate. Tryptic protein digestion was initiated by adding 0.5 µg trypsin and incubated at 37°C overnight; the digestion was stopped by adding 5 µL 10% formic acid. The solution was transferred into an HPLC vial. The tryptic peptides were dried in a Savant SpeedVac concentrator (ThermoFisher) and dissolved in 10 µL 3% acetonitrile in 0.1% formic acid.
For in-gel digestion, Coomassie-stained protein bands were retrieved from SDS-PAGE gels and incubated with trypsin in 25 mM ammonium bicarbonate overnight at 37°C, followed by extraction of peptides with 60% acetonitrile in 0.1% formic acid. The resultant peptides were dried in the SpeedVac concentrator, and then dissolved in 10 µL or 20 µL of 3% acetonitrile in 0.1% formic acid based on the band intensity.
LC-MS/MS analyses were performed on an Agilent 1260 HPLC-Chip nano-electrospray-ionization 6520 Q-TOF tandem MS spectrometry using the data-dependent auto-MS/MS acquisition method in MassHunter software (version B.05.00) as described previously.10 Solvent A was 0.1% formic acid in water and Solvent B was 0.1% formic acid in 95% acetonitrile. Each sample injection was bracketed with solvent A injections to avoid sample carryover. Briefly, for the in-solution digestion samples, 8 µL peptides was injected onto a 500 nL enrichment column in a Zorbax 300A SB-C18 150 mm customizable chip (Agilent, cat. no. G4240-63001 SPQ361) at a flow rate of 2.5 µL/min in 3% Solvent B, then separated at 0.3 μL/min with a 55-minute gradient of 3–25% Solvent B in 35 minutes, 25–40% Solvent B in 10 minutes, 40–90% Solvent B in 5 minutes, and 90% Solvent B for 5 minutes. For the in-gel digestion samples, analytical duplicate injections were performed, where 4 or 8 µL of the 10 or 20 µL samples, respectively, were injected and separated on a ProtID-Chip-150 II 300A C18 150 mm chip (Agilent, Cat. No. G4240-62006) at the same flow rates, but with a short 35-minute gradient of 3–25% Solvent B in 20 minutes, 25–40% Solvent B in 5 minutes, 40–90% Solvent B in 4 minutes, and 90% Solvent B for 6 minutes. Protein identifications were achieved by searching Swiss-Prot Homo sapiens protein database using Spectrum Mill MS Proteomics Workbench software (version B.04.00; Agilent Technologies) with a global false discovery rate of 1%.
Acknowledgments
This work was supported by FDA intramural research funding. We thank Kathleen Clouse for suggestion on including lactated Ringer’s solution in this study. We thank Julianne Twomey, William Bozza and Serge Beaucage for critical reading of the manuscript.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Disclaimer
The comments in this article reflect the views of the author and are based on the experimental data and a survey of the related scientific publications. Those comments should not be construed to represent FDA’s views or policies on the subject matter.
Supplementary material
Supplemental data for this article can be accessed on the publisher’s website.
References
- 1.Khalil DN, Smith EL, Brentjens RJ, Wolchok JD.. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat Rev Clin Oncol. 2016;13:273–11. doi: 10.1038/nrclinonc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tabas I, Glass CK. Anti-inflammatory therapy in chronic disease: challenges and opportunities. Science. 2013;339:166–72. doi: 10.1126/science.1230720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Graham BS, Ambrosino DM. History of passive antibody administration for prevention and treatment of infectious diseases. Curr Opin HIV AIDS. 2015;10:129–34. doi: 10.1097/COH.0000000000000154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weiner GJ. Building better monoclonal antibody-based therapeutics. Nat Rev Cancer. 2015;15:361–70. doi: 10.1038/nrc3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Herceptin prescribing information [cited 2019 Sept 22]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5345lbl.pdf.
- 6.Avastin prescribing information [cited 2019 Sept 22] . https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125085s331lbl.pdf.
- 7.Remicade prescribing information (1998) [cited 2019 Sept 22]: https://www.accessdata.fda.gov/drugsatfda_docs/label/1998/inflcen082498lb.pdf.
- 8.Remicade prescribing information (2018) [cited 2019 Sept 22]: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103772s5385lbl.pdf.
- 9.Arvinte T, Palais C, Green-Trexler E, Gregory S, Mach H, Narasimhan C, Shameem M. Aggregation of biopharmaceuticals in human plasma and human serum: implications for drug research and development. MAbs. 2013;5:491–500. doi: 10.4161/mabs.24245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Luo S, Zhang B. Dextrose-mediated aggregation of therapeutic monoclonal antibodies in human plasma: implication of isoelectric precipitation of complement proteins. MAbs. 2015;7:1094–103. doi: 10.1080/19420862.2015.1087636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kinderman F, Yerby B, Jawa V, Joubert MK, Joh NH, Malella J, Herskovitz J, Xie J, Ferbas J, McBride HJ, et al. Impact of precipitation of antibody therapeutics after subcutaneous injection on pharmacokinetics and immunogenicity. J Pharm Sci. 2019;108:1953–63. doi: 10.1016/j.xphs.2019.01.015. [DOI] [PubMed] [Google Scholar]
- 12.Burgess RR. Protein precipitation techniques. Methods Enzymol. 2009;463:331–42. [DOI] [PubMed] [Google Scholar]
- 13.Maurer RW. Sandler SI and Lenhoff AM. Salting-in characteristics of globular proteins. Biophys Chem. 2011;156:72–78. doi: 10.1016/j.bpc.2011.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Demeule B, Palais C, Machaidze G, Gurny R, Arvinte T. New methods allowing the detection of protein aggregates: a case study on trastuzumab. MAbs. 2009;1:142–50. doi: 10.4161/mabs.1.2.7632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Arvinte T, Poirier E, Palais C. Prediction of aggregation In Vivo by studies of therapeutic proteins in human plasma. In: Rosenberg A, Demeule B, editors. Biobetters: protein engineering to approach the curative. New York: Springer New York; 2015. p. 91–104. [Google Scholar]
- 16.Arvinte T, Cudd A, Palais C, Poirier E. CHAPTER 11 The Formulation of Biological Molecules. Pharmaceutical Formulation: The Science and Technology of Dosage Forms: The Royal Society of Chemistry, 2018:288–316.
- 17.Joubert MK, Hokom M, Eakin C, Zhou L, Deshpande M, Baker MP, Goletz TJ, Kerwin BA, Chirmule N, Narhi LO, et al. Highly aggregated antibody therapeutics can enhance the in vitro innate and late-stage T-cell immune responses. J Biol Chem. 2012;287:25266–79. doi: 10.1074/jbc.M111.330902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz RJ, Dedrick RL, Boretos JW, Oldfield EH. Blacklock JB and Doppman JL. Mixing studies during intracarotid artery infusions in an in vitro model. J Neurosurg. 1986;64:277–83. doi: 10.3171/jns.1986.64.2.0277. [DOI] [PubMed] [Google Scholar]
- 19.Blacklock JB, Wright DC, Dedrick RL, Blasberg RG, Lutz RJ, Doppman JL, Oldfield EH. Drug streaming during intra-arterial chemotherapy. J Neurosurg. 1986;64:284–91. doi: 10.3171/jns.1986.64.2.0284. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Herceptin prescribing information [cited 2019 Sept 22]. https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/103792s5345lbl.pdf.
- Avastin prescribing information [cited 2019 Sept 22] . https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/125085s331lbl.pdf.


