Abstract
Background
Immune checkpoint inhibitors (icis) are increasingly being used in clinical practice, improving outcomes for cancer patients. Preclinical models showed significant interaction between the gut microbiome (gm) and response to icis. However, that interaction remains unclear in clinical practice.
Methods
We performed a systematic review in medline to determine
■ whether antibiotics affect ici efficacy,
■ whether baseline gm composition and ici efficacy show any correlations,
■ whether baseline gm composition and emergence of immune-related adverse events (iraes) show any correlations, and
■ whether gm manipulation can alleviate the iraes.
Included publications had to be written in English or French and had to describe a quantifiable link between gm composition or its modification and the response to icis or the occurrence of iraes, or both.
Results
Of 1451 articles published before December 2018, 13 publications met the inclusion criteria. Five full-text articles and two abstracts highlighted a negative effect of antibiotics on ici efficacy. The composition of the gm was associated with ici efficacy in five full-text articles and one abstract, and with iraes in two full-text articles. In 2 cases, fecal microbiota transplantation was reported to reduce immune colitis.
Conclusions
If possible, antibiotics should be avoided before ici treatment because of their negative effect on ici anticancer efficacy. No specific commensal bacterium was associated with ici efficacy, but an intact gm with high bacterial diversity and a good ratio of “responder-associated” bacteria to “non-responder-associated” bacteria seem to be correlated with better patient outcomes. Fecal microbiota transplantation is a promising technique for reducing ici-associated colitis.
Keywords: Antibiotics, cancer immunotherapy, fecal microbiota transplantation, immune checkpoint inhibitors, microbiome
INTRODUCTION
The human gut microbiome (gm) is composed of more than 100 trillion bacteria1. The gm is highly individual, but can be affected by several external factors such as diet2, antibiotics3,4, and treatment with proton-pump inhibitors5.
The composition of the gm is known to play a key role in the development of multiple diseases6,7 including inflammatory bowel disease8,9, diabetes mellitus10, and obesity2. More recently, the gm composition has also been implicated in the development of cancers such as colorectal cancer11: the presence of certain bacteria, such as Fusobacterium nucleatum appears to be a predictive factor in colorectal cancer development12,13. Furthermore, the gm could be associated with response to chemotherapy. The gm has been shown to promote an anticancer immune response to cyclophosphamide14, and an intact gm was associated with the efficacy of CpG–oligonucleotide immunotherapy and platinum chemotherapy in some cancer models15. The effect of the gm on the immune system is increasingly being explored, particularly in this era of new immune-modulating agents.
Immune checkpoint inhibitors (icis) improve outcomes for patients with cancer. Antibodies targeting ctla-4, PD-1, and PD-L1 are routinely used in multiple cancers, including advanced non-small-cell lung carcinoma (nsclc)16, renal cell carcinoma (rcc)17,18, urothelial carcinoma19,20, melanoma21, and squamous cell carcinoma of the head and neck22. However, objective response rates (orrs) are modest, not exceeding 20%–30%16,17,19,23, and to date, no efficient biomarker to predict the efficacy of icis has been discovered.
Preclinical models show that the composition of the gm and its modification in mouse models can influence the efficacy of icis24,25 or the emergence of immune-related adverse events (iraes)26. Moreover, experimental interventions such fecal microbiota transplantation (fmt) might, in animals, restore the response to icis27,28 and reduce iraes, particularly colitis24. Whether such effects would be observed in humans currently remains unknown. In the present review, we evaluated how gm modification by antibiotics might affect ici efficacy in humans and explored the associations between the composition of the gm and the efficacy and toxicity of icis.
METHODS
This systematic review was performed based on the prisma (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) guidelines29.
The first objective of the review was to evaluate the effect of gm modification by antibiotics on the efficacy of icis, based on orr, progression-free survival (pfs), and overall survival (os) in patients treated for a malignancy with icis (without other cytotoxic agents). The second objective was to analyze the association between the composition of the gm and ici efficacy (based on orr) and toxicity (based on the occurrence of iraes).
We included studies that evaluated icis (anti–ctla-4, anti–PD-1 and anti–PD-L1) in adult patients with solid cancers and that described a quantifiable link between the composition or modification (by antibiotics, probiotics, fmt, etc.) of the gm and the response to the ici or the occurrence of iraes.
To that end, we searched medline using combinations of the terms “cancer immunotherapy” or “immune checkpoint inhibitors” and “microbiome” or “probiotic” or “antibiotic” or “dysbiosis.” Subsequently, the reference lists of included papers were screened to find other studies that met the inclusion criteria. We included only publications written in French or English. All articles published before 9 December 2018 were reviewed. Articles were selected based on a review of the abstract; the full text was subsequently analyzed. The analysis included only full-text articles or abstracts that, through clinical trials or reports, evaluated a link between the gm and icis. Reviews, comments, and expert opinions were excluded, but as already mentioned, reference lists in such items were screened to find other publications.
Only the data published in the article and its supplementary contents were gathered; no verification was sought from the authors of the various studies.
The variables analyzed were found in all the included studies: number of patients, type of icis, cancer type, gm composition, methods used to assess the gm composition, the intervention to the gm (if applicable), and any quantifiable effect of the gm (or its modification) on the efficacy of the ici in terms of orr, pfs, and os, or on the toxicity of the ici in terms of the occurrence of iraes.
The aim of this systematic review was to identify all studies meeting the inclusion criteria, not to perform a quantitative synthesis of the results.
RESULTS
Included Articles
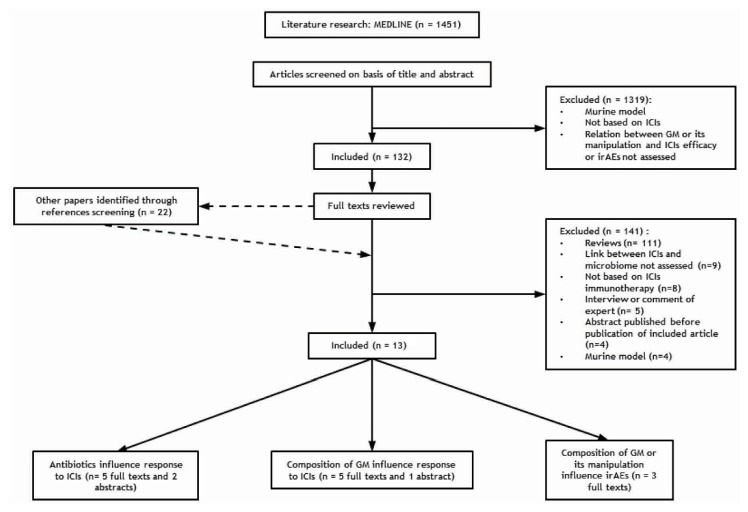
Figure 1 illustrates the selection of the papers as a flow diagram.
FIGURE 1.
Flow diagram of the literature search. ICI = immune checkpoint inhibitor; GM = gut microbiome; irAEs = immune-related adverse events.
We found ten full-text papers and three abstracts that met the inclusion criteria. Five full-text articles27,30–33 and two abstracts34,35 analyzed the influence of antibiotics on ici efficacy; five full-text articles and one abstract evaluated the influence of the gm composition on ici efficacy; and three full-text articles explored the influence of the gm on iraes.
Impact of Antibiotics on ICI Efficacy
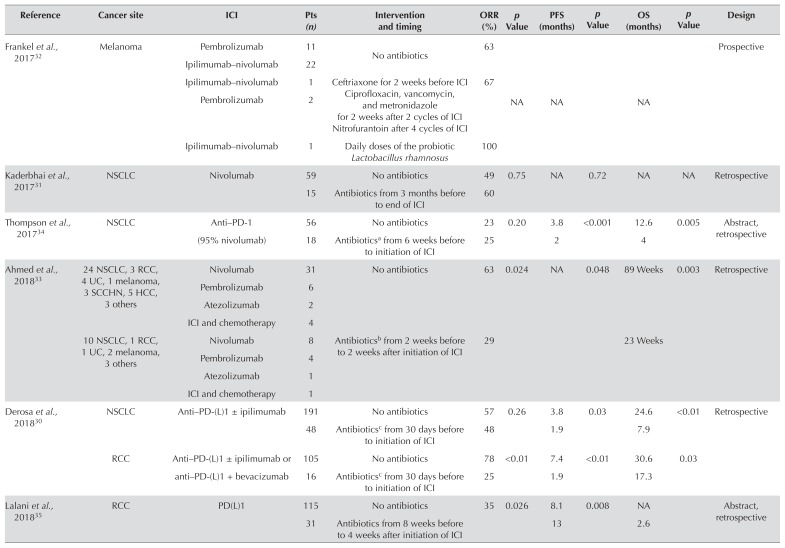
Table I summarizes the articles and abstracts that considered the effect of antibiotics on ici efficacy. One study was prospective32; the remaining studies were retrospective. All publications presented results for two groups, an antibioticnaïve (ABn) group and an antibiotic-treated [ABt (before or during receipt of icis)] group. Patients generally received oral antibiotics for common indications (dental, urinary, and pulmonary infections). Of the 997 patients included in the publications, 784 were in the ABn group, and 213 were in the ABt group. Most of the patients had nsclc (n = 561) or rcc (n = 338). All had received at least one of anti–PD-1 or anti–PD-L1 or anti–ctla-4 therapy.
TABLE I.
Human studies that assess the efficacy of immunotherapy in patients receiving or not receiving antibiotics near in time to the administration of immune checkpoint inhibitors (ICIs)
| Reference | Cancer site | ICI | Pts (n) | Intervention and timing | ORR (%) | p Value | PFS (months) | p Value | OS (months) | p Value | Design |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Frankel et al., 201732 | Melanoma | Pembrolizumab | 11 | No antibiotics | 63 | Prospective | |||||
| Ipilimumab–nivolumab | 22 | ||||||||||
| Ipilimumab–nivolumab | 1 | Ceftriaxone for 2 weeks before ICI | 67 | ||||||||
| Pembrolizumab | 2 | Ciprofloxacin, vancomycin, and metronidazole for 2 weeks after 2 cycles of ICI Nitrofurantoin after 4 cycles of ICI |
NA | NA | NA | ||||||
| Ipilimumab–nivolumab | 1 | Daily doses of the probiotic Lactobacillus rhamnosus | 100 | ||||||||
|
| |||||||||||
| Kaderbhai et al., 201731 | NSCLC | Nivolumab | 59 | No antibiotics | 49 | 0.75 | NA | 0.72 | NA | NA | Retrospective |
| 15 | Antibiotics from 3 months before to end of ICI | 60 | |||||||||
|
| |||||||||||
| Thompson et al., 201734 | NSCLC | Anti–PD-1 | 56 | No antibiotics | 23 | 0.20 | 3.8 | <0.001 | 12.6 | 0.005 | Abstract, retrospective |
| (95% nivolumab) | 18 | Antibioticsa from 6 weeks before to initiation of ICI | 25 | 2 | 4 | ||||||
|
| |||||||||||
| Ahmed et al., 201833 | 24 NSCLC, 3 RCC, 4 UC, 1 melanoma, 3 SCCHN, 5 HCC, 3 others | Nivolumab | 31 | No antibiotics | 63 | 0.024 | NA | 0.048 | 89 Weeks | 0.003 | Retrospective |
| Pembrolizumab | 6 | ||||||||||
| Atezolizumab | 2 | ||||||||||
| 10 NSCLC, 1 RCC, 1 UC, 2 melanoma, 3 others | ICI and chemotherapy | 4 | |||||||||
| Nivolumab | 8 | Antibioticsb from 2 weeks before to 2 weeks after initiation of ICI | 29 | 23 Weeks | |||||||
| Pembrolizumab | 4 | ||||||||||
| Atezolizumab | 1 | ||||||||||
| ICI and chemotherapy | 1 | ||||||||||
|
| |||||||||||
| Derosa et al., 201830 | NSCLC | Anti PD-(L)1 ± ipilimumab | 191 | No antibiotics | 57 | 0.26 | 3.8 | 0.03 | 24.6 | <0.01 | Retrospective |
| 48 | Antibioticsc from 30 days before to initiation of ICI | 48 | 1.9 | 7.9 | |||||||
| RCC | Anti PD-(L)1 ± ipilimumab or anti PD-(L)1 + bevacizumab | 105 | No antibiotics | 78 | <0.01 | 7.4 | <0.01 | 30.6 | 0.03 | ||
| 16 | Antibioticsc from 30 days before to initiation of ICI | 25 | 1.9 | 17.3 | |||||||
|
| |||||||||||
| Lalani et al., 201835 | RCC | PD(L)1 | 115 | No antibiotics | 35 | 0.026 | 8.1 | 0.008 | NA | Abstract, retrospective | |
| 31 | Antibiotics from 8 weeks before to 4 weeks after initiation of ICI | 13 | 2.6 | ||||||||
|
| |||||||||||
| Routy et al., 201827 | NSCLC | Anti PD-(L)1 | 103 | No antibiotics | NA | 2.8 | 0.571 | 15.3 | 0.001 | Retrospective | |
| 37 | Antibioticsd from 2 months before to 1 month after initiation of ICI | 3.5 | 8.3 | ||||||||
| RCC | Anti PD-(L)1 | 47 | No antibiotics | 7.4 | 0.012 | 27.9 | 0.154 | ||||
| 20 | Antibioticsd from 2 months before to 1 month after initiation of ICI | 4.3 | 23.4 | ||||||||
| UC | Anti PD-(L)1 | 30 | No antibiotics | 4.3 | 0.049 | NR | 0.098 | ||||
| 12 | Antibioticsd from 2 months before to 1 month after initiation of ICI | 1.8 | 11.5 | ||||||||
50% Quinolones.
Mostly cephalosporins, then vancomycin, then quinolones.
Mostly beta-lactam ± inhibitors, then quinolones or sulfonamides.
Beta-lactams ± inhibitors, fluoroquinolones, or macrolides.
Pts = patients; ORR = objective response rate; PFS = progression-free survival; OS = overall survival; NA = not assessed; NSCLC = non-small-cell lung carcinoma; RCC = renal cell carcinoma; UC = urothelial carcinoma; SCCHN = squamous cell carcinoma of the head and neck; HCC = hepatocellular carcinoma; anti PD-(L)1 = antibodies against PD-1 or PD-L1; NR = not reached.
Overall, use of antibiotics was associated with lower ici efficacy. In all publications, use of antibiotics in patients with rcc negatively affected pfs (1.9–4.3 months in ABt patients vs. 7.4–8.1 months in ABn patients) and os (17.3–23.4 months in ABt patients vs. 27.9–30.6 months in ABn patients). The orr was also higher in ABn than in ABt patients (35%–78% vs. 13%–25% respectively)27,30,35. In all publications (except for two that lacked os data), use of antibiotics in patients with nsclc negatively affected os (4–7.9 months in ABt patients vs. 12.6–24.6 months in ABn patients); no differences in pfs (1.9–3.5 months vs. 2.8–3.8 months) or orr (25%–60% vs. 23%–63%) were observed27,30,34. Data for patients with urothelial carcinoma were limited to a single article that showed poorer outcomes in ABt patients than in ABn patients in terms of pfs (1.8 months vs. 4.3 months) and os (11.5 months vs. not reached); orr data were not available27. Data for patients with melanoma were similarly limited to one prospective trial in which the response rate to icis was similar in the ABt and ABn groups (67% vs. 63%), and pfs and os data were not available. However, the comparison groups were unbalanced, with just 3 patients in the ABt group and 35 in the ABn group27.
Composition of the GM and Response to ICIs
Table II summarizes the articles and abstracts that considered the relationships between the gm composition and ici efficacy.
TABLE II.
Human studies that assess a link between the gut microbiome and response to immune checkpoint inhibitors (ICIs)
| Reference | Cancer site | ICI | Pts (n) | Assessment method | Sample type | Responders | Non-responders | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| (n) | Associated bacteria | (n) | Associated bacteria | ||||||
| Chaput et al., 201736 | Melanoma | Ipilimumab | 26 | 16S ribosomal RNA sequencing | Fecal | 9 | Firmicutes, Faecalibacterium prausnitzii L2-6, butyrate-producing bacterium L2-21, and Gemmiger formicilis ATCC 27749 | 17 | Bacteroidetes (genus Bacteroides) |
|
| |||||||||
| Ferris et al., 201737 | SCCHN | Nivolumab | 85 | 16S ribosomal RNA sequencing | Salivary | No association | |||
|
| |||||||||
| Frankel et al., 201732 | Melanoma | Pembrolizumab | 13 | Meta-genomic shotgun sequencing | Fecal | 24 | Streptococcus parasanguinis, Bacteroides caccae, Dorea formicigenerans (latter for pembrolizumab only) | 15 | Faecalibacterium prausnitzii, Holdemania filiformis, Bacteroides thetaiotaomicron (phylum Firmicutes) |
| Ipilimumab–nivolumab | 24 | ||||||||
| Nivolumab | 1 | ||||||||
| Ipilimumab | 1 | ||||||||
|
| |||||||||
| Gopalakrishnan et al., 201828 | Melanoma | Anti–PD-1 | 89 | Meta-genomic shotgun sequencing | Fecal | 30 | Clostridiales, Ruminococcaceae, Faecalibacterium, and high alpha diversity | 13 | Bacteroides thetaiotaomicron, Escherichia coli, and Anaerotruncus colihominis, low alpha diversity |
| Salivary | 54 | No association | 32 | Bacteroidales | |||||
|
| |||||||||
| Matson et al., 201838 | Melanoma | Anti–PD-1 | 38 | 16S Ribosomal RNA sequencing, | Fecal | 16 | High Enterococcus faecium, Collinsella aerofaciens, Bifidobacterium adolescentis, Klebsiella pneumoniae, Veillonella parvula, Parabacteroides merdae, Lactobacillus species, and Bifidobacterium longum | 26 | Ruminococcus obeum, Roseburia intestinalis |
| Ipilimumab | 4 | meta-genomic shotgun sequencing, quantitative PCR | |||||||
|
| |||||||||
| Routy et al., 201827 | NSCLC, RCC | Anti PD-(L)1 | 78 | Meta-genomic shotgun sequencing | Fecal | 42 | High Akkermansia muciniphila, Ruminococcus species, Alistipes species, and Eubacterium; low Bifidobacterium adolescentis, Bifidobacterium longum, and Parabacteroides distasonis | 36 | NA |
Pts = patients; ATCC = American Type Culture Collection; SCCHN = squamous cell carcinoma of the head and neck; anti PD-(L)1 = antibodies against PD-1 or PD-L1; PCR = polymerase chain reaction; NSCLC = non-small-cell lung carcinoma; RCC = renal cell carcinoma; NA = not assessed.
The studies analyzed 228 fecal samples and 171 saliva samples from patients who had not yet started icis (anti–ctla-4, anti–PD-1 or anti–PD-L1). Most of the patients providing fecal samples had advanced melanoma (n = 154); the rest had advanced nsclc and rcc. Of the 171 patients who provided saliva samples, 85 had squamous cell carcinoma of the head and neck, and 86 had melanoma. The patients were subsequently classified as responders or non-responders to icis, in most cases using recist (the Response Evaluation Criteria in Solid Tumors). The gm composition was assessed using any one or more of a variety of assays, including meta-genomic shotgun sequencing, quantitative polymerase chain reaction, and 16S ribosomal rna sequencing.
In all publications, authors found a significant association between the commensal microbial composition and clinical response27,28,32,36,38. The species of bacteria identified were different in the reports. For example, Matson et al.38 found that the species more abundant in responder–patients with melanoma included Bifidobacterium longum, Collinsella aerofaciens, and Enterococcus faecium. Routy et al.27 noted correlations between the clinical response to icis and the relative abundance of Akkermansia muciniphila in patients with nsclc and rcc. Gopalakrishnan et al.28 found a relative abundance of bacteria of the Ruminococcaceae family in responder–patients with melanoma. Chaput et al.36 observed longer pfs and os durations in patients with melanoma whose gm contained Faecalibacterium genii and other Firmicutes. In a prospective study, Frankel et al.32 showed that, depending on the ici, commensal f lora could be different in responders. In responders to nivolumab–ipilimumab, the gm was enriched for Faecalibacterium prausnitzii, Bacteroides thetaiotaomicron, and Holdemania filiformis. In responders to pembrolizumab, the gm was enriched for Dorea formicigenerans. Conversely, no association between the oral microbiome and ici efficacy was evident29,30.
Bacteria that have been reported to affect the response to icis are shown by phylum in Table III.
TABLE III.
Gut microbiome bacteria in responders and non-responders to immune checkpoint inhibitors, by phylum
| Responders | Phylum | ||||
|---|---|---|---|---|---|
| Firmicutes | Bacteroidetes | Actinobacteria | Proteobacteria | Verrucomicrobia | |
| Yes | Butyrate-producing bacterium L2-21, Clostridiales, Dorea formicigenerans, Enterococcus faecium, Eubacterium, Faecalibacterium, Faecalibacterium prausnitzii L2-6, Gemmiger formicilis, Lactobacillus, Ruminococcus, Streptococcus parasanguinis, Veillonella parvula | Alistipes, Bacteroides caccae, Parabacteroides merdae | Collinsella aerofaciens, Bifidobacterium adolescentis, Bifidobacterium longum | Klebsiella pneumoniae | Akkermansia muciniphila |
| No | Anaerotruncus colihominis, Faecalibacterium prausnitzii, Holdemania filiformis, Roseburia intestinalis, Ruminococcus obeum | Bacteroides species, Bacteroides thetaiotaomicron, Parabacteroides distasonis | Escherichia coli | ||
Pts = patients; ATCC = American Type Culture Collection.
The GM and irAEs
Table IV summarizes the articles that considered the association between iraes and the gm.
TABLE IV.
Human studies that assess associations of the composition of the gut microbiome
| Reference | Cancer site | ICI | Pts (n) | Sample type | Method | Adverse effect | ||
|---|---|---|---|---|---|---|---|---|
| Pts (n) | Type | Associated bacteria | ||||||
| Dubin et al., 201626 | Melanoma | Ipilimumab | 33 | Fecal | 16S ribosomal RNA sequencing | 13 | Colitis | Low Bacteroidaceae |
| Ipilimumab–nivolumab | 1 | 21 | None | High Bacteroidaceae, Rikenellaceae, Barnesiellaceae | ||||
| Chaput et al., 201736 | Melanoma | Ipilimumab | 26 | Fecal | 16S ribosomal RNA sequencing | 7 | Colitis | High Faecalibacterium prausnitzii L2-6, butyrate-producing bacterium L2-21, Gemmiger formicilis ATCC 27749 |
| 19 | None | High Bacteroides | ||||||
Of three articles, two27,36 found a correlation between the gm composition and the occurrence of ici-mediated colitis in patients with melanoma. Patients experiencing immune-mediated colitis showed a high quantity of Firmicutes in stool samples. In contrast, an abundance of Bacteroidetes was correlated with a low incidence of colitis in ici-treated patients26,36.
An article by Wang et al.39 reported two cases of using fmt to successfully treat ici-mediated colitis.
DISCUSSION
The data presented here strongly attest that use of antibiotics can reduce the efficacy of icis and affect outcomes in patients receiving icis for cancer. Use of antibiotics is associated with poorer orr, pfs, and os, regardless of cancer type. Those data suggest that modification of the gm can negatively affect the course of immunotherapy. Interestingly, proton-pump inhibitors—medications that can also alter the gut microbiota—were not observed by Routy et al.27 to affect pfs or os in patients with cancer, reflecting a specific effect of antibiotics.
The influence of antibiotics on ici efficacy could be explained in various ways. First, as discussed in the present review, modification of the gm by antibiotics could lead to the selection of bacterial species that negatively affect the response to icis. In preclinical mouse models, transplantation of certain species of “favourable” bacteria restored the response to icis after treatment with broad-spectrum antibiotics24,25. Similar research in human patients has not been yet been performed. A second way to elucidate the effect of antibiotics on the response to icis is the intrinsic anti-inflammatory effect of certain antibiotics. Indeed, quinolones lower the levels of pro-inflammatory cytokines (such as tumour necrosis factor α or interleukine 1)40 and macrolides reduce the T cell response, resulting in a potential antagonist effect against icis41. Moreover, independent of ici treatment, some antibiotics might also have an intrinsic negative effect on the clinical course of cancer by favouring carcinogenesis and metastases42.
Currently, determining the type of antibiotics that most strongly affect ici efficacy is difficult, although it seems logical that broad-spectrum antibiotics are likely to have the most significant effect. Indeed, Ahmed et al.33 reported that the orr was significantly lower in patients receiving broad-spectrum antibiotics than in those who were naïve to such antibiotics. In contrast, no difference was observed between patients who did and did not receive narrow-spectrum antibiotics. In addition, questions remain about the optimal time interval that has to pass after a course of antibiotic therapy before icis to treat cancer are started; however, we observed a similar negative effect of antibiotics in the Derosa et al.30 report (antibiotics administered within the 30 days before ici start) and in the Routy et al.27 report (antibiotics administered between 60 days before and 30 days after ici start), suggesting that the effect of antibiotics on the anticancer activity of icis could be deleterious for several months30. All those observations highlight the importance of balancing the benefits and inconveniences of starting antibiotics when considering immunotherapy in a patient.
Preclinical studies in mice demonstrated that certain bacteria are associated with ici efficacy25,26. In the present review, identifying specific species or phyla that are clearly associated with ici efficacy in a specific cancer or a variety of cancers is impossible. All the publications included in the review identified different commensal bacteria. That variation could be explained by the different assays used, the different baseline characteristics of patients, and differences in the medical and infectious history of the patients. Notably, the five major phyla of gm bacteria are present in both responder and non-responder patient groups (Table III). Conversely, the oral microbiome seems to have no correlation with ici efficacy28,37. It might be hypothesized that a gm with a high diversity of commensal bacterial28 and a favourable ratio between high-orr-associated species and low-orr-associated species38 should provide the best clinical outcomes, but would have to be confirmed in future clinical trials.
Even if ici-mediated colitis shares some clinical and histologic features with inflammatory bowel diseases such as Crohn disease, the gm compositions in the two entities are completely different, suggesting that the two diseases cannot be confused9. Specific bacterial species might be associated with development of immune-related adverse events, particularly colitis26,36. It is interesting to note that, as reported by Chaput et al.36, some bacterial species might be associated both with better clinical benefit from icis and with the occurrence of immune-related colitis—an observation that could reflect an epiphenomenon: the well-known positive correlation between ici efficacy and immune-mediated enterocolitis, as reported by Beck et al.40 in patients with rcc or melanoma treated with ipilimumab. However, that hypothesis also requires further prospective clinical trials.
Fecal microbiota transplantation is effective for the treatment of recurrent Clostridium difficile infection41 or ulcerative colitis42. It is a safe technique with a low rate of adverse events41–43. In preclinical models, fmt enriched in Bacteroides24 or Bifidobacterium species25 from responder mice into germ-free or ABt mice increased the efficacy of icis; fmt from non-responder mice did not improve the response to icis27,28. Enrichment in Bifidobacterium was also shown to reduce colitis in mice treated with ctla-4 inhibitors44. No data are available about fmt to improve ici efficacy in human patients. However, Wang et al.39 reported two cases of the use of fmt in ici-treated human patients to alleviate ici-mediated colitis. One patient had developed glucocorticoid-refractory colitis and experienced complete recuperation of symptoms 2 weeks after a single fmt. The second patient, a 78-year-old man, had been enrolled on an immunotherapy trial for prostate cancer. He also developed an immune-related refractory colitis. Complete resolution of symptoms occurred after 2 colonoscopic fmts. Even if that strategy appears promising, further trials are needed to explore the clinical implications of fmt.
The recommended treatments for high-grade iraes are, first, corticosteroids; if corticosteroids fail, biologic agents targeting tumour necrosis factor α are then administered. However, the latter agents can generate many metabolic and immunologic adverse events. In future, fmt might be used as the first-line therapy for high-grade immune-related colitis if that treatment’s efficacy and toxicity profile are proved to be more beneficial than current first-line therapies. Notably, Wang et al. did not specifically prepare or enrich the bacterial content used for their fmt. A major challenge should be to enhance control of immune-related colitis or even the efficacy of ici by the addition of beneficial bacteria species to the material used for fmt or probiotic administration.
Our review of the literature confirmed the negative effect of antibiotics on the anticancer efficacy of icis and highlighted potential correlations between the gm composition and ici efficacy and iraes. However, our work has multiple limitations. First, we searched for publications only in the medline system. However, we hypothesize that most relevant clinical trials were included in our investigation because of our complete scan of the references in the publications (n = 111) found by our initial research algorithm. Second, only papers written in English or French were included, although the number of articles in other languages was low. Third, the included trials focused on various cancers being treated with a variety of therapies (anti–ctla-4, anti–PD-1, and anti–PD-L1), regardless of the patient’s PD-L1 status, prior therapies, and baseline characteristics. Most of the trials were not prospective and included a small number of patients. Given the large number of variables, it is difficult to certify that the ABn and ABt groups were well balanced with respect to baseline characteristics. Furthermore, the types of antibiotics used were often unknown, as were the reasons for their initiation.
CONCLUSIONS
If possible, use of antibiotics must be avoided before or during ici treatment because of their negative effect on the anticancer efficacy of icis and on patient outcomes. However, we cannot precisely define the optimal timing of antibiotic exposure when necessary or prioritize the classes of antibiotics that should be avoided in patients being treated with icis. No specific commensal bacterium was found to be associated with high ici efficacy; however, an intact gm, with high bacterial diversity and a good ratio of “responder-associated” bacteria to “non-responder-associated” bacteria, seems to be associated with beneficial clinical outcomes. Fecal microbiota transplantation is a promising concept to reduce ici-associated colitis, but further investigation into current clinical practice is needed because of the heterogeneity of the relevant studies and the difficulty in obtaining accurate quantitative data.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 2.Tremaroli V, Backhed F. Functional interactions between the gut microbiota and host metabolism. Nature. 2012;489:242–9. doi: 10.1038/nature11552. [DOI] [PubMed] [Google Scholar]
- 3.De La Cochetiere MF, Durand T, Lepage P, Bourreille A, Galmiche JP, Doré J. Resilience of the dominant human fecal microbiota upon short-course antibiotic challenge. J Clin Microbiol. 2005;43:5588–92. doi: 10.1128/JCM.43.11.5588-5592.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME J. 2007;1:56–66. doi: 10.1038/ismej.2007.3. [Erratum in: ISME J 2013;7:456] [DOI] [PubMed] [Google Scholar]
- 5.Imhann F, Bonder MJ, Vich Vila A, et al. Proton pump inhibitors affect the gut microbiome. Gut. 2016;65:740–8. doi: 10.1136/gutjnl-2015-310376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper LV, Littman DR, Macpherson AJ. Interactions between the microbiota and the immune system. Science. 2012;336:1268–73. doi: 10.1126/science.1223490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9:313–23. doi: 10.1038/nri2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kostic AD, Xavier RJ, Gevers D. The microbiome in inflammatory bowel disease: current status and the future ahead. Gastroenterology. 2014;146:1489–99. doi: 10.1053/j.gastro.2014.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank DN, St Amand AL, Feldman RA, Boedeker EC, Harpaz N, Pace NR. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc Natl Acad Sci U S A. 2007;104:13780–5. doi: 10.1073/pnas.0706625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Qin J, Li Y, Cai Z, et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature. 2012;490:55–60. doi: 10.1038/nature11450. [DOI] [PubMed] [Google Scholar]
- 11.Schwabe RF, Jobin C. The microbiome and cancer. Nat Rev Cancer. 2013;13:800–12. doi: 10.1038/nrc3610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kostic AD, Chun E, Robertson L, et al. Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell Host Microbe. 2013;14:207–15. doi: 10.1016/j.chom.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kostic AD, Gevers D, Pedamallu CS, et al. Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res. 2012;22:292–8. doi: 10.1101/gr.126573.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Viaud S, Saccheri F, Mignot G, et al. The intestinal microbiota modulates the anticancer immune effects of cyclophosphamide. Science. 2013;342:971–6. doi: 10.1126/science.1240537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iida N, Dzutsev A, Stewart CA, et al. Commensal bacteria control cancer response to therapy by modulating the tumor microenvironment. Science. 2013;342:967–70. doi: 10.1126/science.1240527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–39. doi: 10.1056/NEJMoa1507643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motzer RJ, Escudier B, McDermott DF, et al. on behalf of the CheckMate 025 investigators. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373:1803–13. doi: 10.1056/NEJMoa1510665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Motzer RJ, Tannir NM, McDermott DF, et al. on behalf of the CheckMate 214 investigators. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378:1277–90. doi: 10.1056/NEJMoa1712126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387:1909–20. doi: 10.1016/S0140-6736(16)00561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Powles T, Eder JP, Fine GD, et al. MPDL3280A (anti–PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature. 2014;515:558–62. doi: 10.1038/nature13904. [DOI] [PubMed] [Google Scholar]
- 21.Hodi FS, O’Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–23. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ferris RL, Blumenschein G, Jr, Fayette J, et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N Engl J Med. 2016;375:1856–67. doi: 10.1056/NEJMoa1602252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–54. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vetizou M, Pitt JM, Daillère R, et al. Anticancer immunotherapy by ctla-4 blockade relies on the gut microbiota. Science. 2015;350:1079–84. doi: 10.1126/science.aad1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sivan A, Corrales L, Hubert N, et al. Commensal Bifidobacterium promotes antitumour immunity and facilitates anti–PD-L1 efficacy. Science. 2015;350:1084–9. doi: 10.1126/science.aac4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dubin K, Callahan MK, Ren B, et al. Intestinal microbiome analyses identify melanoma patients at risk for checkpoint-blockade-induced colitis. Nat Commun. 2016;7:10391. doi: 10.1038/ncomms10391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Routy B, Le Chatelier E, Derosa L, et al. Gut microbiome influences efficacy of PD-1–based immunotherapy against epithelial tumors. Science. 2018;359:91–7. doi: 10.1126/science.aan3706. [DOI] [PubMed] [Google Scholar]
- 28.Gopalakrishnan V, Spencer CN, Nezi L, et al. Gut microbiome modulates response to anti–PD-1 immunotherapy in melanoma patients. Science. 2018;359:97–103. doi: 10.1126/science.aan4236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liberati A, Altman DG, Tetzlaff J, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derosa L, Hellmann MD, Spaziano M, et al. Negative association of antibiotics on clinical activity of immune checkpoint inhibitors in patients with advanced renal cell and non-small-cell lung cancer. Ann Oncol. 2018;29:1437–44. doi: 10.1093/annonc/mdy103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaderbhai C, Richard C, Fumet JD, et al. Antibiotic use does not appear to influence response to nivolumab. Anticancer Res. 2017;37:3195–200. doi: 10.21873/anticanres.11680. [DOI] [PubMed] [Google Scholar]
- 32.Frankel AE, Coughlin LA, Kim J, et al. Metagenomic shotgun sequencing and unbiased metabolomic profiling identify specific human gut microbiota and metabolites associated with immune checkpoint therapy efficacy in melanoma patients. Neoplasia. 2017;19:848–55. doi: 10.1016/j.neo.2017.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ahmed J, Kumar A, Parikh K, et al. Use of broad-spectrum antibiotics impacts outcome in patients treated with immune checkpoint inhibitors. Oncoimmunology. 2018;7:e1507670. doi: 10.1080/2162402X.2018.1507670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson J, Szabo A, Arce-Lara C, Menon S. Microbiome and immunotherapy: antibiotic use is associated with inferior survival for lung cancer patients receiving PD-1 inhibitors [abstract P1.07-008] J Thorac Oncol. 2017;11(suppl 2):S1998. doi: 10.1016/j.jtho.2017.09.926. [DOI] [Google Scholar]
- 35.Lalani AKA, Xie W, Lin X, et al. Antibiotic use and outcomes with systemic therapy in metastatic renal cell carcinoma (mrcc) [abstract 607] J Clin Oncol. 2018;36 doi: 10.1200/JCO.2018.36.6_suppl.607. [Available online at: https://ascopubs.org/doi/10.1200/JCO.2018.36.6_suppl.607; cited 14 September 2019. [DOI] [Google Scholar]
- 36.Chaput N, Lepage P, Coutzac C, et al. Baseline gut microbiota predicts clinical response and colitis in metastatic melanoma patients treated with ipilimumab. Ann Oncol. 2017;28:1368–79. doi: 10.1093/annonc/mdx108. [DOI] [PubMed] [Google Scholar]
- 37.Ferris RL, Blumenschein G, Harrington K, et al. Evaluation of oral microbiome profiling as a response biomarker in squamous cell carcinoma of the head and neck: analyses from CheckMate 141 [abstract CT022] Cancer Res. 2017;77(suppl) [Available online at: https://cancerres.aacrjournals.org/content/77/13_Supplement/CT022; cited 14 September 2019] [Google Scholar]
- 38.Matson V, Fessler J, Bao R, et al. The commensal microbiome is associated with anti–PD-1 efficacy in metastatic melanoma patients. Science. 2018;359:104–8. doi: 10.1126/science.aao3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang Y, Wiesnoski DH, Helmink BA, et al. Fecal microbiota transplantation for refractory immune checkpoint inhibitor–associated colitis. Nat Med. 2018;24:1804–8. doi: 10.1038/s41591-018-0238-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beck KE, Blansfield JA, Tran KQ, et al. Enterocolitis in patients with cancer after antibody blockade of cytotoxic T-lymphocyte–associated antigen 4. J Clin Oncol. 2006;24:2283–9. doi: 10.1200/JCO.2005.04.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassam Z, Lee CH, Yuan Y, Hunt RH. Fecal microbiota transplantation for Clostridium difficile infection: systematic review and meta-analysis. Am J Gastroenterol. 2013;108:500–8. doi: 10.1038/ajg.2013.59. [DOI] [PubMed] [Google Scholar]
- 42.Imdad A, Nicholson MR, Tanner-Smith EE, et al. Fecal transplantation for treatment of inflammatory bowel disease. Cochrane Database Syst Rev. 2018;11:CD012774. doi: 10.1002/14651858.CD012774.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Brandt LJ, Aroniadis OC, Mellow M, et al. Long-term follow-up of colonoscopic fecal microbiota transplant for recurrent Clostridium difficile infection. Am J Gastroenterol. 2012;107:1079–87. doi: 10.1038/ajg.2012.60. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Yin Q, Chen L, Davis MM. Bifidobacterium can mitigate intestinal immunopathology in the context of ctla-4 blockade. Proc Natl Acad Sci U S A. 2018;115:157–61. doi: 10.1073/pnas.1712901115. [DOI] [PMC free article] [PubMed] [Google Scholar]