Abstract
Background
We assessed whether the presence and severity of common cancer symptoms are associated with the health utility score (hus) generated from the EQ-5D (EuroQol Research Foundation, Rotterdam, Netherlands) in patients with cancer and evaluated whether it is possible pragmatically to integrate routine hus and symptom evaluation in our cancer population.
Methods
Adult outpatients at Princess Margaret Cancer Centre with any cancer were surveyed cross-sectionally using the Edmonton Symptom Assessment System (esas) and the EQ-5D-3L, and results were compared using Spearman correlation coefficients and regression analyses.
Results
Of 764 patients analyzed, 27% had incurable disease. We observed mild-to-moderate correlations between each esas symptom score and the hus (Spearman coefficients: −0.204 to −0.416; p < 0.0001 for each comparison), with the strongest associations being those for pain (R = −0.416), tiredness (R = −0.387), and depression (R =−0.354). Multivariable analyses identified pain and depression as highly associated (both p < 0.0001) and tiredness as associated (p = 0.03) with the hus. The ability of the esas to predict the hus was low, at 0.25. However, by mapping esas pain, anxiety, and depression scores to the corresponding EQ-5D questions, we could derive the hus using partial esas data, with Spearman correlations of 0.83–0.91 in comparisons with direct EQ-5D measurement of the hus.
Conclusions
The hus derived from the EQ-5D-3L is associated with all major cancer symptoms as captured by the esas. The esas scores alone could not predict EQ-5D scores with high accuracy. However, esas-derived questions assessing the same domains as the EQ-5D-3L questions could be mapped to their corresponding EQ-5D questions to generate the hus, with high correlation to the directly measured hus. That finding suggests a potential approach to integrating routine symptom and hus evaluations after confirmatory studies.
Keywords: Value in cancer care, patient-reported outcomes
INTRODUCTION
Health utilities constitute a preference-based system for measuring patient-reported health-related quality of life (hrqol) that produces a single value, termed the “health utility score” (hus), ranging from 0.0 (death) to 1.0 (perfect health)1,2. The hus can be generated directly, through complicated trade-off testing specific to a disease or condition3, or indirectly, through instruments such as the EQ-5D (EuroQol Research Foundation, Rotterdam, Netherlands) 4. The EQ-5D measures health-state preferences based on 5 dimensions (mobility, self-care, usual activities, pain or discomfort, and anxiety or depression) that define multiple health states linked to a single preference value4; it is used to assess health interventions in patients with specific health conditions5,6. In cancer, hus data are collected mainly in clinical trials, with examples from breast7, gastrointestinal8,9, prostate10, and lung cancers12, and melanoma11. There is a paucity of routinely collected hus data for patients with cancer in the real-world non-trial setting13, which our previous work sought to correct14.
The Edmonton Symptom Assessment System (esas) is a valid and reliable assessment tool to screen and monitor for the presence and severity of 8 common symptoms experienced by patients with cancer, in addition to a general assessment of well-being. It has been used since the mid-1990s in palliative care, oncology, nephrology, and other disciplines15,16. In 2007, Cancer Care Ontario implemented a large-scale, system-wide approach to symptom screening and assessment across Ontario, based on the esas17. Outpatients with cancer have since routinely completed the esas at Ontario’s 14 regional cancer centres, mostly electronically using touch-screen stations or tablets18. That comprehensive rollout of esas for use in routine screening of cancer symptoms serves a base population of 14.6 million individuals.
The EQ-5D has been mapped to the European Organisation for Research and Treatment of Cancer’s hrqol tool, the 30-question Core Quality of Life Questionnaire in specific cancer sites19–22 and has been correlated with the Functional Assessment of Cancer Therapy—General (FACIT.org, Ponte Vedra, FL, U.S.A.), the Edmonton Functional Assessment Tool (Capital Health Authority, Edmonton, AB), and the Memorial Symptom Assessment Scale–Short Form (Memorial Sloan Kettering Cancer Center, New York, NY, U.S.A.) in the palliative setting23,24. Additionally, the EQ-5D has been successfully mapped to several disease-specific hrqol tools25–34, and the EQ-5D hus has been correlated with other health-utility scoring systems, such as the SF-6D (University of Sheffield, Sheffield, U.K.)35. However, the EQ-5D has not been comprehensively mapped to esas symptoms and certainly not for the entire spectrum of cancers. Because symptoms form such an important component of hrqol, such a correlation would serve to demonstrate the clinical utility of the EQ-5D.
The present study was designed primarily to assess the correlation of esas symptoms self-reported by patients having cancer (any cancer site) with the EQ-5D–derived hus in a large single-institution dataset from the Princess Margaret Cancer Centre (Toronto, ON). A secondary objective was to determine the most pragmatic way to incorporate a hus assessment into routine practice, in a place that had already incorporated the esas into routine practice for symptom assessment and management. In an unpublished pilot survey of a convenience sample of 100 patients with cancer at our institution, we found that, although 83% were willing to routinely complete patient-reported outcome measures, only 42% were willing to answer routine questions that appeared to be repetitive or duplicative. When shown the EQ-5D anxiety, depression, and pain questions followed by the corresponding esas questions, 72% of patients agreed or strongly agreed (on a 5-point Likert scale) that those questions appeared duplicative. We thus had a secondary goal of identifying a pragmatic approach to combining the esas and the EQ-5D, knowing that Cancer Care Ontario’s mandatory use of the esas was unlikely to change.
METHODS
This study was approved by the University Health Network’s institutional review board.
Study Population and Study Design
Adult cancer patients more than 18 years of age having any solid tumour or hematologic malignancy at any disease stage, who attended Princess Margaret Cancer Centre outpatient oncology clinics and agreed to complete the electronic questionnaires, were surveyed cross-sectionally. The primary cohort was assessed from June to September 2015, with a replication cohort of patients being assessed from June to September 2016. Eligibility criteria included any histologically confirmed malignancy, ability to provide informed consent, lack of any significant cognitive deficit, and ability to answer questionnaires on a touch-screen-based system.
Procedures
Consented patients were asked to complete the esas tool to screen for the presence and severity of pain, tiredness, nausea, depression, anxiety, drowsiness, appetite, and shortness of breath, and overall well-being. A visual assessment scale recorded symptoms from 0 (no symptoms) to 10 (worst possible symptoms). At the same time, patients were asked to complete the EQ-5D, which assesses symptoms (pain or discomfort, anxiety or depression) and physical functions (mobility, self-care, daily activities) on a 3-level scale (1, no limitations or symptoms; 2, some limitations or symptoms; 3, severe limitations or symptoms). Using Canadian health state preference weights, the values obtained for the 5 questions of EQ-5D-3L were converted to form a single hus36. Patients also completed a sociodemographic questionnaire.
Statistical Analysis
All analyses were conducted using the SAS software application (version 9.3: SAS Institute, Cary, NC, U.S.A.) or the R software application (version 2.0: The R Foundation for Statistical Computing, Vienna, Austria). All p values less than 0.05 were considered significant.
Descriptive summary statistics are reported to describe sociodemographic variables for the original and replication cohorts. Analyses then fall into 5 parts, in which the first 4 involve only the original cohort, and the last analysis involves both the original and the replication cohorts.
The purpose of the first (primary) analysis was to determine whether, across multiple cancers, the EQ-5D–derived hus could be accounted for, in part, by the physical symptoms (or their lack) commonly experienced by cancer patients. Statistically, we correlated esas scores for individual items or for the global score (sum of all scores) with the EQ-5D–derived hus in the original cohort. Specifically, using Spearman rank-correlation coefficients, individual and summed esas symptom scores were correlated, one by one, with the hus data derived from the EQ-5D37. The EQ-5D and esas values chosen for correlation had to fall within a 14-day period of each other.
The purpose of secondary analyses 2 and 3 was to determine whether the esas is adequate, by itself, to replace the EQ-5D in generating the hus. Theoretically, being able to make use of esas symptom data to derive utilities would then allow for all existing esas data since 2007 in Ontario to be used to derive the real-life hus in multiple new health interventions implemented since 2010; it would also provide proof-of-principle that combining symptom assessments with the EQ-5D would not necessarily require assessments of the same symptoms twice, because pain, depression, and anxiety measured by the EQ-5D are common elements of most symptom assessment tools.
Statistically, for analysis 2, we developed a multivariable model of esas scores and clinico-demographic variables to predict the hus. Using a stepwise selection approach that introduced variables one-by-one if they met a generous p value threshold of less than 0.10, a final model incorporating clinico-demographic and esas symptoms was developed that included all variables at p < 0.05.
Analysis 3 used the coefficient of determination (R2) to quantitatively assess the predictive ability of the model derived in analysis 2 for the hus. Adjusted R2 values accounted for the number of covariates being assessed, and residual plots were also performed. In brief, that method determines how close the data reside to the fitted regression line and compares the explained with the total variation by the factor or factors being evaluated.
Analyses 4 and 5 were to be performed if the predictive ability of the multivariable model of the esas on hus in analysis 3 was poor. To generate an alternative strategy (and based on pilot data showing a lack of patient acceptance of duplicate questioning), we had to avoid asking pain, anxiety, and depression questions twice should the esas and the EQ-5D be used together. In analysis 4, we mapped the individual 11-level esas questions in those 3 dimensions to the corresponding 3-level EQ-5D questions, with the goal of replacing the EQ-5D versions of those dimensions with the esas versions. We determined the best cut-points for shrinking the larger number of levels in the esas to the smaller number of levels in the EQ-5D. In the case of pain, the 11-level esas was mapped to the 3-level EQ-5D; subsequent analyses used mapped pain data to generate the hus, which was then correlated with hus data derived directly from the EQ-5D-3L. For depression and anxiety, either the greatest value of the two, or the sum of their esas scores, was mapped to the 3-level EQ-5D, as with the pain scores.
In analysis 5, using both the original cohort and the independent replication cohort, we determined whether the hus values generated from the mapping process for pain, anxiety, and depression correlated well with the standard hus generated based on the values from the original 5 dimensions.
RESULTS
Table I summarizes the clinico-demographic characteristics of the 764 patients in the original cohort and the 580 in the replication cohort. The overall participation rates for the original and replication cohorts were 81% and 76% respectively. The research ethics board did not permit the collection of any demographic data for the patients who refused to participate. Characteristics of the two cohorts were similar, except that patients in the replication cohort were slightly older, and that cohort also contained slightly fewer white patients, and more patients with thoracic, head-and-neck, and gynecologic malignancies. The original cohort included more patients with breast and gastrointestinal malignancies. Although the 0–10 distribution of esas symptoms was similar in both cohorts (p > 0.20, all comparisons), the mean hus was slightly lower in the replication cohort (median value: 0.78 vs. 0.83 in the original cohort; p = 0.03), possibly reflecting the differences in disease subsites.
TABLE I.
Clinical and demographic characteristics of the original and replication cohorts
| Characteristic | Cohort | p Value | |
|---|---|---|---|
|
| |||
| Original | Replication | ||
| Participants (n) | 764 | 580 | |
|
| |||
| Age (years) | 0.03 | ||
| Mean | 56.9±4.9 | 58.8±14.4 | |
| Median | 58 | 60.5 | |
| Range | 18–91 | 18.9–110 | |
|
| |||
| Sex [n (%)] | 0.78 | ||
| Women | 411 (54) | 317 (55) | |
| Men | 353 (46) | 263 (45) | |
|
| |||
| Education [n (%)] | 0.20 | ||
| No university or college | 267 (36) | 210 (39) | |
| University or college | 481 (64) | 324 (61) | |
| Missing or refused to answer | 16 | 46 | |
|
| |||
| Household income [n (%)] | 0.43 | ||
| ≤$100,000 | 342 (65) | 223 (62) | |
| >$100,000K | 183 (35) | 134 (38) | |
| Missing or refused to answer | 239 | 223 | |
|
| |||
| Marital status [n (%)] | 0.63 | ||
| Married or living with partner | 524 (70) | 376 (69) | |
| Other | 224 (30) | 171 (31) | |
| Missing or refused to answer | 16 | 33 | |
|
| |||
| Ethnicity [n (%)] | 0.02 | ||
| White | 575 (78) | 385 (72) | |
| Non-white | 166 (22) | 151 (28) | |
| Missing or refused to answer | 23 | 44 | |
|
| |||
| Disease site [n (%)] | <0.001 | ||
| Breast | 114 (15) | 71 (12) | |
| Gastrointestinal | 120 (15) | 64 (11) | |
| Genitourinary | 91 (12) | 73 (13) | |
| Gynecologic | 83 (11) | 101 (18) | |
| Hematologic | 110 (14) | 80 (14) | |
| Thoracic and head-and-neck | 174 (23) | 164 (28) | |
| Other | 92 (9) | 27 (5) | |
|
| |||
| Curative status [n (%)] | 0.09 | ||
| Palliative | 206 (27) | 175 (30) | |
For analysis 1, the 8 esas symptoms (pain, tiredness, nausea, depression, anxiety, drowsiness, loss of appetite, shortness of breath) and the global assessment of well-being all individually correlated (Spearman correlation coefficients) with the EQ-5D-3L–derived hus in both cohorts (Table II), with modest-to-moderate, but highly significant values ranging from R2 = −0.204 (for shortness of breath) to R2 = −0.416 (for pain), p < 0.0001 for each comparison). Correlations were strongest for pain, tiredness, and depression. After variable selection and adjustment for clinical variables, symptom scores for pain and depression remained highly associated with the hus (−0.02, p < 0.001); to a lesser extent, tiredness was also so associated (−0.01, p = 0.03, Table II).
TABLE II.
Correlation between each Edmonton Symptom Assessment System (ESAS) symptom and the EQ-5D-3La health utility score (HUS)
| ESAS symptom | Responses (n) | Spearman correlation with HUS | p Value | Univariable regression analysis | p Value | Multivariable regression analysis | p Value | ||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| Value | 95% CI | Value | 95% CI | ||||||
| Pain | 744 | −0.416 | <0.0001 | −0.03 | −0.03 to −0.02 | <0.001 | −0.02 | −0.03 to −0.01 | <0.001 |
|
| |||||||||
| Tiredness | 747 | −0.387 | <0.0001 | −0.02 | −0.03 to −0.02 | <0.001 | −0.01 | −0.02 to −0.001 | 0.03 |
|
| |||||||||
| Nausea | 746 | −0.231 | <0.0001 | −0.03 | −0.03 to −0.02 | <0.001 | 0.0002 | −0.01 to 0.01 | 0.97 |
|
| |||||||||
| Depression | 743 | −0.354 | <0.0001 | −0.03 | −0.03 to −0.02 | <0.001 | −0.02 | −0.02 to −0.01 | <0.001 |
|
| |||||||||
| Anxiety | 741 | −0.311 | <0.0001 | −0.02 | −0.03 to −0.02 | <0.001 | −0.002 | −0.01 to 0.01 | 0.64 |
|
| |||||||||
| Drowsiness | 746 | −0.306 | <0.0001 | −0.02 | −0.02 to −0.02 | <0.001 | 0.002 | −0.005 to 0.01 | 0.53 |
|
| |||||||||
| Appetite | 745 | −0.277 | <0.0001 | −0.02 | −0.03 to −0.02 | <0.001 | −0.002 | −0.01 to 0.004 | 0.50 |
|
| |||||||||
| Well-being | 738 | −0.345 | <0.0001 | −0.02 | −0.02 to −0.01 | <0.001 | 0.003 | −0.003 to 0.01 | 0.30 |
|
| |||||||||
| Shortness of breath | 746 | −0.204 | <0.0001 | −0.02 | −0.02 to −0.01 | <0.001 | 0.002 | −0.005 to 0.01 | 0.62 |
EuroQol Research Foundation, Rotterdam, Netherlands.
CI = confidence interval.
Table III shows the development of a multivariable model in analysis 2 (both univariable and multivariable data). After multivariable linear regression with stepwise selection, only sex and education were found to be statistically significant. Those variables were then used to adjust all subsequent multivariable analyses.
TABLE III.
Regression analysisa of clinico-demographic characteristics of the original cohort
| Characteristic | Univariable analysis | p Valueb | Global p valueb | Multivariable analysis | Global p valueb | ||
|---|---|---|---|---|---|---|---|
|
|
|
||||||
| Estimate | 95% CI | Estimate | 95% CI | ||||
| Age | 0.0001 | −0.0006 to 0.0009 | 0.69 | ||||
|
| |||||||
| Sex | <0.001 | <0.001 | |||||
| Women | Reference | Reference | |||||
| Men | 0.04 | 0.02 to 0.07 | 0.05 | 0.02 to 0.07 | |||
|
| |||||||
| Education | 0.01 | 0.008 | |||||
| No university or college | Reference | Reference | |||||
| University or college | 0.03 | 0.01 to 0.05 | 0.03 | 0.01 to 0.05 | |||
|
| |||||||
| Household income | 0.04 | ||||||
| ≤$100,000 | Reference | ||||||
| >$100,000 | 0.03 | 0.001 to 0.06 | |||||
|
| |||||||
| Marital status | 0.02 | ||||||
| Married or living with partner | Reference | ||||||
| Other | −0.03 | −0.05 to −0.004 | |||||
|
| |||||||
| Ethnicity | 0.08 | ||||||
| White | Reference | ||||||
| Other | −0.02 | −0.05 to 0.002 | |||||
|
| |||||||
| Disease site | 0.04 | ||||||
| Breast | Reference | ||||||
| Gastrointestinal | 0.02 | −0.02 to 0.05 | 0.41 | ||||
| Genitourinary | 0.06 | 0.02 to 0.10 | 0.005 | ||||
| Gynecologic | −0.02 | −0.06 to 0.03 | 0.43 | ||||
| Hematologic malignancies | 0.01 | −0.03 to 0.05 | 0.56 | ||||
| Thoracic and head-and-neck | 0.01 | −0.03 to 0.04 | 0.63 | ||||
| Other | 0.03 | −0.02 to 0.08 | 0.26 | ||||
|
| |||||||
| Curative status | |||||||
| Curative | Reference | ||||||
| Palliative | 0.04 | −0.01 to 0.06 | 0.08 | ||||
In the univariable analysis, linear regression modelling was used to individually compare each variable with the health utility score (HUS). After stepwise selection procedures (entry: 0.10; exit: 0.05), the multivariable model identified only sex and education as being independently associated with the HUS. This multivariable model serves as the baseline model for the analysis of the relationship between the various Edmonton Symptom Assessment System symptom factors and the HUS.
Significant values appear in boldface type.
CI = confidence interval.
Table IV summarizes the analysis 3 results of the predictive ability (coefficient of determination) of the various multivariable models that included sex and education in addition to individual esas symptoms and their combinations. The predictive ability of all 8 esas symptoms plus well-being was 0.25, which could be almost entirely attributed to pain, anxiety, and depression, which together had a predictive ability of 0.24. The predictive ability of each of the individual symptoms was lower, ranging from 0.08 to 0.18. Residual plots and adjusted R2 values confirm the lack of precision (that is, a large prediction interval) with which esas components or the overall esas can predict the hus.
TABLE IV.
Predictive abilitya of Edmonton Symptom Assessment (ESAS) items on the health utility score (HUS)
| ESAS item | R2 |
|---|---|
| Pain | 0.18 |
| Tiredness | 0.14 |
| Nausea | 0.10 |
| Depression | 0.18 |
| Anxiety | 0.13 |
| Drowsiness | 0.11 |
| Appetite | 0.11 |
| Shortness of breath | 0.08 |
| Well-being | 0.12 |
| Pain, depression, and anxiety | 0.24 |
| All items | 0.25 |
Calculation: R2 = Explained variation/Total variation. Each model evaluated the ESAS item as a linear variable and included sex and education as adjustment factors (identified in the multivariable analyses, Table III).
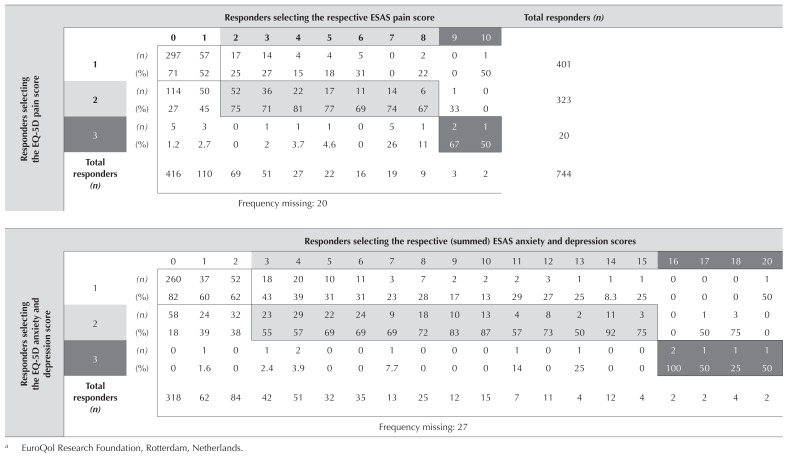
For analysis 4, we mapped the pain variable of the esas with the pain question of the EQ-5D-3L, and the depression and anxiety variables of the esas with the depression or anxiety question of the EQ-5D-3L (Table V). In the case of depression and anxiety, we were mapping two separate questions on the esas to a single question on the EQ-5D. We tried various options: looking at each individual esas question and its correlation with the depression or anxiety EQ-5D question; summing the esas scores for depression and anxiety; and using the higher score for either depression or anxiety as the score that would correlate with the three levels of EQ-5D. We found that the sum of the esas scores for depression and anxiety provided the best mapping to the corresponding single question on the EQ-5D-3L.
TABLE V.
Mapping of the Edmonton Symptom Assessment (ESAS) pain score or the sum of the ESAS anxiety and depression scores to the corresponding EQ-5Da symptom questions
EuroQol Research Foundation, Rotterdam, Netherlands.
Having completed the mapping process, we correlated the “mapped hus” and “standard hus” results, which resulted in Spearman correlation coefficients of 0.95 for the pain variable and 0.90 for the anxiety or depression variable (analysis 5). In a combined comparison mapping both questions, the result was a Spearman correlation coefficient of 0.83 (Table VI). We repeated the mapping of the pain and of the depression or anxiety variables in the replication cohort using a mapping procedure identical to that used for the original cohort. The Spearman coefficients in the replication cohort were at least as good as those in the original cohort, and the mapped hus, which involved the mapping of both symptom questions, resulted in a Spearman correlation coefficient of 0.91.
TABLE VI.
Correlation between the mapped health utility score (HUS) and the standard HUS
| Cohort | Definition of the mapped HUS | Spearman correlation coefficient with standard HUS | p Value | Observations (n) |
|---|---|---|---|---|
| Original | Only ESAS pain scores are mapped to the pain question on the EQ-5Da | 0.95 | <0.0001 | 744 |
| Original | The sum of the Edmonton Symptom Assessment (ESAS) anxiety and depression scores are mapped to the anxiety or depression question on the EQ-5Da | 0.90 | <0.0001 | 737 |
| Original | ESAS pain and sum of the ESAS depression and anxiety scores are mapped, respectively, to the pain and depression or anxiety questions on the EQ-5Da | 0.83 | <0.0001 | 735 |
| Replication | 0.91 | <0.0001 | 568 |
EuroQol Research Foundation, Rotterdam, Netherlands.
DISCUSSION
In this single-institution cross-sectional observational study of adult outpatients with cancer (any site), we compared the patient-reported esas data with scoring on the EQ-5D health utility tool. We first identified modest-to-moderate correlations of the individual symptom domains of the esas with the hus, suggesting that various physical symptoms contribute to the health state preference, but that no individual symptom drove the relationship exclusively, which accords with prior analyses exploring the discriminative ability of various utility assays8,35. We found that pain and depression had the strongest correlations, consistent with the findings of a prior study demonstrating that severity of depression is a significant predictor for lower health utility in patients with cancer (standardized coefficient β = −0.25)38.
With those correlations documented, the next question was whether the esas could ultimately replace the EQ-5D in generating utilities. From the beginning, we felt that that approach was not a good one, because the two measures capture different psychometric concepts. However, when trying to establish the importance of co-administration of the EQ-5D and the esas in routine practice, we were consistently asked by clinicians why we could not simply use one to estimate the other. We realized that, even if the esas is replaced with any other symptom assessment tool, the issue of duplication of symptom assessment will come up again; further, routine, systematic symptom assessment is likely to become common practice, based on a recent publication39. Our assessments document that the physical and emotional symptoms evaluated by the esas provide an incomplete picture of the domains important for health state preference. The lack in the esas tool of an evaluation of physical functioning, which, in contrast, comprises 3 of the 5 EQ-5D questions, helps to explain why the predictive ability of the esas is so low.
An alternative plan was then necessary to obtain the hus from routine clinical management. Although we documented the feasibility and patient acceptance of adding the EQ-5D to our current routine esas administration39, we realized that two major barriers accompany routine EQ-5D administration in the clinical setting. First, 3 of the EQ-5D questions relate to physical functioning, which is not evaluated in the esas. Separate to the present study, we are developing the use of those 3 questions as screening tools for a broader checklist of physical functioning issues that patients with cancer face, thereby generating a potential clinical management reason to ask them. Second, the two remaining questions, about pain or discomfort and depression or anxiety are duplicated in the esas (and other hrqol tools). Clinicians suggested that, despite years of painstaking psychometric evaluation of individual tools, we should simply substitute questions from one tool for questions from the other. The last section of the present manuscript tackles the latter issue.
Our original hypothesis was that we would not be able to substitute individual questions from one tool to the next, and we simply wanted to have relevant empiric evidence. To complicate matters, anxiety and depression are separate questions on the esas, but a single question on the EQ-5D. We were surprised that the mapping process for pain and depression or anxiety generated a hus that had a Spearman correlation of 0.83 in the original cohort and an even better 0.91 in the replication cohort. Thus, given the current results, we can no longer dismiss the possibility of substituting individual questions from one tool for similar questions on the next. We strongly caution against extrapolating the substitution of individual items into routine practice based on a single study; much work is required to determine whether the high correlations observed here hold true in other cancer populations and in different geographic and sociocultural regions.
Our study has limitations. First, this is a single-institution analysis of a very specific comparison between the esas and the EQ-5D. Larger, multi-institutional confirmatory studies are needed. Further, even if the original and replication cohorts were to be combined, the sample is not large enough to allow for sub-segmentation analyses, and those additional analyses are necessary to explore the conditions under which item substitution can take place. Second, we used the EQ-5D-3L because it had Canadian-based reference weights available at the time of study initiation. However, Canadian weights for the EQ-5D-5L are now available. The EQ-5D-5L is a more sensitive and responsive tool that does not demonstrate the ceiling effects demonstrated in the the EQ-5D-3L; future studies should focus on using the EQ-5D-5L to confirm our findings. Third, because the esas is already implemented in the clinic, we had to match the timing of the EQ-5D data collection. We were variably successful, because some patients requested that the “research” component be administered during a separate visit; however, to reduce patient burden, esas data are supposed to be routinely collected not more often than once every month. We decided on a 14-day window between the esas and EQ-5D assessments; however, hrqol and health states can change relatively quickly in patients with cancer. Our post hoc analysis found that 86% of our study’s esas and EQ-5D data points were collected within 3 days, and 77% were captured on the same day. Sensitivity analyses using those subsets of patients identified almost identical relationships in terms of the magnitude and direction of associations. A future replication study should find ways logistically to capture the data on the same day, where the chance of being in the same health state is exceedingly high. Fourth, all studies of this kind experience refusal by a segment of the population to participate, with no way of determining the extent of the participation bias; fortunately, our participation rates were relatively high. We also note that, because of provincial publicity concerning incidents of privacy breach at the time of the replication cohort, more patients in the replication cohort than in the original cohort refused to answer clinico-demographic questions, resulting in less-complete data. Fortunately, those refusals had little impact, because most of the analyses were performed using the original cohort, including the multivariable regression analyses that required complete covariate data. Lastly, although high correlations in the 0.83–0.91 range are promising, the optimal way of collecting utility data is still to use the original, unaltered EQ-5D instrument. In certain circumstances, the potential substitution of some of the variables, wordings, and categorizations with those from another validated instrument should be considered hypothesis-generating and will require multiple validations in various settings before such substitutions could be used in the clinical setting.
In summary, EQ-5D–derived health utility reflects physical and emotional symptoms that are collected through the esas, demonstrating that the EQ-5D is an appropriate tool to assess health preferences in the oncologic setting for multiple cancer sites, disease stages, and treatment intents. The relationships are, at best, representative of moderate correlation, meaning that the esas can never replace a specific tool, such as EQ-5D, that generates a hus. In contrast, by mapping almost identical questions from the esas and the EQ-5D and demonstrating high correlations for those two methods of surveying patients, we have now demonstrated an expected strategy to incorporate hus assessments into routine clinical practice and to base our health utility analyses on real-life data, rather than on clinical trial data alone. Although much research remains to be performed, incorporation into clinical practice could revolutionize the way that economic analyses are performed, because routine collection of patient-reported data could then be used to generate health utilities.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
We have read and understood Current Oncology’s policy on disclosing conflicts of interest, and we declare that we have none.
REFERENCES
- 1.Horsman J, Furlong W, Feeny D, Torrance G. The Health Utilities Index (hui): concepts, measurement properties and applications. Health Qual Life Outcomes. 2003;1:54. doi: 10.1186/1477-7525-1-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Earle CC, Chapman RH, Baker CS, et al. Systematic overview of cost–utility assessments in oncology. J Clin Oncol. 2000;18:3302–17. doi: 10.1200/JCO.2000.18.18.3302. [DOI] [PubMed] [Google Scholar]
- 3.Stiggelbout AM, Kiebert GM, Kievit J, Leer JW, Stoter G, de Haes JC. Utility assessment in cancer patients: adjustment of time tradeoff scores for the utility of life years and comparison with standard gamble scores. Med Decis Making. 1994;14:82–90. doi: 10.1177/0272989X9401400110. [DOI] [PubMed] [Google Scholar]
- 4.Kind P. Measuring the value of quality of life in cancer: an index based on eortc qlq-C30 [abstract 6013] J Clin Oncol. 2005;23 doi: 10.1200/jco.2005.23.16_suppl.6013. [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2005.23.16_suppl.6013; cited 2 November 2019. [DOI] [Google Scholar]
- 5.Flanagan W, McIntosh CN, Le Petit C, Berthelot JM. Deriving utility scores for co-morbid conditions: a test of the multiplicative model for combining individual condition scores. Popul Health Metr. 2006;4:13. doi: 10.1186/1478-7954-4-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gries KS, Regier DA, Ramsey SD, Patrick DL. Preferences for prostate cancer outcomes: a comparison of the patient perspective, the general population perspective, and a population at risk for prostate cancer. Value Health. 2016;19:218–25. doi: 10.1016/j.jval.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Paracha N, Thuresson PO, Moreno SG, MacGilchrist KS. Health state utility values in locally advanced and metastatic breast cancer by treatment line: a systematic review. Expert Rev Pharmacoecon Outcomes Res. 2016;16:549–59. doi: 10.1080/14737167.2016.1222907. [DOI] [PubMed] [Google Scholar]
- 8.Carter GC, King DT, Hess LM, et al. Health state utility values associated with advanced gastric, oesophageal, or gastrooesophageal junction adenocarcinoma: a systematic review. J Med Econ. 2015;18:954–66. doi: 10.3111/13696998.2015.1066380. [DOI] [PubMed] [Google Scholar]
- 9.Doherty MK, Leung Y, Su J, et al. Health utility scores from EQ-5D and health-related quality of life in patients with esophageal cancer: a real-world cross-sectional study. Dis Esophagus. 2018;31 doi: 10.1093/dote/doy058. [DOI] [PubMed] [Google Scholar]
- 10.Bremner KE, Mitsakakis N, Wilson L, Krahn MD. Predicting utility scores for prostate cancer: mapping the Prostate Cancer Index to the Patient-Oriented Prostate Utility Scale (porpus) Prostate Cancer Prostatic Dis. 2014;17:47–56. doi: 10.1038/pcan.2013.44. [DOI] [PubMed] [Google Scholar]
- 11.Nafees B, Lloyd AJ, Dewilde S, Rajan N, Lorenzo M. Health state utilities in non–small cell lung cancer: an international study. Asia Pac J Clin Oncol. 2016;13:e195–20. doi: 10.1111/ajco.12477. [DOI] [PubMed] [Google Scholar]
- 12.Hatswell AJ, Pennington B, Pericleous L, Rowen D, Lebmeier M, Lee D. Patient-reported utilities in advanced or metastatic melanoma, including analysis of utilities by time to death. Health Qual Life Outcomes. 2014;12:140. doi: 10.1186/s12955-014-0140-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Labbe C, Leung Y, Silva Lemes JG, et al. Real-world EQ5D health utility scores for patients with metastatic lung cancer by molecular alteration and response to therapy. Clin Lung Cancer. 2017;18:388–95. doi: 10.1016/j.cllc.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 14.Naik H, Howell D, Su S, et al. EQ-5D health utility scores: data from a comprehensive Canadian cancer centre. Patient. 2017;10:105–15. doi: 10.1007/s40271-016-0190-z. [DOI] [PubMed] [Google Scholar]
- 15.Bruera E, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (esas): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7:6–9. doi: 10.1177/082585979100700202. [DOI] [PubMed] [Google Scholar]
- 16.Hui D, Bruera E. The Edmonton Symptom Assessment System 25 years later: past, present, and future developments. J Pain Symptom Manage. 2017;53:630–43. doi: 10.1016/j.jpainsymman.2016.10.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dudgeon D, King S, Howell D, et al. Cancer Care Ontario’s experience with implementation of routine physical and psychological symptom distress screening. Psychooncology. 2012;21:357–64. doi: 10.1002/pon.1918. [DOI] [PubMed] [Google Scholar]
- 18.Pereira JL, Chasen MR, Molloy S, et al. Cancer care professionals’ attitudes toward systematic standardized symptom assessment and the Edmonton Symptom Assessment System after large-scale population-based implementation in Ontario, Canada. J Pain Symptom Manage. 2016;51:662–72. doi: 10.1016/j.jpainsymman.2015.11.023. [DOI] [PubMed] [Google Scholar]
- 19.Crott R, Briggs A. Mapping the qlq-C30 quality of life cancer questionnaire to EQ-5D patient preferences. Eur J Heal Econ. 2010;11:427–34. doi: 10.1007/s10198-010-0233-7. [DOI] [PubMed] [Google Scholar]
- 20.Crott R, Versteegh M, Uyl-de-Groot C. An assessment of the external validity of mapping qlq-C30 to EQ-5D preferences. Qual Life Res. 2013;22:1045–54. doi: 10.1007/s11136-012-0220-9. [DOI] [PubMed] [Google Scholar]
- 21.Khan I, Morris S, Pashayan N, Matata B, Bashir Z, Maguirre J. Comparing the mapping between EQ-5D-5L, EQ-5D-3L and the eortc-qlq-C30 in non–small cell lung cancer patients. Health Qual Life Outcomes. 2016;14:60. doi: 10.1186/s12955-016-0455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim SH, Jo MW, Kim HJ, Ahn JH. Mapping eortc qlq-C30 onto EQ-5D for the assessment of cancer patients. Health Qual Life Outcomes. 2012;10:151. doi: 10.1186/1477-7525-10-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xia Q, Hwang SS, Chang VT, et al. Validity, reliability and responsiveness of EuroQOL (EQ5D) in patients (Pts) receiving palliative care (pc) [abstract 8082] J Clin Oncol. 2005;23 doi: 10.1200/jco.2005.23.16_suppl.8082. [Available online at: https://ascopubs.org/doi/abs/10.1200/jco.2005.23.16_suppl.8082; cited 2 November 2019. [DOI] [Google Scholar]
- 24.Longworth L, Yang Y, Young T, et al. Use of generic and condition-specific measures of health-related quality of life in nice decision-making: a systematic review, statistical modelling and survey. Health Technol Assess. 2014;18:1–224. doi: 10.3310/hta18090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheung YB, Luo N, Ng R, Lee CF. Mapping the Functional Assessment of Cancer Therapy–Breast (fact-B) to the 5-level EuroQOL group’s 5-dimension questionnaire (EQ-5D-5L) utility index in a multi-ethnic Asian population. Health Qual Life Outcomes. 2014;12:180. doi: 10.1186/s12955-014-0180-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skaltsa K, Longworth L, Ivanescu C, Phung D, Holmstrom S. Mapping the fact-P to the preference-based EQ-5D questionnaire in metastatic castration-resistant prostate cancer. Value Health. 2014;17:238–44. doi: 10.1016/j.jval.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Ivanescu C, Longworth L, Skaltsa K, Holmstrom S. Mapping fact-P to EQ-5D in metastatic castration-resistant prostate cancer (mcrpc): performance of a previously developed algorithm when applied on a sample with a different disease stage. Value Health. 2014;17:A567. doi: 10.1016/j.jval.2014.08.1890. [DOI] [PubMed] [Google Scholar]
- 28.Lee CF, Luo N, Ng R, et al. Comparison of the measurement properties between a short and generic instrument, the 5-level EuroQOL group’s 5-dimension (EQ-5D-5L) questionnaire, and a longer and disease-specific instrument, the Functional Assessment of Cancer Therapy–Breast (fact-B) Qual Life Res. 2013;22:1745–51. doi: 10.1007/s11136-012-0291-7. [DOI] [PubMed] [Google Scholar]
- 29.Hettle R, Borrill J, Suri G, Wulff J. Generating health state utility values from fact–Ovarian data collected in a phase ii maintenance study in platinum sensitive recurrent ovarian cancer (Study 19): a comparison of mapping algorithms (abstract) Value Health. 2014;17:A646. doi: 10.1016/j.jval.2014.08.2341. [DOI] [PubMed] [Google Scholar]
- 30.Diels J, Hamberg P, Ford D, Price PW, Spencer M, Dass RN. Mapping fact-P to EQ-5D in a large cross-sectional study of metastatic castration-resistant prostate cancer patients. Qual Life Res. 2015;24:591–8. doi: 10.1007/s11136-014-0794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu EQ, Mulani P, Farrell MH, Sleep D. Mapping fact-P and eortc qlq-C30 to patient health status measured by EQ-5D in metastatic hormone-refractory prostate cancer patients. Value Health. 2007;10:408–14. doi: 10.1111/j.1524-4733.2007.00195.x. [DOI] [PubMed] [Google Scholar]
- 32.Kind P, Macran S. Eliciting social preference weights for functional assessment of cancer therapy-lung health states. Pharmacoeconomics. 2005;23:1143–53. doi: 10.2165/00019053-200523110-00006. [DOI] [PubMed] [Google Scholar]
- 33.Askew RL, Swartz RJ, Xing Y, et al. Mapping fact-Melanoma quality-of-life scores to EQ-5D health utility weights. Value Health. 2011;14:900–6. doi: 10.1016/j.jval.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 34.Colwell HH, Mathias SD, Turner MP, et al. Psychometric evaluation of the fact Colorectal Cancer symptom index (fcsi-9): reliability, validity, responsiveness, and clinical meaningfulness. Oncologist. 2010;15:308–16. doi: 10.1634/theoncologist.2009-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barton GR, Sach TH, Doherty M, Avery AJ, Jenkinson C, Muir KR. An assessment of the discriminative ability of the EQ-5Dindex, SF-6D, and EQ vas, using sociodemographic factors and clinical conditions. Eur J Health Econ. 2008;9:237–49. doi: 10.1007/s10198-007-0068-z. [DOI] [PubMed] [Google Scholar]
- 36.Bansback N, Tsuchiya A, Brazier J, Anis A. Canadian valuation of EQ-5D health states: preliminary value set and considerations for future valuation studies. PLoS One. 2012;7:e31115. doi: 10.1371/journal.pone.0031115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauke J, Kossowski T. Comparison of values of Pearson’s and Spearman’s correlation coefficients on the same sets of data. Quaestiones Geographicae. 2011;30:87–93. doi: 10.2478/v10117-011-0021-1. [DOI] [Google Scholar]
- 38.Fujisawa D, Inoguchi H, Shimoda H, et al. Impact of depression on health utility value in cancer patients. Psychooncology. 2016;25:491–5. doi: 10.1002/pon.3945. [DOI] [PubMed] [Google Scholar]
- 39.Naik H, Qiu X, Brown MC, et al. Cancer patients willingness to routinely complete the EQ-5D instrument at clinic visits. J Popul Ther Clin Pharmacol. 2016;23:e196–204. doi: 10.22374/1710-6222.23.1.1. [DOI] [PubMed] [Google Scholar]