Abstract
Aims: The ankle-brachial index (ABI) can be a prognostic marker for chronic kidney disease (CKD) in Western populations. Since there is little relevant evidence for Asian populations, we investigated the relationship between ABI and the risk of incident CKD in a general Japanese population.
Methods: The cohort included 5,072 participants aged 30–79 without a history of renal disease or cerebro-cardiovascular disease. Incident CKD, defined as an estimated glomerular filtration rate < 60 (mL/min/1.73 m2) and/or proteinuria (≥ 1 + on urine dipstick), was compared among participants grouped according to baseline ABI: 0.90–0.99, 1.00–1.09, 1.10–1.19, 1.20–1.29, and 1.30–1.39. Hazard ratios for incident CKD were estimated using a Cox proportional hazards model, with the ABI 1.10–1.19 group serving as the reference.
Results: The CKD incidence rate (/100 person-years) was 1.80 during the mean follow-up period of 5.1 years. The CKD incidence rate was 3.04 in the ABI category 0.90–0.99, 1.58 in ABI 1.00–1.09, 1.72 in ABI 1.10–1.19, 2.01 in ABI 1.20–1.29, and 3.33 in ABI 1.30–1.39. The hazard ratios for developing CKD were 2.14 (95% confidence interval 1.16–3.92) in ABI 0.90–0.99, 1.08 (0.83–1.41) in ABI 1.00–1.09, 1.03 (0.83–1.29) in ABI 1.20–1.29, and 1.37 (0.77–2.47) in ABI 1.30–1.39, after adjusting for age, sex, systolic blood pressure, diabetes, and other confounding factors.
Conclusions: In a general Japanese population, an ABI of 0.90–0.99 was associated with an increased risk of incident CKD, independent of traditional cardiovascular risk factors.
Keywords: Ankle-brachial index, Chronic kidney disease, Cohort study, Japanese
See editorial vol. 26: 1043–1044
Introduction and Aim
Chronic kidney disease (CKD) is defined by an estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73 m2, proteinuria, or structural kidney disease1, 2). CKD's prevalence in the Japanese adult population exceeded 13% in 2012, and the number of patients with CKD is expected to increase as the Japanese population ages2). Clarifying risk factors, or predictive markers, for CKD may facilitate earlier decision making for treatment. This would improve adverse outcomes, including end-stage renal failure and cardiovascular diseases, such as coronary heart disease and stroke1–4). Toward this end, multiple studies have evaluated risk factors or predictive markers for CKD5–17).
The ankle-brachial index (ABI) is a well-established, non-invasive test primarily used to screen for peripheral artery disease. Values lower than 0.90 reflect the presence of flow-limiting atherosclerotic stenosis in the lower extremities' arteries, resulting from accumulated plaques in the arterial walls18, 19). Two previous studies reported that ABI < 0.90 predicts future kidney function decline in general Western populations6, 7). In addition to low ABI, very high ABI (> 1.40), caused by arterial stiffness due to medial calcification, is associated with increased cardiovascular events and mortality20). Based on these pathological findings, epidemiological studies have suggested that ABI has a U-shape relationship with cardiovascular disease and all-cause mortality21–26). However, little is known about the relationship between ABI and kidney function in Asian populations, who have different cardiovascular risk profiles from Western populations27). Therefore, we investigated this topic using longitudinal data obtained from a general Japanese population.
Methods
Design and Participants
This retrospective cohort study included 8,828 Japanese individuals with no history of cerebrovascular, cardiovascular, or renal disease. The participants underwent an annual health check-up between January 2003 and December 2010 at Keijinkai Maruyama Clinic, a health check-up facility in Sapporo, Japan. After excluding individuals who were < 30 or > 79 years old, had an eGFR of < 60 ml/min/1.73 m2, and/or had a protein reading of (1+), (2+) or (3+) on a urine dipstick at baseline (Fig. 1), 7,994 participants eligible for a follow-up survey. The primary outcome, CKD development, was defined by an eGFR < 60 ml/min/1.73 m2 and/or proteinuria, determined by a reading of (1+), (2+) or (3+) on urine dipstick1). Participants' annual health check-up data were followed until March 2014. Of the 7,905 participants, 5,072 were eligible for the final analysis (64.2%) after excluding those without follow-up information.
Fig. 1.
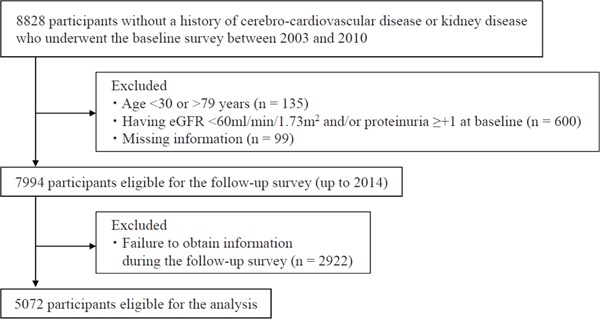
Study flow chart for inclusion of the participants
Baseline Survey
ABI was defined as the ratio of systolic blood pressure in the posterior tibial artery and/or the dorsalis pedis artery to systolic blood pressure in the brachial artery5, 28, 29) We used a validated, automated device (Form series BP-203RPE III; Omron-Colin Co., Tokyo, Japan), which measures blood pressure in the ankle and brachial arteries by the oscillometric method. Licensed clinical laboratory technologists performed all ABI measurements. Participants were asked to lay in the supine position for five minutes before ABI measurement. Brachial systolic blood pressure was collected from both arms, and the higher reading was used as a denominator to calculate ABI. The average of the right and left ABI was used in the analysis. Although the lower ABI from bilateral measurements is used to screen for peripheral artery disease in a primary setting, several studies reported a U-shape relationship between ABI and cardiovascular diseases20, 24, 25, 30). With reference to these findings, our study assessed whether both low and high ABI would predict CKD development.
Blood samples were obtained after overnight fasting. Serum creatinine was measured enzymatically (CicaLiquid S, Kanto Chemical Co., Inc., Tokyo, Japan). The eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation with the coefficient for Japanese populations31, 32). The Modification of Diet in Renal Disease (MDRD) equation with the Japanese coefficient and the Japanese Society of Nephrology Chronic Kidney Disease Initiative (JSN-CKDI) equation were used for the sensitivity analysis33). Proteinuria was assessed by urine dipstick (Uriflet S, ARKRAY, Inc., Kyoto, Japan). Serum total cholesterol, high-density lipoprotein (HDL) cholesterol, fasting blood glucose, and glycated hemoglobin (HbA1c) were also measured. Non-HDL cholesterol was calculated as total cholesterol minus HDL cholesterol. HbA1c was measured with the quality control standard defined by the Japan Diabetes Society and converted to the National Glycohemoglobin Standardization Program (NGSP) value34). Diabetes was defined as a fasting blood glucose ≥ 126 mg/dL, HbA1c (NGSP) ≥ 6.5%, and/or use of medication for diabetes. Blood pressure was measured by trained nurses using a standard mercury sphygmomanometer after patients quietly sat for ten minutes. Body mass index was calculated as weight in kilograms divided by the square of height in meters. A self-administered questionnaire was used to obtain baseline medical history and smoking habits.
Follow-Up Survey
To identify CKD development during follow-up, serum creatinine and urine protein were repeatedly measured until the end of March 2014, as often as participants underwent annual health check-ups. If a participant withdrew from the follow-up survey at mid-study without developing CKD, follow-up was terminated at the latest survey that he/she underwent. The procedures for measuring these variables were consistent throughout the follow-up period. The same definition used to define CKD was used for exclusion at baseline (i.e., eGFR < 60 ml/min/1.73 m2 and/or proteinuria, defined as a reading of (1+), (2+), or (3+) on urine dipstick)1, 2).
Statistical Analysis
The participants were divided into the following five categories according to their ABI values: ABI 0.90–0.99, ABI 1.00–.09, ABI 1.10–1.19, ABI 1.20–1.29, and ABI 1.30–1.39. No participants had ABI < 0.9 or > 1.39 after exclusion. CKD development (based on the CKD-EPI equation for eGFR calculation and urine dipstick) was compared among the five ABI categories. Participants who were lost to follow- up were treated as censored cases after their most recent survey. A Cox proportional hazards model was used to estimate the hazard ratios (HRs) and 95% confidence intervals (CIs) for developing CKD in each ABI category, with ABI 1.10–1.19 acting as the reference category. The reference group was based on the mean ABI values reported by previous studies in middle-aged-to-elderly Asian populations35, 36). The model incorporated the following variables as covariates: age (years as a continuous variable), sex (male or female), baseline eGFR (ml/min/1.73 m2 as a continuous variable), body mass index (kg/m2 as a continuous variable), smoking habits (current, former, or never smoker, using two dummy variables with never smoker as the reference), systolic blood pressure (mmHg as a continuous variable), serum non-HDL cholesterol (mg/dL as a continuous variable), HDL cholesterol (mg/dL as a continuous variable), and diabetic status (present or absent). A sensitivity analysis for developing CKD was conducted based on the MDRD equation and the JSN-CKDI equation for eGFR calculation. Statistical analyses were performed using SPSS Ver 23.0 for Windows (IBM Institute, Tokyo, Japan).
Ethical Considerations
This study was approved by the institutional ethical committee of Hokkaido University Faculty of Medicine and Graduate School of Medicine (I 15–028) and Teine Keijinkai Medical Center. Individual informed consent was waived because of the retrospective nature of the study, and because all surveys had been done under the comprehensive prior consent arrangement before collecting data.
Results
Characteristics of Study Participants
Table 1 shows the 5,072 study participants' baseline characteristics. Lower ABI values were associated with a higher proportion of women, higher levels of non-HDL cholesterol, and higher eGFR. Higher ABI values were associated with older age, higher proportion of current smoking status, higher systolic blood pressure, and higher proportion of diabetic participants.
Table 1. Baseline characteristics of the 5072 participants without CKD, grouped according to ankle-brachial index.
| Ankle-brachial index |
|||||||
|---|---|---|---|---|---|---|---|
| Overall | 0.90–0.99 | 1.00–1.09 | 1.10–1.19 | 1.20–1.29 | 1.30–1.39 | ||
| (n = 5072) | (n = 80) | (n = 843) | (n = 2864) | (n = 1209) | (n = 76) | P | |
| Female, % | 29.0 (1470) | 43.8 (35) | 45.1 (380) | 29.6 (847) | 16.8 (203) | 6.6 (5) | < 0.001 |
| Age (yrs) | 50.6 ± 8.7 | 47.2 ± 10.0 | 47.8 ± 8.9 | 50.7 ± 8.5 | 52.2 ± 8.2 | 53.8 ± 7.5 | < 0.001 |
| Body mass index (kg/m2) | 23.7 ± 3.2 | 23.6 ± 4.9 | 23.2 ± 3.8 | 23.5 ± 3.0 | 24.3 ± 3.1 | 25.3 ± 2.8 | < 0.001 |
| Smoking status, % | |||||||
| Never smoker | 44.1 (2237) | 43.8 (35) | 48.8 (411) | 44.5 (1275) | 40.5 (490) | 34.2 (26) | |
| Ex-smoker | 22.4 (1136) | 25.0 (20) | 17.9 (151) | 21.5 (617) | 26.9 (325) | 30.3 (23) | < 0.001 |
| Current smoker | 33.6 (1699) | 31.3 (25) | 33.3 (281) | 33.9 (972) | 32.6 (394) | 35.5 (27) | |
| Systolic blood pressure (mmHg) | 119.6 ± 15.9 | 116.2 ± 16.5 | 116.2 ± 15.7 | 119.3 ± 15.6 | 122.2 ± 16.1 | 125.8 ± 14.4 | < 0.001 |
| Non-HDL cholesterol (mg/dl) | 152.0 ± 34.5 | 159.0 ± 43.6 | 151.5 ± 36.9 | 152.8 ± 34.3 | 150.1 ± 32.4 | 146.7 ± 32.4 | 0.04 |
| Diabetes, % | 5.1 (261) | 6.3 (5) | 4.4 (37) | 5.3 (151) | 4.9 (59) | 11.8 (9) | 0.08 |
| eGFR (ml/min/1.73 m2) | 84.5 ± 8.2 | 87.5 ± 8.1 | 86.8 ± 8.4 | 84.3 ± 8.1 | 83.4 ± 7.8 | 81.4 ± 8.5 | < 0.001 |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein.
Data are presented as mean ± standard deviation for continuous variables and % (number) for categorical variables.
P values were calculated by one-way analysis of variance for continuous variables and Chi-Square test for categorical variables.
CKD was defined as eGFR < 60 ml/min/1.73m2 and/or proteinuria ≥ + 1 on urine dipstick (reference 1, 2).
GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation with the coefficient for Japanese population (reference 32).
Diabetes was defined as a fasting plasma glucose level ≥ 126 mg/dl and/or HbA1c ≥ 6.5%.
ABI and Incident CKD
During the total follow-up of 25,827.9 person-years, 466 incident cases of CKD were identified (incidence rate 1.80 per 100 person-years). The CKD incidence rate in each ABI category per 100 person-years was 3.04 in ABI 0.90–0.99, 1.58 in ABI 1.00–1.09, 1.72 in ABI 1.10–1.19, 2.01 in ABI 1.20–1.29, and 3.33 in ABI 1.30–1.39. After adjusting potential confounding risk factors, the ABI 0.90–0.99 category had a significantly higher risk of developing CKD than the ABI 1.10–1.19 category: HR, 2.14 (95%CI 1.16–3.92) in ABI 0.90–0.99, 1.08 (95%CI 0.83–1.41) in ABI 1.00–1.09, 1.03 (95%CI 0.83–1.29) in ABI 1.20–1.29, and 1.37 (95%CI 0.77–2.47) in ABI 1.30–1.39 (Table 2). Our sensitivity analysis, using the MDRD and JSN-CKDI equations for eGFR calculation, showed comparable results.
Table 2. Hazard ratio for CKD in the study participants, grouped according to ankle-brachial index at baseline.
| Ankle-brachial index |
|||||
|---|---|---|---|---|---|
| 0.90–0.99 | 1.00–1.09 | 1.10–1.19 | 1.20–1.29 | 1.30–1.39 | |
| (n = 80) | (n = 843) | (n = 2864) | (n = 1209) | (n = 76) | |
| Cases | 11 | 70 | 253 | 120 | 12 |
| Person-years of follow-up | 361.6 | 4443.6 | 14700.5 | 5961.6 | 360.6 |
| Incidence rate (/100 person-years) | 3.04 | 1.58 | 1.72 | 2.01 | 3.33 |
| Age and sex-adjusted HR (95% CI) | 2.09 (1.14–3.82) | 1.08 (0.83–1.41) | Reference | 1.06 (0.85–1.31) | 1.69 (0.94–3.01) |
| Multivariate-adjusted HR (95% CI) | 2.14 (1.16–3.92) | 1.08 (0.83–1.41) | Reference | 1.03 (0.83–1.29) | 1.37 (0.77–2.47) |
Abbreviations: CI, confidence interval; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Multivariate-adjusted model included the following covariates: age, sex, body mass index, smoking status, systolic blood pressure, non-high-density lipoprotein cholesterol, diabetes status, and baseline eGFR.
CKD was defined as eGFR < 60 ml/min/1.73 m2 and/or proteinuria ≥ + 1 on urine dipstick (reference 1, 2).
GFR was estimated using the Chronic Kidney Disease Epidemiology Collaboration equation with the coefficient for Japanese population (reference 32).
Discussion and Conclusion
We found patients with ABI 0.90–0.99 had an approximately two-fold increased risk of developing CKD than patients with ABI 1.10–1.19, independent of age, sex, and potential confounding factors, including smoking, obesity, hypertension, diabetes, dyslipidemia, and baseline eGFR. To the best of our knowledge, our study is the first to demonstrate that low ABI independently predicts CKD development in a general Asian population.
Only two relevant cohort studies in Western countries have investigated the association between ABI and kidney function in a general population6, 7). O'Hare et al. observed that patients with ABI < 0.9 had a significantly higher risk for ≥ 50% increase in serum creatinine after a three-year follow-up period6). Foster et al. observed that patients with ABI < 0.9 had a significantly higher risk for rapid eGFR decline, defined as ≥ 3 mL/min/1.73 m2 decrease per year7). Although these two cohort studies did not find a significant increase of CKD in patients with normal ABI, a normal ABI could still be associated with an increased risk of kidney function decline6, 7). For example, O'Hare et al. observed an odds ratio of 1.9 (95%CI 0.97–3.8) for increased creatinine in the ABI 0.90–0.99 group compared to the ABI ≥ 1 group6). Similarly, Foster et al. observed an odds ratio of 1.32 (95%CI 0.93–1.89) for microalbuminuria in the ABI 0.9–1.1 group compared to the ABI 1.1–1.4 group7). Although there were methodological differences between the studies (ABI categorization, definition of reference group, and outcome measures), the results may support our findings. ABI 0.90–0.99 has been considered normal for diagnosing peripheral artery disease. In this regard, the present study provided notable insights.
Patients with low ABI tend to not only have arterial stenosis in lower extremities, but also have a higher risk of coronary artery disease and stroke due to generalized atherosclerosis20, 24, 25). In addition to the possible link between low ABI and generalized atherosclerosis, several studies assessing the link between kidney damage and atherosclerosis are worthy of attention. Iwakiri et al. found, in his autopsy series, that pathological findings in the renal vasculature, including an increased intima/media layer ratio in the renal artery, increased proportion of renal arteriolar hyalinization, and increased proportion of global glomerulosclerosis, were associated with increased degree of generalized atherosclerosis37). Kasiske et al. also suggested that intrarenal vascular disease and glomerulosclerosis were associated with generalized atherosclerosis38). Tracy et al. found that hyalinized renal arterioles were markers for severe atherosclerosis in coronary arteries39). Nakamura et al. found in their autopsy study that asymptomatic plaques in common iliac arteries are associated with generalized atherosclerosis and renal failure40). Taken together, these studies support our results that low ABI, even within clinically normal values, indicates generalized atherosclerosis and predicts development of renal vascular disease and CKD.
Various other mechanisms associated with an abnormal ABI may contribute to CKD, further supporting our findings. Ozkaramanli Gur et al. reported increased cytokine levels in both low and high ABI groups in patients with previous coronary artery bypass grafting41). Therefore, both low and high ABI groups may have vascular damage in the kidney via circulatory cytokines. Wang et al. showed that exertional leg pain and intermittent claudication were more prevalent in patients with borderline-low ABI (0.91–0.99) and borderline-high ABI (≥ 1.40) compared to those with normal ABI (1.00–1.39)42). Therefore, using pain relief medication (e.g., nonsteroidal anti-inflammatory drug) for symptomatic, lower extremity artery disease may also affect kidney function. Furthermore, using hypertension medications (e.g., renin-angiotensin system blocking agents), which are usually more prevalent in both lower and higher ABI groups42), can deteriorate renal function.
Ishida et al. reported that there is a possible J-shaped relationship between ABI and the risk of presence of proteinuria, but not low eGFR, in a Japanese cross-sectional study42). These findings are consistent with our results demonstrating that the ABI 1.30–1.39 group was likely to have a higher risk of incident CKD, compared to the ABI 1.10–1.19 category. Furthermore, based on our findings, we could not definitively show that there is no link between high ABI and the development of CKD. It is possible that ABI has a U-shaped relationship with incident CKD.
In general, females have lower ABIs than males35), which is consistent with our findings. We showed that the proportion of females decreased with increasing ABI at baseline. Unfortunately, our study did not include enough participants to conduct sex-specific analyses. In preliminary, sex-stratified analyses, we observed a U-shaped relationship between ABI and incident CKD among male participants (data not shown). However, we could not obtain reliable results among female participants because there were few CKD cases in the lowest and highest ABI groups. Future studies, including a large number of participants, are warranted to elucidate the sex-specific effect of ABI on CKD development in Asian populations.
The strength of our study is that it included a large sample size and had a total follow-up period of 24,592.1 person-years with annual data. However, our study had several limitations. First, the study participants consisted solely of health checkup examinees at a single clinic; thus, caution should be exercised when generalizing our results. Additionally, of the health checkup examinees at the clinic, only those who voluntarily underwent ABI measurement were involved in this study. Because these participants were likely to be concerned about health issues, there may have been a selection bias that they had healthier characteristics and, therefore, would be less likely to develop CKD in the future. Second, since they were relatively young and healthy, the original cohorts only included three participants with ABI < 0.9 and one participant with ABI > 1.39 at baseline. After selecting for eligible participants, all ABI levels fell between 0.9 and 1.39 (Fig. 1). Therefore, we were unable to determine such low or high ABI levels' influence on future kidney function. Third, 36.5% (2,922) of the 7,994 participants who underwent the baseline survey were excluded from the analysis due to the lack of follow-up data. Additionally, 23.7% (1145) of the 5,072 eligible participants dropped out during the follow-up period, with a mean follow-up of 1.7 years, but were included in the final analysis. However, baseline characteristics were similar between the participants who were excluded from the study, those who withdrew in mid-course, and those who completed the follow-up. Furthermore, the mean follow-up period was similar across all ABI categories in the 5,072 eligible participants. Fourth, our follow-up observations were based on the results of a single measurement of serum creatinine and urinary protein at annual health check-ups. However, CKD diagnosis usually requires multiple observations made over three months or more. This may limit the accuracy of our outcome measurements. Finally, due to the lack of data on medication for hypertension, dyslipidemia, and diabetes, we were unable to include these conditions in the analysis.
In conclusion, this study found that ABI from 0.90 to 0.99 predicts CKD development, independent of traditional cardiovascular risk factors. In addition to detecting peripheral artery disease and predicting future cardiovascular events, ABI, even at clinically normal values, may be a useful marker for predicting future CKD.
Acknowledgments
The authors thank all the staff and participants for their important contributions.
Notice of Grant
None.
Conflict of Interest
None declared.
Author Contributions
H.S. was responsible for the study concept and design, collected the data, analyzed the data, and drafted the manuscript. K.N. and A.T. interpreted thet results, and made critical revision of the manuscript.
References
- 1). Levey AS, de Jong PE, Coresh J, El Nahas M, Astor BC, Matsushita K, Gansevoort RT, Kasiske BL, Eckardt KU. The definition, classification, and prognosis of chronic kidney disease: a KDIGO Controversies Conference report. Kidney Int, 2011; 80: 17-28 [DOI] [PubMed] [Google Scholar]
- 2). Nephrology JSo ed. Clinical practice guidebook for diagnosis and treatment of chronic kidney disease. Tokyo, Japan: Tokyoigakusha; 2012 [PubMed] [Google Scholar]
- 3). Iseki K, Kinjo K, Iseki C, Takishita S. Relationship between predicted creatinine clearance and proteinuria and the risk of developing ESRD in Okinawa, Japan. Am J Kidney Dis, 2004; 44: 806-814 [PubMed] [Google Scholar]
- 4). Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Chronic Kidney Disease Prognosis C Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int, 2011; 80: 93-104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5). Lin JS, Olson CM, Johnson ES, Whitlock EP. The ankle-brachial index for peripheral artery disease screening and cardiovascular disease prediction among asymptomatic adults: a systematic evidence review for the U.S. Preventive Services Task Force. Ann Intern Med, 2013; 159: 333-341 [DOI] [PubMed] [Google Scholar]
- 6). O'Hare AM, Rodriguez RA, Bacchetti P. Low ankle-brachial index associated with rise in creatinine level over time: results from the atherosclerosis risk in communities study. Arch Intern Med, 2005; 165: 1481-1485 [DOI] [PubMed] [Google Scholar]
- 7). Foster MC, Ghuman N, Hwang SJ, Murabito JM, Fox CS. Low ankle-brachial index and the development of rapid estimated GFR decline and CKD. Am J Kidney Dis, 2013; 61: 204-210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8). Chen FA, Yang CY, Yang WC, Chen JY, Ng YY, Li SY, Liu WS, Cheng ST, Wang YJ, Lin CC. Ankle-brachial index is a powerful predictor of renal outcome and cardiovascular events in patients with chronic kidney disease. ScientificWorldJournal, 2012; 2012: 238494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9). Dong X, Wu D, Jia C, Ruan Y, Feng X, Wang G, Liu J, Shen Y, Li H, Li L. Low ankle-brachial index is associated with early-stage chronic kidney disease in type 2 diabetic patients independent of albuminuria. PLoS One, 2014; 9: e109641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Jin X, Ma JH, Shen Y, Luo Y, Su XF, Chen YY, Qi SK, Wu JD. An analysis of the relationship between ankle-brachial index and estimated glomerular filtration rate in type 2 diabetes. Angiology, 2013; 64: 237-241 [DOI] [PubMed] [Google Scholar]
- 11). Mostaza JM, Suarez C, Manzano L, Cairols M, Garcia-Iglesias F, Sanchez-Alvarez J, Ampuero J, Godoy D, Rodriguez-Samaniego A, Sanchez-Zamorano MA. Relationship between ankle-brachial index and chronic kidney disease in hypertensive patients with no known cardiovascular disease. J Am Soc Nephrol, 2006; 17: S201-205 [DOI] [PubMed] [Google Scholar]
- 12). Tian SL, Tian XK, Han QF, Axelsson J, Wang T. Presence of peripheral arterial disease predicts loss of residual renal function in incident CAPD patients. Perit Dial Int, 2012; 32: 67-72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13). Kshirsagar AV, Coresh J, Brancati F, Colindres RE. Ankle brachial index independently predicts early kidney disease. Ren Fail, 2004; 26: 433-443 [DOI] [PubMed] [Google Scholar]
- 14). Makhdoomi K, Mohammadi A, Yekta Z, Aghasi MR, Zamani N, Vossughian S. Correlation between ankle-brachial index and microalbuminuria in type 2 diabetes mellitus. Iran J Kidney Dis, 2013; 7: 204-209 [PubMed] [Google Scholar]
- 15). Ninomiya T, Kiyohara Y, Tokuda Y, Doi Y, Arima H, Harada A, Ohashi Y, Ueshima H. Japan Arteriosclerosis Longitudinal Study G. Impact of kidney disease and blood pressure on the development of cardiovascular disease: an overview from the Japan Arteriosclerosis Longitudinal Study. Circulation, 2008; 118: 2694-2701 [DOI] [PubMed] [Google Scholar]
- 16). Chronic Kidney Disease Prognosis C. Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet, 2010; 375: 2073-2081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17). Nagata M, Ninomiya T, Kiyohara Y, Murakami Y, Irie F, Sairenchi T, Miura K, Okamura T, Ueshima H, Group E-JR Prediction of cardiovascular disease mortality by proteinuria and reduced kidney function: pooled analysis of 39,000 individuals from 7 cohort studies in Japan. Am J Epidemiol, 2013; 178: 1-11 [DOI] [PubMed] [Google Scholar]
- 18). Carter SA. Indirect systolic pressures and pulse waves in arterial occlusive diseases of the lower extremities. Circulation, 1968; 37: 624-637 [DOI] [PubMed] [Google Scholar]
- 19). Yao ST, Hobbs JT, Irvine WT. Ankle systolic pressure measurements in arterial disease affecting the lower extremities. Br J Surg, 1969; 56: 676-679 [DOI] [PubMed] [Google Scholar]
- 20). Aboyans V, Criqui MH, Abraham P, Allison MA, Creager MA, Diehm C, Fowkes FG, Hiatt WR, Jonsson B, Lacroix P, Marin B, McDermott MM, Norgren L, Pande RL, Preux PM, Stoffers HE, Treat-Jacobson D, American Heart Association Council on Peripheral Vascular D, Council on E, Prevention, Council on Clinical C, Council on Cardiovascular N, Council on Cardiovascular R, Intervention, Council on Cardiovascular S, Anesthesia Measurement and interpretation of the ankle-brachial index: a scientific statement from the American Heart Association. Circulation, 2012; 126: 2890-2909 [DOI] [PubMed] [Google Scholar]
- 21). Allison MA, Hiatt WR, Hirsch AT, Coll JR, Criqui MH. A high ankle-brachial index is associated with increased cardiovascular disease morbidity and lower quality of life. J Am Coll Cardiol, 2008; 51: 1292-1298 [DOI] [PubMed] [Google Scholar]
- 22). O'Hare AM, Katz R, Shlipak MG, Cushman M, Newman AB. Mortality and cardiovascular risk across the ankle-arm index spectrum: results from the Cardiovascular Health Study. Circulation, 2006; 113: 388-393 [DOI] [PubMed] [Google Scholar]
- 23). Su HM, Lin TH, Hsu PC, Chu CY, Lee WH, Chen SC, Lee CS, Voon WC, Lai WT, Sheu SH. Abnormally low and high ankle-brachial indices are independently associated with increased left ventricular mass index in chronic kidney disease. PLoS One, 2012; 7: e44732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24). Criqui MH, McClelland RL, McDermott MM, Allison MA, Blumenthal RS, Aboyans V, Ix JH, Burke GL, Liu K, Shea S. The ankle-brachial index and incident cardiovascular events in the MESA (Multi-Ethnic Study of Atherosclerosis). J Am Coll Cardiol, 2010; 56: 1506-1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25). Fowkes FG, Murray GD, Butcher I, Heald CL, Lee RJ, Chambless LE, Folsom AR, Hirsch AT, Dramaix M, deBacker G, Wautrecht JC, Kornitzer M, Newman AB, Cushman M, Sutton-Tyrrell K, Fowkes FG, Lee AJ, Price JF, d'Agostino RB, Murabito JM, Norman PE, Jamrozik K, Curb JD, Masaki KH, Rodriguez BL, Dekker JM, Bouter LM, Heine RJ, Nijpels G, Stehouwer CD, Ferrucci L, McDermott MM, Stoffers HE, Hooi JD, Knottnerus JA, Ogren M, Hedblad B, Witteman JC, Breteler MM, Hunink MG, Hofman A, Criqui MH, Langer RD, Fronek A, Hiatt WR, Hamman R, Resnick HE, Guralnik J, McDermott MM. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA, 2008; 300: 197-208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26). European Stroke O. Tendera M, Aboyans V, Bartelink ML, Baumgartner I, Clement D, Collet JP, Cremonesi A, De Carlo M, Erbel R, Fowkes FG, Heras M, Kownator S, Minar E, Ostergren J, Poldermans D, Riambau V, Roffi M, Rother J, Sievert H, van Sambeek M, Zeller T, Guidelines ESCCfP ESC Guidelines on the diagnosis and treatment of peripheral artery diseases: Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries: the Task Force on the Diagnosis and Treatment of Peripheral Artery Diseases of the European Society of Cardiology (ESC). Eur Heart J, 2011; 32: 2851-2906 [DOI] [PubMed] [Google Scholar]
- 27). Gijsberts CM, Groenewegen KA, Hoefer IE, Eijkemans MJ, Asselbergs FW, Anderson TJ, Britton AR, Dekker JM, Engstrom G, Evans GW, de Graaf J, Grobbee DE, Hedblad B, Holewijn S, Ikeda A, Kitagawa K, Kitamura A, de Kleijn DP, Lonn EM, Lorenz MW, Mathiesen EB, Nijpels G, Okazaki S, O'Leary DH, Pasterkamp G, Peters SA, Polak JF, Price JF, Robertson C, Rembold CM, Rosvall M, Rundek T, Salonen JT, Sitzer M, Stehouwer CD, Bots ML, den Ruijter HM. Race/Ethnic Differences in the Associations of the Framingham Risk Factors with Carotid IMT and Cardiovascular Events. PLoS One, 2015; 10: e0132321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28). Reed JF, 3rd, Eid S, Edris B, Sumner AD. Prevalence of peripheral artery disease varies significantly depending upon the method of calculating ankle brachial index. Eur J Cardiovasc Prev Rehabil, 2009; 16: 377-381 [DOI] [PubMed] [Google Scholar]
- 29). Allison MA, Aboyans V, Granston T, McDermott MM, Kamineni A, Ni H, Criqui MH. The relevance of different methods of calculating the ankle-brachial index: the multi-ethnic study of atherosclerosis. Am J Epidemiol, 2010; 171: 368-376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30). Stoffers HE, Kester AD, Kaiser V, Rinkens PE, Kitslaar PJ, Knottnerus JA. The diagnostic value of the measurement of the ankle-brachial systolic pressure index in primary health care. J Clin Epidemiol, 1996; 49: 1401-1405 [DOI] [PubMed] [Google Scholar]
- 31). Horio M, Imai E, Yasuda Y, Watanabe T, Matsuo S. Modification of the CKD epidemiology collaboration (CKD-EPI) equation for Japanese: accuracy and use for population estimates. Am J Kidney Dis, 2010; 56: 32-38 [DOI] [PubMed] [Google Scholar]
- 32). Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J, Ckd EPI. A new equation to estimate glomerular filtration rate. Ann Intern Med, 2009; 150: 604-612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33). Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta K, Yamagata K, Tomino Y, Yokoyama H, Hishida A, Collaborators developing the Japanese equation for estimated GFR Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis, 2009; 53: 982-992 [DOI] [PubMed] [Google Scholar]
- 34). Kashiwagi A, Kasuga M, Araki E, Oka Y, Hanafusa T, Ito H, Tominaga M, Oikawa S, Noda M, Kawamura T, Sanke T, Namba M, Hashiramoto M, Sasahara T, Nishio Y, Kuwa K, Ueki K, Takei I, Umemoto M, Murakami M, Yamakado M, Yatomi Y, Ohashi H, Committee on the Standardization of Diabetes Mellitus-Related Laboratory Testing of Japan Diabetes S International clinical harmonization of glycated hemoglobin in Japan: From Japan Diabetes Society to National Glycohemoglobin Standardization Program values. J Diabetes Investig, 2012; 3: 39-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35). Aboyans V, Criqui MH, McClelland RL, Allison MA, McDermott MM, Goff DC, Jr., Manolio TA. Intrinsic contribution of gender and ethnicity to normal ankle-brachial index values: the Multi-Ethnic Study of Atherosclerosis (MESA). J Vasc Surg, 2007; 45: 319-327 [DOI] [PubMed] [Google Scholar]
- 36). Ishida A, Nakachi-Miyagi M, Kinjo K, Iseki K, Ohya Y. A high normal ankle-brachial index is associated with proteinuria in a screened cohort of Japanese: the Okinawa Peripheral Arterial Disease Study. J Hypertens, 2014; 32: 1435-1443 [DOI] [PubMed] [Google Scholar]
- 37). Iwakiri T, Sato Y, Matsuura Y, Hatakeyama K, Marutsuka K, Yamashita A, Fujimoto S, Kitamura K, Asada Y. Association between renal vasculature changes and generalized atherosclerosis: an autopsy survey. J Atheroscler Thromb, 2014; 21: 99-107 [DOI] [PubMed] [Google Scholar]
- 38). Kasiske BL. Relationship between vascular disease and age-associated changes in the human kidney. Kidney Int, 1987; 31: 1153-1159 [DOI] [PubMed] [Google Scholar]
- 39). Tracy RE, Strong JP, Newman WP, 3rd, Malcom GT, Oalmann MC, Guzman MA. Renovasculopathies of nephrosclerosis in relation to atherosclerosis at ages 25 to 54 years. Kidney Int, 1996; 49: 564-570 [DOI] [PubMed] [Google Scholar]
- 40). Nakamura E, Sato Y, Iwakiri T, Yamashita A, Moriguchi-Goto S, Maekawa K, Gi T, Asada Y. Asymptomatic Plaques of Lower Peripheral Arteries and Their Association with Cardiovascular Disease: An Autopsy Study. J Atheroscler Thromb, 2017; 24: 921-927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41). Ozkaramanli Gur D, Gur O, Guzel S, Akyuz A, Gurkan S, Alpsoy S, Gulec NS, Koc F. Inflammatory Mediators Across the Spectrum of Ankle-Brachial Index. J Atheroscler Thromb, 2019; 26: 351-361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42). Wang JC, Criqui MH, Denenberg JO, McDermott MM, Golomb BA, Fronek A. Exertional leg pain in patients with and without peripheral arterial disease. Circulation, 2005; 112: 3501-3508 [DOI] [PMC free article] [PubMed] [Google Scholar]


