Abstract
Aim: Although coronary endothelial vasomotor dysfunction predicts future coronary events, there are few human studies showing the relationship between endothelial vasomotor dysfunction and atheroma plaque progression in the same coronary artery. This study examined whether endothelial vasomotor dysfunction is related to atheroma plaque progression in the infarct-related coronary artery of ST-segment elevation myocardial infarction (STEMI) survivors using serial assessment of coronary plaque size with intravascular ultrasound (IVUS) and coronary vasomotor responses to acetylcholine (ACh).
Methods: This study included 50 patients with a first acute STEMI due to occlusion of the left anterior descending coronary artery (LAD) and successful reperfusion therapy with percutaneous coronary intervention (PCI). IVUS and vasomotor response to ACh in the LAD were measured within two weeks of acute myocardial infarction (AMI) (1st test) and repeated six months (2nd test) after AMI under optimal anti-atherosclerotic therapies.
Results: Percent atheroma volume (PAV) and total atheroma volume (TAV) in the LAD progressed over six months of follow-up in 18 and 14 patients, respectively. PAV and TAV progression was significantly associated with persistent impairment of epicardial coronary artery dilation and coronary blood flow increase in response to ACh at both the 1st and 2nd tests. PAV and TAV progression had no significant association with traditional risk factors, PCI-related variables, medications, and the coronary vasomotor responses to sodium nitroprusside, an endothelium-independent vasodilator.
Conclusions: Persistent impairment of endothelial vasomotor function in the conduit arterial segment and the resistance arteriole was related to atheromatous plaque progression in the infarct-related coronary arteries of STEMI survivors.
Keywords: Coronary atherosclerosis, Coronary endothelial function, Intravascular ultrasound, Myocardial infarction
Introduction
Previous studies showed that coronary endothelial vasomotor dysfunction predicted coronary events1, 2). A decrease in the anti-atherogenic role of endothelium-derived nitric oxide (NO) may explain the association between endothelial dysfunction and coronary events3, 4). However, endothelial dysfunction of either a peripheral or coronary artery is regarded as an integrated index of all atherogenic and atheroprotective factors present in an individual patient5). In this sense, systemic atherosclerotic risk burden, rather than a local reduction of endothelial NO, may mediate the relationship between coronary endothelial dysfunction and coronary events. Thus, whether endothelial vasomotor dysfunction is intimately related to plaque progression in the same coronary artery remains unknown. Also, there are few human studies showing the relationship between endothelial vasomotor dysfunction and atheromatous plaque progression in the same infarct-related coronary artery.
Residual stenosis in the infarct-related coronary artery is associated with a great risk of re-occlusion and may predispose patients to coronary events during follow-up in patients with acute myocardial infarction (AMI)6). High risk patients, such as AMI survivors, have severely impaired coronary endothelial function due to systemic atherosclerotic risk burden7–10). In addition, reperfusion and using a drug-eluting stent (DES) exacerbate endothelial dysfunction in the entire tree of the infarct-related coronary artery in AMI survivors11–14). However, endothelial vasomotor dysfunction in the infarct-related coronary arteries at the acute phase of MI is restored over time, as shown in our previous reports11, 12). Thus, changeable endothelial dysfunction may confound the relationship between endothelial dysfunction and atherosclerotic plaque in the same infarct-related coronary artery. Using serial measurements with IVUS, this study examined the relationship between endothelial vasomotor dysfunction and residual atherosclerotic plaque progression in the infarct-related coronary artery in new-onset ST-segment elevation MI (STEMI) survivors with successful reperfusion therapy.
Methods
Study Patients
This prospective study initially enrolled 210 consecutive patients with a first STEMI due to occlusion of a proximal segment of the left anterior descending coronary artery (LAD), who were admitted to Yamanashi University Hospital between January 2008 and December 2017. All patients received emergency coronary angiography and successful reperfusion therapy, within 12 hours of the onset of symptoms, by primary percutaneous coronary intervention (PCI) using IVUS-guided stenting. STEMI diagnosis was based on the presence of each of the following criteria15): typical chest pain persisting for ≥ 30 minutes, ST-segment elevation of ≥ 0.2 mV in two or more contiguous leads on a standard 12-lead electrocardiogram (ECG), and creatine kinase-MB ≥ two fold of the upper limit of normal or troponin T > 0.1 ng/mL. Among the 210 initially enrolled patients, 132 fulfilled the inclusion criterion of no residual organic stenosis (≥ 30%) in the LAD after PCI (Fig. 1). The exclusion criteria were as follows: 1) previous PCI in the LAD; 2) previous coronary artery bypass surgery; 3) presence of collaterals to the LAD with Rentrop grade ≥ 2; 4) congestive heart failure (New York Heart Association classification IV) at one week after AMI; 5) persistent atrial fibrillation and a paced rhythm; 6) age > 80 years; 7) myocardial bridge in the LAD; 8) valvular heart diseases (aortic stenosis [aortic valve area < 1.0 cm2], aortic regurgitation [Sellers classification III/IV], or mitral regurgitation [Sellers classification III/IV]), secondary hypertension, stroke, renal dysfunction (serum creatinine > 2.0 mg/dL), or other serious disease. After applying the exclusion criteria, the study included 63 STEMI patients (Fig. 1). The study also included 20 age- and sex-matched control patients, who were selected from 25 consecutive patients with atypical chest pain and normal coronary angiograms. The control patients served as a reference group for determination of cut-off values of coronary vasomotor dysfunction. Written informed consent was obtained from all patients and control subjects before the study. The ethics committee of Yamanashi University Hospital approved the study, which conformed to the principles outlined in the 1975 Declaration of Helsinki.
Fig. 1.
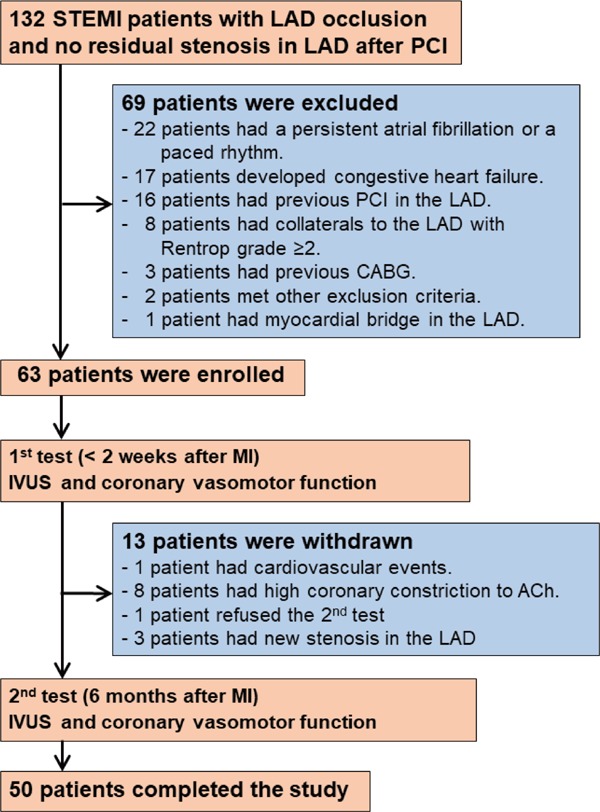
A flow chart of the enrollment of patients with acute anterior STEMI. The 1st test was performed < 2 weeks after MI; the 2nd test was performed 6 months after MI. LAD, left anterior descending coronary artery; PCI, percutaneous coronary intervention; CABG, coronary artery bypass graft.
Study Protocol
After emergency coronary angiography with PCI, followed by IVUS (IVUS at the 1st test) on admission, cardiac catheterization, including the coronary vasomotor function test, was repeated after one to two weeks and six months (coronary vasomotor measurements at the 1st and 2nd tests, respectively) after AMI (Fig. 1). The cardiac catheterization six months after AMI included IVUS (IVUS at the 2nd test) (Fig. 1). Also, measuring the coronary vasomotor response was performed only once in all of the control subjects. Vasodilators, including calcium channel blockers, nitrates, and nicorandil were withdrawn more than three days before coronary angiography in patients with AMI and control subjects. Blood samples from a peripheral vein were obtained at one to two weeks (1st test) and six months after AMI (2nd test). Patients received standard medical treatment after admission16), and this was continued for six months, as outlined in Table 1. The patients followed recommended diet and lifestyle changes throughout the follow-up period.
Table 1. Comparisons of Clinical Characteristics between Patients with and without Progression of Percent Atheroma Volume (PAV) in the Infarct-related Coronary Artery.
| PAV progression |
|||
|---|---|---|---|
| With (n = 18) | Without (n = 32) | p-value | |
| Age, years | 64 ± 8 | 63 ± 13 | 0.77 |
| Sex, male, n (%) | 14 (77.8) | 27 (84.4) | 0.56 |
| Hypertension, n (%) | 12 (66.7) | 21 (65.6) | 0.94 |
| Diabetes mellitus, n (%) | 3 (16.7) | 5 (15.6) | 0.92 |
| Measurements at the 1st test | |||
| BMI (kg/m2) | 24.0 ± 3.2 | 24.9 ± 4.4 | 0.49 |
| Systolic BP (mmHg) | 104 (96–118) | 107 (100–121) | 0.39 |
| FBG (mg/dL) | 98 (91–113) | 97 (89–109) | 0.88 |
| HbA1c (%) | 5.9 (5.6–6.4) | 6.0 (5.6–6.2) | 0.96 |
| Triglyceride (mg/dL) | 138 (112–170) | 149 (107–184) | 0.49 |
| HDL-C (mg/dL) | 44 (38–54) | 40 (34–45) | 0.09 |
| LDL-C (mg/dL) | 142 ± 25 | 138 ± 36 | 0.74 |
| CRP (mg/dL) | 0.5 (0.2–0.9) | 0.6 (0.2–1.7) | 0.52 |
| BNP (pg/mL) | 63 (38–210) | 110 (31–210) | 0.79 |
| LVEF (%) | 54 ± 12 | 56 ± 10 | 0.47 |
| Measurements at the 2nd test | |||
| BMI (kg/m2) | 23.9 ± 3.2 | 24.7 ± 4.2 | 0.49 |
| Systolic BP (mmHg) | 119 (107–132) | 115 (108–125) | 0.58 |
| FBG (mg/dL) | 97 (91–113) | 99 (90–110) | 0.52 |
| HbA1c (%) | 6.0 (5.6–6.2) | 6.0 (5.7–6.4) | 0.68 |
| Triglyceride (mg/dL) | 135 (81–205) | 126 (97–204) | 0.97 |
| HDL-C (mg/dL) | 44 (37–54) | 43 (38–50) | 0.68 |
| LDL-C (mg/dL) | 83 ± 18* | 86 ± 28* | 0.65 |
| CRP (mg/dL) | 0.2 (0.1–0.4)* | 0.1 (0.0–0.1)* | 0.37 |
| BNP (pg/mL) | 32 (20–115)* | 25 (14–76)* | 0.43 |
| LVEF (%) | 54 ± 15 | 61 ± 9 | 0.11 |
| Risk status at the 1st test, n (%) | |||
| Current smoking | 7 (38.9) | 15 (46.9) | 0.59 |
| Pts with BP < 140/90 | 15 (83.3) | 27 (84.4) | 0.92 |
| Pts with HbA1c < 7.0% | 15 (83.3) | 28 (87.5) | 0.69 |
| Pts with LDL-C < 100 | 0 (0.0) | 4 (12.5) | 0.12 |
| Risk status at the 2nd test, n (%) | |||
| Current smoking | 0 (0.0) | 1 (3.1)* | 0.45 |
| Pts with BP < 140/90 | 13 (72.2) | 27 (84.4) | 0.23 |
| Pts with HbA1c < 7.0% | 17 (94.4) | 28 (87.5) | 0.43 |
| Pts with LDL-C < 100 | 13 (72.2)* | 24 (75.0)* | 0.94 |
| Medications at the 1st test, n (%) | |||
| Beta-blocker | 10 (55.6) | 13 (40.6) | 0.31 |
| ACE-I/ARB | 13 (72.2) | 22 (68.8) | 0.80 |
| Statin | 17 (94.4) | 28 (87.5) | 0.43 |
| Biganide | 1 (5.6) | 1 (3.1) | 0.67 |
| Aspirin | 18 (100) | 32 (100) | - |
| Thienopyridines | 18 (100) | 32 (100) | - |
| Medications at the 2nd test, n (%) | |||
| Beta-blocker | 9 (50.0) | 15 (46.9) | 0.83 |
| ACE-I/ARB | 15 (83.3) | 24 (75.0) | 0.50 |
| Statin | 18 (100) | 30 (93.8) | 0.28 |
| Biganide | 1 (5.6) | 1 (3.1) | 0.67 |
| Aspirin | 18 (100) | 31 (96.9) | 0.45 |
| Thienopyridines | 18 (100) | 32 (100) | - |
| AMI variables | |||
| Peak CPK (IU/L) | 2471 ± 1889 | 3340 ± 2310 | 0.22 |
| Use of BMS, n (%) | 4 (22.2) | 7 (21.9) | 0.98 |
| Use of 1st generation DES, n (%) | 3 (16.7) | 5 (15.6) | 0.92 |
| Use of 2nd generation DES, n (%) | 11 (61.1) | 20 (62.5) | 0.98 |
| Stent length (mm) | 28.9 ± 14 | 23.1 ± 9.8 | 0.13 |
| MLD after stenting (mm) | 2.9 ± 0.5 | 3.1 ± 0.4 | 0.40 |
Data are expressed as the mean ± S.D, median (25th–75th percentiles) or the number (%) of patients.
p < 0.05 vs. the respective value at the 1st test.
PAV, percent atheroma volume; Pts, patients; BMI, body mass index; BP, blood pressure; FBG, fasting blood glucose; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BNP, brain natriuretic peptide; CRP, C-reactive protein; LVEF, left ventricular ejection fraction; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AMI, acute myocardial infarction; CPK, creatine phosphokinase; PCI, percutaneous coronary intervention; BMS, bare metal stent; DES, drug eluting stent; MLD, minimal lumen diameter.
Measurement of Epicardial Coronary Diameter and Coronary Blood Flow Response to Acetylcholine (ACh) and Sodium Nitroprusside (SNP)
A quantitative coronary angiography was performed as described in our previous reports10–12). After baseline angiography, incremental doses of acetylcholine chloride (ACh, OVISOT, Daiichi Sankyo, Tokyo) (5, 10 and 50 µg/min) were infused directly into the left coronary artery through the Judkins catheter for 2 min with a 5-min interval between successive doses. After an additional 15 min, intracoronary sodium nitroprusside (SNP) (10 µg/min) was infused in the same manner as ACh.
To assess the epicardial coronary diameter response to ACh, luminal diameter in a segment 15–25 mm from the distal edge of the stent in the LAD was measured quantitatively (Cardio 500, Kontron Instruments, Munich, Germany) before and during each infusion10–12). Each segment analyzed was referenced to a specific anatomic landmark, including the stent for identification, and the analyses at one to two weeks and six months after AMI were examined in parallel to ensure analysis of the identical LAD portion. The luminal diameter at the LAD mid-segments was measured in all of the control subjects in the same manner as in the AMI patients.
Blood flow velocity was measured before and at each infusion using a 0.014-inch wire equipped with a Doppler crystal at its tip (FloWire, Cardiometrics, Mountain View, California)10–12). The wire was carefully advanced through the Judkins catheter, and the wire tip was positioned in a segment of the LAD 5–15 mm from the distal edge of the stent. To calculate flow, the coronary luminal diameter response was also measured in an LAD segment 5–10 mm distal to the tip of the flow wire before and during each infusion, which was the separate measurement for the epicardial coronary diameter response to ACh in the LAD, as described above. Coronary blood flow (mL/min) was estimated from the coronary blood flow velocity and luminal diameter by the following formula: 0.5 × average peak velocity (cm/min) × cross-sectional area (cm2), as described previously10–12).
Responses of the coronary artery diameter and coronary blood flow to ACh and SNP infusions were expressed as percent changes from their respective baseline values taken just before each infusion. These measurements were performed by two observers (Y. W., K. W.) blinded to the study protocol and patient's clinical characteristics. Measurements of the percent changes in coronary diameter and coronary blood flow in response to ACh (10 µg/min) from the respective baseline values by the two independent observers were highly reproducible (r = 0.98 and 0.97, mean difference: 0.91 ± 0.04% and 2.9 ± 0.4%, respectively).
Determination of the Epicardial Coronary Diameter and Coronary Blood Flow Response to ACh in Each Patient and the Cut-Off Values of Impairment of Their Vasomotor Responses
Coronary vasomotor responses to ACh were determined by the net balance between dilation induced mainly by endothelium-derived NO and constriction mediated by muscarinic receptors in coronary smooth muscle. This balance is altered by the dose of ACh injected10–12). In this study, the greatest dilator response from baseline among the responses to 3 ACh doses (5, 10 and 50 µg/min) was selected as the epicardial coronary vasomotor response to ACh for each patient and control subject. The epicardial coronary response with the least constriction from baseline among the 3 ACh doses was selected for each patient who did not have a dilator response to any ACh dose. Similarly, the greatest increase in coronary flow response from baseline among the 3 ACh doses was selected as the coronary flow response to ACh for each patient and control subject. The coronary vasomotor dysfunction cut-off values in response to ACh were arbitrarily defined as the lower 10% of the distribution of coronary vasomotor responses to ACh in control subjects. The yield was 4.9% dilation from baseline for the epicardial coronary dilator response and 133% increase from baseline for coronary flow response in control subjects.
Intravascular Ultrasound (IVUS) Image Acquisition
After intracoronary administration of nitroglycerin, the IVUS catheter was positioned in the distal LAD segment and withdrawn to the coronary ostium using automated pullback at 0.5 mm/sec using 40-MHz IVUS imaging catheters (Galaxy 2, Boston Scientific Corporation, Natick, MA)17). The volumetric IVUS analyses were performed with a computerized planimetry program (EchoPlaque 3.0, INDEC System, Inc., Mountain View, CA) by an independent examiner blinded to the patients' clinical characteristics17). The study analyzed the 3 mm segment with the greatest plaque located within a 5 - 25 mm segment that started 5 mm distal to the LAD's distal stent edge (Fig. 2). External elastic membrane (EEM) and lumen areas were obtained in every frame (0.1 mm thickness) by manual contour detection. The percent atheroma volume (PAV) of the plaque with the 3-mm segment was calculated as ∑ (EEM cross-section area (CSA) – lumen CSA) divided by ∑EEM CSA and multiplied by 100. The total atheroma volume (TAV) of the plaque with the 3-mm segment was calculated as ∑ (EEM CSA — lumen CSA). Each analyzed segment was referenced to a specific anatomic landmark, including the stent for identification. IVUS analyses at admission and six months after AMI were examined in parallel. The exact distance of the targeted segment from the distal edge of the stent was recorded as a landmark on the longitudinal image of IVUS in each patient to ensure that the same targeted segment was measured at the 1st and 2nd tests. Measurements of the PAV and TAV by the two independent observers (D.F., K.N.) were highly reproducible (r = 0.98 and 0.98, mean difference: 0.28 ± 1.16% and 0.40 ± 1.22 mm3, respectively).
Fig. 2.
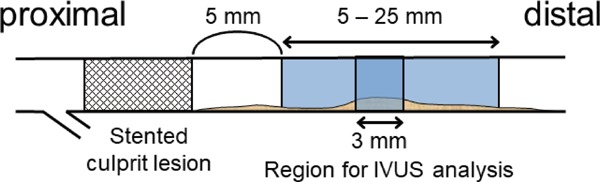
A schematic representation of the coronary segments used for intravascular ultrasound (IVUS) analysis. The study analyzed a 3-mm segment with the greatest plaque located within a 5 - 25 mm segment that started 5 mm distal to the distal edge of the stent in the infarct-related coronary artery (LAD).
Statistical Analysis
Data are expressed as either the mean ± SD, median and interquartile range (25th and 75th percentile), or frequencies (%). Continuous variables were compared using Student's paired or unpaired t-test, as appropriate. The Shapiro-Wilk test showed that systolic blood pressure (BP), fasting blood glucose (FBG), hemoglobin A1c (HbA1c), triglyceride, high-density lipoprotein cholesterol (HDL-C), C-reactive protein (CRP), and brain natriuretic peptide (BNP) were not distributed normally. Therefore, these variables were expressed as the median and interquartile range (25th and 75th percentiles), and were compared after log-transformation. Categorical variables between two groups were compared using a chi-square analysis or Fisher's exact test. Correlation between two groups was examined using linear regression analysis. Logistic regression analysis was used to associate coronary plaque volume progression with coronary vasomotor function and the other clinical data. Statistical significance was defined as p < 0.05. Analyses were assessed in part using STATA 10.0 (StataCorp, College Station, TX, USA).
Based on our preliminary observations in the STEMI patients who had LAD occlusion and successful reperfusion therapy, the progression of PAV during the six-month follow-up period occurred in approximately 50% of patients with impaired endothelial flow response to ACh in the LAD at both 1st and 2nd tests, and in 5% of the remaining patients. To provide our two-sided statistical analyses with sufficient statistical power of 0.90 (β = 0.10 and α = 0.05), 46 patients with STEMI were required to show the statistically significant difference in the prevalence of PAV progression between patients with and without persistent impairment of coronary flow response to ACh at both the 1st and 2nd tests. Sixty-three patients provided this study with sufficient statistical power.
Results
Study Patients
Sixty-three patients were finally included in this study, and they had the 1st test (Fig. 1). Among them, 13 patients were withdrawn from the study after the 1st test (Fig. 1), as one patient had a cardiovascular event between the 1st and 2nd tests, eight had a high constrictor response (> 30% from baseline) of the epicardial segment to ACh at the 1st test, and one refused to take the 2nd test. Three had new angiographic organic stenosis (> 30%) in the lesion proximal to the proximal edge of the stent at the 2nd test. Moderate stenosis (> 30%) in the conduit coronary artery might affect an increase in coronary blood flow18). The remaining 50 patients completed both the 1st and 2nd tests (Fig. 1). This study finally analyzed the 50 patients who completed the study protocol (Fig. 1). Their clinical characteristics are shown in Table 1.
Comparison of Clinical Parameters between Patients with and without Progression of PAV or TAV
As a whole, patients' PAV and TAV at the target lesion decreased over six months (PAV, 41.1 ± 12.7% at the 1st test vs. 38.6 ± 12.0% at the 2nd test, p = 0.02; TAV, 13.6 ± 8.1 mm3 at the 1st test vs. 11.7 ± 7.0 mm3 at the 2nd test, p < 0.01)(Fig. 3). However, PAV and TAV increased over six months in 18 and 14 patients, respectively (Tables 2 and 3). There was no significant difference in traditional risk factors, the rate of achievement of risk factors' target levels, medication use at the 1st and 2nd tests, or PCI-related variables between patients with and without progression of PAV or TAV (Table 1 and Supplemental Table 1). LDL-C and BNP levels significantly decreased from the 1st to the 2nd test in both patients with and without progression of PAV or TAV (Table 1 and Supplemental Table 1). The prevalence of current smoking was reduced, and the number of patients with LDL-C levels < 100 mg/dL increased from the 1st to the 2nd test in both patients with and without progression of PAV or TAV (Table 1 and Supplemental Table 1).
Fig. 3.
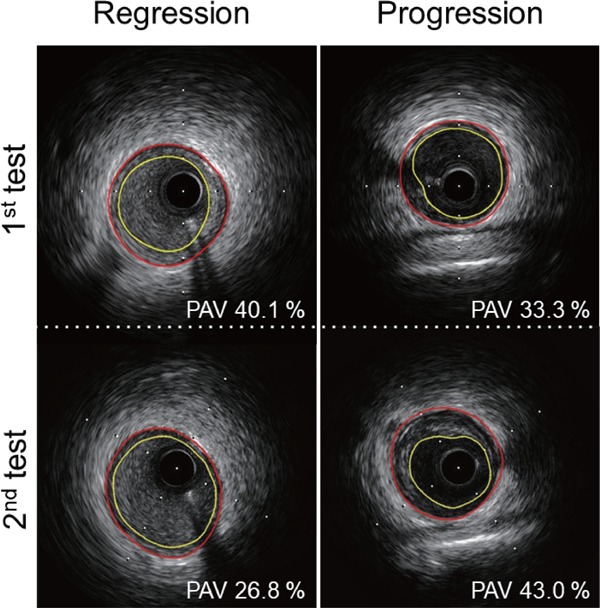
Representative IVUS images of PAV with regression (left panels) and images of PAV with progression (right panels) from the 1st to the 2nd tests. Yellow line indicates lumen; red line indicates the boundary of the lamina elastica externa.
Table 2. Comparisons of Epicardial Coronary Diameter and Coronary Blood Flow at Baseline, Their Responses to ACh and SNP, and IVUS Parameters between Patients with and without PAV Progression in the Infarct-related Coronary Arteries.
| PAV progression |
|||
|---|---|---|---|
| With (n = 18) | Without (n = 32) | p-value | |
| Responses to ACh (%) | |||
| Coronary diameter at the 1st test | 2.5 ± 12.0 | −1.1 ± 10.9 | 0.28 |
| Coronary diameter at the 2nd test | 6.7 ± 18.1 | 14.8 ± 11.4* | 0.10 |
| Coronary flow at the 1st test | 99 ± 95 | 83 ± 70 | 0.49 |
| Coronary flow at the 2nd test | 159 ± 150 | 199 ± 113* | 0.28 |
| Responses to SNP (%) | |||
| Coronary diameter at the 1st test | 15.2 ± 8.1 | 14.9 ± 9.2 | 0.91 |
| Coronary diameter at the 2nd test | 22.0 ± 16.7 | 17.5 ± 10.7 | 0.26 |
| Coronary flow at the 1st test | 108 ± 90 | 144 ± 117 | 0.26 |
| Coronary flow at the 2nd test | 182 ± 129 | 147 ± 98 | 0.29 |
| Baseline measurements before ACh injection | |||
| Coronary diameter at the 1st test (mm) | 1.6 ± 0.2 | 1.7 ± 0.3 | 0.06 |
| Coronary diameter at the 2nd test (mm) | 1.6 ± 0.4 | 1.7 ± 0.3 | 0.07 |
| Coronary flow at the 1st test (mL/min) | 21 ± 9 | 24 ± 9 | 0.28 |
| Coronary flow at the 2nd test (mL/min) | 19 ± 11 | 25 ± 12 | 0.10 |
| IVUS measurements | |||
| Vessel volume at the 1st test (mm3) | 31.3 ± 19.3 | 32.4 ± 10.1 | 0.79 |
| Vessel volume at the 2nd test (mm3) | 28.4 ± 18.5 | 30.4 ± 10.2 | 0.60 |
| Lumen volume at the 1st test (mm3) | 19.2 ± 11.3 | 18.0 ± 6.3 | 0.56 |
| Lumen volume at the 2nd test (mm3) | 16.4 ± 10.3 | 18.9 ± 7.4 | 0.42 |
| PAV at the 1st test (%) | 36.5 ± 10.6 | 43.8 ± 13.3 | 0.06 |
| PAV at the 2nd test (%) | 40.6 ± 10.8* | 37.5 ± 12.7* | 0.39 |
Data are expressed as the mean ± S.D.
p < 0.05 vs. the respective value at the 1st test.
SNP, sodium nitroprusside; IVUS, intravascular ultrasound; other abbreviations are as in Table 1.
Table 3. Comparisons of Epicardial Coronary Diameter and Coronary Blood Flow at Baseline, Their Responses to ACh and SNP, and IVUS Parameters between Patients with and without TAV Progression in the Infarct-related Coronary Arteries.
| TAV progression |
|||
|---|---|---|---|
| With (n = 14) | Without (n = 36) | p-value | |
| Responses to ACh (%) | |||
| Coronary diameter at the 1st test | −0.9 ± 12.5 | 0.6 ± 11.0 | 0.68 |
| Coronary diameter at the 2nd test | 2.3 ± 15.6 | 15.6 ± 12.4* | < 0.01 |
| Coronary flow at the 1st test | 93 ± 100 | 87 ± 71 | 0.80 |
| Coronary flow at the 2nd test | 114 ± 94 | 212 ± 129* | 0.01 |
| Responses to SNP (%) | |||
| Coronary diameter at the 1st test | 12.9 ± 5.2 | 15.8 ± 9.7 | 0.31 |
| Coronary diameter at the 2nd test | 19.0 ± 15.8 | 19.2 ± 12.3 | 0.97 |
| Coronary flow at the 1st test | 104 ± 69 | 142 ± 119 | 0.17 |
| Coronary flow at the 2nd test | 133 ± 85 | 171 ± 118 | 0.28 |
| Baseline measurements before ACh injection | |||
| Coronary diameter at the 1st test (mm) | 1.6 ± 0.3 | 1.7 ± 0.3 | 0.51 |
| Coronary diameter at the 2nd test (mm) | 1.6 ± 0.4 | 1.6 ± 0.4 | 0.41 |
| Coronary flow at the 1st test (mL/min) | 22 ± 8 | 23 ± 10 | 0.91 |
| Coronary flow at the 2nd test (mL/min) | 20 ± 10 | 24 ± 12 | 0.39 |
| IVUS measurements | |||
| Vessel volume at the 1st test (mm3) | 27.0 ± 20.6 | 33.9 ± 10.0 | 0.10 |
| Vessel volume at the 2nd test (mm3) | 28.6 ± 20.0 | 30.1 ± 10.5 | 0.69 |
| Lumen volume at the 1st test (mm3) | 17.2 ± 14.0 | 19.1 ± 6.1 | 0.52 |
| Lumen volume at the 2nd test (mm3) | 17.7 ± 13.4 | 18.3 ± 6.8 | 0.88 |
| TAV at the 1st test (mm3) | 10.1 ± 8.4 | 14.9 ± 7.7 | 0.06 |
| TAV at the 2nd test (mm3) | 11.3 ± 8.5* | 11.8 ± 6.5* | 0.82 |
Supplementary Table 1. Comparisons of Clinical Characteristics between Patients with and without Progaression of Total Atheroma Volume (TAV) in the Infarct-related Coronary Artery.
| TAV progression |
|||
|---|---|---|---|
| With (n = 14) | Without (n = 36) | p-value | |
| Age, years | 63 ± 14 | 63 ± 11 | 0.99 |
| Sex, male, n (%) | 9 (64.3) | 32 (88.9) | 0.83 |
| Hypertension, n (%) | 10 (71.4) | 23 (63.9) | 0.61 |
| Diabetes mellitus, n (%) | 3 (21.4) | 5 (13.9) | 0.51 |
| Measurements at the 1st test | |||
| BMI (kg/m2) | 25.4 ± 6.7 | 24.3 ± 2.3 | 0.56 |
| Systolic BP (mmHg) | 104 (95–116) | 109 (100–120) | 0.19 |
| FBG (mg/dL) | 109 (95–119) | 95 (88–105) | 0.62 |
| HbA1c (%) | 5.9 (5.6–6.6) | 6.0 (5.7–6.3) | 0.13 |
| Triglyceride (mg/dL) | 162 (135–202) | 140 (106–173) | 0.06 |
| HDL-C (mg/dL) | 39 (34–47) | 41 (36–50) | 0.76 |
| LDL-C (mg/dL) | 134 ± 28 | 142 ± 34 | 0.42 |
| CRP (mg/dL) | 0.3 (0.2–1.1) | 0.7 (0.4–1.1) | 0.31 |
| BNP (pg/mL) | 158 (92–249) | 58 (29–153) | 0.04 |
| LVEF (%) | 51 ± 10 | 57 ± 11 | 0.10 |
| Measurements at the 2nd test | |||
| BMI (kg/m2) | 25.2 ± 6.4 | 24.1 ± 2.3 | 0.54 |
| Systolic BP (mmHg) | 118 (103–136) | 117 (108–129) | 0.75 |
| FBG (mg/dL) | 97 (91–124) | 99 (91–110) | 0.38 |
| HbA1c (%) | 6.0 (5.8–6.5) | 6.0 (5.7–6.4) | 0.55 |
| Triglyceride (mg/dL) | 179 (117–264) | 125 (92–168) | 0.07 |
| HDL-C (mg/dL) | 43 (38–48) | 45 (38–50) | 0.84 |
| LDL-C (mg/dL) | 82 ± 30* | 86 ± 23* | 0.62 |
| CRP (mg/dL) | 0.1 (0.0–0.5) | 0.1 (0.0–0.1)* | 0.16 |
| BNP (pg/mL) | 52 (18–158)* | 24 (15–63)* | 0.27 |
| LVEF (%) | 55 ± 13 | 60 ± 11 | 0.19 |
| Risk status at the 1st test, n (%) | |||
| Current smoking | 5 (35.7) | 17 (47.2) | 0.46 |
| Pts with BP < 140/90 | 12 (85.7) | 30 (83.3) | 0.84 |
| Pts with HbA1c < 7.0% | 11 (78.6) | 32 (88.9) | 0.30 |
| Pts with LDL-C < 100 | 2 (14.3) | 2 (5.6) | 0.31 |
| Risk status at the 2nd test, n (%) | |||
| Current smoking | 0 (0.0) | 1 (2.8)* | 0.53 |
| Pts with BP < 140/90 | 10 (71.4) | 30 (83.3) | 0.10 |
| Pts with HbA1c < 7.0% | 11 (78.6) | 34 (94.4) | 0.09 |
| Pts with LDL-C < 100 | 9 (64.3)* | 28 (77.8)* | 0.18 |
| Medications at the 1st test, n (%) | |||
| Beta-blocker | 9 (64.3) | 14 (38.9) | 0.11 |
| ACE-I/ARB | 10 (71.4) | 25 (69.4) | 0.89 |
| Statin | 14 (100) | 31 (86.1) | 0.14 |
| Biganide | 0 (0.0) | 2 (5.6) | 0.37 |
| Aspirin | 14 (100) | 36 (100) | - |
| Thienopyridines | 14 (100) | 36 (100) | - |
| Medications at the 2nd test, n (%) | |||
| Beta-blocker | 9 (64.3) | 15 (41.7) | 0.15 |
| ACE-I/ARB | 13 (92.9) | 26 (72.2) | 0.11 |
| Statin | 14 (100) | 34 (94.4) | 0.37 |
| Biganide | 0 (0.0) | 2 (5.6) | 0.37 |
| Aspirin | 14 (100) | 35 (97.2) | 0.53 |
| Thienopyridines | 14 (100) | 36 (100) | - |
| AMI variables | |||
| Peak CPK (IU/L) | 2808 ± 1739 | 3112 ± 2357 | 0.66 |
| Use of BMS, n (%) | 4 (28.6) | 7 (19.4) | 0.48 |
| Use of 1st generation DES, n (%) | 1 (7.1) | 7 (19.4) | 0.51 |
| Use of 2nd generation DES, n (%) | 9 (64.3) | 22 (61.1) | 0.65 |
| Stent length (mm) | 28.7 ± 12.6 | 23.8 ± 11.2 | 0.16 |
| MLD after stenting (mm) | 2.9 ± 0.5 | 3.1 ± 0.4 | 0.19 |
Data are expressed as the mean ± S.D, median (25th–75th percentiles) or the number (%) of patients.
p < 0.05 vs. the respective value at the 1st test.
TAV, total atheroma volume; Pts, patients; BMI, body mass index; BP, blood pressure; FBG, fasting blood glucose; HbA1c, hemoglobin A1c; HDL-C, high-density lipoprotein cholesterol; LDL-C, low-density lipoprotein cholesterol; BNP, brain natriuretic peptide; CRP, C-reactive protein; LVEF, left ventricular ejection fraction; BP, blood pressure; ACE-I, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; AMI, acute myocardial infarction; CPK, creatine phosphokinase; PCI, percutaneous coronary intervention; BMS, bare metal stent; DES, drug eluting stent; MLD, minimal lumen diameter.
Comparison of Coronary Vasomotor Functions and IVUS Parameters between Patients with and without Progression of PAV or TAV
The responses of epicardial coronary diameter and coronary flow to ACh, but not SNP, improved significantly from the 1st to the 2nd test in patients without progression of PAV or TAV (Tables 2 and 3). The epicardial coronary diameter and coronary flow responses to ACh at the 1st test were similar between patients with and without progression of PAV or TAV (Tables 2 and 3). The vasomotor responses to ACh at the 2nd test were significantly lower in patients with progression of TAV (Table 3). The vasomotor responses to ACh at the 2nd test tended to be lower in patients with progression of PAV, but there was no statistical significance (Table 2). The coronary vasomotor responses to SNP at either the 1st or 2nd test were comparable between patients with and without PAV or TAV progression (Tables 2 and 3). The baseline coronary diameter and flow before the injection of ACh or SNP were similar between the 1st and 2nd tests in patients with and without progression of PAV or TAV (Tables 2 and 3). Pre-existing PAV and TAV of the coronary lesion at the 1st test were similar between patients with and without progression of PAV or TAV (Tables 2 and 3).
Logistic Regression Analysis for Association of Progression of PAV and TAV with Traditional Risk Factors, Coronary Vasomotor Function, and Their Changes
The progression of PAV or TAV over six months had no significant association with traditional risk factors at either the 1st or 2nd test (Table 4). The epicardial coronary diameter and coronary flow responses to ACh at the 2nd test were significantly associated with progression of TAV. In contrast, the responses to ACh at the 1st test were not significantly associated with PAV and TAV progression. The epicardial coronary diameter and flow responses to SNP at both the 1st and 2nd tests were not associated with progression of PAV or TAV. We classified patients into two groups according to the epicardial coronary diameter response to ACh: patients who had epicardial coronary vasomotor dysfunction at both the 1st and 2nd tests (persistently impaired group); and patients who had epicardial vasomotor dysfunction at either the 1st or the 2nd test, or neither of the two tests (other group). Similarly, patients were classified to the two groups according to their coronary flow response to ACh. The progression of PAV or TAV over six months was significantly associated with persistent impairment of epicardial coronary diameter and the coronary flow responses to ACh (Table 4).
Table 4. Logistic Regression Analysis for Association of Progression of PAV and TAV with Clinical Parameters.
| PAV progression |
TAV progression |
|||
|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |
| Age | 1.01 (0.96–1.06) | 0.76 | 1.00 (0.95–1.06) | 0.99 |
| Male gender | 1.54 (0.36–6.68) | 0.56 | 4.44 (0.98–20.09) | 0.06 |
| DES | 1.02 (0.25–4.10) | 0.98 | 1.66 (0.40–6.88) | 0.49 |
| Hypertension | 0.96 (0.28–3.24) | 0.94 | 0.71 (0.19–2.71) | 0.61 |
| Diabetes mellitus | 0.93 (0.19–4.43) | 0.93 | 0.59 (0.12–2.89) | 0.51 |
| Current smoking at the 1st test | 1.39 (0.43–4.49) | 0.59 | 1.61 (0.45–5.76) | 0.46 |
| LDL-C at the 1st test | 1.00 (0.99–1.02) | 0.73 | 0.99 (0.97–1.01) | 0.41 |
| LDL-C at the 2nd test | 0.99 (0.97–1.02) | 0.64 | 0.99 (0.97–1.02) | 0.55 |
| BNP at the 1st test | 0.99 (0.98–1.01) | 0.65 | 1.01 (0.99–1.01) | 0.21 |
| BNP at the 2nd test | 1.00 (0.99–1.01) | 0.99 | 1.01 (0.99–1.01) | 0.10 |
| CRP at the 1st test | 0.60 (0.27–1.33) | 0.21 | 0.91 (0.59–1.41) | 0.68 |
| CRP at the 2nd test | 1.04 (0.74–1.47) | 0.38 | 1.11 (0.79–1.57) | 0.54 |
| Responses to ACh | ||||
| Coronary diameter at the 1st test | 1.03 (0.98–1.09) | 0.28 | 0.99 (0.94–1.04) | 0.68 |
| Coronary diameter at the 2nd test | 0.96 (0.92–1.01) | 0.07 | 0.93 (0.87–0.98) | < 0.01 |
| Coronary flow at the 1st test | 1.00 (0.99–1.01) | 0.49 | 1.00 (0.99–1.01) | 0.79 |
| Coronary flow at the 2nd test | 0.99 (0.98–1.01) | 0.28 | 0.99 (0.98–0.99) | 0.02 |
| Responses to SNP | ||||
| Coronary diameter at the 1st test | 1.00 (0.94–1.08) | 0.90 | 0.96 (0.89–1.04) | 0.31 |
| Coronary diameter at the 2nd test | 1.03 (0.98–1.07) | 0.25 | 0.99 (0.95–1.05) | 0.97 |
| Coronary flow at the 1st test | 1.00 (0.99–1.01) | 0.26 | 0.99 (0.99–1.01) | 0.27 |
| Coronary flow at the 2nd test | 1.00 (0.99–1.01) | 0.29 | 0.99 (0.99–1.01) | 0.28 |
| Persistent impairment of responses to ACh | ||||
| Coronary diameter | 6.15 (1.35–28.13) | 0.02 | 6.00 (1.36–26.45) | 0.02 |
| Coronary flow | 4.33 (1.20–15.61) | 0.03 | 5.52 (1.44–21.14) | 0.01 |
Linear Correlation of the Percent Changes in PAV and TAV with the Difference in Epicardial Coronary Diameter and Coronary Flow Responses to ACh from the 1st to the 2nd test
The change in PAV or TAV over six months had a significant inverse correlation with the difference in epicardial coronary diameter and coronary flow response to ACh from the 1st to 2nd test (Fig. 4). In contrast, the changes in PAV or TAV over six months had no significant correlation with the difference in the coronary responses to SNP between the 1st and 2nd tests (changes in PAV, r = −0.06 and 0.11, respectively, with the difference in epicardial coronary diameter and flow response; changes in TAV, r = −0.06 and −0.01, respectively, with the difference in epicardial coronary diameter and flow response).
Fig. 4.
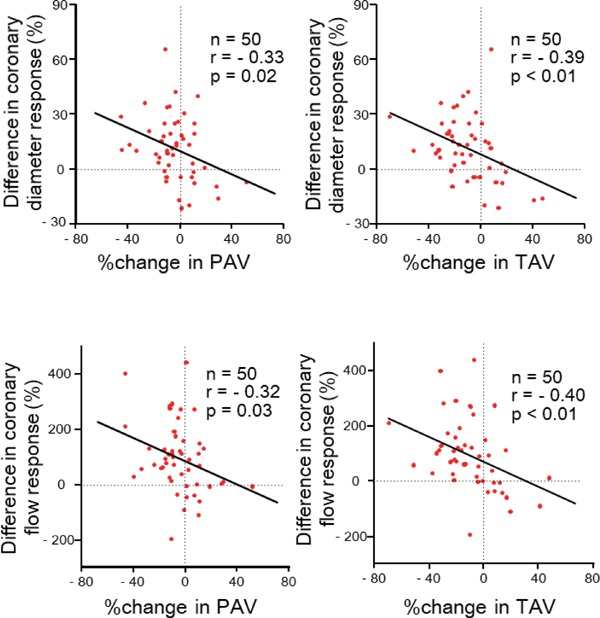
Correlations of the percent changes in PAV (left panels) and TAV (right panels) over six months from the 1st to 2nd test with the difference in responses of epicardial coronary diameter (upper panels) and coronary flow (lower panels) to ACh from the 1st to the 2nd test.
Discussion
The present study showed that the percent changes in PAV or TAV over six months of follow-up were inversely correlated with the changes in epicardial coronary diameter and coronary flow responses to ACh, but not SNP, an endothelium-independent vasodilator, in the infarct-related coronary artery in the STEMI patients. In addition, the progression of PAV and TAV was associated with persistently impaired coronary vasomotor responses to ACh. This is the first study to show that persistently impaired endothelial vasomotor function was related to atheroma plaque progression in the entire infarct-related coronary artery tree of STEMI survivors. The longer endothelial dysfunction persisted, the greater the progression of atherosclerotic plaque in the infarct-related coronary artery of STEMI survivors.
Endothelial vasomotor function at baseline (1st test) did not predict plaque progression in the infarct-related coronary artery in the present study. A single assessment of endothelial vasomotor function may not necessarily reflect later atherosclerotic risk burden in the coronary artery because anti-atherosclerotic treatments were initiated after MI onset. In addition, reperfusion injury and DES transiently exacerbate endothelial dysfunction at baseline11, 12). Changeable endothelial function after the baseline measurement may explain the association of coronary plaque progression with the change in the endothelial vasomotor function over time, but not with the baseline endothelial function in the infarct-related coronary artery in the present study. Coronary arterial remodeling affects the measurement of PAV but not TAV. Coronary vasomotor responses similarly related to PAV and TAV, a marker of coronary atherosclerotic burden, which may strengthen our hypothesis that coronary endothelial dysfunction is related to the progression of coronary atherosclerotic burden.
The present study showed that systemic atherosclerotic risk burden at both the 1st and 2nd tests was not associated with coronary plaque progression over six months, a relatively short follow-up period, in the infarct-related coronary artery. Thus, the systemic atherosclerotic risk burden was unlikely to mediate the relationship between coronary endothelial dysfunction and plaque progression in the infarct-related coronary artery in STEMI survivors. Although the traditional atherosclerotic risk factors were not appropriately treated in some study patients, the rate of target level achievement of the risk factors and the atherosclerotic risk-factor burden at the 2nd test were similar in patients with and without coronary plaque progression. Undetermined residual risks or genetic predisposition may also exist in patients who did not respond to interventions to reduce atherosclerotic risk factors. These non-responders are at considerable risk of further coronary events19).
Residual atherosclerotic plaques in the infarct-related coronary artery are associated with a greater risk of re-occlusion and may predispose to coronary events at follow-up6). To prevent progression of atherosclerotic plaque in the infarct-related coronary artery, attenuating reperfusion-induced vascular injury and DES-induced endothelial dysfunction may be required in addition to anti-atherosclerotic therapies. We previously showed that the cypher stent inhibited recovery of reperfusion-induced endothelial dysfunction in the infarct-related coronary artery11). Second generation stents are currently recommended, as they have less of a suppressive effect on endothelial function20).
Study Limitations
First, this study excluded patients with residual organic stenosis (> 30% stenosis) or a high constrictor response (> 30% from baseline) of the epicardial segment to ACh in the LAD. In addition, the study excluded patients who had new lesions (> 30% stenosis) in the LAD during the follow-up period. This exclusion was required because even moderate stenosis (> 30%) in the conduit coronary artery may increase coronary blood flow in response to ACh18). Second, risk-factor modification was not optimal for some of present study's patients. However, it was unlikely that insufficient risk-factor reduction was the main cause of plaque progression in the present patients, because the rate of attainment of target levels of risk factors was similar between patients with and without coronary plaque progression. Third, we arbitrarily determined the cut-off value for impaired coronary vasomotor responses from the distribution of these responses in control subjects. There are few previous studies using cut-off values for coronary endothelial vasomotor dysfunction. The methods of measuring coronary vasomotor response to ACh vary among studies. Future studies are needed to confirm whether the cut-off values used in this study are appropriate. Fourth, the coronary segment analyzed for epicardial coronary vasomotion by quantitative coronary angiography was not necessarily identical to the segment analyzed for plaque progression by IVUS because of technical issues with quantitative coronary angiography in some patients. Fifth, this study did not analyze the relationship of coronary vasomotor responses with coronary plaque composition. Sixth, approximately 20% of the patients were excluded after the 1st test. This exclusion may confound the results.
Conclusion
Persistent impairment of endothelial vasomotor function in the conduit arterial segment and the resistance arteriole was related to atheromatous plaque progression in the infarct-related coronary arteries of STEMI survivors.
Acknowledgment
None.
Notice of Grant Support
This research was supported by the Ministry of Education, Culture, Sports, Science, and Technology, Health, Tokyo, Japan (grants-in-aid for B2-19390209 and B-22390158).
Conflicts of Interests
K.K. has received scholarship donations from Takeda, Daiichi Sankyo, Astellas, Boehringer Ingelheim, MSD, Boston Scientific Japan, Abbott, Medtronic, Biotronik Japan, and ST Jude Medical. The other authors declare no conflicts of interest.
References
- 1). Schachinger V, Britten MB, Zeiher AM: Prognostic impact of coronary vasodilator dysfunction on adverse long-term outcome of coronary heart disease. Circulation, 2000; 101: 1899-1906 [DOI] [PubMed] [Google Scholar]
- 2). Halcox JP, Schenke WH, Zalos G, Mincemoyer R, Prasad A, Waclawiw MA, Nour KR, Quyyumi AA: Prognostic value of coronary vascular endothelial dysfunction. Circulation, 2002; 106: 653-658 [DOI] [PubMed] [Google Scholar]
- 3). Meredith I, Anderson T, Uehata A, Yeung A, Selwyn A, Ganz P: Role of Endothelium in lschemic Coronary Syndromes. Am J Cardiol, 1993; 72: 27C-31C [DOI] [PubMed] [Google Scholar]
- 4). Vanhoutte PM: Endothelial dysfunction: the first step toward coronary arteriosclerosis. Circ J, 2009; 73: 595-601 [DOI] [PubMed] [Google Scholar]
- 5). Bonetti PO, Lerman LO, Lerman A: Endothelial dysfunction: a marker of atherosclerotic risk. Arterioscler Thromb Vasc Biol, 2003; 23: 168-175 [DOI] [PubMed] [Google Scholar]
- 6). Chen L, Crook JR, Tousoulis D, Chester MR, Kaski JC: Complex stenosis morphology predicts late reocclusion during follow-up after myocardial infarction in patients with patent infarct-related coronary arteries. Am Heart J, 1998; 136: 877-883 [DOI] [PubMed] [Google Scholar]
- 7). Okumura K, Yasue H, Matsuyama K, Ogawa H, Morikami Y, Obata K, Sakaino N: Effect of acetylcholine on the highly stenotic coronary artery: Difference between the constrictor response of the infarct-related coronary artery and that of the noninfarct-related artery. Journal of the American College of Cardiology, 1992; 19: 752-758 [DOI] [PubMed] [Google Scholar]
- 8). Vita JA, Treasure CB, Nabel EG, McLenachan JM, Fish RD, Yeung AC, Vekshtein VI, Selwyn AP, Ganz P: Coronary vasomotor response to acetylcholine relates to risk factors for coronary artery disease. Circulation, 1990; 81: 491-497 [DOI] [PubMed] [Google Scholar]
- 9). Quyyumi AA, Dakak N, Andrews NP, Husain S, Arora S, Gilligan DM, Panza JA, Cannon RO, 3rd: Nitric oxide activity in the human coronary circulation. Impact of risk factors for coronary atherosclerosis. J Clin Invest, 1995; 95: 1747-1755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10). Kugiyama K, Doi H, Motoyama M, Soejima H, Misumi K, Kawano H, Nakagawa O, Michihiro Yoshimura M, Ogawa H, Matsumura T, Sugiyama S, Nakano T, Nakajima K, Yasue H: Association of Remnant Lipoprotein Levels With Impairment of Endothelium-Dependent Vasomotor Function in Human Coronary Arteries. Circulation, 1998; 97: 2519-2526 [DOI] [PubMed] [Google Scholar]
- 11). Obata JE, Kitta Y, Takano H, Kodama Y, Nakamura T, Mende A, Kawabata K, Saitoh Y, Fujioka D, Kobayashi T, Yano T, Kugiyama K: Sirolimus-eluting stent implantation aggravates endothelial vasomotor dysfunction in the infarct-related coronary artery in patients with acute myocardial infarction. J Am Coll Cardiol, 2007; 50: 1305-1309 [DOI] [PubMed] [Google Scholar]
- 12). Obata JE, Nakamura T, Kitta Y, Kodama Y, Sano K, Kawabata K, Saitoh Y, Fujioka D, Kobayashi T, Yano T, Watanabe Y, Watanabe K, Kugiyama K: Treatment of acute myocardial infarction with sirolimus-eluting stents results in chronic endothelial dysfunction in the infarct-related coronary artery. Circ Cardiovasc Interv, 2009; 2: 384-391 [DOI] [PubMed] [Google Scholar]
- 13). Ku DD: Coronary vascular reactivity after acute myocardial ischemia. Science, 1982; 218: 576-578 [DOI] [PubMed] [Google Scholar]
- 14). VanBenthuysen KM, McMurtry IF, Horwitz LD: Reperfusion after acute coronary occlusion in dogs impairs endothelium-dependent relaxation to acetylcholine and augments contractile reactivity in vitro. J Clin Invest, 1987; 79: 265-274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15). Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M, Hochman JS, Krumholz HM, Kushner FG, Lamas GA, Mullany CJ, Ornato JP, Pearle DL, Sloan MA, Smith SC, Jr., Alpert JS, Anderson JL, Faxon DP, Fuster V, Gibbons RJ, Gregoratos G, Halperin JL, Hiratzka LF, Hunt SA, Jacobs AK, American College of Cardiology/American Heart Association Task Force on Practice G : ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction--executive summary: a report of the American College of Cardiology/ American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1999 Guidelines for the Management of Patients With Acute Myocardial Infarction). Circulation, 2004; 110: 588-636 [DOI] [PubMed] [Google Scholar]
- 16). Expert Panel on Detection E and Treatment of High Blood Cholesterol in A: Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III). JAMA, 2001; 285: 2486-2497 [DOI] [PubMed] [Google Scholar]
- 17). Futamata M, Matsuoka S, Shimizu T, Yoshizaki T, Obata JE, Nakamura T, Fujioka D, Watanabe Y, Nakamura K, Watanabe K, Kugiyama K: Echolucency of the carotid artery is associated with short-term plaque progression and positive remodeling in the culprit coronary artery in AMI survivors. J Cardiol, 2017; 70: 438-445 [DOI] [PubMed] [Google Scholar]
- 18). Gould KL, Lipscomb K: Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol, 1974; 34: 48-55 [DOI] [PubMed] [Google Scholar]
- 19). Flammer AJ, Anderson T, Celermajer DS, Creager MA, Deanfield J, Ganz P, Hamburg NM, Luscher TF, Shechter M, Taddei S, Vita JA, Lerman A: The assessment of endothelial function: from research into clinical practice. Circulation, 2012; 126: 753-767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20). Kim JW, Seo HS, Park JH, Na JO, Choi CU, Lim HE, Kim EJ, Rha SW, Park CG, Oh DJ: A prospective, randomized, 6-month comparison of the coronary vasomotor response associated with a zotarolimus- versus a sirolimus-eluting stent: differential recovery of coronary endothelial dysfunction. J Am Coll Cardiol, 2009; 53: 1653-1659 [DOI] [PubMed] [Google Scholar]


