Abstract
Amplified inflammatory reaction has been observed to be involved in cardiometabolic diseases such as obesity, insulin resistance, diabetes, dyslipidemia, and atherosclerosis. The complement system was originally viewed as a supportive first line of defense against microbial invaders, and research over the past decade has come to appreciate that the functions of the complement system extend beyond the defense and elimination of microbes, involving in such diverse processes as clearance of the immune complexes, complementing T and B cell immune functions, tissue regeneration, and metabolism. The focus of this review is to summarize the role of the activation of complement system and the initiation and progression of metabolic disorders including obesity, insulin resistance and diabetes mellitus. In addition, we briefly describe the interaction of the activation of the complement system with diabetic complications such as diabetic retinopathy, nephropathy and neuropathy, highlighting that targeting complement system therapeutics could be one of possible routes to slow down those aforementioned diabetic complications.
Keywords: Inflammation, Complement activation, Metabolic disorders, Obesity, Insulin resistance, Type 2 diabetic mellitus
Core tip: Inflammatory reaction is involved in cardiometabolic diseases such as obesity, insulin resistance, and diabetes. The complement system, a key component of innate immunity, was viewed as the first line of defense against microbial. Recent research has come to appreciate that the complement system is involved in such diverse processes as clearance of the immune complexes, complementing immune functions, tissue regeneration, and metabolism. The review is to update the role of the activation of complement system and the progression of metabolic disorders. We provided a paradigm that targeting complement therapeutics could be one of possible routes to slow down diabetic complications.
INTRODUCTION
The prevalence of type 2 diabetes mellitus (T2DM) is rapidly increasing globally and affects approximately one third of the worldwide population. The etiology of T2DM revolves around obesity and insulin resistance. Metabolic disorders like obesity[1-4], insulin resistance and diabetes mellitus[5-8] all possess inflammatory components. Although we are still in the early stage of fully understanding the relationship between inflammation and metabolic diseases, a wealth of data points to the fundamental role of inflammation in the initiation, development and progression of those metabolic disorders[9]. Activation of pattern recognition receptors (PRRs) that include NOD-like receptors (NLRs) and Toll-like receptors (TLRs) can initiate the immune responses by sensing both endogenous damage-associated molecular patterns (DAMPs) and exogenous pathogen-associated molecular patterns (PAMPs). Obesity-associated PAMPs and DAMPs have been revealed to trigger the NF-κB pro-inflammation pathway, and are associated with NLRP3 inflammasome activation in adipose tissue[10,11]. Several studies have revealed that metabolic inflammatory signaling can transcriptionally and post-transcriptionally interfere with insulin action and modulate metabolic pathways that promote insulin resistance[12,13]. The complement system is a central component of innate immunity and contributes substantially to homeostasis by eliminating infectious microbes, cellular debris, complementing immunological and inflammatory processes, and sending “danger” signals. The complement system was originally regarded as a supportive first line of defense against microbial invaders; however, research over the past decade has come to realize that the functions of the complement system extend far beyond the defense and elimination of microbes, partaking in such diverse processes including the clearance of immune complexes, mobilization of hematopoietic stem-progenitor cells, tissue regeneration, synapse maturation, angiogenesis, and lipid metabolism[14].
In this review paper we will look into the roles of several key components of the complement system in metabolic disorders and provide an updated overview of the complement system in the pathophysiology and development of obesity, insulin resistance, diabetic mellitus, and diabetes-related complications. In this way, we aim to elucidate the function of the complement system in the pathogenesis of metabolic disorders as well as highlight the complement system as a target for future potential therapeutics.
COMPLEMENT SYSTEM
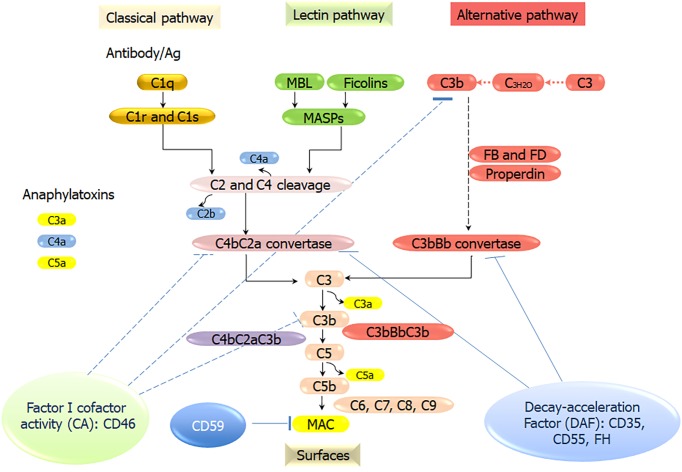
The complement system, a crucial part of the human innate immune system, comprises of more than 50 soluble protein receptors and regulators in the plasma and cell membrane that act in a highly coordinated way to kill microbes, send danger signals, and expedite the elimination of apoptotic cells without damaging the healthy host cells. The complement system can be activated through PRRs that have evolved to recognize PAMPs and DAMPs, which include specific antibodies, mannan-binding lectin (MBL), ficolins, C-reactive proteins (CRP), C1q, and natural IgM. These PRRs activate three major complement pathways: The classical pathway (CP), the mannose-binding lectin pathway (LP), and the alternative pathway (AP). CP activation is initiated by the association of C1q with immune complexes (IgG or IgM) to form a complex with serine proteases C1r and C1s. The complex cleaves C4, then associates with the cleavage product C4b forming a new complex, which cleaves C2 (into C2a and C2b) to form the C3 convertase C4bC2b. The LP activation starts by the association of MBL/ficolins/collectin 11 to the mannose residues on microbial surfaces. MBL-associated serine proteases (MASP)-1, MASP-2, and MASP-3 are activated by the binding of MBL to microbial surfaces, which function like C1r and C1s, and cleave C4 and C2 to form the same C3 convertase as that of CP. The AP is distinctive as it is activated without PRRs. Instead, a “tick over” mechanism starts with the spontaneous hydrolysis of C3 into C3(H2O), which acts similar to C3b and binds factor B. The AP C3 convertase (C3bBb) is generated through a chain of reactions involving factor D and properdin[15]. C3 convertase cleaves C3 into C3a and C3b. C3b then binds to the C3 convertase to form C5 convertases (C4bC2bC3b or C3bBbC3b), which cleaves C5 into C5a and C5b. C5b binds C6, C7, C8, and C9 molecules to form the membrane attack complex (MAC), the lytic machinery of the complement system (Figure 1). The complement system is finely tuned by positive and negative regulators, which can avoid a potentially misdirected or excessive activation of the system. However, due to dysfunction of the complement system, this defense system can be inappropriately triggered to attack host cells, which contributes to a broad range of immune, inflammatory, and age-related diseases. For example, when the dysfunction of the complement system occurs in the regulatory proteins, Factor H, the powerful cell-killing property of the complement system can be turned against the host self, causing diseases like atypical Hemolytic uremic syndrome, and paroxysmal nocturnal hemoglobinuria[14,16-18]. Unrestrained or undue complement activation is now recognized as one of key pathogenic drivers in a wide variety of immune-mediated and inflammatory diseases, including hematological and ageing-related ocular pathologies, cancer, autoimmunity, oral dysbiotic diseases, neuroinflammatory, neurodegenerative, and metabolic disorders[17].
Figure 1.
Overview of the activation and regulation of the complement pathways. Three different recognition and activation pathways, including classical pathway (CP), lectin pathway (LP), and alternative pathway (AP). The CP is triggered by the formation of antigen-antibody immune complexes that bind to the C1 complex (C1q, C1r2, C1s2). The LP is activated by the binding of mannan-binding lectin (MBL), ficolins, or collectin 11 to carbohydrates/mannan/other pathogen-associated molecular patterns. MBL-associated serine proteases (MASP)-1, MASP-2, and MASP-3 are activated. Serine protease C1s/C1r (CP) or MASPs (LP) sequentially cleave C4 and C2 to form C3 convertase C4bC2b. The AP C3 convertase (C3bBb) is generated through a chain of reactions involving factor B, factor D and properdin. C3b then binds with the C3 convertase to form C5 convertases (C4bC2bC3b or C3bBbC3b), which cleaves C5 into C5a and C5b. C5b binds C6, C7, C8, and C9 molecules to form the membrane attack complex (MAC), the lytic machinery of the complement system. MBL: Mannan-binding lectin; MASP: Mannan-binding lectin-associated serine proteases; FB: Factor B; FD: Factor D; MAC: Membrane attack complex.
Complement system and inflammation
Acute and chronic inflammation is a recognized hallmark of many diseases. It has been understood now that the complement system acts as a sensor of pathogens and a contributor in various inflammatory diseases as well, especially those disorders with complement imbalances[17,18]. Insufficient control or excessive activation of the complement system can cause a malicious cycle between the complement system, inflammatory cells and tissue injury[16,17]. New findings have revealed an interesting cross-talk between the complement system and the inflammatory network[14,16,19-21]. During certain diseases and injuries, the complement and coagulation act in a coordinated fashion. Some enzymes of the coagulation system directly cleave and activate complement component C3 and C5; the products by such activation can affect coagulation. It was demonstrated that C5a, C5 activation fragment could stimulate tissue factor[22-24]. It has been recently recognized that the complement system and TLR, both described as “first line of defense” systems, are cooperated. The cross-talk between complement components CR3, C5aR, CD46, CD55, and gC1qR and TLR, CD14, and MyD88 of TLR system has been revealed to be important for antimicrobial defense but also contributes to inflammatory diseases[25-27]. It has been well described that the complement system essentially contributes to autoimmune and other diseases by the maturation and stimulation of B cells via CD21[28]. By directly acting on T cells or antigen presenting cells (APC), the complement system can modulate different phases of T cell immunity. Locally generated C3a and C5a stimulate a shift toward Th1 immunity by enhancing T cell proliferation and cytokine release on APC. In addition, complement regulator protein, CD46 was recognized as a key regulatory player in the IL-2-dependent conversion of Th1 cells[29,30]. C5aR and CD46 signaling were revealed to be associated with the function of γδT cells[31,32]. Conversely, the interaction of T cells and APC induces the enhanced secretion of C3, C5, Factor B, and Factor D with the down-regulation of CD55, which causes complement activation on immune cells. In addition, stimulation of C5aR promotes the expression of activated FcγR, by binding to autoantibodies to induce the local secretion of C5, hence activating the C5a/C5aR signaling pathway, revealing a positive feedback loop in cooperation of complement with FcγR to remove immune complexes[33]. Recent study discovered that high-galactose immune complexes associated with inhibitory FcγRIIB and lectin receptor dectin-1 could induce a signaling pathway that counteracts C5aR signaling pathway[34]. Studies have also shown that C1q is involved in age-related inflammation and tissue injury via upregulating the Wnt pathway that enhances age-related fibrotic muscle changes in mice skeletal muscle[35]. Lastly, the complement system has been demonstrated to regulate the functions of NKT and NK cells[36], myeloid-derived suppressor cells[37] and mast cells[38]. All these discoveries highlight the crucial functions of the complement system in the inflammatory network and indicate that dysregulation of the complement system may result in substantial clinical consequences by shifting the functions of T, B, and APC immune cells.
COMPLEMENT ACTIVATION IN METABOLIC DISORDERS
Complement activation in obesity
Many complement components can influence the biology of adipose tissue. T2MD arises from the setting of inflammation and is especially targeted in obesity-related adipose tissue, particularly in white adipose tissue (WAT), which is also the site of numerous substances involved in pro-inflammatory pathways[39]. WAT secretes active cytokines such as TNFα, CRP, interleukins, plasminogen activator/inhibitor, fibrinogen, monocyte chemoattractant protein-1 and the anti-inflammatory factor adiponectin. Obesity induces adipocytes to go through hypertrophy and hyperplasia and gets invaded by macrophages and other immune cells[40]. This leads to a shift leaning towards the production of more pro-inflammatory than anti-inflammatory adipokines that results in chronic, low-grade inflammation[41]. Adipocytes are a primary source of human factor D, which plays an important role in the activation of alternative complement pathway. Adipsin, the mouse homolog of factor D, is essential for the differentiation of pre-adipocytes, revealing that the function of the complement system is far beyond the defense against microbial intruders. Further studies demonstrated that components from alternative complement pathways, C3, Factor B, properdin, Factor H, and Factor I are expressed in adipose tissue, emphasizing the hypothesis that local complement activation can substantially affect adipose tissue biology. In an aged population, complement C3 has been described as a strong marker of insulin resistance[42]. New studies have shown that adipose tissue can activate the alternative complement pathway in T2DM, which exhibits characteristic of the low-grade inflammation[43,44]. Increased C3 levels are associated with inflammation via cross-talk with TLR4 action of enhancing the production of pro-inflammatory cytokines via C3aR and C5aR signaling pathways[45,46]. Additionally, significantly increased levels of other complement components C3 and Factor D were found significantly in obese individuals as well[43,47,48]. The expression of complement in adipocyte can be used as a proxy measure of adipose tissue insulin resistance[49]. C3adesArg, identical to serum-derived acylation stimulating factor (ASP) has an important role in adipose tissue biology[50]. C3adesArg enhances adipocyte triglyceride synthesis, and increases plasma triglyceride levels, which are expected to be part of the causes in increasing insulin resistance and leading to the development of metabolic syndromes[51]. Reduced glucose tolerance and delayed postprandial triglyceride and non-esterified fatty acids clearance were reported in both C3- and Factor B-deficient mice as compared with wild-type mice on low-fat and high-fat diets[52]. Moreover, increased plasma levels of C3adesArg are also observed in obese individuals and patients suffering from type II diabetes[14,53]. Furthermore, receptors for C3a (C3aR) and C5a (C5aR1 and C5aR2) have been documented to be expressed in adipocytes[49]. Thus, adipose, in addition to producing complement components, is a potential target of complement action as well. Upon high-fat diet feeding, C3aR expression is increased in WAT. Both macrophage and adipose tissue within WAT express significant amount of C3aR. C3aR-/- mice on high fat diet transiently resist diet-induced obesity during 8-wk period compared with wild type mice. Improvement in glucose tolerance and insulin resistance are revealed in C3aR-/- mice. Furthermore, macrophage from C3-/- mice were polarized to the M1 phenotype, which demonstrated a considerable decrease in pro-inflammatory functions. Obesity-associated PAMPs and DAMPs have also been shown to activate nucleotide-binding domain and leucine-rich repeat-containing (NLR) protein NLRP3 inflammasome in adipose tissue[54]. In summary, the effects of complement components ASP, Factor B, C3a, C5a, and of their receptors in adipose tissue play essential roles in adipose tissue biology as well as the development of metabolic disorder.
Complement activation in insulin resistance
Insulin resistance is linked with elevated circulating complement factor C3 level, which involves the terminal and alternative complement pathways. Accumulating evidence revealed that anaphylatoxin C3a interaction with its receptor C3aR plays a role in metabolic disorders such as diabetes, obesity, and atherosclerosis. Animal studies revealed that improper complement stimulation exacerbates high-fat-diet-induced insulin resistance. C3 is particularly studied in insulin resistance due to its cleavage product, C3adesArg, also known as ASP, which has insulin-like properties by stimulating the uptake of glucose and the synthesis of triglycerides in adipose tissue[55]. The development of insulin resistance can be further explained by understanding ASP resistance in adipose tissue. Obesity-induced adipose tissue activates the alternative complement pathway, thereby creating C3adesArg, among other factors. This leads to a pro-inflammatory state due to an influx of pro-inflammatory biomarkers leading to ASP resistance, which contributes to the development of insulin resistance[55]. Another explanation could be that activated macrophages from adipose tissue, which can produce pro-inflammatory cytokines, like IL-6 and TNF-α, which can inhibit the insulin receptor functions and thereby induce insulin resistance[56]. The increased levels of C3 production from adipocytes also leads to increased macrophage infiltration into adipose tissue, thereby exacerbating insulin resistance[57]. Additionally, it was found that C1q in the CP could cause adipocyte apoptosis, which in turn leads to the infiltration of macrophages into adipose tissue and causes insulin resistance[58,59]. Clearly the components from complement system, especially complement C3, play crucial roles in causing insulin resistance. Thus, inhibition of the C1 and/or C3 complement activation and/or its regulators could be potential therapeutics for insulin resistance.
Complement activation in diabetic mellitus
The role of complement activation in diabetes mellitus has not been fully investigated. Some components of the complement system are thought to be involved in both Type I and II diabetes mellitus. Nevertheless, accumulating experimental and clinical evidence demonstrates a connection between the activation of the complement system and the pathogenesis of diabetic mellitus[60-63]. Pro-inflammatory cytokines IL-6 and IL-1 enhance complement C3 production from the liver[64,65]. The C3 gene is also observed highly expressed in adipose tissue[66]. Pro-inflammatory cytokines generated in adipose tissue, an organ with endocrine functions, have been associated with insulin resistance and impaired glucose uptake[67-69]. Systemic low-grade inflammation with the actions cytokines might explain the connection between C3 and the incidence of diabetes. Another possible link between C3 and diabetes could be due to C3adesArg or ASP, the proteolytic fragment of C3, which is a paracrine metabolic factor that can stimulate glucose uptake and lipid storage in adipose tissue[70,71]. ASP insufficiency was linked to the reduction of body weight in mice[72], and the correlation of high ASP levels and insulin resistance was observed in nondiabetic subjects[43,73]. However, C3-knockout mice have shown only modest alterations in insulin function and glucose metabolism, suggesting the activation of the C3-ASP system might function differentially in human and mice[74]. Complement C3 also provides a key link between inflammation and thrombosis in diabetes. It was reported that complement C3 interacts with fibrin leading to prolonged fibrinolysis in patients with T1DM and T2DM[75,76]. Another study has found that levels of C4 and C3 are both increased in patients with diabetes[77]. Complement C4 has been less studied in metabolic disorders. Nevertheless, accumulated data suggested that C4 plays a role in T1DM[78-80]. TLR4 can interact with free fatty acids or free acid carrier (fetuin-A) to trigger the NF-κB pro-inflammation pathway in cells, which promotes adipose tissue inflammation and insulin resistance in diet-induced obesity[81,82]. Recent study demonstrated that C3a can increase insulin secretion[83]. To confirm this result, the study used complement factor D knockout mice which responded poorly to glucose tolerance due to a defect in insulin production[84]. In the same population, the authors of the study restored serum factor D levels, which increased plasma insulin levels, then additionally reversed this via treatment with a C3aR antagonist. Such findings allow for the conclusion that factor D is involved in the alternative complement pathway activation and C3a signaling, ultimately allowing for insulin secretion[84]. CD59 is a known complement inhibitor, but recent studies have shown a specialized function involving the secretion of insulin in β cells[84,85]. Specifically, CD59 seems to play a role in maintaining lipid raft stability in β cells to maintain basal insulin release rates. In addition, CD59 facilitates recycling of exocytotic core proteins to allow for the secretion of insulin granules[85]. Collectively, the components in the complement system, such as, C3, C4, factor D, and CD59 were shown to be involved in the pathogenesis of diabetic mellitus. Regulation of the activation of complement system might slow the pathological process of diabetes mellitus.
Complement in diabetic complications
The pathophysiology of T2DM occurs in the setting of baseline and/or excessive chronic inflammation, contributing to the progression of diabetes. Emerging evidence has shown that complement-mediated chronic inflammation is linked to diabetic microvascular complications. In T2DM patients, the end product of complement activation, sC5b-9 and the MASP-2 were elevated. Such an environment contributes to endothelial dysfunction and reactive oxidative species production that plays a major role in the pathophysiology of diabetic complications. High concentration of complement C3 is reported to be connected to the increased risk of diabetic retinopathy, nephropathy, and neuropathy[86]. A study by Fujita et al[87] revealed the possible connection between complement activation, inflammation, and the development of atherosclerosis in diabetic conditions. Obesity-induced adipocytes overproduce C3desArg (ASP) from C3 and factor B. In this case, C3 is converted to C3a and C3b in the process C3 “tick over” AP. Then C3a is converted into C3desArg (ASP), which plays a role in insulin resistance as well as chronic inflammation. C3b goes through the terminal pathway and generates the MAC, which contributes to the formation of atheroma. The continuous activation of the complement AP in T2DM with obesity and dyslipidemia significantly contributes to microvascular complications[87]. This study suggests that significantly increased plasma ASP/C3a levels in severe microangiopathy may cause to the acceleration of such complications. Another recent study showed that a high concentration of complement C3 was associated with an increased risk of diabetic complications, such as retinopathy, nephropathy and neuropathy[86,88]. The complement system and inflammation are closely interconnected. Studies have shown that key complement proteins (such as C3, C4, MBL and Factor D) are correlated with a known marker of inflammation, like CRP[60,61,89]. Adipocytokines released from obesity-activated adipocytes induce the production of pro-inflammatory cytokines, causing the dysfunction of vascular endothelial cells and organ damage. After analyzing the relationship between complement-mediated inflammation and diabetic microangiopathy in obese T2DM patients vs normal individuals, plasma levels of C3, C4, iC3b, C3desArg (ASP), Factor B, and Bb were found to be significantly increased in T2DM patients. Furthermore, the level of complement components and their activation intermediates in plasma was tested with respect to the level of complication of the disease. The results of study revealed that the early phase of the alternative complement pathway was excessively activated, suggesting complement-mediated inflammation may contribute to the acceleration of diabetic microangiopathy. A statistical study revealed a close correlation between C3desArg (ASP), highly sensitive CRP, and body mass index. In the macroalbuminuric and proliferative retinopathy patient groups, plasma C3desArg (ASP) was significantly elevated. Diabetic retinopathy complement activation occurs in choriocapillaries of those with diabetic retinopathy. It was found in the eyes of patients with diabetic retinopathy with intense positive staining for MAC deposits, C3d and C5b-9[90,91]. Additionally, there was a reduction of CD55 and CD59 which are inhibitors of the complement pathway in the vessels of those with diabetic retinopathy[92]. Such findings may point to the activation of the AP in diabetic retinopathy[91]. The enhanced expression of C3 mRNA and protein in the glomeruli was positively correlated with the disease status of diabetic kidneys[93]. The increased levels of the complement components (such as C1q, MBL, MASP-2, factor B, C3 and C5b-9) were found in diabetic nephropathy in rat, suggesting that the complement system is involved in aggravating the pathophysiology of this disease[94]. Specifically, most of these factors were found in the renal tubules, suggesting that complement also plays a role in tubular injury, not just in glomerular damage in diabetic nephropathy[94]. MAC was increased in diabetic patients’ blood vessels as well as their kidneys. MAC insertion into cell membranes and into the glomerular mesangium will activate several intracellular signaling pathways leading to the production of reactive oxygen species[62,95-97]. Several other downstream effects of the insertion of MAC include cell proliferation and thrombosis formation. MAC can upregulate fibroblast growth factor and platelet-derived growth factor in glomerular mesangium causing the proliferation of mesangial cell, upregulation of TGF-β, MCP-1 and activation of collagen IV. Moreover, pro-inflammatory molecules such as P-selectin, vascular cell molecule 1, and E-selectin leak out through the MAC complex[98]. These effects of MAC could further explain the pathogenesis of diabetic nephropathy. Normally, MAC is inhibited by membrane proteins including Decay-acceleration factor (DAF) and CD59. Thus the decreased level of DAF or CD59 could result in increased level of MAC in diabetics. In the hyperglycemic state, the glycation of CD59 would increase the activation of MAC and then increase the release of the downstream effects of MAC, which contributes to the development of atherosclerosis and diabetic nephropathy[62,96]. Complement activation has been reported to cause nerve damage in peripheral neuropathies[99]. A recent study demonstrated that a single missense mutation of the CD59 gene, which results in the deficiency of the complement regulatory protein CD59 on the cell surface, causes chronic peripheral neuropathy in children[100]. It is also reported that there were activated complement proteins and MAC neoantigen are found in sural nerve biopsies from individuals with diabetes[101]. Moreover, the co-localization of MAC and CD59 was observed in the same nerve biopsies analyzed in that study[95]. In summary, the activation of the complement system is clearly involved in the diabetic complications, including nephropathy, retinopathy and neuropathy.
PERSPECTIVES AND THERAPEUTICS
Recent studies have revealed the functional link between immune and metabolic systems, which are two central pillars of survival pathways evolved from common ancestors. Human complement system is a global mediator of our innate immune system. It is recently understood that the complement system not only contributes substantially to homeostasis by eliminating infectious microbes, and cellular debris, complementing immunological and inflammatory processes and immune surveillance, but also contributes to various immune, inflammatory-related diseases. Undoubtedly, complement system may not be the chief driving force in these conditions, but it may still be a vital factor that can tip the scales between the initiation and resolution of inflammatory processes. It has been receiving increasing attention that the complement system plays a major regulatory role in metabolism and metabolic disorders. Accumulating evidence suggests that metabolic disorders like diabetes, obesity, insulin resistance, and arthrosclerosis possess important inflammatory components. In Table 1, we highlight those functions of some key complement components in obesity, insulin resistance, and diabetes mellitus (Table 1). In the past few years there is increasing attention in the development of therapeutic modulators of the complement system[17]. Such therapy should modify the complement system to sufficiently and precisely control the aberrant levels while allowing for the beneficial players to continue contributing to immune surveillance[91]. Some drugs are in clinical use for diseases other than metabolic diseases or diabetic complications. Given the strong etiologic components of the complement system activation, those medications might be worth to be evaluated for their ability to slow or halt the progression of metabolic disorders or related complications. Future studies are warranted to test the efficacy of long-term inhibition of activation of the complement system in the context of progression of T2DM and its related complications.
Table 1.
The roles of complement proteins in obesity, insulin resistance, diabetes
| Complement protein | Obesity | Insulin resistance | Diabetes |
| C3 | Increases adipose tissue inflammation[45,46]; Highly expressed in adipose tissue[70,71] | Marker of insulin resistance[42] | Proinflammatory cytokine causing systemic low-grade inflammation, insulin resistance and impaired glucose uptake[67-69]; Link between inflammation and thrombosis[75,76]; Higher concentrations associated with increased risk of diabetic complications[86] |
| C3a and C3aR | Increased levels of C3 in obese individuals[43,47,48]; Increased C3aR in white adipose tissue[49] | Improved glucose tolerance and insulin resistance in C3aR-/- mice[54,55,84] | Proinflammatory cytokine enhanced by cross-talk with TLR4 and C3[45,46]; C3a augments insulin secretion from mouse pancreatic beta cells[83] |
| C3adesArg (ASP) | Increased levels found in obese individuals[14,53]; Obesity induced adipose tissue creates this product leading to a pro-inflammatory state and ASP resistance[55] | Insulin-like properties by stimulating uptake of glucose and synthesis of triglycerides in adipose tissue[55] | Stimulating glucose uptake and lipid storage in adipose tissue[70,71]; Plays a role in chronic inflammation and contribute to microvascular complications[87] |
| C5a and C5aR | Expressed in adipocytes[49]; Play role in adipose tissue biology and development of metabolic disorders | Affect coagulation and contribute to inflammation[22-27] | |
| Factor I and H | Increased in adipose tissue, activated by complement activation and affecting adipose tissue biology[41,42] | ||
| sC5b-9 and MASP-2 | Elevated in T2DM; Contributing to endothelial dysfunction and ROS production in diabetes pathophysiology[62,95-97]; MASP-2 contributes to complications of diabetes[94] | ||
| CD 59 and MAC | Decreased levels of CD 59 in diabetics[62]; Increased levels of MAC found in diabetic patients and contributes to pathophysiology of complications[62,95-97] |
MAC: Membrane attack complex; ROS: Reactive oxygen species; MASP: Mannan-binding lectin-associated serine proteases; ASP: Acylation stimulating factor; T2DM: Type 2 diabetes mellitus; TLR: Toll-like receptors.
Footnotes
Conflict-of-interest statement: Authors declare no conflict of interests for this article.
Manuscript source: Unsolicited manuscript
Peer-review started: August 3, 2019
First decision: September 28, 2019
Article in press: November 26, 2019
Specialty type: Endocrinology and metabolism
Country of origin: United States
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bolshakova GB, Hassan A, Nakhoul F, Su CC S-Editor: Yan JP L-Editor: A E-Editor: Wu YXJ
Contributor Information
Kyumin Shim, Department of Basic Science, California Northstate University College of Medicine, Elk Grove, CA 95757, United States.
Rayhana Begum, Department of Pharmacy, Primeasia University, Dhaka 1213, Bangladesh.
Catherine Yang, Department of Basic Science, California Northstate University College of Medicine, Elk Grove, CA 95757, United States; California Northstate University College of Graduate Studies, Elk Grove, CA 95757, United States.
Hongbin Wang, Department of Basic Science, California Northstate University College of Medicine, Elk Grove, CA 95757, United States; California Northstate University College of Graduate Studies, Elk Grove, CA 95757, United States; Department of Pharmaceutical and Biomedical Sciences, California Northstate University College of Pharmacy, Elk Grove, CA 95757, United States. hongbin.wang@cnsu.edu.
References
- 1.Chatzigeorgiou A, Karalis KP, Bornstein SR, Chavakis T. Lymphocytes in obesity-related adipose tissue inflammation. Diabetologia. 2012;55:2583–2592. doi: 10.1007/s00125-012-2607-0. [DOI] [PubMed] [Google Scholar]
- 2.Donath MY. Inflammation as a sensor of metabolic stress in obesity and type 2 diabetes. Endocrinology. 2011;152:4005–4006. doi: 10.1210/en.2011-1691. [DOI] [PubMed] [Google Scholar]
- 3.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chawla A, Nguyen KD, Goh YP. Macrophage-mediated inflammation in metabolic disease. Nat Rev Immunol. 2011;11:738–749. doi: 10.1038/nri3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donath MY, Böni-Schnetzler M, Ellingsgaard H, Ehses JA. Islet inflammation impairs the pancreatic beta-cell in type 2 diabetes. Physiology (Bethesda) 2009;24:325–331. doi: 10.1152/physiol.00032.2009. [DOI] [PubMed] [Google Scholar]
- 7.Hotamisligil GS. Endoplasmic reticulum stress and the inflammatory basis of metabolic disease. Cell. 2010;140:900–917. doi: 10.1016/j.cell.2010.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 9.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 10.Stienstra R, Tack CJ, Kanneganti TD, Joosten LA, Netea MG. The inflammasome puts obesity in the danger zone. Cell Metab. 2012;15:10–18. doi: 10.1016/j.cmet.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 11.Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, Ravussin E, Stephens JM, Dixit VD. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat Med. 2011;17:179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- 13.Ozes ON, Akca H, Mayo LD, Gustin JA, Maehama T, Dixon JE, Donner DB. A phosphatidylinositol 3-kinase/Akt/mTOR pathway mediates and PTEN antagonizes tumor necrosis factor inhibition of insulin signaling through insulin receptor substrate-1. Proc Natl Acad Sci USA. 2001;98:4640–4645. doi: 10.1073/pnas.051042298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ricklin D, Hajishengallis G, Yang K, Lambris JD. Complement: a key system for immune surveillance and homeostasis. Nat Immunol. 2010;11:785–797. doi: 10.1038/ni.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hourcade DE. The role of properdin in the assembly of the alternative pathway C3 convertases of complement. J Biol Chem. 2006;281:2128–2132. doi: 10.1074/jbc.M508928200. [DOI] [PubMed] [Google Scholar]
- 16.Ricklin D, Lambris JD. Progress and Trends in Complement Therapeutics. Adv Exp Med Biol. 2013;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013;190:3839–3847. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ricklin D, Reis ES, Lambris JD. Complement in disease: a defence system turning offensive. Nat Rev Nephrol. 2016;12:383–401. doi: 10.1038/nrneph.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leslie M. Immunology. The new view of complement. Science. 2012;337:1034–1037. doi: 10.1126/science.337.6098.1034. [DOI] [PubMed] [Google Scholar]
- 20.Markiewski MM, Lambris JD. The role of complement in inflammatory diseases from behind the scenes into the spotlight. Am J Pathol. 2007;171:715–727. doi: 10.2353/ajpath.2007.070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Cordoba SR, Tortajada A, Harris CL, Morgan BP. Complement dysregulation and disease: from genes and proteins to diagnostics and drugs. Immunobiology. 2012;217:1034–1046. doi: 10.1016/j.imbio.2012.07.021. [DOI] [PubMed] [Google Scholar]
- 22.Oikonomopoulou K, Ricklin D, Ward PA, Lambris JD. Interactions between coagulation and complement--their role in inflammation. Semin Immunopathol. 2012;34:151–165. doi: 10.1007/s00281-011-0280-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Brückner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–5636. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ritis K, Doumas M, Mastellos D, Micheli A, Giaglis S, Magotti P, Rafail S, Kartalis G, Sideras P, Lambris JD. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J Immunol. 2006;177:4794–4802. doi: 10.4049/jimmunol.177.7.4794. [DOI] [PubMed] [Google Scholar]
- 25.Wang M, Krauss JL, Domon H, Hosur KB, Liang S, Magotti P, Triantafilou M, Triantafilou K, Lambris JD, Hajishengallis G. Microbial hijacking of complement-toll-like receptor crosstalk. Sci Signal. 2010;3:ra11. doi: 10.1126/scisignal.2000697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hajishengallis G, Lambris JD. Crosstalk pathways between Toll-like receptors and the complement system. Trends Immunol. 2010;31:154–163. doi: 10.1016/j.it.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krauss JL, Potempa J, Lambris JD, Hajishengallis G. Complementary Tolls in the periodontium: how periodontal bacteria modify complement and Toll-like receptor responses to prevail in the host. Periodontol 2000. 2010;52:141–162. doi: 10.1111/j.1600-0757.2009.00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Carroll MC, Isenman DE. Regulation of humoral immunity by complement. Immunity. 2012;37:199–207. doi: 10.1016/j.immuni.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kwan WH, van der Touw W, Heeger PS. Complement regulation of T cell immunity. Immunol Res. 2012;54:247–253. doi: 10.1007/s12026-012-8327-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Heeger PS, Kemper C. Novel roles of complement in T effector cell regulation. Immunobiology. 2012;217:216–224. doi: 10.1016/j.imbio.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 31.Cardone J, Le Friec G, Vantourout P, Roberts A, Fuchs A, Jackson I, Suddason T, Lord G, Atkinson JP, Cope A, Hayday A, Kemper C. Complement regulator CD46 temporally regulates cytokine production by conventional and unconventional T cells. Nat Immunol. 2010;11:862–871. doi: 10.1038/ni.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Han G, Geng S, Li Y, Chen G, Wang R, Li X, Ma Y, Shen B, Li Y. γδT-cell function in sepsis is modulated by C5a receptor signalling. Immunology. 2011;133:340–349. doi: 10.1111/j.1365-2567.2011.03445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kumar V, Ali SR, Konrad S, Zwirner J, Verbeek JS, Schmidt RE, Gessner JE. Cell-derived anaphylatoxins as key mediators of antibody-dependent type II autoimmunity in mice. J Clin Invest. 2006;116:512–520. doi: 10.1172/JCI25536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karsten CM, Pandey MK, Figge J, Kilchenstein R, Taylor PR, Rosas M, McDonald JU, Orr SJ, Berger M, Petzold D, Blanchard V, Winkler A, Hess C, Reid DM, Majoul IV, Strait RT, Harris NL, Köhl G, Wex E, Ludwig R, Zillikens D, Nimmerjahn F, Finkelman FD, Brown GD, Ehlers M, Köhl J. Anti-inflammatory activity of IgG1 mediated by Fc galactosylation and association of FcγRIIB and dectin-1. Nat Med. 2012;18:1401–1406. doi: 10.1038/nm.2862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Naito AT, Sumida T, Nomura S, Liu ML, Higo T, Nakagawa A, Okada K, Sakai T, Hashimoto A, Hara Y, Shimizu I, Zhu W, Toko H, Katada A, Akazawa H, Oka T, Lee JK, Minamino T, Nagai T, Walsh K, Kikuchi A, Matsumoto M, Botto M, Shiojima I, Komuro I. Complement C1q activates canonical Wnt signaling and promotes aging-related phenotypes. Cell. 2012;149:1298–1313. doi: 10.1016/j.cell.2012.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fusakio ME, Mohammed JP, Laumonnier Y, Hoebe K, Köhl J, Mattner J. C5a regulates NKT and NK cell functions in sepsis. J Immunol. 2011;187:5805–5812. doi: 10.4049/jimmunol.1100338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Markiewski MM, DeAngelis RA, Benencia F, Ricklin-Lichtsteiner SK, Koutoulaki A, Gerard C, Coukos G, Lambris JD. Modulation of the antitumor immune response by complement. Nat Immunol. 2008;9:1225–1235. doi: 10.1038/ni.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol Lett. 2010;128:36–45. doi: 10.1016/j.imlet.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cancello R, Clément K. Is obesity an inflammatory illness? Role of low-grade inflammation and macrophage infiltration in human white adipose tissue. BJOG. 2006;113:1141–1147. doi: 10.1111/j.1471-0528.2006.01004.x. [DOI] [PubMed] [Google Scholar]
- 41.Suganami T, Ogawa Y. [Role of chronic inflammation in adipose tissue in the pathophysiology of obesity] Nihon Rinsho. 2013;71:225–230. [PubMed] [Google Scholar]
- 42.Muscari A, Antonelli S, Bianchi G, Cavrini G, Dapporto S, Ligabue A, Ludovico C, Magalotti D, Poggiopollini G, Zoli M Pianoro Study Group. Serum C3 is a stronger inflammatory marker of insulin resistance than C-reactive protein, leukocyte count, and erythrocyte sedimentation rate: comparison study in an elderly population. Diabetes Care. 2007;30:2362–2368. doi: 10.2337/dc07-0637. [DOI] [PubMed] [Google Scholar]
- 43.Weyer C, Tataranni PA, Pratley RE. Insulin action and insulinemia are closely related to the fasting complement C3, but not acylation stimulating protein concentration. Diabetes Care. 2000;23:779–785. doi: 10.2337/diacare.23.6.779. [DOI] [PubMed] [Google Scholar]
- 44.Phieler J, Garcia-Martin R, Lambris JD, Chavakis T. The role of the complement system in metabolic organs and metabolic diseases. Semin Immunol. 2013;25:47–53. doi: 10.1016/j.smim.2013.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rudilla F, Fayolle C, Casares N, Durantez M, Arribillaga L, Lozano T, Villanueva L, Pio R, Sarobe P, Leclerc C, Prieto J, Lasarte JJ. Combination of a TLR4 ligand and anaphylatoxin C5a for the induction of antigen-specific cytotoxic T cell responses. Vaccine. 2012;30:2848–2858. doi: 10.1016/j.vaccine.2012.02.052. [DOI] [PubMed] [Google Scholar]
- 46.Mogilenko DA, Kudriavtsev IV, Trulioff AS, Shavva VS, Dizhe EB, Missyul BV, Zhakhov AV, Ischenko AM, Perevozchikov AP, Orlov SV. Modified low density lipoprotein stimulates complement C3 expression and secretion via liver X receptor and Toll-like receptor 4 activation in human macrophages. J Biol Chem. 2012;287:5954–5968. doi: 10.1074/jbc.M111.289322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Błogowski W, Budkowska M, Sałata D, Serwin K, Dołęgowska B, Łokaj M, Prowans P, Starzyńska T. Clinical analysis of selected complement-derived molecules in human adipose tissue. J Transl Med. 2013;11:11. doi: 10.1186/1479-5876-11-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hermsdorff HH, Volp AC, Puchau B, Barbosa KB, Zulet MA, Bressan J, Martínez JA. Contribution of gender and body fat distribution to inflammatory marker concentrations in apparently healthy young adults. Inflamm Res. 2012;61:427–435. doi: 10.1007/s00011-011-0429-z. [DOI] [PubMed] [Google Scholar]
- 49.Hertle E, van Greevenbroek MM, Arts IC, van der Kallen CJ, Geijselaers SL, Feskens EJ, Jansen EH, Schalkwijk CG, Stehouwer CD. Distinct associations of complement C3a and its precursor C3 with atherosclerosis and cardiovascular disease. The CODAM study. Thromb Haemost. 2014;111:1102–1111. doi: 10.1160/TH13-10-0831. [DOI] [PubMed] [Google Scholar]
- 50.Baldo A, Sniderman AD, St-Luce S, Avramoglu RK, Maslowska M, Hoang B, Monge JC, Bell A, Mulay S, Cianflone K. The adipsin-acylation stimulating protein system and regulation of intracellular triglyceride synthesis. J Clin Invest. 1993;92:1543–1547. doi: 10.1172/JCI116733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Copenhaver M, Yu CY, Hoffman RP. Complement Components, C3 and C4, and the Metabolic Syndrome. Curr Diabetes Rev. 2019;15:44–48. doi: 10.2174/1573399814666180417122030. [DOI] [PubMed] [Google Scholar]
- 52.Paglialunga S, Fisette A, Yan Y, Deshaies Y, Brouillette JF, Pekna M, Cianflone K. Acylation-stimulating protein deficiency and altered adipose tissue in alternative complement pathway knockout mice. Am J Physiol Endocrinol Metab. 2008;294:E521–E529. doi: 10.1152/ajpendo.00590.2007. [DOI] [PubMed] [Google Scholar]
- 53.Faraj M, Havel PJ, Phélis S, Blank D, Sniderman AD, Cianflone K. Plasma acylation-stimulating protein, adiponectin, leptin, and ghrelin before and after weight loss induced by gastric bypass surgery in morbidly obese subjects. J Clin Endocrinol Metab. 2003;88:1594–1602. doi: 10.1210/jc.2002-021309. [DOI] [PubMed] [Google Scholar]
- 54.Lukens JR, Dixit VD, Kanneganti TD. Inflammasome activation in obesity-related inflammatory diseases and autoimmunity. Discov Med. 2011;12:65–74. [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed M, Neville MJ, Edelmann MJ, Kessler BM, Karpe F. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity (Silver Spring) 2010;18:27–34. doi: 10.1038/oby.2009.208. [DOI] [PubMed] [Google Scholar]
- 56.Osborn O, Oh DY, McNelis J, Sanchez-Alavez M, Talukdar S, Lu M, Li P, Thiede L, Morinaga H, Kim JJ, Heinrichsdorff J, Nalbandian S, Ofrecio JM, Scadeng M, Schenk S, Hadcock J, Bartfai T, Olefsky JM. G protein-coupled receptor 21 deletion improves insulin sensitivity in diet-induced obese mice. J Clin Invest. 2012;122:2444–2453. doi: 10.1172/JCI61953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mamane Y, Chung Chan C, Lavallee G, Morin N, Xu LJ, Huang J, Gordon R, Thomas W, Lamb J, Schadt EE, Kennedy BP, Mancini JA. The C3a anaphylatoxin receptor is a key mediator of insulin resistance and functions by modulating adipose tissue macrophage infiltration and activation. Diabetes. 2009;58:2006–2017. doi: 10.2337/db09-0323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fraser DA, Laust AK, Nelson EL, Tenner AJ. C1q differentially modulates phagocytosis and cytokine responses during ingestion of apoptotic cells by human monocytes, macrophages, and dendritic cells. J Immunol. 2009;183:6175–6185. doi: 10.4049/jimmunol.0902232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alkhouri N, Gornicka A, Berk MP, Thapaliya S, Dixon LJ, Kashyap S, Schauer PR, Feldstein AE. Adipocyte apoptosis, a link between obesity, insulin resistance, and hepatic steatosis. J Biol Chem. 2010;285:3428–3438. doi: 10.1074/jbc.M109.074252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Engström G, Hedblad B, Janzon L, Lindgärde F. Weight gain in relation to plasma levels of complement factor 3: results from a population-based cohort study. Diabetologia. 2005;48:2525–2531. doi: 10.1007/s00125-005-0021-6. [DOI] [PubMed] [Google Scholar]
- 61.Engström G, Hedblad B, Eriksson KF, Janzon L, Lindgärde F. Complement C3 is a risk factor for the development of diabetes: a population-based cohort study. Diabetes. 2005;54:570–575. doi: 10.2337/diabetes.54.2.570. [DOI] [PubMed] [Google Scholar]
- 62.Acosta J, Hettinga J, Flückiger R, Krumrei N, Goldfine A, Angarita L, Halperin J. Molecular basis for a link between complement and the vascular complications of diabetes. Proc Natl Acad Sci USA. 2000;97:5450–5455. doi: 10.1073/pnas.97.10.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wlazlo N, van Greevenbroek MM, Ferreira I, Feskens EJ, van der Kallen CJ, Schalkwijk CG, Bravenboer B, Stehouwer CD. Complement factor 3 is associated with insulin resistance and with incident type 2 diabetes over a 7-year follow-up period: the CODAM Study. Diabetes Care. 2014;37:1900–1909. doi: 10.2337/dc13-2804. [DOI] [PubMed] [Google Scholar]
- 64.Ritchie RF, Palomaki GE, Neveux LM, Navolotskaia O, Ledue TB, Craig WY. Reference distributions for complement proteins C3 and C4: a practical, simple and clinically relevant approach in a large cohort. J Clin Lab Anal. 2004;18:1–8. doi: 10.1002/jcla.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Moshage H. Cytokines and the hepatic acute phase response. J Pathol. 1997;181:257–266. doi: 10.1002/(SICI)1096-9896(199703)181:3<257::AID-PATH756>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 66.Gabrielsson BG, Johansson JM, Lönn M, Jernås M, Olbers T, Peltonen M, Larsson I, Lönn L, Sjöström L, Carlsson B, Carlsson LM. High expression of complement components in omental adipose tissue in obese men. Obes Res. 2003;11:699–708. doi: 10.1038/oby.2003.100. [DOI] [PubMed] [Google Scholar]
- 67.Pickup JC, Crook MA. Is type II diabetes mellitus a disease of the innate immune system? Diabetologia. 1998;41:1241–1248. doi: 10.1007/s001250051058. [DOI] [PubMed] [Google Scholar]
- 68.Hotamisligil GS. The role of TNFalpha and TNF receptors in obesity and insulin resistance. J Intern Med. 1999;245:621–625. doi: 10.1046/j.1365-2796.1999.00490.x. [DOI] [PubMed] [Google Scholar]
- 69.Yudkin JS, Stehouwer CD, Emeis JJ, Coppack SW. C-reactive protein in healthy subjects: associations with obesity, insulin resistance, and endothelial dysfunction: a potential role for cytokines originating from adipose tissue? Arterioscler Thromb Vasc Biol. 1999;19:972–978. doi: 10.1161/01.atv.19.4.972. [DOI] [PubMed] [Google Scholar]
- 70.Cianflone K. Acylation stimulating protein and triacylglycerol synthesis: potential drug targets? Curr Pharm Des. 2003;9:1397–1410. doi: 10.2174/1381612033454784. [DOI] [PubMed] [Google Scholar]
- 71.Van Harmelen V, Reynisdottir S, Cianflone K, Degerman E, Hoffstedt J, Nilsell K, Sniderman A, Arner P. Mechanisms involved in the regulation of free fatty acid release from isolated human fat cells by acylation-stimulating protein and insulin. J Biol Chem. 1999;274:18243–18251. doi: 10.1074/jbc.274.26.18243. [DOI] [PubMed] [Google Scholar]
- 72.Xia Z, Sniderman AD, Cianflone K. Acylation-stimulating protein (ASP) deficiency induces obesity resistance and increased energy expenditure in ob/ob mice. J Biol Chem. 2002;277:45874–45879. doi: 10.1074/jbc.M207281200. [DOI] [PubMed] [Google Scholar]
- 73.Koistinen HA, Remitz A, Gylling H, Miettinen TA, Koivisto VA, Ebeling P. Dyslipidemia and a reversible decrease in insulin sensitivity induced by therapy with 13-cis-retinoic acid. Diabetes Metab Res Rev. 2001;17:391–395. doi: 10.1002/dmrr.222. [DOI] [PubMed] [Google Scholar]
- 74.Murray I, Havel PJ, Sniderman AD, Cianflone K. Reduced body weight, adipose tissue, and leptin levels despite increased energy intake in female mice lacking acylation-stimulating protein. Endocrinology. 2000;141:1041–1049. doi: 10.1210/endo.141.3.7364. [DOI] [PubMed] [Google Scholar]
- 75.Howes JM, Richardson VR, Smith KA, Schroeder V, Somani R, Shore A, Hess K, Ajjan R, Pease RJ, Keen JN, Standeven KF, Carter AM. Complement C3 is a novel plasma clot component with anti-fibrinolytic properties. Diab Vasc Dis Res. 2012;9:216–225. doi: 10.1177/1479164111432788. [DOI] [PubMed] [Google Scholar]
- 76.Hess K, Alzahrani SH, Mathai M, Schroeder V, Carter AM, Howell G, Koko T, Strachan MW, Price JF, Smith KA, Grant PJ, Ajjan RA. A novel mechanism for hypofibrinolysis in diabetes: the role of complement C3. Diabetologia. 2012;55:1103–1113. doi: 10.1007/s00125-011-2301-7. [DOI] [PubMed] [Google Scholar]
- 77.McMillan DE. Elevation of complement components in diabetes mellitus. Diabete Metab. 1980;6:265–270. [PubMed] [Google Scholar]
- 78.Bertrams J, Hintzen U, Schlicht V, Schoeps S. C4: another marker for type I diabetes. Lancet. 1982;1:41. doi: 10.1016/s0140-6736(82)92577-6. [DOI] [PubMed] [Google Scholar]
- 79.Mason MJ, Speake C, Gersuk VH, Nguyen QA, O'Brien KK, Odegard JM, Buckner JH, Greenbaum CJ, Chaussabel D, Nepom GT. Low HERV-K(C4) copy number is associated with type 1 diabetes. Diabetes. 2014;63:1789–1795. doi: 10.2337/db13-1382. [DOI] [PubMed] [Google Scholar]
- 80.Cooper ME, Duff R, Buchanan R, McPherson J, Jerums G. Low serum C4 concentrations and microangiopathy in type I and type II diabetes. Br Med J (Clin Res Ed) 1986;292:801. doi: 10.1136/bmj.292.6523.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shi H, Kokoeva MV, Inouye K, Tzameli I, Yin H, Flier JS. TLR4 links innate immunity and fatty acid-induced insulin resistance. J Clin Invest. 2006;116:3015–3025. doi: 10.1172/JCI28898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pal D, Dasgupta S, Kundu R, Maitra S, Das G, Mukhopadhyay S, Ray S, Majumdar SS, Bhattacharya S. Fetuin-A acts as an endogenous ligand of TLR4 to promote lipid-induced insulin resistance. Nat Med. 2012;18:1279–1285. doi: 10.1038/nm.2851. [DOI] [PubMed] [Google Scholar]
- 83.Lo JC, Ljubicic S, Leibiger B, Kern M, Leibiger IB, Moede T, Kelly ME, Chatterjee Bhowmick D, Murano I, Cohen P, Banks AS, Khandekar MJ, Dietrich A, Flier JS, Cinti S, Blüher M, Danial NN, Berggren PO, Spiegelman BM. Adipsin is an adipokine that improves β cell function in diabetes. Cell. 2014;158:41–53. doi: 10.1016/j.cell.2014.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.King BC, Blom AM. Non-traditional roles of complement in type 2 diabetes: Metabolism, insulin secretion and homeostasis. Mol Immunol. 2017;84:34–42. doi: 10.1016/j.molimm.2016.12.009. [DOI] [PubMed] [Google Scholar]
- 85.Krus U, King BC, Nagaraj V, Gandasi NR, Sjölander J, Buda P, Garcia-Vaz E, Gomez MF, Ottosson-Laakso E, Storm P, Fex M, Vikman P, Zhang E, Barg S, Blom AM, Renström E. The complement inhibitor CD59 regulates insulin secretion by modulating exocytotic events. Cell Metab. 2014;19:883–890. doi: 10.1016/j.cmet.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 86.Rasmussen KL, Nordestgaard BG, Nielsen SF. Complement C3 and Risk of Diabetic Microvascular Disease: A Cohort Study of 95202 Individuals from the General Population. Clin Chem. 2018;64:1113–1124. doi: 10.1373/clinchem.2018.287581. [DOI] [PubMed] [Google Scholar]
- 87.Fujita T, Hemmi S, Kajiwara M, Yabuki M, Fuke Y, Satomura A, Soma M. Complement-mediated chronic inflammation is associated with diabetic microvascular complication. Diabetes Metab Res Rev. 2013;29:220–226. doi: 10.1002/dmrr.2380. [DOI] [PubMed] [Google Scholar]
- 88.Rasmussen KL, Nordestgaard BG, Frikke-Schmidt R, Nielsen SF. An updated Alzheimer hypothesis: Complement C3 and risk of Alzheimer's disease-A cohort study of 95,442 individuals. Alzheimers Dement. 2018;14:1589–1601. doi: 10.1016/j.jalz.2018.07.223. [DOI] [PubMed] [Google Scholar]
- 89.Onat A, Can G, Rezvani R, Cianflone K. Complement C3 and cleavage products in cardiometabolic risk. Clin Chim Acta. 2011;412:1171–1179. doi: 10.1016/j.cca.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 90.Gerl VB, Bohl J, Pitz S, Stoffelns B, Pfeiffer N, Bhakdi S. Extensive deposits of complement C3d and C5b-9 in the choriocapillaris of eyes of patients with diabetic retinopathy. Invest Ophthalmol Vis Sci. 2002;43:1104–1108. [PubMed] [Google Scholar]
- 91.Xu H, Chen M. Targeting the complement system for the management of retinal inflammatory and degenerative diseases. Eur J Pharmacol. 2016;787:94–104. doi: 10.1016/j.ejphar.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang J, Gerhardinger C, Lorenzi M. Early complement activation and decreased levels of glycosylphosphatidylinositol-anchored complement inhibitors in human and experimental diabetic retinopathy. Diabetes. 2002;51:3499–3504. doi: 10.2337/diabetes.51.12.3499. [DOI] [PubMed] [Google Scholar]
- 93.Woroniecka KI, Park AS, Mohtat D, Thomas DB, Pullman JM, Susztak K. Transcriptome analysis of human diabetic kidney disease. Diabetes. 2011;60:2354–2369. doi: 10.2337/db10-1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Huang Y, Xu J, Wu X, Chen X, Bai X, Zhuang Y, Fang J, Lin X. High Expression of Complement Components in the Kidneys of Type 2 Diabetic Rats With Diabetic Nephropathy. Front Endocrinol (Lausanne) 2019;10:459. doi: 10.3389/fendo.2019.00459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Qin X, Goldfine A, Krumrei N, Grubissich L, Acosta J, Chorev M, Hays AP, Halperin JA. Glycation inactivation of the complement regulatory protein CD59: a possible role in the pathogenesis of the vascular complications of human diabetes. Diabetes. 2004;53:2653–2661. doi: 10.2337/diabetes.53.10.2653. [DOI] [PubMed] [Google Scholar]
- 96.Acosta J, Qin X, Halperin J. Complement and complement regulatory proteins as potential molecular targets for vascular diseases. Curr Pharm Des. 2004;10:203–211. doi: 10.2174/1381612043453441. [DOI] [PubMed] [Google Scholar]
- 97.Acosta JA, Benzaquen LR, Goldstein DJ, Tosteson MT, Halperin JA. The transient pore formed by homologous terminal complement complexes functions as a bidirectional route for the transport of autocrine and paracrine signals across human cell membranes. Mol Med. 1996;2:755–765. [PMC free article] [PubMed] [Google Scholar]
- 98.Ghosh P, Sahoo R, Vaidya A, Chorev M, Halperin JA. Role of complement and complement regulatory proteins in the complications of diabetes. Endocr Rev. 2015;36:272–288. doi: 10.1210/er.2014-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kaida K, Kusunoki S. Antibodies to gangliosides and ganglioside complexes in Guillain-Barré syndrome and Fisher syndrome: mini-review. J Neuroimmunol. 2010;223:5–12. doi: 10.1016/j.jneuroim.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 100.Nevo Y, Ben-Zeev B, Tabib A, Straussberg R, Anikster Y, Shorer Z, Fattal-Valevski A, Ta-Shma A, Aharoni S, Rabie M, Zenvirt S, Goldshmidt H, Fellig Y, Shaag A, Mevorach D, Elpeleg O. CD59 deficiency is associated with chronic hemolysis and childhood relapsing immune-mediated polyneuropathy. Blood. 2013;121:129–135. doi: 10.1182/blood-2012-07-441857. [DOI] [PubMed] [Google Scholar]
- 101.Rosoklija GB, Dwork AJ, Younger DS, Karlikaya G, Latov N, Hays AP. Local activation of the complement system in endoneurial microvessels of diabetic neuropathy. Acta Neuropathol. 2000;99:55–62. doi: 10.1007/pl00007406. [DOI] [PubMed] [Google Scholar]