This editorial refers to ‘Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF’†, by S.J. Shah et al., on page 3439.
There is increasing documentation that coronary microvascular dysfunction (CMD)-related subendocardial ischaemia contributes to left ventricular remodeling, diastolic and systolic dysfunction, and subsequent heart failure with preserved ejection fraction (HFpEF).1 To date, prevalence of microvascular dysfunction in heart failure with preserved ejection fraction (PROMIS-HFpEF) (n=202) is the largest prospective, multicentre study (Scandinavia, USA, and Singapore) evaluating coronary flow reserve (CFR), a diagnostic measure of CMD, in subjects with known HFpEF.2 In this study, 75% of the subjects had evidence of CMD, defined as CFR < 2.5 measured by adenosine stress transthoracic Doppler echocardiography. The investigators found that low CFR is associated with elevated N-terminal pro b-type natriuretic peptide (NTproBNP), and abnormal cardiac structure and function including left atrial and right ventricular (RV) dysfunction. Shah et al. also report that low CFR is associated with peripheral endothelial dysfunction, as measured by reactive hyperemia index (RHI). While other studies have reported peripheral vascular dysfunction in patients with HFpEF,3 the PROMIS-HFpEF investigators are the first to document an association with CFR. Specifically, low CFR is more strongly associated with low RHI in obesity, suggesting that HFpEF is a systemic vascular disorder that potentially impacts other organs.
The PROMIS-HFpEF study comes at a critical juncture to address key knowledge gaps that adversely impact women, the majority of CMD and HFpEF victims; indeed, 55% of the study sample are women. We and others have conducted multiple pharmacological probe trials for CMD with observed benefit only with angiotensin converting enzyme inhibitors (ACE-Is) and alpha–beta-blockers,4 while therapeutic HFpEF trials to date including ACE-Is, angiotensin receptor blockers (ARBs), digoxin, aldosterone antagonists, phosphodiesterase type 5 inhibitor (PDE5 inhibitor), and nitrates have not demonstrated benefit in HFpEF, with the exception of one small study evaluating the beta-blocker propranolol.5 These findings suggest that while identification of CMD in HFpEF may be an important link, targeting CMD alone as a therapeutic HFpEF target may prove ineffective, e.g. prior established endothelial therapies (statins and ACE-I) trials in HFpEF have failed. New hypotheses and approaches to mechanistic understanding are needed to address remaining knowledge gaps, and to design clinical trials for evidence-based HFpEF guidelines.
Our and other investigations to date suggest that risk factor conditions (hypertension, obesity, dyslipidaemia, dysglycaemia, oestrogen loss, inactivity etc.) promote a pro-inflammatory, pro-oxidative state, rendering the coronary microvasculature and myocardium vulnerable to: (i) adverse left ventricular (LV) remodeling, (ii) subendocardial ischaemia, (iii) focal and diffuse fibrosis, (iv) cardiac steatosis, and (iv) vascular stiffness4. Based on these observations, we propose that CMD plays a critical role in initiating pre-HFpEF, and LV and RV dysfunction in patients with ischaemia and no obstructive coronary arteries (INOCA).6 We further hypothesize that this syndrome is associated with a microvascular-related ischaemia–myocardial dysfunctional state, characterized by abnormal diastolic and systolic function, which may progress to HFpEF. That these conditions lead to actual myocardial damage is evidenced by a recent study7 showing that CMD is associated with increases in high-sensitivity cardiac troponin (hs-cTnI), and that hs-cTnI elevations predict future HFpEF.8 However, how CMD-related ischaemic damage contributes to HFpEF development is an important knowledge gap. Investigations identifying specific mechanistic pathways are therefore critically needed if we are going to make any therapeutic advancements.
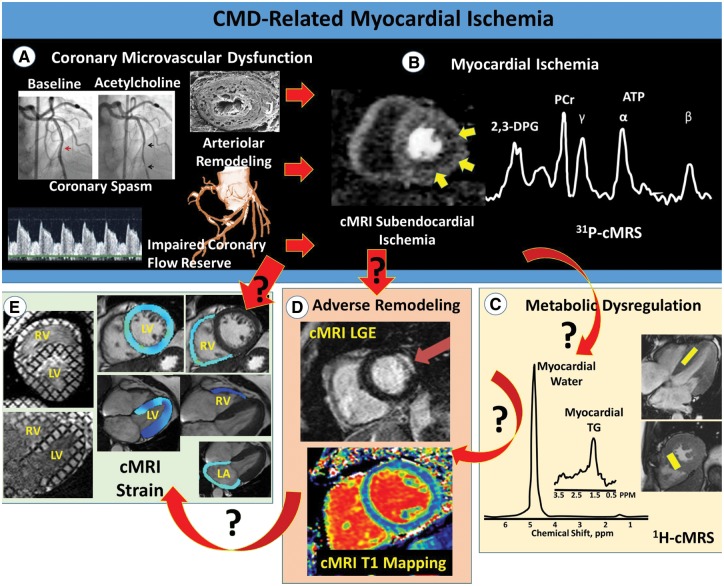
Cardiac magnetic resonance imaging/magnetic resonance spectroscopy (cMRI/MRS) is a powerful clinical tool that has the potential to non-invasively measure the key pathophysiological endpoints in this cascade linking CMD to HFpEF. In addition to being the most precise and reproducible imaging modality to assess cardiac morphology and global systolic function, cMRI/MRS provides a quantitative platform for the evaluation of regional tissue deformation (strain), myocardial perfusion reserve, bioenergetics, and tissue characteristics (Figure 1). Indeed, cMRI-derived markers are able to assess both the intramyocardial microvascular network and the extravascular myocardium. Consistent with our overarching hypothesis, we reason that chronic ischaemia (impaired flow reserve) related to the dysfunction and/or rarefaction of the intramyocardial microvasculature in CMD leads to repeat bouts of myocardial damage, which in turn is manifested as diffuse interstitial fibrosis, myocyte hypertrophy, and/or myocardial steatosis, ultimately culminating in diastolic and systolic dysfunction with myocardial stiffening, emblematic of HFpEF. Similar to the Doppler left anterior descending (LAD) approach used by Shah et al.,2 cMRI-derived myocardial perfusion reserve index is a surrogate measure of impaired coronary reactivity to adenosine and increased microvascular resistance.9,10 In contrast, however, cMRI enables direct assessment of subendocardial perfusion, a more sensitive measure of microvascular dysfunction/rarefaction. Further, focal fibrosis can be detected using late gadolinium-enhancement cMRI, which, in a recent study of women with INOCA, has been shown to have a prevalence of 8%.11 Moreover, cMRI-derived myocardial T1 is elevated in women with INOCA compared to matched controls, a finding that may be indicative of adverse intramyocardial remodeling including diffuse fibrosis.12 Finally, in similar INOCA cohorts, myocardial steatosis, along with both subclinical systolic and diastolic dysfunction, have also been detected using cMRS and cMRI, respectively.13,14 Taken together, cMRI/MRS provides a wide array of pathophysiological endpoints, each representing novel potential treatment targets. Following confirmatory studies, these pathways could be targeted by innovative metabolic, inflammatory, and fibrosis strategies currently being tested in other organ systems, such as non-alcoholic steatohepatitis.
Figure 1.
Potential pathophysiological mechanisms linking CMD to HFpEF. The power of cardiac magnetic resonance imaging/magnetic resonance spectroscopy to define key pathophysiological mechanisms linking coronary microvascular dysfunction-related myocardial ischaemia to heart failure with preserved ejection fraction. Top illustrates traditional invasive measures of CMD (A), and resultant subendocardial ischaemia measured by first-pass perfusion magnetic resonance imaging and altered myocardial bioenergetics measured by 31P magnetic resonance spectroscopy (B). (C–E) illustrate three proposed mechanistic pathways linking coronary microvascular dysfunction-related ischaemia to heart failure with preserved ejection fraction, including cardiac steatosis-induced lipotoxicity (C), adverse remodeling (D), and impaired systolic and diastolic function (E). The red arrows indicate directionality (cause and effect), with black question marks highlighting knowledge gaps for investigation. Note both the simple cause–effect relationship in some cases (e.g. ischaemia-related adverse remodeling), as well as more complex relationships involving multiple pathways. Importantly, cardiac magnetic resonance imaging/magnetic resonance spectroscopy is ideally situated to address all of the proposed mechanistic pathways. 2,3-DPG: 2-3-diphosphoglycerate; ATP: adenosine triphosphate; CMD, coronary microvascular dysfunction; cardiac magnetic resonance imaging/spectroscopy, HFpEF, heart failure with preserved ejection fraction; LA, left atrium; LGE, late gadolinium enhancement; LV, left ventricle; PCr, phosphocreatine; RV, right ventricle; TG, triglyceride.
In summary, Shah et al.2 have provided an important contribution to our pathophysiological understanding of HFpEF, providing some of the strongest evidence to date of a CMD–HFpEF relationship. Further investigation is now needed to better define the specific mechanisms and temporal relationship, linking CMD-related myocardial ischemia, myocellular damage, and HFpEF. cMRI/MRS may provide the necessary depth and breadth to address these knowledge gaps, improve HFpEF subgroup phenotyping, and identify new treatment targets. Only then can mechanistically supported HFpEF intervention trials be considered, including: (i) anti-ischaemic/scar therapies (intensive statin/ACE-I/ARB combination, alpha–beta-blockers, and nitric oxide-cyclic GMP), (ii) strain/remodeling therapies (sacubitril/valsartan), and/or (iii) anti-fibrotic therapies (galectin 3, peptidyl arginine deiminase type IV inhibitor, stress-activated kinase-1 inhibitor, protein kinase G, and fibroblast growth factor).
Footnotes
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
† doi:10.1093/eurheartj/ehy531.
Funding
Research reported in this publication was supported by the National Heart, Lung and Blood Institute (NHLBI) under grant numbers N01HV68161, N01HV68162, N01HV-68163, N01HV68164, U01HL64829, U01HL649141, U01HL649241, T32HL69751, R00HL124323; the National Institute on Aging (NIA) under grant number R03AG032631; the National Institute of Child Health and Human Development under grant number K12HD051959; the National Center for Research Resources (NCRR) under grant number M01RR000425; and grants from the Gustavus and Louis Pfeiffer Research Foundation, Danville, NJ, The Women’s Guild of Cedars-Sinai Medical Center, Los Angeles, CA, The Ladies Hospital Aid Society of Western Pennsylvania, Pittsburgh, PA, and QMED, Inc., Laurence Harbor, NJ, the Edythe L. Broad Women’s Heart Research Fellowship, Cedars-Sinai Medical Center, Los Angeles, California, and the Barbra Streisand Women’s Cardiovascular Research and Education Program, Cedars-Sinai Medical Center, Los Angeles, American Heart Association grant 16SDG27260115, and the Harry S. Moss Heart Trust.
Conflict of interest: The authors have no conflicts of interest to disclose.
References
- 1. Nelson MD, Wei J, Bairey Merz CN.. Coronary microvascular dysfunction and heart failure with preserved ejection fraction as female-pattern cardiovascular disease: the chicken or the egg? Eur Heart J 2018;39:850–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Shah SJ, Lam CSP, Svedlund S, Saraste A, Hage C, Tan R-S, Beussink-Nelson L, Fermer ML, Broberg MA, Gan L-M, Lund LH. Prevalence and correlates of coronary microvascular dysfunction in heart failure with preserved ejection fraction: PROMIS-HFpEF. Eur Heart J 2018;39:3439–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, Redfield MM.. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol 2010;56:845–854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bairey Merz CN, Pepine CJ, Walsh MN, Fleg JL.. Ischemia and no obstructive coronary artery disease (INOCA): developing evidence-based therapies and research agenda for the next decade. Circulation 2017;135:1075–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zheng SL, Chan FT, Nabeebaccus AA, Shah AM, McDonagh T, Okonko DO, Avis S.. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart 2018;104:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Pepine CJ, Petersen JW, Bairey Merz CN.. A microvascular-myocardial diastolic dysfunctional state and risk for mental stress ischemia: a revised concept of ischemia during daily life. JACC Cardiovasc Imaging 2014;7:362–365. [DOI] [PubMed] [Google Scholar]
- 7. Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, Hainer J, Bibbo CF, Dorbala S, Blankstein R, Di Carli MF.. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J 2018;39:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Evans JDW, Dobbin SJH, Pettit SJ, Di Angelantonio E, Willeit P.. High-sensitivity cardiac troponin and new-onset heart failure: a systematic review and meta-analysis of 67,063 patients with 4,165 incident heart failure events. JACC Heart Fail 2018;6:187–197. [DOI] [PubMed] [Google Scholar]
- 9. Liu A, Wijesurendra RS, Liu JM, Forfar JC, Channon KM, Jerosch-Herold M, Piechnik SK, Neubauer S, Kharbanda RK, Ferreira VM.. Diagnosis of microvascular angina using cardiac magnetic resonance. J Am Coll Cardiol 2018;71:969–979. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 10. Thomson LE, Wei J, Agarwal M, Haft-Baradaran A, Shufelt C, Mehta PK, Gill EB, Johnson BD, Kenkre T, Handberg EM, Li D, Sharif B, Berman DS, Petersen JW, Pepine CJ, Bairey Merz CN.. Cardiac magnetic resonance myocardial perfusion reserve index is reduced in women with coronary microvascular dysfunction. A National Heart, Lung, and Blood Institute-sponsored study from the Women's Ischemia Syndrome Evaluation. Circ Cardiovasc Imaging 2015;8:e002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wei J, Bakir M, Darounian N, Li Q, Landes S, Mehta PK, Shufelt CL, Handberg EM, Kelsey SF, Sopko G, Pepine CJ, Petersen JW, Berman DS, Thomson LEJ, Bairey Merz CN.. Myocardial scar is prevalent and associated with subclinical myocardial dysfunction in women with suspected ischemia but no obstructive coronary artery disease: From the Women's Ischemia Syndrome Evaluation-Coronary Vascular Dysfunction study. Circulation 2018;137:874–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shaw JL, Nelson MD, Wei J, Motwani M, Landes S, Mehta PK, Thomson LEJ, Berman DS, Li D, Bairey Merz CN, Shariff B.. Inverse association of MRI-derived native myocardial T1 and perfusion reserve index in women with evidence of ischemia and no obstructive CAD: a pilot study. Int J Cardiol: doi: 10.1016/j.ijcard.2018.06.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nelson MD, Sharif B, Shaw JL, Cook-Weins G, Shufelt C, Mehta PK, Thomson LEJ, Berman DS, Thompson RB, Handberg EM, Pepine CJ, Li D, Bairey Merz CN.. Myocardial tissue deformation is reduced in subjects with coronary microvascular dysfunction but not rescued by treatment with ranolazine. Clin Cardiol 2017;40:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wei J, Nelson MD, Szczepaniak EW, Smith L, Mehta PK, Thomson LE, Berman DS, Li D, Bairey Merz CN, Szczepaniak LS.. Myocardial steatosis as a possible mechanistic link between diastolic dysfunction and coronary microvascular dysfunction in women. Am J Physiol Heart Circ Physiol 2016;310:H14–H19. [DOI] [PMC free article] [PubMed] [Google Scholar]