Enzyme-linked immunosorbent and microneutralization assays of 180 Ebola convalescent plasma specimens were highly concordant and predictive for detection of antibody by 50% plaque reduction neutralization test. Viral load decreased following infusion of antibody-containing plasma in 2 Ebola virus disease patients.
Keywords: Ebola virus disease, therapy, blood plasma, Ebola virus, Ebola antibodies
Abstract
Background
Ebola virus (EBOV) neutralizing antibody in plasma may reduce viral load following administration of plasma to patients with Ebola virus disease (EVD), but measurement of these antibodies is complex.
Methods
Anti-EBOV antibody was measured by 2 neutralization and 2 enzyme-linked immunosorbent assays (ELISAs) in convalescent plasma (ECP) from 100 EVD survivor donors in Liberia. Viral load was assessed repetitively in patients with EVD participating in a clinical trial of enhanced standard of care plus ECP.
Results
All 4 anti-EBOV assays were highly concordant for detection of EBOV antibody. Antibodies were not detected in plasma specimens obtained from 15 of 100 donors, including 7 with documented EBOV-positive reverse-transcription polymerase chain reaction during EVD. Viral load was reduced following each dose in the 2 clinical trial participants who received ECP with higher antibody levels but not in the 2 who received ECP with lower antibody levels.
Conclusions
Recovery from EVD can occur with absence of detectable anti-EBOV antibody several months after disease onset. ELISAs may be useful to select ECP donors or identify ECP units that contain neutralizing antibody. ECP with higher anti-EBOV antibody levels may have greater effect on EBOV load—an observation that requires further investigation.
Clinical Trials Registration
Ebola virus disease (EVD) produces rapidly progressive multiorgan system failure and is fatal in 40%–90% of patients. The survival advantage of an early humoral immune response to Ebola virus (EBOV) was documented during the 1996 Gabon outbreak, in which survivors manifested increasing levels of immunoglobulin G (IgG) against virus nucleoprotein [1]; in contrast, EBOV-specific IgG was not detected in fatal cases. This recognition of a correlation between humoral response and survival has led to attempts to treat acute EVD with whole blood or plasma collected from survivors—a precedent established with pathogen-specific immunoglobulin therapy for hepatitis B virus infection [2], influenza [3, 4], Argentine hemorrhagic fever [5], and severe acute respiratory syndrome [6].
Anecdotal reports of EVD patients treated with convalescent whole blood from the 1995 Kikwit Ebola outbreak suggested improved survival compared with untreated patients [7]. This finding, however, was confounded by the use of historical controls and a higher level of supportive care among those transfused. We collected Ebola convalescent plasma (ECP) by apheresis from EVD survivors in Liberia in 2014–2015, measured anti-EBOV antibodies using 4 different assays, and examined the virologic and clinical outcomes of acute EVD patients treated with ECP or enhanced standard of care in a controlled clinical trial.
METHODS
ECP was collected from EVD survivors and administered to EVD patients at the Eternal Love Winning Africa (ELWA) Hospital and the ELWA Ebola Treatment Unit-2 (ELWA-2) in Paynesville, Liberia. Clinical protocols for plasma collection, specimen analysis, and the treatment trial (ClinicalTrials.gov NCT02333578) sponsored by Clinical Research Management, Inc, were reviewed and approved by the University of Liberia Pacific Institute for Research and Evaluation; the Liberia Medicines and Health Products Regulatory Authority; and the institutional review boards of the University of North Carolina, Duke University, and the US Army Medical Research and Materiel Command. All ECP donors and clinical trial participants provided written, informed consent. Studies were conducted in accordance with the Declaration of Helsinki, Good Clinical Practices, and applicable laws and regulations.
Collection of ECP and Analysis of Anti-EBOV Antibodies
Collection of ECP
As previously described [8], ECP was collected by apheresis from EVD survivor donors with Ebola treatment unit discharge certificates who met World Health Organization–recommended criteria for blood donation. ECP (650 mL) was treated with amotosalen and illuminated with ultraviolet light (INTERCEPT, Cerus) prior to distribution into six 100-mL bags. Bags of ECP were frozen within 6 hours of collection and stored below –18°C until thawed to ambient temperature immediately prior to infusion.
Collection of Blood for Antibody Levels
Blood for determination of anti-EBOV antibody levels was collected in ethylenediaminetetraacetic acid (EDTA) tubes at screening and ECP donation visits. Specimen tubes were processed within 24 hours at the Liberian Institute of Biomedical Research (LIBR), where they were centrifuged, and plasma was divided into 0.5-mL aliquots, which were frozen and maintained at –20°C until analysis for anti-EBOV antibody levels at the US Army Medical Research Institute of Infectious Diseases.
Quantitative ELISAs
Survivor plasma samples, naive human serum (negative control), and EBOV convalescent nonhuman primate (NHP) serum (positive control) were serially diluted and added to plates coated with either recombinant EBOV glycoprotein (GP) or irradiated whole EBOV antigen. Reference standard was serially diluted in accordance with protocols specific for each antigen of interest. Following room temperature incubation, plates were washed and incubated with horseradish peroxidase–labeled anti-human IgG secondary antibody. Again, plates were washed following room temperature incubation and 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) substrate was added. Absorbance values were measured at 405 nm using a plate reader. The absorbance value of each test sample dilution that fell within the acceptable range (based on coefficient of variation and percentage recovery) of the reference standard curve was used to calculate the amount (units/mL) of EBOV GP- or EBOV-specific antibody. Cutoff values for each ELISA were defined as the mean + 3 times the standard deviation (SD) titer of negative control serum collected from uninfected individuals in Uganda.
Neutralization Assays
Survivor plasma samples and naive human serum were diluted 1:10, 1:20, and 1:40 in cell culture media, in duplicate. EBOV-specific monoclonal antibody KZ52 was used as the positive control. Diluted test specimens and controls were mixed with EBOV and incubated at 37°C for 1 hour before adding to Vero E6 cells. Unbound virus was washed away after a further 1-hour incubation. For the microneutralization assay (MNA), Vero growth media was added back to the cells. Cells were fixed 48 hours postinfection and infected cells were detected using EBOV-specific monoclonal antibody and fluorescently labeled secondary antibodies. The percentage of infected cells was determined using an Operetta high-content imaging system and Harmonia software. Results were expressed as MNA50 titer, the highest plasma dilution that neutralized ≥50% of virus. For the 50% plaque reduction neutralization test (PRNT50), after removal of unbound virus, Vero cells were overlaid with agarose. Plaque reduction was assessed as previously described [9] and results expressed as PRNT50 titer; percentage neutralization at each plasma dilution was also recorded.
Clinical Trial of ECP
Objectives
The primary trial objective was correlation of anti-EBOV IgG and neutralization titers in ECP with kinetics of plasma viral load in ECP-treated participants. Secondary objectives were to describe kinetics of plasma viral load in participants treated with and without ECP and compare kinetics of viral load between groups; compare survival up to time of hospital discharge of participants treated for EVD with and without ECP; and assess safety of treatment with and without ECP.
Participants
Eligible participants were patients at ELWA-2 Ebola Treatment Unit (ETU) at least 18 years of age with EVD confirmed by RT-PCR who had been admitted to the confirmed section of the ETU no more than 48 hours prior to enrollment, had adequate vascular access, and were able to provide informed consent or had a family member who could consent for them.
Treatment
Participants were allocated to receive either enhanced standard of care alone (control group) or enhanced standard of care plus ECP (ECP group). Enhanced standard of care included aggressive intravenous hydration and electrolyte replacement guided by handheld point-of-care diagnostic testing (iSTAT, Abbott Diagnostics). In addition, participants routinely received prophylactic ceftriaxone intravenously, oral antimalarial therapy with chloroquine or artemether/lumefantrine, and oral selenium as a nutritional supplement.
Following enrollment, participants were assigned, in order of ETU admission, to the ECP group if ABO-compatible ECP was available or, if ABO-compatible ECP was not available, to the control group. Participants in the ECP group received 200 mL of ECP intravenously (100 mL from 1 donor followed immediately by 100 mL from another donor) per dose. This dose could be repeated every 48 hours using the same lots of ECP used for the first dose for up to 3 doses unless participants were considered by the principal investigator to have improved and had been afebrile for at least 36 hours.
Assessments
Participants in both groups were followed with frequent vital signs and assessment of adverse events. Safety laboratory tests were performed at least every other day and more often if clinically indicated and feasible. Blood was collected into EDTA tubes for viral load at enrollment, immediately prior to and 4 hours after completion of each 200-mL ECP treatment, and daily on noninfusion days for the ECP group; control group specimens were collected every 48 hours.
Plasma viral load was measured at LIBR using the Department of Defense EZ1 real-time RT-PCR assay [10] and reported as cycle threshold (Ct). Ct values were converted to log10 genomic equivalent (GE)/mL by a formula derived from the assay validation: log10 GE/mL = log10 800 + (Ct – 41.16) / –3.36, where 800 = sample dilution factor, 41.16 = y-intercept, and –3.36 = slope of Ct (y) vs log10 GE (x).
Statistical Methods
Laboratory values were summarized by descriptive statistics (mean, median, and SD). Between-group differences were assessed by Fisher exact test or t test. Pearson correlation was performed on ELISA and neutralization results. EBOV GP ELISA results below the limit of detection were assigned a value of 0.001 U, the lowest measured value in the assay. If ELISA tests were repeated (3 specimens), the median value was used for calculations. Sensitivity and specificity testing were performed using MedCalc (medcalc.org).
RESULTS
Anti-EBOV Antibody Titers in EVD Survivors
One hundred donor candidate survivors of EVD provided 190 specimens. Of these, 9 specimens were unsatisfactory for testing. All 4 antibody assays were conducted on 180 specimens; 1 specimen was analyzed by all tests except the PRNT50. Table 1 summarizes the results of all 4 tests from the first specimen collected from each donor, and from the complete set of 180 specimens. At least 2 specimens were obtained from 41 donors at times ranging from 53 to 252 days after their discharge from an ETU (Supplementary Table 1). In all but 1 donor, the PRNT50 serological status (ie, ≥1:10 or <1:10) in the second sample remained unchanged from the first specimen analyzed. In this donor (donor 83, Supplementary Table 1), PRNT50 changed from 1:20 in the first specimen to <1:10 in a specimen collected 5 days later, while the ELISAs and the MNA50 remained positive, suggesting that the PRNT50 result of the second test was erroneous. Relative to the PRNT50 as the gold standard (including the potentially erroneous value noted above), both ELISAs were highly sensitive and specific (Table 1) and were highly concordant with each other for positive or negative serological status using cutoffs derived from normal serum specimens (P < .001, Fisher exact test). The quantitative relationship (Pearson) between the 2 ELISA results for each specimen was highly significant but weak (P < .001 and r2 = 0.22), as were relationships between either ELISA and percentage neutralization at each plasma dilution (1:10, 1:20, and ≥1:40) in PRNT50 (P < .001 for all comparisons, with r2 = 0.15–0.21).
Table 1.
Performance Characteristics of Enzyme-Linked Immunosorbent Assays and 50% Microneutralization Assay Relative to 50% Plaque Reduction Neutralization Test
| Initial Specimen (N = 100) | ||||||
|---|---|---|---|---|---|---|
| PRNT50 Positive (n = 85) | PRNT50 Negative (n = 15) | |||||
| Test | Test Positive, No. | Test Negative, No. | Test Negative, No. | Test Positive, No. | Sensitivity, % (95% CI) |
Specificity, % (95% CI) |
| MNA50 | 83 | 2 | 15 | 0 | 97.65 (91.76–99.71) | 100.00 (78.20–100.00) |
| EBOV GP ELISA | 85 | 0 | 15 | 0 | 100.00 (95.75–100.00) | 100.00 (78.20–100.00) |
| EBOV ELISA | 84 | 1 | 15 | 0 | 98.82 (93.62–99.97) | 100.00 (78.20–100.00) |
| Complete Specimen Set (N = 180) | ||||||
| PRNT50 Positive (n = 141) | PRNT50 Negative (n = 39) | |||||
| Test | Test Positive, No. | Test Negative, No. | Test Negative, No. | Test Positive, No. | Sensitivity, % (95% CI) |
Specificity, % (95% CI) |
| MNA50 | 138 | 3 | 38 | 1 | 97.87 (93.91–99.56) | 97.44 (86.52–99.94) |
| EBOV GP ELISA | 141 | 0 | 37 | 2 | 100.00 (97.42–100.00) | 94.87 (82.68–99.37) |
| EBOV ELISA | 139 | 2 | 37 | 2 | 98.58 (94.97–99.83) | 94.87 (82.68–99.37) |
Abbreviations: CI, confidence interval; EBOV ELISA, enzyme-linked immunosorbent assay detecting whole Ebola virus; EBOV GP ELISA, enzyme-linked immunosorbent assay detecting Ebola virus glycoprotein; ELISA, enzyme-linked immunosorbent assay; MNA50, 50% microneutralization assay; PRNT50, 50% plaque reduction neutralization test.
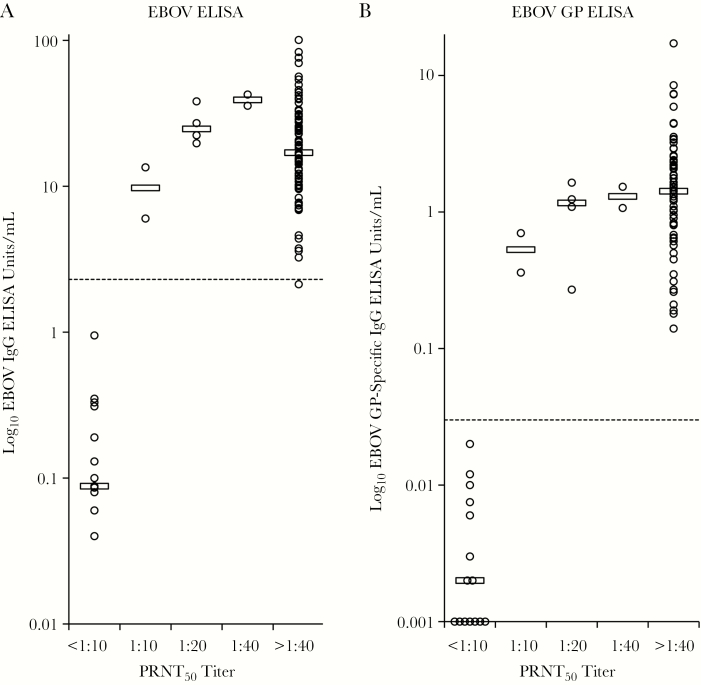
Figures 1A and 1B show the distributions of anti-EBOV GP and EBOV ELISA values from the first 100 specimens according to their PRNT50 titer. ELISA-negative specimens clustered with specimens having PRNT50 <1:10, but there was no clear dose-response relationship between the ELISA values and the semiquantitative PRNT50 results in antibody-positive specimens. Similar relationships were noted in the complete set of 180 specimens (data not shown).
Figure 1.
A and B, Distributions of anti-Ebola virus (EBOV) and EBOV glycoprotein (GP) enzyme-linked immunosorbent assay (ELISA) values from the first 100 specimens according to their 50% plaque reduction neutralization test (PRNT50) titer. The first blood specimen collected from each plasma donor was assayed by immunoglobulin G (IgG) EBOV ELISA, IgG EBOV GP ELISA, and PRNT50. ELISA units per milliliter in each specimen were derived using a standard curve prepared with a reference antibody. Open circles depict ELISA results at each PRNT50 titer. Open horizontal bars denote median ELISA values. Dotted line indicates upper cutoff for negative ELISA values.
The MNA50 was also highly sensitive and specific for predicting positive and negative PRNT50 results (Table 1). Titers for the MNA50 tended to be lower than for the PRNT50 (Supplementary Table 1). No consistent change in antibody levels over time was observed in donors who provided repeated specimens (Supplementary Table 1).
For 7 of the antibody-negative donors, ETU records were available and included a laboratory report of a positive RT-PCR test for EBOV. These are identified as PCR-confirmed cases and listed separately from the nonconfirmed cases (Supplementary Table 1). IgM ELISAs on the initial IgG antibody–negative specimens were negative and total IgG levels were normal (data not shown). Comparison of age, sex, vital signs, blood group, prevalence of positive tests for transfusion-transmitted infections, and hemoglobin levels showed no differences between PCR-confirmed antibody-negative, all antibody-negative, and antibody-positive donors (data not shown). The PCR-confirmed antibody-negative donor group had a significantly shorter mean interval between ETU discharge date and first specimen collection than did the antibody-negative nonconfirmed and antibody-positive groups (80, 157, and 144 days, respectively), reflecting the better availability of records in the PCR-confirmed group (Supplementary Table 1).
Clinical Trial Enrollment, Baseline Characteristics, and Treatment Allocation
The clinical trial, which began in December 2014, was designed to enroll 70 acute EVD participants. By March 2015, only 6 were enrolled as the incidence of EVD in Liberia dropped rapidly. Four participants (T1–T4) were assigned to the ECP group and 2 (C1 and C2) to the control group.
Demographic and baseline clinical findings are shown in Supplementary Table 2. Typically, participants presented late in their course, often with diarrhea and conjunctival and gingival bleeding. Clinical laboratory results, viral load at enrollment, and survival outcome for each participant are shown in Supplementary Table 3. With the exception of participant T2, who had normal clinical laboratory values except for a creatinine level of 1.7 mg/dL, all participants had profound metabolic abnormalities on admission. Mean viral load at first determination was 8.0 ± 0.8 log10 GE (range, 6.8–8.9).
Clinical Course and Ebola Viral Load
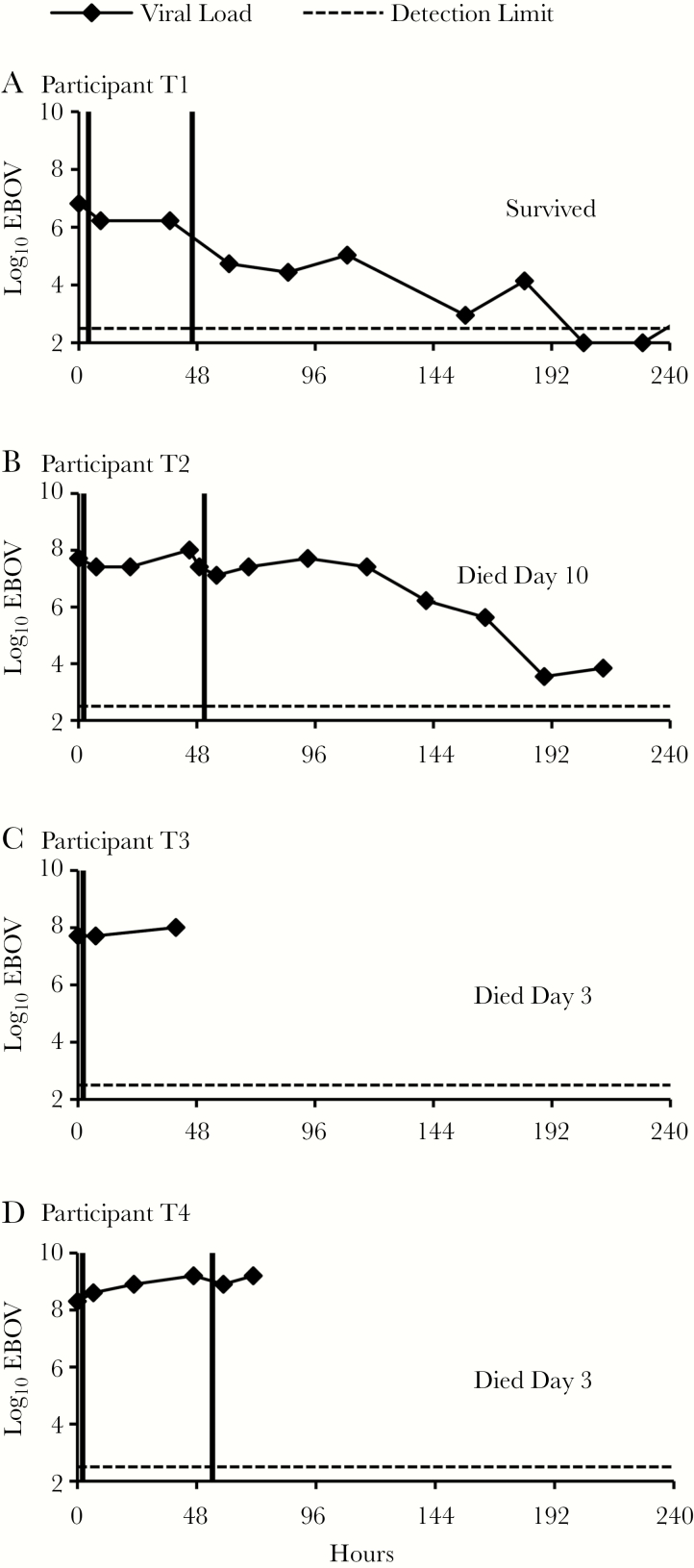
We observed no adverse effects of treatment with ECP. ECP anti-EBOV levels were not known during the trial; as a consequence, the antibody content of ECP varied. Two participants (T1 and T2) each received ECP units from 2 donors with EBOV-specific ELISA values near the median of all donor specimen values; MNA50 and PRNT50 were >1:40 (Table 2). Participants T1 and T2 each received 2 doses of ECP 48 hours apart; per protocol, neither received a third dose because they were considered to be improving at the scheduled 96-hour time point. On each occasion of ECP treatment, plasma viral load declined between the pre- and first postinfusion sampling point in both participants (Figure 2). Participant T1’s viral load decreased 4-fold after the first dose and a further 31-fold after the second dose. Participant T2’s viral load decreased 2-fold following each dose, rebounded at 93 hours back to baseline, and slowly decreased thereafter. Despite severe clinical and laboratory manifestations of EVD (Supplementary Tables 2 and 3), Participant T1 gradually improved and survived. Participant T2 had less severe clinical laboratory abnormalities on admission, but had a history of human immunodeficiency virus infection and hypertension (Supplementary Tables 2 and 3) and progressively increasing creatinine level. Participant T2 deteriorated clinically on day 6 with pallor and epigastric tenderness, and died on day 10.
Table 2.
Anti–Ebola Virus Antibodies in Infused Ebola Convalescent Plasma
| Participants | ECP | Anti-EBOV GP IgG, U/mL | Anti-EBOV IgG, U/mL | MNA50 Titer | PRNT50 Titer |
|---|---|---|---|---|---|
| T1 and T2 | Donation Aa | 12.57 | 1.39 | >1:40 | >1:40 |
| Donation B | 19.07 | 1.64 | >1:40 | >1:40 | |
| Mean unit 1b | 15.82 | 1.52 | |||
| T3 and T4 | Donation C | 8.36 | 0.28 | 1:10 | 1:40 |
| Donation D | 0.31 | 0.002 | <1:10 | <1:10 | |
| Mean unit 2 | 4.34 | 0.14 |
Abbreviations: EBOV GP, Ebola virus glycoprotein; ECP, Ebola convalescent plasma; IgG, immunoglobulin G; MNA50, 50% microneutralization assay; PRNT50, 50% plaque reduction neutralization test; U/mL, laboratory units per milliliter of Ebola virus glycoprotein and Ebola virus enzyme-linked immunosorbent assays.
aDonations indicate specimens for antibody assay from donors obtained at the time of ECP donation.
bAt each dosing time point, clinical trial participants received 100 mL of ECP from each indicated donor one after the other, to make up a unit of ECP.
Figure 2.
A–D, Ebola virus (EBOV) load in participants treated with Ebola convalescent plasma. Participants were treated with enhanced standard of care. In addition, Ebola convalescent plasma was infused (vertical lines) within 4 hours after collection of preinfusion blood specimens for viral load. Specimens obtained at the time points indicated (squares) were assayed for EBOV by reverse-transcription polymerase chain reaction and converted to log10 genomic equivalents/mL by a formula established at assay validation. Dashed line indicates lower limit of virus detection. Zero hours indicates time of first preinfusion specimen collection.
Participants T3 and T4 received ECP units containing much lower levels of Ebola-specific IgG and neutralizing activity than either of the ECP units provided to participants T1 and T2 (Table 2). The viral load in participant T3, who received only 1 infusion of ECP, did not change between the pre- and first postinfusion sampling time points (Figure 2), but increased afterward approximately 2-fold prior to death on day 3. Participant T4’s viral load increased 2-fold after the first infusion and declined 2-fold after the second infusion (Figure 2), but then increased 4-fold. Participant T4 died before the scheduled 96-hour third infusion.
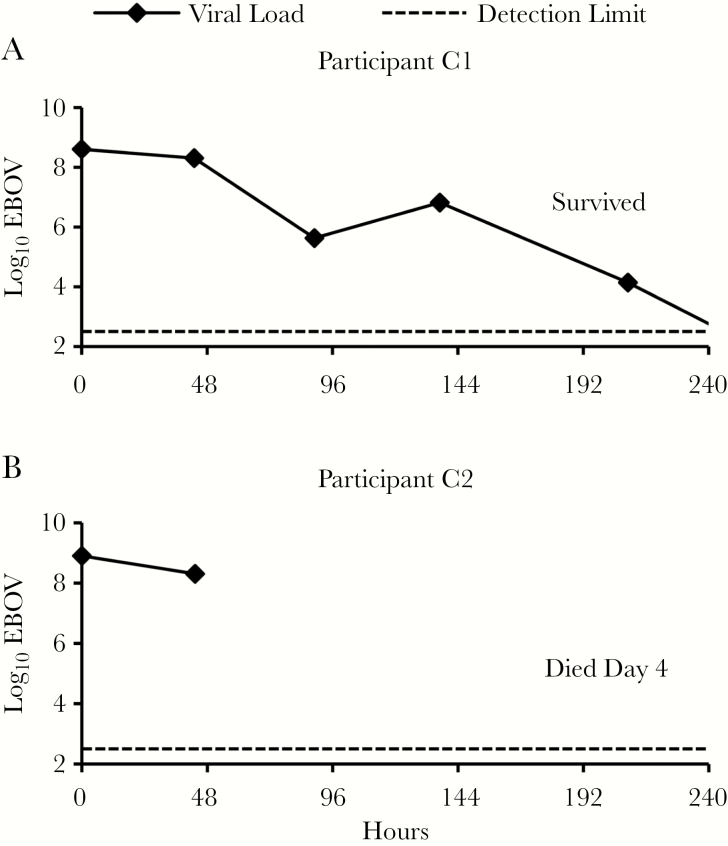
No ABO-compatible ECP was available for control participants C1 and C2 (Figure 2). The viral load in participant C1, who survived, declined 2-fold over the first 48 hours and 645-fold over the first 89 hours. The viral load in participant C2, who died on day 4, declined 4-fold over 43 hours. All 3 participants who survived for at least 8 days had detectable viral RNA in blood at least through day 7 (Figures 2 and 3).
Figure 3.
A and B, Ebola virus (EBOV) load in control participants. Participants were treated with enhanced standard of care. Blood specimens obtained at the time points indicated (squares) were assayed for EBOV by reverse-transcription polymerase chain reaction and converted to log10 genomic equivalents per milliliter by a formula established at assay validation. Dashed line indicates lower limit of virus detection. Zero hours indicates time of first specimen collection after enrollment.
DISCUSSION
We found a high concordance between 4 different assays (2 ELISAs and 2 neutralization tests) for categorization of EVD survivors (candidate ECP donors) by anti-EBOV antibody status. Using PRNT50 results as a gold standard, the MNA50, EBOV ELISA, and EBOV GP ELISA were all highly sensitive and specific tests. These data indicate that the ELISAs are excellent candidates for identification of EVD survivors who have neutralizing antibody, although the quantitative relationship between percentage neutralization and ELISA values and between ELISAs was weak. Compared to neutralization assays, ELISAs for EBOV do not require virus culture, are much less labor-intensive, and can be conducted in diagnostic laboratories with lower biosafety levels.
Given a previous report that failure to develop anti-EBOV antibodies predicted high mortality from EVD [1], the proportion of participants with negative tests for anti-EBOV antibody led us to question whether these donor candidates were truly EVD survivors, even though selection required a history of EVD and a certificate of discharge from an ETU with a diagnosis of EVD. Although a positive RT-PCR result was found in ETU records for only 7 participants, most of the participants in this study had been personally cared for in the ELWA-2 ETU by 1 of the authors (J. F. B.); their disease presentation and hospital course were believed at the time of treatment and discharge to be that of EVD and patients were not transferred to the confirmed section of the ETU unless a positive RT-PCR result had been received. It is possible, but unlikely, that the RT-PCR test results used for confirmatory diagnosis of EVD were erroneous. These findings suggest that between 7% and 15% of the ECP donors had severe EVD and survived, while failing to mount a specific humoral immune response to EBOV. The negative IgM ELISAs and normal concentrations of plasma IgG in the first specimen suggested that defective class switching or immunoglobulin deficiency do not explain this failure. Negative tests for neutralizing antibody in 20% of 85 plasma units from 58 donors were observed in the Ebola-Tx trial [11]. Additional prospective studies should be conducted to examine the course of humoral and cellular immune responses during acute EVD and to confirm the proportion of seronegative individuals among both survivors and fatalities.
The temporal relationships of ECP treatment and change in viral load in the 2 participants treated with ECP containing higher anti-EBOV levels suggest that ECP treatment may decrease viral load. Although the data are limited by the small number of enrolled participants and severity of disease in all enrollees, the results provide support for further assessment of ECP or immunoglobulin for therapy of EVD and are consistent with results of the Ebola-Tx trial [11, 12]. In the present study, EBOV viral load levels were assessed approximately 4 hours following the end of ECP infusion and daily to assess kinetics of any neutralizing activity. The reduced viral load at the first postinfusion time point in the 2 high-dose participants, but rebound at later time points, suggests that sampling for viral load at time points proximate to the end of ECP infusion may be informative in future studies. We note that since our expression of viral load as GE was based on validation curves of the RT-PCR assay and the RT-PCR assay as performed at LIBR did not include an extraction control or standard curve, the absolute GE reported in this study should be considered an estimate.
The impact of ECP treatment on clinical outcomes cannot be assessed from these data, given the small number of participants and the severity of their disease, limitations also encountered in other EVD treatment trials [13–15]. Overall, participants in this trial comprised a group with predicted high mortality based on virologic, clinical laboratory, and clinical criteria [16–20].
The study’s strengths include careful assessment of Ebola viral load dynamics over multiple time points and the characterization of the potential potency of the ECP collected and administered. The trial also identified opportunities for enhancement to protocol design that could inform future trials of ECP efficacy. Had the anti-EBOV levels in donor ECP been available prior to treatment, ECP with higher antibody titers may have provided a better assessment of the effects of ECP on viremia. Donors with high anti-EBOV titers have now been identified in Liberia and other Ebola-affected countries, and have provided sufficient ECP to support further investigation should another outbreak occur in the near future. The excellent sensitivity and specificity of the ELISAs for predicting neutralizing activity reported here suggest that either of these tests could be used to screen donors to provide additional high-titer ECP in future trials.
We infused 200 mL of ECP (100 mL from each of 2 donors) at each dose, an amount that could be prepared by centrifugation from a single whole blood unit, as used by Mupapa et al [7]. A larger dose of ECP, as used in the Ebola-Tx trial with no apparent adverse effects [12], may be advisable in future trials. Similarly, consistent treatment with 3 doses rather than allowing for omission of the third scheduled dose based on clinical improvement may be advisable in a future study to increase the number of data points and prolong the active treatment period. Data from NHP studies suggest that repetitive administration of antibody in the form of immunoglobulin is important for successful treatment [21]; a series of 3 infusions was used in the Partnership for Research on Ebola Virus in Liberia II trial [14] of anti-EBOV monoclonal antibody cocktail. Convalescent whole blood from NHPs [22] and serum from humans [23] did not enhance survival of NHPs challenged with 1000 plaque-forming units of EBOV, which routinely leads to death 8–9 days after exposure.
In conclusion, this study showed that 7%–15% of EVD survivors who planned to donate convalescent plasma were negative by 4 tests for anti-EBOV antibody. Further studies are needed to assess the frequency of antibody negativity in the overall survivor population and to evaluate innate and cellular immune responses that may mediate recovery in the absence of detectable antibodies. Minimum thresholds for positivity in the EBOV or EBOV GP ELISA may be useful to predict the presence of plasma virus neutralizing activity and select ECP donors for further clinical trials. A temporally related decline in plasma EBOV load following treatment with ECP containing neutralizing antibody observed in this study supports previous observations and justifies further evaluation of this intervention. The selection of ECP units with high anti-EBOV titers, use of a larger dose volume of ECP, administration of at least 3 repeated doses of ECP unless contraindicated, and frequent sampling for viral load in both controls and ECP-treated participants should be considered in the design of future trials.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We gratefully acknowledge the intense individual effort and support from many sources to make this study possible and the contributions of plasma donors and clinical trial participants. Korto Pewu, Edwina Reeves, and Darlington Komosee cared for patients in the ETU; Galakpai Gorvego and Uriah Glaybo provided ECP donor care and technical assistance. Many other ELWA and Clinical Research Management staff and management contributed selflessly.
Disclaimer. Opinions, conclusions, interpretations, and recommendations are those of the authors and are not necessarily endorsed by the US Army. The mention of trade names or commercial products does not constitute endorsement or recommendation for use by the Department of the Army or the Department of Defense.
Financial support. This work was supported by the Bill & Melinda Gates Foundation; Greenbaum Foundation; Paul G. Allen Family Foundation; US Defense Threat Reduction Agency (CB10166); World Food Programme; Cerus Corporation; Abbott; B. Braun Medical; Haemonetics; Helmer Scientific; Fisher Scientific; Welch Allyn; BD; and PDC Healthcare. C. K. C. received support from the Duke University Center for AIDS Research, a National Institutes of Health–funded program (award number 5P30 AI064518).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Baize S, Leroy EM, Georges-Courbot MC, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med 1999; 5:423–6. [DOI] [PubMed] [Google Scholar]
- 2. Surgenor DM, Chalmers TC, Conrad ME, et al. Clinical trials of hepatitis B immune globulin. Development of policies and materials for the 1972-1975 studies sponsored by the National Heart and Lung Institute. N Engl J Med 1975; 293:1060–2. [DOI] [PubMed] [Google Scholar]
- 3. Hung IF, To KK, Lee CK, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis 2011; 52:447–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment?Ann Intern Med 2006; 145:599–609. [DOI] [PubMed] [Google Scholar]
- 5. Maiztegui JI, Fernandez NJ, de Damilano AJ. Efficacy of immune plasma in treatment of Argentine haemorrhagic fever and association between treatment and a late neurological syndrome. Lancet 1979; 2:1216–7. [DOI] [PubMed] [Google Scholar]
- 6. Mair-Jenkins J, Saavedra-Campos M, Baillie JK, et al. Convalescent Plasma Study Group The effectiveness of convalescent plasma and hyperimmune immunoglobulin for the treatment of severe acute respiratory infections of viral etiology: a systematic review and exploratory meta-analysis. J Infect Dis 2015; 211:80–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mupapa K, Massamba M, Kibadi K, et al. Treatment of Ebola hemorrhagic fever with blood transfusions from convalescent patients. International Scientific and Technical Committee. J Infect Dis 1999; 179(Suppl 1):S18–23. [DOI] [PubMed] [Google Scholar]
- 8. Brown JF, Rowe K, Zacharias P, et al. Apheresis for collection of Ebola convalescent plasma in Liberia. J Clin Apher 2017; 32:175–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Howell KA, Qiu X, Brannan JM, et al. Antibody treatment of Ebola and Sudan virus infection via a uniquely exposed epitope within the glycoprotein receptor-binding site. Cell Rep 2016; 15:1514–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Trombley AR, Wachter L, Garrison J, et al. Comprehensive panel of real-time TaqMan polymerase chain reaction assays for detection and absolute quantification of filoviruses, arenaviruses, and New World hantaviruses. Am J Trop Med Hyg 2010; 82:954–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. van Griensven J, Edwards T, Baize S; Ebola-Tx Consortium Efficacy of convalescent plasma in relation to dose of Ebola virus antibodies. N Engl J Med 2016; 375:2307–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. van Griensven J, Edwards T, de Lamballerie X, et al. Ebola-Tx Consortium Evaluation of convalescent plasma for Ebola virus disease in Guinea. N Engl J Med 2016; 374:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Dunning J, Sahr F, Rojek A, et al. RAPIDE-TKM Trial Team Experimental treatment of Ebola virus disease with TKM-130803: a single-arm phase 2 clinical trial. PLoS Med 2016; 13:e1001997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. PREVAIL II Writing Group; Multi-National PREVAIL II Study Team ; Davey RT Jr, Dodd L, Proschan MA, et al. A randomized, controlled trial of ZMapp for Ebola virus infection. N Engl J Med 2016; 375:1448–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sissoko D, Laouenan C, Folkesson E, et al. JIKI Study Group Experimental treatment with favipiravir for Ebola virus disease (the JIKI Trial): a historically controlled, single-arm proof-of-concept trial in Guinea. PLoS Med 2016; 13:e1001967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Towner JS, Rollin PE, Bausch DG, et al. Rapid diagnosis of Ebola hemorrhagic fever by reverse transcription-PCR in an outbreak setting and assessment of patient viral load as a predictor of outcome. J Virol 2004; 78:4330–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Schieffelin JS, Shaffer JG, Goba A, et al. KGH Lassa Fever Program; Viral Hemorrhagic Fever Consortium; WHO Clinical Response Team Clinical illness and outcomes in patients with Ebola in Sierra Leone. N Engl J Med 2014; 371:2092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. de La Vega MA, Caleo G, Audet J, et al. Ebola viral load at diagnosis associates with patient outcome and outbreak evolution. J Clin Invest 2015; 125:4421–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Fitzpatrick G, Vogt F, Moi Gbabai OB, et al. The contribution of Ebola viral load at admission and other patient characteristics to mortality in a Medecins Sans Frontieres Ebola case management centre, Kailahun, Sierra Leone, June-October 2014. J Infect Dis 2015; 212:1752–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li J, Duan HJ, Chen HY, et al. Age and Ebola viral load correlate with mortality and survival time in 288 Ebola virus disease patients. Int J Infect Dis 2016; 42:34–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dye JM, Herbert AS, Kuehne AI, et al. Postexposure antibody prophylaxis protects nonhuman primates from filovirus disease. Proc Natl Acad Sci U S A 2012; 109:5034–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jahrling PB, Geisbert JB, Swearengen JR, Larsen T, Geisbert TW. Ebola hemorrhagic fever: evaluation of passive immunotherapy in nonhuman primates. J Infect Dis 2007; 196(Suppl 2):S400–3. [DOI] [PubMed] [Google Scholar]
- 23. Mire CE, Geisbert JB, Agans KN, et al. Passive immunotherapy: assessment of convalescent serum against Ebola virus Makona infection in nonhuman primates. J Infect Dis 2016; 214:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.