Abstract
Background
Informed by the Pediatric Self-Management Model, the present study tested relationships between parent and family functioning, sickle cell disease (SCD) self-management, and health outcomes for children with SCD.
Method
83 children with SCD and a parent completed baseline data as part of a larger investigation of a family-based, problem-solving intervention for children with SCD (M age = 8.47). Youth and parents completed a measure of child health-related quality of life (HRQOL), and parents completed measures of family efficacy, parenting stress, and SCD self-management. SCD pain episodes and urgent health utilization information over the past year were obtained via medical chart review.
Results
SCD self-management mediated the relationship between parent-reported family efficacy and parent proxy HRQOL, as well as the relationship between parenting stress and child and parent proxy HRQOL. Mediation models were nonsignificant for outcomes beyond HRQOL, including SCD pain episodes and urgent health utilization.
Conclusion
Fostering family efficacy and reducing parenting stress may be meaningful intervention targets for improving SCD self-management and child HRQOL among school-aged children. Although findings were consistent with the Pediatric Self-Management Model in terms of HRQOL, the model was not supported for pain episodes or urgent health utilization, highlighting the need for multi-method, longitudinal research on the SCD self-management behaviors that are linked to preventable health outcomes.
Keywords: adherence, family functioning, hematology, medical self-management, parent stress, sickle cell disease
Sickle cell disease (SCD) is an inherited group of hematological disorders that affect red blood cells and is found in approximately 1 in 365 African American children and 1 in 16,300 Hispanic/Latino American children in the United States (CDC, 2016). SCD is associated with significant health complications, including vaso-occlusive pain episodes, respiratory infections, chronic anemia, compromised spleen functioning, slow growth, and stroke; all of which require daily care and monitoring (Berkelhammer et al., 2007; Platt, 2000; Rees, Williams, & Gladwin, 2010). Treatment guidelines emphasize the role of parents in the daily management of SCD, including administering prophylactic antibiotics, pain medications, folic acid, hydroxyurea, and oral iron chelation therapy, and encouraging frequent hydration, adequate nutrition, and rest (Beyer & Simmons, 2004; Platt, 2008; Raphael, Bernhardt, Mahoney, & Mueller, 2009; Yawn et al., 2014). While these treatments are expected to decrease morbidities and increase the life span of those with SCD, documented rates of adherence to SCD treatment are suboptimal (Elliott, Morgan, Day, Mollerup, & Wang, 2001; Witherspoon & Drotar, 2006), with objective measures (i.e., urinary assays) finding roughly 50% of families are nonadherent to prescribed medications (Walsh et al., 2014). Poor SCD self-management may precipitate health crises, including vaso-occlusive pain episodes and pneumococcal infections, which necessitate urgent and costly healthcare interventions (Gil et al., 1993; Gil, Williams, Thompson, & Kinney, 1991; Rapoff, 2009).
A necessary first step toward improving the health of pediatric patients with SCD is to better understand barriers to self-management for this population. Medical self-management refers to any behavior conducted by a child or family member in relation to caring for a chronic illness (Modi et al., 2012), and thus, medical self-management relates to adherence behaviors (e.g., developing a routine for taking medications), as well as other processes beyond adherence (e.g., coping skills for managing pain). Evidenced-based models of pediatric medical self-management have yet to be tested or adapted to fit the complex needs of youth with SCD, who face distinctive challenges owing to unique characteristics of the larger SCD population, such as racial and socioeconomic discrimination (Loiselle et al., 2016) and health-related stigma (Wakefield et al., 2017), as well as the preventative nature of SCD self-management. The Pediatric Self-Management Model underscores the modifiable and non-modifiable influences on medical self-management behaviors and health outcomes (Modi et al., 2012). This ecological framework is grounded in evidence from the larger pediatric psychology literature that demonstrates the multiple individual (e.g., child age), family (e.g., family functioning), community (e.g., social support), and health system (e.g., resources) influences that may affect medical self-management. The Pediatric Self-Management Model also describes the impact of self-management behaviors on health outcomes, including disease complications, urgent health utilization, and child health-related quality of life (HRQOL).
Among the levels of influence proposed by the Pediatric Self-Management Model, the relationships between family-level variables and medical self-management have been well established in other pediatric populations, such as youth with diabetes (Anderson et al., 2002; Caccavale, Weaver, Chen, Streisand, & Holmes, 2015; Hilliard, Guilfoyle, Dolan, & Hood, 2011; Wu et al., 2014; Wysocki et al., 1999), but less studied in pediatric SCD. Existing research has found links between lower family problem-solving and more passive parent coping with poorer SCD self-management, although measurement of SCD self-management has varied (Barakat, Smith-Whitley, & Ohene-Frempong, 2002; Treadwell et al., 2005). Further, the implications of poor SCD self-management on health outcomes remain unclear. One study found that higher adherence related to better child HRQOL (Fisak, Belkin, Von Lehe, & Bansal, 2012), whereas another investigation found an inverse relationship between adherence and child HRQOL (Barakat, Lutz, Smith-Whitley, & Ohene-Frempong, 2005). A separate study found poorer health outcomes when parents reported higher stress and lower family functioning (Barakat, Patterson, Weinberger, et al., 2007), suggesting possible relationships between family factors and child health. Clarifying the family influences on SCD self-management and health outcomes is critical, as family-based interventions are particularly potent for improving disease management among youth with other chronic health conditions (Hommel et al., 2012; Kahana, Drotar, & Frazier, 2008).
Guided by the Pediatric Self-Management Model (Modi et al., 2012), the current study tested relationships between parent and family functioning, SCD self-management, and, ultimately, health outcomes (i.e., child HRQOL, pain episodes, and urgent health utilization) in children with SCD. We hypothesized that higher family efficacy and lower parenting stress would relate to better child HRQOL, fewer pain episodes, and fewer urgent health utilization. When accounting for SCD self-management, we hypothesized that the relationship between family functioning and health outcomes would be significantly reduced (i.e., SCD self-management would mediate the relationship).
Methods
This study was a secondary analysis of baseline data from a family-based problem-solving skills intervention for school-aged children with SCD. Participants were English-speaking children with any SCD hemoglobinopathy who were currently engaged in SCD follow-up care and between the ages of 6 and 12 years and a parent. Exclusion criteria included the presence of a developmental disability or significant neurocognitive deficits based on the medical record. Eligible families were provided with a letter and a flyer explaining the study at two SCD centers. If the family expressed interest in participating, the research coordinator contacted the family in clinic or via telephone to schedule a baseline assessment either at the family’s home or in the medical clinic.
Of the 208 families approached, 172 agreed to participate and 36 refused. The most common reasons for refusal included schedule too busy (n = 13) and intervention viewed as not needed (n = 10). Of the 172 families that agreed to participate, 78 passively refused (e.g., did not return telephone messages) and we were unable to locate 11 families after initial recruitment (recruitment rate was 40%). Age and gender were similar for those who passively/actively refused the study (M age = 9.19, % female = 51.7) compared with those who participated in the study (M age = 8.47, % female = 49.4). Although the recruitment rate was not ideal, it was similar to prior psychosocial clinical trials in pediatric SCD (Barakat, Schwartz, Salamon, & Radcliffe, 2010; Kaslow et al., 2000) and observational studies of pediatric SCD adherence (Loiselle et al., 2016). Of note, a peer-patient navigator improved retention of participants in the intervention (52% of families who met with the navigator attended the intervention, compared with 42% who did not meet the navigator). We extensively discussed these recruitment and retention challenges in a prior publication (Daniel et al., 2015).
Family Functioning Measures
Family Efficacy
The Perceived Collective Family Efficacy Scale (FES) is a 20-item questionnaire, along a 7-point Likert scale, that measures parents’ beliefs in the family’s capability to work together as a family system to accomplish tasks necessary for adaptive family functioning (e.g., “Agree to decisions that require some sacrifice of personal interests”; Bandura, Caprara, Barbaranelli, Regalia, & Scabini, 2011; Caprara, Regalia, Scabini, Barbaranelli, & Bandura, 2004). The FES has demonstrated adequate construct validity, as evidenced by significant correlations with other well-established indicators of family functioning (family satisfaction, open communication, parental monitoring, and management of conflict; Caprara et al., 2004). This study utilized the total FES score by summing item responses (higher scores representing higher perceived family efficacy). The internal consistency of this measure was adequate (α = .94).
Parenting Stress
The Pediatric Inventory for Parents (PIP) is a reliable and valid measure of parenting stress related to caring for a child with a chronic illness (Streisand, Braniecki, Tercyak, & Kazak, 2001). The PIP includes 43 items related to communication, emotional functioning, medical care, and role functioning (e.g., “Arguing with family members” and “Difficulty sleeping”). Parents rated each item along a 5-point Likert scale for the item’s frequency and level of difficulty over the past week. Frequency and difficulty scores were summed for a total frequency score and a total difficulty score (higher scores indicate greater frequency and difficulty). There was a high correlation between the PIP frequency and difficulty total scores (r = .84), which is consistent with the initial validation sample (Streisand et al., 2001). Thus, to reduce the number of variables under investigation, this research used the frequency total score only (α = .94).
Disease Management Measure
SCD Self-Management
The Self Care Inventory-Sickle Cell (SCI-SC) is a 13-item parent-report questionnaire in which parents rate how well the family follows recommended SCD self-management tasks on a 5-point Likert scale (Hilker, Sytsma Jordan, Jensen, Elkin, & Iyer, 2006). The SCI-SC has demonstrated adequate internal consistency (αs ranging from .71 to .92; Hilker et al., 2006; Jensen et al., 2005) and initial construct validity (children with poorer SCD self-management required more frequent medical visits; Hilker et al., 2006). Subscales for the SCI-SC include general health behaviors (6 items; e.g., eat a healthy diet), sickle cell management (4 items; e.g., take medications given by a doctor), and pain management (3 items; e.g., use deep breathing to relax when in pain). The present study evaluated subscale mean scores and a total score (calculated by summing the three mean subscale scores). Higher scores indicate better SCD self-management. Internal consistency was acceptable for the total score (α = .77), general health behaviors subscale (α = .77), and pain management subscale (α = .78). Owing to poor internal consistency (α = .21), we did not utilize the sickle cell management subscale separately, though these items were included in the total score.
Health Outcome Measures
Child Health-Related Quality of Life
The Pediatric Quality of Life Inventory (PedsQL) is a 23-item parent-proxy report with a parallel child-report form used to assess the child HRQOL across four areas of functioning: physical, emotional, social, and school (Varni, Seid, & Kurtin, 2001). The generic core and SCD version have been validated in children with SCD (Dampier et al., 2011; Panepinto & Bonner, 2012; Panepinto, Pajewski, Foerster, & Hoffmann, 2008). Notably, the SCD version of the PedsQL had not yet been developed at the time of current study recruitment (Panepinto, Torres, & Varni, 2012). Items asked the child or parent to identify how much of a problem the child had with specific physical/psychosocial items over the past month (e.g., “I have low energy” or “I feel angry”). Internal consistency was adequate for parent-proxy report (α = .90) and child self-report (α = .88).
Disease Outcome Variables
Trained research assistants conducted medical chart review using a structured file review form and obtained information on sickle cell genotype, acute chest syndrome, transfusion therapy, lifetime stroke/cerebrovascular accident (CVA) occurrence, hydroxyurea use, pain episodes, and urgent health utilization in the year prior to study enrollment. For this study, we evaluated the number of pain episodes and urgent health utilization over the past year. The urgent health utilization variable consisted of the number of emergency department visits and inpatient hospitalizations for fever or pain. We assessed pain episodes by reviewing clinic notes and hospital discharge summaries for any mention of a pain episode. Dates of pain episodes were recorded and analyzed to ensure that a single pain episode was not erroneously counted as two or more pain episodes. For example, we recorded “one pain episode” when a participant presented to the clinic with pain that resulted in a subsequent inpatient admission with pain.
Demographic/Neurocognitive Information
Child IQ and Demographics
The Wechsler Abbreviated Scale of Intelligence (WASI) is a brief measure of intelligence for individuals aged 6 to 89 years (Wechsler, 1999). We utilized the two-subtest form, which includes Vocabulary and Matrix Reasoning subtests. Parents completed a demographic form, which included information on the child’s age, gender, and race/ethnicity, as well as parent race/ethnicity, education, and income.
Data Analytic Plan
Mediation analyses were conducted using the SPSS macro PROCESS (Preacher, Rucker, & Hayes, 2007). First, bivariate correlations were conducted to examine relationships among the variables of interest and demographic variables. Consistent with the Pediatric Self-Management Model, we examined correlations between non-modifiable demographic (child age and family income) and neurocognitive variables (child IQ) and main study variables. Demographic variables that were significantly related to any of the predictor or outcome variables were controlled for as covariates in the subsequent models. We conducted mediation analyses using bootstrapping to determine if overall SCD self-management mediated family/parent functioning → health outcome relationships (eight analyses). Multiple mediation models tested whether subscales on SCD self-management measure (general health behaviors and pain management) also mediated these relationships (eight analyses). As recommended by Preacher and Hayes (2008), we generated 5,000 bootstrapped samples and considered results as significant if the 95% confidence interval did not contain zero. Assuming a power of .80, an alpha of .05, and an estimated R2 of .15 (a medium effect size), a sample of 84 was required for the analyses with up to four independent variables (Cohen, 1992). Therefore, the current study had enough power to detect medium to large effects.
Results
The final study sample consisted of 83 children (M age = 8.47; see Table 1). Means, standard deviations, and scale ranges for demographic/medical information and variables utilized in the analyses are presented in Table 1. Medical chart review indicated that the majority of children were diagnosed with HbSS (60%). Parents consisted of primarily mothers (90%; 6% fathers, 4% other), of whom 26.5% had graduated from high school and 40% attended some college. Most of the sample reported monthly family income under $3,333, with over 31.3% of the sample falling below the national poverty level at that time (U.S. Department of Health and Human Services, 2009).
Table I.
Demographic Information and Means, Standard Deviations, and Ranges for Family, Sickle Cell Management, and Health Outcome Variables
| Variable (n = 83) | n (% when applicable) | M | SD | Min–Max |
|---|---|---|---|---|
| Demographic/Disease variables | ||||
| Child age | 83 | 8.47 | 2.11 | 6.00–12.00 |
| Gender (female) | 41 (49.4) | – | – | – |
| Child ethnicity, non-Hispanic | 78 (94.0) | – | – | – |
| Child race, African American | 78 (94.0) | – | – | – |
| Genotype: | ||||
| HbSS | 50 (60.2) | – | – | – |
| HbSC | 24 (28.9) | – | – | – |
| HbSß+ | 6 (7.2) | – | – | – |
| HbSß0 | 1 (1.2) | – | – | – |
| Other | 2 (2.4) | – | – | – |
| Acute chest syndrome past year | 13 (15.5) | 0.17 | 0.41 | 0.00–2.00 |
| Receiving transfusion therapy | 24 (28.6) | – | – | – |
| Stroke/CVA lifetime occurrence | 11 (13.1) | – | – | – |
| Prescribed hydroxyurea | 15 (17.9) | – | – | – |
| Child IQ | 83 | 93.43 | 12.10 | 65.00–121.00 |
| Family variables | ||||
| Family efficacy (P) | 83 | 112.37 | 17.45 | 60.00–140.00 |
| Frequency of stressful Events (P) | 83 | 94.30 | 29.32 | 42.00–157.00 |
| Sickle cell disease management | ||||
| Pain management (subscale) | 83 | 3.13 | 1.18 | 1.00–5.00 |
| General health behaviors (subscale) | 83 | 4.12 | 0.69 | 2.00–5.00 |
| Overall self-management (total) | 83 | 11.41 | 1.94 | 7.67–15.00 |
| Health outcome variables | ||||
| Child HRQOL (C) | 82 | 68.10 | 16.73 | 26.09–100.00 |
| Child HRQOL (P) | 83 | 70.10 | 17.14 | 33.70–96.74 |
| Pain episodes | 83 | 1.69 | 1.94 | 0.00–8.00 |
| Urgent health utilization | 83 | 3.06 | 3.69 | 0.00–17.00 |
Notes. SD = standard deviation; HRQOL = health-related quality of life; P = Parent report; C = Child report. No data reported on SCD subscale owing to inadequate internal consistency.
Preliminary Analyses
Pearson correlation analyses showed that older child age was associated with higher child-reported HRQOL (r = .27, p = .02; see Table 2). Higher family income was associated with less frequent stressful events (r = −.35, p = .001). As a result of these significant relationships, child age and family income were controlled in all subsequent analyses. Among main study variables, higher family efficacy related to better SCD self-management (total score on the SCI-SD; r = .31, p = .004), general health behaviors (subscale on the SCI-SCD; r = .31, p = .004), and pain management (subscale on the SCI-SCD; r = .27, p = .01). More frequent stressful events were associated with lower child-reported (r = −.28, p = .01) and parent-proxy (r = −.34, p = .002) HRQOL, as well as poorer general health behaviors (r = −.35, p = .001). Higher parent-proxy child HRQOL related to better overall SCD self-management (r = .34, p = .002), general health behaviors (r = .36, p = .001), and pain management (r = .27, p = .01), and fewer pain episodes (r = −.33, p = .002) and urgent health utilization (r = −.26, p = .02). More pain episodes were associated with higher urgent health utilization (r = .79, p < .001).
Table II.
Pearson Correlations for Demographic and Main Study Predictor and Outcome Variables
| Variables | 1. | 2. | 3. | 4. | 5. | 6. | 7. | 8. | 9. | 10. | 11. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Child age | 1.00 | ||||||||||
| 2. Family income | −.16 | 1.00 | |||||||||
| 3. Family efficacy (P) | .13 | .03 | 1.00 | ||||||||
| 4. PIP—Frequency of stressful events (P) | −.05 | −.35** | −.17 | 1.00 | |||||||
| 5. Overall SCD self-management (P) | .02 | .12 | .31** | −.16 | 1.00 | ||||||
| 6. General health behaviors subscale (P) | .02 | .20 | .32** | −.35** | .70** | 1.00 | |||||
| 7. Pain management subscale (P) | .12 | .08 | .27* | −.11 | .86** | .27* | 1.00 | ||||
| 8. Child HRQOL (P) | −.10 | .21 | .21 | −.34** | .34** | .36** | .27* | 1.00 | |||
| 9. Child HRQOL (C) | .27* | −.18 | .16 | −.28* | −.01 | .26* | −.11 | .15 | 1.00 | ||
| 10. Urgent health utilization in past year | −.17 | −.18 | −.01 | .21 | .07 | −.10 | .05 | −.26* | −.05 | 1.00 | |
| 11. Pain episodes in past year | −.13 | −.10 | −.02 | .16 | .02 | −.01 | −.05 | −.33** | −.06 | .80** | 1.00 |
Notes. MANOVAs showed that SCD genotype (HbSS, HbSC, HbSβ+, HbSβ0, Other) did not significantly relate to main study predictor or outcome variables (ps > .05). Owing to significant correlations with main study predictor and outcome variables, child age and family income were included as covariates for all subsequent analyses. *p < .05, **p < .01; P = Parent report; C = Child report; PIP = The Pediatric Inventory for Parents.
Mediation Predicting Health Outcomes
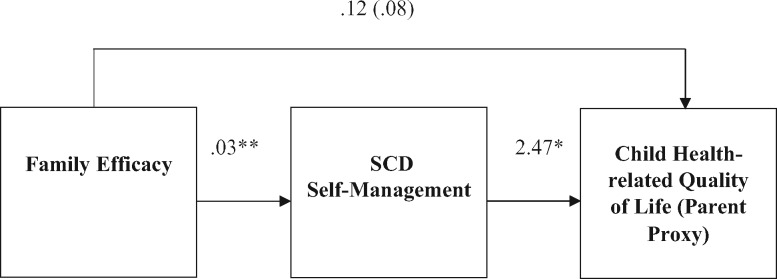
Utilizing a bootstrapping model while controlling for child age and family income, family efficacy was positively related to overall SCD self-management, which in turn related to higher parent-proxy HRQOL, as evidenced by the indirect effect of the confidence interval that did not contain zero (95% CI = .02, .20). Figure 1 shows the mediation model and identifies the bootstrapped estimate of the indirect effect as well as the unstandardized B weights for path coefficients. Mediation models predicting the remaining health outcomes (child-reported HRQOL, urgent health utilization, and pain episodes) were nonsignificant.
Figure 1.
Cross-sectional mediation model for family efficacy predicting SCD self-management and parent-proxy child health-related quality of life.
Notes. Controlled for child age and family income. Path coefficients are unstandardized B weights. Estimate of the indirect effect = (.08); bootstrapped at 95% confidence interval = (.0195, 1971). *p < .05. **p < .01.
For multiple mediation analyses, the total indirect effect of general health behaviors and pain management to parent-proxy HRQOL was significant, as demonstrated by the confidence interval that did not contain zero (see Table 3). The specific indirect effect of general health behaviors mediating the relationship between family efficacy and parent-proxy HRQOL was also significant, such that increased family efficacy was positively related to general health behaviors, which in turn positively related to parent-proxy HRQOL. General health behaviors also mediated relationships between parenting stress and child-reported HRQOL and parent-proxy HRQOL. That is, less frequent parenting stress was associated with better general health behaviors, which in turn related to better child HRQOL. All other mediation models were nonsignificant.
Table III.
Significant Cross-Sectional Multiple Mediation
| Mediation Model 1: | |||
|---|---|---|---|
| Predictor: Family efficacy | |||
| Mediators: General health behaviors and pain management | |||
|
Outcome variable: Parent-proxy child HRQOL | |||
|
BCa 95% bootstrapped CI |
|||
|
Point estimate |
Lower |
Upper |
|
| Total indirect effect | .116a | .038 | .240 |
| General health behaviors | .077a | .018 | .180 |
| Pain management | .040 | −.005 | .141 |
|
Mediation Model 2: | |||
| Predictor: PIP—Frequency of stressful events | |||
| Mediators: General health behaviors and pain management | |||
|
Outcome variable: Parent-proxy child HRQOL | |||
|
BCa 95% bootstrapped CI |
|||
|
Point estimate |
Lower |
Upper |
|
| Total indirect effect | −.046 | −.120 | .003 |
| General health behaviors | −.038a | −.114 | −.002 |
| Pain management | −.008 | −.061 | .013 |
|
Mediation Model 3: | |||
| Predictor: PIP—Frequency of stressful events | |||
| Mediators: General health behaviors and pain management | |||
|
Outcome variable: Child-reported HRQOL | |||
|
BCa 95% bootstrapped CI |
|||
|
Point estimate |
Lower |
Upper |
|
| Total indirect effect | −.043 | −.127 | .013 |
| General health behaviors | −.053a | −.144 | −.004 |
| Pain management | .010 | −.021 | .063 |
Confidence intervals that do not contain zero are deemed to be significant; BCa are bias-corrected and accelerated; PIP = The Pediatric Inventory for Parents; HRQOL = Health-related quality of life.
Discussion
Guided by the Pediatric Self-Management Model (Modi et al., 2012), this study evaluated the impact of modifiable family factors on SCD self-management and, ultimately, health outcomes. We tested whether SCD self-management mediated relationships between family efficacy and parenting stress and health outcomes (child and parent proxy HRQOL, pain episodes, and urgent health utilization). After controlling for child age and family income, higher family efficacy and less frequent parenting stress related to better SCD self-management (overall SCD self-management and general health behaviors), which in turn had positive implications for child HRQOL. Of note, preliminary analyses showed a correlation between lower income and more frequent stressful events, suggesting that disease-related parenting stress may be exacerbated by economic disadvantages. This study offered further evidence that positive family interactions and lower parenting stress may promote adaptive SCD self-management and improve child HRQOL, which is consistent with existing studies in SCD (Badawy, Thompson, Lai, et al., 2017; Barakat et al., 2002; Raphael et al., 2013; Treadwell et al., 2005) and growing literature on the protective influence of family efficacy on risky health behaviors and medical adherence (Guilfoyle, Goebel, & Pai, 2011; Kao, Lupiya, & Clemen-Stone, 2014; Kao & Caldwell, 2015).
Contrary to the Pediatric Self-Management Model, SCD self-management did not mediate pain episodes and urgent health utilization. It is possible that these health outcomes were influenced by other variables that may not be within the family’s control (e.g., health complications exacerbated by cold weather). A recent meta-analysis reported small effect sizes between aspects of pediatric SCD self-management and health outcomes, suggesting remaining gaps in the literature about how to best measure SCD self-management and understand its association with preventable health outcomes (Loiselle et al., 2016). Nonetheless, HRQOL represents an important patient-reported outcome for intervention and observational studies of children with SCD (Dampier et al., 2010). Thus, testing theoretically informed and modifiable variables (such as family functioning and disease self-management) in relation to child and parent proxy HRQOL is a valuable contribution to the literature.
The inclusion of the child-reported measure of HRQOL is another important strength of this investigation, as we found similarities (parenting stress relating to child and parent proxy HRQOL) and differences (family efficacy relating to parent proxy but not child HRQOL) across reporters. Past research has found both positive and negative relations between aspects of SCD self-management and HRQOL (Barakat et al., 2005; Fisak et al., 2012), and we offer further evidence that better SCD self-management may promote HRQOL for school-aged children with SCD. To thoroughly test the applicability of the Pediatric Self-Management Model, rigorous longitudinal and multi-method research is needed. Attention to each ecological level of the Pediatric Self-Management Model, including community and health system influences (e.g., limited access to health care providers and insurance barriers; Treadwell et al., 2011), is essential for bringing consensus to the SCD literature
Current findings highlight possible intervention targets for improving HRQOL for children with SCD. Such interventions may include strategies to minimize the frequency of stressful events for parents (e.g., problem-solving interventions that address items endorsed on the PIP) and strategies to promote the family’s capability to work together as a system. Family-based interventions demonstrate high acceptability in a pediatric SCD samples (Barakat et al., 2010; Daniel et al., 2015) and adapting existing interventions may be beneficial. For example, adapting a culturally sensitive, family-based psychoeducational intervention that improved caregiver and child SCD knowledge (Kaslow et al., 2000). Of note, caregiver SCD knowledge has been positively associated with SCD adherence (Barakat et al., 2002; Jensen et al., 2005; Witherspoon & Drotar, 2006). Parent problem-solving training is an efficacious intervention for reducing distress among mothers of children with cancer (Sahler et al., 2013) and may be generalized to decrease parenting stress while navigating problems with SCD self-management. Using strengths-based and culturally sensitive strategies (Schwartz, Radcliffe, & Barakat, 2007) to incorporate family efficacy into health promotion interventions may also be advantageous, though health providers should consider that the priorities for family efficacy may vary across diverse patient populations. For instance, one study found that families from ethnic and socioeconomic minority backgrounds prioritized pragmatic (e.g., setting boundaries and parental monitoring) and value-laden family efficacy (e.g., ethnic pride and religious faith) compared with relational family efficacy (e.g., communication) to protect children from risky health behaviors (Kao & Caldwell, 2015).
While the present study evaluated school-aged children with SCD, older children and adolescents with SCD may be at greater risk for poor psychosocial HRQOL (Panepinto, O'Mahar, DeBaun, Loberiza, & Scott, 2005). Across a variety of pediatric chronic disease groups, researchers have documented deteriorations in disease self-management during adolescence (Hommel, Ramsey, Rich, & Ryan, 2017). As adolescents with SCD establish increased personal responsibility for their health (Patterson, McDonald, Zebrack, & Medlow, 2015; Wiebe et al., 2014), while also negotiating other developmental tasks (such as greater affiliation with peers), the protective influence of supportive family dynamics may become even more salient. At the same time, as adolescent patients acquire more responsibility with SCD self-management tasks, it will be important to further investigate individual (e.g., disease knowledge and self-efficacy) and peer factors (e.g., peer acceptance) that may promote or hinder their acquisition of adaptive self-management skills. Emerging studies are beginning to focus on the transition to adult-centered care among adolescents and young adults with SCD (Crosby, Quinn, & Kalinyak, 2015; McPherson, Thaniel, & Minniti, 2009; Treadwell, Telfair, Gibson, Johnson, & Osunkwo, 2011). A related and important area of future research is the study of how parents transfer SCD medical responsibilities to their children, and the family dynamics that support or hinder youth’s early development of independent SCD self-management skills.
Relationships between SCD self-management and other health outcomes may have been significant if our models included an objective measure of adherence. Although self-report measures of disease self-management possess key advantages, our parent-reported measure may have been inflated owing to social desirability (Bender et al., 2000; Stirratt et al., 2015). Future studies should also incorporate multiple informants (e.g., child, parent, and provider) into SCD self-management assessments (Duncan, Mentrikoski, Wu, & Fredericks, 2014). Owing to poor internal consistency for the sickle cell management subscale, we were unable to examine direct links between SCD-specific behaviors and health outcomes. The poor internal consistency of the sickle cell management subscale was surprising given similarities between our sample and the initial validation sample, but suggests a possible need for a revised SCD self-management measure. Furthermore, although this study focused on self-management behaviors that are pertinent to most children with SCD, the measure did not include all relevant SCD tasks (e.g., chelation therapy).
There are several other limitations that should be addressed in future work. First, as is typical in studies of pediatric SCD, the sample size in this study was small and the recruitment rate was suboptimal. Second, the majority of the respondents in this study were mothers, which posed risks for common-method variance. Third, the cross-sectional nature of this secondary analysis did not allow for an examination of the temporal ordering of the variables. Thus, the directionality and influence of family functioning on SCD self-management and health outcomes across time cannot be determined with these data. For example, it is possible that parenting stress influenced child HRQOL or poor child HRQOL influenced parenting stress. Moreover, the use of cross-sectional data to test for mediation is less than ideal. Operationalizing pain episodes through electronic health record review represents another limitation, as many individuals with SCD experience and manage pain exclusively from home (Bakshi, Smith, Ross, & Krishnamurti, 2017; Smith et al., 2008). Thus, we may have obtained an underestimate of the frequency of pain episodes. Measuring pain in real-world settings through validated daily diary methods and ecological momentary assessment may allow for a more fine-grained temporal assessment of SCD pain episodes and related social-ecological factors (Bakshi et al., 2015).
In summary, several study findings were aligned with the Pediatric Self-Management Model, which emphasizes the family context as one of several critical influences for supporting pediatric self-management and HRQOL across childhood. Although family-based interventions are typically acceptable to parents and may improve SCD self-management and HRQOL, recruitment and retention remain difficult (Daniel et al., 2015). Future research may adopt peer-patient navigators, which improved retention of participants in our larger intervention trial, as well as test patient-centered intervention delivery methods that may reduce perceived burden (e.g., meeting with families during routine clinic visits, mobile health strategies). Although nonsignificant for this study, in other pediatric populations, neurocognitive factors were important predictors of disease self-management (McNally, Rohan, Pendley, Delamater, & Drotar, 2010; O’Hara & Holmbeck, 2013; Perez et al., 2017; Psihogios, Murray, Zebracki, Acevedo, & Holmbeck, 2017) and in SCD, recall barriers were associated with worse hydroxyurea adherence (Badawy, Thompson, Penedo, et al., 2017). Future work should rigorously evaluate how characteristic neurocognitive challenges among children with SCD, such as deficits in IQ, executive functioning, and attention for patients with and without documented stroke or silent infarct (Brown et al., 2000; Schatz, Finke, Kellett, & Kramer, 2002), affect SCD self-management. Prospective research is also needed to detect how early patterns of family functioning and disease management affect health outcomes over time. Such work is especially salient for promoting adherence during adolescence, which is a developmental period characterized by inadequate medical self-management across many chronic illness populations (La Greca & Mackey, 2009), poorer HRQOL (Dampier et al., 2011), and higher parenting stress in SCD (Barakat, Patterson, Tarazi, & Ely, 2007).
Funding
This work was supported in part by a grant from the National Heart, Lung, and Blood Institute (U54 HL070585). The writing of this article was supported by a Postdoctoral Fellowship from the American Cancer Society (PF-16-166-01-CPPB) to A.M.P. This study is part of a larger investigation of a family-based, problem-solving skills intervention for school-aged children with SCD. The authors express their gratitude to participating children and families.
Conflicts of interest: None declared.
References
- Anderson B., Vangsness L., Connell A., Butler D., Goebel‐Fabbri A., Laffel L. (2002). Family conflict, adherence, and glycaemic control in youth with short duration type 1 diabetes. Diabetic Medicine, 19, 635–642. [DOI] [PubMed] [Google Scholar]
- Badawy S. M., Thompson A. A., Lai J. S., Penedo F. J., Rychlik K., Liem R. I. (2017). Health‐related quality of life and adherence to hydroxyurea in adolescents and young adults with sickle cell disease. Pediatric Blood and Cancer, 64, 608–614. [DOI] [PubMed] [Google Scholar]
- Badawy S. M., Thompson A. A., Penedo F. J., Lai J. S., Rychlik K., Liem R. I. (2017). Barriers to hydroxyurea adherence and health‐related quality of life in adolescents and young adults with sickle cell disease. European Journal of Haematology, 98, 608–614. [DOI] [PubMed] [Google Scholar]
- Bakshi N., Smith M. E., Ross D., Krishnamurti L. (2017). Novel metrics in the longitudinal evaluation of pain data in sickle cell disease. The Clinical Journal of Pain, 33, 517–527. [DOI] [PubMed] [Google Scholar]
- Bakshi N., Stinson J. N., Ross D., Lukombo I., Mittal N., Joshi S. V., Belfer I., Krishnamurti L. (2015). Development, content validity, and user review of a web-based multidimensional pain diary for adolescent and young adults with sickle cell disease. The Clinical Journal of Pain, 31, 580–590. [DOI] [PubMed] [Google Scholar]
- Bandura A., Caprara G. V., Barbaranelli C., Regalia C., Scabini E. (2011). Impact of family efficacy beliefs on quality of family functioning and satisfaction with family life. Applied Psychology, 60, 421–448. [Google Scholar]
- Barakat L. P., Lutz M., Smith-Whitley K., Ohene-Frempong K. (2005). Is treatment adherence associated with better quality of life in children with sickle cell disease? Quality of Life Research, 14, 407–414. [DOI] [PubMed] [Google Scholar]
- Barakat L. P., Patterson C. A., Tarazi R. A., Ely E. (2007). Disease-related parenting stress in two sickle cell disease caregiver samples: Preschool and adolescent. Families, Systems, and Health, 25, 147–161. [Google Scholar]
- Barakat L. P., Patterson C. A., Weinberger B. S., Simon K., Gonzalez E. R., Dampier C. (2007). A prospective study of the role of coping and family functioning in health outcomes for adolescents with sickle cell disease. Journal of Pediatric Hematology/Oncology, 29, 752–760. [DOI] [PubMed] [Google Scholar]
- Barakat L. P., Schwartz L. A., Salamon K. S., Radcliffe J. (2010). A family-based randomized controlled trial of pain intervention for adolescents with sickle cell disease. Journal of Pediatric Hematology/Oncology, 32, 540–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat L. P., Smith-Whitley K., Ohene-Frempong K. (2002). Treatment adherence in children with sickle cell disease: Disease-related risk and psychosocial resistance factors. Journal of Clinical Psychology in Medical Settings, 9, 201–209. [Google Scholar]
- Bender B., Wamboldt F., O'connor S. L., Rand C., Szefler S., Milgrom H., Wamboldt M. Z. (2000). Measurement of children's asthma medication adherence by self report, mother report, canister weight, and Doser CT. Annals of Allergy, Asthma and Immunology, 85, 416–421. [DOI] [PubMed] [Google Scholar]
- Berkelhammer L. D., Williamson A. L., Sanford S. D., Dirksen C. L., Sharp W. G., Margulies A. S., Prengler R. A. (2007). Neurocognitive sequelae of pediatric sickle cell disease: A review of the literature. Child Neuropsychology, 13, 120–131. [DOI] [PubMed] [Google Scholar]
- Beyer J. E., Simmons L. E. (2004). Home treatment of pain for children and adolescents with sickle cell disease. Pain Management Nursing, 5, 126–135. [DOI] [PubMed] [Google Scholar]
- Brown R. T., Davis P. C., Lambert R., Hsu L., Hopkins K., Eckman J. (2000). Neurocognitive functioning and magnetic resonance imaging in children with sickle cell disease. Journal of Pediatric Psychology, 25, 503–513. [DOI] [PubMed] [Google Scholar]
- Caccavale L. J., Weaver P., Chen R., Streisand R., Holmes C. S. (2015). Family density and SES related to diabetes management and glycemic control in adolescents with type 1 diabetes. Journal of Pediatric Psychology, 40, 500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara G. V., Regalia C., Scabini E., Barbaranelli C., Bandura A. (2004). Assessment of filial, parental, marital, and collective family efficacy beliefs. European Journal of Psychological Assessment, 20, 247–261. [Google Scholar]
- CDC. (2016, August 2016). Sickle Cell Disease (SCD). Retrieved from https://www.cdc.gov/ncbddd/sicklecell/data.html (September 22, 2016)
- Cohen J. (1992). A power primer. Psychological Bulletin, 112, 155–159. [DOI] [PubMed] [Google Scholar]
- Crosby L. E., Quinn C. T., Kalinyak K. A. (2015). A biopsychosocial model for the management of patients with sickle-cell disease transitioning to adult medical care. Advances in Therapy, 32, 293–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampier C., LeBeau P., Rhee S., Lieff S., Kesler K., Ballas S., Rogers Z., Wang W.; Comprehensive Sickle Cell Centers (CSCC) Clinical Trial Consortium (CTC) Site Investigators. (2011). Health‐related quality of life in adults with sickle cell disease (SCD): A report from the comprehensive sickle cell centers clinical trial consortium. American Journal of Hematology, 86, 203–205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dampier C., Lieff S., LeBeau P., Rhee S., McMurray M., Rogers Z., Smith-Whitley K., Wang W.; Comprehensive Sickle Cell Centers (CSCC) Clinical Trial Consortium (CTC). (2010). Health‐related quality of life in children with sickle cell disease: A report from the Comprehensive Sickle Cell Centers Clinical Trial Consortium. Pediatric Blood and Cancer, 55, 485–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniel L. C., Li Y., Smith K., Tarazi R., Robinson M. R., Patterson C. A., Smith-Whitley K., Stuart M., Barakat L. P. (2015). Lessons learned from a randomized controlled trial of a family-based intervention to promote school functioning for school-age children with sickle cell disease. Journal of Pediatric Psychology, 40, 1085–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan C. L., Mentrikoski J. M., Wu Y. P., Fredericks E. M. (2014). Practice-based approach to assessing and treating non-adherence in pediatric regimens. Clinical Practice in Pediatric Psychology, 2, 322–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott V., Morgan S., Day S., Mollerup L. S., Wang W. (2001). Parental health beliefs and compliance with prophylactic penicillin administration in children with sickle cell disease. Journal of Pediatric Hematology/Oncology, 23, 112–116. [DOI] [PubMed] [Google Scholar]
- Fisak B., Belkin M., Von Lehe A., Bansal M. (2012). The relation between health‐related quality of life, treatment adherence and disease severity in a paediatric sickle cell disease sample. Child: Care, Health and Development, 38, 204–210. [DOI] [PubMed] [Google Scholar]
- Gil K. M., Thompson R. J., Keith B. R., Tota-Faucette M., Noll S., Kinney T. R. (1993). Sickle cell disease pain in children and adolescents: Change in pain frequency and coping strategies over time. Journal of Pediatric Psychology, 18, 621–637. [DOI] [PubMed] [Google Scholar]
- Gil K. M., Williams D. A., Thompson R. J., Kinney T. R. (1991). Sickle cell disease in children and adolescents: The relation of child and parent pain coping strategies to adjustment. Journal of Pediatric Psychology, 16, 643–663. [DOI] [PubMed] [Google Scholar]
- Guilfoyle S. M., Goebel J. W., Pai A. L. (2011). Efficacy and flexibility impact perceived adherence barriers in pediatric kidney post-transplantation. Families, Systems, and Health, 29, 44–54. [DOI] [PubMed] [Google Scholar]
- Hilker K. A., Sytsma Jordan S., Jensen S., Elkin T. D., Iyer R. (2006). Development of a screening instrument of adherence in pediatric sickle cell disease. Children's Health Care, 35, 235–246. [Google Scholar]
- Hilliard M. E., Guilfoyle S. M., Dolan L. M., Hood K. K. (2011). Prediction of adolescents' glycemic control 1 year after diabetes-specific family conflict: The mediating role of blood glucose monitoring adherence. Archives of Pediatrics and Adolescent Medicine, 165, 624–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel K. A., Hente E. A., Odell S., Herzer M., Ingerski L. M., Guilfoyle S. M., Denson L. A. (2012). Evaluation of a group-based behavioral intervention to promote adherence in adolescents with inflammatory bowel disease. European Journal of Gastroenterology and Hepatology, 24, 64–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommel K. A., Ramsey R. R., Rich K. L., Ryan J. L. (2017). Adherence to Pediatric Treatment Regimens. In Roberts M. C., Steele R. G. (Eds). Handbook of Pediatric Psychology, Fifth Edition (pp.119–133). New York, NY: The Guilford Press. [Google Scholar]
- Jensen S. A., Elkin T. D., Hilker K., Jordan S., Iyer R., Smith M. G. (2005). Caregiver knowledge and adherence in children with sickle cell disease: Knowing is not doing. Journal of Clinical Psychology in Medical Settings, 12, 333–337. [Google Scholar]
- Kahana S., Drotar D., Frazier T. (2008). Meta-analysis of psychological interventions to promote adherence to treatment in pediatric chronic health conditions. Journal of Pediatric Psychology, 33, 590–611. [DOI] [PubMed] [Google Scholar]
- Kao T. S. A., Caldwell C. H. (2015). Family efficacy within ethnically diverse families: A qualitative study. Family Process, 56, 217–233. [DOI] [PubMed] [Google Scholar]
- Kao T. S. A., Lupiya C. M., Clemen-Stone S. (2014). Family efficacy as a protective factor against immigrant adolescent risky behavior: A literature review. Journal of Holistic Nursing, 32, 202–216. [DOI] [PubMed] [Google Scholar]
- Kaslow N. J., Collins M. H., Rashid F. L., Baskin M. L., Griffith J. R., Hollins L., Eckman J. E. (2000). The efficacy of a pilot family psychoeducational intervention for pediatric sickle cell disease (SCD). Families, Systems, and Health, 18, 381–404. [Google Scholar]
- La Greca A. M., Mackey E. R. (2009). Type 1 diabetes mellitus In Behavioral Approaches to Chronic Disease in Adolescence (pp. 85–100). New York, NY: Springer. [Google Scholar]
- Loiselle K., Lee J. L., Szulczewski L., Drake S., Crosby L. E., Pai A. L. (2016). Systematic and meta-analytic review: Medication adherence among pediatric patients with sickle cell disease. Journal of Pediatric Psychology, 41, 406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNally K., Rohan J., Pendley J. S., Delamater A., Drotar D. (2010). Executive functioning, treatment adherence, and glycemic control in children with type 1 diabetes. Diabetes Care, 33, 1159–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McPherson M., Thaniel L., Minniti C. P. (2009). Transition of patients with sickle cell disease from pediatric to adult care: Assessing patient readiness. Pediatric Blood and Cancer, 52, 838–841. [DOI] [PubMed] [Google Scholar]
- Modi A. C., Pai A. L., Hommel K. A., Hood K. K., Cortina S., Hilliard M. E., Guilfoyle S. M., Gray W. N., Drotar D. (2012). Pediatric self-management: A framework for research, practice, and policy. Pediatrics, 129, e473–e485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hara L. K., Holmbeck G. N. (2013). Executive functions and parenting behaviors in association with medical adherence and autonomy among youth with spina bifida. Journal of Pediatric Psychology, 38, 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto J. A., Bonner M. (2012). Health‐related quality of life in sickle cell disease: Past, present, and future. Pediatric Blood and Cancer, 59, 377–385. [DOI] [PubMed] [Google Scholar]
- Panepinto J. A., O'mahar K. M., DeBaun M. R., Loberiza F. R., Scott J. (2005). Health‐related quality of life in children with sickle cell disease: Child and parent perception. British Journal of Haematology, 130, 437–444. [DOI] [PubMed] [Google Scholar]
- Panepinto J. A., Pajewski N. M., Foerster L. M., Hoffmann R. G. (2008). The performance of the PedsQL™ generic core scales in children with sickle cell disease. Journal of Pediatric Hematology/Oncology, 30, 666–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panepinto J. A., Torres S., Varni J. W. (2012). Development of the PedsQL™ sickle cell disease module items: Qualitative methods. Quality of Life Research, 21, 341–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson P., McDonald F. E., Zebrack B., Medlow S. (2015). Emerging issues among adolescent and young adult cancer survivors. Seminars in Oncology Nursing, 31, 53–59. [DOI] [PubMed] [Google Scholar]
- Perez K. M., Patel N. J., Lord J. H., Savin K. L., Monzon A. D., Whittemore R., Jaser S. S. (2017). Executive function in adolescents with type 1 diabetes: Relationship to adherence, glycemic control, and psychosocial outcomes. Journal of Pediatric Psychology, 42, 636–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt O. S. (2000). Sickle cell anemia as an inflammatory disease. The Journal of Clinical Investigation, 106, 337–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt O. S. (2008). Hydroxyurea for the treatment of sickle cell anemia. New England Journal of Medicine, 358, 1362–1369. [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Hayes A. F. (2008). Asymptotic and resampling strategies for assessing and comparing indirect effects in multiple mediator models. Behavior Research Methods, 40, 879–891. [DOI] [PubMed] [Google Scholar]
- Preacher K. J., Rucker D. D., Hayes A. F. (2007). Addressing moderated mediation hypotheses: Theory, methods, and prescriptions. Multivariate Behavioral Research, 42, 185–227. [DOI] [PubMed] [Google Scholar]
- Psihogios A. M., Murray C., Zebracki K., Acevedo L., Holmbeck G. N. (2017). Testing the utility of a bio-neuropsychosocial model for predicting medical adherence and responsibility during early adolescence in youth with spina bifida. Journal of Pediatric Psychology, 42, 910–921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raphael J. L., Bernhardt M. B., Mahoney D. H., Mueller B. U. (2009). Oral iron chelation and the treatment of iron overload in a pediatric hematology center. Pediatric Blood and Cancer, 52, 616–620. [DOI] [PubMed] [Google Scholar]
- Raphael J. L., Butler A. M., Rattler T. L., Kowalkowski M. A., Mueller B. U., Giordano T. P. (2013). Parental information, motivation, and adherence behaviors among children with sickle cell disease. Pediatric Blood and Cancer, 60, 1204–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapoff M. A. (2009). Adherence to Pediatric Medical Regimens. Springer Science & Business Media. [Google Scholar]
- Rees D. C., Williams T. N., Gladwin M. T. (2010). Sickle-cell disease. Lancet, 376, 2018–2031. [DOI] [PubMed] [Google Scholar]
- Sahler O. J. Z., Dolgin M. J., Phipps S., Fairclough D. L., Askins M. A., Katz E. R., Noll R. B., Butler R. W. (2013). Specificity of problem-solving skills training in mothers of children newly diagnosed with cancer: Results of a multisite randomized clinical trial. Journal of Clinical Oncology, 31, 1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schatz J., Finke R. L., Kellett J. M., Kramer J. H. (2002). Cognitive functioning in children with sickle cell disease: A meta-analysis. Journal of Pediatric Psychology, 27, 739–748. [DOI] [PubMed] [Google Scholar]
- Schwartz L. A., Radcliffe J., Barakat L. P. (2007). The development of a culturally sensitive pediatric pain management intervention for African American adolescents with sickle cell disease. Children's Healthcare, 36, 267–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith W. R., Penberthy L. T., Bovbjerg V. E., McClish D. K., Roberts J. D., Dahman B., Aisiku I. P., Levenson J. L., Roseff S. D. (2008). Daily assessment of pain in adults with sickle cell disease. Annals of Internal Medicine, 148, 94–101. [DOI] [PubMed] [Google Scholar]
- Stirratt M. J., Dunbar-Jacob J., Crane H. M., Simoni J. M., Czajkowski S., Hilliard M. E., Aikens J. E., Hunter C. M., Velligan D. I., Huntley K., Ogedegbe G., Rand C. S., Schron E., Nilsen W. J. (2015). Self-report measures of medication adherence behavior: Recommendations on optimal use. Translational Behavioral Medicine, 5, 470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streisand R., Braniecki S., Tercyak K. P., Kazak A. E. (2001). Childhood illness-related parenting stress: The Pediatric Inventory for Parents. Journal of Pediatric Psychology, 26, 155–162. [DOI] [PubMed] [Google Scholar]
- Treadwell M., Telfair J., Gibson R. W., Johnson S., Osunkwo I. (2011). Transition from pediatric to adult care in sickle cell disease: Establishing evidence‐based practice and directions for research. American Journal of Hematology, 86, 116–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadwell M. J., Law A. W., Sung J., Hackney-Stephens E., Quirolo K., Murray E., Glendenning G. A., Vichinsky E. (2005). Barriers to adherence of deferoxamine usage in sickle cell disease. Pediatric Blood and Cancer, 44, 500–507. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. (2009). The 2009 HHS poverty guidelines. Federal Register, 74, 4199–4201. [Google Scholar]
- Varni J. W., Seid M., Kurtin P. S. (2001). PedsQL™ 4.0: Reliability and validity of the Pediatric Quality of Life Inventory™ version 4.0 generic core scales in healthy and patient populations. Medical Care, 39, 800–812. [DOI] [PubMed] [Google Scholar]
- Wakefield E. O., Popp J. M., Dale L. P., Santanelli J. P., Pantaleao A., Zempsky W. T. (2017). Perceived racial bias and health-related stigma among youth with sickle cell disease. Journal of Developmental and Behavioral Pediatrics, 38, 129–134. [DOI] [PubMed] [Google Scholar]
- Walsh K. E., Cutrona S. L., Kavanagh P. L., Crosby L. E., Malone C., Lobner K., Bundy D. G. (2014). Medication adherence among pediatric patients with sickle cell disease: A systematic review. Pediatrics, 134, 1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. (1999). Wechsler Abbreviated Scale of Intelligence. Psychological Corporation. [Google Scholar]
- Wiebe D. J., Chow C. M., Palmer D. L., Butner J., Butler J. M., Osborn P., Berg C. A. (2014). Developmental processes associated with longitudinal declines in parental responsibility and adherence to type 1 diabetes management across adolescence. Journal of Pediatric Psychology, 39, 532–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witherspoon D., Drotar D. (2006). Correlates of adherence to prophylactic penicillin therapy in children with sickle cell disease. Children's Health Care, 35, 281–296. [Google Scholar]
- Wu Y. P., Rausch J., Rohan J. M., Hood K. K., Pendley J. S., Delamater A., Drotar D. (2014). Autonomy support and responsibility-sharing predict blood glucose monitoring frequency among youth with diabetes. Health Psychology, 33, 1224–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wysocki T., Miller K. M., Greco P., Harris M. A., Harvey L. M., Taylor A., Danda C. E., McDonell K., White N. H. (1999). Behavior therapy for families of adolescents with diabetes: Effects on directly observed family interactions. Behavior Therapy, 30, 507–525. [Google Scholar]
- Yawn B. P., Buchanan G. R., Afenyi-Annan A. N., Ballas S. K., Hassell K. L., James A. H., Jordan L., Lanzkron S. M., Lottenberg R., Savage W. J., Tanabe P. J., Ware R. E., Murad M. H., Goldsmith J. C., Ortiz E., Fulwood R., Horton A., John-Sowah J. (2014). Management of sickle cell disease: Summary of the 2014 evidence-based report by expert panel members. JAMA, 312, 1033–1048. [DOI] [PubMed] [Google Scholar]