Abstract
A prospective cohort of women with reverse transcription polymerase chain reaction (RT-PCR) confirmed Zika virus infection aged 18–39 years in Puerto Rico found that pregnant women have about a 3-fold longer estimated median detection of Zika virus RNA in serum, which can increase definitive diagnosis of infection and facilitate timely and appropriate clinical management.
Zika virus (ZIKV) infection in pregnant women has been associated with congenital Zika syndrome, which includes microcephaly and severe brain anomalies and developmental disabilities in the infant [1–3]. Several case reports have demonstrated longer-than-expected detection of ZIKV RNA in pregnant women, including a case study that reported detection of ZIKV RNA in serum 10 weeks post symptom onset [4, 5]. However, existing evidence has been limited to case reports which lack nonpregnant controls and are unable to determine if pregnancy is independently associated with prolonged detection of ZIKV RNA. Understanding the risk for prolonged detection of ZIKV RNA may inform guidelines for diagnostic testing and clinical counseling for pregnant women.
A preliminary analysis of data collected during the Zika Virus Persistence Study (ZiPer) found that the median time to loss of ZIKV RNA detection in serum among all symptomatic ZIKV-infected participants was 13.9 days (95% confidence interval [CI]: 11.2, 16.6) [6]. We used data from the ZiPer study to test if symptomatic pregnant women have longer detection of ZIKV RNA than symptomatic, nonpregnant women of similar ages.
MATERIALS AND METHODS
Data Source
ZiPer is an ongoing prospective cohort study in Puerto Rico that began in May 2016 and includes rigorous participant follow-up. ZiPer participants included males and females of all ages with ZIKV infection diagnosed by RT-PCR, the methods of which have been previously described [6]. Briefly, symptomatic patients who presented for care and tested positive for ZIKV by RT-PCR (index participants) were offered enrollment. Household members of index participants were invited to participate regardless of the presence or absence of disease, and those who tested positive for ZIKV infection by RT-PCR were eligible to join the prospective cohort. Upon enrollment, participants completed a survey including pregnancy status and information on Zika-like illnesses (ie, fever, conjunctivitis, rash, or arthralgia) during the prior 30 days. Serum, urine, saliva, semen, and vaginal secretions (the last 2 in adults only and self-collected) were collected weekly for the first month and at 2, 4, and 6 months post-enrollment. For participants in whom ZIKV RNA was detected in any specimen at week 4, biweekly collection continued until all specimens tested negative. Detailed instructions were given to participants that self-collected vaginal secretions or semen samples. Specimens were tested by Trioplex RT-PCR assay to detect dengue, chikungunya, and ZIKV RNA [7]. All participants received a $50 reimbursement per visit. The ZiPer protocol was approved by the Centers for Disease Control and Prevention (CDC) Institutional Review Board and all participants gave informed consent.
Only women aged 18–39 years who reported a recent Zika-like illness were included in this analysis. We summarized participants’ demographic characteristics, and described detection of ZIKV RNA in serum by days post symptom onset of specimen collection. “Time-to-loss” of ZIKV RNA was defined as the number of days between onset of ZIKV symptoms and the first nonpositive (negative or below the threshold of detection) RT-PCR result in serum that was sustained for the duration of the study. For analysis purposes, we assumed that all infected participants had ZIKV RNA in serum at symptom onset. For participants who had intermittent shedding of ZIKV (defined as the detection of viral RNA that was followed by a lack of detection and then subsequent detection, regardless of the interval between specimen collections), we used the first negative result after the final recorded test result that was positive by RT-PCR.
Statistical Analysis
We used Fisher exact test to assess if characteristics were associated with pregnancy and t-test to compare continuous variables. Because participants were not tested daily we could not identify the precise day when women lost detectable ZIKV RNA. Consequently, to estimate time-to-loss of ZIKV RNA in serum we used nonparametric maximum-likelihood Turnbull estimator, which accounts for interval censoring due to loss of detection occurring between 2 visit dates [6]. The lower interval was the last specimen collected with a positive ZIKV result by RT-PCR and the upper interval was the first specimen collected with a nonpositive ZIKV result by RT-PCR immediately following the lower interval. We used general linear model to compare the distribution of the length of the intervals (in days) by pregnancy status to assess if the intervals could bias the Turnbull model estimator. We used Turnbull regression models to conduct a sensitivity analysis to address uncertainty resulting from the interval-censored data that conservatively assumed that ZIKV RNA was no longer detectable the day after the last positive ZIKV RT-PCR test. We also conducted a sensitivity analyses to assess if the intermittent positive results influenced the estimated time-to-loss of ZIKV RNA detection. For this we ignored any lone RT-PCR positive result that occurred after 1 or more negative result. We adjusted the upper interval to be the first specimen collected with a nonpositive ZIKV result by RT-PCR and the lower interval to be the previous specimen collected with a positive result and we applied the same nonparametric maximum-likelihood Turnbull estimator. All statistical analyses were performed using SAS version 9.4 and α = 0.05.
RESULTS
During May 2016–April 2017, a total of 300 participants were recruited into ZiPer, including 58 symptomatic women aged 18–39 years, 9 of whom were pregnant. Age and days post symptom onset at enrollment were not different between pregnant and non-pregnant women, and 55.2% of study participants were recruited within 2 days of symptom onset (see Supplementary Table 1). There were no differences in frequency of reported symptoms of rash, arthralgia, and conjunctivitis, but nonpregnant women were more likely to report fever than pregnant women (78% vs. 22%, respectively; P = .003).
Pregnant women provided an average of 9.9 serum specimens compared to 8.2 among nonpregnant women (P = .08) and both groups provided the same number of specimens after the last ZIKV RNA positive specimen (6.7 vs. 6.7) (see Supplementary Table 1). The last serum specimen collected from pregnant women was, on average, 169 days post illness onset, whereas nonpregnant women provided serum until 152 days post illness onset on average (P = .26).
At some point during the study, ZIKV RNA was detected in the serum of all 9 pregnant women and among almost all nonpregnant women (96%). Detecting ZIKV RNA in urine at any point during the study was more common among nonpregnant women (49%) than pregnant women (11%) but was not significantly (P = .06). ZIKV RNA was detected in the saliva of 1 participant. Due to infrequent detection of ZIKV RNA in urine and saliva, we were unable to estimate time-to-loss in these specimens. ZIKV RNA was not detected in any vaginal secretion specimens.
The Turnbull model derived median time-to-loss of detectable ZIKV RNA in serum was about 3 times longer among pregnant women than nonpregnant women (40 days vs. 14 days; P < .001) (see supplemental Table 2 and supplemental Figure 1). Similarly, the sensitivity analysis using the last-positive RT-PCR result to estimate duration showed that pregnant women had significantly longer duration of detectable ZIKV RNA than non-pregnant women (see supplemental Table 2). Two pregnant women (Figure 1) and four nonpregnant women were intermittent shedders. When removing the positive time points after negative RT-PCR results from the analyses, the difference in duration between pregnant and nonpregnant women remained statistically significant (P = .01). The median interval between the last specimen collected that tested positive for ZIKV RNA and the first subsequent specimen that tested nonpositive for ZIKV RNA among pregnant women (14 days; range; 7, 57) was not significantly different from the interval among nonpregnant women (17 days; 1, 56) (P = .85). The similar distribution of the intervals between pregnant and nonpregnant women suggests that differences in estimated ZIKV RNA duration were not due to differences in testing frequency.
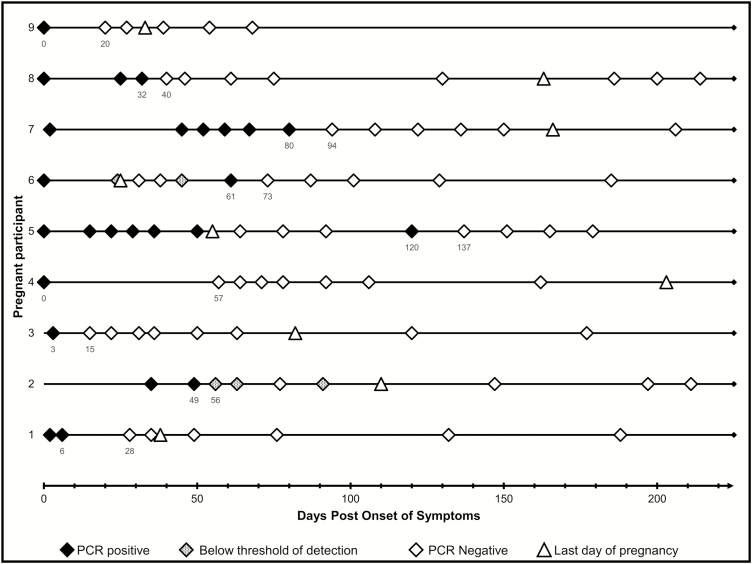
Figure 1.
Detection of Zika virus RNA in the serum of pregnant women aged 18–39 years (n = 9) enrolled in the Zika Virus Persistence Study in Puerto Rico, 2016–2017. Numbers below the PCR results indicate the days post symptom onset of specimen collection. Last day of pregnancy for line 3 is an estimate within one week. The lower and upper intervals used in statistical modeling are shown for each pregnant woman. PCR positive results = CT value <38; Below threshold of detection = CT value >38–≤40; PCR negative = CT value >40. Abbreviations: CT, cycle threshold; PCR, polymerase chain reaction.
A detailed description of all of the RT-PCR testing results of the specimens collected from the 9 pregnant women and the last day of the pregnancy is include in Figure 1. The last day of the pregnancy for 1 participant was 55 days after symptom onset, was followed by 3 negative ZIKV test results, and then a specimen that tested positive for ZIKV RNA 120 days after symptom onset (participant 5 in Figure 1). Participant 6 also had a specimen test positive for ZIKV RNA 36 days after the last day of her pregnancy. All other pregnant women had no specimens test positive for ZIKV RNA after the last day of their pregnancies. Birth outcomes for all pregnancies, other than one, were normal births with infants with apparently no dysmorphic features. One pregnancy (participant 5), resulted in fetal death at 24 weeks gestation.
DISCUSSION
This prospective cohort study provides evidence that time-to-loss of ZIKV RNA in serum among pregnant women can be longer than among nonpregnant women of similar ages. Three different models consistently demonstrated a significantly longer median duration among pregnant women. Only 1 pregnant woman had detectable ZIKV RNA in urine or saliva after enrollment. This study contributes to the body of knowledge by systematically collecting serum samples from all participants, allowing for statistical modeling to estimate duration, and was the first to provide a comparison group of nonpregnant women.
The mechanism of prolonged ZIKV RNA detection in the serum of pregnant women is unknown, but several hypotheses have been proposed. Placental trophoblasts have been observed to be infected during pregnancy and support in vitro growth of ZIKV, indicating that they may act as a viral reservoir [8, 9]. Additionally, the fetus has been suggested as another possible ZIKV reservoir [5]. Last, altered immunity during pregnancy has been postulated as a reason for delayed immune clearance of viruses [10]. Two of the 9 pregnant women in our study had a specimen positive for ZIKV RNA after the last day of their pregnancies. This may have been left over viral particles as opposed to live virus or may suggest reservoirs other than the placenta or fetus. Further research to identify definitively the mechanism of prolonged ZIKA RNA detection could assist in development of interventions to reduce risk of fetal infection.
This study has limitations. First, a small number of pregnant women were enrolled, and we could not identify the precise day when women lost detectable ZIKV RNA, which resulted in imprecision around the median duration of persistence of ZIKV RNA in serum. Nonetheless, all models demonstrated that time-to-loss of ZIKV RNA among pregnant women is significantly longer than among nonpregnant women. Pregnant women were tested slightly more often, and their last specimen collected was longer post illness onset compared to nonpregnant women, but neither of these were significantly different. Slightly increased testing among pregnant women is likely an artifact of the protocol, which required more frequent testing until ZIKV RNA was no longer detected. Additionally, both groups had equal numbers of specimens collected after their last ZIKV RNA positive test. Consequently, more and longer testing among pregnant women likely do not introduce bias into the results. Last, as this analysis only included symptomatic women, results may not be generalizable to women with asymptomatic infections.
To facilitate timely diagnosis of ZIKV infection and appropriate clinical management of pregnancies, the Centers for Disease Control and Prevention (CDC) currently recommends nucleic acid testing (such as RT-PCR) of symptomatic pregnant women within 12 weeks of illness onset [11]. The findings of this study support CDC’s current recommendations. Additionally, CDC recommends that healthcare providers consider nucleic acid testing at least once per trimester, unless a previous test has been positive, for pregnant women who live in or frequently travel to areas with ZIKV transmission [11]. Clinicians managing pregnant women with evidence of confirmed ZIKV infection should follow CDC guidelines, including considering serial ultrasounds every 3–4 weeks to assess fetal anatomy and growth [11].
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The views expressed in this article are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Funding. This work was supported by the Centers for Disease Control and Prevention and the Center for AIDS Research at Emory University [NIH P30AI050409].
Potential conflict of interest. E. S. R.’s previous institution, Emory University, received grant funding from the Centers for Disease Control and Prevention for work related to this manuscript. For activities outside of this manuscript, E. S. R. was paid for consultancy by Medidata Inc.; received royalties from Cengage Learning and his previous and current institutions, Emory University and SUNY University at Albany; received grants from the National Institutes of Health and the Centers for Diseases Control and Prevention. No other authors have conflicts of interest to report. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Portions of this work were accepted as an abstract and presented at IDWeek 2017 Conference October 7, 2017.
References
- 1. Costa F, Sarno M, Khouri R, et al. Emergence of congenital Zika syndrome: viewpoint from the front lines. Ann Intern Med 2016; 164:689–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Miranda-Filho Dde B, Martelli CM, Ximenes RA, et al. Initial description of the presumed congenital Zika syndrome. Am J Public Health 2016; 106:598–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rasmussen SA, Jamieson DJ, Honein MA, Petersen LR. Zika virus and birth defects–reviewing the evidence for causality. N Engl J Med 2016; 374:1981–7. [DOI] [PubMed] [Google Scholar]
- 4. Driggers RW, Ho CY, Korhonen EM, et al. Zika virus infection with prolonged maternal viremia and fetal brain abnormalities. N Engl J Med 2016; 374:2142–51. [DOI] [PubMed] [Google Scholar]
- 5. Meaney-Delman D, Oduyebo T, Polen KN, et al. ; U.S. Zika Pregnancy Registry Prolonged Viremia Working Group Prolonged detection of Zika virus RNA in pregnant women. Obstet Gynecol 2016; 128:724–30. [DOI] [PubMed] [Google Scholar]
- 6. Paz-Bailey G, Rosenberg ES, Doyle K, et al. Persistence of Zika virus in body fluids : preliminary report. N Engl J Med 2017. doi: 10.1056/NEJMoa1613108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Food and Drug Administration. Zika virus emergency use authorization. Silver Spring, MD, 2016. [Google Scholar]
- 8. Bhatnagar J, Rabeneck DB, Martines RB, et al. Zika virus RNA replication and persistence in brain and placental tissue. Emerg Infect Dis 2017; 23:405–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Aagaard KM, Lahon A, Suter MA, et al. Primary human placental trophoblasts are permissive for Zika virus (ZIKV) replication. Sci Rep 2017; 7:41389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Granovsky MO, Minkoff HL, Tess BH, et al. Hepatitis C virus infection in the mothers and infants cohort study. Pediatrics 1998; 102:355–9. [DOI] [PubMed] [Google Scholar]
- 11. Oduyebo T, Polen KD, Walke HT, et al. Update: interim guidance for health care providers caring for pregnant women with possible Zika virus exposure —United States (Including US Territories), July 2017. MMWR Morb Mortal Wkly Rep 2017; 66:781–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.