Abstract
Background
In 2002, a previously healthy 69-year-old man travelled to France from the United States and presented to our hospital with a febrile illness that subsequently was determined to be babesiosis. The blood isolated from this patient served as a source for propagation of the Babesia microti R1 strain with subsequent sequencing and annotation of the parasite genome.
Methods
Upon admission, we obtained a medical history, performed a physical examination, and examined his blood for the presence of a blood borne pathogen by microscopy, PCR and indirect immunofluorescence antibody testing. Once the diagnosis of babesiosis was made, we reviewed the literature to assess the distribution of B. microti-associated babesiosis cases in immunocompetent patients from outside the USA.
Results
The patient recalled a tick bite during the previous month on Cape Cod, Massachusetts. The diagnosis was confirmed by identification of Babesia-infected red blood cells on blood smears, amplification of B. microti DNA in blood by PCR and the presence of B. microti antibody in the serum. This strain was the first isolate of B. microti to be fully sequenced and its annotated genome serves as a reference for molecular and cell biology studies aimed at understanding B. microti pathophysiology and developing diagnostic tests and therapies. A review of babesiosis cases demonstrates a worldwide distribution of B. microti and identifies potential emerging endemic areas where travelers may be at risk of contracting B. microti infection.
Conclusion
This case provides clinical information about the patient infected with the R1 isolate and a review of travel risk, diagnosis and treatment of babesiosis in endemic and non-endemic areas.
Keywords: Babesiosis, Babesia microti, R1 strain, tick-borne disease
Introduction
Babesia species are intra-erythrocytic protozoan parasites that cause babesiosis, a tick-borne infectious disease that occurs worldwide.1 These parasites belong to the phylum Apicomplexa, which encompasses other protozoan parasites such as those that cause malaria, toxoplasmosis and cryptosporidiosis. Babesia microti, the causative agent of most human babesiosis cases, is transmitted to humans by hard bodied (Ixodes) tick vectors, the same ticks that transmit the agents of Lyme disease (Borrelia burgdorferi) and human granulocytic anaplasmosis (Anaplasma phagocytophilum).2Babesia microti also can be acquired through blood transfusion and is the most common transfusion-transmitted pathogen in the United States.3 Transfusion-transmitted babesiosis (TTB) is a risk to the blood supply in endemic and non-endemic areas because infected blood from asymptomatic donors from endemic regions may be transported to non-endemic areas and donors that are asymptomatically infected in an endemic area may return to a non-endemic area and donate blood.4,5 Various screening strategies of blood supply have been implemented and have helped to reduce TTB incidence.3–6 Human babesiosis due to B. microti is endemic in the northeastern and northern midwestern United States and sporadically reported in Asia, Australia, Europe and South America.2,7–18 In 2011, the Institute of Medicine in the United States designated babesiosis an emerging health threat19 and the United States Centers for Disease Control and Prevention (CDC) designated babesiosis as a nationally notifiable disease.20 In 2012, the sequencing and annotation of the full B. microti genome was completed, an important advance in our understanding of B. microti biology and evolution.21–23 The R1 strain that was sequenced is currently used as a reference genome to characterize the genetic diversity and pathogenicity of B. microti strains and to develop new diagnostic tests and therapies.24 This manuscript describes the clinical case that led to the isolation of the B. microti R1 isolate and provides important information about babesiosis management in endemic and non-endemic areas.
Case Presentation
A 69-year-old previously healthy Caucasian male was in good health until he developed chills during a flight from the United States to France in the summer of 2002. Despite self-administration of non-steroidal anti-inflammatory drugs (NSAIDs), he subsequently developed a frontal headache and purpuric rash, which continued until presentation to the Centre Hospitalier Bretagne Atlantique in Vannes, Brittany, France. He was a resident of New York City with a second residence in Cape Cod, Massachusetts. Over the course of 30 years the patient had traveled to Europe, South America, Asia and Australia. One month prior to hospitalization, he spent 2 weeks at his Cape Cod residence where he reported multiple tick bites. The patient was on no medications. His past medical history included appendectomy.
On physical examination, the patient was awake, alert and oriented to person, place and time and in no acute distress. He was febrile to 105 °F (rectal) with normal blood pressure and heart rate. There was no evidence of meningismus. Eye exam revealed no icterus. Skin examination revealed diffuse purpura of the lower extremities. The remainder of the patient’s clinical examination was unremarkable.
Initial laboratory findings showed anemia with hemoglobin of 12.2 g/dl; a normal mean corpuscular volume (MCV); thrombocytopenia with platelet count of 77 000/mm3; normal leukocyte and neutrophil counts of 4800/mm3 and 2450/mm3, respectively, mild lymphopenia of 1250/mm3 with 16% activated lymphocytes, reticulocytosis of 3% (119 100/mm3) and an elevated erythrocyte sedimentation rate (ESR) of 100 mm/h. Prothrombin time (PT) was slightly prolonged with an INR of 1.3. Serum electrolytes revealed a sodium of 134 mmol/l and potassium of 3.4 mmol/l. Serum creatinine was 1.37 mg/dl. Liver enzymes alanine aminotransferase (ALT), gamma-glutamyl transpeptidase (GGT), alkaline phosphatase and total bilirubin were in normal range, and haptoglobin was undetectable. Aspartate transaminase (AST: 75 U/l) and lactate dehydrogenase (LDH: 700 U/l) levels were elevated. Marked elevations were noted in the acute phase protein fibrinogen (6600 mg/dl) and C-reactive protein (CRP: 286 mg/l).
Three blood cultures were performed and were negative. Lumbar puncture analysis revealed clear CSF with no cells and normal total protein and glucose. CSF culture revealed no growth of organisms. Urine analysis showed no evidence of leukocytes, nitrites or erythrocytes. Electrocardiography was normal. Abdominal echography showed a normal spleen and no other abnormalities except a hemangioma of the liver in segment VI. Tranthoracic echocardiography revealed normal ejection fraction, morphology and valvular function without evidence of pericardial effusion. Chest radiography demonstrated a mild interstitial infiltrate at the right lung base.
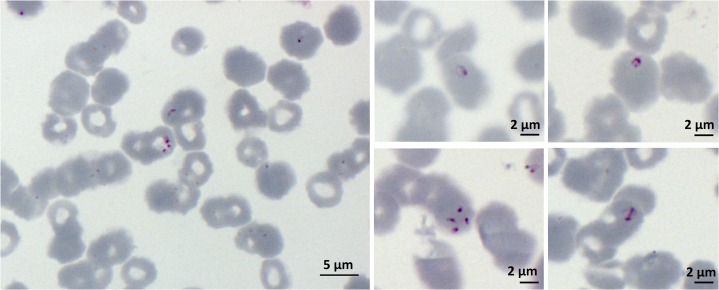
An initial peripheral blood smear suggested Plasmodium falciparum with a 3% parasitemia. Re-evaluation of the blood smear and morphology of parasites within the patient’s red blood cells Babesia infection (Figure 1). Babesia microti DNA was amplified from blood using a Babesia PCR using the primer pair PIRO-A (5′AATACCCAATCCTGACACAGGG 3′) and antisense oligonucleotide primer PIRO-B (5′TTAAATACGAATGCCCCCAAC 3′) amplifying a variable section of the 18S encoding gene.25 Sequencing of the PCR product showed 95% homology to the 18S rDNA sequence of B. microti.
Figure 1.
Peripheral blood smear of R1 isolate infected patient. The patient was treated at the Centre Hospitalier Bretagne Atlantique in Vannes, Brittany, France. He had a residence in Cape Cod, Massachusetts
Several Babesia serologic tests were performed using indirect immunofluorescence antibody tests (IFT) with polyvalent anti-human secondary antibody (Sigma, F6506) to discriminate between antibodies to different Babesia species. Markedly elevated antibody titer was detected for B. microti (1:16 400) with the presumption that reactivity to Babesia divergens (titer: 1:2048) and Babesia canis (titer: 1:512) were a result of cross-reactivity of B. microti antibody to antigens of these other Babesia species. The initial serological analysis for malaria showed negative IgM but positive IgG. The results of antibody testing for ehrlichiosis, syphilis (Treponema pallidum) and for HIV were negative. Lyme disease serology was performed to assess possible coinfection with B. burgdorferi. Borrelia burgdorferi IgG antibody was detected but no IgM. The patient had no previous history of Lyme disease. A diagnosis of acute Lyme disease was ruled out based on the absence of an erythema migrans rash and the absence of IgM antibody confirmed by western blot analysis.
The patient was diagnosed with severe babesiosis caused by B. microti and was given intravenous quinine (20 mg/kg body weight loading dose, followed by 10 mg/kg body weight every 8 h) for 4 days. The patient experienced a rapid defervescence following treatment with resolution of thrombocytopenia but experienced persistent hemolysis and mild renal insufficiency. After completion of 4 days of intravenous quinine, he was given oral quinine 500 mg thrice daily and clindamycin 300 mg thrice daily for another 10 days. By the end of the fifth hospital day, the patient recovered well with resolution of hemolysis and mild renal dysfunction. He was discharged with no residual symptoms.
Review and Discussion
The primary objective of this report is to describe the clinical presentation, diagnosis and therapy of human babesiosis case that led to the isolation of the R1 isolate, which provided the first completed genome sequence of B. microti.21 The sequencing, assembly and annotation of the genome of this isolate was performed in collaboration between the Genoscope (Evry, France) and our teams at University of Montpellier in France and Yale University in the USA. The patient traveled to France where he was diagnosed to have babesiosis at the Centre Hospitalier Bretagne Atlantique in Vannes, Brittany. This report further highlights frequently encountered problems in the diagnosis of babesiosis, particularly in non-endemic regions of the world.
B. microti R1 genome analysis: biological and clinical implications
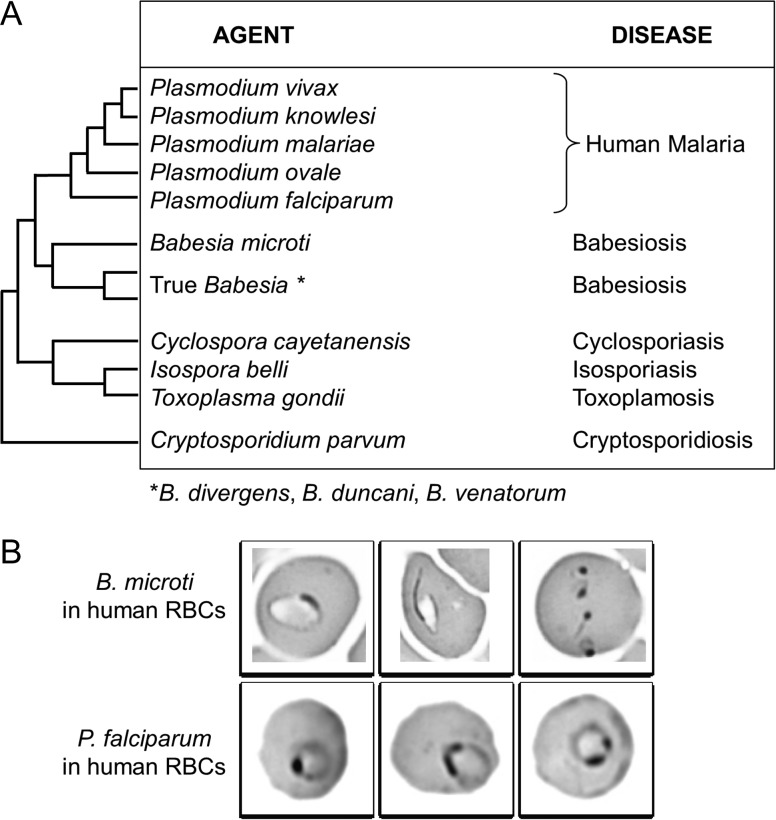
The assembly and annotation of the B. microti R1 genome made it possible to understand the metabolic functions of the parasite, identify new antigens for development of diagnostic assays, and identify targets for development of novel therapies.24,26 The parasite was first isolated from the blood of the patient and propagated in gerbils or hamsters.21 The genome was then sequenced, annotated and made available on public genome databases.21 Subsequent analyses included whole genome comparison between R1 and Gray strain isolates using optical mapping, determination of the structure and composition of the mitochondrial and apicoplast genomes.22,23 The size of the B. microti R1 nuclear genome is 6.5 MB, making it the smallest nuclear genome among apicomplexa.21,22 The genome consists of 4-chromosomes, has a G + C content of 36% and encodes 3567 protein-coding genes, most of which are expressed during parasite development in mammalian red blood cells. Phylogenetic analyses reveal that B. microti represents a new lineage within the phylum Apicomplexa, distinct from lineages encompassing Plasmodium sp. and true Babesia sp. (Figure 2A). Despite its distinct evolution and until future renaming, the genus Babesia is still used for B. microti taxonomy and closely related species like B. rodhaini and B. felis. Other Babesia species (including those that infect humans, such as B. divergens) are phylogenetically related and are designated as Babesia sensu stricto or true Babesia sp. as the group encompasses type species used to specify the genera. Comparison of 18S rDNA sequences revealed that the B. microti clade is diverse with evidence of differences in mammalian host specificity among different groups.27 Following sequencing of the R1 isolate, further efforts by our group and others have led to the sequencing of several isolates from human, ticks and infected animals.28–30 The overall size of the genomes of the B. microti isolates sequenced so far ranges between 6.3 and 6.9 MB. Available data suggest that sub-population of B. microti might have evolved new properties that allow infection of humans.28,29
Figure 2.
Difficulty in distinguishing between Babesia microti and Plasmodium falciparum on blood smear. (A) Babesia microti and P. falciparum are the only challenging apicomplexa. Plasmodium falciparum is only species for which confusion was described among all Plasmodium species responsible for human malaria. Dendogram shows clade organization of human infecting apicomplexa. Babesia microti is the type species of parasites that get separated early in piroplasmida evolution. Babesia divergens, B. duncani and B. venatorum are the major species infecting human among the major branch of piroplasmida encompassing true Babesia sp. Plasmodium and piroplasmida are hemoparasites. Other apicomplexa are intestinal parasites. (B) Challenging diagnosis of B. microti vs P. falciparum. Blood smears from patients with babesiosis or malaria are similar. Similarity is true for B. microti during human infection only
Areas of travel risk for babesiosis
Human babesiosis is endemic in the United States and China,1,31 while sporadic cases occur in Europe (Figure 3). B. microti infection is endemic in the northeastern and northern Midwestern United States with the number of cases increasing significantly since the disease has become nationally notifiable in 2011.2 In 2016, more than 1600 were reported to CDC. Babesiosis is not a notifiable disease outside the USA and incidence of this disease is likely to be underestimated. Babesia divergens is the most common etiologic agent of babesiosis in Europe and splenectomized persons are at greater risk.1 Most B. microti cases reported in Europe are found in travelers coming from the USA.7,8,11–13,16 Serosurveys suggest that local transmission of B. microti could happen in Europe, but with little to no clinical signs.1–3,32–36 Indigenous B. microti infection has been observed in an immunocompromised patient in Germany.37 Surveys in local areas, often linked with poverty and presence of rodents have revealed B. microti infection in China, Mongolia and Bolivia.38–40 Case of B. microti in South America was reported for a traveler coming back in the USA.17 A patient traveling from Uruguay to Spain presented mild symptoms of B. microti infection a few days after he return to his home but the origin of the parasite remains uncertain.18Babesia venatorum infection is endemic in Heilongjiang province, the most northeastern province of China.31,41 Both B. microti infection and malaria are found in Yunnan province in southwestern China along the Myanmar border.31,38 There is concern that babesiosis may be misidentified as resistant malaria, given that both Plasmodium and Babesia parasites have a similar appearance on microscopic examination following Giemsa staining (Figure 2B) and that the Babesia parasites are less sensitive to chloroquine and mefloquine than Plasmodium parasites.34 Physicians and other health care workers in areas where both babesiosis and malaria are endemic should order Babesia and malaria PCR, in addition to blood smears.17,42
Figure 3.
Endemic areas and sporadic cases of B. microti-human babesiosis and relationship with malaria transmission areas. Most travelers in Europe that experimence babesiosis due to B. microti acquired their infections in the USA. Babesiosis is not a notifiable disease outside the USA. We refer only to B. microti cases concerning immunocompetent patients. Babesia divergens, B. duncani, B. microti and B. venatorum are the major species infecting human across the world. Reference number is given in brackets
Babesiosis diagnosis
Babesiosis presents as a malaria-like illness without an easily recognizable sign. The diagnosis is therefore often delayed, especially in non-endemic regions. Because the malaria-like symptoms are non-specific (see below), health care workers in non-endemic areas often will not think of babesiosis and the diagnosis may first be made by laboratory personnel who recognize Babesia on a thin blood smear that was ordered as part of a complete blood count (CBC) or a peripheral smear requested for workup of malaria. Consequently, health care workers and laboratory personnel must include babesiosis in their differential diagnosis, especially because of the worldwide emergence of the disease and the increase in international travel.
Babesiosis should be considered in any patient experiencing a malaria-like illness, including fever, fatigue, chills, sweats, headache and/or muscle aches. Other symptoms include anorexia, nausea, arthralgia and emotional lability.1,2 There are few findings on physical examination other than fever and occasional splenomegaly and/or hepatomegaly. A thorough travel history is important to obtain in such patients, including travel within any Babesia-endemic areas. It is also important to ask about blood transfusion within the previous 6 months because of the possibility of having received a transfusion of Babesia contaminated blood. Babesia microti is the most common pathogen transmitted by blood transfusion in the USA.1–3 Due to the absence of routine screening of blood for Babesia infection and the transport of blood products from endemic to non-endemic areas, Babesia infection from blood transfusion can occur in both endemic and non-endemic areas.4–6
Useful laboratory screening tests for patients include a CBC that usually shows hemolytic anemia and thrombocytopenia.43 Liver enzymes are often elevated. Of note, thrombocytopenia and elevated transaminases also may be observed during A. phagocytophilum infection. Specific diagnosis is made by identification of Babesia on a Giemsa or Wright-stained thin blood smear, or PCR amplification of Babesia DNA with specific primers. There is a risk of mis-interpretation of intra-erythrocytic microorganisms on blood smear as B. microti or P. falciparum (Figure 2B). Distinguishing features of Babesia include tetrad merozoite forms (‘Maltese Cross’) that are pathognomonic for Babesia and the absence of pigment inclusions and visible gametocytes. Again, a travel history is often useful in distinguishing between babesiosis and malaria. PCR is more sensitive and specific than blood smear so that infections with as few as three parasites per 100µl of blood can yield a positive result.44–47 PCR usually is performed with two specific primers design in the 18S gene such as piroA/piroB,25 as used for our patient or BAB1/BAB4.44 Use of specific PCR primers or DNA sequencing of PCR products is necessary to identify various Babesia species such as B. microti, B. divergens, B. duncani and B. venatorum. Serology with IFA or ELISA is another useful laboratory test that may be positive at presentation because diagnosis is often delayed, giving time for production of antibody. Alternatively, the presence of IgG antibody may be due to past infection. In the case of the R1 patient, the serological analysis for B. microti was positive for IgM and IgG while that for malaria was negative for IgM but positive for IgG, most likely due to cross-reactivity of IgG against P. falciparum antigens. Cross-reactivity between antigens of different hemoparasites has previously been described for Babesia bovis and P. falciparum.48
Babesiosis therapy: Current therapy for the treatment of B. microti-human babesiosis consists primarily of the combination of atovaquone and azithromycin.49,50 Clindamycin and quinine is used for severe disease. This combination is recommended for babesiosis resulting from B. divergens infections which are almost always severe.50 The recommended duration of therapy is 7–10 days in most cases. Clinical improvement is usually observed within 48–72 h. In highly immunocompromised patients with severe disease who do not respond well to a standard duration of therapy, a more extended course of at least 6 weeks (including 2 weeks with repeatedly negative blood smears) is recommended.1,2,51 Such patients include those with malignancy and rituximab treatment (especially B cell lymphoma and asplenia), HIV/AIDS, autoimmune disease treated with rituximab and organ transplantation treated with immunosuppressive agents.51 The risk–benefit ratio of more prolonged therapy must be considered, especially with quinine that can lead to gastrointestinal distress, hearing impairment including tinnitis and/or cardiac impairment.2,49 Intravenous quinine or quinidine treatment may be given in the early phase of severe infection but should only be given in an ICU with cardiac monitoring. Our patient initially had insufficient improvement on quinine monotherapy so that addition of clindamycin to the quinine regimen was used to clear the infection.
The current antibiotics used for treatment of babesiosis generally are effective but newer antimicrobial regimens are needed, especially for severe disease. Although quinine is often used for treatment of severe babesiosis, the side effects associated with this drug, the lack of evidence that B. microti degrades hemoglobin, and the inability of the compound to inhibit growth of the parasite in mice all suggest that its use for babesiosis treatment should be reevaluated. Atovaquone is a useful anti-Babesia drug with fewer side effects. The drug irreversibly binds to the mitochondrial cytochrome bc1 complex and blocks the electron flux in the mitochondrial inner membrane, which plays a central role in parasite physiology. However, resistance alleles have been found in the cytochrome b (cytb) gene on the mitochondrial genome.48 The combination of atovaquone with endochin-like quinolone (ELQ) has shownpromise in preclinical studies52 and awaits clinical evaluation. Azithromycin and clindamycin are suspected to target the ribosome of the apicoplast or the mitochondria, and are used in separate combination therapy regimens. Sensitivity of B. microti to artemisinin has been demonstrated in rodent models but efficacy in humans is still unknown.53
Partial or complete exchange blood transfusion is recommended in patients with high grade parasitemia (≥10%), significant hemolysis, or renal, hepatic or pulmonary compromise. Patients who are susceptible to severe babesiosis include those with asplenia, cancer, organ transplantation, HIV, hemoglobinopathies, those who acquire babesiosis through blood transfusion, are on immunosuppressive drugs, have chronic heart, lung or liver disease, or are neonates or elderly. Mortality rates of many such patients are about 20% even with antibiotic therapy.1,51
Coinfection in tick-borne disease complicates both diagnosis and therapy
Ixodid ticks transmit an array of pathogens including A. phagocytophilum, B. microti, B. burgdorferi, Borrelia miyamotoi, Borrelia mayonii, Ehrlichia muris-like agent, and Powassan virus.54–57 A single tick can carry more than one agent. Accordingly, several reports have shown evidence of coinfection in reservoir hosts and humans. The distribution of these agents varies geographically. Patient travel history and knowledge about tick-borne diseases prevalent in the patient’s place of residence are thus very important and may help better understand the clinical presentation and determine best therapeutic strategies. Coinfection may have a significant impact in natural hosts and in humans. Recent evidence suggests that B. burgdorferi coinfection in the natural reservoir host (Peromyscus leucopus mice) increases B. microti parasitemia and the transmission of B. microti to other mice and to humans.58,59 Lyme disease coinfection may therefore enhance the emergence of babesiosis. Lyme disease patients who are coinfected with either B. microti or A. phagocytophilum experience a greater number of symptoms for longer duration than those with Lyme disease alone.60 Coinfection should be considered in patients with more severe Lyme disease or patients who do not respond well to standard Lyme disease therapy. Finally, gene transfer between coinfecting microbes may occur. Genome sequencing of the B. microti R1 isolate provided evidence for lateral transfer of a Bartonella gene encoding a putative thiamin pyrophosphokinase into the B. microti genome.21 The possible impacts of these genetic events on clinical severity or antibiotic resistance remains unknown.
The diagnosis of Lyme disease and coinfection with babesiosis was considered in the R1 patient because of the detection of B. burgdorferi IgG antibody. The absence of an erythema migrans rash and IgM antibody indicated that the patient did not have acute Lyme disease but had experienced Lyme disease in the past. Simultaneous infection with Babesia and non tick-borne pathogens may occur.
Conclusions
The B. microti R1 parasite isolated from the blood of the patient described in this report sat the stage for the genomic analysis of this parasite, which in turns has helped advance our understanding of the parasite’s biology and pathogenesis and identify new targets for development of more sensitive diagnostic assays and more effective therapies. The diagnosis of babesiosis in non-endemic areas requires knowledge of the disease among health care workers and microbiology laboratory personnel. A travel history or history of blood transfusion within the 6 months preceding clinical evaluation is often important in making a correct diagnosis. Health care workers should consider coinfection with other Ixodes-borne pathogens in any patient with babesiosis. Severe disease may occur in immunocompromised hosts and those who acquire the infection through blood transfusion. Current treatment of babesiosis consists of atovaquone and azithromycin or clindamycin and quinine as an alternative treatment for severe disease. Current therapy for babesiosis is generally effective but new therapies, especially for severe disease are needed.
Abbreviations
- ALT
alanine transaminase
- AST
aspartate transaminase
- CDC
Center for Disease Control
- CSF
cerebrospinal fluid
- HIV
human immunodefiency virus
- ICU
intensive care unit
- LDH
lactate dehydrogenase
- NSAID
non-steroidal anti-inflammatory drug
- PCR
polymerase chain reaction
- TTB
transfusion-transmitted babesiosis
- WB
western blot
Funding
Emmanuel CORNILLOT was supported by the Agence Nationale de la Recherche “Investissements d’avenir/Bioinformatique”: ANR-11-BINF-0002 “Institut de Biologie Computationnelle”. In memoriam of Fabrice Legros, former director of the Centre National de Référence des Maladies d’Importation.
Conflict of interest: None declared.
Contributors
Y.P. and V.C. were the primary physicians and managed the patient. P.P. made the diagnosis of babesiosis. P.S. wrote the initial report. L.B. set up Figure 1. B.E. reviewed initial manuscript and provided data for Figure 2. E.C., C.B.M. and P.J.K. collected all information and wrote the manuscript. All co-authors participated in the writing and editing of the report.
References
- 1. Vannier E, Krause PJ. Human babesiosis. N Engl J Med 2012; 366: 2397–407. [DOI] [PubMed] [Google Scholar]
- 2. Vannier EG, Diuk-Wasser MA, Ben Mamoun C, Krause PJ. Babesiosis. Infect Dis Clin North Am 2015; 29: 357–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Herwaldt BL, Linden JV, Bosserman E et al. . Transfusion-associated babesiosis in the United States: a description of cases. Ann Intern Med 2011; 155: 509–19. [DOI] [PubMed] [Google Scholar]
- 4. Leiby DA. Transfusion-associated babesiosis: shouldn’t we be ticked off? Ann Intern Med 2011; 155: 556–7. [DOI] [PubMed] [Google Scholar]
- 5. Young C, Chawla A, Berardi V et al. . Preventing transfusion-transmitted babesiosis: preliminary experience of the first laboratory-based blood donor screening program. Transfusion 2012; 52: 1523–9. [DOI] [PubMed] [Google Scholar]
- 6. Moritz ED, Winton CS, Tonnetti L et al. . Screening for Babesia microti in the U.S. Blood Supply. N Engl J Med 2016; 375: 2236–45. [DOI] [PubMed] [Google Scholar]
- 7. Humiczewska M, Kuźna-Grygiel W. A case of imported human babesiosis in Poland. Wiad Parazytol 1997; 43: 227–9. [PubMed] [Google Scholar]
- 8. Nohýnková E, Kubek J, Mĕst’ánková O et al. . A case of Babesia microti imported into the Czech Republic from the USA. Cas Lek Cesk 2003; 142: 377–81. [PubMed] [Google Scholar]
- 9. Saito-Ito A, Tsuji M, Wei Q et al. . Transfusion-acquired, autochthonous human babesiosis in Japan: isolation of Babesia microti-like parasites with hu-RBC-SCID mice. J Clin Microbiol 2000; 38: 4511–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Senanayake SN, Paparini A, Latimer M et al. . First report of human babesiosis in Australia. Med J Aust 2012; 196: 350–2. [DOI] [PubMed] [Google Scholar]
- 11. Berens-Riha N, Zechmeister M, Hirzmann J et al. . Babesiose by einem spenektomierten reisenden aus dem USA. FTR 2012; 19: 113–5. [Google Scholar]
- 12. Holler JG, Roser D, Nielsen HV et al. . A case of human babesiosis in Denmark. Travel Med Infect Dis 2013; 11: 324–8. [DOI] [PubMed] [Google Scholar]
- 13. Poisnel E, Ebbo M, Berda-Haddad Y et al. . Babesia microti: an unusual travel-related disease. BMC Infect Dis 2013; 13: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou X, Xia S, Yin SQ, Zhou XN. Emergence of babesiosis in China-Myanmar border areas. Parasit Vectors 2015; 8: 390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bullar JM, Ahsanuddin AN, Perry AM et al. . The first case of locally acquired tick-borne Babesia microti infection in Canada. Can J Infect Dis Med Microbiol 2014; 25: e87–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Merino A. Blood film findings in severe babesiosis. Br J Haematol 2016; 172: 839. [DOI] [PubMed] [Google Scholar]
- 17. Al Zoubi M, Kwak T, Patel J, Kulkarni M, Kallal CA. Atypical challenging and first case report of babesiosis in Ecuador. IDCases 2016; 4: 15–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Arsuaga M, Gonzalez LM, Lobo CA et al. . First report of Babesia microti-caused Babesiosis in Spain. Vector Borne Zoonotic Dis 2016; 16: 677–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Institute of Medicine of the National Academies Committee on Lyme disease and other tick-borne diseases. Critical needs and gaps in understanding. Prevention, amelioration, and resolution of Lyme and other tick-borne diseases. The Short-term and long-term outcomes. Washington, DC: The National Academies Press, 2011. http://www.nap.edu. [PubMed] [Google Scholar]
- 20. CDC Nationally Notifiable Infectious Conditions, United States 2011, Babesiosis, 2011.
- 21. Cornillot E, Hadj-Kaddour K, Dassouli A et al. . Sequencing of the smallest Apicomplexan genome from the human pathogen Babesia microti. Nucleic Acids Res 2012; 40: 9102–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cornillot E, Dassouli A, Garg A et al. . Whole genome mapping and re-organization of the nuclear and mitochondrial genomes of Babesia microti isolates. PLoS One 2013; 8: e72657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Garg A, Stein A, Zhao W et al. . Sequence and annotation of the apicoplast genome of the human pathogen Babesia microti. PLoS POne 2014; 9: e107939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cornillot E, Dassouli A, Pachikara N et al. . A targeted immunomic approach identifies diagnostic antigens in the human pathogen Babesia microti. Transfusion 2016; 56: 2085–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Olmeda AS, Armstrong PM, Rosenthal BM et al. . A subtropical case of human babesiosis. Acta Trop 1997; 67: 229–34. [DOI] [PubMed] [Google Scholar]
- 26. Lawres LA, Garg A, Kumar V et al. . Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J Exp Med 2016; 213: 1307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Baneth G, Florin-Christensen M, Cardoso L, Schnittger L. Reclassification of Theileria annae as Babesia vulpes sp. nov. Parasit Vectors 2015; 8: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lemieux JE, Tran AD, Freimark L et al. . A global map of genetic diversity in Babesia microti reveals strong population structure and identifies variants associated with clinical relapse. Nat Microbiol 2016; 1: 16079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Carpi G, Walter KS, Mamoun CB et al. . Babesia microti from humans and ticks hold a genomic signature of strong population structure in the United States. BMC Genomics 2016; 17: 888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silva JC, Cornillot E, McCracken C et al. . Genome-wide diversity and gene expression profiling of Babesia microti isolates identify polymorphic genes that mediate host-pathogen interactions. Sci Rep 2016; 6: 35284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Zhou X, Huang J, Tambo E et al. . Human babesiosis, an emerging tick-borne disease in the People’s Republic of China. Parasit Vectors 2014; 7: 509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hunfeld K-P, Lambert A, Kampen H et al. . Seroprevalence of Babesia infection in human exposed to ticks in midwestern Germany. J Clin Microbiol 2002; 40: 2431–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Foppa IM, Krause PJ, Spielman A et al. . Entomologic and serologic evidence of zoonotic transmission of Babesia microti, eastern Switzerland. Emerg Infect Dis 2002; 8: 722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Genchi C. Human babesiosis, an emerging zoonosis. Parassitologia 2007; 49: 29. [PubMed] [Google Scholar]
- 35. Lempereur L, Shiels B, Heyman P et al. . A retrospective serological survey on human babesiosis in Belgium. Clin Microbiol Infect 2015; 21: 96.e1. [DOI] [PubMed] [Google Scholar]
- 36. Moniuszko-Malinowska A, Swiecicka I, Dunaj J et al. . Infection with Babesia microti in humans with non-specific symptoms in North East Poland. Infect Dis (Lond) 2016; 48: 537–43. [DOI] [PubMed] [Google Scholar]
- 37. Hildebrandt A, Hunfeld KP, Baier M et al. . First confirmed autochthonous case of human Babesia microti infection in Europe. Eur J Clin Microbiol Infect Dis 2007; 26: 595–601. [DOI] [PubMed] [Google Scholar]
- 38. Zhou X, Li SG, Chen SB et al. . Co-infections with Babesia microti and Plasmodium parasites along the China-Myanmar border. Infect Dis Poverty 2013; 2: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hong SH, Anu D, Jeong YI et al. . Molecular detection and seroprevalence of Babesia microti among stock farmers in Khutul City, Selenge Province, Mongolia. Korean J Parasitol 2014; 52: 443–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Gabrielli S, Totino V, Macchioni F et al. . Human Babesiosis, Bolivia, 2013. Emerg Infect Dis 2016; 22: 1445–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jiang JF, Zheng YC, Jiang RR et al. . Epidemiological, clinical, and laboratory characteristics of 48 cases of ‘Babesia venatorum’ infection in China: a descriptive study. Lancet Infect Dis 2015; 15: 196–203. [DOI] [PubMed] [Google Scholar]
- 42. Warren T, Lau R, Ralevski F et al. . Fever in a visitor to Canada: a case of mistaken identity. J Clin Microbiol 2015; 53: 1783–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Krause PJ. Babesiosis diagnosis and treatment. Vector Borne Zoonotic Dis 2003; 3: 45–51. [DOI] [PubMed] [Google Scholar]
- 44. Persing DH, Mathiesen D, Marshall WF et al. . Detection of Babesia microti by polymerase chain reaction. J Clin Microbiol 1992; 30: 2097–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Armstrong PM, Katavolos P, Caporale DA et al. . Diversity of Babesia infecting deer ticks (Ixodes dammini). Am J Trop Med Hyg 1998; 58: 739–42. [DOI] [PubMed] [Google Scholar]
- 46. Rollend L, Bent SJ, Krause PJ et al. . Quantitative PCR for detection of Babesia microti in Ixodes scapularis ticks and in human blood. Vector Borne Zoonotic Dis 2013; 13: 784–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wilson M, Glaser KC, Adams-Fish D et al. . Development of droplet digital PCR for the detection of Babesia microti and Babesia duncani. Exp Parasitol 2015; 149: 24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. James MA, Montenegro-James S, Fajfar-Whetstone C et al. . Antigenic relationship between Plasmodium falciparum and Babesia bovis: reactivity with antibodies to culture-derived soluble exoantigens. J Protozool 1987; 34: 328–32. [DOI] [PubMed] [Google Scholar]
- 49. Krause PJ, Lepore T, Sikand VK et al. . Atovaquone and azithromycin for the treatment of babesiosis. N Engl J Med 2000; 343: 1454–8. [DOI] [PubMed] [Google Scholar]
- 50. Kletsova EA, Spitzer ED, Fries BC, Marcos LA. Babesiosis in Long Island: review of 62 cases focusing on treatment with azithromycin and atovaquone. Ann Clin Microbiol Antimicrob 2017; 16: 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Krause PJ, Gewurz BE, Hill D et al. . Persistent and relapsing babesiosis in immunocompromised patients. Clin Infect Dis 2008; 46: 370–6. [DOI] [PubMed] [Google Scholar]
- 52. Lawres LA, Garg A, Kumar V et al. . Radical cure of experimental babesiosis in immunodeficient mice using a combination of an endochin-like quinolone and atovaquone. J Exp Med 2016; 213: 1307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Goo YK, Terkawi MA, Jia H et al. . Artesunate, a potential drug for treatment of Babesia infection. Parasitol Int 2010; 59: 481–6. [DOI] [PubMed] [Google Scholar]
- 54. Diuk-Wasser MA, Vannier E, Krause PJ. Coinfection by Ixodes tick-borne pathogens: ecological, epidemiological, and clinical consequences. Trends parasitol 2016; 32: 30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Nelder MP, Russell CB, Sheehan NJ et al. . Human pathogens associated with the blacklegged tick Ixodes scapularis: a systematic review. Parasit Vectors 2016; 9: 265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Saito-Ito A, Kasahara M, Kasai M et al. . Survey of Babesia microti infection in field rodents in Japan: records of the Kobe-type in new foci and findings of a new type related to the Otsu-type. Microbiol Immunol 2007; 51: 15–24. [DOI] [PubMed] [Google Scholar]
- 57. Yabsley MJ, Shock BC. Natural history of Zoonotic Babesia: role of wildlife reservoirs. Int J Parasitol Parasites Wildl 2013; 2: 18–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Dunn JM, Krause PJ, Davis S et al. . Borrelia burgdorferi promotes the establishment of Babesia microti in the northeastern United States. PloS One 2014; 9: e115494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Hersh MH, Ostfeld RS, McHenry DJ et al. . Co-infection of blacklegged ticks with Babesia microti and Borrelia burgdorferi is higher than expected and acquired from small mammal hosts. PloS One 2014; 9: e99348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Knapp KL, Rice NA. Human coinfection with Borrelia burgdorferi and Babesia microti in the United States. J Parasitol Res 2015; 2015: 587131. [DOI] [PMC free article] [PubMed] [Google Scholar]